Abstract
Seawater pH lowering, known as ocean acidification, is considered among the major threats to marine environment. In this study, post-spawning adults of the sea urchin Paracentrotus lividus were maintained at three pH values (8.0, 7.7, 7.4) for 60 days. Physiological, biochemical, cellular, behavioural and reproductive responses were evaluated in males and females. Significant differences between sexes were observed, with higher ammonia excretion and lower catalase activity in males. Respiration rate (after 21 days), catalase activity in gonads and total coelomocyte count showed the same increasing trend in males and females under low pH. Ammonia excretion, gonadosomatic index and lysozyme activity exhibited opposite responses to low pH, with an increasing trend in males and decreasing in females. Results demonstrated that exposure to low pH could result in different response strategies of male and female sea urchins at a physiological, biochemical and immunological level. Reduced female gonadosomatic index under low pH suggested decreased energy investment in reproduction.
Electronic supplementary material
The online version of this article (10.1007/s11356-020-10040-7) contains supplementary material, which is available to authorized users.
Keywords: Sea urchins, Ocean acidification, Sex, Biomarkers, Physiological parameters, Gonadosomatic index, Righting time
Introduction
Ocean acidification (OA) is a phenomenon of lowering seawater pH, due to the dissolution of rising atmospheric CO2. Alterations of atmospheric gas composition observed during the last two centuries are mainly originated from anthropogenic activities, first from all fossil fuels’ combustion. Since pre-industrial time, ocean surface pH has decreased by approximately 0.1 units (IPCC 2013). The present average pH value for shallow and surface seawaters is 8.1, and predicted global surface pH reduction is of 0.06–0.32 units by the year 2100 and 0.7 units by 2300 (Hartin et al. 2016; IPCC 2019).
Responses to OA represent a species-specific phenomenon, and the effects detected in laboratory experiments are dependent on geographic area and life-history stages of the studied species (Hall-Spencer et al. 2015), as well as on the duration of the experimental exposure (Suckling et al. 2015). There are evidence that OA can differentially affect physiology, reproduction, biochemistry and survival of marine invertebrates (McClellan-Green et al. 2007; Ellis et al. 2014; Lane et al. 2015). When exposed to OA, organisms may change their resource energy allocation and due to higher production cost of eggs compared to sperm, females could be deemed more vulnerable to this stressor. Notwithstanding, Ellis et al. (2017) reported in their review that only 3.77% of 511 OA studies published between January 2008 and May 2016 tested gender-related responses in fish, crustaceans, echinoderms and molluscs. When tested, sex significantly influenced the response to OA, suggesting that sex has to be considered in order to correctly evaluate the impact at the population level (Ellis et al. 2017).
Among aquatic species, echinoderms are considered as model organisms to assess the effects of changing environmental conditions. Echinoderms include exclusively marine species, such as sea urchins, which build calcareous skeleton in both larval and adult phase. Skeletal rods in larvae and test, teeth and spines in adults are formed from magnesium calcite that is one of the most soluble forms of calcium carbonate (Morse et al. 2006). In long-term experimental exposures to OA, sea urchins revealed mostly negative but sub-lethal effects, with diminished calcification as principal impairment in both adult and larval stages. Although sea urchin species inhabiting low pH environments such as upwelling regions, intertidal pools, and CO2 vents exhibited great potential to adapt to OA, in about 20 species of echinoplutei from several world regions and habitats, reduction in growth and increased alteration of body morphology were observed (Byrne and Hernández 2020). Long-lasting experiments can allow us to inspect organisms’ capability to acclimate to low pH and to produce more relevant data for predicting long-term consequences of OA. It was demonstrated that short-term exposures to low pH values could lead to hypercapnic conditions in sea urchin coelomic fluid (Miles et al. 2007; Spicer et al. 2011; Dupont and Thorndyke 2012; Spicer and Widdicombe 2012; Stumpp et al. 2012; Holtmann et al. 2013; Kurihara et al. 2013). This effect could represent a “shock” response (Byrne 2012; Queirós et al. 2015) possibly related to both the absence of respiratory pigments and low capability of sea urchins to regulate ions. Generally, in most species, with few exceptions (Kurihara et al. 2013), the acid-base balance is recovered in some days or weeks (Calosi et al. 2013; Dupont and Thorndyke 2012; Stumpp et al. 2012; Holtman et al. 2013; Moulin et al. 2014). Experiments on Paracentrotus lividus (from 6 days to 2 months long) showed an increased coelomic fluid buffer capacity under low pH. However, in this species, coelomic fluid pH seems to be only partially compensated at extreme pH condition (7.4 pH). This compensation of the coelomic fluid pH in P. lividus was not dependent on skeleton dissolution, indeed skeletal mechanical properties were not affected at 7.7 pH (Catarino et al. 2012; Collard et al. 2013, 2014, 2016; Cohen-Rengifo et al. 2019). The acid-base regulation capability allows sea urchins to maintain appropriate extracellular and intracellular pH conditions, but the processes involved in this regulation are energy-consuming and lead to increased oxygen uptake. Therefore, other processes such as growth, reproduction and behaviour could be compromised if energy acquisition is not increased (Dupont et al. 2013).
It is supposed that increased levels of CO2 cause oxidative stress directly by increasing the production of ROS and/or indirectly by lowering internal pH, which may induce the release of chelated transition metals such as Fe2+ from intracellular compartments and enhance the Fenton reaction (Tomanek et al. 2011). Induction of oxidative stress by low pH has been scarcely investigated in marine echinoderms (Migliaccio et al. 2019), but it was evaluated and detected in other taxa, such as marine bivalves (Tomanek et al. 2011; Matozzo et al. 2013; Benedetti et al. 2016; Velez et al. 2016; Freitas et al. 2017a; Nardi et al. 2017; Sui et al. 2017; Huang et al. 2018; Munari et al. 2018), crustaceans (Priya et al. 2017; Rato et al. 2017; Glippa et al. 2018), polychaetes (Freitas et al. 2017b), gastropods (Cardoso et al. 2017), corals (Soriano-Santiago et al. 2013) and fish larvae (Pimentel et al. 2015). In all these studies, the exposure to OA conditions lasted from at least 72 h to a maximum of 1 month. For the sea urchin P. lividus, seasonal changes in biomarker responses were observed in specimens from the Gulf of Annaba (Algeria), with an increase in antioxidant enzymes’ activity during the reproductive period (spring), suggesting that for this species natural physiological cycle as well as biotic and abiotic factors could influence oxidative stress responses (Amri et al. 2017).
As demonstrated in Lytechinus variegatus, Echinometra lucunter and Strongylocentrotus droebachiensis, ocean acidification affects adult sea urchin immune system after a short-term exposure (from 24 h to 7 days). Alterations of immunological parameters seem to be mainly linked to pH decrease in coelomic liquid and appeared reversible when natural values were re-established (Dupont and Thorndyke 2012; Leite Figueiredo et al. 2016).
Effects of OA on sea urchin fecundity was evaluated mainly by measurement of gonadic mass production (Siikavuopio et al. 2007; Stumpp et al. 2012; Kurihara et al. 2013; Taylor et al. 2014; Uthicke et al. 2014; Mos et al. 2016; Dworjanyn and Byrne 2018), as well as by determination of egg number and size, gamete performance or RNA/DNA ratio in gonads (Catarino et al. 2012; Cohen-Rengifo et al. 2013; Dupont et al. 2013; Uthicke et al. 2013; Suckling et al. 2014; Graham et al. 2015; Campbell et al. 2016; Karelitz et al. 2020).
In the present study, adults of the sea urchin P. lividus were used as model organism. This species is widely distributed in the Mediterranean and in the north-eastern Atlantic (Boudouresque and Verlaque 2013), where it plays a dominant role as a grazer and acts as a keystone species in controlling dynamic, structure and composition of infralittoral macroalgal assemblages (Hereu 2006; Privitera et al. 2008; Boudouresque and Verlaque 2013). During a 60-day experiment, post-spawning adults of P. lividus were exposed to control pH (8.0) and to two reduced pH values, 7.7 and 7.4, according to projections for shallow and surface seawaters by 2100 and 2300, respectively (Hartin et al. 2016; IPCC 2019). A number of physiological, biochemical, cellular, behavioural and reproductive responses were investigated in both males and females. The hypotheses we tested were as follows: (i) OA affects the biological responses measured in P. lividus; (ii) males and females respond differently to OA.
Materials and methods
Specimen collection
About 200 adult specimens of P. lividus, with a live weight of 36.7 ± 11.4 g and a test diameter of 4.5 ± 0.5 cm, were collected by SCUBA divers at approximately 5 m depth in the southern basin of the Venice Lagoon (NW Adriatic Sea, Italy) between February and March 2017. The sampling area is close to the southern inlet of the lagoon where pollution levels are generally low (Parolini et al. 2010; Parolini et al. 2012; Cassin et al. 2018; Zonta et al. 2018). In the laboratory, animals were acclimated in flow-through aquaria for 2 weeks at least, at 18 ± 0.5 °C temperature and 34 ± 1 salinity, and fed with Ulva sp. In order to recognize male and female sea urchins and to obtain individuals in the same very early stage of gametogenesis, all specimens were induced to spawn, by injecting 0.5 ml of 0.5 M KCl solution into the coelom, through the peristome membrane (Gago and Luís 2011). After that, males and females were maintained in separate aquaria and allowed to recover for a week in the previously reported conditions. Prior to exposure, the sea urchins were acclimatized to the experimental conditions by gradually reducing the natural pH values by about − 0.3 or − 0.6 units (approximately 0.1 reduction per day).
Experimental sea urchin culture system
Post-spawning adults of P. lividus were kept under three pH values: 8.0, 7.7 and 7.4. Each experimental condition was assessed in triplicate 60 l tanks, each containing at least six males and nine females separated by a plastic grid. Tanks were supplied with filtered seawater (5 μm) at a flow rate of 300 ml min−1 and were equipped with an aerator. In each individual low pH (7.7 and 7.4) tank, the pH value was maintained by bubbling CO2 using an electronic control system (Aquarium Controller Evolution, mod. ACQ110, Aquatronica, Italy) connected to a pH electrode (ACQ310N-pH by Aquatronica, Italy). To verify and adjust pH electrode measurements, in each experimental tank pH value was checked twice a day at least using a benchtop pH-meter Basic 20+ (Crison, Spain) calibrated daily with Crison buffer solutions.
In order to promote the gonadal maturation, sea urchins were fed ad libitum with fresh Ulva sp., and maintained at 18 °C temperature (Grosjean et al. 1998), and 9 h light: 15 h dark photoperiod (Spirlet et al. 2000). Salinity values were in the same range measured during the acclimation period. Throughout the experiment, no spontaneous spawning event was observed.
At each experimental condition, sea urchins were randomly sampled within the tanks and physiological effects were evaluated after 7, 14, 21 and 40 days through measurements of respiration rate, ammonia production rate and assimilation rate. Due to some technical inconveniences, data were not collected at day 40 for ammonia production and at days 21 and 40 for assimilation. For each parameter measured, four males and four females per experimental condition were used. After 40 days, righting time (see below for details) was also measured on six males and six females per experimental condition. Physiological and behavioural measurements were performed at the same pH, temperature and salinity used during exposure. After 60 days of exposure to differing pH values, six males and six females were used to evaluate gonadosomatic index, superoxide dismutase (SOD) and catalase (CAT) activity in gonads and digestive tract, coelomocyte number and volume (TCC and CV respectively) and lysozyme activity in coelomic fluid and coelomocytes.
Seawater chemistry
On days 30, 50 and 60, seawater samples from each experimental condition were collected into 250 ml polypropylene bottles. In order to halt the biological activity, samples were immediately poisoned with 100 μl of saturated mercuric chloride solution (HgCl2) and were stored at 4 °C in the dark until analysis.
Total alkalinity (TA) was determined via potentiometric titration using an automatic titrator (836 Titrando, Metrohom). Each seawater sample was thermostated at 25 °C (HAAKE C25P Phoenix II, ENCO) before titration. A synthetic alkalinity standard was prepared following the method of Dickson et al. (2003) and used as a reference. The estimate of the extended uncertainty interval was 4.9% with respect to the TA value of the standard. All TA values obtained with the synthetic alkalinity standard in the analysis of the real samples were compliant. The 4.3% of the titrations of the seawater samples were repeated more than two times to meet the relative precision specifications of 2% obtained in the validation of the TA standard.
The dissolved inorganic carbon (DIC) content, carbonate concentration values and CO2 partial pressure (pCO2) in seawater were computed at the sampling temperature, salinity and pHT using the TA values. All thermodynamic equilibrium constants were computed according to Millero (1979), Millero (1995) and Millero et al. (2006). The solubility values of calcite and aragonite were obtained from Mucci (1983) and from Ingle (1975). The saturation states of calcite (Ωca) and aragonite (Ωar) were computed based on the solubility products reported in Millero (1979). Results are reported in Table 1.
Table 1.
Seawater carbonate chemistry variables (mean values ± SD; n = 9) recorded during the experiment
| Tanks | pHT | DIC (μmol kg−1) | TA (μmol kg−1) | pCO2 (μ Atm) | ΩCa | ΩAr |
|---|---|---|---|---|---|---|
| 8.0 pH | 8.02 ± 0.03 | 2564.97 ± 110.81 | 2772.47 ± 120.47 | 544.02 ± 59.42 | 5.65 ± 0.12 | 3.68 ± 0.09 |
| 7.7 pH | 7.69 ± 0.02 | 2703.25 ± 25.32 | 2780.86 ± 27.43 | 1263.29 ± 41.99 | 2.95 ± 0.17 | 1.92 ± 0.11 |
| 7.4 pH | 7.38 ± 0.02 | 2783 ± 43.71 | 2757.13 ± 42.10 | 3643.17 ± 88.35 | 1.47 ± 0.05 | 0.95 ± 0.03 |
TA, total alkalinity; DIC, total dissolved inorganic carbon; pCO2, CO2 partial pressure; Ωca, calcite saturation state; Ωar, aragonite saturation state
Respiration rate
Since feeding increases the buffer capacity and non-fed individuals can undergo severe metabolic acidosis (Stumpp et al. 2012; Collard et al. 2013), sea urchins were not fasted before respiration measurements to reproduce the environmental conditions more faithfully and to avoid additional stress. To measure oxygen consumption, individual sea urchins were placed in 0.8 l plexiglas respirometry chambers provided with a magnetic stirrer bar placed under a perforated plate. Chambers were filled with air-saturated 0.45 μm-filtered seawater at each pH studied and placed on the multi-position magnetic stirrer. During measurements, the chambers were kept at 18 °C using a thermostatic bath. For each batch, a chamber without sea urchin was used as a control. Oxygen concentration was measured after 0, 30, 60 and 90 min using an optical oxygen meter (fibre-optic oxygen meter‑Piccolo2, Pyro Science GmbH, Aachen, Germany). Oxygen saturation never fell below 70% during the trial. Oxygen uptake (μmolO2 h−1 g−1) was calculated by multiplying the slope of the oxygen depletion curve by the volume of seawater inside the chamber and dividing by the live sea urchin weight. The volume of water was determined by subtracting the volume of each sea urchin from the total volume in the chamber.
Ammonia excretion
Ammonia excretion (μmolN-NH3 h−1 g−1) was measured in water samples collected from each respirometry chamber after 90 min. Ammonia was determined spectrophotometrically according to the method of Solorzano (1969). Ammonia excretion was calculated from the difference in ammonia concentration between the chambers with and without animals and referred to sea urchin live weight.
Assimilation efficiency
To measure assimilation efficiency, sea urchins were placed individually in 2.5-l beakers filled with filtered seawater (0.45 μm) for 24 h and during this period they were not fed. Faeces from each beaker were drawn off, filtered on pre-ashed and weighed glass fibre filters (Whatman GFC), and rinsed with distilled water to remove the salt. Filters were then dried in an oven at 60 °C, weighed after 24 h, ashed in a muffle furnace at 450 °C for 4 h and re-weighed (Conover 1966; Reid et al. 2010). Weight determinations were performed using a Mettler Toledo, XS105 Dual Range analytical balance (0.01 mg readability). The same procedure was applied in triplicate on diet samples. In faeces and algae, organic content (OC) was calculated as ash-free dry weight obtained as the difference between dry and ash weight. Absorption efficiency (AE) was determined according to Conover (1966): AE = [(DietOC − FaecesOC) / (1 − FaecesOC) × DietOC] × 100, where DietOC and FaecesOC are the organic fractions in algae and in faeces, respectively.
Superoxide dismutase and catalase activity
Gonads and digestive tract were dissected from six males and six females and aliquots of the individual tissues were placed in tubes and immediately frozen in liquid nitrogen and stored at − 80 °C until analysis. The gonads and digestive tract were thawed on ice and homogenized (1:4, w:v) in 0.1 M Tris–HCl buffer (pH 7.5) containing 0.15 M KCl, 0.5 M sucrose, 1 mM EDTA and 1 mM dithiothreitol (DTT, Sigma). Homogenates were centrifuged at 12,000g for 45 min at 4 °C and supernatants (SN) were collected to measure antioxidant enzyme activities. To this aim, widely validated methods in sea urchins were used in this study (Zuo et al. 2018; Klein et al. 2019; Zapata-Vivenes and Aparicio 2019).
Total SOD activity was measured in SN of gonads and digestive tract with the xanthine oxidase/cytochrome C method in accordance with Crapo et al. (1978). The cytochrome C reduction by superoxide anion generated by xanthine oxidase/hypoxanthine reaction was detected using a Beckman Coulter (DU® Series 730) spectrophotometer at 550 nm at room temperature (20 °C). Enzyme activity was expressed as U mg−1 of protein, 1 unit of SOD is defined as the amount of sample producing 50% inhibition of cytochrome C reduction in the assay conditions. The reaction mixture contained 46.5 μM KH2PO4/K2HPO4 (pH 8.6), 0.1 mM EDTA, 195 μM hypoxanthine, 16 μM cytochrome c and 2.5 μU xanthine oxidase.
CAT activity was measured in tissue SN following the method described in Aebi (1984). Decreases in absorbance of a 50-mM H2O2 solution (Ɛ = − 0.0436 mM−1 cm−1) in 50 mM phosphate buffer (pH 7.8) and 10 μl of tissue SN were continuously recorded at 240 nm and at 10-s intervals for 1 min. The results were expressed as U mg−1 of protein, 1 unit of CAT being defined as the amount of enzyme that catalyses the dismutation of 1 μmol of H2O2 min−1.
For both SOD and CAT assays, SN protein concentrations were quantified in accordance with Bradford (1976).
Coelomic fluid collection
Two millitres of coelomic fluid were collected from the peristomial membrane of each animal, with a plastic syringe, and stored in ice. Coelomic fluid (1 ml) was used to determine the total coelomocyte count (TCC) and coelomocyte volume (CV), while 1 ml was used to measure both lysozyme activity and total protein concentration in coelomocyte lysate (CL) and cell-free coelomic fluid (CFC).
Total coelomocyte count and coelomocyte volume
A Coulter counter (Z2 mod., Beckman Coulter) was used to determine TCC and CV after adding 1 ml of coelomic fluid to 19 ml of 0.45 μm-filtered seawater. TCC results were expressed as the number of coelomocytes (× 106) ml coelomic fluid−1. The haemocyte volume was expressed in femtolitres (fl).
Lysozyme activity
Lysozyme activity was quantified in both CFC and CL, according to Santarém et al. (1994) and Fernández-Boo et al. (2018). Coelomic fluid from each sea urchin was centrifuged at 780g for 10 min. The supernatant, corresponding to CFC, was collected, whereas the coelomocytes were resuspended in distilled water and sonicated at 4 °C for 1 min to obtain CL. CL and CFC were frozen and stored at − 80 °C before analyses. Fifty microlitres of CL and CFC were added to 950 μl of a 0.15% suspension of Micrococcus lysodeikticus (Sigma) in 66 mM phosphate buffer, pH 6.2; and the decrease in absorbance (ΔA min−1) was continuously recorded at 450 nm for 5 min at room temperature. Standard solutions containing 1, 2.5, 5 and 10 μg lysozyme per ml of 66 mM phosphate buffer, pH 6.2, were prepared from crystalline hen egg-white lysozyme (Sigma). The average decrease in absorbance per minute was determined for each enzyme solution, and a standard curve of enzyme concentration versus ΔA min−1 was drawn. Results were expressed as μg lysozyme mg protein−1. CL and CFC protein concentrations were also quantified according to Bradford (1976).
Righting time
Each animal was tested three times in water from its exposure tank. At the beginning of the trial, the sea urchin was placed on its aboral surface and the time used by each animal to right itself completely was recorded. The test was carried out using a 5 l plastic rectangular container with a smooth surface and large enough to avoid contact between vertical walls and the animal. After the first experimental trial, water was completely removed from the container in order to detach the sea urchin without disturbing it. Each individual was maintained in its experimental tank for 1 h before repeating the measurement.
Gonadosomatic index
Live sea urchins were weighed using a digital balance (± 0.01 g) and dissected to obtain the gonads. Dissected gonads were weighed and GSI was calculated as the percentage of fresh weight of gonads respect to the live weight of the animal.
Statistical analysis
For all the parameters considered, significant effects due to pH, sex and pH*sex interaction were assessed by linear mixed models with a tank as a random effect and followed by the Tukey post-hoc correction. The threshold for significance was set at p < 0.05.
Lastly, a canonical correlation analysis (CCA) was performed using a data matrix made up on all biomarkers’ measurements (i.e., respiration rate, SOD and CAT activity in gonads and digestive tract, total coelomocyte number and volume, lysozyme activity in CL and CFC, righting time and gonadosomatic index) detected in males and females after at least 40 days of exposure. The set of variables included are pH and gender vs the measured physiological, cellular and biochemical parameters. All statistical analyses were performed using package R (R Core Team 2019, Austria) with the CCA package (González and Déjean 2012) and r41sqrt10 package (Finos 2020).
Results
Detailed statistical results for all biomarkers measured are shown in the Supplementary material.
Physiological parameters
No effects of the experimental conditions tested were recorded in respiration rate after 7 and 40 days of exposure (Fig. 1a, Table 2). After 14 days, this parameter was significantly affected by gender and showed higher values in males compared with females. A significant effect of pH was recorded after 21 days of exposure, with a significant increase in respiration rate at 7.4 pH respect to 7.7 pH in both males and females.
Fig. 1.
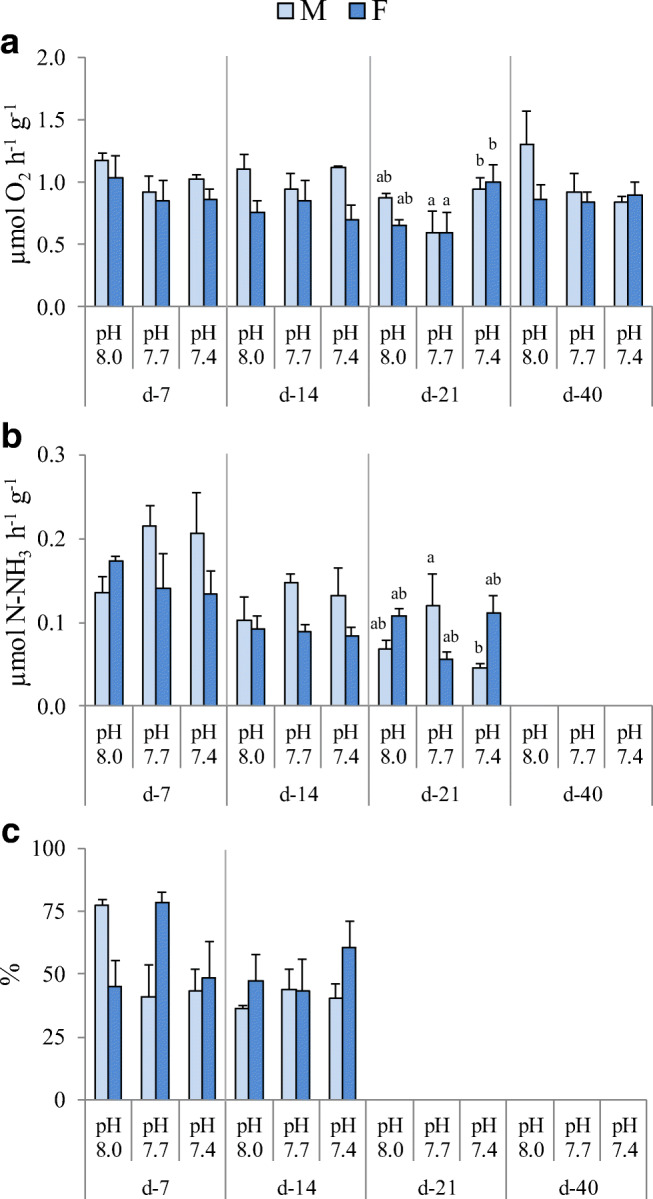
Respiration rate (a), ammonia excretion (b) and assimilation efficiency (c) of P. lividus males (M) and females (F) after 7, 14, 21 and 40-day exposure to 8.0, 7.7 and 7.4 pH. Values are the means ± SD (n = 3). Significant differences among the various experimental conditions are presented with lower case letters (a, b). Due to some technical inconveniences, data are not available at day 40 for ammonia production and at days 21 and 40 for assimilation
Table 2.
Linear mixed model results for respiration rate, ammonia production and assimilation efficiency in P. lividus, after 7, 14, 21 and 40 days of exposure to 8.0, 7.7 and 7.4 pH. Due to some technical inconveniences, data are not available at day 40 for ammonia production and at days 21 and 40 for assimilation. Significant effects are in italics
| Factor | d-7 | d-14 | d-21 | d-40 | ||
|---|---|---|---|---|---|---|
| Respiration rate | pH | p | 0.341 | 0.892 | 0.000 | 0.182 |
| sex | p | 0.336 | 0.005 | 0.386 | 0.214 | |
| pH*sex | p | 0.914 | 0.256 | 0.350 | 0.268 | |
| Ammonia production | pH | p | 0.855 | 0.806 | 0.799 | |
| sex | p | 0.353 | 0.043 | 0.354 | ||
| pH*sex | p | 0.303 | 0.677 | 0.001 | ||
| Assimilation efficiency | pH | p | 0.322 | 0.660 | ||
| sex | p | 0.689 | 0.220 | |||
| pH*sex | p | 0.008 | 0.593 |
No effects of the variables considered on ammonia excretion were observed at 7 days (Table 2). As in respiration rate, a significant effect of sex was observed after 14 days, with higher values in males than in females. After 21 days, ammonia excretion pH*sex interaction was significant. In details, a marked decrease in ammonia excretion was recorded in males kept at 7.4 pH, whereas the same was observed in females maintained at 7.7 pH (Fig. 1b). A significant effect of interaction between pH and sex on assimilation efficiency was detected after 7 days of exposure (Table 2). Under low pH values, this parameter showed decreased values in males, while an increase was observed in females, mostly at 7.7 pH (Fig. 1c).
Antioxidant enzyme activities
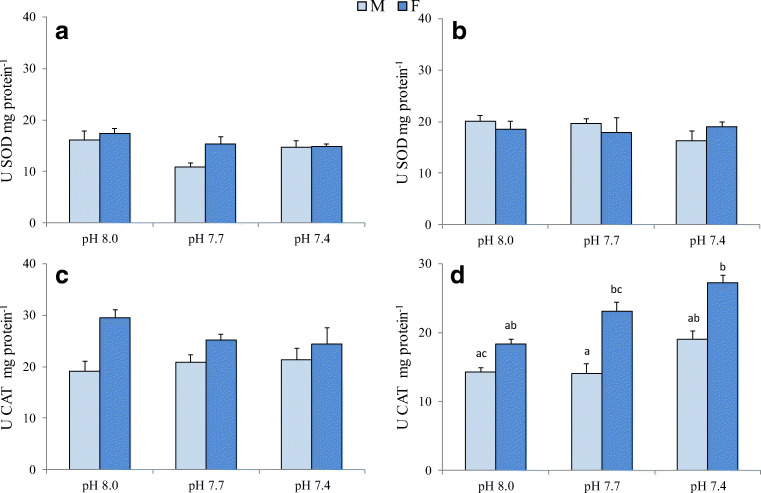
In both gonads and digestive tract, pH, sex and their interaction did not affect significantly SOD activity (Fig. 2a, b, Table 3). Conversely, CAT activity in gonads was significantly affected by sex (Table 3), showing higher values in females than in males (Fig. 2c). In the digestive tract, both pH and sex influenced significantly CAT activity (Table 3). In these tissues, an increasing trend in CAT activity was observed at low pH with higher values in females. In particular, the activity was significantly higher in females at 7.4 pH with respect to males at 8.0 and 7.7 pH (Fig. 2d).
Fig. 2.
SOD and CAT activity in gonads (a, c) and digestive tract (b, d) of P. lividus males (M) and females (F) after 60-day exposure at 8.0, 7.7 and 7.4 pH. Values are the means ± SD (n = 3). Significant differences among the various experimental conditions are presented with lower case letters (a, b)
Table 3.
Linear mixed model results for superoxide dismutase (SOD) and catalase (CAT) activities in gonads and digestive tract, total coelomocyte count (TCC) and coelomocyte volume (CV), lysozyme activity (Lyso) in cell-free coelomic fluid (CFC) and coelomocytes (CL), righting time (RT) and gonadosomatic index (GSI) in P. lividus. Significant effects are in italics
| SOD | CAT | Lyso | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Factor | Gonad | Dig. tract | Gonad | Dig. tract | TCC | CV | CFC | CL | RT | GSI | |
| pH | p | 0.159 | 0.637 | 0.887 | 0.011 | 0.011 | 0.801 | 0.110 | 0.511 | 0.256 | 0.429 |
| sex | p | 0.198 | 0.902 | 0.017 | 0.00 | 0.381 | 0.465 | 0.459 | 0.884 | 0.375 | 0.232 |
| pH*sex | p | 0.534 | 0.363 | 0.440 | 0.506 | 0.239 | 0.588 | 0.002 | 0.034 | 0.361 | 0.002 |
Coelomocyte and coelomic fluid parameters
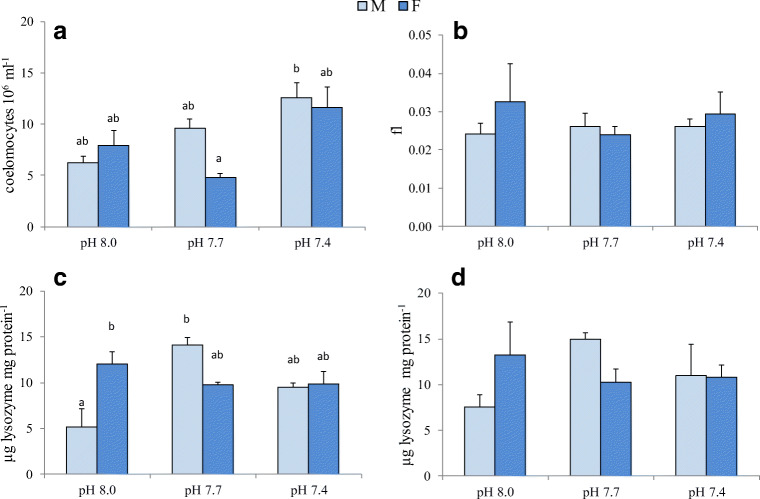
TCC was significantly affected by pH (Table 3). Higher TCC values were observed at 7.4 pH in both sexes, in particular males at 7.4 pH showed significantly higher coelomocyte number compared to females at 7.7 pH (Fig. 3a). On the contrary, CV values were not significantly influenced by the experimental conditions tested (Fig. 3b, Table 3). In both CFC and CL, lysozyme activity was significantly affected by pH*sex interaction (Table 3). Interestingly, lysozyme activity showed the same pattern of variation in CFC and CL (Fig. 3c, d). At 8.0 pH, females showed significantly higher CFC lysozyme activity compared to males. No pH-induced variations in CFC lysozyme activity were observed in females, whereas in males a significant increase of the enzyme activity was observed at 7.7 pH respect to control (Fig. 3c).
Fig. 3.
Total coelomocyte count, TCC (a), coelomocyte volume, CV (b), lysozyme activity in CFC (c) and in CL (d) in P. lividus males (M) and females (F) after 60-day exposure to 8.0, 7.7 and 7.4 pH. Values are the means ± SD (n = 3). Significant differences among the various experimental conditions are presented with lower case letters (a, b)
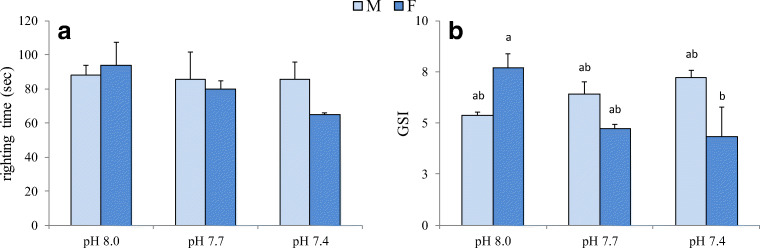
Righting time and gonadosomatic index
Although no significant effects of the experimental conditions were found (Table 3), a slight decrease in righting time was shown in females under reduced pH, but not in males (Fig. 4a). GSI values were significantly influenced by pH*sex interaction (Table 3). At decreasing pH, GSI showed an opposite trend in the two sexes, slightly increasing in males and markedly decreasing in females (Fig. 4b). Females maintained at 7.4 pH showed a significantly lower GSI compared with females from the control condition.
Fig. 4.
Righting time (a) and gonadosomatic index (b) of P. lividus males (M) and females (F) after 60-day exposure to 8.0, 7.7 and 7.4 pH. Values are the means ± SD (n = 3). Significant differences among the various experimental conditions are presented with lower case letters (a, b)
Canonical correlation analysis
The CCA performed on the whole dataset of biomarkers measured from day 40 to day 60 is shown in Fig. 5. The explained correlation is 99% for both canonical correlations. The CCA biplot reveals two clear pairs of canonical components. The first canonical component is roughly associated with the pH level, while the second is associated with the gender. There is a clear separation of pH 7.4 in both males and females. In particular, control females and those at 7.7 pH are well discriminated, while there is a less evident separation between the same pH conditions in males.
Fig. 5.
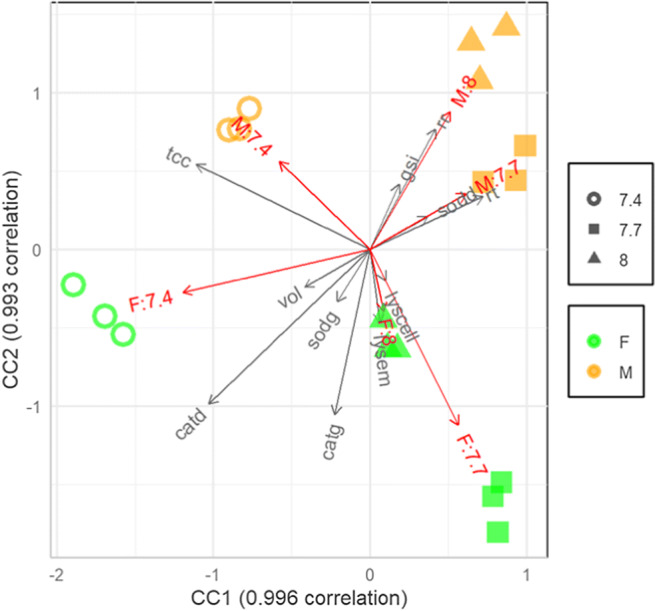
Canonical correlation analysis of the biomarker dataset. M:8.0, M:7.7, M:7.4: males at pH 8.0, 7.7 and 7.4, respectively; F:8.0, F:7.7, F:7.4: females at pH 8.0, 7.7 and 7.4, respectively. Abbreviations: respiration rate (rr), superoxide dismutase (sod), catalase (cat), gonads (g), digestive tract (d), total coelomocyte count (tcc), coelomocyte volume (vol), lysozyme (lys), cell-free coelomic fluid (em), coelomocytes (cell), righting time (rt) and gonadosomatic index (gsi)
Discussion
The increased CO2 concentration in seawater can affect marine organisms both directly, as CO2 enters the organisms by diffusion inducing hypercapnia (i.e., CO2 accumulation in the internal fluids) and indirectly through acidosis (i.e., internal pH decrease). In sea urchins, regulation of internal pH under seawater acidification is documented in both laboratory and natural conditions (Catarino et al. 2012; Dupont and Thorndyke 2012; Collard et al. 2013; Leite Figueiredo et al. 2016; Lewis et al. 2016; Migliaccio et al. 2019). Maintenance of acid-base homeostasis is attained by increasing bicarbonate levels in the coelomic fluid, as demonstrated in P. lividus and S. droebachiensis exposed to reduced pH (Collard et al. 2013; Stumpp et al. 2012). However, this is an energy-requiring process possibly leading to a reduction in the energy that can be allocated to other processes, such as growth and reproduction (Melzner et al. 2009; Collard et al. 2013). In this context, variations in physiological responses as a proxy for metabolic expenditure have been assessed in our study. After 21 days of exposure to reduced pH, P. lividus respiration rate decreased at 7.7 and increased at 7.4 pH, the difference resulting significant between the two reduced pH conditions in both males and females. In the same species, increased respiration rate was observed by Catarino et al. (2012) after 19 days of exposure to reduced pH (7.7 and 7.4) at a temperature of 10 °C, whereas no differences respect to controls were found at 16 °C. Although in our study pH significantly influenced respiration rate after 21 days, no significant effects of pH were observed after 40 days of exposure. This suggested that both male and female P. lividus have the potential to acclimate at low pH conditions after prolonged exposure, as already observed for the same species, both in a laboratory 2-months exposure (Cohen-Rengifo et al. 2019) and in specimens from CO2 vents at Ischia Island (Migliaccio et al. 2019). Similarly, in other species, namely E. mathaei, Strongylocentrotus fragilis, Strongylocentrotus droebachiensis, and Echinometra sp. A, respiration rate was not affected by reduced pH following long-term exposures (from 49 to 140 days) (Stumpp et al. 2012; Moulin et al. 2014; Taylor et al. 2014; Uthicke et al. 2014). In addition, results obtained after prolonged exposure (more than 5 months) highlighted the capability of Hemicentrotus pulcherrimus and Sterechinus neumayeri to acclimate to low pH conditions (Kurihara et al. 2013; Suckling et al. 2015). However, significant effects of low pH on sea urchin metabolic rate are reported in the literature, following exposures that lasted no longer than 5 months. For example, in Echinometra mathaei and Anthocidaris crassispina, oxygen uptake was found significantly reduced under OA conditions lasted 42 and 140 days, respectively (Uthicke et al. 2013; Wang et al. 2013). Conversely, in Sterechinus neumayeri and Heliocidaris erythrogramma oxygen consumption increased after exposure for 30, and 60 days to reduced pH (Suckling et al. 2015; Carey et al. 2016). Overall, these data highlight that capability of sea urchins to acclimate to low pH is species- and exposure duration-dependent.
Ammonia production is an indicator of protein metabolism and it was hypothesized that under acidified conditions increased ammonia excretion could act as an additional acid extrusion mechanism in mussels (Thomsen and Melzner 2010) and sea urchins (Stumpp et al. 2012). In this study, after 14 days of exposure, ammonia production was not significantly affected by pH, but it was significantly different between sexes with higher values in males. However, after 21 days, the effect of pH was significant, with decreasing trend at low pH conditions in both sexes, even though ammonia excretion decreased at 7.4 pH in males and at 7.7 pH in females. Unfortunately, ammonia excretion data from 40-day-exposed sea urchins lack in our study. Consequently, a more exhaustive conclusion about such physiological parameter cannot be formulated. In this regard, it is important to highlight that exposure for 70 days to 860–940 μAtm CO2 did not affect ammonia production in Echinometra sp. A (Uthicke et al. 2014). Similar results were obtained in P. lividus from the vent and non-vent areas at Ischia Island (Migliaccio et al. 2019).
In our study, assimilation efficiency was the only physiological parameter significantly affected after 7 days of exposure. Indeed, a significant interaction between pH and sex was found, suggesting higher assimilation of organic matter from the food by females when exposed to low pH, while an opposite tendency was observed in males. Similarly to what was observed in males in this study, Siikavuopio et al. (2007) reported decreased assimilation in S. droebachiensis exposed for 56 days to 6.98 pH. Conversely, in the same species Stumpp et al. (2012) found that the assimilation efficiency was not affected after 10- and 45-days exposure to moderate (1007–1431 μAtm) and high (2800–3800 μAtm) pCO2.
Maintenance of antioxidant and immune defence is essential to ensure animal health in acidified seawater. SOD and CAT are considered the primary antioxidant enzymes. They prevent oxidative damage removing reactive oxygen species (ROS) produced during normal metabolism and after oxidative injury. In particular, SOD dismutates superoxide anion (O2−) to hydrogen peroxide (H2O2) and O2, and CAT is the most important H2O2 scavenger in cells and reduces H2O2 to water and O2. In this study, SOD activity in both gonads and digestive tract did not vary significantly owing to either pH exposure or animal sex. In the study of Amri et al. (2017), a seasonal assessment of antioxidant activities in gonads of P. lividus showed highest levels of SOD activity in spring, when GSI reached its maximum value. Conversely, our results did not highlight a similar relationship between SOD activity and GSI. Unlike SOD, CAT activity was significantly different between sexes, with higher values in both gonads and digestive tract from females. Moreover, gonad CAT activity and GSI exhibited a similar pattern of variation in both females and males, suggesting increasing enzyme activity with increasing gonad development. The important role of CAT in gonad antioxidant defence is mirrored in higher activity levels respect to SOD, as observed in this and in previous studies on P. lividus (Perez-Trigo et al. 1995). A prevailing role of CAT against oxidative stress is also confirmed in digestive tract results. Indeed, in both sexes, the enzyme activity increases with increasing stress conditions due to low pH. Contrary to what was observed by Amri et al. 2017, in this study increased CAT activity does not match increased SOD activity. Interestingly, under low pH and high-temperature values, a general increase in CAT activity was also shown in two bivalves, the clam Chamelea gallina and the mussel Mytilus galloprovincialis (Matozzo et al. 2013). To support the statement of responsiveness of CAT activity to environmental stressors, it is important to highlight that increased enzyme activity was found in gonads of P. lividus from areas subject to several industrial activities, and the increase was higher in male specimens (Boussoufa et al. 2017). Although sex-related differences in oxidative stress responses to increased temperature and reduced pH have recently been reported for the marine gastropod Trochus histrio (Grilo et al. 2018), similar information is lacking for sea urchins, to our knowledge at least.
The sea urchin coelomic fluid contains coelomocytes, circulating cells that have various roles, ranging from immunity to metabolite transport (Endean 1966). Coelomocytes are involved in the immune defence through several processes, such as phagocytosis, coagulation, encapsulation, cytotoxicity and production of antimicrobial agents and other humoral factors (Silva 2013). Coelomocyte number and cell type proportion, as well as many functional responses, vary with the species and the physiological conditions of individuals, as a response to environmental factors, pollutants, pathogens or accidental injuries (Matranga et al. 2000; Pinsino et al. 2007; Ramírez-Gomez et al. 2010). Exposure of P. lividus to pollutants, such as lindane and zinc, or accidental injuries induced an increase in red spherula cells, considered as primary cells possibly affected by stressful conditions (Matranga et al. 2000; Pagliara and Stabili 2012; Stabili and Pagliara 2015).
Among environmental stressors, the effects of exposure to near-future OA on the sea urchins’ immune parameters have been investigated in some recent studies. Alterations in coelomocyte proportions/number and immune functions, such as phagocytic capacity, cell spreading, bacterial growth inhibition capacity, total antioxidant capacity, and nitric oxide production, have been reported (Dupont and Thorndyke 2012; Brothers et al. 2016; Leite Figueiredo et al. 2016; Migliaccio et al. 2019). In this study, no differences in coelomocyte number and volume between sexes were found, but 60-day exposure induced a significant increase in coelomocyte number of both males and females in the extreme experimental condition tested (7.4 pH). Similarly, a significant increase in coelomocyte number with no differences in cell-type proportions was observed in L. variegatus exposed for 5 days to 7.3 pH (Leite Figueiredo et al. 2016). Despite coelomocyte number remained unchanged in Echinometra lucunter and E. droebachiensis, exposure to low pH induced an increase in phagocytic amoebocytes and a decrease in vibratile cells (Dupont and Thorndyke 2012; Leite Figueiredo et al. 2016). No differences in coelomocyte number and type proportion were found in P. lividus from CO2 vents and control sites at Ischia Island, even though enhanced defensive abilities were revealed in specimens living under reduced pH (Migliaccio et al. 2019). Among immunomarkers, lysozyme, one of the most important lysosomal hydrolase, is synthesized in coelomocytes and released into the coelomic fluid as a defence mechanism against pathogens and other foreign substances (Stabili et al. 1996). In this study, in both cell-free coelomic fluid and coelomocytes, a significant interaction between pH and sex induced an opposite pattern of variation in males and females when exposed to low pH, with increased activity in males, at 7.7 pH in particular, and decreased activity in females. In control conditions, higher lysozyme activity in females respect to males suggested a better immunosurveillance possibly related to different reproductive requirements in the two sexes. Our results match those of Arizza et al. (2013) reporting higher levels of immune activities (cytotoxic, haemolytic and agglutinating) in females of P. lividus, together with a higher number of coelomocytes. Since in our study TCC did not differ significantly in females and males, higher lysozyme activity in females seems to be constitutive. Under reduced pH, increased levels in male CFC lysozyme activity in the absence of pathogen challenge could be due to reduced membrane stability of coelomocytes, even though attempts to increase immunosurveillance at the peripheral level cannot be excluded. Unlike males, females exposed to low pH showed decreased lysozyme activity levels in both CL and CFC, suggesting a reduction in energy expenditure through decreased lysozyme secretion. Different strategies in males and females coping with ocean acidification likely occur because of different constitutive levels of the enzyme in the two sexes.
The righting response reflects the general physiological state of the echinoderms when subject to environmental changes and it has been used as an indicator of stress and organism well-being (Lawrence and Cowell 1996). This behaviour is neuromuscular-mediated and represents the coordination ability between tube foot and spine (Bayed et al. 2005). In P. lividus, righting time was shown to be possibly related to the reproductive status, with increasing values during gonad development and decreasing values after spawning (Bayed et al. 2005). Other authors highlighted the presence of sublethal effects on P. lividus righting time, due to oil pollution (Axiak and Saliba 1981). In our experiment, at the control condition, both male and female righting time was close to the reference value (100 s) reported by Axiak and Saliba (1981) for P. lividus. At low pH values, in males righting time remained unchanged, while in females it was reduced on average to 75 s at 7.7 pH, and 69 s at 7.4 pH. However, the pH effect observed in females was not statistically significant suggesting that reduced pH does not affect righting time in P. lividus, as observed in juveniles and adults of L. variegatus (Challener and McClintock 2013; Emerson et al. 2017). Only extremely low pH (6.6) negatively affected righting time in S. fragilis (Taylor et al. 2014). Other behavioural aspects in P.lividus were recently investigated by Cohen-Rengifo et al. (2019). They showed that podia adhesion strength was not influenced by reduced pH and highlighted positive synergistic effects of ocean acidification and ocean warming on sea urchin moving velocity at 7.7 pH, but not at 7.4 pH. It was hypothesized that the observed behavioural modifications were ascribed to the accumulation of HCO3− in extracellular fluid, as protection against acidosis, and to the compensatory reduction of Cl− (Stumpp et al. 2012; Collard et al. 2014). Alterations in the concentration of these ions could influence GABA receptors, which are involved in neurological mechanisms, such as information processing (Cohen-Rengifo et al. 2019). Potential modifications of sea urchin behaviour under reduced pH deserve further attention in future studies. Interestingly, in our study female righting time and GSI exhibited the same pattern of variation with decreasing pH value.
According to Luís et al. (2005), 2 months were enough to allow gonad maturation and spawning in P. lividus after a previous KCl spawning induction, and the observed GSI values were in the range reported for ripe sea urchins in natural populations (Gago et al. 2003). In sea urchins, gonad development is affected by various abiotic and biotic factors, such as temperature (Delorme and Sewell 2016; Zhao et al. 2016; Yeruham et al. 2019), pH (Stumpp et al. 2012; Kurihara et al. 2013; Taylor et al. 2014), photoperiod (Shpigel et al. 2004), food availability and quality (Murillo-Navarro and Jiménez-Guirado 2012; Prato et al. 2018), hydrodynamism (Gianguzza et al. 2013) and pollutants (Schäfer and Köhler 2009; Rouane-Hacene et al. 2018). Sea urchin gonads are very plastic organs that can be used as energy storage: they can be filled or depleted depending on animal conditions. Under stressful conditions, the effects observed are often species-specific and mostly dependent on the type and duration of the stress occurred. Although no differences in GSI are generally reported in males and females from natural populations (Guettaf and San Martin 1995; Gago et al. 2003; Luís et al. 2005), there is increasing evidence of sex-related differences under stressful conditions. Accordingly, in this study, no difference between sexes was found at 8.0 pH; however, an opposite effect on GSI was observed in the two sexes at low pH, with a significant decrease at 7.4 pH in females and an increasing trend at low pH values in males. In a previous study, RNA/DNA ratio, an indicator of gonadal production, was higher in female than in male gonads of P. lividus, but in both sexes no differences were observed after exposure for 19 days to 8.0, 7.7 and 7.4 pH (Catarino et al. 2012). Conversely, in E. mathaei maintained for 6 weeks at similar pH values, a reduced spawning ability was observed in males kept at low pH, whereas in females both spawning ability and oocyte size did not change (Uthicke et al. 2013). In Echinometra sp. A, gonad index was not affected by a 77-day exposure at low pH, but a synergistic effect of decreased pH and increased temperature was revealed in both sexes, and males appeared more sensitive to low pH (Uthicke et al. 2014). After 9-month exposure to elevated pCO2, Hemicentrotus pulcherrimus showed a 1-month delay in gonad maturation and spawning, even though the maximum number of eggs was not affected (Kurihara et al. 2013). Gonad growth was reduced after 45- and 56-day exposure at low pH in S. droebachiensis (Siikavuopio et al. 2007; Stumpp et al. 2012) and after 140 days in S. fragilis (Taylor et al. 2014). Although several studies reported negative effects of reduced pH on sea urchin gonad growth, GSI reduction under low pH observed in this study in P. lividus females could be indicative of more detrimental effects arising from greater energy request to maintain homeostasis, with a consequent reduction in energy to invest in reproduction.
Overall, this study highlighted the presence of differences between sexes under control conditions and male and female sea urchins often responded differently to OA. As shown in CCA results, at 8.0 pH, sexes are clearly separated along the second canonical component, differing in CAT activity in both the digestive tract and gonads (higher in females), coelomocyte parameters (lysozyme activity in both coelomocytes and haemolymph, and coelomocyte volume higher in females) and metabolism (higher in males). In both sexes, a clear spatial distribution was observed according to pH values along the first canonical component. With increasing seawater acidity, enhanced CAT activity and decreased metabolism were observed in females. Females appeared more sensitive to pH variations with a clear separation between controls and reduced pH treatments. In females, low pH, 7.4 in particular, led to enhanced antioxidant defence in the digestive tract (with increased CAT activity), modified behaviour (with a reduction of righting time) and reduced energy investment in reproduction (with a gonadosomatic index decrease). Since the pattern of separation was opposite in females and males, the latter showed reduced antioxidant activity in gonads, reduced coelomocyte volume and lysozyme activity and increased metabolism. At extremely low pH condition (7.4), both males and females exhibited the highest value of coelomocyte number.
Conclusions
In this study, different responses to OA were observed in males and females of P. lividus. However, responses appeared to be mostly influenced by basal differences between genders. Males exhibited lower protective levels in antioxidant and immune defence, and when subject to reduced pH they appeared to tackle this deficiency by somehow reducing metabolic expenditure. Conversely, females having a better basal protection against stress differently modulated antioxidant and immune-related responses but reduced their reproductive potential. Sex-specific differences, likely reflecting adaptive mechanisms of gametes with different life span, very short in sperm and long in eggs, can be important drivers affecting responses to environmental stress. For this reason, further research is needed to shed more light on the strengths and weaknesses of male and female sea urchins under global change scenarios.
Electronic supplementary material
(PDF 943 kb)
Acknowledgements
Open access funding provided by Università degli Studi di Padova within the CRUI-CARE Agreement. The authors wish to thank Prof. Livio Finos for his valuable support in the statistical analyses of data. Thanks are also extended to Mr. Mohamad Sofi Abu Hassan and Mrs. Nik Nurasyikin Nik Mohmmad Azmi for their technical assistance in biochemical analyses.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aebi H. Catalase in vitro. Method Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Amri S, Samar MF, Sellem F, Ouali K. Seasonal antioxidant responses in the sea urchin Paracentrotus lividus (Lamarck 1816) used as a bioindicator of the environmental contamination in the South-East Mediterranean. Mar Pollut Bull. 2017;122:392–402. doi: 10.1016/j.marpolbul.2017.06.079. [DOI] [PubMed] [Google Scholar]
- Arizza V, Vazzana M, Schillaci D, Russo D, Giaramita FT, Parrinello N. Gender differences in the immune system activities of sea urchin Paracentrotus lividus. Comp Biochem Physiol. 2013;164A:447–455. doi: 10.1016/j.cbpa.2012.11.021. [DOI] [PubMed] [Google Scholar]
- Axiak V, Saliba LJ. Effects of surface and sunken crude oil on the behaviour of a sea urchin. Mar Pollut Bull. 1981;12:14–19. [Google Scholar]
- Bayed A, Quiniou F, Benrha A, Guillou M. The Paracentrotus lividus populations from the northern Moroccan Atlantic coast: growth, reproduction and health condition. J Mar Biol Assoc UK. 2005;85:999–1007. [Google Scholar]
- Benedetti M, Lanzoni I, Nardi A, d’Errico G, Di Carlo M, Fattorini D, Nigro M, Regoli F. Oxidative responsiveness to multiple stressors in the key Antarctic species, Adamussium colbecki: interactions between temperature, acidification and cadmium exposure. Mar Environ Res. 2016;121:20–30. doi: 10.1016/j.marenvres.2016.03.011. [DOI] [PubMed] [Google Scholar]
- Boudouresque CF, Verlaque M. Paracentrotus lividus. In: Lawrence JM, editor. Sea urchins: biology and ecology, developments in aquaculture and fisheries science. Amsterdam: Elsevier; 2013. pp. 297–327. [Google Scholar]
- Boussoufa D, Chalouati H, Feriel G, Safa B, El Cafsi M. Assessment of sex-related variability of biomarkers in sea urchin (Paracentrotus lividus) from Bizerte lagoon, Tunisia. Toxicol Lett. 2017;280(Suppl. 1):S213. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brothers CJ, Harianto J, McClintock JB, Byrne M. Sea urchins in a high-CO2 world: the influence of acclimation on the immune response to ocean warming and acidification. Proc R Soc B. 2016;283:20161501. doi: 10.1098/rspb.2016.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne M. Global change ecotoxicology: identification of early life history bottlenecks in marine invertebrates, variable species responses and variable experimental approaches. Mar Environ Res. 2012;76:3–15. doi: 10.1016/j.marenvres.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Byrne M, Hernández JC. Sea urchins in a high CO2 world: impacts of climate warming and ocean acidification across life history stages. In: Lawrence JM, editor. Sea urchins: biology and ecology, developments in aquaculture and fisheries science. Amsterdam: Elsevier; 2020. pp. 281–297. [Google Scholar]
- Calosi P, Rastrick SPS, Graziano M, Thomas SC, Baggini C, Carter HA, Hall-Spencer JM, Milazzo M, Spicer JI. Distribution of sea urchins living near shallow water CO 2 vents is dependent upon species acid–base and ion-regulatory abilities. Mar Pollut Bull. 2013;73:470–484. doi: 10.1016/j.marpolbul.2012.11.040. [DOI] [PubMed] [Google Scholar]
- Campbell AL, Levitan DR, Hosken DJ, Lewis C. Ocean acidification changes the male fitness landscape. Sci Rep. 2016;6:31250. doi: 10.1038/srep31250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso PG, Grilo TF, Dionísio G, Aurélio M, Lopes AR, Pereira R, Pacheco M, Rosa R. Short-term effects of increased temperature and lowered pH on a temperate grazer-seaweed interaction (Littorina obtusata/Ascophyllum nodosum) Estuar Coast Shelf Sci. 2017;197:35–44. [Google Scholar]
- Carey N, Harianto J, Byrne M. Urchins in a high CO2 world: partitioned effects of body-size, ocean warming and acidification on metabolic rate. J Exp Biol. 2016;219:1178–1186. doi: 10.1242/jeb.136101. [DOI] [PubMed] [Google Scholar]
- Cassin D, Dominik J, Botter M, Zonta R. PAH and PCB contamination in the sediments of the Venice Lagoon (Italy) before the installation of the MOSE flood defence works. Environ Sci Pollut Res. 2018;25:24951–24964. doi: 10.1007/s11356-018-2524-y. [DOI] [PubMed] [Google Scholar]
- Catarino AI, Bauwens M, Dubois P. Acid–base balance and metabolic response of the sea urchin Paracentrotus lividus to different seawater pH and temperatures. Environ Sci Pollut Res. 2012;19:2344–2353. doi: 10.1007/s11356-012-0743-1. [DOI] [PubMed] [Google Scholar]
- Challener RC, McClintock JB. Exposure to extreme hypercapnia under laboratory conditions does not impact righting and covering behavior of juveniles of the common sea urchin Lytechinus variegatus. Mar Fresh Behav Physiol. 2013;46:191–199. [Google Scholar]
- Cohen-Rengifo M, García E, Hernández CA, Hernández JC, Clemente S (2013) Global warming and ocean acidification affect fertilization and early development of the sea urchin Paracentrotus lividus. Cah Biol Mar 54:667–675
- Cohen-Rengifo M, Agüera A, Bouma T, M’Zoudi S, Flammang P, Dubois P. Ocean warming and acidification alter the behavioral response to flow of the sea urchin Paracentrotus lividus. Ecol Evol. 2019;9:12128–12143. doi: 10.1002/ece3.5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collard M, Laitat K, Moulin L, Catarino AI, Grosjean P, Dubois P. Buffer capacity of the coelomic fluid in echinoderms. Comp Biochem Physiol. 2013;166A:199–206. doi: 10.1016/j.cbpa.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Collard M, Dery A, Dehairs F, Dubois P. Euechinoidea and Cidaroidea respond differently to ocean acidification. Comp Biochem Physiol. 2014;174(A):45–55. doi: 10.1016/j.cbpa.2014.04.011. [DOI] [PubMed] [Google Scholar]
- Collard M, Rastrick SPS, Calosi P, Demolder Y, Dille J, Findlay HS, Hall-Spencer JM, Milazzo M, Moulin L, Widdicombe S, Dehairs F, Dubois P. The impact of ocean acidification and warming on the skeletal mechanical properties of the sea urchin Paracentrotus lividus from laboratory and field observations. ICES J Mar Sci. 2016;73:727–738. [Google Scholar]
- Conover RJ. Assimilation of organic matter by zooplankton. Limnol Oceanogr. 1966;11:338–345. [Google Scholar]
- Crapo JD, McCord JM, Fridovich I. Preparation and assay of superoxide dismutases. Method Enzymol. 1978;53:382–393. doi: 10.1016/s0076-6879(78)53044-9. [DOI] [PubMed] [Google Scholar]
- Delorme NJ, Sewell MA. Effects of warm acclimation on physiology and gonad development in the sea urchin Evechinus chloroticus. Comp Biochem Physiol. 2016;198A:33–40. doi: 10.1016/j.cbpa.2016.03.020. [DOI] [PubMed] [Google Scholar]
- Dickson AG, Afghan JD, Anderson GC. Reference materials for oceanic CO2 analysis: a method for the certification of total alkalinity. Mar Chem. 2003;80:185–197. [Google Scholar]
- Dupont ST, Thorndyke MS. Relationship between CO2-driven changes in extracellular acid–base balance and cellular immune response in two polar echinoderm species. J Exp Mar Biol Ecol. 2012;424–425:32–37. [Google Scholar]
- Dupont S, Dorey N, Stumpp M, Melzner F, Thorndyke M. Long-term and trans-life-cycle effects of exposure to ocean acidification in the green sea urchin Strongylocentrotus droebachiensis. Mar Biol. 2013;160:1835–1843. [Google Scholar]
- Dworjanyn SA, Byrne M. Impacts of ocean acidification on sea urchin growth across the juvenile to mature adult life-stage transition is mitigated by warming. Proc R Soc B Biol Sci. 2018;285:1–10. doi: 10.1098/rspb.2017.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis RP, Spicer JI, Byrne J, Sommer U, Viant MR, White D, Widdicombe S. 1H NMR metabolomics reveals contrasting response by male and female mussels exposed to reduced seawater pH, increased temperature and a pathogen. Environ Sci Technol. 2014;48:7044–7052. doi: 10.1021/es501601w. [DOI] [PubMed] [Google Scholar]
- Ellis RP, Davison W, Queirós AM, Kroeker KJ, Calosi P, Dupont S, Spicer JI, Wilson RW, Widdicombe S, Urbina MA. Does sex really matter? Explaining intraspecies variation in ocean acidification responses. Biol Lett. 2017;13:20160761. doi: 10.1098/rsbl.2016.0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson CE, Reinardy HC, Bates NR, Bodnar AG. Ocean acidification impacts spine integrity but not regenerative capacity of spines and tube feet in adult sea urchins. R Soc Open Sci. 2017;4:170140. doi: 10.1098/rsos.170140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endean R. The coelomocytes and coelomic fluids. In: Boolootian RA, editor. Physiology of Echinodermata. New York: Interscience Publishers; 1966. pp. 301–328. [Google Scholar]
- Fernández-Boo S, Pedrosa-Oliveira MH, Afonso A, Arenas F, Rocha F, Valente LMP, Costa B. Annual assessment of the sea urchin (Paracentrotus lividus) humoral innate immune status: tales from the north Portuguese coast. Mar Environ Res. 2018;141:128–137. doi: 10.1016/j.marenvres.2018.08.007. [DOI] [PubMed] [Google Scholar]
- Finos L (2020) r41sqrt10: livio’s sandbox. R package version 0.1
- Freitas R, De Marchi L, Bastos M, Moreira A, Velez C, Chiesa S, Wrona FJ, Figueira E, Soaresa AMVM. Effects of seawater acidification and salinity alterations on metabolic, osmoregulation and oxidative stress markers in Mytilus galloprovincialis. Ecol Indic. 2017;79:54–62. [Google Scholar]
- Freitas R, De Marchi L, Moreira A, Pestana JLT, Wrona FJ, Figueira E, Soares AMVM. Physiological and biochemical impacts induced by mercury pollution and seawater acidification in Hediste diversicolor. Sci Total Environ. 2017;595:691–701. doi: 10.1016/j.scitotenv.2017.04.005. [DOI] [PubMed] [Google Scholar]
- Gago J, Luís OJ. Comparison of spawning induction techniques on Paracentrotus lividus (Echinodermata: Echinoidea) broodstock. Aquacult Int. 2011;19:181–191. [Google Scholar]
- Gago J, Range P, Luís OJ. Growth, reproductive biology and habitat selection of the sea urchin Paracentrotus lividus in the coastal waters of Cascais, Portugal. In: Féral JP, David B, editors. Echinoderm research 2001. Lisse: Swets & Zeitlinger; 2003. pp. 269–276. [Google Scholar]
- Gianguzza P, Bonaviri C, Prato E, Fanelli G, Chiantore M, Privitera D, Luzzu F, Agnetta D. Hydrodynamism and its influence on the reproductive condition of the edible sea urchin Paracentrotus lividus. Mar Environ Res. 2013;85:29–33. doi: 10.1016/j.marenvres.2012.12.007. [DOI] [PubMed] [Google Scholar]
- Glippa O, Engström-Öst J, Kanerva M, Rein A, Vuori K. Oxidative stress and antioxidant defense responses in Acartia copepods in relation to environmental factors. PLoS One. 2018;13(4):e0195981. doi: 10.1371/journal.pone.0195981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González I, Déjean S (2012) CCA: canonical correlation analysis. R package version 1.2 http://CRAN.R-project.org/package=CCA
- Graham H, Rastrick SPS, Findlay HS, Bentley MG, Widdicombe S, Clare AS, Caldwell GS. Sperm motility and fertilisation success in an acidified and hypoxic environment. ICES J Mar Sci. 2015;73(3):783–790. [Google Scholar]
- Grilo TF, Lopes AR, Sampaio E, Rosa R, Cardoso PG. Sex differences in oxidative stress responses of tropical topshells (Trochus histrio) to increased temperature and high pCO2. Mar Pollut Bull. 2018;131:252–259. doi: 10.1016/j.marpolbul.2018.04.031. [DOI] [PubMed] [Google Scholar]
- Grosjean P, Spirlet C, Gosselin P, Vaitilingon D, Jangoux M. Land-based, closed-cycle echiniculture of Paracentrotus lividus (Lamarck) (Echinoidea: Echinodermata): a long-term experiment at a pilot scale. J Shellfish Res. 1998;17:1523–1531. [Google Scholar]
- Guettaf M, San Martin GA. Étude de la variabilité de l’indice gonadique de l’oursin comestible Paracentrotus lividus (Echinodermata: Echinidae) en Méditerranée Nord-Occidentale. Vie Milieu. 1995;45:129–137. [Google Scholar]
- Hall-Spencer JM, Thorndyke M, Dupont S. Impact of ocean acidification on marine organisms-unifying principles and new paradigms. Water. 2015;7:5592–5598. [Google Scholar]
- Hartin CA, Bond-Lamberti B, Patel P, Mundra A. Ocean acidification over the next three centuries using a simple global climate carbon-cycle model: projections and sensitivities. Biogeosciences. 2016;13:4329–4342. [Google Scholar]
- Hereu B. Depletion of palatable algae by sea urchins and fishes in a Mediterranean subtidal community. Mar Ecol Prog Ser. 2006;313:95–103. [Google Scholar]
- Holtmann WC, Stumpp M, Gutowska MA, Syré S, Himmerkus N, Melzner F, Bleich M. Maintenance of coelomic fluid pH in sea urchins exposed to elevated CO2: the role of body cavity epithelia and stereom dissolution. Mar Biol. 2013;160:2631–2645. [Google Scholar]
- Huang X, Liu Z, Xie Z, Dupont S, Huang W, Wu F, Kong H, Liu L, Sui Y, Lin D, Lu W, Hu M, Wang Y. Oxidative stress induced by titanium dioxide nanoparticles increases under seawater acidification in the thick shell mussel Mytilus coruscus. Mar Environ Res. 2018;37:49–59. doi: 10.1016/j.marenvres.2018.02.029. [DOI] [PubMed] [Google Scholar]
- Ingle SE. Solubility of calcite in the ocean. Mar Chem. 1975;3:301–319. [Google Scholar]
- IPCC . Climate change 2013: the physical science basis. In: Stocker TF, Qin D, Plattner GK, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM, editors. Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change. Cambridge: Cambridge University Press; 2013. p. 1535. [Google Scholar]
- IPCC (2019) Summary for policymakers. In: Pörtner HO, Roberts DC, Masson-Delmotte V, Zhai P, Tignor M, Poloczanska E, Mintenbeck K, Alegría A, Nicolai M, Okem A, Petzold J, Rama B, Weyer NM (eds.) IPCC Special report on the ocean and cryosphere in a changing climate. In press
- Karelitz S, Lamare M, Patel F, Gemmell N, Uthicke S. Parental acclimation to future ocean conditions increases development rates but decreases survival in sea urchin larvae. Mar Biol. 2020;167:2. doi: 10.1007/s00227-019-3610-5. [DOI] [Google Scholar]
- Klein RD, Nogueira LS, Domingos-Moreira FXV, Gomes Costa P, Bianchini A, Wood CM. Effects of sublethal Cd, Zn, and mixture exposures on antioxidant defense and oxidative stress parameters in early life stages of the purple sea urchin Strongylocentrotus purpuratus. Aquat Toxicol. 2019;217:105338. doi: 10.1016/j.aquatox.2019.105338. [DOI] [PubMed] [Google Scholar]
- Kurihara H, Yin R, Nishihara GN, Soyano K, Ishimatsu A. Effect of ocean acidification on growth, gonad development, and physiology: of the sea urchin Hemicentrotus pulcherrimus. Aquat Biol. 2013;18:281–292. [Google Scholar]
- Lane A, Campanati C, Dupont S, Thiyagarajan V. Trans-generational responses to low pH depend on parental gender in a calcifying tubeworm. Sci Rep. 2015;5:10847. doi: 10.1038/srep10847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence JM, Cowell BC. The righting response as an indication of stress in Stichaster striatus (Echinodermata, Asteroidea) Mar Fresh Behav Physiol. 1996;27:239–248. [Google Scholar]
- Leite Figueiredo DA, Branco PC, dos Santos DA, Emerenciano AK, Iunes RS, Shimada Borges JC, Machado Cunha da Silva JR. Ocean acidification affects parameters of immune response and extracellular pH in tropical sea urchins Lytechinus variegatus and Echinometra luccunter. Aquat Toxicol. 2016;180:84–94. doi: 10.1016/j.aquatox.2016.09.010. [DOI] [PubMed] [Google Scholar]
- Lewis C, Ellis RP, Vernon E, Elliot K, Newbatt S, Wilson RW. Ocean acidification increases copper toxicity differentially in two key marine invertebrates with distinct acid-base responses. Sci Rep. 2016;6:21554. doi: 10.1038/srep21554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luís O, Delgado F, Gago J. Year-round captive spawning performance of the sea urchin Paracentrotus lividus: relevance for the use of its larvae as live feed. Aquat Living Resour. 2005;18:45–54. [Google Scholar]
- Matozzo V, Chinellato A, Munari M, Bressan M, Marin MG. Can the combination of decreased pH and increased temperature values induce oxidative stress in the clam Chamelea gallina and the mussel Mytilus galloprovincialis? Mar Pollut Bull. 2013;72:34–40. doi: 10.1016/j.marpolbul.2013.05.004. [DOI] [PubMed] [Google Scholar]
- Matranga V, Toia G, Bonaventura R, Muller WEG. Cellular and biochemical responses to environmental and experimentally induced stress in sea urchin coelomocytes. Cell Stress Chaperon. 2000;5:158–165. doi: 10.1379/1466-1268(2000)005<0113:cabrte>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClellan-Green P, Romano J, Oberdörster E. Does gender really matter in contaminant exposure? A case study using invertebrate models. Environ Res. 2007;104:183–191. doi: 10.1016/j.envres.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Melzner F, Gutowska MA, Langenbuch M, Dupont S, Lucassen M, Thorndyke MC, Bleich M, Pörtner HO. Physiological basis for high CO2 tolerance in marine ectothermic animals: preadaptation through lifestyle and ontogeny? Biogeosciences. 2009;6:2313–2331. [Google Scholar]
- Migliaccio O, Pinsino A, Maffioli E, Smith AM, Agnisola C, Matranga V, Nonnis S, Tedeschi G, Byrne M, Gambi MC, Palumbo A. Living in future ocean acidification, physiological adaptive responses of the immune system of sea urchins resident at a CO2 vent system. Sci Total Environ. 2019;672:938–950. doi: 10.1016/j.scitotenv.2019.04.005. [DOI] [PubMed] [Google Scholar]
- Miles H, Widdicombe W, Spice JI, Hall-Spencer J. Effects of anthropogenic seawater acidification on acid–base balance in the sea urchin Psammechinus miliaris. Mar Pollut Bull. 2007;54:89–96. doi: 10.1016/j.marpolbul.2006.09.021. [DOI] [PubMed] [Google Scholar]
- Millero FJ. The thermodynamics of the carbonic acid system in seawater. Geochim Cosmochim Acta. 1979;43:1651–1661. [Google Scholar]
- Millero FJ. Thermodynamics of the carbon dioxide system in the oceans. Geochim Cosmochim Acta. 1995;59:661–677. [Google Scholar]
- Millero FJ, Graham TB, Huang F, Bustos-Serrano H, Pierrot D. Dissociation constants of carbonic acid in seawater as a function of salinity and temperature. Mar Chem. 2006;100:80–94. [Google Scholar]
- Morse JW, Andersson AJ, Mackenzie FT. Initial response of carbonate-rich shelf sediments to rising atmospheric pCO2 and “ocean acidification”: role of high Mg-calcites. Geochim Cosmochim Acta. 2006;70:5814–5830. [Google Scholar]
- Mos B, Byrne M, Dworjanyn SA. Biogenic acidification reduces sea urchin gonad growth and increases susceptibility of aquaculture to ocean acidification. Mar Environ Res. 2016;113:39–48. doi: 10.1016/j.marenvres.2015.11.001. [DOI] [PubMed] [Google Scholar]
- Moulin L, Grosjean P, Leblud J, Batigny A, Dubois P. Impact of elevated pCO2 on acid–base regulation of the sea urchin Echinometra mathaei and its relation to resistance to ocean acidification: a study in mesocosms. J Exp Mar Biol Ecol. 2014;457:97–104. [Google Scholar]
- Mucci A. The solubility of calcite and aragonite in seawater at various salinities, temperatures, and one atmosphere total pressure. Am J Sci. 1983;283:780–799. [Google Scholar]
- Munari M, Matozzo V, Gagné F, Chemello G, Riedl V, Finos L, Pastore P, Badocco D, Marin MG. Do seawater acidification and diclofenac induce oxidative stress in marine bivalves? A comparison study with the mussel Mytilus galloprovincialis and the clam Ruditapes philippinarum. Environ Pollut. 2018;240:925–937. doi: 10.1016/j.envpol.2018.05.005. [DOI] [PubMed] [Google Scholar]
- Murillo-Navarro R, Jiménez-Guirado D. Relationships between algal food and gut and gonad conditions in the Mediterranean sea urchin Paracentrotus lividus (Lam.) Mediterr Mar Sci. 2012;13:227–238. [Google Scholar]
- Nardi A, Mincarelli LF, Benedetti M, Fattorini D, d’Errico G, Regoli F. Indirect effects of climate changes on cadmium bioavailability and biological effects in the Mediterranean mussel Mytilus galloprovincialis. Chemosphere. 2017;169:493–502. doi: 10.1016/j.chemosphere.2016.11.093. [DOI] [PubMed] [Google Scholar]
- Pagliara P, Stabili L. Zinc effect on the sea urchin Paracentrotus lividus immunological competence. Chemosphere. 2012;89:563–568. doi: 10.1016/j.chemosphere.2012.05.052. [DOI] [PubMed] [Google Scholar]
- Parolini M, Binelli A, Matozzo V, Marin MG. Persistent organic pollutants in sediments from the Lagoon of Venice—a possible hazard for sediment-dwelling organisms. J Soils Sediments. 2010;10:1362–1379. [Google Scholar]
- Parolini M, Binelli A, Marin MG, Matozzo V, Masiero L, Provini A. New evidences in the complexity of contamination of the lagoon of Venice: polybrominated diphenyl ethers (PBDEs) pollution. Environ Monit Assess. 2012;184:2001–2015. doi: 10.1007/s10661-011-2095-6. [DOI] [PubMed] [Google Scholar]
- Perez-Trigo E, Garcia-Martinez P, Catoira JL, Mosquera G. Subcellular distribution of antioxidant enzymes in the gonads of the sea urchin, Paracentrotus lividus Lmk, from Rìa Ares-Betanzos, NW Spain. In: Emson R, Smith A, Campbell A, editors. Echinoderm research 1995. Rotterdam: Balkema; 1995. pp. 51–55. [Google Scholar]
- Pimentel MS, Faleiro F, Diniz M, Machado J, Pousão-Ferreira P, Peck MA, Pörtner HO, Rosa R. Oxidative stress and digestive enzyme activity of flatfish larvae in a changing ocean. PLoS One. 2015;10(7):e0134082. doi: 10.1371/journal.pone.0134082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinsino A, Thorndyke MC, Matranga V. Coelomocytes and post-traumatic response in the common sea star Asterias rubens. Cell Stress Chaperon. 2007;12:331–341. doi: 10.1379/CSC-288.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prato E, Fanelli G, Angioni A, Biandolino F, Parlapiano I, Papa L, Denti G, Secci M, Chiantore M, Kelly MS, Ferranti MP, Addis P. Influence of a prepared diet and a macroalga (Ulva sp.) on the growth, nutritional and sensory qualities of gonads of the sea urchin Paracentrotus lividus. Aquaculture. 2018;493:240–250. [Google Scholar]
- Privitera D, Chiantore M, Mangialajo L, Glavic N, Kozul V, Cattaneo-Vietti R. Inter- and intra-specific competition between Paracentrotus lividus and Arbacia lixula in resource-limited barren areas. J Sea Res. 2008;60:184–192. [Google Scholar]
- Priya RJ, Anand M, Maruthupandy M, Beevi AH. Biomarker response of climate change induced ocean acidification and hypercapnia studies on brachyurian crab Portunus pelagicus. Global J Environ Sci Manage. 2017;3:165–176. [Google Scholar]
- Queirós AM, Fernandes JA, Faulwetter S, Nunes J, Rastrick SPS, Mieszkowska N, Artioli Y, Yool A, Calosi P, Arvanitidis C, Findlay HS, Barange M, Cheung WWL, Widdicombe S. Scaling up experimental ocean acidification and warming research: from individuals to the ecosystem. Glob Chang Biol. 2015;21:130–143. doi: 10.1111/gcb.12675. [DOI] [PubMed] [Google Scholar]
- R Core Team (2019) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org
- Ramírez-Gomez F, Aponte-Rivera F, Mendez-Castaner L, García-Arraras JE. Changes in holothurian coelomocyte populations following immune stimulation with different molecular patterns. Fish Shellfish Immunol. 2010;29:175–185. doi: 10.1016/j.fsi.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rato LD, Novais SC, Lemos MFL, Alves LMF, Leandro SM. Homarus gammarus (Crustacea: Decapoda) larvae under an ocean acidification scenario: responses across different levels of biological organization. Comp Biochem Physiol. 2017;203C:29–38. doi: 10.1016/j.cbpc.2017.09.002. [DOI] [PubMed] [Google Scholar]
- Reid GK, Liutkus M, Bennett A, Robinson SMC, MacDonald B, Page F. Absorption efficiency of blue mussels (Mytilus edulis and M. trossulus) feeding on Atlantic salmon (Salmo salar) feed and fecal particulates: implications for integrated multi-trophic aquaculture. Aquaculture. 2010;299:165–169. [Google Scholar]
- Rouane-Hacene O, Boutiba Z, Benaissa M, Belhaouari B, Francour P, Guibbolini-Sabatier ME, Risso-De Faverney C. Seasonal assessment of biological indices, bioaccumulation, and bioavailability of heavy metals in sea urchins Paracentrotus lividus from Algerian west coast, applied to environmental monitoring. Environ Sci Pollut Res. 2018;25:1238–11251. doi: 10.1007/s11356-017-8946-0. [DOI] [PubMed] [Google Scholar]
- Santarém MM, Robledo JAF, Figueras A. Seasonal changes in hemocytes and serum defense factors in the blue mussel Mytilus galloprovincialis. Dis Aquat Org. 1994;18:217–222. [Google Scholar]
- Schäfer S, Köhler A. Gonadal lesions of female sea urchin (Psammechinus miliaris) after exposure to the polycyclic aromatic hydrocarbon phenanthrene. Mar Environ Res. 2009;68:128–136. doi: 10.1016/j.marenvres.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Shpigel M, McBride SC, Marciano S, Lupatsch I. The effect of photoperiod and temperature on the reproduction of European sea urchin Paracentrotus lividus. Aquaculture. 2004;232:343–355. [Google Scholar]
- Siikavuopio SI, Mortensen A, Dale T, Foss A. Effects of carbon dioxide exposure on feed intake and gonad growth in green sea urchin, Strongylocentrotus droebachiensis. Aquaculture. 2007;266:97–101. [Google Scholar]
- Silva JRMC. Immunology in sea urchins. In: Lawrence JM, editor. Sea urchins: biology and ecology, developments in aquaculture and fisheries science. Amsterdam: Elsevier; 2013. pp. 187–194. [Google Scholar]
- Solorzano L. Determination of ammonia in natural waters by the phenolhypochlorite method. Limnol Oceanogr. 1969;14:799–801. [Google Scholar]
- Soriano-Santiago OS, Liñán-Cabello MA, Delgadillo-Nuño MA, Ortega-Ortiz C, Cuevas-Venegas S. Physiological responses to oxidative stress associated with pH variations in host tissue and zooxanthellae of hermatypic coral Pocillopora capitata. Mar Freshw Behav Physiol. 2013;46:275–286. [Google Scholar]
- Spicer J, Widdicombe S. Acute extracellular acid–base disturbance in the burrowing sea urchin Brissopsis lyrifera during exposure to a simulated CO2 release. Sci Total Environ. 2012;427-428:203–207. doi: 10.1016/j.scitotenv.2012.02.051. [DOI] [PubMed] [Google Scholar]
- Spicer J, Widdicombe S, Needham H, Berge J. Impact of CO2-acidified seawater on the extracellular acid–base balance of the northern sea urchin Strongylocentrotus dröebachiensis. J Exp Mar Biol Ecol. 2011;407:19–25. [Google Scholar]
- Spirlet C, Grosjean P, Jangoux M. Optimization of gonad growth by manipulation of temperature and photoperiod in cultivated sea urchins, Paracentrotus lividus (Lamarck Echinodermata) Aquaculture. 2000;185:85–99. [Google Scholar]
- Stabili L, Pagliara P. The sea urchin Paracentrotus lividus immunological response to chemical pollution exposure: the case of lindane. Chemosphere. 2015;134:60–66. doi: 10.1016/j.chemosphere.2015.04.006. [DOI] [PubMed] [Google Scholar]
- Stabili L, Pagliara P, Roch P. Antibacterial activity in the coelomocytes of the sea urchin Paracentrotus lividus. Comp Biochem Physiol. 1996;113B:639–644. doi: 10.1016/0305-0491(95)02080-2. [DOI] [PubMed] [Google Scholar]
- Stumpp M, Trübenbach K, Brennecke D, Hu MY, Melzner F. Resource allocation and extracellular acid–base status in the sea urchin Strongylocentrotus droebachiensis in response to CO2 induced seawater acidification. Aquat Toxicol. 2012;110–111:194–207. doi: 10.1016/j.aquatox.2011.12.020. [DOI] [PubMed] [Google Scholar]
- Suckling CC, Clark MS, Beveridge C, Brunner L, Hughes AD, Harper EM, Cook EJ, Davies AJ, Peck LS. Experimental influence of pH on the early life-stages of sea urchins II: increasing parental exposure times gives rise to different responses. Invertebr Reprod Dev. 2014;58:61–175. [Google Scholar]
- Suckling CC, Clark MS, Richard J, Morley SA, Thorne MAS, Harper EM, Peck LS. Adult acclimation to combined temperature and pH stressors significantly enhances reproductive outcomes compared to short-term exposures. J Anim Ecol. 2015;84:773–784. doi: 10.1111/1365-2656.12316. [DOI] [PubMed] [Google Scholar]
- Sui Y, Hu M, Shang Y, Wu F, Huang X, Dupont S, Storch D, Pörtner HO, Lia J, Lu W, Wang Y. Antioxidant response of the hard shelled mussel Mytilus coruscus exposed to reduced pH and oxygen concentration. Ecotoxicol Environ Safe. 2017;137:94–102. doi: 10.1016/j.ecoenv.2016.11.023. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Lovera C, Whaling PJ, Buck KR, Pane EF, Barry JP. Physiological effects of environmental acidification in the deep-sea urchin Strongylocentrotus fragilis. Biogeosciences. 2014;11:1413–1423. [Google Scholar]
- Thomsen J, Melzner F. Seawater acidification does not elicit metabolic depression in the blue mussel Mytilus edulis. Mar Biol. 2010;157:2667–2676. [Google Scholar]
- Tomanek L, Zuzow MJ, Ivanina AV, Beniash E, Sokolova IM. Proteomic response to elevated PCO2 level in eastern oysters, Crassostrea virginica: evidence for oxidative stress. J Exp Biol. 2011;214:1836–1844. doi: 10.1242/jeb.055475. [DOI] [PubMed] [Google Scholar]
- Uthicke S, Soars N, Foo S, Byrne M. Effects of elevated pCO2 and the effect of parent acclimation on development in the tropical Pacific Sea urchin Echinometra mathaei. Mar Biol. 2013;160:1913–1926. [Google Scholar]
- Uthicke S, Liddy M, Nguyen HD, Byrne M. Interactive effects of near-future temperature increase and ocean acidification on physiology and gonad development in adult Pacific sea urchin. Echinometra sp. A. Coral Reefs. 2014;33:831–845. [Google Scholar]
- Velez C, Figueira E, Soares AMVM, Freitas R. Combined effects of seawater acidification and salinity changes in Ruditapes philippinarum. Aquat Toxicol. 2016;176:141–150. doi: 10.1016/j.aquatox.2016.04.016. [DOI] [PubMed] [Google Scholar]
- Wang G, Yagi M, Yin R, Lu W, Ishimatsu A. Effects of elevated seawater CO2 on feed intake, oxygen consumption and morphology of Aristotle’s lantern in the sea urchin Anthocidaris crassispina. J Mar Sci Technol. 2013;21:192–200. [Google Scholar]
- Yeruham E, Abelson A, Rilov G, Ben Ezra D, Shpigel M. Energy budget of cultured Paracentrotus lividus under different temperatures. Aquaculture. 2019;501:7–13. [Google Scholar]
- Zapata-Vivenes E, Aparicio G. Antioxidant defenses in the coelomic fluid of Echinometra lucunter (Linnaeus, 1758) stimulated with bacterial inoculums. Rev Mar Cost. 2019;11:27–42. [Google Scholar]
- Zhao C, Sun P, Wei J, Zhang L, Zhang W, Song J, Chang Y. Larval size and metamorphosis are significantly reduced in second generation of inbred sea urchins Strongylocentrotus intermedius. Aquaculture. 2016;452:402–406. [Google Scholar]
- Zonta R, Botter M, Cassin D, Bellucci LG, Pini R, Dominik J. Sediment texture and metal contamination in the Venice Lagoon (Italy): a snapshot before the installation of the MOSE system. Estuar Coast Shelf S. 2018;205:131–151. [Google Scholar]
- Zuo RT, Li M, Ding J, Chang YQ. Higher dietary arachidonic acid levels improved the growth performance, gonad development, nutritional value, and antioxidant enzyme activities of adult sea urchin (Strongylocentrotus intermedius) J Ocean Univ China. 2018;17:932–940. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 943 kb)