Abstract
Cancer has the ability to escape the immune system using different molecular actors. Adenosine is known to be involved in mechanisms which control inflammatory reactions and prevent excessive immune response. This purine nucleoside can be translocated from the cell or produced in the extracellular space by 5′-ectonucleotidases. Once bound to its receptors on the surface of immune effector cells, adenosine activates various molecular pathways, which lead to functional inhibition of the cell or its death. Some tumors are infiltrated by the different cells of immune system but are able to use adenosine as an immunosuppressive molecule and thus inhibit immune anticancer response. This mechanism is well described on adaptive cells, but much less on innate cells. This review outlines major effects of adenosine on innate immune cells, its consequences on cancer progression, and possible ways to block the adenosine-dependent immunosuppressive effect.
Keywords: Adenosine, Cancer, Microenvironment, Inflammation, Macrophages, NK cells, Neutrophils, Mast cells, Dendritic cells
Introduction
Cancer is a complex disease, caused by multiple cellular dysregulations. For a long time, it was believed that these dysregulations exclusively concerned the cancer cell and mainly its genetic material, but today we know it involves not only molecular but also cellular actors beside the cancer cell.
The immune system is involved in all stages of cancer progression. In a healthy organism, the immune system eliminates abnormal cells. Consequently, immune deficiencies favor cancer occurrence. When cancer cells escape this immune surveillance and initiate tumor growth, innate and adaptive immune cells infiltrate the tumor and can modulate the disease progression [1]. These tumor-infiltrating immune cells often correlate with patient’s outcome in a positive or negative manner since their activity can be pro- or anti-tumoral [2]. Several studies also show a very strong link between chronic inflammation (consequent to viral or bacterial infection) and cancer development.
The tumor microenvironment contains a large number of cancer-associated molecules with various biological effects. Most of them provide a cancer-supportive effect by acting directly on cancer cells or by acting on the surrounding cells. The purine nucleoside adenosine (ADO) has been identified among these factors. ADO is a biologically active molecule engaged in multiple intracellular and extracellular processes [3]. Within the cell, its nucleotide forms (ATP, ADP, or AMP) participate in various signaling pathways (i.e., cAMP, enzymatic reactions, DNA synthesis) and form the cell’s energy pool. In the extracellular space, these molecules can have paracrine and autocrine activities. Therefore, the extracellular concentration of ADO is finely regulated, and its misbalance is reported in different pathologies such as in immunodeficiencies due to a deficit in ADO deaminase, an enzyme responsible for the degradation of ADO into inosine [4]. Nucleotide availability in general is directly related to cell survival as the disequilibrium of dNTP pool leads to genomic instability and mutagenesis and can provoke cell death [5]. Cancers usually show increased levels of extracellular ADO, which has an overall inhibitory effect on effector immune cells found in tumor microenvironment [3]. This inhibits tumor-specific response provided by adaptive immune cells but also affects pro-inflammatory abilities of innate immune cells. As the current literature is rich on data and reviews on ADO effects on adaptive immune cells, we will focus on how extracellular ADO influence innate immunity cells.
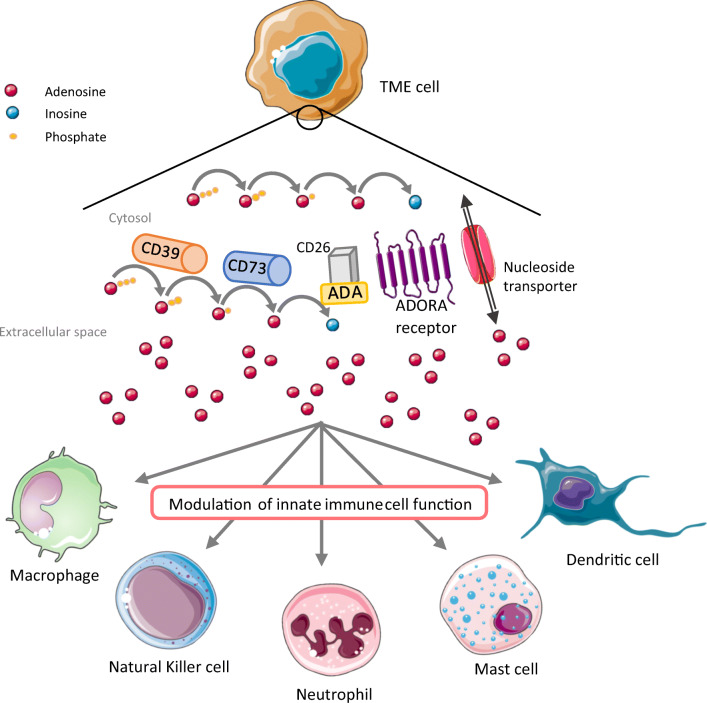
The extracellular ADO concentration increases after tissue injury caused by tumor growth, hypoxia, or inflammation [3]. Extracellular ADO has two different origins, and it can be translocated from within the cell by equilibrative nucleoside transporters (ENT) after its generation by intracellular enzymes or be directly synthesized using its precursors (ATP, ADP, AMP, or nicotinamide derivatives) in the extracellular space by ectonucleotidases (CD39, TNAP, CD73, and PAP) expressed by different cells (Fig. 1) [6, 7]. CD39 and TNAP dephosphorylate ATP or ADP, and resulting AMP undergoes the conversion into ADO by CD73. Once synthesized, ADO can pass inside the cell using specific nucleoside transporters (ENT or concentrative transporters, CNT) and ensure signaling roles or be phosphorylated to increase the nucleotide pools, or it can bind extracellular ADO receptors (A1, A2A, A2B, and A3) which results in activation of coupled G proteins, corresponding molecular pathways, and expression of target genes [8]. CD38 and CD203a can substitute the activity of CD39 and generate AMP using NAD+ in an acid environment [9]. This alternative mechanism was first described in human T cells and recently in myeloma cells. As explained, in the case of multiple myeloma, ADO in bone marrow is mainly provided by CD38, CD203a, and CD73 expressed by different cells within the bone morrow. In view of this novel insight, it is important to consider the origin of ADO in order to efficiently block its production.
Fig. 1.
Metabolism and signaling of adenosine in tumor microenvironment. Adenosine in the tumor microenvironment derives from hydrolysis of ATP. Within the tumor microenvironment, different cells can participate in increasing the ADO concentration, such as cancer cells, fibroblasts, adipocytes, endothelial cells, and adaptive and innate immune cells. Meanwhile, the distribution of metabolic enzymes is not the same on all cells and can vary and depends on tumor localization, hypoxia, and extrinsic signals from other cells. Increased levels of adenosine in the tumor microenvironment have an immunosuppressive effect on innate immune cells via ADORA-dependent signaling. Resulting modulation of innate immune cells function manifests by decreased local inflammation and increased pro-tumoral effect of innate immune cells (Table 1). ADA, adenosine deaminase; ADORA, adenosine receptor; TME, tumor microenvironment
Derived from various cell types, ADO has an immunosuppressing effect in the tumor microenvironment. We will review in the next paragraphs how this purine nucleoside affects different innate immune cells within the tumor microenvironment and how it can influence disease progression.
Adenosine and macrophages
Macrophages are innate immune cells derived from monocytes involved in tissue homeostasis. They remove apoptotic and/or senescent cells and extracellular pathogens by phagocytosis. Besides this clearance activity, these cells can modulate an immune response by secreting inflammatory mediators and initiate the adaptive response by presenting antigens to T lymphocytes. In the tumor microenvironment (TME), infiltrating macrophages are called tumor-associated macrophages (TAMs). To date, this cell type is the best characterized infiltrating innate cells, and two types are described: classically activated macrophages (M1) and alternatively activated macrophages (M2) [10]. Current results suggest that M1 macrophages predominately express A2A receptors, while A2B receptor is responsible of alternative macrophages activation into M2 phenotype [11].
M1 macrophage polarization is driven by danger signals such as ATP and cytokines secreted by Th1 lymphocytes. These macrophages have an anti-tumoral effect. Their functions are limited to presenting antigens on major histocompatibility complex (MHC) class II to T helper cells after phagocytosis of cell debris and to producing pro-inflammatory cytokines, which help to attract effector cells. ADO has multiple suppressive effects on M1 macrophages functions. Indeed, A2 receptor signaling can block the differentiation of monocytes into macrophages [12] and abolish the phagocytic activity of monocytes and macrophages [13]. Macrophages produce different intracellularly active molecules like nitrogen oxide (NO) using inducible NO synthase (iNOS) that has anti-tumoral and bactericide effect. It has been shown that ADO can regulate the production of NO by decreasing the production of iNOS [14]. Moreover, A2B receptor activation by ADO or agonists blocks IFNγ production by Th1 cells and thus IFNγ-induced MHC type II expression on macrophages [15]. Consequently, these antigen-presenting cells display less MHC-peptide complexes on their surface, leading to a less efficient adaptive response toward the tumor. ADO also negatively regulates M1 macrophages by modulating their differentiation toward a less pro-inflammatory phenotype. Indeed, there is a certain plasticity in macrophage polarization, and macrophages can switch between their pro- or anti-tumoral phenotype according to extrinsic signals. This is the reason why most of TAMs have M2-like phenotypes, which are acquired in the TME by the action of different pro-tumoral factors. For example, activation of A2 receptors decreases the inflammatory reaction by blocking the production of pro-inflammatory cytokines in macrophages [14]. Thus, target genes such as TNFα are no longer expressed, while the secretion of IL-10 increases. The latter has an anti-inflammatory function and prevents excessive inflammatory response, thereby protecting normal tissue homeostasis. Nevertheless, this factor has a pro-tumoral effect in the TME, promoting inhibition of effector cells and metastasis development.
M2 macrophages are believed to have pro-tumoral functions because of their decreased production of pro-inflammatory factors and low phagocytic activity. M2 macrophages can be divided into M2a, M2b, M2c, and M2d subsets in regard to surface markers and secreted cytokines. The polarization into different subsets is driven by different anti-inflammatory cytokines. Notably, M2d macrophages can be induced by ADO signaling in a cytokine-independent way via A2A receptor [16, 17]. In consequence, M2d macrophages secrete factors such as VEGF and IL-10, which have pro-angiogenic function. Moreover, IL-10 promotes Th17 and Treg polarization, the latter being involved in regulation and inhibition of inflammatory reactions. Along with other factors such as IL-6, TGF-β, and metalloproteinase-9 (MMP-9), M2 macrophages can be involved in tissue repair and matrix remodeling, thus promoting tumor growth, angiogenesis, and metastasis development [18, 19].
Adenosine and natural killer cells
Natural killer (NK) cells are cytotoxic cells of the innate immunity. They display cytotoxic functions, but unlike CD8+ cells, they do not need to be activated by specific antigens. Their particularity consists in being inhibited by normal cells, via MHC class I expression. An abnormal cell, for example, a transformed cancer cell, tends to decrease MHC class I expression, thus escaping lysis by CD8+ T cells. NK cells sense these MCH class I altered expression and kill the cell. They also contribute to the immune response in immunotherapy. They express Fc receptors (CD16 molecule) and are thus able to bind the constant fragment of antibodies that target cancer cells. This interaction allows to stabilize the physical interaction between NK cells and their targets, facilitating the FAS/FAS-L interaction. Moreover, Fc receptor/antibody interaction results in antibody-dependent cell cytotoxicity (or ADCC) activation and kills the cell by degranulation of cytotoxic factors.
In the tumor microenvironment, ADO inhibits NK activity, and it can result in NK cell death. NK cells require a maturation stage to be fully activated, and this process can be interrupted by A2A receptor signaling as it was demonstrated that A2AR-deficient mice have an increased pool of mature NK cells in the periphery and a reduced fraction of immature NK cells [20]. In addition, mature NK cells maintain their proliferation ability in A2AR-deficient mice, and they have in general greater capacity to control tumor initiation and growth.
The tumor-associated ADO can also promote NK cell dysfunction through the accumulation of cAMP within the cell [21]. cAMP activates PKA, which in turn will activate various targets and modify cellular functions; this results in decreased cytotoxicity and cytokine production. This was observed with NK cells incubated with CADO, a stable ADO-analogue, which binds A2A receptor. Moreover, cAMP/PKA signaling pathway can activate caspase-dependent apoptosis, thus promoting cell death.
Another effect of ADO is to inhibit TNFα secretion by NK cells [22]. As said before, TNFα is required in many functions (inflammation, apoptosis, and others). Therefore, inhibited NK cells are unable to stimulate other cells like macrophages and neutrophils.
Neo et al. recently showed that tumor cells modulate infiltrated NK cells by the engagement of 4-1BBL (CD137) on their surface, inducing the transport of intracellular vesicles with CD73 to the cell surface of NK cells and upregulate the production and secretion of IL-10 and TGF-β in STAT3-dependent manner [23]. Once in the TME, IL-10 inhibits the proliferation of CD4+ T cells and its IFNγ production, thus blocking adaptive anti-tumor response, and CD73 on NK cells increase ADO concentration within the TME, which will in turn suppress immune response.
Adenosine and neutrophils
Neutrophils are the most abundant circulating innate cells. Their lifespan is short, but these cells are very effective in pathogen elimination. When infiltrated in the tumor, neutrophils are called tumor-associated neutrophils (TANs). They are capable of phagocytosis and degranulation and can form neutrophil extracellular traps (NET). Neutrophils are increasingly recognized as important actors in the cross talk between immune cells and cancer cells by modulating the recruitment of adaptive cells via cytokine and chemokine production [24]. As for macrophages, neutrophils can undergo two different types of polarization and acquire anti-tumor phenotype (N1) or pro-tumor (N2) phenotype. The major difference between these two cell types is their capacity in production of pro- or anti-inflammatory cytokines and the fact that N2 cells are pro-angiogenic and prometastatic.
Several studies on TANs report that ADO can inhibit their adhesion to endothelial cells, thus blocking the migration of neutrophils from bloodstream to inflammatory site [25]. ADO, via its A2A receptor, can modulate the capacity of neutrophils to produce pro-inflammatory cytokines [26]. Indeed, it was shown that the activation of A2AR on neutrophils inhibits the production of pro-inflammatory cytokines and chemokines such as TNFα, CCL3, CCL4, and others. In the TME, decreased levels of these molecules result in ineffective recruitment of innate cells such as NK cells, monocytes, and others. Moreover, several teams showed the involvement of A2 and A3 receptors in inhibition of neutrophil degranulation, which decreases pro-inflammatory potential of neutrophils [27, 28].
Adenosine and mast cells
In recent studies, scientists investigated the function of mast cells in the TME. These cells originate from myeloid progenitor cells and have an important function in various immune responses. Mature cells reside in peripheral tissues and are commonly activated by antigen-specific antibodies. Beside their role in allergic reactions and inflammation, mast cells can influence angiogenesis and wound healing, making them attractive cells to modulate TME in a pro-tumoral way. Gorzalczany et al. investigated mast cell activation in the TME and found that they can be activated by direct contact with cancer cell membranes when they infiltrate the tumor [29] or rather by cancer-derived extracellular vesicles when they locate in the TME periphery [30]. This activation results in IL-8, IL-6, and VEGF secretion [26]. Combined together, these mediators promote neo-angiogenesis, and new blood vessels will supply the tumor with nutrition and oxygen and promote metastasis development. Both mechanisms involve CD73 activation on mast cell and autocrine signaling of ADO via A3 receptor [29, 30].
Adenosine and dendritic cells
Dendritic cells (DC) are professional antigen-presenting cells responsible of activation of naive T cells and induction of their differentiation into effector cells. Thus, DCs assure the link between innate and adaptive immune systems by recognition, phagocytosis, and presentation of antigens to adaptive cells. DCs originate from bone morrow monocyte-dendritic cell progenitor (MDP) which differentiate into common DC precursor (CDP) and then into one of the two major DC subsets. It was also shown, both in vivo and in vitro, that monocytes can give rise to DC, called monocyte-derived DC [31]. This occurs during inflammatory reactions caused by cancer or infection.
Panther et al. investigated how ADO affects normal function of DCs. They showed that DCs still express A2A receptor after maturation, and exposure of mature DCs to ADO or A2A receptor agonist resulted in increased levels of intracellular cAMP and inhibited production of IL-12 [32]. In addition, their results suggest that DCs maturated in the presence of ADO produce IL-10 and have a reduced capacity of induction of Th1 [33]. Novitskiy et al. performed multiple assays of differentiation of human monocytes and mouse peritoneal macrophages and hematopoietic progenitor cells (HPCs) into myeloid DCs in the presence of increased levels of ADO [34]. Their results show impaired activation and function of resulting DCs as they fail to activate naive T cells and they produce anti-inflammatory, angiogenic, and tolerogenic factors. This ADO effect is due to the activation of A2B receptor [34]. These findings suggest that ADO not only affects innate and adaptive cells but also the only cell type capable of activation of effector cells.
Future perspectives
Targeting ADO in TME has become a very promising strategy in cancer treatment since this will allow to enhance the immune response against cancer cells. The therapeutic approaches currently studied to block ADO functions are the inhibition of ADO-producing enzymes (CD39 and CD73) or ADO receptors. Ongoing studies investigate the effect of anti-ADO strategies on effector cells of the adaptive immune system, since these cells assure specific anticancer immune response [35–38]. However, these studies usually do not describe how anti-ADO strategies in cancer influence innate immune cells. Considering that functional maintenance of anticancer activity of innate immune cells would result in efficient activation of adaptive immune cells, leading to global and multi-effector tumor control, it seems fair to include the innate compartment in such studies.
Little is known about the effect of ADO-targeting treatments on innate immune cells. However, this can, at least in part, be deduced from these cells’ activities and their ADORA expression profiles (Table 1). Indeed, as innate immune cells can directly trigger cancer cell death and/or recruit and activate adaptive immune cells [39], their regulation by ADO-targeting drugs will most certainly have an impact on the anticancer activity of these compounds. These effects can also be indirect as exemplified by the studies focusing on M2 macrophages reprogramming toward a less pro-tumoral phenotype [40, 41] or modulating NK cells activity [42]. Knowing the role of ADO in the differentiation or maturation of these cells, these processes could be reinforced by ADO analogs or receptor antagonists. Moreover, DC-based anticancer vaccines [43] consist in educating these cells in vitro to present the tumor antigens on their surface and reinject them into the patient in order to activate tumor-specific T cells. The use of ADO signaling-targeting agents during the reinjection of DCs could support a favorable cytokine context and maintain the potency of these cells. Other cancer strategies consist in blocking TGFβ to enhance neutrophil recruitment and anticancer activation [44]. It was recently demonstrated that CD73/ADO signaling could trigger TGFβ expression in cervical cancer [45]. Thus, targeting the adenosinergic pathway on cancer cells themselves may be an interesting way to indirectly act on neutrophil recruitment and polarization. In addition, this study suggests that targeting TGFβ-1 in cancer could also impact the ADO cascade.
Table 1.
Effect of adenosine on different innate immune cells
| Innate immune cells | ADORA receptor | Effect of adenosine |
|---|---|---|
| Macrophages | A2A, A2B, A3 | Decrease in pro-inflammatory cytokine/chemokine production |
| A2B | Inhibition of phagocytosis | |
| A1, A2A, A2B | Inhibition of superoxide production (anti-microbial and anti-tumoral activity) | |
| A1, A2A, A3 | Increase in anti-inflammatory cytokines production | |
| A2B | Inhibition MHC-II production | |
| A3 | Induction of MMP-9 production | |
| A2A, A2B | Induction of M2-like macrophages polarization | |
| Natural killer cells (NK cells) | A2A | Suppression of NK maturation in TME |
| Suppression of cell activity by induction of cAMP/PKA pathway | ||
| Increase in TNFα release | ||
| Neutrophils | A2 | Inhibition of adhesion and transmigration |
| A2A | Inhibition of production of pro-inflammatory cytokines | |
| A2, A3 | Inhibition of degranulation | |
| Mast cells | A3 | Production of various cytokines (IL-8, IL-6, VEGF) → promotion of tumor growth and metastasis |
| Dendritic cells | A2B | Polarization of monocytes into aberrant DCs unable to activate T cells |
| A2A | Decrease in pro-inflammatory cytokine production | |
| Increase in anti-inflammatory cytokines production |
On the other hand, due to the diversity of ADO-mediated effects on different cells in the TME (Table 1), ADO receptor targeting drugs should be used carefully, in order to maintain a balance that favors global anti-tumoral functions [8]. ADO-dependent immunosuppression is a physiological barrier which controls inflammatory and immune response in the organism. Thus, it should be defined to what extent this axis can safely be modified. The alteration of multiple components of the TME such as adipose and vascular tissues or fibroblasts must be taken into account because of their interactions with the immune system or their direct involvement in cancer progression. Further understanding the complex interplay between ADO axis-targeting strategies and conventional chemotherapies, immunotherapies, or targeted therapies on different molecular and cellular actors within the TME is needed in order to optimize appropriate anticancer responses.
In conclusion, as reviewed in this paper, ADO has diverse and important biological roles on various cells of the innate immune system. The knowledge about these processes is of main importance in the clinical settings where ADO signaling is targeted.
Funding information
LPJ receives funding from Olav Raagholt og Gerd Meidel Raagholts stiftelse for forskning.
Compliance with ethical standards
Conflict of interest
Regina Strakhova declares that she has no conflict of interest.
Octavia Cadassou declares that she has no conflict of interest.
Emeline Cros-Perrial declares that she has no conflict of interest.
Lars Petter Jordheim declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Regina Strakhova, Email: regina.strakhova@etu.univ-lyon1.fr.
Octavia Cadassou, Email: octavia.cadassou@uclouvain.be.
Emeline Cros-Perrial, Email: emeline.perrial@univ-lyon1.fr.
Lars Petter Jordheim, Email: lars-petter.jordheim@univ-lyon1.fr.
References
- 1.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnes TA, Amir E. HYPE or HOPE: the prognostic value of infiltrating immune cells in cancer. Br J Cancer. 2017;117:451–460. doi: 10.1038/bjc.2017.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar V. Adenosine as an endogenous immunoregulator in cancer pathogenesis: where to go? Purinergic Signal. 2013;9:145–165. doi: 10.1007/s11302-012-9349-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karmouty-Quintana H, Xia Y, Blackburn MR. Adenosine signaling during acute and chronic disease states. J Mol Med. 2013;91:173–181. doi: 10.1007/s00109-013-0997-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bester AC, Roniger M, Oren YS, Im MM, Sarni D, Chaoat M, Bensimon A, Zamir G, Shewach DS, Kerem B. Nucleotide deficiency promotes genomic instability in early stages of cancer development. Cell. 2011;145:435–446. doi: 10.1016/j.cell.2011.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boison D, Yegutkin GG. Adenosine metabolism: emerging concepts for cancer therapy. Cancer Cell. 2019;36:582–596. doi: 10.1016/j.ccell.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zylka MJ, Sowa NA, Taylor-Blake B, Twomey MA, Herrala A, Voikar V, Vihko P. Prostatic acid phosphatase is an ectonucleotidase and suppresses pain by generating adenosine. Neuron. 2008;60:111–122. doi: 10.1016/j.neuron.2008.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borea PA, Gessi S, Merighi S, Vincenzi F, Varani K. Pharmacology of adenosine receptors: the state of the art. Physiol Rev. 2018;98:1591–1625. doi: 10.1152/physrev.00049.2017. [DOI] [PubMed] [Google Scholar]
- 9.Horenstein AL, Bracci C, Morandi F, Malavasi F. CD38 in adenosinergic pathways and metabolic re-programming in human multiple myeloma cells: in-tandem insights from basic science to therapy. Front Immunol. 2019;10:760. doi: 10.3389/fimmu.2019.00760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haskó G, Pacher P. Regulation of macrophage function by adenosine. Arterioscler Thromb Vasc Biol. 2012;32:865–869. doi: 10.1161/ATVBAHA.111.226852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Najar HM, Ruhl S, Bru-Capdeville AC, Peters JH. Adenosine and its derivatives control human monocyte differentiation into highly accessory cells versus macrophages. J Leukoc Biol. 1990;47:429–439. doi: 10.1002/jlb.47.5.429. [DOI] [PubMed] [Google Scholar]
- 13.Belikoff BG, Hatfield S, Georgiev P, Ohta A, Lukashev D, Buras JA, Remick DG, Sitkovsky M. A2B adenosine receptor blockade enhances macrophage-mediated bacterial phagocytosis and improves polymicrobial sepsis survival in mice. JI. 2011;186:2444–2453. doi: 10.4049/jimmunol.1001567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haskó G, Szabó C, Németh ZH, et al. Adenosine receptor agonists differentially regulate IL-10, TNF-alpha, and nitric oxide production in RAW 264.7 macrophages and in endotoxemic mice. J Immunol. 1996;157:4634–4640. [PubMed] [Google Scholar]
- 15.Xaus J, Mirabet M, Lloberas J, et al. IFN-gamma up-regulates the A2B adenosine receptor expression in macrophages: a mechanism of macrophage deactivation. J Immunol. 1999;162:3607–3614. [PubMed] [Google Scholar]
- 16.Ferrante CJ, Pinhal-Enfield G, Elson G, Cronstein BN, Hasko G, Outram S, Leibovich SJ. The adenosine-dependent angiogenic switch of macrophages to an M2-like phenotype is independent of interleukin-4 receptor alpha (IL-4Rα) signaling. Inflammation. 2013;36:921–931. doi: 10.1007/s10753-013-9621-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pinhal-Enfield G, Ramanathan M, Hasko G, Vogel SN, Salzman AL, Boons GJ, Leibovich SJ. An angiogenic switch in macrophages involving synergy between toll-like receptors 2, 4, 7, and 9 and adenosine A2A receptors. Am J Pathol. 2003;163:711–721. doi: 10.1016/S0002-9440(10)63698-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Velot E, Haas B, Léonard F, Ernens I, Rolland-Turner M, Schwartz C, Longrois D, Devaux Y, Wagner DR. Activation of the adenosine-A3 receptor stimulates matrix metalloproteinase-9 secretion by macrophages. Cardiovasc Res. 2008;80:246–254. doi: 10.1093/cvr/cvn201. [DOI] [PubMed] [Google Scholar]
- 19.Csóka B, Selmeczy Z, Koscsó B, Németh ZH, Pacher P, Murray PJ, Kepka-Lenhart D, Morris SM, Jr, Gause WC, Leibovich SJ, Haskó G. Adenosine promotes alternative macrophage activation via A 2A and A 2B receptors. FASEB J. 2012;26:376–386. doi: 10.1096/fj.11-190934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Young A, Ngiow SF, Gao Y, Patch AM, Barkauskas DS, Messaoudene M, Lin G, Coudert JD, Stannard KA, Zitvogel L, Degli-Esposti MA, Vivier E, Waddell N, Linden J, Huntington ND, Souza-Fonseca-Guimaraes F, Smyth MJ. A2AR adenosine signaling suppresses natural killer cell maturation in the tumor microenvironment. Cancer Res. 2018;78:1003–1016. doi: 10.1158/0008-5472.CAN-17-2826. [DOI] [PubMed] [Google Scholar]
- 21.Lokshin A, Raskovalova T, Huang X, Zacharia LC, Jackson EK, Gorelik E. Adenosine-mediated inhibition of the cytotoxic activity and cytokine production by activated natural killer cells. Cancer Res. 2006;66:7758–7765. doi: 10.1158/0008-5472.CAN-06-0478. [DOI] [PubMed] [Google Scholar]
- 22.Miller JS, Cervenka T, Lund J, et al. Purine metabolites suppress proliferation of human NK cells through a lineage-specific purine receptor. J Immunol. 1999;162:7376–7382. [PubMed] [Google Scholar]
- 23.Neo SY, Yang Y, Julien R, et al. CD73 immune checkpoint defines regulatory NK-cells within the tumor microenvironment. J Clin Investig. 2019;130:1185–1198. doi: 10.1172/JCI128895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu S, Awaji S. Tumor-associated neutrophils in cancer: going pro. Cancers. 2019;11:564. doi: 10.3390/cancers11040564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Felsch A, Stöcker K, Borchard U. Phorbol ester-stimulated adherence of neutrophils to endothelial cells is reduced by adenosine A2 receptor agonists. J Immunol. 1995;155:333–338. [PubMed] [Google Scholar]
- 26.McColl SR, St-Onge M, Dussault A-A, et al. Immunomodulatory impact of the A 2A adenosine receptor on the profile of chemokines produced by neutrophils. FASEB J. 2006;20:187–189. doi: 10.1096/fj.05-4804fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bouma MG, Jeunhomme TM, Boyle DL, et al. Adenosine inhibits neutrophil degranulation in activated human whole blood: involvement of adenosine A2 and A3 receptors. J Immunol. 1997;158:5400–5408. [PubMed] [Google Scholar]
- 28.Gessi S, Varani K, Merighi S, Cattabriga E, Iannotta V, Leung E, Baraldi PG, Borea PA. A(3) adenosine receptors in human neutrophils and promyelocytic HL60 cells: a pharmacological and biochemical study. Mol Pharmacol. 2002;61:415–424. doi: 10.1124/mol.61.2.415. [DOI] [PubMed] [Google Scholar]
- 29.Gorzalczany Y, Akiva E, Klein O, Merimsky O, Sagi-Eisenberg R. Mast cells are directly activated by contact with cancer cells by a mechanism involving autocrine formation of adenosine and autocrine/paracrine signaling of the adenosine A3 receptor. Cancer Lett. 2017;397:23–32. doi: 10.1016/j.canlet.2017.03.026. [DOI] [PubMed] [Google Scholar]
- 30.Gorzalczany Y, Merimsky O, Sagi-Eisenberg R. Mast cells are directly activated by cancer cell–derived extracellular vesicles by a CD73- and adenosine-dependent mechanism. Transl Oncol. 2019;12:1549–1556. doi: 10.1016/j.tranon.2019.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coillard A, Segura E. In vivo differentiation of human monocytes. Front Immunol. 2019;10:1907. doi: 10.3389/fimmu.2019.01907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Panther E, Idzko M, Herouy Y, et al. Expression and function of adenosine receptors in human dendritic cells. FASEB J. 2001;15:1963–1970. doi: 10.1096/fj.01-0169com. [DOI] [PubMed] [Google Scholar]
- 33.Panther E, Corinti S, Idzko M, Herouy Y, Napp M, la Sala A, Girolomoni G, Norgauer J. Adenosine affects expression of membrane molecules, cytokine and chemokine release, and the T-cell stimulatory capacity of human dendritic cells. Blood. 2003;101:3985–3990. doi: 10.1182/blood-2002-07-2113. [DOI] [PubMed] [Google Scholar]
- 34.Novitskiy SV, Ryzhov S, Zaynagetdinov R, Goldstein AE, Huang Y, Tikhomirov OY, Blackburn MR, Biaggioni I, Carbone DP, Feoktistov I, Dikov MM. Adenosine receptors in regulation of dendritic cell differentiation and function. Blood. 2008;112:1822–1831. doi: 10.1182/blood-2008-02-136325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ohta A, Gorelik E, Prasad SJ, Ronchese F, Lukashev D, Wong MKK, Huang X, Caldwell S, Liu K, Smith P, Chen JF, Jackson EK, Apasov S, Abrams S, Sitkovsky M. A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci. 2006;103:13132–13137. doi: 10.1073/pnas.0605251103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fong L, Hotson A, Powderly JD, Sznol M, Heist RS, Choueiri TK, George S, Hughes BGM, Hellmann MD, Shepard DR, Rini BI, Kummar S, Weise AM, Riese MJ, Markman B, Emens LA, Mahadevan D, Luke JJ, Laport G, Brody JD, Hernandez-Aya L, Bonomi P, Goldman JW, Berim L, Renouf DJ, Goodwin RA, Munneke B, Ho PY, Hsieh J, McCaffery I, Kwei L, Willingham SB, Miller RA. Adenosine 2A receptor blockade as an immunotherapy for treatment-refractory renal cell cancer. Cancer Discov. 2020;10:40–53. doi: 10.1158/2159-8290.CD-19-0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Antonioli L, Yegutkin GG, Pacher P, Blandizzi C, Haskó G. Anti-CD73 in cancer immunotherapy: awakening new opportunities. Trends in Cancer. 2016;2:95–109. doi: 10.1016/j.trecan.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perrot I, Michaud H-A, Giraudon-Paoli M, et al. Blocking antibodies targeting the CD39/CD73 immunosuppressive pathway unleash immune responses in combination cancer therapies. Cell Rep. 2019;27:2411–2425.e9. doi: 10.1016/j.celrep.2019.04.091. [DOI] [PubMed] [Google Scholar]
- 39.Woo S-R, Corrales L, Gajewski TF. Innate immune recognition of cancer. Annu Rev Immunol. 2015;33:445–474. doi: 10.1146/annurev-immunol-032414-112043. [DOI] [PubMed] [Google Scholar]
- 40.Vidyarthi A, Khan N, Agnihotri T, Negi S, Das DK, Aqdas M, Chatterjee D, Colegio OR, Tewari MK, Agrewala JN. TLR-3 stimulation skews M2 macrophages to M1 through IFN-αβ signaling and restricts tumor progression. Front Immunol. 2018;9:1650. doi: 10.3389/fimmu.2018.01650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang F, Parayath NN, Ene CI, Stephan SB, Koehne AL, Coon ME, Holland EC, Stephan MT. Genetic programming of macrophages to perform anti-tumor functions using targeted mRNA nanocarriers. Nat Commun. 2019;10:3974. doi: 10.1038/s41467-019-11911-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zaghi E, Calvi M, Marcenaro E, Mavilio D, di Vito C. Targeting NKG2A to elucidate natural killer cell ontogenesis and to develop novel immune-therapeutic strategies in cancer therapy. J Leukoc Biol. 2019;105:1243–1251. doi: 10.1002/JLB.MR0718-300R. [DOI] [PubMed] [Google Scholar]
- 43.Saxena M, Bhardwaj N. Re-emergence of dendritic cell vaccines for cancer treatment. Trends Cancer. 2018;4:119–137. doi: 10.1016/j.trecan.2017.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.García-Rocha R, Monroy-García A, Hernández-Montes J, Weiss-Steider B, Gutiérrez-Serrano V, del Carmen Fuentes-Castañeda M, Ávila-Ibarra LR, Don-López CA, Torres-Pineda DB, de Lourdes Mora-García M. Cervical cancer cells produce TGF-β1 through the CD73-adenosine pathway and maintain CD73 expression through the autocrine activity of TGF-β1. Cytokine. 2019;118:71–79. doi: 10.1016/j.cyto.2018.09.018. [DOI] [PubMed] [Google Scholar]