Abstract
Cyanobacteria produce a wide range of lipopeptides that exhibit potent membrane-disrupting activities. Laxaphycins consist of two families of structurally distinct macrocyclic lipopeptides that act in a synergistic manner to produce antifungal and antiproliferative activities. Laxaphycins are produced by range of cyanobacteria but their biosynthetic origins remain unclear. Here, we identified the biosynthetic pathways responsible for the biosynthesis of the laxaphycins produced by Scytonema hofmannii PCC 7110. We show that these laxaphycins, called scytocyclamides, are produced by this cyanobacterium and are encoded in a single biosynthetic gene cluster with shared polyketide synthase enzymes initiating two distinct non-ribosomal peptide synthetase pathways. The unusual mechanism of shared enzymes synthesizing two distinct types of products may aid future research in identifying and expressing natural product biosynthetic pathways and in expanding the known biosynthetic logic of this important family of natural products.
Keywords: synergy, biosynthesis, natural product, laxaphycin, scytocyclamide, antifungal, cupin, dehydrobutyrine
Introduction
Natural products are small molecules produced by living organisms (Newman and Cragg, 2016). Research interest in natural products is focused on the discovery of new molecules with pharmaceutical applications (Spainhour, 2005; Newman and Cragg, 2016). Natural products often have complex chemical structures with rare chemical moieties that allow them to react with specific molecular targets and to kill or inhibit the growth of other organisms (Rodrigues et al., 2016). Cyanobacteria produce a wide variety of natural products with potent bioactivities (Demay et al., 2019; Huang and Zimba, 2019). Characterization of new natural products offers starting material for drug design as new active structures (Rodrigues et al., 2016). Characterization of the biosynthesis of these products advance methods in production of the structures through combinatorial biosynthesis (Kim et al., 2015). Many microbial and cyanobacterial natural products are synthesized by polyketide synthases (PKS) and non-ribosomal peptide synthetases (NRPS) (Kehr et al., 2011; Dittmann et al., 2015). PKS and NRPS enzymes often act together and are encoded in joint biosynthetic gene clusters producing hybrid PKS/NRPS products (Miyanaga et al., 2018). PKS and NRPS enzymes allow the production of complex structures with characteristic non-proteinogenic amino acids and the combination of non-ribosomal peptides (NRP) with polyketide chains and decorations (Evans et al., 2011). NRPS and PKS biosynthesis typically follows a colinearity rule, where the number and order of the catalytic domains correspond to the amino acid number, order and structure in the product (Guenzi et al., 1998; Callahan et al., 2009).
Laxaphycins are cyanobacterial cyclic lipopeptides that fall in two distinct structural macrocycles consisting of either 11 amino acids (known as A-type laxaphycins) or 12 amino acids (known as B-type laxaphycins) (Frankmölle et al., 1992a; Luo et al., 2015). Both types include β-aminooctanoic acid (Aoa) or β-aminodecanoic acid (Ada) (Table 1). Eleven- and 12-residue laxaphycins have strong synergistic activity in antifungal and antiproliferative bioactivity assays (Frankmölle et al., 1992b; MacMillan et al., 2002; Cai et al., 2018). Laxaphycins are hypothesized to be produced by the PKS/NRPS hybrid pathway (Bornancin et al., 2015, 2019). However, the biosynthetic origins of members of the laxaphycin family remains unclear. Despite sharing the same name, they are chemically distinct and are anticipated to be produced by distinct pathways (Bornancin et al., 2015, 2019). The nomenclature of laxaphycins is complicated due to the two distinct core types addressed as a single family combined with naming new members after the producing organisms and distinguishing variants with lettering complicates (Table 1). Therefore, we refer to the two types as 11- and 12-residue laxaphycins. There are 30 diverse members assigned to the laxaphycin family reported to date (Table 1). The first laxaphycins to exhibit synergistic effects were described from Anabaena laxa (Frankmölle et al., 1992a,b). Here, we focused on laxaphycin variants called scytocyclamides produced by Scytonema hofmannii PCC 7110 (Grewe, 2005). S. hofmannii PCC 7110 was previously studied by our group and a methanol crude extract of the cells was antifungal but the active agent was not identified (Shishido et al., 2015).
TABLE 1.
Amino acid sequence of laxaphycin variants.
| Amino acid residue | Ref. | ||||||||||||
| 11-residue laxaphycins | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | ||
| Laxaphycin A | Aoa | Hse | Dhb | OHPro | HSe | Phe | Leu | Ile | Ile | Leu | Gly | 1 | |
| Laxaphycin A2 | Aoa | Hse | Dhb | OHPro | HSe | Phe | Leu | Val | Ile | Leu | Gly | 10 | |
| Laxaphycin E | Ada | Hse | Dhb | OHPro | HSe | Phe | Leu | Ile | Ile | Leu | Gly | 1 | |
| Hormothamnin A | Aoa | Hse | Dhb | OHPro | HSe | Phe | Leu | Ile | Ile | Leu | Gly | 2 | |
| Lobocyclamide A | Aoa | Ser | Dhb | OHPro | HSe | Tyr | Leu | Ile | Ile | Leu | Gly | 3 | |
| Trichormamide A | Ada | Ser | Ser | Pro | Ser | Tyr | Leu | Ile | Ile | Pro | Gly | 7 | |
| Trichormamide D | Ada | Gln | Dhb | Pro | Ser | Tyr | Leu | Val | Phe | Leu | Gly | 8 | |
| Scytocyclamide A | Aoa | Gln | Dhb | OHPro | HSe | Phe | Leu | Ile | Ile | Leu | Gly | 4 | |
| [L-Val8]laxaphycin A | Aoa | Hse | Dhb | OHPro | HSe | Phe | Leu | Val | Ile | Leu | Gly | 11 | |
| [D-Val9]laxaphycin A | Aoa | Hse | Dhb | OHPro | HSe | Phe | Leu | Ile | Val | Leu | Gly | 11 | |
| Acyclolaxaphycin A | H-Aoa | Hse | Dhb | OHPro | HSe | Phe | Leu | Ile | Ile | Leu | Gly-OH | 11 | |
| [des-Gly11] acyclolaxaphycin A | H-Aoa | Hse | Dhb | OHPro | HSe | Phe | Leu | Ile | Ile | Leu-OH | 11 | ||
| [des-(Leu10-Gly11)] acyclolaxaphycin A | H-Aoa | Hse | Dhb | OHPro | HSe | Phe | Leu | Ile | Ile-OH | 11 | |||
| 12-residue laxaphycins | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | Ref. |
| Laxaphycin B | Ada | Val | OHLeu | Ala | OHLeu | Gln | NMe-Ile | OHAsn | Thr | Pro | Leu | Thr | 1 |
| Laxaphycin B2 | Ada | Val | OHLeu | Ala | Leu | Gln | NMe-Ile | OHAsn | Thr | Pro | Leu | Thr | 5 |
| Laxaphycin B3 | Ada | Val | OHLeu | Ala | OHLeu | Gln | NMe-Ile | OHAsn | Thr | OHPro | Leu | Thr | 5 |
| Laxaphycin B4 | Ada | Val | OHLeu | Hse | OHLeu | Gln | NMe-Ile | OHAsn | Thr | OHPro | Leu | Thr | 10 |
| Laxaphycin B5 | Ada | Ile | OHLeu | Val | OHLeu | Gln | NMe-Ile | Asn | Thr | Pro | Tyr | Thr | 12 |
| Laxaphycin B6 | Ada | Ile | OHLeu | Val | Leu | Gln | NMe-Ile | Asn | Thr | Pro | Tyr | Thr | 12 |
| Laxaphycin D | Aoa | Val | OHLeu | Ala | OHLeu | Gln | NMe-Ile | OHAsn | Thr | Pro | Leu | Thr | 1 |
| Lobocyclamide B | Ada | Val | OHLeu | Ala | OHLeu | Gln | NMe-Ile | OHThr | Thr | OHPro | Leu | Thr | 3 |
| Lobocyclamide C | Aoa | Val | OHLeu | Ala | OHLeu | Gln | NMe-Ile | OHThr | Thr | OHPro | Leu | Thr | 3 |
| Lyngbyacyclamide A | Ada | Val | OHLeu | Hse | Leu | Gln | NMe-Ile | OHAsn | Thr | Pro | Phe | Thr | 6 |
| Lyngbyacyclamide B | Ada | Val | OHLeu | Hse | Leu | Gln | NMe-Ile | OHAsn | Thr | OHPro | Phe | Thr | 6 |
| Trichormamide B | Ada | Ile | OHLeu | Hse | OHLeu | Gln | NMe-Ile | Ser | Thr | Pro | Tyr | Thr | 7 |
| Trichormamide C | Ada | Val | OHLeu | Ala | OHLeu | Gln | NMe-Ile | Asn | Thr | Pro | Leu | Thr | 8 |
| Acyclolaxaphycin B | Ada | Val | OHLeu-OH | H-Ala | OHLeu | Gln | NMe-Ile | OHAsn | Thr | Pro | Leu | Thr | 9 |
| Acyclolaxaphycin B3 | Ada | Val | OHLeu-OH | H-Ala | OHLeu | Gln | NMe-Ile | OHAsn | Thr | OHPro | Leu | Thr | 9 |
| Scytocyclamide B | Aoa | Val | OHLeu | Ala | OHLeu | Gln | NMe-Ile | OHAsn | Thr | Pro | Leu | Thr | 4 |
| Scytocyclamide C | Aoa | Val | OHLeu | Ala | Leu | Gln | NMe-Ile | OHAsn | Thr | Pro | Leu | Thr | 4 |
1 Frankmölle et al. (1992b), 2 Gerwick et al. (1992), 3 MacMillan et al. (2002), 4 Grewe (2005), 5 Bonnard et al. (2007), 6 Maru et al. (2010), 7 Luo et al. (2014), 8 Luo et al. (2015), 9 Bornancin et al. (2015), 10 Cai et al. (2018), 11 Bornancin et al. (2019), 12 Sullivan et al. (2020). Aoa – β-aminooctanoic acid, Ada - β-aminodecanoic acid, Hse - Homoserine, Dhb - Dehydrobutyrine, NMe-Ile – N-Methyl Isoleucine, OHPro – 4-hydroxyproline, OHAsn – 3-hydroxyasparagine, OHLeu – 3-hydroxyleucine, OHThr – 4-hydroxythreonine.
Here we describe the biosynthetic pathways responsible for the biosynthesis of scytocyclamides from S. hofmannii PCC 7110. We show that the two types of scytocyclamides are synthesized by a branched NRPS/PKS biosynthetic pathway. These pathways encode shared loading PKS enzymes that initiate two distinct NRPS pathways exceptionally to the colinearity rule. We also report the synergistic antifungal activity of scytocyclamides and three new laxaphycin variants (scytocyclamides A2, B2, and B3).
Materials and Methods
Scytocyclamide Purification
Scytonema hofmannii PCC 7110 was grown in 5-L Erlenmeyer flasks with 2.7 L modified Z8 medium without source of combined nitrogen (Supplementary Table S1) at 20–21°C with photon irradiation of 3–7 μmol m−2 s−1 with constant sterilized air bubbling for 3–5 weeks. Cells were collected by decanting excess media and centrifugation at 8000 × g for 5 min. Cells were frozen at −80°C and freeze-dried with CHRIST BETA 2–8 LSC plus freeze drier with a LYO CUBE 4–8 chamber. The total amount of freeze-dried biomass was 4 g.
For each gram of dry cells, 30 ml of methanol was used and the mixture was homogenized with Heidolph Silent crusher M at 20 000 rpm for 30 s. The suspension was centrifuged 10,000 × g for 5 min and supernatant was collected. Extraction of the precipitate was repeated with 30 ml of methanol. Chromatorex (Fuji-Davison Chemical Ltd., Aichi, Japan) chromatography silica ODS powder (10 ml) was added to the supernatant pool and the mixture was dried with rotary evaporator Büchi Rotavapor R-200 at 30°C. Solid phase extraction (SPE) was performed with Phenomenex SPE strata SI-1 silica 5 g/20 ml column, preconditioned with 20 ml isopropanol and 20 ml of heptane. Silica ODS powder with the dry extract was added to the column and extracted with heptane, ethyl acetate, acetone, acetonitrile, and methanol with each fraction collected individually. Fractions were dried with nitrogen gas flow and re-dissolved in 1 ml of methanol for bioactivity assays. The active methanol fraction was further fractionated with liquid chromatography. Chromatography was performed with an Agilent 1100 Series liquid chromatograph with a Phenomenex Luna 5 μm C18(2) (150 × 10 mm, 100 Å) column. The sample was injected in 100-μl batches and eluted with acetonitrile/isopropanol 1:1 (solvent B) and 0.1% HCOOH (solvent A) with a flow rate of 3 ml min–1 in the following four stages: 1, isocratic stage of 43% solvent B in A for 15 min; 2, a linear gradient of solvent B from 43% to 60% in 10 min; 3, a linear gradient of solvent B from 60% to 81% in 5 min; and 4, a linear gradient of solvent B from 81% to 100% in 6 min. Six scytocyclamide fractions were collected, dried with nitrogen, and weighed.
3-Hydroxyleucine Feeding Experiment
Scytonema hofmannii PCC 7110 was grown in 100-mL Erlenmeyer flasks with 41 mL modified Z8 medium without a source of combined nitrogen with 40 μM of racemic 3-OHLeu mixture of all four isomers (2-Amino-3-hydroxy-4-methylpentanoic acid, ABCR) to determine if 3-OHLeu is utilized as a substrate in scytocyclamide production. Control cultivations were grown on the same medium without added 3-OHLeu. For both media, three duplicates were cultivated at 20–21°C with photon irradiation of 3–7 μmol m−2 s−1 for 17 days. Cells were collected by decanting excess media and centrifugation 8000 × g for 5 min. Cells were frozen at −80°C and freeze-dried with CHRIST BETA 2–8 LSC plus with a LYO CUBE 4–8 freeze drier. Freeze-dried biomass was weighed and extracted with 0.5 ml methanol and glass beads (0.5-mm glass beads, Scientific Industries Inc., United States) using a FastPrep cell disrupter two times for 25 s at a speed of 6.5 m s−1. Samples were centrifuged at room temperature for 5 min at 10 000 × g and supernatant was collected.
Peptide Identification by LC-MS
Scytonema hofmannii PCC 7110 was grown in 500-mL Erlenmeyer flasks of with 250 mL modified Z8 medium without a source of combined nitrogen at 20–21°C with photon irradiation of 3–7 μmol m−2 s−1 with constant sterilized air bubbling for 4 weeks. Cells were collected by decanting excess media and centrifugation at 8000 × g for 5 min. Cells were frozen at −80°C and freeze-dried with CHRIST BETA 2–8 LSC plus with a LYO CUBE 4–8 freeze drier. Freeze-dried cells (100 mg) were extracted with 1 ml methanol and glass beads (0.5-mm glass beads, Scientific Industries Inc., United States) using a FastPrep cell disrupter two times for 25 s at a speed of 6.5 m s−1. Samples were centrifuged at room temperature for 5 min at 10 000 × g. The supernatant was collected and extraction was repeated with 1 ml of methanol.
Extracts and purified scytocyclamide methanol solutions were analyzed with UPLC-QTOF (Acquity I-Class UPLC-Synapt G2-Si HR-MS, Waters Corp., Milford, MA, USA) equipped with a Kinetex C8 column (2.1 × 50 or 100 mm, 1.7 μm, 100 Å, Phenomenex, Torrance, CA, United States). The equipment was injected with 0.5 or 1 μl samples, eluted at 40°C with 0.1% HCOOH in water (solvent A) and acetonitrile/isopropanol 1:1, + 0.1% HCOOH (solvent B) with a flow rate of 0.3 ml min–1. Two solvent gradients were used. 5% solvent B to 100% solvent B in 5 min, maintained for 2 min, back to 5% B in 0.50 min, and maintained for 2.50 min before next run. Alternatively, 10% solvent B to 70% of solvent B in 5 min, then to 95% of solvent B in 0.01 min, maintained for 1.99 min, then back to 10% of solvent B in 0.5 min, and finally maintained for 2.5 min before the next run. QTOF was calibrated using sodium formate and Ultramark 1621, which yielded a calibrated mass range from m/z 91 to 1921. Leucine Enkephalin was used at 10-s intervals as a lock mass reference compound. Mass spectral data were accumulated in positive electrospray ionization resolution mode. The MSE Trap Collision Energy Ramp Started from 40.0 eV and ended at 70.0 eV.
Bioactivity Assays
The same S. hofmannii PCC 7110 methanol extract used for peptide identification LC-MS was used for antimicrobial activity screening. The screening was performed with fungal and bacterial strains (Table 2). The following samples were pipetted directly on spots on agar: 50 μl cyanobacterial cellular methanol extract, 50 μl negative control (methanol), and 10 μl positive control (nystatin) (Nystatin, Streptomyces noursei, EMD Millipore Corp, Germany) solution 5 mg/ml in methanol for fungi and 10 μl ampicillin (Ampicillin sodium salt, Sigma, Israel) 50 mg/ml in 70% ethanol for bacteria. Solvents were allowed to evaporate, leaving the extracts diffused in the agar. Inocula were prepared by growing the fungi for 2–14 days on PDA (Potato Dextrose Agar) media at 28°C and bacteria for two days on BHI (Brain Heart Infusion) agar at 37°C. Cell mass was transferred with a cotton swab from the agar to 3 ml of sterile 5 M NaCl solution or sterile water in the case of A. flavus. The inocula were spread on the agar with cotton swabs. Fungal plates were incubated at 28°C and bacterial plates at 37°C for 2 days and analyzed for inhibition zones.
TABLE 2.
Strains used in bioassays.
| Organism | Strain | Medium | Incubation temperature |
| Fungi | |||
| Candida albicans | FBCC 2462 | PDA | 28°C |
| Candida guilliermondii | FBCC 2457 | PDA | 28°C |
| Candida krusei | FBCC 2464 | PDA | 28°C |
| Candida parapsilosis | FBCC 2465 | PDA | 28°C |
| Filobasidiella neoformans | FBCC 2466 | PDA | 28°C |
| Aspergillus niger | FBCC 2467 | PDA | 28°C |
| Aspergillus parasiticus | FBCC 2500 | PDA | 28°C |
| Aspergillus flavus | FBCC 2467 | PDA | 28°C |
| Bacteria | |||
| Staphylococcus aureus | HAMBI 66 | BHI | 37°C |
| Enterococcus faecium | HAMBI 1821 | BHI | 37°C |
| Bacillus cereus | HAMBI 1881 | BHI | 37°C |
| Micrococcus luteus | HAMBI 2688 | BHI | 37°C |
| Pseudomonas aeruginosa | HAMBI 25 | BHI | 37°C |
| Escherichia coli | HAMBI 1723 | BHI | 37°C |
| Acinetobacter baumannii | HAMBI 1760 | BHI | 37°C |
| Enterobacter aerogenes | HAMBI 1898 | BHI | 37°C |
| Salmonella enterica | HAMBI 2331 | BHI | 37°C |
The antifungal activity of purified scytocyclamide fractions dissolved in methanol were tested with A. flavus performed as with the cellular extract. Disk diffusion assays were performed with purified scytocyclamides as follows. Paper disks (Blank monodiscs, Abtek biologicals Ltd., United Kingdom) were prepared with methanol solutions of the peptides, methanol as a negative control, and nystatin as a positive control. A. flavus inoculum was prepared as previously and spread on the plate. Disks were placed on agar and the plates were incubated at 28°C for 2 days and analyzed.
Biosynthetic Gene Cluster Analysis
The S. hofmannii PCC 7110 draft genome sequence (ANNX02) was analyzed with AntiSMASH 4.1 (Blin et al., 2017) to identify the scytocyclamide biosynthetic gene clusters. AntiSMASH recognized 9 NRPS/PKS coding regions in the draft genome. The NRPS gene domain organization was compared to the scytocyclamide structure and neighboring candidate pathways for scytocyclamide biosynthesis were identified. Flanking genes with the same orientation to the NRPSs were included in the candidate biosynthetic gene cluster between 3,716,086- 3,812,822 bp. The biosynthetic gene cluster is limited from both sides by genes with opposite orientation. Adenylation domain substrate specificity prediction was performed by combining differring AntiSMASH 4.1 and AntiSMASH 5.1.2 (Blin et al., 2019) results. The scytocyclamide biosynthetic gene cluster was visualized using Artemis (Rutherford et al., 2000) and functional annotations (Supplementary Table S2) were manually refined using a combination of BLASTp and CDD database searches.
The condensation domain of NRPS module LxaC3 was analyzed with Natural Product Domain Seeker NaPDos (Ziemert et al., 2012) to study the role of the condensation domain in the biosynthesis of Dhb. The phylogenetic comparison was made with condensation domains with a similar position to Dhb in hassallidin biosynthesis (Vestola et al., 2014) and nodularin biosynthesis (Jokela et al., 2017) with the condensation domains of HasO2 and NdaA1, respectively.
Results
Structure of Scytocyclamides
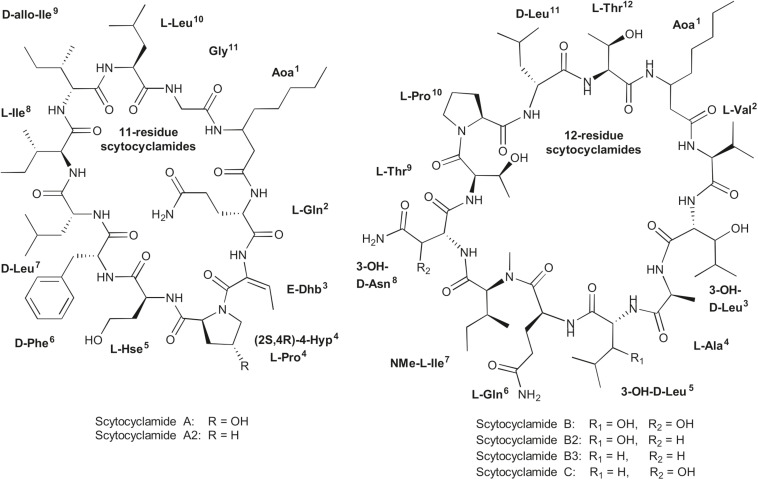
UPLC-QTOF analysis of S. hofmannii PCC 7110 methanol extract yielded six peaks corresponding to six scytocyclamide variants (Supplementary Figures S1, S2). Three of these (scytocyclamides A-C) have been previously characterized with spectrometric methods, including NMR. Three new less abundant variants, scytocyclamides A2, B2, and B3 appeared to be less hydroxylated (Figure 1, Table 3). The protonated masses and relative intensities for each compound are shown in Table 4. Product ion spectra (MSE) of protonated scytocyclamides A-C showed that the amino acid sequence could be generated from high-intensity ions in which Pro is N-terminal (Supplementary Figures S3, S4). Application of this fragmentation behavior to product ion spectra (MSE) of the new scytocyclamides A2, B2, and B3 clearly showed the amino acids lacking a hydroxyl group (Supplementary Figures S3, S4). Scytocyclamides A and A2 fall in 11-residue laxaphycins and scytocyclamides B-C fall in 12-residue laxaphycins. The yields for each compound were 1 mg (A), 1 mg (A2), 3 mg (B), 0.8 mg (C), 0.4 mg (B2), and 0.4 mg (B3).
FIGURE 1.
Structures of 11- and 12-residue laxaphycin variants scytocyclamides.
TABLE 3.
Structures of scytocyclamides from S. hofmannii PCC 7110, with new variants A2, B2, and B3.
| 11-residue Laxaphycins | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | ||
| Scytocyclamide A | Aoa | L-Gln | E-Dhb | L-OHPro | L-HSe | D-Phe | D-Leu | L-Ile | D-allo-Ile | L-Leu | Gly | ||
| Scytocyclamide A2 | Aoa | L-Gln | E-Dhb | L-Pro | L-HSe | D-Phe | D-Leu | L-Ile | D-allo-Ile | L-Leu | Gly | ||
| 12-residue Laxaphycins | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| Scytocyclamide B | Aoa | L-Val | D-OHLeu | L-Ala | D-OHLeu | L-Gln | NMe-L-Ile | D-OHAsn | L-Thr | L-Pro | D-Leu | L-Thr | |
| Scytocyclamide B2 | Aoa | L-Val | D-OHLeu | L-Ala | D-OHLeu | L-Gln | NMe-L-Ile | D-Asn | L-Thr | L-Pro | D-Leu | L-Thr | |
| Scytocyclamide B3 | Aoa | L-Val | D-OHLeu | L-Ala | D-Leu | L-Gln | NMe-L-Ile | D-Asn | L-Thr | L-Pro | D-Leu | L-Thr | |
| Scytocyclamide C | Aoa | L-Val | D-OHLeu | L-Ala | D-Leu | L-Gln | NMe-L-Ile | D-OHAsn | L-Thr | L-Pro | D-Leu | L-Thr | |
Stereochemistry according to epimerase location in the biosynthetic gene cluster modules and Grewe (2005).
TABLE 4.
Scytocyclamides A–C from S. hofmannii PCC 7110.
| tR |
[M + H]+ |
||||
| 11-residue laxaphycins | (min) | Exp (m/z) | Δ (ppm) | Formula | RI (%) |
| Scytocyclamide A | 3.46 | 1223.7399 | 0,0 | C61H99N12O14 | 98 |
| Scytocyclamide A2 | 3.56 | 1207.7422 | −2.3 | C61H99N12O13 | 2 |
| 12-residue laxaphycins | |||||
| Scytocyclamide B | 3.10 | 1367.8173 | 2.1 | C63H111N14O19 | 50 |
| Scytocyclamide B2 | 3.14 | 1351.8169 | −2.0 | C63H111N14O18 | 18 |
| Scytocyclamide B3 | 3.27 | 1335.8228 | −1.4 | C63H111N14O17 | 10 |
| Scytocyclamide C | 3.23 | 1351.8190 | −0.4 | C63H111N14O18 | 22 |
Retention times (tR), experimental (Exp) mass of protonated scytocyclamides, difference (Δ) to calculated mass, chemical formula, and relative intensity (RI) of pronated scytocyclamides.
Scytocyclamide Biosynthetic Gene Cluster
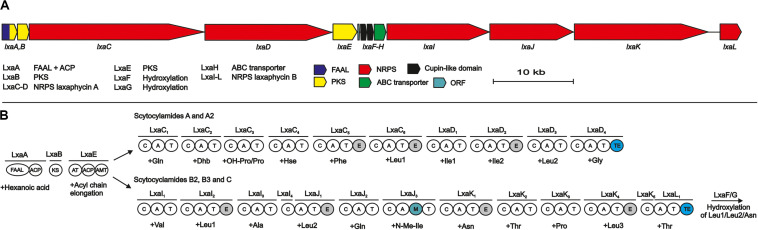
Analysis of the public 12.3-Mb draft genome of S. hofmannii PCC 7110 identified 15 putative NRPS/PKS pathways in 9 regions recognized by AntiSMASH (Table or figure reference). Two sets of NRPSs with domain architecture matching the amino acid sequences of the two scytocyclamide types were found, separated from each other by a 9-kb region encoding 5 ORFs (Figure 2). Surprisingly, just a single candidate enzyme for the initiation of the biosynthetic pathways was found encoded with the NRPS biosynthetic genes (Figure 2). Both types of scytocyclamides contain β-aminooctanoic acid (Aoa) in their structures, and we predict that the two compounds share the initiating biosynthetic enzymes for the production of Aoa (Figure 2). The 96-kb biosynthetic gene cluster encodes 13 reading frames that were annotated lxaA-L, and ORF1 (Figure 2, Supplementary Table S2).
FIGURE 2.
The scytocyclamide (lxa) biosynthetic gene cluster and putative biosynthetic scheme. (A) Organization of predicted scytocyclamide biosynthetic genes. (B) Proposed biosynthetic pathway of scytocyclamides. NRPS, Non-ribosomal peptide synthetase, PKS, Polyketide synthase, FAAL, Fatty acyl AMP Ligase, ACP, acyl carrier protein, KS, ketosynthase, AT, acyltransferase, AMT, aminotransferase, C, condensation domain, A, adenylation domain, T, thiolation domain, M, methylation domain, TE, thioesterase domain.
The predicted biosynthesis of both scytocyclamide types is initiated by the LxaA enzyme containing FAAL and ACP domains and is predicted to activate and load a hexanoic acid (Figure 2). The hexyl group is passed to the PKS enzymes LxaB and LxaE (Figure 2). LxaB contains a single ketosynthase (KS) domain and LxaE is composed of acyl transferase (AT), ACP, and aminotransferase (AMT) domains (Figure 2). These PKS domains elongate the hexyl chain with an acyl group to octyl chain and the aminotransferase acts on the carbonyl in the β position adding the amino group (Figure 2). We predict that β-aminooctanoic acid has two alternative branched pathways, the 11- or 12-residue scytocyclamide NRPSs (Figure 2). In 11-residue scytocyclamide synthesis the LxaC-D NRPSs and in 12-residue scytocyclamides the LxaA-D NRPS enzymes incorporate the amino acids (Figure 2). Both pathways have a terminal thioesterase (TE) that head-to-tail cyclize and release the scytocyclamides. Each module of LxaC-D and LxaI-L enzymes bears a condensation (C), adenylation (A), and thiolation (T) domain (Figure 2). In addition, LxaC5, LxaC6, LxaD2 and LxaI2, LxaJ1, LxaJ3, and LxaK4 modules contain epimerase domains and LxaJ3 contains an N-methylation domain (Figure 2). The biosynthetic gene cluster encodes just a single LxaH ABC-transporter, which is characteristic of NRPS biosynthetic gene clusters.
The predicted adenylation domain substrate specificities of LxaC-D and LxaI-L match with the amino acids incorporated to scytocyclamides with some modifications (Supplementary Table S3). The scytocyclamide chemical structures contain 3-OHLeu, 3-OHAsn, 4-OHPro, and Dhb (Table 3). Scytocyclamide chemical variants with hydroxylations are the most abundant products produced by S. hofmannii PCC 7110 (Table 4). The Leu-binding pockets are identical (DAWFLGNVVK) for each of the four predicted Leu-activating adenylation domains (position 10 in 11-residue scytocyclamides and positions 3, 5, and 11 in 12-residue scytocyclamides) with the possible exception of a gap in the adenylation domain amino aci sequence of position 3 (—FLGNVVK) (Supplementary Table S3). Cultivation of S. hofmannii PCC 7110 in modified growth medium containing racemic 3-OHLeu did not result in an increase of the relative amounts of hydroxylated Leu-containing laxaphycin variants (Supplementary Figure S5). This could indicate that LxaI2 and LxaJ1 adenylation domains incorporate Leu and not 3-OHLeu, assuming that 3-OHLeu is transported into the cell. S. hofmannii PCC 7110 incorporated the non-proteinogenic amino acids (2S,4R)-4-MePro, (2R,4R)-4-MePro, (2S,4S)-4-MePro, (2S,4S)-4-OHPro, and (2S,4R)-4-OHPro in parallel cultivation experiments (data not shown). We predict that the cupin 8 family proteins LxaF-G hydroxylates Leu and the Asn after incorporation of the proteinogenic amino acids into the peptide intermediate by the corresponding adenylation domain (Figure 2). We did not find suitable candidate enzymes for synthesis of 4-OHPro encoded in the biosynthetic gene cluster.
The modified AA clade of condensation domains is proposed to play an active role in Thr dehydration during biosynthesis of non-ribosomal peptides (Tillett et al., 2000; Moffitt and Neilan, 2004). The Dhb-tailoring condensation domains LxaC3, HasO2, and NdaA1 were most similar to the modified AA clade of condensation domains in phylogenetic analysis performed using NaPDoS (Supplementary Figure S6).
Antimicrobial Activity
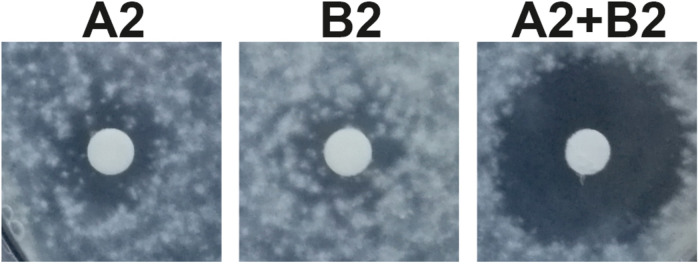
Antimicrobial activity of S. hofmannii PCC 7110 methanol extracts was studied with several fungal and bacterial strains (Table 2). The extracts inhibited only the growth of A. flavus FBCC 2467. Disk diffusion assays were performed after purification of the scytocyclamides from the extract. Inhibition of fungal growth was observed with individual scytocyclamides as a hazy inhibition zone and synergy was observed between 11-residue and 12-residue compounds as a noticeably increased clear inhibition zone (Figure 3). Scytocyclamide amounts and inhibition zone diameters are shown in Supplementary Table S4. Cross-contamination between purified scytocyclamides A-D was from <1% to 5% and 15% for E (Supplementary Figure S7).
FIGURE 3.
Inhibition of growth of Aspergillus flavus by scytocyclamides. Scytocyclamide A2 (200 μg), scytocyclamide B2 (85 μg), and scytocyclamides A2 + B2 (100 μg + 43 μg). Disk diameter is 5 mm.
Discussion
We described an unusual natural product biosynthetic gene cluster for producing structurally distinct scytocyclamides. Our analysis suggests that scytocyclamides have branched biosynthesis and share loading modules LxaA-B and LxaE (Figure 2 and Supplementary Table S2). These shared loading modules initiate biosynthesis to produce the β-amino acid Aoa, which is the only common amino acid in the peptide sequence of the two structural distinct types of scytocyclamides. The biosynthesis then branches to two NRPS pathways (Figure 2). The organization of the catalytic domains in the NRPS enzymes LxaC-D matches the structure of 11-residue scytocyclamides A and A2 and NRPSs LxaI-L match the structure of 12-residue scytocyclamides B, B2, B3, and C (Figure 2), as analyzed in this study and reported earlier (Grewe, 2005). Such branching is exceptional because natural product biosynthetic gene clusters are typically self-contained and act independently following the colinearity rule of PKS/NRPS biosynthesis (Guenzi et al., 1998; Callahan et al., 2009; Baral et al., 2018). However, there are other known exceptions to this rule. Modules can be skipped, as in the case of anabaenopeptin and namalide synthesis in Nostoc sp. CENA543, where the two compounds are produced by the same gene cluster, but a shorter product namalide is produced when three modules are skipped (Shishido et al., 2017). For example, PKS domain skipping occurs in the synthesis of leinamycin (Tang et al., 2006). Alternative starter modules have been found in the synthesis of anabaenopeptins (Rouhiainen et al., 2010) and puwainaphycins and minutissamides (Mareš et al., 2019). Gene clusters have also been shown to share enzymes in producing non-proteinogenic amino acids as in the case of anabaenopeptin and spumigin (Lima et al., 2017) and aeruginosin and spumigin, which results in the side product pseudoaeruginosin (Liu et al., 2015). Crosstalk between NRPS clusters has also been found in erythrochelin biosynthesis with two separate clusters sharing essential biosynthetic enzymes (Lazos et al., 2010). Some NRPSs incorporate multiple residues of the same amino acid iteratively, as in enterobactin synthesis (Shaw-Reid et al., 1999). Shared loading modules in laxaphycin biosynthesis are now presented as a new exception to the colinearity rule of NRPS/PKS synthesis.
Twelve-residue scytocyclamides have 3-OHLeu in positions 3 and 5 and 3-OHAsn in position 8. However, the adenylation domain substrate specificity predictions are for proteinogenic Leu and Asn with a 100% match (Supplementary Table S3). We propose that the proteinogenic amino acids act as substrates for the NRPS enzymes and the hydroxylation occurs after peptide-bond formation. We propose that hydroxylation of Leu and Asn residues in all scytocyclamides is performed by cupin 8-like proteins encoded in the biosynthetic gene cluster. The JmjC-like cupin 8 family (pfam13621) of proteins are Fe(II) or Zn(II) and α-ketoglutarate (α-KG) dependent oxygenases and act as hydroxylases and demethylases (Hewitson et al., 2002; Markolovic et al., 2016). There are examples of hydroxylation of Asn, Asp, His, Lys, Arg, and RNA in human and animal proteins (Wilkins et al., 2018). The activity of cupin 8 is specific to the amino acid position in the peptide. Their location within the biosynthetic gene cluster suggests a role in the biosynthesis of scytocyclamides. To our knowledge, this activity has not been previously reported cupin 8 proteins. The hydroxylated amino acids occur in modules with epimerase domains Figure 2. This suggests that the enzymes hydroxylating the residues are specific to D-amino acids or that the epimerase domains play a role in the hydroxylation Figure 2. Other mechanisms have previously been found to introduce 3-hydroxylated amino acids to NRPS products (Hou et al., 2011). α-KG-dependent oxygenases hydroxylate L-Arg in viomycin (Yin and Zabriskie, 2004), L-Asn in daptomycin-like peptide (Strieker et al., 2007), and D-Glu in kutzneride (Strieker et al., 2009) biosyntheses. No homologs to these enzymes were found near the scytocyclamide cluster.
Dhb is enzymatically produced from Thr recognized by the adenylation domain (Challis et al., 2000). In the case of microcystin and nodularin synthesis, the dehydration has been proposed to occur due to the active role of the following condensation domain in the process (Tillett et al., 2000; Moffitt and Neilan, 2004) and bleomycin synthesis (Du et al., 2000). These microcystin and bleomycin condensation domains have been assigned to their own clade of condensation domains as “modified AA” C-domains (Ziemert et al., 2012; Bloudoff and Schmeing, 2017). When the LxaC3 condensation domain was analyzed by NaPDoS, it grouped with these modified AA condensation domains (Supplementary Figure S6). The similarity of these domains with direct contact to the modified amino acid suggests that the Dhb and Dha dehydration could be indeed catalyzed by the condensation domains in these cases. For the Hse residues, no prediction was given by AntiSMASH 5.1. However, a previous version, antiSMASH 4.1.0, did recognize the corresponding binding pocket sequence for DLKNFGSDVK as Hse based on the Stachelhaus code. Hse as an amino acid in NRPS products is less common and in cyanobacteria has been previously seen in laxaphycin family peptides and nostocyclopeptide M1 (Jokela et al., 2010). However, the biosynthesis and adenylation domains for this product have not been published. OHPro has been found in other cyanobacterial natural products, such as nostoweipeptins W1-W7 and nostopeptolides L1-L4 (Liu et al., 2014). The process of incorporating the OHPro or hydroxylating the prolyl residue remain unclear.
The catalytic domain organization of the scytocyclamide gene cluster matches the laxaphycin family compound structures reported earlier. The epimerizations are conserved in 11-residue laxaphycins in positions 6, 7, and 9 and in 12-residue laxaphycins in positions 3, 5, 8, and 11. The N-methylation of the amino acid in position 7 of the 12-residue laxaphycins is also conserved. Dhb3 is conserved in the structures of 11-residue laxaphycins. The 3-OHLeu3 is conserved in 12-residue laxaphycins and 3-OHLeu5 and 3-OHAsn8 are common in 12-residue laxaphycins (Table 1). Bornancin et al. (2019) predicted that laxaphycin gene clusters should have FAAL and PKS modules to initiate biosynthesis, because the 11-residue acyclic acyclolaxaphycins have a break just before the Aoc and cyclization would be the last step of synthesis. Bornancin et al. (2015) found acyclic 11-residue laxaphycin variants with a gap between the second and third amino acid in sequence starting with the Adc. They proposed that this gap could be where the synthesis is finished and the cyclization occurs, or that the compounds they found were cleaved by environmental agents. Our results confirm the discovered acyclic 11-residue variants could be immature products of the pathway, as the linear peptide follows the biosynthetic organization we have described. With the acyclic 12-residue variants, the gap in the sequence occurs within a predicted NRPS gene and the proposed mechanism of other agents or enzymes in the environment cleaving the products would seem more reasonable.
Cyanobacteria are abundant primary producers in aquatic environments and are targeted to grazing by higher organisms, such as sea hares (Cruz-Rivera and Paul, 2007). Cyanobacteria produce a wide range of bioactive natural products (Dittmann et al., 2015; Demay et al., 2019) that seem to be produced to deter the grazing fauna in the environment (Leão et al., 2012; Mazard et al., 2016). Potential competitors to cyanobacteria are also other microbes such as chytrids, which are fungi parasitic to cyanobacteria (Agha et al., 2018). Some cyanobacterial natural products have reached clinical trials and are approved as cancer drugs (Luesch et al., 2001; Deng et al., 2013). Cyclic lipopeptides are common among the cyanobacterial natural products and typically contain a single fatty acid as in laxaphycins (Galica et al., 2017) that confers membrane-disruptive properties (Humisto et al., 2019). Laxaphycin family peptides have been shown to be toxic to or inhibit the growth of multiple organisms and cell lines (Gerwick et al., 1989; Frankmölle et al., 1992b; Bonnard et al., 1997, 2007; MacMillan et al., 2002; Maru et al., 2010; Luo et al., 2014, 2015; Dussault et al., 2016; Cai et al., 2018; Bornancin et al., 2019). We observed antifungal activity of scytocyclamides toward A. flavus (Figure 3 and Supplementary Table S4). In an earlier report by Grewe (2005), no activity against C. albicans was detected for scytocyclamides A, B, and C, which was also observed in this study. Synergistic antifungal activity between 11- and 12-residue laxaphycins has been previously reported (Frankmölle et al., 1992b; MacMillan et al., 2002). The same synergistic activity was observed between 11- and 12-residue scytocyclamides (Figure 3 and Supplementary Table S4). According to previous studies and our results, the 12-residue laxaphycins are typically more potent on their own than 11-residue laxaphycins. Our previous study on S. hofmannii PCC 7110 failed to identify the antifungal agent in the extract, when purified fractions lacked activity. We now conclude that the antifungal activity was most probably caused by scytocyclamides, but the purified fractions had insufficient amounts of material to produce the inhibitory effect without a synergistic partner (Shishido et al., 2015).
It is probable that the other type of laxaphycins originally existed without a synergistic partner peptide in the cells, as many laxaphycins have antimicrobial activity by themselves. Through recombination events, a synergistically acting peptide has emerged to enhance the activity of the original peptide. One possibility is that the two peptides had individual gene clusters, but the initiating enzymes have been subject to an elimination event when two distinct starter enzymes were no longer necessary. It is clear that the synergistic bioactivity and shared biosynthesis of laxaphycins go together. Similar colocalization with co-regulation of distinct synergistic biosynthetic gene clusters has been previously observed in the streptomycetal antibiotics griseoviridin and viridogrisein (Xie et al., 2012). The mechanism behind the synergistic action is usually two different compounds acting on two different targets, thus combining their activity (Caesar and Cech, 2019). It is possible that one compound makes the target cell vulnerable to the other, such as via damage to the cell wall. The colocalization of genes and shared biosynthesis suggest simultaneous regulation and expression of the synergistic products to act on a single cellular target through different mechanisms.
Data Availability Statement
All datasets presented in this study are included in the manuscript/Supplementary Material.
Author Contributions
LH, KS, JJ, MW, and DF designed the study. AJ, LH, and MW performed the experiments. LH, JJ, and DF analyzed and interpreted the data. LH, DF, JJ, and KS wrote the manuscript, which was corrected, revised, and approved by all authors.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Lyudmila Saari for maintaining the cyanobacterial strain retrieved from The Pasteur Culture Collection of Cyanobacteria.
Footnotes
Funding. This work was supported by a grant awarded to KS from the Jane and Aatos Erkko Foundation.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2020.578878/full#supplementary-material
References
- Agha R., Gross A., Rohrlack T., Wolinska J. (2018). Adaptation of a chytrid parasite to Its cyanobacterial host is hampered by host intraspecific diversity. Front. Microbiol. 9 921–921. 10.3389/fmicb.2018.00921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baral B., Akhgari A., Metsä-Ketelä M. (2018). Activation of microbial secondary metabolic pathways: Avenues and challenges. Synth. Syst. Biotechnol. 3 163–178. 10.1016/j.synbio.2018.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blin K., Shaw S., Steinke K., Villebro R., Ziemert N., Lee S. Y., et al. (2019). antiSMASH 5.0: updates to the secondary metabolite genome mining pipeline. Nucleic Acids Res. 47 W81–W87. 10.1093/nar/gkz310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blin K., Wolf T., Chevrette M. G., Lu X. W., Schwalen C. J., Kautsar S. A., et al. (2017). antiSMASH 4.0-improvements in chemistry prediction and gene cluster boundary identification. Nucleic Acids Res. 45 W36–W41. 10.1093/nar/gkx319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloudoff K., Schmeing T. M. (2017). Structural and functional aspects of the nonribosomal peptide synthetase condensation domain superfamily: discovery, dissection and diversity. Biochim. Biophys. Acta 1865(11 Pt. B), 1587–1604. 10.1016/j.bbapap.2017.05.010 [DOI] [PubMed] [Google Scholar]
- Bonnard I., Rolland M., Francisco C., Banaigs B. (1997). Total structure and biological properties of laxaphycins A and B, cyclic lipopeptides from the marine cyanobacterium Lyngbya majuscula. Lett. Pept. Sci. 4 289–292. 10.1007/bf02442891 [DOI] [Google Scholar]
- Bonnard I., Rolland M., Salmon J. M., Debiton E., Barthomeuf C., Banaigs B. (2007). Total structure and inhibition of tumor cell proliferation of laxaphycins. J. Med. Chem. 50 1266–1279. 10.1021/jm061307x [DOI] [PubMed] [Google Scholar]
- Bornancin L., Alonso E., Alvarino R., Inguimbert N., Bonnard I., Botana L. M., et al. (2019). Structure and biological evaluation of new cyclic and acyclic laxaphycin-A type peptides. Bioorg. Med. Chem. 27 1966–1980. 10.1016/j.bmc.2019.03.046 [DOI] [PubMed] [Google Scholar]
- Bornancin L., Boyaud F., Mahiout Z., Bonnard I., Mills S. C., Banaigs B., et al. (2015). Isolation and synthesis of Laxaphycin B-type Peptides: a case study and clues to their biosynthesis. Mar. Drugs 13 7285–7300. 10.3390/md13127065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caesar L. K., Cech N. B. (2019). Synergy and antagonism in natural product extracts: when 1+1 does not equal 2. Nat. Prod. Rep. 36 869–888. 10.1039/c9np00011a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W. J., Matthew S., Chen Q. Y., Paul V. J., Luesch H. (2018). Discovery of new A- and B-type laxaphycins with synergistic anticancer activity. Bioorg. Med. Chem. 26 2310–2319. 10.1016/j.bmc.2018.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan B., Thattai M., Shraiman B. I. (2009). Emergent gene order in a model of modular polyketide synthases. Proc. Natl. Acad. Sci. U.S.A. 106 19410–19415. 10.1073/pnas.0902364106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challis G. L., Ravel J., Townsend C. A. (2000). Predictive, structure-based model of amino acid recognition by nonribosomal peptide synthetase adenylation domains. Chem.Biol. 7 211–224. 10.1016/S1074-5521(00)00091-0 [DOI] [PubMed] [Google Scholar]
- Cruz-Rivera E., Paul V. J. (2007). Chemical deterrence of a cyanobacterial metabolite against generalized and specialized grazers. J. Chem. Ecol. 33 213–217. 10.1007/s10886-006-9212-y [DOI] [PubMed] [Google Scholar]
- Demay J., Bernard C., Reinhardt A., Marie B. (2019). Natural products from cyanobacteria: focus on beneficial activities. Mar. Drugs 17:49 10.3390/md17060320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng C., Pan B., O’Connor O. A. (2013). Brentuximab Vedotin. Clin. Cancer Res. 19 22–27. 10.1158/1078-0432.CCR-12-0290 [DOI] [PubMed] [Google Scholar]
- Dittmann E., Gugger M., Sivonen K., Fewer D. P. (2015). Natural product biosynthetic diversity and comparative genomics of the cyanobacteria. Trends Microbiol. 23 642–652. 10.1016/j.tim.2015.07.008 [DOI] [PubMed] [Google Scholar]
- Du L., Sánchez C., Chen M., Edwards D. J., Shen B. (2000). The biosynthetic gene cluster for the antitumor drug bleomycin from Streptomyces verticillus ATCC15003 supporting functional interactions between nonribosomal peptide synthetases and a polyketide synthase. Chem. Biol. 7 623–642. 10.1016/S1074-5521(00)00011-9 [DOI] [PubMed] [Google Scholar]
- Dussault D., Vu K. D., Vansach T., Horgen F. D., Lacroix M. (2016). Antimicrobial effects of marine algal extracts and cyanobacterial pure compounds against five foodborne pathogens. Food Chem. 199 114–118. 10.1016/j.foodchem.2015.11.119 [DOI] [PubMed] [Google Scholar]
- Evans B. S., Robinson S. J., Kelleher N. L. (2011). Surveys of non-ribosomal peptide and polyketide assembly lines in fungi and prospects for their analysis in vitro and in vivo. Fungal Genet. Biol. 48 49–61. 10.1016/j.fgb.2010.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankmölle W. P., Knubel G., Moore R. E., Patterson G. M. L. (1992a). Antifungal cyclic peptides from the terrestrial blue-green alga Anabaena laxa. 2. Structures of laxaphycin A, laxaphycin B, laxaphycin D and laxaphycin E. J. Antibiot 45 1458–1466. 10.7164/antibiotics.45.1458 [DOI] [PubMed] [Google Scholar]
- Frankmölle W. P., Larsen L. K., Caplan F. R., Patterson G. M. L., Knubel G., Levine I. A., et al. (1992b). Antifungal cyclic peptides from the terrestrial blue-green alga Anabaena laxa. 1. Isolation Biol. Properties 45 1451–1457. 10.7164/antibiotics.45.1451 [DOI] [PubMed] [Google Scholar]
- Galica T., Hrouzek P., Mares J. (2017). Genome mining reveals high incidence of putative lipopeptide biosynthesis NRPS/PKS clusters containing fatty acyl-AMP ligase genes in biofilm-forming cyanobacteria. J. Phycol. 53 985–998. 10.1111/jpy.12555 [DOI] [PubMed] [Google Scholar]
- Gerwick W. H., Jiang Z. D., Agarwal S. K., Farmer B. T. (1992). Total structure of hormothamnin A, A toxic cyclic undecapeptide from the tropical marine cyanobacterium Hormothamnion enteromorphoides. Tetrahedron 48 2313–2324. 10.1016/S0040-4020(01)88753-6 [DOI] [Google Scholar]
- Gerwick W. H., Mrozek C., Moghaddam M. F., Agarwal S. K. (1989). Novel cytotoxic peptides from the tropical marine cyanobacterium Hormothamnion enteromorphoides 1. Discovery, isolation and initial chemical and biological characterization of the hormothamnins from wild and cultured material. Experientia 45 115–121. 10.1007/BF01954842 [DOI] [PubMed] [Google Scholar]
- Grewe J. C. (2005). Cyanopeptoline und Scytocyclamide: Zyklische Peptide aus Scytonema hofmanni PCC 7110 Struktur und biologische Aktivität. Dissertation thesis, Albert-Ludwigs-Universität Freiburg im Breisgau, Fahnenbergplatz. [Google Scholar]
- Guenzi E., Galli G., Grgurina I., Pace E., Ferranti P., Grandi G. (1998). Coordinate transcription and physical linkage of domains in surfactin synthetase are not essential for proper assembly and activity of the multienzyme complex. J. Biol. Chem. 273 14403–14410. 10.1074/jbc.273.23.14403 [DOI] [PubMed] [Google Scholar]
- Hewitson K. S., McNeill L. A., Riordan M. V., Tian Y. M., Bullock A. N., Welford R. W., et al. (2002). Hypoxia-inducible factor (HIF) asparagine hydroxylase is identical to factor inhibiting HIF (FIH) and is related to the cupin structural family. J. Biol. Chem. 277 26351–26355. 10.1074/jbc.C200273200 [DOI] [PubMed] [Google Scholar]
- Hou J., Robbel L., Marahiel Mohamed A. (2011). Identification and characterization of the lysobactin biosynthetic gene cluster reveals mechanistic insights into an unusual termination module architecture. Chem Biol 18 655–664. 10.1016/j.chembiol.2011.02.012 [DOI] [PubMed] [Google Scholar]
- Huang I. S., Zimba P. V. (2019). Cyanobacterial bioactive metabolites-A review of their chemistry and biology. Harmful Algae 83 42–94. 10.1016/j.hal.2018.11.008 [DOI] [PubMed] [Google Scholar]
- Humisto A., Jokela J., Teigen K., Wahlsten M., Permi P., Sivonen K., et al. (2019). Characterization of the interaction of the antifungal and cytotoxic cyclic glycolipopeptide hassallidin with sterol-containing lipid membranes. Biochim. Biophys. Acta Biomembr. 1861 1510–1521. 10.1016/j.bbamem.2019.03.010 [DOI] [PubMed] [Google Scholar]
- Jokela J., Heinilä L. M. P., Shishido T. K., Wahlsten M., Fewer D. P., Fiore M. F., et al. (2017). Production of high amounts of hepatotoxin nodularin and new protease inhibitors Pseudospumigins by the Brazilian benthic Nostoc sp. CENA543. Front. Microbiol. 8:14 10.3389/fmicb.2017.01963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokela J., Herfindal L., Wahlsten M., Permi P., Selheim F., Vasconcelos V., et al. (2010). A novel cyanobacterial nostocyclopeptide is a potent antitoxin against microcystins. Chem. Bio. Chem. 11 1594–1599. 10.1002/cbic.201000179 [DOI] [PubMed] [Google Scholar]
- Kehr J.-C., Gatte Picchi D., Dittmann E. (2011). Natural product biosyntheses in cyanobacteria: a treasure trove of unique enzymes. Beilstein J. Org. Chem. 7 1622–1635. 10.3762/bjoc.7.191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E., Moore B. S., Yoon Y. J. (2015). Reinvigorating natural product combinatorial biosynthesis with synthetic biology. Nat. Chem. Biol. 11 649–659. 10.1038/nchembio.1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazos O., Tosin M., Slusarczyk A. L., Boakes S., Cortés J., Sidebottom P. J., et al. (2010). Biosynthesis of the putative siderophore erythrochelin requires unprecedented crosstalk between separate nonribosomal peptide gene clusters. Chem Biol 17 160–173. 10.1016/j.chembiol.2010.01.011 [DOI] [PubMed] [Google Scholar]
- Leão P. N., Engene N., Antunes A., Gerwick W. H., Vasconcelos V. (2012). The chemical ecology of cyanobacteria. Nat. Prod. Rep. 29 372–391. 10.1039/c2np00075j [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima S. T., Alvarenga D. O., Etchegaray A., Fewer D. P., Jokela J., Varani A. M., et al. (2017). Genetic organization of anabaenopeptin and spumigin biosynthetic gene clusters in the cyanobacterium Sphaerospermopsis torques-reginae ITEP-024. ACS Chem. Biol. 12 769–778. 10.1021/acschembio.6b00948 [DOI] [PubMed] [Google Scholar]
- Liu L., Budnjo A., Jokela J., Haug B. E., Fewer D. P., Wahlsten M., et al. (2015). Pseudoaeruginosins, nonribosomal peptides in Nodularia spumigena. ACS Chem. Biol. 10 725–733. 10.1021/cb5004306 [DOI] [PubMed] [Google Scholar]
- Liu L. W., Jokela J., Herfindal L., Wahlsten M., Sinkkonen J., Permi P., et al. (2014). 4-Methylproline guided natural product discovery: co-ccurrence of 4-Hydroxy- and 4-Methylprolines in nostoweipeptins and nostopeptolides. ACS Chem. Biol. 9 2646–2655. 10.1021/cb500436p [DOI] [PubMed] [Google Scholar]
- Luesch H., Yoshida W. Y., Moore R. E., Paul V. J., Corbett T. H. (2001). Total structure determination of apratoxin A, a potent novel cytotoxin from the marine cyanobacterium Lyngbya majuscula. J. Am. Chem. Soc 123 5418–5423. 10.1021/ja010453j [DOI] [PubMed] [Google Scholar]
- Luo S. W., Kang H. S., Krunic A., Chen W. L., Yang J. L., Woodard J. L., et al. (2015). Trichormamides C and D, antiproliferative cyclic lipopeptides from the cultured freshwater cyanobacterium cf. Oscillatoria sp. UIC 10045. Bioorg. Med. Chem. 23 3153–3162. 10.1016/j.bmc.2015.04.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo S. W., Krunic A., Kang H. S., Chen W. L., Woodard J. L., Fuchs J. R., et al. (2014). Trichormamides A and B with antiproliferative activity from the cultured freshwater cyanobacterium Trichormus sp. UIC 10339. J. Nat. Prod. 77 1871–1880. 10.1021/np5003548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMillan J. B., Ernst-Russell M. A., de Ropp J. S., Molinski T. F. (2002). Lobocyclamides A-C, lipopeptides from a cryptic cyanobacterial mat containing Lyngbya confervoides. J. Org. Chem. 67 8210–8215. 10.1021/jo0261909 [DOI] [PubMed] [Google Scholar]
- Mareš J., Hájek J., Urajová P., Kust A., Jokela J., Saurav K., et al. (2019). Alternative biosynthetic starter units enhance the structural diversity of cyanobacterial lipopeptides. Genet. Mol. Biol. 85:e2675-18 10.1128/AEM.02675-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markolovic S., Leissing T. M., Chowdhury R., Wilkins S. E., Lu X., Schofield C. J. (2016). Structure-function relationships of human JmjC oxygenases - demethylases versus hydroxylases. Curr. Opin. Struct. Biol. 41 62–72. 10.1016/j.sbi.2016.05.013 [DOI] [PubMed] [Google Scholar]
- Maru N., Ohno O., Uemura D. (2010). Lyngbyacyclamides A and B, novel cytotoxic peptides from marine cyanobacteria Lyngbya sp. Tetrahedron Lett. 51 6384–6387. 10.1016/j.tetlet.2010.06.105 [DOI] [Google Scholar]
- Mazard S., Penesyan A., Ostrowski M., Paulsen I. T., Egan S. (2016). Tiny microbes with a big impact: the role of cyanobacteria and their metabolites in shaping our future. Mar. Drugs 14:97 10.3390/md14050097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyanaga A., Kudo F., Eguchi T. (2018). Protein–protein interactions in polyketide synthase–nonribosomal peptide synthetase hybrid assembly lines. Nat. Prod. Rep. 35 1185–1209. 10.1039/C8NP00022K [DOI] [PubMed] [Google Scholar]
- Moffitt M. C., Neilan B. A. (2004). Characterization of the nodularin synthetase gene cluster and proposed theory of the evolution of cyanobacterial hepatotoxins. Appl. Environ. Microbiol. 70 6353–6362. 10.1128/aem.70.11.6353-6362.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman D. J., Cragg G. M. (2016). Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 79 629–661. 10.1021/acs.jnatprod.5b01055 [DOI] [PubMed] [Google Scholar]
- Rodrigues T., Reker D., Schneider P., Schneider G. (2016). Counting on natural products for drug design. Nat. Chem. 8 531–541. 10.1038/nchem.2479 [DOI] [PubMed] [Google Scholar]
- Rouhiainen L., Jokela J., Fewer D. P., Urmann M., Sivonen K. (2010). Two alternative starter modules for the non-ribosomal biosynthesis of specific anabaenopeptin variants in Anabaena (Cyanobacteria). Chem. Biol. 17 265–273. 10.1016/j.chembiol.2010.01.017 [DOI] [PubMed] [Google Scholar]
- Rutherford K., Parkhill J., Crook J., Horsnell T., Rice P., Rajandream M. A., et al. (2000). Artemis: sequence visualization and annotation. Bioinformatics 16 944–945. 10.1093/bioinformatics/16.10.944 [DOI] [PubMed] [Google Scholar]
- Shaw-Reid C. A., Kelleher N. L., Losey H. C., Gehring A. M., Berg C., Walsh C. T. (1999). Assembly line enzymology by multimodular nonribosomal peptide synthetases: the thioesterase domain of E. coli EntF catalyzes both elongation and cyclolactonization. Chem. Biol. 6 385–400. 10.1016/S1074-5521(99)80050-7 [DOI] [PubMed] [Google Scholar]
- Shishido T. K., Humisto A., Jokela J., Liu L. W., Wahlsten M., Tamrakar A., et al. (2015). Antifungal compounds from cyanobacteria. Mar. Drugs 13 2124–2140. 10.3390/md13042124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shishido T. K., Jokela J., Fewer D. P., Wahlsten M., Fiore M. F., Sivonen K. (2017). Simultaneous production of anabaenopeptins and namalides by the cyanobacterium Nostoc sp CENA543. ACS Chem. Biol. 12 2746–2755. 10.1021/acschembio.7b00570 [DOI] [PubMed] [Google Scholar]
- Spainhour C. B. (2005). Natural Products: Source of Potential Drugs. New Jersey: John Wiley & Sons, Inc. [Google Scholar]
- Strieker M., Kopp F., Mahlert C., Essen L.-O., Marahiel M. A. (2007). Mechanistic and structural basis of stereospecific Cβ-hydroxylation in calcium-dependent antibiotic, a daptomycin-type lipopeptide. ACS Chem. Biol. 2 187–196. 10.1021/cb700012y [DOI] [PubMed] [Google Scholar]
- Strieker M., Nolan E. M., Walsh C. T., Marahiel M. A. (2009). Stereospecific synthesis of threo- and erythro-β-Hydroxyglutamic acid during kutzneride biosynthesis. J. Am. Chem. Soc 131 13523–13530. 10.1021/ja9054417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan P., Krunic A., Burdette J. E., Orjala J. (2020). Laxaphycins B5 and B6 from the cultured cyanobacterium UIC 10484. J. Antibiotics 73 526–533. 10.1038/s41429-020-0301-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang G.-L., Cheng Y.-Q., Shen B. (2006). Polyketide chain skipping mechanism in the biosynthesis of the hybrid nonribosomal peptide-polyketide antitumor antibiotic leinamycin in Streptomyces atroolivaceus S-140. J Nat Prod 69 387–393. 10.1021/np050467t [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillett D., Dittmann E., Erhard M., von Dohren H., Borner T., Neilan B. A. (2000). Structural organization of microcystin biosynthesis in Microcystis aeruginosa PCC7806: an integrated peptide-polyketide synthetase system. Chem. Biol. 7 753–764. 10.1016/s1074-5521(00)00021-1 [DOI] [PubMed] [Google Scholar]
- Vestola J., Shishido T. K., Jokela J., Fewer D. P., Aitio O., Permi P., et al. (2014). Hassallidins, antifungal glycolipopeptides, are widespread among cyanobacteria and are the end-product of a nonribosomal pathway. Res. Output 111:E1909 10.1073/pnas.1320913111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins S. E., Islam S., Gannon J. M., Markolovic S., Hopkinson R. J., Ge W., et al. (2018). JMJD5 is a human arginyl C-3 hydroxylase. Nat. Commun. 9:12 10.1038/s41467-018-03410-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y., Wang B., Liu J., Zhou J., Ma J., Huang H., et al. (2012). Identification of the biosynthetic gene cluster and regulatory cascade for the synergistic antibacterial antibiotics griseoviridin and viridogrisein in Streptomyces griseoviridis. Chem. Bio. chem 13 2745–2757. 10.1002/cbic.201200584 [DOI] [PubMed] [Google Scholar]
- Yin X., Zabriskie T. M. (2004). VioC is a non-heme iron, α-Ketoglutarate-dependent oxygenase that catalyzes the formation of 3S-Hydroxy-L-Arginine during viomycin biosynthesis. Chem. Bio. chem 5 1274–1277. 10.1002/cbic.200400082 [DOI] [PubMed] [Google Scholar]
- Ziemert N., Podell S., Penn K., Badger J. H., Allen E., Jensen P. R. (2012). The natural product domain seeker NaPDoS: a phylogeny based bioinformatic tool to classify secondary metabolite gene diversity. PLoS One 7:e34064 10.1371/journal.pone.0034064 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All datasets presented in this study are included in the manuscript/Supplementary Material.