Abstract
Parkinson’s disease (PD) signs and symptoms regularly include tremor. Interestingly, the nucleoside guanosine (GUO) has already proven to be effective in reducing reserpine-induced tremulous jaw movements (TJMs) in rodent models, thus becoming a promising antiparkinsonian drug. Here, we aimed at revealing the mechanism behind GUO antiparkinsonian efficacy by assessing the role of adenosine A1 and A2A receptors (A1R and A2AR) on GUO-mediated anti-tremor effects in the reserpinized mouse model of PD. Reserpinized mice showed elevated reactive oxygen species (ROS) production and cellular membrane damage in striatal slices assessed ex vivo and GUO treatment reversed ROS production. Interestingly, while the simultaneous administration of sub-effective doses of GUO (5 mg/kg) and SCH58261 (0.01 mg/kg), an A2AR antagonist, precluded reserpine-induced TJMs, these were ineffective on reverting ROS production in ex vivo experiments. Importantly, GUO was able to reduce TJM and ROS production in reserpinized mouse lacking the A2AR, thus suggesting an A2AR-independent mechanism of GUO-mediated effects. Conversely, the administration of DPCPX (0.75 mg/kg), an A1R antagonist, completely abolished both GUO-mediated anti-tremor effects and blockade of ROS production. Overall, these results indicated that GUO anti-tremor and antioxidant effects in reserpinized mice were A1R dependent but A2AR independent, thus suggesting a differential participation of adenosine receptors in GUO-mediated effects.
Keywords: Guanosine, Tremor, Reserpine, Adenosine receptors
Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disorder worldwide. It is mainly characterized by the progressive loss of dopaminergic neurons within the nigrostriatal pathway, which leads to a debilitating motor dysfunction [1]. The cardinal motor symptoms of Parkinsonism include akinesia, bradykinesia, rigidity, and a resting tremor [1]. Tremor can be defined as “a rhythmic and involuntary oscillation of a body part, caused by reciprocal innervations of a muscle, which leads to repetitive, stereotyped contractions with regular frequency and amplitude” [2]. The tremulous jaw movement (TJM) behavior is an extensively validated rodent model of tremor [3]. TJM is characterized by rapid vertical deflections of the lower jaw that resemble chewing but are not directed at any particular stimulus [4]. TJMs are induced by conditions that also lead to parkinsonism in humans (i.e., striatal dopamine depletion, dopamine antagonism, and cholinomimetic activity) [5]. Among them, reserpine, an inhibitor of vesicular monoamine transporter (VMAT-2) that causes monoamine neurotransmitters depletion, induces motor disturbances as hypolocomotion, muscle rigidity, and TJM. Therefore, reserpine administration can be used as a model for screening drugs with potential antiparkinsonian effect [6].
The purine nucleoside guanosine (GUO), which is able to cross the blood-brain-barrier [7], is an important extracellular signaling molecule at the central nervous system [8]. Accordingly, GUO has been shown to display trophic effects in neural cells and significant neuroprotective effects [9]. Nonetheless, GUO also exerts some behavioral effects in rodents. In line with this, it has been reported that GUO can display anticonvulsive [10], antinociceptive [11], anxiolytic-like [12], and antidepressant-like effects [13]. For these reasons, we have investigated the potential effect of GUO in animal models of parkinsonism. Interestingly, in unilaterally 6-hydroxidopamine-(6-OHDA)-lesioned rats, GUO increased L-DOPA sub-maximal response and decreased L-DOPA-induced dyskinesia (LID). Similarly, GUO also reversed reserpine-induced TJM and catalepsy in mice [14], showing it may be effective for reversing parkinsonian motor impairments. Besides that, GUO also showed protective effects against in vitro cellular models of PD [15–17].
Although the antiparkinsonian-like effects of GUO have been already evaluated, the mechanism of action of this molecule is still unknown. Based on some data reporting anti-ischemic effects of GUO in hippocampal slices and cortical astrocytes, a possible role for adenosine receptors has been suggested [18, 19]. In fact, adenosinergic transmission has been pointed out as a promising therapeutic strategy for motor symptoms of PD [20, 21]. This therapeutic potential is mainly due to the fact that adenosine A1 and A2A receptors (A1R and A2AR) are largely expressed in the striatum and have a key role in modulating dopaminergic neurotransmission [22–28].
Here, we aimed to investigate the potential role of A1R and A2AR mediating GUO effects in the reserpinized mice by evaluating the behavioral and biochemical effects of GUO in the presence of selective A1R and A2AR antagonists.
Materials and methods
Animals
Male Swiss mice (central animal facility of Federal University of Santa Catarina) and A2AR knock-out (A2AR−/−) mice developed in a CD-1 genetic background (animal facility of University of Barcelona) (30–50 g) were used. Animals were housed and tested in compliance with the guidelines described in the Guide for the Care and Use of Laboratory Animals [29] and following the European Union directives (2010/63/EU), FELASA and ARRIVE guidelines. The animals were conventionally housed in groups of 4 or 5 in a temperature-controlled (22 °C) and humidity-controlled (66%) environment under a 12-h/12-h light/dark cycle, where food and water intake was ad libitum. The study protocol was approved by the Ethical Committee on Animal Use and Care of the University of Barcelona (CEEA/UB) and Federal University of Santa Catarina (CEUA/UFSC, Protocol PP00955).
Drugs
Reserpine, guanosine (GUO), 1,3-dipropyl-8-cyclopentylxanthine (DPCPX) - A1R antagonist, 5-amino-7-(2-phenylethyl)-2-(2-furyl)-pyrazolo(4,3-e)-1,2,4-triazolo(1,5-c)pyrimidine (SCH58261) - A2AR antagonist, were from Sigma Chemical, St. Louis, MO.
Reserpine treatment
To induce the TJM behavior, we use a previous standardized protocol [30, 31], where mice were injected twice (every other day) with reserpine (1.0 mg/kg) subcutaneously (s.c.). Reserpine was dissolved in glacial acetic acid and then diluted to a final concentration of 0.1% acetic acid with saline (NaCl 0.9%). Controls were injected with a saline in 0.1% acetic acid solution.
Pharmacological treatment
Guanosine treatment
GUO was dissolved in saline (NaCl 0.9%) and administered in effective or sub-effective doses (7.5 or 5 mg/kg, respectively; [14]) by oral route (p.o.) 20 min prior the behavioral tests and 24 h after the last injection of reserpine. GUO doses were selected from our own group experience [14]. Controls were treated with saline (p.o.).
A2AR experiments
To evaluate the involvement of A2AR on GUO-induced antidyskinetic effect, a dose response of SCH58261 was initially performed. SCH58261 was dissolved in dimethylsulfoxide (DMSO) then in saline to the final desired concentrations, and the behavioral analysis was carried out after 30 min. To analyze a putative potentiation effect with GUO and SCH58261 treatment, they were administered in their sub-effective doses (5 mg/kg p.o. and 0.01 mg/kg i.p., respectively) with 10 min treatment interval. Behavioral tests were conducted 30 min after SCH58261 and 20 min after GUO treatments and 24 h after the last injection of reserpine.
In the A2AR-KO mice protocol, mice were treated with GUO (5 or 7.5 mg/kg) 20 min prior the tests and 24 h after the last injection of reserpine.
A1R experiments
To evaluate the involvement of A1R on GUO-induced antidyskinetic effect, DPCPX (0.75 mg/kg; dissolved in DMSO then in saline) was injected via intraperitoneal (i.p.) 30 min prior the GUO active dose administration (7.5 mg/kg, p.o.). The dose of DPCPX was selected on the basis of literature data on oral tremor [32].
Tremulous jaw movements
Tremulous jaw movements (TJMs) were defined as rapid vertical deflections of the lower jaw that resembled chewing but were not directed at any particular stimulus [4]. This protocol was initially standardized to rats [4, 33] and we adapted the protocol to mice based on previous published studies [30, 31]. To quantify the occurrence of this orofacial dyskinesia, mice were placed individually in a glass cylinder (13 cm diameter) and hand-operated counters were employed to score TJM frequencies. Mirrors were placed under the floor and behind the back wall of the cylinder to allow observation when the animal was faced away from the observer. If TJM occurred during a period of grooming, they were not taken into account. The incidence of these oral movements was measured continuously for 10 min.
Brain slices
Animals were euthanized by decapitation and brains were quickly removed and the cerebral cortex, hippocampus, and striatum were rapidly dissected in ice-cold KREBS ringer buffer (KRB) (122 mM NaCl, 3 mM KCl, 1.2 mM MgSO4, 1.3 mM CaCl2, 0.4 mM KH2PO4, 25 mM NaHCO3, and 10 mM D-glucose, bubbled with 95% O2/5% CO2 up to pH 7.4) [19]. For the biochemical assays, slices (0.4 mm) were prepared using a Mcllwain Tissue Chopper (The Mickle Laboratory Engineering Co. ltd., England) and separated in KRB at 4 °C. After sectioning, slices were incubated in KRB for 30 min, at 37 °C, for recovery.
ROS levels
ROS production was measured by using the molecular probe 2,7-dichlorofluorescein diacetate (H2DCFDA, Sigma Chemical, St. Louis, MO.). H2DCFDA diffuses through the cell membrane and is hydrolyzed by intracellular esterases to the non-fluorescent form 2′,7′-dichlorofluorescein (DCFH). DCFH reacts with intracellular ROS (such as H2O2) to form dichlorofluorescein (DCF), a green fluorescent dye. DCF fluorescence intensity is proportional to the amount of ROS. Brain slices were incubated with 80 μM of H2DCFDA for 30 min at 37 °C and then washed in KRB. Fluorescence was read with the multifunctional microplate reader Infinite M200 (Tecan Group Ltd., Mannedorf, Switzerland), using excitation and emission wavelengths of 480 and 525 nm, respectively [19].
Membrane integrity evaluation
Membrane integrity was assessed by evaluating the uptake of the fluorescent exclusion dye, propidium iodide (PI, Sigma Aldrich, St Louis, MO, USA), which is a polar compound that enters only in cells with damaged membranes. Once inside the cells, PI complexes with DNA and emits an intense red fluorescence (630 nm) when excited by green light (495 nm) [34]. Slices were incubated with PI (7 μg/mL) for 30 min at 37 °C, and then washed with KRB for analysis on fluorescence microplate reader Infinite M200 from Tecan®.
Mitochondrial membrane potential
Mitochondrial membrane potential was measured by using the molecular probe tetramethylrhodamine ethyl ester (TMRE, Sigma Chemical, St. Louis, MO.) 100ηM for 30 min at 37 °C. Fluorescence was measured with the multifunctional microplate reader Infinite M200 from Tecan®, using wavelengths of excitation and emission of 550 and 590 ηm, respectively [35].
MTT reduction assay
Cellular viability in slices was quantified by measuring the reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Sigma Chemical, St. Louis, MO.) to a dark violet formazan product by dehydrogenases. Slices were incubated with MTT (0.5 mg/mL) in KRB buffer for 20 min at 37 °C, the formazan produced was solubilized by replacing the medium with 200 μL of DMSO, resulting in a colored compound which was quantified spectrophotometrically at a wavelength of 550 ηm. Absorbance was measured with the multifunctional microplate reader Infinite M200 from Tecan®. The results are expressed and normalized as percentages relative to the control conditions.
Data analysis
Data are represented as means ± S.E.M. Normalized data from multiple experiments were averaged and statistical analysis was carried out as described in the figure legends. Data with two groups were analyzed by Student’s t test, and other data used one-way or two-way ANOVA followed by Tukey’s post hoc. Statistical difference was accepted when P < 0.05.
Results
Guanosine effects on the reserpinized mice
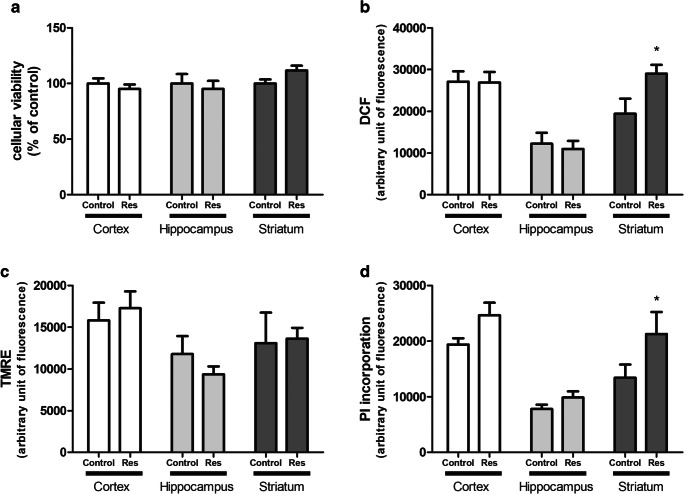
Initially, cortical, hippocampal, and striatal slices were used to biochemical evaluations, as cellular viability of slices, ROS production, mitochondrial membrane potential, and cell membrane permeabilization. Reserpine administration caused no alteration in cortical or hippocampal slices (Fig. 1a–d). In striatal slices, reserpine administration did not alter cellular viability and mitochondrial membrane potential (Fig. 1a, c). However, striatal slices showed an increase of 50% in ROS production (P = 0.017) and an increase of 58% in PI incorporation (P = 0.046) by reserpine treatment as compared with control (Fig. 1b, d).
Fig. 1.
Evaluation of reserpine (Res, 1 mg/kg) neurotoxicity in cortical, hippocampal, and striatal slices. a Cellular viability measured by MTT reduction, expressed as percentage of control. b ROS measurement through fluorescence of DCF dye. c Evaluation of mitochondrial membrane potential with TMRE fluorescent dye. d Membrane integrity evaluation due to PI incorporation. Fluorescence data are shown as arbitrary fluorescent unit. Results are presented as means ± SEM (*P < 0.05 vs control; Student’s t test; n = 7)
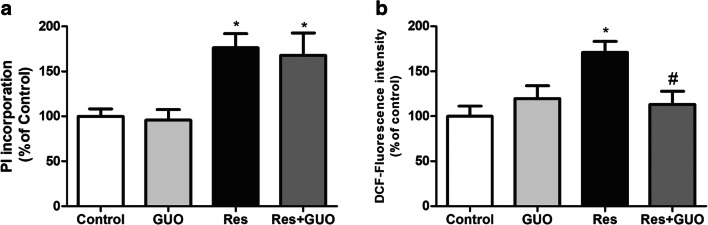
Accordingly, we obtained striatal slices and assessed GUO effects reversing reserpine-induced ROS production, and PI incorporation. The dose of 7.5 mg/kg of GUO was used based on a previous study, in which we described that GUO (7.5 mg/kg) decreased TJM frequency in reserpinized mice [14]. Interestingly, we could observe that GUO totally reversed reserpine-induced ROS production in striatal slices (P = 0.031), while it failed to block PI incorporation (P = 0.982) (Fig. 2a, b).
Fig. 2.
Guanosine protective ex vivo effect in reserpinized mice. a GUO (7.5 mg/kg) effect on ROS production and b cellular membrane permeability through PI incorporation in striatal slices of mice treated with reserpine. Results are presented as means ± SEM.; #P < 0.05 vs reserpine (two-way ANOVA with Tukey’s post hoc test; n = 5)
Involvement of adenosine A2AR on guanosine-mediated TJM and ROS decrease
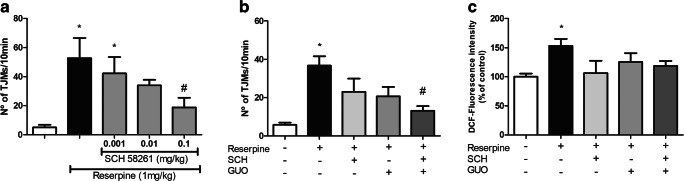
It is known that A2AR antagonism has effects on motor disturbances related to PD [32, 36–38]. Therefore, we aimed to see if GUO effect of reducing TJM and ROS generation in the striatum could be related to antagonism of A2AR. Firstly, a dose-response curve was performed with SCH58261 (Fig. 3a). The highest dose of SCH58261 (0.1 mg/kg) fully reversed the TJM by reserpine (P = 0.042) while the lowest dose (0.001 mg/kg) had no effect (P = 0.893). The 0.01 mg/kg dose was sub-effective (i.e., presented statistical difference either from control or to reserpine group). Then, we evaluated the effect of co-treatment of SCH58261 and GUO sub-effective doses. We previously showed that 5 mg/kg GUO presented a sub-effective effect on reserpine-induced TJM [14]. The co-administration of sub-effective doses of SCH58261 (0.01 mg/kg) and GUO (5 mg/kg) completely reversed the reserpine-induced orofacial tremor (P = 0.004) (Fig. 3b). Regarding ROS generation, treatment with sub-effective dose of SCH58261 (0.01 mg/kg), or with sub-effective GUO dose (5 mg/kg), displayed no statistically significant effect of decreasing ROS production (P = 0.111 and 0.553, respectively). Co-administration of SCH58261 and GUO also showed no significant difference from reserpine-treated animals (P = 0.287) (Fig. 3c).
Fig. 3.
Involvement of A2AR on reserpine-induced TJM and ROS production. a Dose-response curve of A2AR antagonist SCH58261 (0.001; 0.01 and 0.1 mg/kg) in reserpine-induced oral tremor (TJM). b SCH58261 (0.01 mg/kg) plus GUO (5 mg/kg) effect on reserpine-induced TJM. c SCH58261 (0.01 mg/kg) and GUO (5 mg/kg) effect on ROS increase in striatal slices of reserpinized mice. Results are presented as means ± SEM (*P < 0.05 vs control; #P < 0.05 vs reserpine; one-way ANOVA with Tukey’s post hoc test; n = 8–10)
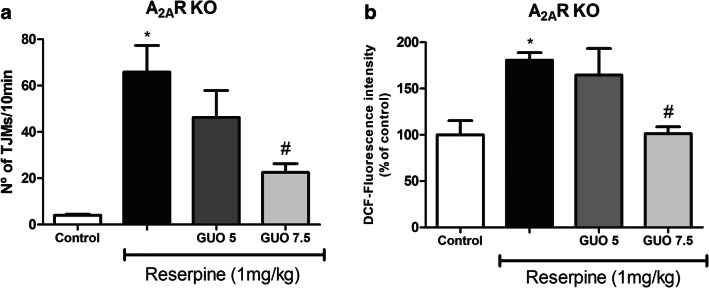
To clarify the role of A2AR on GUO effect in the reserpine-induced TJM and striatal ROS production, genetic-modified mice A2AR deficient (A2AR-KO) were used. Animals were subjected to the same protocol of reserpine and GUO treatment. The sub-effective GUO dose (5 mg/kg) had no effect against reserpine in these animals, both in the TJM quantification (P = 0.362) and ROS measurement (P = 0.807) (Fig. 4a). On the other hand, GUO at 7.5 mg/kg dose presented an effect of reversing the reserpine induction of TJM (P = 0.017) and ROS increase (P = 0.040) (Fig. 4b), indicating that presence of A2AR is not necessary to GUO effect in reserpinized mice.
Fig. 4.
Effect of guanosine on reserpine-induced TJM and ROS production in A2AR-deficient (A2AR-KO) mice. a TJM in mice treated with GUO sub-effective (5 mg/kg) or effective (7.5 mg/kg) doses (n = 6). b ROS production in striatal slices of mice treated GUO (5 mg/kg or 7.5 mg/kg, n = 3). Results are presented as means ±SEM (*P < 0.05 vs control; #P < 0.05 vs reserpine; one-way ANOVA with Tukey’s post hoc test)
Involvement of adenosine A1R on guanosine-mediated TJM and ROS decrease
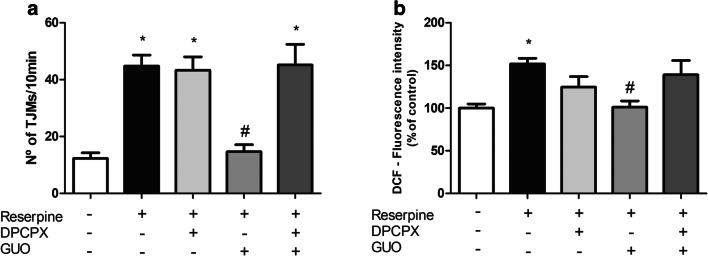
To test the involvement of A1R, 24 h after the last reserpine administration, mice were treated with the A1R antagonist DPCPX (0.75 mg/kg i.p. [32], 30 min prior the GUO administration. DPCPX treatment did not alter reserpine-induced TJM (P = 0.999), but it completely blocked the effect of GUO on TJM frequency (P = 0.0003) (Fig. 5a).
Fig. 5.
Effect of A1R blockade on reserpine-induced TJM and ROS production in mouse striatal slices. a Effect of A1R antagonist DPCPX (0.75 mg/kg i.p.) plus GUO (7.5 mg/kg) on TJM of mice. b Evaluation of GUO (7.5 mg/kg) plus DPCPX (0.75 mg/kg) effect on ROS increase in striatal slices of reserpinized mice. Results are presented as means ± SEM (*P < 0.05 vs control; #P < 0.05 vs reserpine; one-way ANOVA with Tukey’s post hoc test; n = 5–6)
As GUO (7.5 mg/kg) showed effect through reverting ROS increase by reserpine, we aimed to see if this effect is related to A1R. Prior treatment with the A1R antagonist DPCPX (0.75 mg/kg) did not significantly alter ROS increase induced by reserpine (P = 0.383) but it prevented the reversion of GUO (P = 0.912) (Fig. 5b). These data suggest a strong dependence of A1R for GUO behavioral and biochemical effects.
Discussion
GUO treatment shows a promising effect on animal models of motor disorders. We already shown that in unilaterally 6-hydroxidopamine-(6-OHDA)-lesioned rats, GUO increased L-DOPA sub-maximal response and decreased LID. Also, GUO reversed reserpine-induced TJM and catalepsy in mice [14]. In this study, we investigated the mechanisms behind this GUO effect of reducing the orofacial tremor and the striatal oxidative damage evoked by reserpine, by assessing the possible involvement of adenosine receptors in the GUO effects.
The mechanism related to the induction of oral tremor is multifaceted, with multiple neurotransmitters, including GABA, serotonin, adenosine, and acetylcholine, interacting with dopamine in the regulation of basal ganglia motor functions [4, 39–43]. In this study, we focused in a possible therapeutic approach towards adenosinergic transmission.
A2AR antagonists have emerged as a potential treatment of parkinsonian motor impairments as they can exert allosteric modulations upon D2R ligands [44]. Also, A2AR is highly expressed in the striatum and it was shown that its antagonism reduces oral tremor in different rodent models [32, 36, 45–48]. Accordingly, SCH58261, the A2AR antagonist tested in this study, exhibited effect in the reserpine-induced TJM in mice. Moreover, SCH58261 and GUO sub-effective doses potentiated each other’s effect on TJM behavior. Despite the potentiated effect observed with SCH58261 and GUO co-treatment on reserpine-induced TJM, when GUO was tested in genetic-modified animals lacking adenosine A2A receptors (A2AR-KO), it was observed that this receptor is not essentially involved in the GUO antidyskinetic effect. Therefore, we decided to test the participation of A1R on behavioral and biochemical GUO effects.
It is known that adenosine A1R can antagonistically modulate D1R responses and that the stimulation of A1R could inhibit the D1R stimulation [25, 49–51]. Interestingly, rats treated with reserpine (1 mg/kg; s.c.) for 5 days showed an increase in the responsiveness of adenylate cyclase after D1R stimulation [52]. Also, rats treated with one single dose of reserpine (1 mg/kg; i.p.) showed upregulation of the transduction mechanism associated with D1R, without changing the activity of D2R [53]. Evidence from our results suggests that GUO motor effect could be through A1R stimulation. In fact, as results with A1R stimulation for oral tremor are lacking in the literature, we tested reserpinized mice with 2-chloro-N-6-cyclopentyladenosine (CCPA), an A1R agonist, and a potent antidyskinetic effect was observed (in the doses 0.0125, 0.025, and 0.05 mg/kg). However, we also observed that CCPA promotes an important sedative response in these animals (data not shown) that precludes its use as a treatment against motor impairments. In this sense, A1R stimulation promoted by GUO could be inhibiting the overstimulation of D1R in reserpinized mice, and then decreasing the oral tremor.
Besides the motor disturbance, we also investigated biochemical changes in reserpinized mice in different cerebral structures (i.e., cerebrocortex, hippocampus, and striatum). It is well known that the inhibition of dopamine vesicular storage leads to an increase in ROS; this occurs because dopamine metabolism intrinsically results in ROS formation [54]. The major area affected was the striatum, where it was seen an increase in ROS production and permeabilization of the cellular membrane. Thus, oxidative and cell membrane damage in the striatum might sum up to the monoamine depletion to impair motor performance. This increase in ROS nearby the cell membrane could cause its oxidation and lead to an injury in the membrane lipids as it was seen on incorporation of PI. In fact, some studies with the same reserpine protocol have already shown an increase on lipid peroxidation in striatum [30, 55]. This increase in ROS production and cell membrane permeabilization may reflect early events of toxicity by reserpine but surprisingly, we did not see alteration in the cell reductive capacity, assessed by MTT reduction method. Accordingly, the reserpine toxicity in this protocol does not affect the mitochondrial membrane potential. More important, GUO acutely administrated was able to reverse ROS increase induced by reserpine, and this effect was dependent on A1R and not A2AR.
To our knowledge, our previous study was the first to identify GUO treatment as an antiparkinsonian agent in a rodent model of orofacial tremor [14]. Although other studies have shown the protective effect of GUO in cellular models of PD [15, 17] or in vivo rodent models of PD [56], none of them has assessed the molecular targets related to GUO effects. Despite this, evidence from other brain disease models has pointed to GUO effect via adenosine receptor modulation. In an in vitro ischemia model, hippocampal slices subjected to oxygen/glucose deprivation presented increased ROS production prevented by GUO, but this effect is abolished by pre-incubation with DPCPX [19]. These data corroborate with the idea that GUO effect of preventing an oxidative damage is A1R dependent. Notwithstanding, in the same ischemia protocol, not only DPCPX but also an A2AR agonist (CGS21680) blunted the protective effect of GUO in hippocampal slices [19] and in cortical astrocytes [18]. Likewise, the ischemia model in A2AR-KO animals implies GUO-protective effects upon A2AR in the hippocampus [57]. As different results obtained with A2AR-KO mice may be dependent on the cerebral area analyzed, there is still controversy regarding GUO effects via adenosine A1R or A2AR interaction, and additionally, the possibility of GUO interaction with adenosine receptor heteromers. More important, a recent study from our group shed some light on this issue of GUO interaction on A1R and/or A2AR. We showed that GUO-induced effects may require both A1R and A2AR co-expression in transfected HEK293 cells, indicating that GUO acts on adenosine receptors in an oligomeric conformation, i.e., the A1R-A2AR heteromer [57]. Once GUO acts upon A1R-A2AR heteromer formation, its effects on other receptor oligomeric organization of A1R and/or A2AR are possible and were still not evaluated. Thus, it is feasible to speculate that in the striatum, GUO can interact with A1R and then modulated D1R or A1R-D1R heteromer interaction, and further investigations are necessary to clarify GUO mechanism on motor control.
In conclusion, our results strengthen the demonstration of extracellular actions of GUO and the dependence of adenosine A1R activation to the motor-related effect of GUO. Considering the GUO-mediated motor improvement differs mechanistically from classic adenosine receptor modulators, it is important to understand the mechanisms behind GUO effects.
Funding information
The research performed at the Universidade Federal de Santa Catarina was supported by the Brazilian funding agencies, CAPES (CAPES/PAJT), CNPq (INCT-EN for Excitotoxicity and Neuroprotection), and FAPESC (NENASC/PRONEX) to C.I.T. The research performed at the Universitat de Barcelona was supported by FEDER/Ministerio de Ciencia, Innovación y Universidades–Agencia Estatal de Investigación (SAF2017-87349-R) and ISCIII (PIE14/00034), the Catalan government (2017 SGR 1604), Fundació la Marató de TV3 (Grant 20152031), and FWO (SBO-140028) to F.C. Also, CAPES-PDSE (47/2017) provided doctoral fellowship to C.M.M. We thank LAMEB/UFSC team work for experimental support.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Ethical approval
The study protocol was approved by the Ethical Committee on Animal Use and Care of the University of Barcelona (CEEA/UB) and Federal University of Santa Catarina (CEUA/UFSC, Protocol PP00955).
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hirsch EC, Mouatt A, Faucheux B, Bonnet AM, Javoy-Agid F, Graybiel AM, Agid Y. Dopamine, tremor, and Parkinson’s disease. Lancet. 1992;340:125–126. doi: 10.1016/0140-6736(92)90457-e. [DOI] [PubMed] [Google Scholar]
- 2.Deuschl G, Raethjen J, Baron R, Lindemann M, Wilms H, Krack P. The pathophysiology of parkinsonian tremor: a review. J Neurol. 2000;247(Suppl 5):V33–V48. doi: 10.1007/pl00007781. [DOI] [PubMed] [Google Scholar]
- 3.Collins-Praino LE, Paul NE, Rychalsky KL, Hinman JR, Chrobak JJ, Senatus PB, Salamone JD. Pharmacological and physiological characterization of the tremulous jaw movement model of parkinsonian tremor: potential insights into the pathophysiology of tremor. Front Syst Neurosci. 2011;5:49. doi: 10.3389/fnsys.2011.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salamone JD, Mayorga AJ, Trevitt JT, Cousins MS, Conlan A, Nawab A. Tremulous jaw movements in rats: a model of parkinsonian tremor. Prog Neurobiol. 1998;56:591–611. doi: 10.1016/s0301-0082(98)00053-7. [DOI] [PubMed] [Google Scholar]
- 5.Duma SR, Fung VS. Drug-induced movement disorders. Aust Prescr. 2019;42:56–61. doi: 10.18773/austprescr.2019.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colpaert FC. Pharmacological characteristics of tremor, rigidity and hypokinesia induced by reserpine in rat. Neuropharmacology. 1987;26:1431–1440. doi: 10.1016/0028-3908(87)90110-9. [DOI] [PubMed] [Google Scholar]
- 7.Jiang S, Ballerini P, Buccella S, Giuliani P, Jiang C, Huang X, Rathbone MP. Remyelination after chronic spinal cord injury is associated with proliferation of endogenous adult progenitor cells after systemic administration of guanosine. Purinergic Signal. 2008;4:61–71. doi: 10.1007/s11302-007-9093-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmidt AP, Lara DR, Souza DO. Proposal of a guanine-based purinergic system in the mammalian central nervous system. Pharmacol Ther. 2007;116:401–416. doi: 10.1016/j.pharmthera.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 9.Lanznaster D, Mack JM, Coelho V, Ganzella M, Almeida RF, Dal-Cim T, Hansel G, Zimmer ER, Souza DO, Prediger RD, Tasca CI (2016) Guanosine prevents anhedonic-like behavior and impairment in hippocampal glutamate transport following amyloid-β1–40 administration in mice. Mol Neurobiol [DOI] [PubMed]
- 10.Lara DR, Schmidt AP, Frizzo ME, Burgos JS, Ramírez G, Souza DO. Effect of orally administered guanosine on seizures and death induced by glutamatergic agents. Brain Res. 2001;912:176–180. doi: 10.1016/s0006-8993(01)02734-2. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt AP, Böhmer AE, Schallenberger C, Antunes C, Tavares RG, Wofchuk ST, Elisabetsky E, Souza DO. Mechanisms involved in the antinociception induced by systemic administration of guanosine in mice. Br J Pharmacol. 2010;159:1247–1263. doi: 10.1111/j.1476-5381.2009.00597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Almeida RF, Comasseto DD, Ramos DB, Hansel G, Zimmer ER, Loureiro SO, Ganzella M, Souza DO (2016) Guanosine anxiolytic-like effect involves adenosinergic and glutamatergic neurotransmitter systems. Mol Neurobiol [DOI] [PubMed]
- 13.Bettio LE, Freitas AE, Neis VB, Santos DB, Ribeiro CM, Rosa PB, Farina M, Rodrigues AL. Guanosine prevents behavioral alterations in the forced swimming test and hippocampal oxidative damage induced by acute restraint stress. Pharmacol Biochem Behav. 2014;127:7–14. doi: 10.1016/j.pbb.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 14.Massari CM, López-Cano M, Núñez F, Fernández-Dueñas V, Tasca CI, Ciruela F. Antiparkinsonian efficacy of guanosine in rodent models of movement disorder. Front Pharmacol. 2017;8:700. doi: 10.3389/fphar.2017.00700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marques NF, Massari CM, Tasca CI. Guanosine protects striatal slices against 6-OHDA-induced oxidative damage, mitochondrial dysfunction, and ATP depletion. Neurotox Res. 2019;35:475–483. doi: 10.1007/s12640-018-9976-1. [DOI] [PubMed] [Google Scholar]
- 16.Giuliani P, Ballerini P, Buccella S, Ciccarelli R, Rathbone MP, Romano S, D'Alimonte I, Caciagli F, Di Iorio P, Pokorski M. Guanosine protects glial cells against 6-hydroxydopamine toxicity. Adv Exp Med Biol. 2015;837:23–33. doi: 10.1007/5584_2014_73. [DOI] [PubMed] [Google Scholar]
- 17.Giuliani P, Romano S, Ballerini P, Ciccarelli R, Petragnani N, Cicchitti S, Zuccarini M, Jiang S, Rathbone MP, Caciagli F, Di Iorio P. Protective activity of guanosine in an in vitro model of Parkinson’s disease. Panminerva Med. 2012;54:43–51. [PubMed] [Google Scholar]
- 18.Dal-Cim T, Poluceno GG, Lanznaster D, de Oliveira KA, Nedel CB, Tasca CI (2019) Guanosine prevents oxidative damage and glutamate uptake impairment induced by oxygen/glucose deprivation in cortical astrocyte cultures: involvement of A. Purinergic Signal [DOI] [PMC free article] [PubMed]
- 19.Dal-Cim T, Ludka FK, Martins WC, Reginato C, Parada E, Egea J, López MG, Tasca CI. Guanosine controls inflammatory pathways to afford neuroprotection of hippocampal slices under oxygen and glucose deprivation conditions. J Neurochem. 2013;126:437–450. doi: 10.1111/jnc.12324. [DOI] [PubMed] [Google Scholar]
- 20.Yabe I, Kitagawa M, Takahashi I, Matsushima M, Sasaki H. The efficacy of istradefylline for treating mild wearing-off in Parkinson disease. Clin Neuropharmacol. 2017;40:261–263. doi: 10.1097/WNF.0000000000000249. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki K, Miyamoto T, Miyamoto M, Uchiyama T, Hirata K. Could istradefylline be a treatment option for postural abnormalities in mid-stage Parkinson's disease? J Neurol Sci. 2018;385:131–133. doi: 10.1016/j.jns.2017.12.027. [DOI] [PubMed] [Google Scholar]
- 22.T. M. Palmer and G. L. Stiles, "Adenosine receptors," Neuropharmacology, vol. 34, pp. 683–694, 1995 [DOI] [PubMed]
- 23.Krügel U, Kittner H, Franke H, Illes P. Purinergic modulation of neuronal activity in the mesolimbic dopaminergic system in vivo. Synapse. 2003;47:134–142. doi: 10.1002/syn.10162. [DOI] [PubMed] [Google Scholar]
- 24.Ferré S, Fuxe K, von Euler G, Johansson B, Fredholm BB. Adenosine-dopamine interactions in the brain. Neuroscience. 1992;51:501–512. doi: 10.1016/0306-4522(92)90291-9. [DOI] [PubMed] [Google Scholar]
- 25.Popoli P, Giménez-Llort L, Pezzola A, Reggio R, Martínez E, Fuxe K, Ferré S. Adenosine A1 receptor blockade selectively potentiates the motor effects induced by dopamine D1 receptor stimulation in rodents. Neurosci Lett. 1996;218:209–213. doi: 10.1016/s0304-3940(96)13143-8. [DOI] [PubMed] [Google Scholar]
- 26.Fuxe K, Ferré S, Canals M, Torvinen M, Terasmaa A, Marcellino D, Goldberg SR, Staines W, Jacobsen KX, Lluis C, Woods AS, Agnati LF, Franco R. Adenosine A2A and dopamine D2 heteromeric receptor complexes and their function. J Mol Neurosci. 2005;26:209–220. doi: 10.1385/JMN:26:2-3:209. [DOI] [PubMed] [Google Scholar]
- 27.Fernández-Dueñas V, Gómez-Soler M, Valle-León M, Watanabe M, Ferrer I, Ciruela F (2019) Revealing adenosine A. Int J Mol Sci 20 [DOI] [PMC free article] [PubMed]
- 28.Ferré S, Ciruela F. Functional and neuroprotective role of striatal adenosine a. J Caffeine Adenosine Res. 2019;9:89–97. doi: 10.1089/caff.2019.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clark JD, Gebhart GF, Gonder JC, Keeling ME, Kohn DF. Special report: the 1996 guide for the care and use of laboratory animals. ILAR J. 1997;38:41–48. doi: 10.1093/ilar.38.1.41. [DOI] [PubMed] [Google Scholar]
- 30.Burger ME, Alves A, Callegari L, Athayde FR, Nogueira CW, Zeni G, Rocha JB. Ebselen attenuates reserpine-induced orofacial dyskinesia and oxidative stress in rat striatum. Prog Neuro-Psychopharmacol Biol Psychiatry. 2003;27:135–140. doi: 10.1016/s0278-5846(02)00344-5. [DOI] [PubMed] [Google Scholar]
- 31.Cunha AS, Matheus FC, Moretti M, Sampaio TB, Poli A, Santos DB, Colle D, Cunha MP, Blum-Silva CH, Sandjo LP, Reginatto FH, Rodrigues AL, Farina M, Prediger RD. Agmatine attenuates reserpine-induced oral dyskinesia in mice: role of oxidative stress, nitric oxide and glutamate NMDA receptors. Behav Brain Res. 2016;312:64–76. doi: 10.1016/j.bbr.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 32.Collins LE, Galtieri DJ, Brennum LT, Sager TN, Hockemeyer J, Müller CE, Hinman JR, Chrobak JJ, Salamone JD. Oral tremor induced by the muscarinic agonist pilocarpine is suppressed by the adenosine A2A antagonists MSX-3 and SCH58261, but not the adenosine A1 antagonist DPCPX. Pharmacol Biochem Behav. 2010;94:561–569. doi: 10.1016/j.pbb.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 33.Salamone J, Baskin P. Vacuous jaw movements induced by acute reserpine and low-dose apomorphine: possible model of parkinsonian tremor. Pharmacol Biochem Behav. 1996;53:179–183. doi: 10.1016/0091-3057(95)00164-6. [DOI] [PubMed] [Google Scholar]
- 34.Ludka FK, Cunha MP, Dal-Cim T, Binder LB, Constantino LC, Massari CM, Martins WC, Rodrigues AL, Tasca CI (2016) Atorvastatin protects from Aβ1–40-induced cell damage and depressive-like behavior via ProBDNF cleavage. Mol Neurobiol [DOI] [PubMed]
- 35.Ludka FK, Dal-Cim T, Binder LB, Constantino LC, Massari C, Tasca CI (2016) Atorvastatin and fluoxetine prevent oxidative stress and mitochondrial dysfunction evoked by glutamate toxicity in hippocampal slices. Mol Neurobiol [DOI] [PubMed]
- 36.Pinna A, Ko WK, Costa G, Tronci E, Fidalgo C, Simola N, Li Q, Tabrizi MA, Bezard E, Carta M, Morelli M. Antidyskinetic effect of A2A and 5HT1A/1B receptor ligands in two animal models of Parkinson’s disease. Mov Disord. 2016;31:501–511. doi: 10.1002/mds.26475. [DOI] [PubMed] [Google Scholar]
- 37.Kondo T, Mizuno Y, J. I. S. Group A long-term study of istradefylline safety and efficacy in patients with Parkinson disease. Clin Neuropharmacol. 2015;38:41–46. doi: 10.1097/WNF.0000000000000073. [DOI] [PubMed] [Google Scholar]
- 38.Gandía J, Morató X, Stagljar I, Fernández-Dueñas V, Ciruela F. Adenosine A2A receptor-mediated control of pilocarpine-induced tremulous jaw movements is Parkinson’s disease-associated GPR37 receptor-dependent. Behav Brain Res. 2015;288:103–106. doi: 10.1016/j.bbr.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 39.Mayorga AJ, Carriero DL, Cousins MS, Gianutsos G, Salamone JD. Tremulous jaw movements produced by acute tacrine administration: possible relation to parkinsonian side effects. Pharmacol Biochem Behav. 1997;56:273–279. doi: 10.1016/s0091-3057(96)00225-0. [DOI] [PubMed] [Google Scholar]
- 40.Mayorga AJ, Trevitt JT, Conlan A, Gianutsos G, Salamone JD. Striatal and nigral D1 mechanisms involved in the antiparkinsonian effects of SKF 82958 (APB): studies of tremulous jaw movements in rats. Psychopharmacology (Berl) 1999;143:72–81. doi: 10.1007/s002130050921. [DOI] [PubMed] [Google Scholar]
- 41.Trevitt J, Kawa K, Jalali A, Larsen C. Differential effects of adenosine antagonists in two models of parkinsonian tremor. Pharmacol Biochem Behav. 2009;94:24–29. doi: 10.1016/j.pbb.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 42.Carlson BB, Behrstock S, Tobin AJ, Salamone JD. Brain implantations of engineered GABA-releasing cells suppress tremor in an animal model of Parkinsonism. Neuroscience. 2003;119:927–932. doi: 10.1016/s0306-4522(03)00218-5. [DOI] [PubMed] [Google Scholar]
- 43.Carlson BB, Wisniecki A, Salamone JD. Local injections of the 5-hydroxytryptamine antagonist mianserin into substantia nigra pars reticulata block tremulous jaw movements in rats: studies with a putative model of Parkinsonian tremor. Psychopharmacology (Berl) 2003;165:229–237. doi: 10.1007/s00213-002-1247-3. [DOI] [PubMed] [Google Scholar]
- 44.Ferré S, Bonaventura J, Tomasi D, Navarro G, Moreno E, Cortés A, Lluís C, Casadó V, Volkow ND. Allosteric mechanisms within the adenosine A2A-dopamine D2 receptor heterotetramer. Neuropharmacology. 2016;104:154–160. doi: 10.1016/j.neuropharm.2015.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Svenningsson P, Le Moine C, Kull B, Sunahara R, Bloch B, Fredholm BB. Cellular expression of adenosine A2A receptor messenger RNA in the rat central nervous system with special reference to dopamine innervated areas. Neuroscience. 1997;80:1171–1185. doi: 10.1016/s0306-4522(97)00180-2. [DOI] [PubMed] [Google Scholar]
- 46.Dixon AK, Gubitz AK, Sirinathsinghji DJ, Richardson PJ, Freeman TC. Tissue distribution of adenosine receptor mRNAs in the rat. Br J Pharmacol. 1996;118:1461–1468. doi: 10.1111/j.1476-5381.1996.tb15561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salamone JD, Collins-Praino LE, Pardo M, Podurgiel SJ, Baqi Y, Müller CE, Schwarzschild MA, Correa M. Conditional neural knockout of the adenosine A(2A) receptor and pharmacological A(2A) antagonism reduce pilocarpine-induced tremulous jaw movements: studies with a mouse model of parkinsonian tremor. Eur Neuropsychopharmacol. 2013;23:972–977. doi: 10.1016/j.euroneuro.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 48.Collins-Praino LE, Paul NE, Ledgard F, Podurgiel SJ, Kovner R, Baqi Y, Müller CE, Senatus PB, Salamone JD. Deep brain stimulation of the subthalamic nucleus reverses oral tremor in pharmacological models of parkinsonism: interaction with the effects of adenosine A2A antagonism. Eur J Neurosci. 2013;38:2183–2191. doi: 10.1111/ejn.12212. [DOI] [PubMed] [Google Scholar]
- 49.Ferré S, Popoli P, Giménez-Llort L, Finnman UB, Martínez E, Scotti de Carolis A, Fuxe K. Postsynaptic antagonistic interaction between adenosine A1 and dopamine D1 receptors. Neuroreport. 1994;6:73–76. doi: 10.1097/00001756-199412300-00020. [DOI] [PubMed] [Google Scholar]
- 50.Ferre S, Popoli P, Tinner-Staines B, Fuxe K. Adenosine A1 receptor-dopamine D1 receptor interaction in the rat limbic system: modulation of dopamine D1 receptor antagonist binding sites. Neurosci Lett. 1996;208:109–112. doi: 10.1016/0304-3940(96)12577-5. [DOI] [PubMed] [Google Scholar]
- 51.Ismayilova N, Crossman A, Verkhratsky A, Brotchie J. Effects of adenosine A1, dopamine D1 and metabotropic glutamate 5 receptors-modulating agents on locomotion of the reserpinised rats. Eur J Pharmacol. 2004;497:187–195. doi: 10.1016/j.ejphar.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 52.Missale C, Nisoli E, Liberini P, Rizzonelli P, Memo M, Buonamici M, Rossi A, Spano P. Repeated reserpine administration up-regulates the transduction mechanisms of D1 receptors without changing the density of [3H]SCH 23390 binding. Brain Res. 1989;483:117–122. doi: 10.1016/0006-8993(89)90041-3. [DOI] [PubMed] [Google Scholar]
- 53.Liberini P, Nisoli E, Missale C, Memo M, Buonamici M, Rossi A, Spano PF. Differential effect of acute reserpine administration on D-1 and D-2 dopaminergic receptor density and function in rat striatum. Neurochem Int. 1989;14:61–64. doi: 10.1016/0197-0186(89)90010-7. [DOI] [PubMed] [Google Scholar]
- 54.Meiser J, Weindl D, Hiller K. Complexity of dopamine metabolism. Cell Commun Signal. 2013;11:34. doi: 10.1186/1478-811X-11-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Teixeira AM, Reckziegel P, Müller L, Pereira RP, Roos DH, Rocha JB, Bürger ME. Intense exercise potentiates oxidative stress in striatum of reserpine-treated animals. Pharmacol Biochem Behav. 2009;92:231–235. doi: 10.1016/j.pbb.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 56.Su C, Elfeki N, Ballerini P, D'Alimonte I, Bau C, Ciccarelli R, Caciagli F, Gabriele J, Jiang S. Guanosine improves motor behavior, reduces apoptosis, and stimulates neurogenesis in rats with parkinsonism. J Neurosci Res. 2009;87:617–625. doi: 10.1002/jnr.21883. [DOI] [PubMed] [Google Scholar]
- 57.Lanznaster D, Massari CM, Marková V, Šimková T, Duroux R, Jacobson KA, Fernández-Dueñas V, Tasca CI, Ciruela F (2019) Adenosine A. Cells 8 [DOI] [PMC free article] [PubMed]