Abstract
Gliomas, the most common primary brain cancer, are highly infiltrative and extremely difficult to treat. Despite advancements, current treatment is limited, with patients surviving for a median of 14–15 months post-diagnosis. Previous research has demonstrated the upregulation of a purinergic receptor, P2X7R, in human gliomas. P2X7R is expressed on both glioma cells and microglia within the glioma microenvironment. It is hypothesized that P2X7R contributes to tumour growth and proliferation via immune-mediated mechanisms involving tumour cells and surrounding microglia. We sought to elucidate the role of P2X7R in a human glioblastoma cell line (U251) and on surgically resected human glioma samples. We treated U251 and human glioma cultures for 72 h with P2X7R antagonists, Brilliant Blue G (BBG), oxidized ATP (oATP) and AZ10606120. Cell counting via fluorescence confocal microscopy was conducted to assess tumour proliferation. We observed no significant reductions in tumour cell numbers following P2X7R antagonism with BBG (20 μM) and oATP (250 μM) in both U251 cells and human glioma samples. Interestingly, there was a significant reduction in tumour cell number in both U251 cells (p = 0.0156) and human glioma samples (p = 0.0476) treated with varying concentrations of AZ10606120. When compared with the conventional chemotherapeutic agent, temozolomide, AZ10606120 was also found to more effectively inhibit tumour proliferation in U251 cells (p < 0.0001). Our pilot results demonstrate a potential trophic role of P2X7R where its inhibition by AZ10606120, a potent antagonist, hinders glioma growth directly or through the inactivation of microglia. This sheds new light on P2X7R as a therapeutic target for human gliomas.
Keywords: Glioma, Glioblastoma, P2X7R, P2X7 receptor, Cancer
Introduction
Gliomas are the most common primary brain tumour and account for approximately 80% of all brain malignancies [1]. These tumours predominantly manifest as high-grade glioblastomas that are vastly infiltrative and extremely difficult to treat. Glioblastomas kill 225,000 people worldwide annually, with patients surviving a median of 14–15 months post-diagnosis [1].
The current standard of care for high-grade gliomas involves maximal safe surgical resection, chemotherapy and radiotherapy. Following surgery, chemotherapy with the oral DNA alkylating agent, temozolomide, is administered. These treatments only improve survival by a few months albeit with many associated side effects, and despite advances in radiotherapy and the production of targeted biologicals [2], the prognosis for high-grade glioma (glioblastoma) patients remains bleak. Malignant gliomas are a continual burden to global health.
The pathogenesis of glioma tumours is driven by a range of factors that encompass molecular and genetic alterations, growth factor upregulation and the loss of cell-cycle inhibitors [3]. Importantly, the P2X7 receptor (P2X7R) has been implicated in glioma pathophysiology [4–11]. Studies have previously shown P2X7R involvement in a variety of cancers, including melanoma [12], uterine epithelial cancer [13], leukaemia [14] and lung cancer [15]. P2X7R is a purinergic ATP-sensing receptor expressed on cells of haemopoietic and immunological origin. Additional to ATP, synthetic compounds, including 2’3’-O-(4-benzoylbenzoyl)-ATP (bzATP), are known to function as agonists of the receptor [6, 11]. P2X7R has two conductance states. Transient agonist stimulation induces the opening of a highly selective channel that facilitates cation transport across the cell membrane. Alternatively, maximal stimulation fosters the opening of a non-selective pore that forms a passage for larger molecules of up to 900 Da in size [16]. The exact role of P2X7R in a physiological setting is unclear, but it has been implicated in cell trophism and cytokine release.
In gliomas, P2X7R is expressed on both glioma cells and microglia, the main cellular infiltrates of the glioma microenvironment [4]. Glioma-associated microglia exhibit an immunomodulatory phenotype that is likely induced by P2X7R activation [17]. We have previously demonstrated P2X7R upregulation in gliomas [4]. Despite this, the precise functions of P2X7R in the glioma microenvironment remain unclear. In vitro studies have shown that P2X7R antagonism promoted the growth of glioma cells in the rat C6 [5] and mouse GL261 [6] glioma cell line, suggesting a cytolytic role of P2X7R. In contrast, multiple studies have demonstrated reductions in glioma proliferation following P2X7R antagonism [7–10], indicative of trophic P2X7R activity. A further study using rat C6 glioma cells demonstrated increased tumour cell motility following P2X7R stimulation [11]. It is clear that there is significant P2X7R involvement in gliomas; however, limited studies have focused on human glioma samples. The contradictions of the above findings prompted our study on human tumour samples, which would comparatively present a more ‘ideal’ representation of physiological glioma microenvironment.
Here, we show a significant reduction in tumour cells in both the U251 human glioblastoma cell line and surgically resected human glioma samples following P2X7R inhibition with the potent antagonist, AZ10606120. We also confirm P2X7R expression in human glioma. It is hypothesized that P2X7R serves a pro-tumourigenic role in gliomas, which can be prevented by P2X7R antagonism. Our pilot data further support the trophic and therapeutic potential of P2X7R in human gliomas.
Materials and methods
Human tumour sample cultures
We collected 12 histologically diagnosed high-grade (grade III and IV) glioma samples from patients undergoing surgery for routine tumour resection by a qualified neurosurgeon at the Royal Melbourne Hospital, Victoria, Australia. All patients were consented with an ethically approved protocol (Melbourne Health, Ethics Committee) prior to surgery. Upon removal, tumour samples were immediately transported to the laboratory for cell culture. Within a class II biosafety cabinet, the tumour tissue was fragmented with a scalpel and placed in Earle’s Balanced Salt Solution (EBSS; ThermoFisher Scientific Cat# 14155063) containing Papain from papaya latex (200 units; Sigma-Aldrich Cat# P3125) at 37 °C for 35 min. The sample was then washed in triplicate and subsequently dissociated into a single-cell suspension with Minimum Essential Medium (MEM, 1X; ThermoFisher Scientific Cat# 10370021) supplemented with 1 mM D-glucose (Sigma-Aldrich Cat# G7021), 2 mM L-glutamine (Gibco Cat# 25030081), 50 units/mL penicillin-streptomycin (ThermoFisher Scientific Cat# 15070063), 10% heat inactivated foetal bovine serum (FBS; ThermoFisher Scientific Cat# 10100147) and Corning® MITO+ Serum Extender (Sigma-Aldrich Cat# DLW355006). Tumour cells were cultured at a density of approximately 1 × 1055 cells per well into 12-well cell culture plates (Sigma-Aldrich Cat# SIAL0512) containing 18 mm coverslips (Thermo Fisher Scientific Cat# CB00180RA020MNT0) pre-coated with poly-D-lysine hydrobromide (PDL; Sigma-Aldrich Cat# P6407). Cell cultures were maintained in a humidified incubator at 37 °C, 5%CO2, and grown to 80% confluency.
U251 human glioblastoma cell line cultures
Human U251 glioblastoma cells (Sigma-Aldrich Cat# 09063001, RRID: CVCL_0021) were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM; Lonza Cat# 12-604F) supplemented with 1 mM sodium pyruvate (ThermoFisher Scientific Cat# 11360070), 50 units/mL penicillin-streptomycin, 10% FBS and 1% non-essential amino acids (NEAA; ThermoFisher Scientific Cat# 11140050). Cells were maintained in a humidified incubator at 37 °C, 5%CO2, and routinely passaged at 80% confluency.
Cell treatments
Human glioma samples and U251 cells at 80% confluency were treated for 72 h with the following P2X7R antagonists: Brilliant Blue G (BBG; 20 μM; Sigma-Aldrich Cat# B0770), oxidized ATP (oATP; 250 μM; Sigma-Aldrich Cat# 5.05758) or AZ10606120 (Tocris Biosciences Cat# 3323). We used AZ10606120 concentrations of 5 μM and 25 μM for U251 cells and 15 μM for human glioma samples. For comparison of P2X7R antagonism efficacy with conventional glioma treatment, cells were also treated with the chemotherapeutic agent, temozolomide (50 μM; Sigma-Aldrich Cat# T2577).
Immunocytochemistry
Human glioma samples and U251 cells were fixed in a solution of 1:1 acetone-methanol at − 20 °C for 15 min, prior to experiments. Cells were incubated with primary antibody diluted in phosphate buffered saline (PBS; ThermoFisher Scientific Cat# 14190144) and 0.1% Triton X-100 (Sigma-Aldrich Cat# X100) for 72 h at 4 °C. The following primary antibodies were used: rabbit anti-GFAP (1:400; Dako Cat# Z0334, RRID: AB_10013382), mouse anti-CD11b (1:200; Abcam Cat# ab1211, RRID: AB_442947) and goat anti-P2X7R (1:200; Quantum Scientific Cat#, RRID). Cells were subsequently washed in triplicate with PBS and immersed in secondary antibody conjugates at 4 °C for 24 h, including donkey anti-goat Alexa Fluor 488 (1:200; Thermo Fisher Scientific Cat# A-11055, RRID: AB_2534102), goat anti-mouse Texas Red-X (1:200; Thermo Fisher Scientific Cat# T-862, RRID: AB_2556781) and goat anti-rabbit Texas Red-X (1:200; Thermo Fisher Scientific Cat# T-6391, RRID: AB_2556779). Stained cells were mounted using fluorescence mounting medium (Dako Cat# S3023) and viewed via fluorescent confocal microscopy.
Fluorescence confocal microscopy
Mounted glioma samples and U251 cells were viewed with a Zeiss LSM 510 META fluorescent confocal microscope equipped with 488 nm argon and 543 and 633 nm green and red helium/neon lasers. A 63× IR-Achromat (N.A. 0.80) water immersion objective was used for image acquisition.
Cell counts
After 72 h of treatment, cells were fixed with 1:1 acetone-methanol solution at − 20 °C for 15 min. Fixed cells were subsequently stained with 5 μM of DAPI nuclear counterstain (Thermo Fisher Scientific Cat# D1306) at 25 °C for 1 h. Cells were viewed with an Olympus IX-81 fluorescence microscope, and a cell count was conducted based on the number of DAPI-positive nuclei. Images were acquired with a 40× LucPlan FI Olympus (N.A. 0.6) air objective, and nuclei were manually counted using MetaMorph (Universal Imaging Corporation®). For each sample/passage, a total of 16 random fields per treatment were imaged and quantified. To account for the variability between cell numbers of each sample, changes in tumour cell numbers were expressed as a ratio of cells in the treatment group compared with the corresponding control. For U251 cells, the magnitude of change was measured using the ratio of cells in each group compared with that of the average cell count of the control group.
Statistical analysis
All statistical tests were conducted using the GraphPad Prism 7 software (San Diego, California). For comparison of multiple treatments, a one-way analysis of variance (ANOVA) was used to quantify statistical significance, with Tukey’s multiple comparisons post hoc where relevant. Unpaired t-tests were used to quantify the statistical significance of experiments comparing two groups. The significance level was determined as p < 0.05, and data are expressed as mean ± SEM.
Results
P2X7R is widely expressed in U251 cells
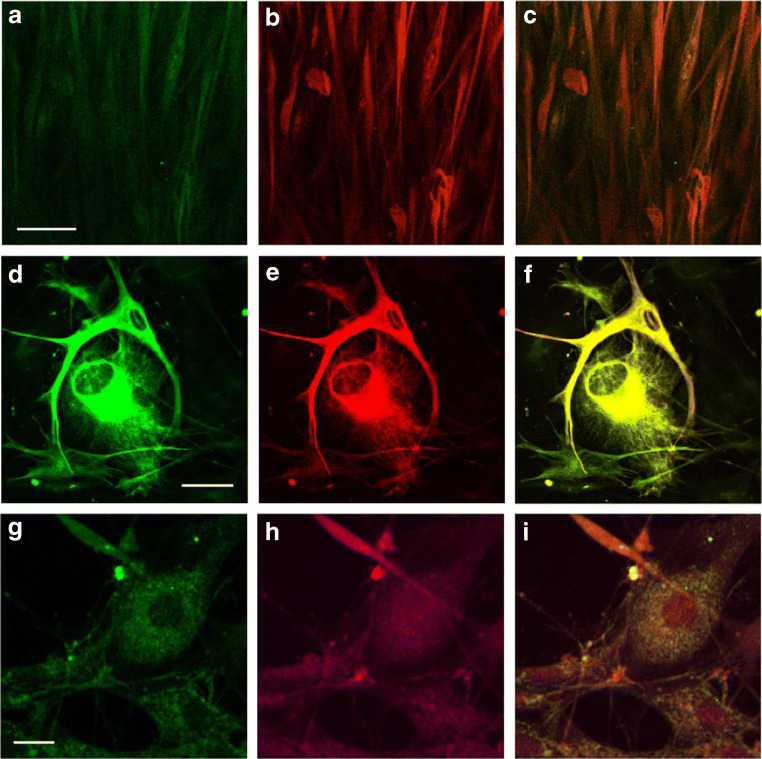
We used GFAP as a marker for glioma cells. GFAP has been documented as a reliable marker for tumour cells of glial origin [18]. The U251 human glioblastoma cell line has previously been shown to express moderate levels of GFAP [18, 19]. We first sought to confirm GFAP expression in U251 cells via immunocytochemistry, and as expected, staining for GFAP was positive in all samples. P2X7R was also present in U251 cells and appeared to be co-localized with GFAP expression (Fig. 1a–c). Taken together, these results confirm both GFAP and P2X7R expression in the U251 human glioblastoma cell line.
Fig. 1.
P2X7 receptor (P2X7R) expression on the human U251 glioblastoma cell line and human high-grade glioma samples. a–c Co-localization of glial fibrillary acidic protein (GFAP) and P2X7R in U251 cells. Cultured U251 cells at 80% confluency were fixed in a solution of acetone-methanol and stained with the following antibodies: primary goat anti-P2X7R and secondary Alexa Fluor 488 (green), followed by primary polyclonal rabbit anti-GFAP and secondary Texas Red-X (red). Shown here are the fluorescent immunocytochemical images depicting a P2X7R expression, b GFAP expression and c P2X7R and GFAP co-localisation. Scale bar = 5 μM. d–f Co-localization of GFAP and P2X7R in human high-grade glioma samples. Cultured human tumour samples at 80% confluency were fixed in a solution of acetone-methanol and stained with the following antibodies: primary anti-P2X7 and secondary Alexa Fluor 488, followed by primary anti-GFAP and secondary Texas Red-X. Shown here are the fluorescent immunocytochemical images depicting d P2X7R expression, e GFAP expression and f P2X7R and GFAP co-localisation. Scale bar = 5 μM. g–i Co-localization of CD11b and P2X7R in human high-grade glioma samples. Cultured human tumour samples at 80% confluency were fixed in a solution of acetone-methanol and stained with the following antibodies: primary anti-P2X7 and secondary Alexa Fluor 488, followed by primary anti-CD11b and secondary Texas Red-X. Shown here are the fluorescent immunocytochemical images depicting g P2X7R expression, h CD11b expression and i P2X7R and CD11b co-localisation. Scale bar = 2 μM
P2X7R is widely expressed in glioma cells and microglia of human glioma samples
Given that P2X7R expression was positive in U251 cells, we analysed human glioma samples for P2X7R expression via immunocytochemistry. Specifically, we characterized P2X7R expression on both glioma cells (GFAP-positive; Fig. 1d–f) and microglia (CD11b-positive; Fig. 1g–i). As expected, P2X7R was present on both cell types and co-localized with GFAP and CD11b expression. These observations indicate widespread expression of P2X7R in tumour cells as well as microglia.
The effect of P2X7R antagonism by BBG and oATP versus conventional therapy on U251 cells and human glioma samples
We next determined the direct effect of P2X7R inhibition by BBG and oATP on U251 cell proliferation. Untreated control cells and cells treated with BBG and oATP for 72 h were fixed and subsequently stained with DAPI for manual quantification (n = 12). A one-way ANOVA yielded no statistically significant difference in cell numbers (n = 12; F(2, 33) = 2.99, p = 0.0643), between each of the treatment groups, as well as the control (Fig. 2a–d).
Fig. 2.
Effect of P2X7 receptor (P2X7R) antagonism by Brilliant Blue G (BBG) and oxidized ATP (oATP) on tumour growth in the human U251 glioblastoma cell line (a–d) and human high-grade glioma samples (e–h). U251 glioma cells and cultured human glioma samples were treated with the P2X7R antagonists, Brilliant Blue G (BBG; 20 μM) and oxidized ATP (oATP; 250 μM). Cells were stained with DAPI nuclear stain and fixed 72 h post-treatment. Human tumour samples were pre-stained with glial fibrillary acidic protein (GFAP) for identification of tumour cells. A manual cell count was conducted thereafter, and the mean cell count was generated from a total of 16 random fields per treatment. The magnitude of change was measured using the ratio of cells in the each group compared with that of the average cell count of the control group. a U251 cell count data. There was no significant reduction in tumour cell numbers between cells treated with BBG and oATP and the control; n = 12, one-way ANOVA, p = 0.0643. Data are represented as mean ± SEM. Example U251 cell count images are provided for b control, c BBG and d oATP. e Human glioma sample cell count data. There was no significant reduction in tumour cell numbers between cells treated with BBG and oATP and the control; n = 5, one-way ANOVA, p = 0.284. Cell counts were expressed as the ratio of treated cells to the control to account for any variability in initial culture cell numbers between tumours. Data are represented as mean ± SEM. Example human glioma sample cell count images are provided for f control, g BBG and h oATP. Scale bar = 5 μM
In the human glioma samples, a one-way ANOVA for the differences in tumour cell (GFAP-positive) counts between BBG and oATP versus control yielded a p value of 0.284 (n = 5 tumours; F(2, 12) = 1.402), which was not statistically significant (Fig. 2e–h). These results collectively indicate that P2X7R inhibition with BBG (20 μM) and oATP (250 μM) did not significantly inhibit glioma cell proliferation in both U251 cells and human glioma samples.
P2X7R antagonism by AZ10606120 significantly inhibited U251 cell proliferation
In separate experiments, we also explored the effect of AZ10606120 on glioma cell proliferation. AZ10606120 is a well-documented, potent and highly selective P2X7R antagonist [20]. U251 cells were treated with 5 μM or 25 μM of AZ10606120 for 72 h and compared with untreated cells. There was a significant difference between the three groups (n = 6; F(2, 13) = 17.52, p = 0.0002), which was then confirmed by Tukey’s multiple comparisons test to be between the control and cells treated with 5 μM (mean ratio to the control = 0.819 ± 0.03; p = 0.0079; 95% CI, 0.742–0.896) and 25 μM (mean ratio to the control = 0.706 ± 0.028; p = 0.0001; 95% CI, 0.634–0.778) of AZ10606120 (Fig. 3a–d). AZ10606120 at a concentration of 25 μM appeared to more effectively reduce U251 cell counts compared with the lower dose of 5 μM; however, these results, when compared with each other, were not statistically significant (p = 0.059) and thus prompts further investigation. Taken together, these data show that treatment with AZ10606120 at both 5 μM and 25 μM for 72 h significantly inhibited U251 cell proliferation.
Fig. 3.
The P2X7 receptor (P2X7R) antagonist, AZ10606120, significantly inhibits tumour growth in U251 glioma cells and was more effective than the conventional chemotherapeutic agent, temozolomide. a Manual cell quantification of U251 cells treated with AZ10606120 (5 μM and 25 μM) compared with control. A total of 16 fields were counted per treatment for n = 6. Both concentrations of AZ10606120 significantly inhibited tumour growth, compared with the control; one-way ANOVA with Tukey’s HSD, **p = 0.0079 compared with control, ***p = 0.0001 compared with control. Data are represented as mean ± SEM. Example U251 cell count images are provided for b control, c AZ10606120 5 μM and d AZ10606120 25 μM. e Manual cell quantification of U251 cells treated with AZ10606120 (15 μM) and temozolomide (50 μM) compared with control. A total of 16 fields were counted per treatment for n = 12. Both AZ10606120 and temozolomide significantly inhibited tumour growth, compared with the control. Cell counts were also significantly lower in cells treated with 15 μM AZ10606120 compared with temozolomide. One-way ANOVA with Tukey’s HSD, ***p = 0.0003, ****p < 0.0001. Data are represented as mean ± SEM. Example U251 cell count images are provided for f control, g AZ10606120 15 μM and h temozolomide 50 μM. The magnitude of change was measured using the ratio of cells in each group compared with that of the average cell count of the control group. Scale bar = 5 μM
P2X7R antagonism by AZ10606120 significantly inhibited cell proliferation in human glioma samples
Human glioma samples (n = 6) were similarly treated with AZ10606120 for 72 h at a concentration of 15 μM (median concentration that was used in the U251 cell line). To assess the effect of AZ10606120 on tumour cell proliferation, GFAP-positive (glioma) cells were quantified in the presence and absence of AZ10606120. Per tumour sample, the average cell count over 16 randomly selected fields was expressed as a ratio to the corresponding control to account for variability in cell numbers between samples. Results show a significant reduction in tumour cells following treatment with 15 μM of AZ10606120, compared with the control (mean ratio to the control = 0.624 ± 0.16; t(10) = 2.352; p = 0.0405; 95% CI, 0.214–1.035; Fig. 4). Therefore, treatment with 15 μM of AZ10606120 for 72 h significantly inhibited tumour proliferation in human glioma samples.
Fig. 4.

The P2X7 receptor (P2X7R) antagonist, AZ10606120, significantly inhibits tumour growth in human high-grade glioma samples. Example cell count images are provided for a control and b AZ10606120 15 μM, scale bar = 8 μM. c Manual cell quantification of human glioma samples treated with AZ10606120 (15 μM), compared with control. Cells were stained with DAPI nuclear stain and fixed 72 h post-treatment. Human tumour samples were pre-stained with glial fibrillary acidic protein (GFAP) for identification of tumour cells. A total of 16 fields were counted per treatment for 6 tumours. Cell counts were expressed as the ratio of treated cells to the control to account for any variability in initial culture cell numbers between tumours. AZ10606120 at 15 μM significantly inhibited tumour proliferation, compared with the control; unpaired t-test, *p = 0.0405. Data are represented as mean ± SEM
P2X7R antagonism by AZ10606120 was more effective at inhibiting tumour cell proliferation than conventional chemotherapy in U251 cells
Given that treatment with AZ10606120 was found to significantly reduce tumour cell numbers in both U251 cells and human glioma samples, we conducted an additional experiment investigating the effects of AZ10606120 versus the conventional chemotherapeutic agent, temozolomide, in U251 cells. Temozolomide is the most common chemotherapeutic agent used in the treatment of high-grade gliomas [21]. U251 cells were treated for 72 h with either AZ10606120 (15 μM) or temozolomide (50 μM). Following treatment, cells were fixed and manually quantified using fluorescence microscopy based on the number DAPI-positive nuclei. A one-way ANOVA yielded a statistically significant difference between groups, indicated by a p value of less than 0.0001 (n = 12; F(2,33) = 64.87; Fig. 3e–h). Specifically, both AZ10606120 (mean ratio to the control = 0.48 ± 0.02; p < 0.0001; 95% CI, 0.901–1.099) and temozolomide (mean ratio to the control = 0.795 ± 0.028; p = 0.0003; 95% CI, 0.735–0.856) significantly reduced tumour cell numbers, when compared with untreated cells. Notably, 15 μM of AZ10606120 was found to be more effective at inhibiting tumour cell proliferation than 50 μM of temozolomide in U251 cells (p < 0.0001; Fig. 3e–h). These results indicate that AZ10606120 shows potential to be used adjunctively or in place of conventional chemotherapy in the near future.
Discussion
Here, we investigated the role of P2X7R antagonism in human gliomas. High-grade gliomas are vastly invasive tumours with a high mortality rate. Currently, these tumours are treated with maximal surgical resection, followed by radiotherapy and chemotherapy [22, 23]. Temozolomide, a DNA chelating agent, is the approved chemotherapy agent for these tumours but only increases survival rate by a few months [21] with significant associated side effects. Currently, there is no cure or effective therapy for human gliomas and the prognosis for those diagnosed remains dismal. The enigmatic role of P2X7R in gliomas is still being unfolded. What is known thus far is that it likely contributes to microglial activation and recruitment of these cells to the glioma microenvironment and, importantly, the release of various cytokines and chemokines that ultimately shape the pro- and anti-tumour response [8, 9, 11, 24]. Recent work from our laboratory documented the importance of the P2X7R pore conductance state in modulating the release of IL-1β, a cytokine present within the glioma microenvironment that interestingly serves as a trophic activator of microglia [25]. We showed in that study that P2X7R is critical in microglial activation and proliferation and that the proliferative response is mediated by the cytokine, IL-1β. Multiple studies have reported that glioma-associated microglia are polarized into a phenotype that largely supports tumour growth and angiogenesis [26, 27]. Butovsky and colleagues (2013) further demonstrated that microglia in high-grade gliomas had increased expression of immunosuppressive and tissue repair genes, but not those that are immunostimulatory [28]. In fact, the release of many immunosuppressive mediators in the glioma microenvironment is likely to be governed by P2X7R activation [8, 9, 11]. In the recent years, many studies have reported findings that support a trophic, tumour-promoting role for P2X7R activation, rather than a cytolytic and tumour-inhibiting role [7, 9, 10]. These speculations formed the basis of our overarching hypothesis that P2X7R promotes tumour growth in high-grade gliomas and its antagonism will be an effective means of inhibiting glioma progression. In this study, we characterized P2X7R expression on both the U251 glioma cell line and human glioma samples and investigated the effect of P2X7R inhibition by various antagonistic agents on tumour proliferation.
As part of this investigation, we confirmed P2X7R expression on both U251 glioma cells and human tumour samples. Specifically, we characterized its expression on glioma cells and microglia, as indicated by GFAP and CD11b expression, respectively. P2X7R was found to be expressed on U251 cells and on both glioma cells and microglia of human tumour samples. The latter observation was consistent with our previous work that showed generalized expression of P2X7R on glioma cells and associated microglia [4]. Ubiquitous expression of P2X7R in the glioma microenvironment suggests that the receptor is likely important in mediating a range of tumourigenic and immunomodulatory functions. P2X7R overexpression has been reported in various cancers, including thyroid papillary cancer [29], pancreatic ductal adenocarcinoma [30] and leukaemia [31]. In gliomas, studies have interestingly demonstrated increased P2X7R expression in infiltrating microglia, compared with glioma cells per se [4, 7, 32]. While P2X7R expression has been relatively well defined, few studies have quantified the differences in expression between glioma cells and microglia. This serves as a continual area of interest for future research.
The idea of targeting P2X7R in gliomas has only recently come into light. The current literature has reported both tumour-promoting [7, 11] and tumour-inhibiting [6, 33] effects, following P2X7R stimulation in the glioma microenvironment, but the roles of P2X7R on tumour growth remain unclear. In this study, treatment with BBG and oATP did not significantly inhibit tumour proliferation in both U251 glioma cells and human glioma samples. We propose several theories to explain these findings. Firstly, the treatment period of 72 h may have been too long, at which time, the reagents, in particular BBG and oATP, could have been degraded. The 72 h timeframe might have also been sufficient to allow the generation of new ‘unblocked’ P2X7R, overriding the effectiveness of treatment. Alternatively, treatment doses may have been too low and not sufficient to induce a cytotoxic effect. Nonetheless, these experiments should be repeated in larger studies that test the above reagents at varying timeframes and concentrations.
This study is one of the first to trial the potent and highly selective P2X7R antagonist, AZ10606120, in U251 glioblastoma cell lines and human glioma samples. AZ10606120 has been shown to reduce numbers and growth of pancreatic stellate cells in pancreatic ductal adenocarcinoma [34]. Furthermore, a study by Adinolfi et al. (2012) demonstrated a substantial reduction in tumour growth in both mouse melanoma B16 cells and human neuroblastoma ACN cells after P2X7R blockade with AZ10606120 [35]. P2X7R inhibition with AZ10606120 also reduced VEGF production and tumour angiogenesis in mice [35]. No previous studies have reported the effects of AZ10606120 in human gliomas, and our study to our knowledge is the first to report such a finding. Our results show a significant inhibition of tumour proliferation in both U251 cells and human glioma samples after 72 h of treatment with AZ10606120. In U251 cells, AZ10606120 at a concentration of both 5 μM and 25 μM was effective at reducing cell numbers. A greater treatment concentration of 25 μM appeared to inhibit tumour cell proliferation to a greater extent, but the effect was not statistically significant. The overall significance of this data may have been affected by the small sample size and should be repeated in larger experiments. Given this, we trialled an AZ10606120 concentration of 15 μM on human tumour samples, which was found to significantly suppress tumour proliferation by almost 40%. Interestingly, AZ10606120 at 15 μM was also more effective than temozolomide at inhibiting tumour proliferation in U251 cells. While this data is preliminary and should be repeated for human glioma samples, AZ10606120 may have the potential to be taken in substitute of temozolomide, as chemotherapeutic agent has been coupled to only a modest (2.5 months) increase in survival time [21] and an array of adverse effects [36]. Taken together, these findings shine the spotlight on AZ10606120 as a potential anti-tumour candidate for further study and, ultimately, development into human clinical trials. Future studies on AZ10606120 should focus on its effect on P2X7R channel and pore function, its efficacy when compared with conventional treatment as well as its mechanism of inhibition and action.
While BBG, oATP and AZ10606120 are all documented P2X7R antagonists, the differences in inhibitory activity as observed in this study may be attributed to differences in the properties, potency and pharmacodynamics of each antagonist. A notable anti-tumour effect was seen in both U251 cells and human glioma samples treated with AZ10606120, but not BBG and oATP. AZ10606120 serves as a non-competitive, negative allosteric P2X7R inhibitor that binds P2X7R with an IC50 of approximately 10 nM [20]. The antagonistic activity of the compound has been additionally shown to bind P2X7R with over 1000-fold selectivity than other P2X receptors [20]. BBG is similarly a non-competitive inhibitor of P2X7R; however, it binds the receptor with a comparatively lower IC50 of 200 nM [37]. In contrast, oATP is an irreversible antagonist that inhibits P2X7R with an IC50 of 100 μM [38]. This is at a substantially lower potency than both AZ10606120 and BBG. Interestingly, the effects of oATP may not be exclusive to P2X7R [39]. Data has highlighted potential anti-inflammatory effects of the reagent that might contribute to tumour progression. oATP has indeed been demonstrated to reduce interleukin-8 (IL-8) release in non-P2X7R-expressing cells [39]. IL-8 is essential for neutrophil chemotaxis and recruitment into the tumour microenvironment [40]. Importantly, neutrophils are implicated in glioma pathogenesis as neutrophil infiltration has been positively correlated with increasing glioma malignancy [41]. In the current study, downstream neutrophil recruitment is an unlikely explanation for the comparatively low efficacy of P2X7R antagonism by oATP; however, it should be considered in future in vivo oATP experiments. As a result, AZ10606120 binds P2X7R with considerably more potency and specificity when compared with both BBG and oATP.
Collectively, we highlight an important aspect of P2X7R involvement in human gliomas. P2X7R was shown to be widely expressed in U251 glioma cells and both microglia and tumour cells of human glioma samples. Importantly, P2X7R antagonism with AZ10606120 significantly inhibited tumour growth in both U251 glioma cells and human glioma samples, supporting a trophic role of the P2X7R receptor in human gliomas. We are aware that small sample size was a limitation of this study and propose to repeat our experiments in a larger investigation. Further studies are required to characterize the effect of P2X7R antagonism on P2X7R channel and pore function, P2X7R antagonism on glioma-associated microglia and the efficacy of AZ10606120-mediated P2X7R inhibition versus conventional treatment. Nonetheless, our study is the first to report that AZ10606120 inhibition in human glioma cultures reduces glioma cell proliferation. Interestingly the effect in U251 glioma cell line was comparatively better than the conventional chemotherapeutic, temozolomide. This sheds new light on P2X7R as a therapeutic target for human gliomas and warrants the development of AZ10606120 for future in vivo animal studies and ultimately for human clinical trials.
Abbreviations
- ANOVA
Analysis of variance
- BBG
Brilliant Blue G
- bzATP
2’3’-O-(4-benzoylbenzoyl)-ATP
- DMEM
Dulbecco’s Modified Eagle’s Medium
- EBSS
Earle’s Balanced Salt Solution
- FBS
Foetal bovine serum
- MEM
Minimum Essential Medium
- NEAA
Non-essential amino acids
- oATP
Oxidized ATP
- P2X7R
P2X7 receptor
- PBS
Phosphate buffered saline
- PDL
Poly-D-lysine hydrobromide
Funding information
Brain Foundation Australia
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hanif F, Muzaffar K, Perveen K, Malhi SM, Simjee SU. Glioblastoma multiforme: a review of its epidemiology and pathogenesis through clinical presentation and treatment. Asian Pac J Cancer Prev. 2017;18(1):3–9. doi: 10.22034/APJCP.2017.18.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Meir EG, Hadjipanayis CG, Norden AD, Shu HK, Wen PY, Olson JJ. Exciting new advances in neuro-oncology: the avenue to a cure for malignant glioma. CA-Cancer J Clin. 2010;60(3):166–193. doi: 10.3322/caac.20069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gladson CL, Prayson RA, Liu WM. The pathobiology of glioma tumors. Annu Rev Pathol. 2010;5:33–50. doi: 10.1146/annurev-pathol-121808-102109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monif M, O’Brien TJ, Drummond KJ, Reid CA, Liubinas SV, Williams DA. P2X7 receptors are a potential novel target for anti-glioma therapies. J Inflamm. 2014;11(1):25. [Google Scholar]
- 5.Fang J, Chen X, Zhang L, Chen J, Liang Y, Li X, Xiang J, Wang L, Guo G, Zhang B, Zhang W. P2X7R suppression promotes glioma growth through epidermal growth factor receptor signal pathway. Int J Biochem Cell Biol. 2013;45(6):1109–1120. doi: 10.1016/j.biocel.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 6.Tamajusuku AS, Villodre ES, Paulus R, et al. Characterization of ATP-induced cell death in the GL261 mouse glioma. J Cell Biochem. 2010;109(5):983–991. doi: 10.1002/jcb.22478. [DOI] [PubMed] [Google Scholar]
- 7.Ryu JK, Jantaratnotai N, Serrano-Perez MC, McGeer PL, McLarnon JG. Block of purinergic P2X7R inhibits tumor growth in a C6 glioma brain tumor animal model. J Neuropathol Exp Neurol. 2011;70(1):13–22. doi: 10.1097/NEN.0b013e318201d4d4. [DOI] [PubMed] [Google Scholar]
- 8.Fang KM, Wang YL, Huang MC, Sun SH, Cheng H, Tzeng SF. Expression of macrophage inflammatory protein-1alpha and monocyte chemoattractant protein-1 in glioma-infiltrating microglia: involvement of ATP and P2X7 receptor. J Neurosci Res. 2011;89(2):199–211. doi: 10.1002/jnr.22538. [DOI] [PubMed] [Google Scholar]
- 9.Bergamin LS, Braganhol E, Figueiro F, et al. Involvement of purinergic system in the release of cytokines by macrophages exposed to glioma-conditioned medium. J Cell Biochem. 2015;116(5):721–729. doi: 10.1002/jcb.25018. [DOI] [PubMed] [Google Scholar]
- 10.Ji Z, Xie Y, Guan Y, Zhang Y, et al. Involvement of P2X7 receptor in proliferation and migration of human glioma cells. Biomed Res Int. 2018;2018:8591397. doi: 10.1155/2018/8591397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei W, Ryu JK, Choi HB, McLarnon JG. Expression and function of the P2X(7) receptor in rat C6 glioma cells. Cancer Lett. 2008;260(1–2):79–87. doi: 10.1016/j.canlet.2007.10.025. [DOI] [PubMed] [Google Scholar]
- 12.White N, Butler PE, Burnstock G. Human melanomas express functional P2 X(7) receptors. Cell Tissue Res. 2005;321(3):411–418. doi: 10.1007/s00441-005-1149-x. [DOI] [PubMed] [Google Scholar]
- 13.Li X, Zhou L, Feng YH, Abdul-Karim FW, Gorodeski GI. The P2X7 receptor: a novel biomarker of uterine epithelial cancers. Cancer Epidemiol Biomark Prev. 2006;15(10):1906–1913. doi: 10.1158/1055-9965.EPI-06-0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang X, Meng L, He B, Chen J, Liu P, Zhao J, Zhang Y, Li M, An D. The role of P2X7 receptor in ATP-mediated human leukemia cell death: calcium influx-independent. Acta Biochim Biophys Sin Shanghai. 2009;41(5):362–369. doi: 10.1093/abbs/gmp016. [DOI] [PubMed] [Google Scholar]
- 15.Boldrini L, Giordano M, Ali G, et al. P2X7 protein expression and polymorphism in non-small cell lung cancer (NSCLC) J Negat Results Biomed. 2014;13:16. doi: 10.1186/1477-5751-13-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burnstock G, Knight GE. The potential of P2X7 receptors as a therapeutic target, including inflammation and tumour progression. Purinergic Signal. 2018;14(1):1–18. doi: 10.1007/s11302-017-9593-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monif M, Reid CA, Powell KL, Smart ML, Williams DA. The P2X7 receptor drives microglial activation and proliferation: a trophic role for P2X7R pore. J Neurosci. 2009;29(12):3781–3791. doi: 10.1523/JNEUROSCI.5512-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Restrepo A, Smith CA, Agnihotri S, Shekarforoush M, Kongkham PN, Seol HJ, Northcott P, Rutka JT. Epigenetic regulation of glial fibrillary acidic protein by DNA methylation in human malignant gliomas. Neuro-Oncology. 2011;13(1):42–50. doi: 10.1093/neuonc/noq145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mondal S, Dirks P, Rutka JT. Immunolocalization of fascin, an actin-bundling protein and glial fibrillary acidic protein in human astrocytoma cells. Brain Pathol. 2010;20(1):190–199. doi: 10.1111/j.1750-3639.2008.00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allsopp RC, Dayl S, Schmid R, Evans RJ. Unique residues in the ATP gated human P2X7 receptor define a novel allosteric binding pocket for the selective antagonist AZ10606120. Sci. 2017;7(1):725. doi: 10.1038/s41598-017-00732-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO, European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups. National Cancer Institute of Canada Clinical Trials Group Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 22.Coniglio SJ, Segall JE. Review: molecular mechanism of microglia stimulated glioblastoma invasion. Matrix Biol. 2013;32(7):372–380. doi: 10.1016/j.matbio.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 23.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359(5):492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 24.Nduom EK, Weller M, Heimberger AB. Immunosuppressive mechanisms in glioblastoma. Neuro Oncol. 2015;17(suppl_7):vii9–vii14. doi: 10.1093/neuonc/nov151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monif M, Reid CA, Powell KL, Drummond KJ, O'Brien TJ, Williams DA. Interleukin-1beta has trophic effects in microglia and its release is mediated by P2X7R pore. J Neuroinflamm. 2016;13(1):173. doi: 10.1186/s12974-016-0621-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Komohara Y, Ohnishi K, Kuratsu J, Takeya M. Possible involvement of the M2 anti-inflammatory macrophage phenotype in growth of human gliomas. J Pathol. 2008;216(1):15–24. doi: 10.1002/path.2370. [DOI] [PubMed] [Google Scholar]
- 27.Hussain SF, Yang D, Suki D, Aldape K, Grimm E, Heimberger AB. The role of human glioma-infiltrating microglia/macrophages in mediating antitumor immune responses. Neuro-Oncology. 2006;8(3):261–279. doi: 10.1215/15228517-2006-008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Butovsky O, Jedrychowski MP, Moore CS, et al. Identification of a unique TGF-β–dependent molecular and functional signature in microglia. Nat Neurosci. 2013;17:131. doi: 10.1038/nn.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Solini A, Cuccato S, Ferrari D, Santini E, Gulinelli S, Callegari MG, Dardano A, Faviana P, Madec S, di Virgilio F, Monzani F. Increased P2X7 receptor expression and function in thyroid papillary cancer: a new potential marker of the disease? Endocrinology. 2008;149(1):389–396. doi: 10.1210/en.2007-1223. [DOI] [PubMed] [Google Scholar]
- 30.Giannuzzo A, Pedersen SF, Novak I. The P2X7 receptor regulates cell survival, migration and invasion of pancreatic ductal adenocarcinoma cells. Mol Cancer. 2015;14:203. doi: 10.1186/s12943-015-0472-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang XJ, Zheng GG, Ma XT, Yang YH, Li G, Rao Q, Nie K, Wu KF. Expression of P2X7 in human hematopoietic cell lines and leukemia patients. Leuk Res. 2004;28(12):1313–1322. doi: 10.1016/j.leukres.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 32.McLarnon JG. Roles of purinergic P2X7 receptor in glioma and microglia in brain tumors. Cancer Lett. 2017;402:93–99. doi: 10.1016/j.canlet.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 33.Fang J, Chen X, Wang S, Xie T, du X, Liu H, Wang S, Li X, Chen J, Zhang B, Liang H, Yang Y, Zhang W. The expression of P2X7 receptors in EPCs and their potential role in the targeting of EPCs to brain gliomas. Cancer Biol Ther. 2015;16(4):498–510. doi: 10.1080/15384047.2015.1016663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giannuzzo A, Saccomano M, Napp J, Ellegaard M, Alves F, Novak I. Targeting of the P2X7 receptor in pancreatic cancer and stellate cells. Int J Cancer. 2016;139(11):2540–2552. doi: 10.1002/ijc.30380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adinolfi E, Raffaghello L, Giuliani AL, Cavazzini L, Capece M, Chiozzi P, Bianchi G, Kroemer G, Pistoia V, di Virgilio F. Expression of P2X7 receptor increases in vivo tumor growth. Cancer Res. 2012;72(12):2957–2969. doi: 10.1158/0008-5472.CAN-11-1947. [DOI] [PubMed] [Google Scholar]
- 36.Quail DF, Joyce JA. The microenvironmental landscape of brain tumors. Cancer Cell. 2017;31(3):326–341. doi: 10.1016/j.ccell.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang LH, Mackenzie AB, North RA, Surprenant A. Brilliant blue G selectively blocks ATP-gated rat P2X(7) receptors. Mol Pharmacol. 2000;58:82–88. [PubMed] [Google Scholar]
- 38.Nemeth ZH, Csoka B, Spolarics Z, DiFazio LT, Rolandelli RH, Hasko G. Extracellular adenosine triphosphate protects against sepsis by enhancing the intracellular killing of bacteria. J Am Coll Surg. 2017;225(4):e15. [Google Scholar]
- 39.Di Virgilio F. Novel data point to a broader mechanism of action of oxidized ATP: the P2X7 receptor is not the only target. Br J Pharmacol. 2003;140(3):441–443. doi: 10.1038/sj.bjp.0705469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bickel M. The role of interleukin-8 in inflammation and mechanisms of regulation. J Periodontol. 1993;64(5 Suppl):456–460. [PubMed] [Google Scholar]
- 41.Fossati G, Ricevuti G, Edwards SW, Walker C, Dalton A, Rossi ML. Neutrophil infiltration into human gliomas. Acta Neuropathol. 1999;98(4):349–354. doi: 10.1007/s004010051093. [DOI] [PubMed] [Google Scholar]