Abstract
The role played by mesenchymal stem cells (MSCs) in contributing to adult tissue homeostasis and damage repair thanks to their differentiation capabilities has raised a great interest, mainly in bone regenerative medicine. The growth/function of these undifferentiated cells of mesodermal origin, located in specialized structures (niches) of differentiated organs is influenced by substances present in this microenvironment. Among them, ancestral and ubiquitous molecules such as adenine-based purines, i.e., ATP and adenosine, may be included. Notably, extracellular purine concentrations greatly increase during tissue injury; thus, MSCs are exposed to effects mediated by these agents interacting with their own receptors when they act/migrate in vivo or are transplanted into a damaged tissue. Here, we reported that ATP modulates MSC osteogenic differentiation via different P2Y and P2X receptors, but data are often inconclusive/contradictory so that the ATP receptor importance for MSC physiology/differentiation into osteoblasts is yet undetermined. An exception is represented by P2X7 receptors, whose expression was shown at various differentiation stages of bone cells resulting essential for differentiation/survival of both osteoclasts and osteoblasts. As well, adenosine, usually derived from extracellular ATP metabolism, can promote osteogenesis, likely via A2B receptors, even though findings from human MSCs should be implemented and confirmed in preclinical models. Therefore, although many data have revealed possible effects caused by extracellular purines in bone healing/remodeling, further studies, hopefully performed in in vivo models, are necessary to identify defined roles for these compounds in favoring/increasing the pro-osteogenic properties of MSCs and thereby their usefulness in bone regenerative medicine.
Keywords: Mesenchymal stem cells/multipotent stromal cells, Bone regenerative medicine, Osteogenic differentiation, Bone repair, Purine receptors
Introduction
Bone is a dynamic tissue undergoing a continuous remodeling to respond to the needs of the body during growth and adult life. While upon fractures bone is repaired by healing without formation of scar tissue, large innate osseous defects fail to heal [1]. Additionally, aging as well as immune/degenerative diseases can alter bone structure and, in these conditions, the local environment is often unfavorable to osteogenesis due to damage to the surrounding soft tissues and vasculature [2, 3].
A new perspective in the cure of bone dysfunctions is offered by the use mesenchymal stem cells (MSCs), which show good self-renewal and differentiation potential, in particular toward osteogenesis. Therefore, these cells are intensely studied, representing a possible new therapeutic tool in bone regenerative medicine. The acronym “MSCs” has also been used to indicate the same cells as multipotent stromal cells [4, 5] and, more recently, as medicinal signaling cells, to emphasize their property to secrete bioactive factors with trophic/regenerative properties at the sites of injury [6]. Accordingly, preclinical and clinical studies demonstrated that the differentiation capabilities of MSCs can be modulated by endogenous substances, even produced by the same cells, or related drugs. Among these, we focused on purines, which are ancestral molecules synthesized and released from all cells, including MSCs that are provided with a large variety of membrane receptors responsive to purines [7]. Noteworthy, the extracellular concentration of these compounds greatly increases during tissue injury. Thereby, MSCs are likely exposed to them when they migrate in vivo or are transplanted into a damaged tissue. Thus, there is a growing interest to explore how endogenous/exogenous purines influence MSC properties by interacting with their own receptors.
Based on these premises, the aim of this review is to summarize the principal events occurring during osteogenesis and bone repair, focusing on the role played by MSCs and purines in these processes. Several excellent reviews have addressed similar topics over the past 5 years [8–11]. Here, we tried to highlight the positive data obtained so far, but also some limitations related to the research on the use of MSCs and of purine compounds in regenerative bone medicine.
MSC involvement in osteogenesis and bone repair
Bone components
Bone is a heterogeneous tissue formed by two components in close relationship, which are as follows:
the extracellular matrix (ECM), composed of a mineral phase (mainly hydroxyapatite crystals, 50–70%) and an organic phase (20–40%), constituted by collagen type I fibers and non-collagenous proteins including growth factors (GFs), cytokines, and proteoglycans [12];
the bone cells including:
osteoblasts, the bone-forming cells deriving from MSCs [13];
osteocytes, the most abundant cells in adult bone [14], in turn deriving from terminally differentiated osteoblasts, which, contrary to osteoblasts, can survive throughout the life of an individual [15] and play critical roles in the regulation of differentiation and function of osteoblasts and osteoclasts [16];
osteoclasts generated in the bone marrow from mononuclear monocyte-macrophage precursors derived from the hematopoietic lineage, which resorb bone, thereafter undergoing apoptosis [17]
Continuous bone remodeling, which allows the skeleton to grow, adapt, and repair itself, is due to a constant balance between formation and resorption processes carried out by osteoblasts and osteoclasts, respectively.
MSCs and osteogenesis
Osteogenesis is the process of development and formation of bone tissue. Undifferentiated MSCs, present in the bone matrix, start to differentiate into mesenchymal osteoblasts (MOBL), which secrete collagen throughout the matrix, forming a woven structure, and into surface osteoblasts (SOBL), which secrete collagen fibrils creating the highly oriented lamellar bone. Once this process finishes, osteoblasts mature into osteocytes surrounded by collagen matrix [18]. MSCs likely receive biochemical stimuli from neighboring cells able to influence their differentiation into bone precursors. About this, in vitro co-cultures of MSCs with osteocytes show a greater osteogenic differentiation than those with osteoblasts [19].
Multiple molecular pathways play important roles in regulating MSC proliferation, differentiation, and apoptosis in bone. In particular:
Wnt signaling is recognized as a key regulator of bone homeostasis and remodeling so that gene mutation of factors in this pathway may lead to abnormal bone formation [20]. Accordingly, β-catenin inactivation in MSCs can inhibit osteogenic differentiation [21–23];
the adenylate cyclase/cAMP system also affects osteogenesis in human—but not in rodent-derived MSCs; in particular, increase in cAMP levels can either stimulate or inhibit osteogenesis in human MSCs, depending on the duration, rather than the strength of the signal [24, 25];
the activation of extracellular-regulated kinases 1 and 2 (ERK1/2) has also been demonstrated by studies in bone marrow-derived MSCs (BMSCs) committed toward osteoblast differentiation in response to various mechanical stimuli [26, 27].
MSC osteogenic differentiation is also regulated by several transcription factors, including Runx-2 (Runt-related transcription factor 2) [28], Osterix (Osx) [29], Mouse segment homeobox 1/2 (Msx1/2) [30], and T cell factor/lymphoid enhancer factor (TCF/LEF) [31], which are interdependent and closely linked with each other to form a network in the mentioned signaling pathways.
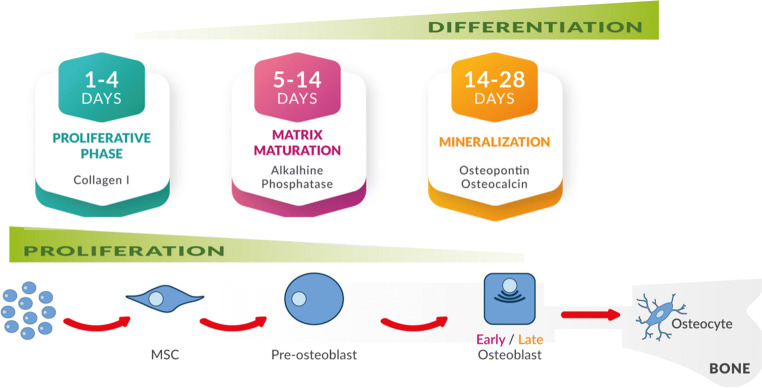
The MSC commitment toward an osteogenic phenotype has long been studied in vitro. This process is induced by the presence of dexamethasone, ascorbic acid, and β-glycerol phosphate in the culture medium [32] and has been divided into three stages [33] along which MSCs express markers typically present in bone forming osteoblasts [34, 35] (see Fig. 1).
Fig. 1.
Steps in the osteogenic differentiation of MSCs: MSCs actively proliferate during the initial stages of osteogenesis and produce collagen [34]. This is followed by early cell differentiation (days from 5 to 14), during which MSCs show a spindle-shaped aspect and decreased proliferation rate, while they start expressing osteogenic markers such as alkaline phosphatase (ALP) secreted by early osteoblasts (matrix maturation phase). After an initial peak, ALP level tends to decline and in the final stage (days from14 to 28) a high expression of osteocalcin and osteopontin, secreted by late osteoblasts, is observed, followed by calcium and phosphate deposition (mineralization phase) [33, 35]. At the end of the bone forming phase, osteoblasts become osteocytes, the most abundant cells in adult bone, with a small cell body and numerous long, dendritic-like cytoplasmic prolongations forming a canalicular system inside bone [14], or bone lining cells (not shown)
MSCs and bone regeneration
The intrinsic bone regeneration capacity plays a substantial role in the repair process in response to injury [36]. Periosteal progenitors are the primary cell source that gives rise directly to fracture callus, whereas bone marrow MSCs (BMSCs), which seem to be fairly abundant in normal and pathological bone in vivo [37], are crucial in supporting healing through paracrine secretion of trophic and immunomodulatory factors [38]. However, in bone diseases such as osteoporosis and non-union, the regeneration process fails. Thus, different strategies are used to improve the impaired or “insufficient” bone repair, including autologous bone graft, considered to be the gold standard procedure, free fibula vascularized graft, allograft implantation, and use of GFs, osteoconductive scaffolds, distraction osteogenesis and, also, injection/use of osteoprogenitor cells [39]. The latter application is supported by a large number of scientific articles, mostly reported in the following chapters.
The first studies on MSC properties and characteristics were carried out on pluripotent cells derived from embryos in the earliest stages of maturation (blastocysts). However, the use of these pluripotent cells raised numerous ethical issues precluding subsequent applications for clinical purposes. Thus, investigations were directed toward adult somatic cells induced to become pluripotent stem cells (iPS) [40]. Unfortunately, both embryonic and iPS cells showed the worrying ability to form teratomas and resulted to be tumorigenic [41, 42], which was an insurmountable obstacle to proceed in investigating their clinical applicability. In the early seventies, after the seminal paper by Friedenstein et al. [43] demonstrating that in bone marrow there are resident cells with stemness features, many other papers pointed out that this kind of cells, characterized as MSCs, are present in almost all adult tissues and are responsible for cellular regeneration and tissue homeostasis. The most important advantages in the potential use of these cells in comparison with embryonic or iPS cells are that they do not rise ethical issues, show no evident antigenicity when used for allogenic transplantation, and have a very low tumorigenic potential, even though some reports indicated that a risk exists [44, 45].
The current approach for delivering osteogenic cells in humans directly to the regeneration site includes use of bone-marrow aspirate from the iliac crest, which also contains GFs [46]. However, the concentration and quality of MSCs may vary significantly, depending on the individual (especially in older people), the aspiration sites, and techniques used. Thus, alternative sources of MSCs are under extensive research.
Availability of MSCs from different sources
MSCs are located in specialized niches of differentiated organs and are deputed to maintain tissue homeostasis [47]. Accordingly, they can be isolated from diverse human adult tissues (i.e., bone marrow, adipose, or dental tissues), but clearly also from embryonic annexes (umbilical cord, placenta) or fluids (amniotic liquid). Even in the adult brain, there are specific zones, mainly the subventricular one, in which MSCs are present, assuring a renewal of neural cells, although in a limited manner, in physiological and pathological conditions [48, 49].
Regardless of their tissue origin, MSCs can differentiate into cells of the mesenchymal lineage, which includes adipocytes, osteoblasts, chondrocytes, tenocytes, skeletal myocytes, and cells of the visceral mesoderm [50, 51], but also into cells of ectodermal and endodermal origin, such as hepatocytes [52], neurons [53], and cardiomyocytes [54, 55]. However, MSC differentiation potential has mainly been investigated toward cells of the mesenchymal lineage [56].
Although in 2006, the Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy defined four criteria as follows: (i) ability to adhere to plastics when placed under standard culture conditions and (ii) to differentiate into osteoblasts, chondrocytes, and adipocytes; iii) presence of CD73, CD90, and CD105 markers, and iv) absence of hematopoietic markers CD14, CD11b, CD34, CD45, CD19, and CD79α) as needed to classify/define a cell as MSC [57], the phenotypic characterization of MSCs still remains a field of investigation due to the lack of unique distinctive markers for their analysis and isolation. From literature it is evident that (i) isolation of MSCs from easily accessible sources and with a great potential of cell expansion and multi-lineage differentiation remains the ideal goal; (ii) MSCs from bone marrow and adipose tissue are the most widely studied and being tested in in vitro and in vivo studies of regenerative medicine and cell-based therapy. In the paragraphs reported below, we summarized data on MSCs isolated from different tissues/fluids.
MSCs from bone marrow
BMSCs, in addition to being the first ones to be investigated as multipotent cells to be directed toward osteogenic differentiation, are considered the reference cell type with which MSCs deriving from other sources are compared. One limiting factor for their use is, in general, their availability, since they are present at low rate, which further decreases with aging [58, 59]. Moreover, cell collection requires an invasive procedure associated with pain and morbidity.
MSCs from neonatal tissues and amniotic fluid
Neonatal tissues such as umbilical cord, placenta, and amniotic membrane are other sources of MSCs with high proliferation rates and potent differentiation capacities, as evaluated in preclinical models [60, 61]. Additionally, human term amniotic fluid could be an ideal source of MSCs. Indeed, preclinical studies recently demonstrated the following: (i) the feasibility of collecting significant volumes of this fluid, otherwise discarded, from a wide number of potential donors; (ii) the high yield of MSCs obtainable; (iii) the properties of these MSCs, given their neonatal origin; and (iv) their suitability to be reprogrammed into the pluripotent state. Thus, these MSCs are a promising and plentiful resource for further evaluation in bio-banking, cell therapy, disease modeling, and regenerative medicine applications [62, 63].
MSCs from dental tissues
MSCs from dental tissues (DSCs) also show self-renewal capacity and multi-differentiation potential. So far, eight main DSCs have caught the interest of researchers, namely dental pulp stem cells (DPSCs), which were the first to be isolated and studied, stem cells from human exfoliated deciduous teeth (SHED), periodontal ligament stem cells (PDLSCs), dental follicle progenitor cells (DFPCs), alveolar bone-derived MSCs (ABMSCs), stem cells from apical papilla (SCAP), tooth germ progenitor cells (TGPCs), and gingival MSCs (GMSCs) [64]. Among them, DPSCs and SHED have been more extensively investigated as autologous stem cells to use for regenerative purposes and tissue engineering therapies (reviewed by [65]).
MSCs from adipose tissue
MSCs can be isolated from subcutaneous (SAT) and visceral (VAT) adipose tissue [66]. Of particular interest is SAT that consists mature adipocytes and a heterogeneous cell population, called stromal vascular fraction (SVF), comprising fibroblasts, pericytes, pre-adipocytes, endothelial cells, blood cells, and adipose stem cells (ASCs) [67]. Noteworthy, ASCs shows easy accessibility and can be harvested also via liposuction under local anesthesia, which is a painless and reasonably comfortable way for patients to provide a good amount of autologous stem cells [68]. Moreover, they are more abundant and provided with higher proliferation rate than BMSCs, although both cell types have a similar multi-lineage differentiation potential [66, 68–72]. Interestingly, the ASC secretome includes active molecules such as numerous GFs [73]. Thus, ASCs could be ideal cells in regenerative medicine [74, 75]. However, there are still some barriers to overcome: (i) a precisely defined source population of ASCs, (ii) isolation procedures according to regulatory clinical standards, (iii) better understanding of the behavior of ASCs in their niche and of their interaction with scaffolds, (iv) evaluation of their safety and efficacy in clinical applications, and (v) long-term follow-up studies on the consequences in patients [73, 75, 76].
Preclinical and clinical applications of MSCs in bone repair
Preclinical studies
Even though undifferentiated MSCs, left in a normal culture medium for a long period (> 30 days), spontaneously differentiate into osteoblasts [77], compelling evidence showed the MSCs are committed toward osteogenesis under suitable culture conditions [78, 79]. The use of this model allowed to characterize the principal stemness features of MSCs from various sources, their differentiation potential, and distinctive properties, such as their immune-modulatory effect, the ability to provide trophic support to other cells, to slow down degenerative processes, to secrete biochemical substances (GFs, cytokines, neurotransmitters, and extracellular matrix compounds), and to recruit endogenous stem/precursor cells to defective sites for new tissue regeneration/repair [80]. Additionally, it has also been demonstrated that MSC osteogenic differentiation can be supported by suitable biomaterial properties (i.e., adhesivity, stiffness, nanocrystalline structure, and degradability) [81] and improved by the associate use of specific GFs and selected scaffolds [82].
Fundamental preclinical evidence on the possible MSC therapeutic applications to treat human diseases has also been achieved by using rodents or large animal experimental models, including companion (dogs, cats, and horses) and farm animals (cows, sheep, goats, and pigs). These studies pointed out the improvement obtained by the MSC use in bone healing and outcome from fracture or segmental defect repair [83, 84] as well as in congenital defects such as spina bifida [85, 86] and chest wall defects [87]. A particular MSC application has been done in experimental dentistry to regenerate oral/dental tissues [88–90] and to cure inflamed periodontium [91, 92]. These models represent an important translational paradigm that closely mimics the clinical environment of humans [93].
Unfortunately, although articles on MSC-based bone reconstruction techniques are numerous, there is a multitude of experimental protocols leading to a wide range of parameters and data, so that it is still difficult to achieve significant conclusions as to the “most predictable” model in stem cell reconstruction [94]. Consequently, there is need for additional studies with more homogenous experimental designs and data analysis in order to gain useful advances in the use of MSCs for bone reconstruction.
Clinical studies
As above reported, in complicated fractures, tumor resections, spinal arthodesis, or large bone defects, healing process may fail. In these cases, clinicians have adopted different therapeutic strategies [95, 96], but clinical trials including MSC use mostly concerns transplantation of human autologous cells, alone or in combination with scaffolds to treat different bone diseases [97–103]. Similar techniques favor spinal fusion following spinal arthrodesis performed in the treatment of spinal trauma, degenerative diseases, tumors, or infections [84]. Additionally, phase I/II clinical studies performed on patients with spinal cord injury (SCI) at the cervical or thoracic level, showed that (i) injection of BMSCs had significant regenerative effects within a therapeutic window of 10–30 days after SCI [104, 105] and (ii) autologous MSC transplantation may be a safe procedure, leading to improvements in motor-sensory functions and tissue regeneration in almost half of patients, thus enhancing their quality of life [106].
The clinical effectiveness of stem cell therapies has also been evaluated in dentistry, mainly for alveolar ridge augmentation preceding the insertion of dental implants, in which BMSCs from the iliac crest have been the most well characterized and commonly used for their osteogenic ability [107], whereas periosteum-derived stem/osteoprogenitor cells, ASCs, and DSCs have been applied for orofacial bone regeneration [108, 109]. Finally, the possible successful MSC applications in pediatric diseases such as osteogenesis imperfecta, juvenile idiopathic arthritis, simple bone cysts, and osteonecrosis of the femoral head have been reported [110], representing an encouraging premise for MSC application to treat incurably ill pediatric patients. As for ASCs, their clinical application ranges from various areas of regenerative medicine, such as hair [111] or peripheral nerve regeneration [112], cardiovascular [113], pancreatic [114] or hepatic repair [115], plastic surgery, and esthetic medicine [75], to treatment of chronic limb defects and diabetic wounds [116, 117]. Additionally, intra- or periarticular ASC injection can support cartilage regeneration in patients suffering from osteoarthritis, chondromalacia, osteonecrosis of the femoral head, or a meniscus tear. In contrast, use of ASCs in bone tissue engineering is still entirely experimental [118] while suggesting its potential benefits in various orthopedic applications [119]. Noteworthy, the ASC paracrine secretion of GFs and the co-culture of these cells with endothelial cells (ECs) may accelerate their phenotypic maturation into ECs [120], thus making them suitable for vascular prosthesis seeding.
In conclusion, numerous clinical studies demonstrated the potential use of human autologous MSCs for bone tissue engineering strategies. However, the major part of them emphasizes that further studies are still needed to improve cell-based therapy in bone regeneration and to decipher the biological and molecular mechanisms that are involved.
It is also to emphasize that a new research avenue has been opened concerning the use of MSC-derived conditioned medium (CM) that is rich of factors able to increase tissue synthetic activity. A recent review [121] reported that a discrete number of in vitro and in vivo investigations has already been performed using MSC-derived CM in bone and periodontal defects, and one study, carried out in patients needing alveolar bone regeneration, showed that MSC-derived CM caused bone formation without systemic or local complications. This obviously means that the identification/characterization of the factors involved in the promotion of the osteogenic differentiation and bone repair becomes extremely important.
Purines and MSC osteogenic differentiation
In bone regeneration, the significance and the experimental therapeutic use of MSCs is ever more investigated. As well, the involvement of local signals during fracture healing and repair has been demonstrated by various studies, which highlighted the role of GFs and related signaling pathways during this process [122]. Thus, it is important to consider that MSCs, if used for bone regenerative medicine, will likely meet an environment rich of GFs and also of other kinds of substances released from cells during tissue injury that could influence MSC ability to support bone repair. Among these agents, we focused on purines.
A brief overview on purines
Many excellent reviews have given a comprehensive picture of the significance of purines inside the cells, the various mechanisms of their release from cells into the extracellular fluid, the carriers involved in the recovery and sometimes also in the release of nucleosides and nucleobases, and the enzymes deputed to their interconversion or degradation (for a review see ref. 123]. Therefore, here we reported a brief introductory overview of the main general findings on purines to then deepen some aspects about them in MSCs.
Purines together with pyrimidines are ancestral and ubiquitous molecules present in animal and plant cells as constituents of nucleic acids or participants in biochemical reactions and energy transport inside the cell [124, 125]. Moreover, agents such as cAMP and cGMP act as important second messengers at intracellular level during signal transduction, whereas guanine-based purines behave as modulators of intracellular processes, especially in relation to the G protein activity in signal transduction [126]. However, purines and some pyrimidine compounds (UTP, UDP, and UDP-glucose) also act at extracellular level, being the release of endogenous nucleotides into the extracellular space a critical step for the initiation of purinergic signaling events. Nonlytic release of ATP, UTP, UDP-glucose, and other nucleotides occurs in all cell types and tissues via both constitutive mechanisms, that is in the absence of external stimuli and, to a greater extent, as a response to biochemical or mechanical/physical stimuli [127]. In particular, ATP and ADP and also uridine-based nucleotides are released from different cell types by cargo-vesicle trafficking and secretory granule exocytosis [9, 128]. The physiological release of adenosine has also been reported in some cell types, including neuronal cells, kidney cells, cardiomyocytes, and immune cells [129], whereas in particular stress conditions, adenosine can be released into the extracellular milieu by ubiquitously expressed transporters, which, however, are mostly deputed to uptake adenosine inside the cells [130]. Finally, all endogenous nucleotides and nucleosides can be discharged into the extracellular space due to different cell stressors causing damage to the plasma membrane [128, 129, 131]. Thus, ATP efflux can occur under various more or less noxious stimuli, including shear stress, hypotonic swelling, stretching, hydrostatic pressure, hypoxia /hypoglycemia/ischemia, and the consequent cellular energy deprivation, as well as in response to Ca2+ agonists [128]. Even though less known, also GTP and related compounds are found in the pericellular fluid and behave as extracellular modulators affecting the glutamatergic activity and exhibiting behavioral and trophic effects on neuronal cells [132].
The concentration of extracellular purines and pyrimidines is tightly controlled by multiple nucleotide and nucleoside-metabolizing enzymes with either hydrolyzing or trans-phosphorylating activities, which predominantly function as membrane-bound enzymes (ecto-enzymes) rather than as soluble or microvesicle-associated ones [10, 11, 133]. In addition, there is a system to remove nucleobases and also nucleosides from the extracellular fluid, represented by specific transport proteins usually functioning to take nucleotide derivatives up [134]. More in detail, two major classes of nucleoside transport systems have been described in mammalian cells, the equilibrative and the concentrative transport systems. The former mediates nucleoside transport in both directions, this depending on the nucleoside concentration gradient across the plasma membrane, while the latter is Na+-dependent and the movement of the nucleoside, regardless of its concentration gradient, is coupled to that of the sodium ion. However, nucleosides, nucleobases, and their analogs are also substrates for other carrier proteins such as organic anion and cation transporters and ABC transporter proteins that mostly function as efflux transporters.
Once released into the extracellular space, purines and pyrimidines activate different types of specific receptors, which are classified based on their molecular structure and agonist specificity as P1 receptors (P1R), selective for adenosine (A1, A2A, A2B, and A3 receptor subtypes) and coupled to G proteins [10, 135], and P2 receptors (P2R), mostly sensitive to ATP, in turn divided into eight metabotropic P2Y and seven ionotropic P2X receptors on the basis of mechanism of action, pharmacology, and molecular cloning [136]. Of note, some P2Y receptors, namely P2Y2, 4, 6, and 14, are mainly sensitive to uridine-derived nucleotides [137]. Each of these receptors is widely but differentially expressed in virtually all cell types [9, 136]. Noteworthy are as follows:
Purine-metabolizing ecto-enzymes are responsible for the nucleotide signal termination and for maintaining the balance of purine concentration in the extracellular environment [10]. This sophisticated mechanism enables a fine-tuning regulation of purine levels and can be altered in pathological conditions [138];
purinergic signaling is involved in the regulation of different processes such as cell proliferation and differentiation, chemotaxis, cytokine release, formation of reactive oxygen species, cell fusion, phagocytosis and induction of cell death for apoptosis or necrosis, inflammation, and cancer [135, 139].
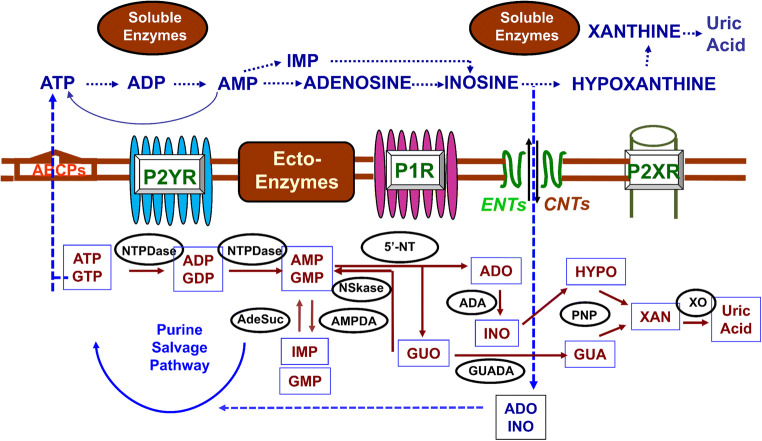
An overall picture of purine turnover at intra- and extracellular levels is depicted in Fig. 2.
Fig.2.
Synthesis, release, and metabolism of intra- and extra-cellular purines. Purine nucleotides are produced in cells by de novo synthesis and the salvage pathway, which recycles nucleosides and purine bases, also deriving from the turnover of nucleic acids, especially RNA [125]. Nucleotides (here, we consider mainly ATP) are released from virtually all cells in physiological conditions by different types of stimulation; additionally, cell damage and membrane leakage determine a huge purine increase in the extracellular fluid [127–129]. Besides secretory mechanisms typical of nervous cells, ATP release is mediated by connexin/pannexin hemichannels, facilitated diffusion by nucleotide-specific ATP-binding cassette transporters, and multiple organic anion transporters. Also, adenosine, in particular stress conditions, is released into the extracellular fluid by transporters; the principal function of which is, however, to bring adenosine back into the cells. The nucleoside transport system comprises equilibrative (ENTs) and concentrative transporters (CNTs) [131, 135] in mammalian cells. Thus, nucleosides are transported by ENTs in both directions depending on their concentration gradient across the plasma membrane, whereas the transport by CNTs is coupled to the concentration gradient of sodium ions. Finally, outside the cells, purines are catabolized thanks to the activity of membrane (ecto-enzymes) and soluble enzymes similar to those involved in the metabolism and salvage pathway of intracellular purines. Among the cell surface-expressed enzymes, the ecto-nucleotidase 1 (also known as CD39) efficiently hydrolyzes ATP to AMP, whereas the ecto-nucleotidases 2 or 3 and 8 lead to a sustained or transient ADP accumulation, respectively. Then, AMP produced from ATP and ADP degradation is further metabolized by ecto-5′-nucleotidase (also known as CD73) into adenosine. Finally, adenosine is inactivated via inosine into hypoxanthine by the tandem action of cell surface-located adenosine deaminase and purine nucleoside phosphorylase or, in part, is transported into the cell to replenish adenine nucleotide pool [132, 134]. Abbreviations: ABCPs, ATP-binding cassette proteins; ADA, adenosine deaminase; AdeSuc, adenyl-succinate synthase; HGPRT, hypoxanthine/guanine ribosyltransferase; NSkase, nucleoside kinase; NTPDase, Nucleotidase; 5-NT, 5’-nucleotidase; AMPDA, AMP deaminase; PNP, purine nucleoside phosphorylase; GUADA, guanine deaminase; XO, xanthine oxidase
In the next paragraphs, we reported more detailed information about the purine system in MSCs. These data have mainly been desumed from primary and/or immortalized cell lines cultures, grown in basal conditions, and/or in classic medium favoring the osteogenic differentiation [32]. As for primary cell cultures, it is to underline that in most articles, the number of cell passages in vitro was hardly reported, even though it is known that use of some MSCs after the 6th–7th passage makes them less prone to differentiate [140]. Moreover, while the influence of purines on MSC osteogenic differentiation has been evaluated in MSCs deriving from different animal and human tissue sources, investigation on differences existing in the expression of purine receptors as well as in their responsiveness to purines ligands did not received similar attention and is still poor. Thus, the results are not always easily comparable (see in the next paragraphs).
Release of ATP during osteogenic differentiation and bone remodeling
In recent years, purines are emerged as key players also in the context of bone remodeling. Indeed, nucleotides are important local factors in the osteogenic differentiation of MSCs/osteoprogenitors, from which are constitutively released under basal and/or stressful conditions through the same mechanisms listed above for all cell types. Among them, exocytosis seems to be a major way for releasing ATP from osteoblasts [141–143], but other findings suggest that maxi-anion channels [144], connexins, namely connexin 43 [145], and the P2X7 receptors (P2X7R) themselves through membrane pore opening [146] contribute to this event. Release mechanisms and total amounts of extracellular ATP are highly dependent on cell differentiation stage, mature bone-forming cells releasing up to sevenfold more ATP than immature proliferating cells [143]. In particular, the expression of connexin 43, which is predominant in bone cells, increases with osteoblast differentiation, possibly allowing gap junction hemichannels to become prevalent and to exert an important functional role in bone cell mechano-transduction [147]. Noteworthy, extracellular nucleotide concentrations are low in basal condition, mainly due to the presence of ubiquitous ecto-nucletotidases (e-NTPDases) and other ecto-phosphatases. Consequently, purinergic signaling is influenced by multiple factors, including the activity of different receptor subtypes, nucleotide releasing pathways, and ecto-nucleotidases [10, 148]. However, the interplay between purinoceptors and ecto-enzymes is still largely unknown in bone cells, leading to a gap in our knowledge regarding as to how they are organized in the control of bone remodeling.
Additionally, many studies showed increments of released ATP in response to external stimuli, namely hypoxia [143] and mechanical stress [149]. In the case of hypoxia, this stimulus results in an increased ATP release from osteoblasts up to 2.5-fold without affecting cell viability, being the mechanism mainly driven by exocytosis [143]. Ultrasound stimulation, a known method to accelerate fracture healing, was also found to promote ATP release from osteoblasts [150]. These and many other studies reported below suggest that the release of nucleotides in response to different stimuli may represent a local trigger of osteogenesis in bone forming niches [151].
Expression and role of purine ecto-enzymes in MSC biological functions
Like virtually all cells, MSCs express different e-NTPDases on their membrane surface, which metabolize extracellular ATP and other nucleotides, thus contributing to modulate the purinergic receptor signals [10, 11]. This evidence has been reported in undifferentiated MSCs from mouse bone marrow and human umbilical cord blood [152, 153], in which these enzymes showed substantial differences as for their expression level, activity, and substrate specificity, likely depending on the tissue of origin and the biological MSC potential. For instance, the e-NTPDase activity is negligible in murine MSCs [154]. In contrast, ecto-5′-nucleotidase (e-5’NT), also known as CD73 and hydrolyzing AMP to adenosine, is highly expressed on the plasma membranes of undifferentiated MSCs from adult [155] and embryo tissues [152] and is considered one of the mesenchymal stem cell markers [57]. Since adenosine increases the proliferation rate of animal and human MSCs, likely by A2A receptor activations [155, 156], it is conceivable that the high proliferative potential of these cells is supported by adenosine present in the extracellular environment thanks to the e-5’NT activity.
Interestingly, the purine enzyme pattern seems to be different in MSCs committed toward differentiation, in which the expression of e-NTPDase or adenosine kinase (the enzyme favoring adenosine phosphorylation and AMP formation) coding genes increases. Since, in general, high levels of extracellular ATP, which accumulates during bone fracture [157], may result dangerous/toxic to cells, the major activity of these enzymes guarantees a tight control of the extracellular nucleotide levels to assure prevalent protective/reparative effects of ATP at low concentrations [158] and to regulate MSC migration in vitro or their homing capability in vivo [159, 160]. Moreover, also high expression levels of e-5’NT were evidenced in human osteoprogenitor cell lines as well as in human BMSCs and mesenchymal progenitor cells in human trabecular bone [161–163]. The activity of this enzyme contributes to the osteogenic activity induced by transforming growth factor ß (TGFß) in human amniotic fluid stem cells [164]. The high expression/function of e-5’NT is coupled to a reduced expression of adenosine deaminase, the enzyme converting adenosine into inosine, thus assuring appropriate levels and prolonged activity of the extracellular adenosine in MSCs [162].
Expression of purinergic receptors in MSCs
As stated above, purinergic receptors are expressed by almost every cell type and are among the first expressed neurotransmitter receptors during development [136]. They have gained considerable interest since their discovery, and many groups have investigated their potential use as therapeutic targets and/or biomarkers for an array of diseases. P2YR have been deeply explored in the context of thrombosis and heart diseases, whereas P2XR have been studied in inflammatory disorders [159] but also in bone homeostasis [165]. Indeed, a number of studies have demonstrated that, once released, purines bind to the purinoceptors aforementioned, which are present also on MSCs, osteoblasts and osteoclasts, and behave as autocrine/paracrine regulators of bone remodeling [166].
More in detail, the expression of all P1R has been reported in human and rodent MSCs (see Table 1), with a major evidence obtained in BMSCs [162, 172–174]. Apart from a nearly general agreement on the prevalent expression of A2B receptors (A2BR) in MSCs deriving from different sources and committed toward osteogenesis, there may be differences in the expression of the other adenosine receptors. For instance, Hajjawi et al. [176] showed that (i) mRNAs for the A1 receptors (A1R) and A2BR were expressed by all primary rat and mouse osteoblasts, (ii) the expression of A2AR was limited to rat bone marrow and mouse calvarial osteoblasts, and (iii) A3 receptors (A3R) were mainly found in rat bone marrow osteoblasts. In humans, Costa et al. [162] showed a decreased expression of A1R and A2AR along the osteogenic differentiation of MSCs from human aged bone marrow in comparison with that of A2B receptors, being the A3R the less expressed in these cells. More recently, Kotova et al. [197] identified transcripts for A1R, A2AR, and A2BR in human undifferentiated ASCs, whereas no evidence for expression of the A3R was obtained.
Table 1.
Principal P1 and P2 purinoreceptor activity in MSCs and bone-related cells
| Receptors | Role and function in bone | References | Animal/human Species | Cell origin | Culture conditions | Use of “natural” or “stable” purine compounds |
|---|---|---|---|---|---|---|
| A1 | Enhance osteoclastogenesis | [167] | Animal | Bone marrow | Primary cell cultures | Stable |
| Promote osteogenic differentiation | [22] | Human | Dental pulp | Primary cell cultures | Stable | |
| [161] | Human | Bone marrow | Primary cell cultures and immortalized cell lines (HCC1) | Stable | ||
| [162] | Human | Bone marrow | Primary cell cultures | Stable | ||
| A2A | Reduce inflammatory osteolysis | [168] | Animal | Bone marrow | Primary cell cultures | Stable |
| Increase osteoblast number while decreasing osteoclast number and activity in inflamed bone | [169] | Animal | Bone marrow | Primary cell cultures | Stable | |
| Stable | ||||||
| Increase the proliferation/development of MSCs | [170] | Animal | Bone marrow | Primary cell cultures | ||
| Inhibit osteogenic differentiation | [162] | Human | Bone marrow | Primary cell cultures | Stable | |
| Enhance osteogenic differentiation | [171] | Animal | Bone marrow | Primary cell cultures and immortalized cell lines (MC3T3-E1 cells) | Stable | |
| A2B | Enhance osteogenic differentiation | [172] | Human | Bone marrow | Primary cell cultures | Stable |
| [173, 174] | Animal | Bone marrow | Primary cell cultures | Stable | ||
| Promote osteogenic marker expression | [161] | Human | Bone marrow | Primary cell cultures and immortalized cell lines (HCC1) | Stable | |
| Enhance osteogenic differentiation | [175] | Human | Bone marrow | Primary cell cultures | Natural/stable | |
| Increase osteogenic differentiation | [162] | Human | Bone marrow | Primary cell cultures | Stable | |
| No effect on osteoblasts or osteoclasts | [176] | Animal | Calvaria bone | Primary cell cultures | Natural/stable | |
| A3 | No activity on osteoblast differentiation | [161] | Human | Bone marrow | Primary cell cultures and immortalized cell lines (HCC1) | Stable |
| Decrease osteoclast differentiation improving bone cell survival and proliferation | [177] | Animal | In vivo experiments | Stable | ||
| P2X1 | Decrease bone mineralization and ALP activity | [178] | Animal | Osteoblasts | Primary cell cultures | Stable |
| Decrease osteogenic differentiation | [179] | Human | Adipose tissue | Primary cell cultures | Stable | |
| P2X2 | Promote bone resorption, increasing osteoclast activity | [180] | Animal | Long bone | Mature osteoclast cultures | Natural |
| Decrease osteogenic marker expression in cells from ovariectomized mice | [181] | Animal | Bone marrow | Primary cultures | Stable | |
| P2X3 | Decrease bone mineralization | [178] | Animal | Osteoblasts | Primary cell cultures | Stable |
| Decrease osteogenic differentiation | [179] | Human | Adipose tissue | Primary cell cultures | Stable | |
| P2X4 | Support pore membrane formation together with P2X7R | [181] | Human | Osteosarcoma | Cell lines | Native and stable |
| P2X5 | Stimulate osteoblast proliferation | [182] | Animal | Calvaria bone | Primary cultures | Natural |
| [183] | Human | Osteosarcoma | Immortalized cell cultures (MG-63) | Natural/stable | ||
| P2X6 | Role largely unknown | [10][178] | ||||
| P2X7 | Physiological stimulation promotes whereas prolonged simulation inhibits osteogenesis | [179] | Human | Adipose tissue | Primary cell cultures | Stable |
|
Enhance osteoblast apoptosis Decrease ALP activity |
[184] | Human | Osteosarcoma and normal bone | Cells lines and primary cell cultures | Natural/stable | |
| Decrease bone formation | [185] | Animal | Calvaria bone | Primary cell cultures | Natural/stable | |
| Cause membrane blebbing and bone formation | [186] | Human | Trabecular bone | Primary cell cultures | Stable | |
| [187] | Human | Bone marrow from post-menopausal women | Primary cell cultures | Stable | ||
| Short (7 days) or prolonged (14 days) cell stimulation increases or decreased ALP activity and osteogenic marker expression | [188] | Animal | Calvaria bone | MC3T3-E1 preosteoblast cell line | Natural/Stable | |
| P2Y1 | Modulate osteoblast response to PTH | [189] | Human | Bone marrow/osteosarcoma | Primary cell cultures/ Sa-OS2 cell lines | Natural |
| Favor adipogenic cell differentiation | [175] | Human | Bone marrow | Primary cell cultures | Natural and stable | |
| Inhibit osteogenesis | [148] | Human | Bone marrow | Primary cell cultures | Native (UTP, UDP) and stable | |
| P2Y2 | No activity on osteogenic differentiation | [148] | Human | Bone marrow | Primary cell cultures | Native (UTP, UDP) and stable |
| Inhibit osteogenic differentiation | [190] | Animal | Calvaria bone | Primary cultures | Native and stable | |
| Inhibit bone mineralization and ALP activity favoring adipogenic differentiation via early ERK1/2 activation | [191] | Animal | Bone marrow | Primary cell cultures | Native and stable | |
| P2Y4 | Inhibit bone mineralization | [148] | Human | Bone marrow | Primary cell cultures | Native and stable |
| Favor adipogenic cell differentiation | [175] | Human | Bone marrow | Primary cell cultures | Stable | |
| [191] | Animal | Bone marrow | Primary cell cultures | Natural | ||
| P2Y6 | Increase ALP activity over culture time and intracellular calcium levels | [148] | Human | Bone marrow (young and post-menopausal females) | Primary cell cultures | Native (UTP, UDP) and stable |
| Promote early ALP activity and increase of osteogenic marker expression | [188] | Animal | Calvaria bone | Immortalized pre-osteoblast cell lines | Native (UTP) and stable | |
| P2Y11 | Largely unknown | [192] | Human | Osteosarcoma | HOS cell lines | - |
| P2Y12 | Inhibit bone mineralization, slow osteoblast proliferation and decrease cell viability. | [193] | Animal | Calvaria bone | Primary cell cultures | Stable (clopidogrel) |
| Decrease mineralization in vivo | Animal | Femoral bone and whole body | In vivo model | Stable (clopidogrel) | ||
| P2Y13 | Promote osteogenic differentiation | [194] | Animal | Bone marrow | Primary cell cultures | Stable |
| Increase ALP activity and osteogenic marker expression | ||||||
| P2Y14 | Protect hematopoietic stem cells and may likely enhance osteoclast formation | [195] | KO Animal | Embryos | Total animal or cells derived from them | Radiation |
| Likely control MSC commitment decreasing osteogenic differentiation | [196] | Human | Adipose tissue | Primary cell cultures | Stable |
As for P2R in general, it has been reported that osteoblast response to nucleotides increases along the osteogenic process, at least in rat MSCs [182]. Looking at the P2X receptors (P2XR), their expression has been found in human MSCs and also in hematopoietic stem cells (HSCs), the extent depending on the stem cell source and the phenotypical commitment toward a specific lineage [196]. In particular, mRNAs for P2X3R, P2X4R, P2X5R, P2X6R, and P2X7R and all P2Y receptors (P2YR) were found in human MSCs, with some peculiarities [196]. For instance, the expression levels of P2Y4R and P2Y12R mRNAs differed between ASCs and ecto-mesenchymal dental follicle cells. Moreover, in studies mainly regarding rat ASCs, P2X6R, and P2Y11R proteins are upregulated upon adipogenic differentiation, while those for P2Y4R and P2Y14R are down-regulated. In contrast, P2X6R mRNA and protein levels decrease in the same ASCs committed toward osteogenesis, so that such a variable expression may be critical for early MSCs commitment to differentiate [198]. In more recent studies, in which human ASCs were again investigated, transcripts for multiple purinoreceptors were found, confirming the presence of P2R previously detected [199], besides most adenosine receptors [197]. In these cells, the effects of specific agonists and antagonists suggested that both P2Y1R and P2Y13R were mainly responsive to ADP, while P2Y4R and P2Y11R served as primary UTP and ATP receptors, respectively. Finally, extracellular NAD+ stimulated Ca2+ signal in ATP-responsive MSCs by interaction with P2Y11.
Influence of P1R on human MSC differentiation
Adenosine modulates different physiological processes in all cells [126]. Its extracellular levels are around 1 μM under physiological conditions. In case of hypoxia, ischemia, trauma, inflammation, or tumor, this level can increase up to 100-fold [170]. Acting via P1R, adenosine participates in a wide number of processes involved in bone homeostasis such as osteoblastogenesis and osteclastogenesis [197], as listed below.
A1R
A1R have been investigated mainly in relation to their promotion of osteoclast differentiation. Indeed, A1R selective antagonist, rolofylline, inhibited macrophage colony stimulating factor (M-CSF)/receptor activator of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) ligand (RANKL) or tumor necrosis factor (TNF) receptor-associated factor 6 (TRAF6), and TGF-beta-activated kinase 1 (TAK1)-induced osteoclastogenesis of mouse bone marrow cells [167]. Moreover, A1R blockade or deletion may increase bone density and prevent ovariectomy-induced bone loss (reviewed in [200]). However, these findings were mostly obtained using rodent MSCs and in vivo animal models.
Focusing on human MSCs, our group [22] and Costa et al. [162] pointed out a proliferative and pro-osteogenic activity of A1R in human MSCs derived from dental pulp or bone marrow, respectively. In particular, while Costa et al. [162] demonstrated a prolonged proliferative effect caused by A1R stimulation along cell osteogenic differentiation, D’Alimonte and colleagues [22] showed that A1R enhanced DPSC proliferation only during the first period along cell differentiation. However, both papers reported that A1R stimulation could also favor the commitment of human MSCs toward osteogenesis. Likely, differences in A1R-mediated effects on cell proliferation could be due to the tissue source from which MSCs derived and the age of patients from whom tissues were obtained (aged vs young people, respectively). It is also to underline that in the case of DPSCs, the authors showed that the enhanced ALP activity, extracellular matrix mineralization, and mRNA expression of Runx2, a key transcription factor in osteogenesis, was coupled to activation of the Wnt signal. Since other studies suggested that the activation of the canonical Wnt pathways could inhibit the differentiation of MSCs [198], they proposed that Wnt signaling may sustain cell differentiation when activated during the early part of this process, inhibiting differentiation when activated later on [23, 201, 202]. More recently, the same authors demonstrated that pro-osteogenic activity of A1R is also evident in human ASCs in which, however, the effect was lower than that observed in DPSCs, since the A1R are expressed to a lesser extent and later during the osteogenic differentiation as compared with DPSCs [70]. Therefore, it seems that the efficacy of A1R stimulation on MSC osteogenic differentiation may depend on (i) the source from which cells derive, which in turn conditions the receptor expression, and (ii) the experimental models used to obtain results. That A1R may influence also the activity of osteoclasts does not represent an obstacle since the receptor activity should be considered in a more general context in which, in example, the effects exerted by the activity of these receptors on osteoblast and osteoclast functions may be regarded as an attempt by the same ligand (adenosine) to balance the tasks of the two cell types in bone remodeling.
A2AR and A2BR
A2AR activation displays different effects as compared with those consequent to A1R stimulation, A2AR being able to inhibit mouse osteoclast differentiation and to enhance bone density, likely through disruption of M-CSF/RANKL ratio, decrease of interleukin 1β (IL-1β) and TNF-alpha levels, and inhibition of cAMP/protein kinase A (PKA)/ERK1/2 signaling pathway [168]. A2AR may also play an active role in mouse BMSC development [155] and their stimulation reduces inflammatory osteolysis, increasing osteoblast numbers in inflamed bone [169]. As well, in a bone defect condition, A2AR help restore bone homeostasis by increasing osteoblasts while decreasing osteoclast number and activity [203]. In contrast, He and colleagues observed that activation or downregulation of the A2AR did not affect human osteoblast differentiation and mineralization [172], whereas Costa et al. [162] demonstrated an inhibitory A2AR effect on the osteogenic differentiation of BMSCs from osteoporotic female patients. Recently, Borhani et al. [171] showed in a murine osteoblast precursor cell line (MC3T3-E1) and in primary mouse osteoblasts that A2AR activation promotes Akt signal, enhancing β-catenin nuclear translocation that may likely increase cell osteogenic differentiation. Once again, the results about A2AR function and bone plasticity have mostly been obtained using animal MSCs/models. Thus, the role of this receptor in osteoblast biology remains ambiguous and not clarified and, overall, further evidence is needed in humans.
Differently from A2AR, several research groups demonstrated that adenosine enhances osteoblast differentiation mainly by interacting with the A2BR [162, 172–174]. Even though A2BR show a low affinity for the endogenous ligand adenosine, the observed increase in extracellular adenosine concentration as well as sensitization/upregulation of A2BR expression under conditions of hypoxia and inflammation suggests the A2BR could be an interesting therapeutic target in different pathological states, including bone disease. In 2006, Evans et al. demonstrated that A2BR were the functionally dominant P1 receptors in human osteoprogenitor cells and also in a human immortalized cell line [161], which were able to secrete adenosine in vitro. A2BR activation enhanced cell osteogenic differentiation by upregulating the expression of typical osteogenic markers like Runx2. Accordingly, BMSCs from A2BR knockout mice showed a reduced osteogenic potential and the same knockout animals exhibited a lower bone density and a delayed fracture repair [204]. Stimulation of A2BR also attenuated bone loss in ovariectomized mice, highlighting the potential of A2BR as a target to treat osteoporosis consequent to estrogen deficiency [205]. Thus, A2BR deserve a particular attention, since they result to be the most effective among adenosine-responsive receptors in increasing the osteogenic differentiation of MSCs, both in those derived from aging females [162] or rodents [173, 174]. However, all findings are related to MSCs from bone marrow; thus, information on other MSC types are lacking. A2BR stimulation in BMSCs may also mediate MSC differentiation by modulating interleukin 6 (IL-6) levels. In the first stage of differentiation, A2BR reduce IL-6, which favors MSC commitment to osteoblasts, but, in the last phase, these receptors cause release of IL-6, which then promotes survival of differentiated MSCs. For this reason, IL-6 restoration by A2BR agonists could promote osteoblast viability and may be used in regeneration and tissue repair. In contrast to the general consensus on the proosteogenic role played by A2BR, Hajjawi and colleagues showed that rodent osteoblast numbers and differentiation were unaffected by high/not physiological concentrations of adenosine or an A2BR agonist (BAY606583). The authors argued that differences in culture methods may contribute to these findings [176].
A3R
Among the adenosine receptors, the role of A3R in osteoblast differentiation remains widely unknown. Our group has demonstrated the expression of A3R in human DPSCs [22]. Evans et al. also showed that A3R are expressed by human osteoblast precursors and osteoblasts [161], even though the use of the A3R agonist, N6-(3-iodo benzyl)adenosine-5′-N-methyluronamide (IB-MECA) did not affect osteoblast cell differentiation measured in terms of ALP activity. In contrast, A3AR activation has been correlated to decreased osteoclast differentiation due to phosphoinositide 3-kinase (PI3K), NFkB, and RANKL downregulation [177]. In addition, IB-MECA modulated the expression of proteins which control survival and apoptosis resulting in the improvement of the inflammatory process and the preservation of bone mass in adjuvant induced arthritis rats. Since these receptors play a major role in inflammatory diseases, it is likely that the experimental conditions to study their involvement in MSC differentiation still need to be better identified.
In conclusion, adenosine receptor activation is involved in bone remodeling/repair, being A2BR and likely A1R able to promote and to favor, respectively, MSC osteogenic differentiation. Therefore, they represent a potential tool that should be, however, better explored for bone regenerative therapies.
Influence of P2R on human MSC differentiation
As mentioned above, nucleotide receptors play important roles in the functions of both osteoblasts and osteoclasts. In vitro cultures have been used to elicit the activity of endogenous ATP in MSCs and HSCs, respectively [165]. Thus, it was shown that low-intensity pulsed ultrasound stimulation treatment, which induces ATP release from osteoblasts, also caused a subsequent activation of P2R-mediated proliferation [150]. As well, bisphosphonates, used for the treatment of osteoporosis, promote nonlytic release of ATP and also the activation of its receptors, namely P2Y1R and P2Y2R, which in turn lead to activation of ERKs [206]. ATP release may be induced also by P2Y2R stimulation by uridine-based nucleotides, even though this has been shown only in osteoclasts [207]. Additionally, an increasing number of findings are showing the ability of these receptors to modulate the osteogenic differentiation of MSCs, thus providing new avenues by which they can be manipulated for tissue regenerative strategies.
Concerning metabotropic P2YR, many authors have demonstrated different effects in bone remodeling, as follows:
the P2Y1R were shown to modulate human osteoblast responses to systemic factors, such as parathyroid hormone (PTH) [189]; however, other reports indicated that the stimulation of these receptors led to inhibition of osteogenesis in human ASCs and BMSCs [148, 187, 196], even favoring the adipogenic differentiation of the latter [175];
regarding P2Y2R and P2Y4R, Hoebertz et al. found that ATP and UTP at concentrations ≥ 1μM are potent inhibitors of mineralization in cultured primary rat calvarial osteoblasts [190]. Recently, Du et al. [208] reported that overexpressing or silencing of P2Y2R impaired or promoted osteogenic differentiation of mouse MSCs, respectively. Since the expression of P2Y2R was much higher in osteoblasts from ovariectomized mice in comparison with control, the authors suggest that their suppression might be an effective strategy to attenuate the estrogen deficiency-induced osteoporosis. Data in human BMSCs showed an inhibitory activity of P2Y2R on cell osteogenic differentiation [148], and are in agreement with findings obtained in rat BMSCs [191], being these receptors and not P2Y4R able to favor the adipogenic differentiation of both cells;
the expression of the P2Y6R is fairly constant throughout maturation of human cultured BMSCs from postmenopausal women, and their stimulation promotes ALP activity and increase in intracellular [Ca2+] [148]; similar findings have been obtained from immortalized cell lines of mouse calvaria bone [188];
the expression of the P2Y11R was detected by RT-PCR in a human osteosarcoma cell line (HOS cells), but their function remains elusive [188];
the ADP-sensitive P2Y12R are usually associated to bone nodule formation [192]. This association also came from the clinical use of a selective P2Y12R antagonist (clopidogrel) that was able to inhibit in vitro mineralization in a concentration-dependent manner, slowing osteoblast proliferation and cell viability, together with a reduction in ALP activity and collagen formation [193];
the ADP-sensitive P2Y13R might be involved in bone remodeling. In fact, ADP activation of P2Y13R+/+ (but not P2Y13R-/-) adherent BMSCs from mice significantly increases the number of alkaline phosphatase-colony-forming units (CFU-ALP) as well as the expression of some osteoblast markers (Osx, ALP and type I collagen) [194];
the role of P2Y14R in bone remodeling also remains still elusive. Some authors suggested that these receptors preserve hematopoietic stem/progenitor cell function [195], whereas others indicated that, acting together with P2X6R and P2Y4R, they may control human ASC commitment toward both adipogenic and osteogenic differentiation. In bone cells, the P2Y14R expression decreases along the passage from undifferentiated to osteoblast-differentiated cells, suggesting an important role of them at early differentiation stages [reviewed by [196].
Furthermore, there are many reports elucidating the role played by ionotropic P2XR on MSC osteogenic differentiation. In particular:
Orriss et al. [178] demonstrated that, in osteoblasts from neonatal Sprague–Dawley rats, P2X1R and P2X3R are involved in the prevention of bone nodule mineralization in vitro, since the P2X1R and P2X3R agonists, α,β-meATP, and β,γ-meATP, respectively, inhibited bone mineralization. More recently, Carluccio et al. [179] confirmed the inhibitory activity of both receptors. Indeed, they demonstrated in human ASCs committed toward osteogenesis the expression of both receptor types, even though to a lower extent as compared with P2X7R (see below), also present in the same cells; moreover, cell exposure to 2′(3′)-O-(4-benzoylbenzoyl) adenosine 5′-triphosphate (Bz-ATP) decreased ASC osteogenic differentiation interacting with P2X1/3R.
On the other hand, the P2X2R seem to be more important in rodent osteoclasts than in osteoblasts, as they promote bone resorption [180]. More recently, Kanaya et al. [181] also showed a decrease in osteogenic marker expression using BMSCs from ovariectomized mice.
The P2X4R are expressed in osteoblasts, however derived from human osteosarcoma cell lines, and seem to be involved in membrane pore formation. Indeed, in these cells, the non-selective P2X7R agonist, BzATP, induced the incorporation of a fluorescent dye (a powerful indicator of pore formation). However, P2X7R antagonists were ineffective to completely block pore formation, thus suggesting that P2X4R may be also involved in this mechanism [209].
Nakamura et al. showed that ATP, acting on P2X5R, among others, can stimulate osteoblast-like (MG-63) cell proliferation, via a mechanism that is dependent on MAPK pathway [183]. Accordingly, Orriss et al. [182] reported a similar observation using MSCs from rat calvaria bone.
The expression of the P2X6R has also been detected in primary cultures of rat and human (SaM-1) osteoblast-like cells, but their role in bone formation is largely unknown [210].
Finally, the role of the P2X7R in osteoblast function appears important but controversial. Early studies suggested that P2X7R activation caused enhanced human osteoblast apoptosis [184]. In contrast, more recent studies indicate that P2X7R stimulation leads to increased membrane blebbing of mouse MSCs and bone formation [211–213]. These roles are supported by P2X7R knockout murine models, where decreased bone mass has been reported as compared with wild-type animals [214]. Moreover, a great number of functional single nucleotide polymorphisms (SNPs) [215], leading to either gain or loss of function of the P2X7R protein, has been identified. Many of these SNPs have been associated with bone alterations in humans. Indeed, gain-of-function polymorphisms of P2X7R have been coupled to increased bone mass [216], whereas loss-of-function polymorphisms of this receptor have been associated with increased fracture risk, reduced bone mineral density and osteoporosis in postmenopausal women [185, 217, 218], and stress fracture injury in military personnel and elite athletes [219].
Summarizing, as for P1R, also for P2R, the studies highlighted a number of interactions between nucleotide signaling and MSCs within the context of bone development and homeostasis. However, the results were once again obtained in cells in vitro, and mostly in cells derived from rodent tissues. Thereby, the translation of these results to in vivo models is compelling.
Finally, it is to recall that, since pathological processes underlying osteoporosis and other bone defects may be due to an imbalance between osteogenesis and adipogenesis, A2AR, within the P1R family, enhance the MSC adipogenic differentiation, whereas A2BR inhibit this process and A1R stimulation mainly controls adipocyte lipogenesis [173, 220]. P2R may also direct MSCs toward an adipogenic differentiation. P2Y11R are important players in this process, and also, P2Y1R and P2Y4R may stimulate MSC adipogenesis [175], whereas most P2R substantially favor osteogenesis and inhibit the adipogenic differentiation (see [11]).
In Table 1 the main findings are summarized concerning P1R and P2R functions in MSCs submitted to differentiation, with a particular attention toward the osteogenic one.
In conclusion, the most promising receptors favoring MSC osteogenic differentiation seem to be A2BR, P2Y6R, and mainly P2X7R. While data related to the first two receptor types are still few and essentially limited to in vitro studies, in the next pages we reported more detailed experimental observations on P2X7R and osteogenesis, since there is a great interest in the role of these receptors in modulating bone formation and resorption processes and studies have been conducted in preclinical animal models and in humans. However, due to the great effervescence in this field, results so far obtained are numerous but often conflicting. Thus, in the next chapter, we tried to arrange a unifying vision of the activity of these receptors in bone homeostasis/repair, based also on our personal experience.
Focus on P2X7R
P2X7R are very particular ATP-gated, non-selective cation channels belonging to the family of ionotropic P2XR. P2RX7 gene encodes a 595-aa protein (the P2X7R subunit or monomer) that assembles into a trimeric complex to form the functional P2X7R [221]. Interestingly, human P2X7R exhibit a number of non-synonymous SNPs, which results in a change in amino acid sequence and expression of different human P2X7R variants, further increasing the structural diversity of these receptors [222]. In humans, seven naturally occurring splice variants were initially reported and their monomeric subunits were termed as P2X7B up to P2X7H [223], with P2X7A referring to the original full-length 595 amino acid protein [224]. Among them, there are truncated variants with an alternate short C terminus (P2X7B, C, E, and G), variants that lack exons responsible for coding parts of the extracellular domain (P2X7C–F) and variants with additional exons, thought to result in P2X7 protein lacking the first transmembrane domain (P2X7G and H) [225]. Two additional naturally occurring splice variants have been identified in humans, termed P2X7I and P2X7J [226]. These ten variants of human P2X7R subunits differ in distribution, functional characteristics, and predicted physiologic roles [227]. Of these, P2X7B is the best studied and, when assembled into a homomeric P2X7BR, it is able to form functional channels but not the large pores that are often associated with inflammation and cell death [228]. Interestingly, P2X7R polymorphisms are associated with low mineral density of lumbar spines and increased bone loss in postmenopausal women [229].
Several pharmacological differences exist between human and rodent P2X7R, including varying EC50 values for the agonists ATP and BzATP (10- to 25-fold higher for humans, in whom P2X7R are activated by millimolar concentrations of extracellular ATP) and sensitivity to other compounds. The agonist profile for human P2X7R is BzATP>>ATP> 2MeSATP>ATPγS>>ADP [230]. Upon ligand binding, P2X7R facilitates the influx of Ca2+ and Na+ and the efflux of K+. However, P2X7R overstimulation by elevated ATP concentrations, like those found during inflammatory events, triggers the opening of a non-selective plasma membrane pore, known as a “macropore,” which allows fluxes of large hydrophilic molecules. This can lead to diverse responses including activation of MAPKs and gene transcription, activation of the inflammasome, as well as the subsequent processing and release of the important inflammatory cytokine IL-1β, or sometimes activation of caspases and apoptosis depending on the given cell type and the experimental conditions [231, 232].
Role of P2X7Rs in bone cell formation and function
Studies so far performed pointed out that the expression and function of P2X7R vary depending on the stage and on the species of bone cells and that P2X7R are able to either promote or inhibit bone mineralization in osteoblasts [233].
Specifically, ATP stimulation of P2X7R can lead to the induction and/or activation of the activating protein-1 (AP-1) transcription factors, JunB, c-Fos, FosB, and ΔFosB [234]. The AP-1 transcription factors, in addition to Runx2, Distal-less homeobox 5 (Dlx5), Msx2, and Osx, play critical roles in osteoclast and osteoblast formation [235]. These gene regulatory proteins are known to be modulated by several components of the Wnt signaling pathway involved in bone formation. Wnt signaling occurs via both canonical (Wnt3a/β-catenin) and non-canonical molecular pathways. It has been reported that the non-selective P2X7R agonist, BzATP, inhibits β-catenin nuclear translocation, significantly decreases ALP activity in primary rat calvarial osteoblasts [185], and reduces nodule formation in primary human trabecular osteoblasts [186]. Moreover, P2X7R activation inhibits human mandibular-derived osteoblast differentiation by down-regulating the canonical Wnt signaling pathway [236].
In contrast, P2X7R were also found to have a stimulating, rather than an inhibiting effect on in vitro mineralization [179, 187, 188, 211]. Promotion of cell osteogenic differentiation both in animal and human tissue-derived MSCs by P2X7R was mediated by activation of molecular pathways leading to the production of lipid mediators such as lysophosphatidic acid and prostaglandin E2 [211] as well as to cell membrane blebbing (zeiosis) [187, 211] in a Rho kinase-dependent manner. In BMSCs obtained from human post-menopausal female, this mechanism has also been linked to activation of phospholipase C and PKC [187].
The differences emerged from the examined papers as for the response of MSC osteogenic differentiation to P2X7R activation could reside on issues such as:
different experimental models, in terms of animal species and tissue sources from which MSCs were obtained. As above reported, cells under investigation were obtained from animals or humans. From those studies, it could be inferred that cells deriving from rodent [211] or human bone marrow (healthy young or aged diseased females) [187] responded to prolonged P2X7R stimulation with an increased osteogenic differentiation, whereas those from rodent [188] or human [179, 236] tissues different from bone marrow underwent a decrease in their osteogenic differentiation potential. These findings could be likely related to a diverse expression and/or function of P2X7R in those cells [179, 187] or, also, to a different inactivation of the P2X7R agonist, BzATP, by ecto-NTDPases [10];
different concentrations of agonists assayed in the experiments. In this concern, the use of ATP or BzATP concentrations [179, 186, 236], unable to selectively stimulate only P2X7R, could elicit the activity of other P2R with negative influence on osteogenic differentiation.
Anyway, today, it seems that the pro-osteogenic activity mediated by P2X7R is better characterized. Thus, in patients with nonunion bone fractures, shockwave therapy enhanced bone healing via ATP release from hMSCs and the subsequent activation of P2X7R, responsible for hMSCs osteogenic differentiation as well as osteoblast proliferation and mineralization. Interestingly, pyridoxalphosphate-6-azophenyl-2',4'-disulfonic acid (PPADS), a pan P2X antagonist, the human P2X7 antagonist (4-[(2S)-2-[(5-isoquinolinylsulfonyl)methylamino]-3-oxo-3-(4-phenyl-1-piperazinyl)propyl] phenyl isoquinolinesulfonic acid ester (KN-62) and a P2X7R-specific siRNA strongly reduced or completely abolished these effects. Shockwave-induced effects were also prevented by apyrase, supporting a role for endogenously released ATP [237]. In addition, the truncated P2X7R isoform, P2X7BR, when co-expressed with the full variant P2X7AR, demonstrates a significant enhancement of mineralization in human osteosarcoma cell line [238], thus consolidating a positive role for the fully functional P2X7R in maintenance of bone strength.
The molecular mechanism underlying this beneficial effect would include calcium mobilization [239] and activation of MAPK/c-Jun NH2-terminal kinase (JNK) signaling cascade [240]. Indeed, several studies support a role for the P2X7R and MAPK signaling in osteogenic differentiation of human MSCs [241]. Cell treatment with KN-62, which, besides P2X7R also blocks Ca2+/calmodulin-dependent kinase type II, or with the non-selective P2R antagonist, PPADS, suppressed p38/MAPK dependent osteogenic differentiation of MSCs in response to shockwaves and ATP treatment [237].
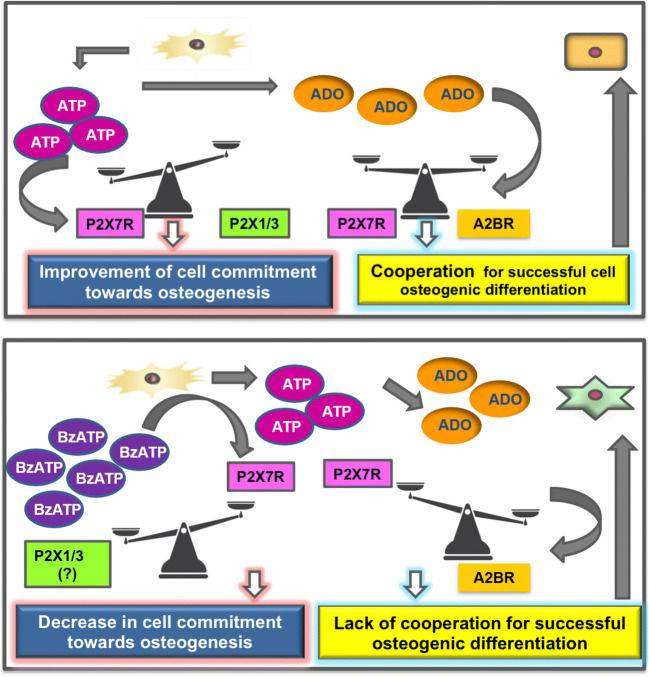
The involvement of P2X7R and endogenous ATP was also shown in murine MC3T3-E1 osteoblastic cells subjected to tension force, in which the P2X7R antagonist 3-[[5-(2,3-dichlorophenyl)-1H-tetrazol-1-yl]methyl]pyridine hydrochloride (A438079) blocked the expression of Runx2 [242]. However, studies by Ciciarello and colleagues [175] demonstrated that Runx2 mRNA levels are lower in differentiated human BMSCs treated with inhibitors against the ecto-NTPDase/CD39 and the ecto-5′NT/CD73. This finding indicates that Runx2 expression may be regulated by the ATP breakdown product adenosine instead of ATP. Accordingly, Carluccio et al. [179] recently reported that the P2X7R antagonist A438079, when administered alone to human ASCs (derived from healthy young females, BMI < 25) first decreased, whereas then increased their osteogenic differentiation. This latter effect was similar to that shown in the same cells by apyrase, an enzyme accelerating the conversion of nucleotides into adenosine. In an attempt to find a unifying explanation, it is conceivable that the interaction of endogenous ATP with P2X7R, the expression of which significantly increased during ASC differentiation, underlays early commitment of these cells toward osteogenesis, whereas adenosine, derived from ATP metabolism, is crucial in the second phase of this process. The pro-osteogenic activity of adenosine (delayed in ASCs) might be reasonably related to the nucleoside interaction with A1R and mainly with A2BR, as previously reported [70, 173, 174]. If confirmed in other MSCs, this would mean that ATP and adenosine may cooperate in concerted manner over time to assure the correct/complete differentiation of engaged MSCs into osteoblasts (see Fig. 3).
Fig. 3.
Scheme for a possible cooperation between endogenos ATP and adenosine in modulating the osteogenic differentiation of ASCs. a Endogenous ATP, released from normal and even more from injured tissue, may interact with P2X7R; the expression of which is significantly increased during ASC osteogenic differentiation, thus supporting this process. The activity of adenosine, derived from ATP metabolism, is more evident in a second time, in which the nucleoside should interact with its receptors (likely of A2BR subtype [173, 174]). These data, if confirmed in MSCs from other human sources, would suggest a temporal cooperation between endogenous ATP and adenosine to assure the correct/complete differentiation of engaged MSCs into osteoblasts. b In contrast, BzATP, a non-selective P2X7R agonist, when administered along cell differentiation, inhibits ASC commitment toward ostogenesis. This inhibitory effect could be either due to its interaction with other P2XR (i.e., P2X1R and P2X3R, mainly expressed in ASCs during the first phase of their osteogenic differentiation) or to a decreased P2X7R expression [179]. In this consition, endogenous adenosine is no longer able to increase the ASCs osteogenic differentiation
It is also to underline that during ASC osteogenic differentiation, the expression of P2X7R A and B splice variants significantly increased. This finding can represent a turning point in the investigation on the role played by P2X7R in ostogenesis, since the co-expression of A and B subunits of the P2X7R may favor the assembly of a heterotrimeric receptor with a greater sensitivity toward ligands, which may be able to better support the energy supply to cells [232] and likely their function. Conversely, ASC exposure to a prolonged pharmacological stimulation with the non-selective agonist BzATP (added to cells under osteogenic differentiation at each medium change, that is, every 3 days, along a period of 28 days) decreased both P2X7R expression and osteogenic differentiation. Such a P2X7R downregulation, already observed by Rodrigues-Ribeiro et al. [188], should be not considered in contrast to the often reported non-desensitization of P2X7R, even to high agonist concentrations, since these data are generally referred to cell exposure to P2X7R agonist for a brief period (minutes) and in relation to ion channel/pore opening [243]. In our case, the drug addition (BzATP 100 μM) to ASCs was repeated several times along a period of 28 days. Thus, the decreased P2X7R expression can be regarded as a defensive tentative by cells against such a repeated, potentially toxic, pharmacological treatment. In agreement with the overall data by Carluccio et al. [179], a number of studies today support the idea that while the basal/transient activation of P2X7R is osteogenic, sustained stimulation could inhibit their expression/function and, thereby, their activity on bone-forming cells [244], favoring negative effects by other P2R such as P2X1/3R, as previously mentioned.
Conclusions
Summarizing data herein reported have highlighted that purines and their receptors can affect MSC proliferation/differentiation potential. But, in our opinion, the scenario in which MSCs and purines move is complex, so that many factors should be taken into consideration.
First of all, it is still difficult to identify the best way to pursue for a successful use of MSCs in bone repair [245]. In our opinion, a general agreement is needed within the scientific community both to standardize the techniques and methods to obtain “safe” MSCs to use for osteogenic purposes and to identify preclinical models suitable to obtain reliable results that can be translated to humans for regenerative medicine. This aspect is crucial and without this agreement, research will inevitably proceed in a sparse way and it will be difficult to arrive to a clear understanding of its usefulness [246]. Obviously, data obtained from the use of immortalized cell lines are of interest, but only as producing pivotal findings, which should be confirmed in normal (possibly human) MSCs.
As for purines, findings so far obtained in relation to their modulation of MSC ostogenic differentiation have been regarded as promising, but, also from data herein reported, it is evident the need to implement studies in vitro on human MSCs from different tissue sources and mainly in vivo, to achieve a more uniform body of evidence, especially for those purine receptors mostly involved in promoting osteogenesis and bone repair processes.
As well, in future studies, it should also be taken into consideration the tight functional interplay existing among intra- and extracellular levels of purines, their receptors, degrading inside- and ecto-enzymes and bidirectional transport systems, all together forming the so purine called “purinome” [10, 138]. This network should be better elucidated in different MSCs so far evaluated during the differentiation processes, to understand whether dysfunction in this system may alter MSC contribution to osteogenesis and, consequently, to adopt therapeutic strategies to correct eventual derangements. The purinome should be also investigated in MSCs either moving from endogenous bone niches or exogenously implanted in the injured/inflamed tissue, in which the extracellular purine amount is increased by different mechanisms (see previous paragraphs). Clearly, in these conditions, the machinery to degrade purines is activated, but its function should be controlled to assure adequate quantities of adenine nucleotides and nucleosides able to promote pro-osteogenic and anti-inflammatory stimuli [200].
Finally, recent preclinical and clinical studies on stem cell-based therapy are pointing out that the success of this application in regenerative medicine is mostly due to factors secreted from exogenous MSCs rather than to differentiation of implanted stem cells in targeted tissues [140, 247, 248]. These substances represent the so called “secretome,” which is defined as the set of factors/molecules secreted from cells to the extracellular space, including, among others, soluble proteins, free nucleic acids, lipids, and extracellular vesicles, which are further classified into distinct groups (exosomes and microvesicles) and are attracting a growing interest [249]. The secretome of individual cells and tissues is specific and changes in response to fluctuations in physiological states or pathological conditions [250].
As a consequence, the investigations so far performed on the purine ability to affect MSC proliferation/osteogenic differentiation potential should be regarded more as a tentative to explain the influence of purines on endogenous MSCs in bone physiology/diseases rather than a real attempt to improve the use of exogenous MSCs in bone regenerative medicine. Thus, it is reasonable to foresee that in the next future the research on purines should be addressed to understand if purines are important compounds within the secretome and how purines, by interacting with their receptors, may affect substances released from MSCs able to favor bone healing/repair. In other words, it has become imperative to evaluate purine involvement in and interactions with MSC secretome, the knowledge of which is at the very beginning [251, 252].
The road ahead is still long but, hopefully, studies in this direction should be more useful to discover the real potentialities of using both MSCs and purines.
Acknowledgments
This work was supported by funds for research to RC and PDI from the University of Chieti-Pescara.
Abbreviations
- A438079
3-[[5-(2,3-dichlorophenyl)-1H-tetrazol-1-yl]methyl]pyridine hydrochloride
- ABMSCs
alveolar bone-derived MSCs
- AP-1
activating protein-1
- ALP
alkaline phosphatase
- A1R
A2AR, A2BR, A3R, adenosine A1, A2A, A2B, A3 receptors
- ASCs
adipose tissue-derived stem cells
- ATP, GTP, UTP, ADP
adenosine, guanosine, uridine tri- or di-phosphate
- BDNF
brain-derived growth factor
- BMSCs
bone marrow-derived stem cells
- BzATP
2′(3′)-O-(4-benzoylbenzoyl)adenosine 5′-triphosphate triethylammonium
- cAMP
adenosine 3',5' cyclic monophosphate
- cGMP
guanosine 3',5' cyclic monophosphate
- CM
conditioned medium
- DFPCs
dental follicle progenitor cells
- DPSCs
dental pulp stem cells
- DSCs
dental stem cells
- ECM
extracellular matrix
- ECs
endothelial cells
- e-5’NT
ecto-5’ nucleotidase
- ERK1/2
extracellular signal-regulated kinase 1 and 2
- G-CSF
granulocyte colony-stimulating factor
- GFs
growth factors
- GMSCs
gingival MSCs
- HGF
hepatocyte growth factor
- HSCs
hematopoietic stem cells
- JNK
c-Jun NH2-terminal kinase
- KN-62
[(2S)-2-[(5-isoquinolinylsulfonyl)methylamino]-3-oxo-3-(4-phenyl-1-piperazinyl)propyl] phenyl isoquinolinesulfonic acid ester
- IB-MECA
N6-(3-iodobenzyl)adenosine-5′-N-methyluronamide
- Il-1, -6, -8
interleukin 1, 6 and 8
- iPS
pluripotent stem cells
- MAPK
mitogen activated prion kinase
- M-CSF
macrophage colony stimulating factor
- MOBL
mesenchymal osteoblasts
- MSCs
mesenchymal stem cells
- Msx1/2
mouse segment homeobox 1/2
- NF-kB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NTPDase
nucleoside triphosphate diphosphohydrolase
- Osx
osterix
- PI3K
phosphoinositide 3-kinase
- PKA
protein kinase A
- PDLSCs
periodontal ligament stem cells
- P1R-P2R-P2XR-P2YR
P1-P2-P2X-P2Y receptors
- PPADS
pyridoxalphosphate-6-azophenyl-2',4'-disulfonic acid
- PTH
parathyroid homone
- P2X7R
P2X7 receptors
- RUNX2
runt-related transcription factor 2
- SAT
subcutaneous adipose tissue
- SCAP
stem cells from apical papilla
- SCI
spinal cord injury
- SHED
exfoliated deciduous teeth
- SOBL
surface osteoblasts
- SVF
stromal vascular fraction
- TCF/LEF
T-cell factor/lymphoid enhancer factor
- TNF
tumor necrosis factor
- TGF-β
transforming growth factor beta
- TGPCs
tooth germ progenitor cells
- VAT
visceral adipose tissue
Authors’ contributions
MC drafted the manuscript together with RC. SZ, MZ, and PG collected papers for the different sections of the manuscript. PDI and FC contributed to analyze data from collected papers. All authors read and approved the final manuscript.
Compliance with ethical standards
Conflict of interest statement
The authors declare no existing conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Marzia Carluccio, Email: marzia.carluccio@unich.it.
Sihana Ziberi, Email: sihana.ziberi@unich.it.
Mariachiara Zuccarini, Email: mariachiara.zuccarini@unich.it.
Patricia Giuliani, Email: patricia.giuliani@unich.it.
Francesco Caciagli, Email: f.caciagli@unich.it.
Patrizia Di Iorio, Email: patrizia.diiorio@unich.it.
Renata Ciccarelli, Email: renata.ciccarelli@unich.it.
References
- 1.Verrier S, Alini M, Alsberg E, et al. Tissue engineering and regenerative approaches to improving the healing of large bone defects. Eur Cell Mater. 2016;32:87–110. doi: 10.22203/ecm.v032a06. [DOI] [PubMed] [Google Scholar]
- 2.Ganguly P, El-Jawhari JJ, Giannoudis PV, et al. Age-related changes in bone marrow mesenchymal stromal cells: a potential impact on osteoporosis and osteoarthritis development. Cell Transplant. 2017;26(9):1520–1529. doi: 10.1177/0963689717721201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lechner J, Aschoff J, Rudi T. The vitamin D receptor and the etiology of RANTES/CCL-expressive fatty-degenerative osteolysis of the jawbone: an interface between osteoimmunology and bone metabolism. Int J Gen Med. 2018;11:155–166. doi: 10.2147/IJGMS152873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amini AR, Laurencin CT, Nukavarapu SP. Bone tissue engineering: recent advances and challenges. Crit Rev Biomed Eng. 2012;40:363–408. doi: 10.1615/critrevbiomedeng.v40.i5.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oryan A, Kamali A, Moshiri A, Baghaban Eslaminejad M. Oryan A et al. Role of mesenchymal stem cells in bone regenerative medicine: what is the evidence? Cells Tissues Organs. 2017;204(2):59–83. doi: 10.1159/000469704. [DOI] [PubMed] [Google Scholar]
- 6.Caplan AI. Mesenchymal stem cells: time to change the name! Stem Cells Transl Med. 2017;6:1445–1451. doi: 10.1002/sctm17-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cavaliere F, Donno C, D'Ambrosi N. Purinergic signaling: a common pathway for neural and mesenchymal stem cell maintenance and differentiation. Front Cell Neurosci. 2015;9:211. doi: 10.3389/fncel201500211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang LH, Hao Y, Mousawi F, Peng H, Yang X. Expression of P2 purinergic receptors in mesenchymal stem cells and their roles in extracellular nucleotide regulation of cell functions. J Cell Physiol. 2017;232:287–297. doi: 10.1002/jcp25484. [DOI] [PubMed] [Google Scholar]
- 9.Kaebisch C, Schipper D, Babczyk P, Tobiasch E. The role of purinergic receptors in stem cell differentiation. Comput Struct Biotechnol J. 2015;13:75. doi: 10.1016/jcsbj201411003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noronha-Matos JB, Correia-De-Sá P. Mesenchymal stem cells ageing: targeting the “purinome” to promote osteogenic differentiation and bone repair. J Cell Physiol. 2016;231(9):1852–1861. doi: 10.1002/jcp25303. [DOI] [PubMed] [Google Scholar]
- 11.Roszek K, Wujak M. How to influence the mesenchymal stem cells fate? Emerging role of ectoenzymes metabolizing nucleotides. J Cell Physiol. 2019;234:320–334. doi: 10.1002/jcp.26904. [DOI] [PubMed] [Google Scholar]
- 12.Schönherr E, Hausser HJ. Extracellular matrix and cytokines: a functional unit. Dev Immunol. 2000;7(2–4):89–101. doi: 10.1155/2000/31748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clarke B. Normal bone anatomy and physiology. Clin J Am Soc Nephrol CJASN. 2008;3(Suppl 3):S131–S139. doi: 10.2215/CJN.04151206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schaffler MB, Cheung WY, Majeska R, Kennedy O. Osteocytes: master orchestrators of bone. Calcif Tissue Int. 2014;94(1):5–24. doi: 10.1007/s00223-013-9790-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manolagas SC, Parfitt AM. What old means to bone. Trends Endocrinol Metab. 2010;21(6):369–374. doi: 10.1016/jtem201001010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonewald LF. The amazing osteocyte. J Bone Miner Res. 2011;26(2):229–238. doi: 10.1002/jbmr320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Infante A, Rodríguez CI. Osteogenesis and aging: lessons from mesenchymal stem cells. Stem Cell Res Ther. 2018;9:244. doi: 10.1186/s13287-018-0995-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shapiro F. Bone development and its relation to fracture repair. The role of mesenchymal osteoblasts and surface osteoblasts. Eur Cell Mater. 2008;15:53–76. doi: 10.22203/ecm.v015a05. [DOI] [PubMed] [Google Scholar]
- 19.Birmingham E, Niebur GL, McHugh PE, et al. Osteogenic differentiation of mesenchymal stem cells is regulated by osteocyte and osteoblast cells in a simplified bone niche. Eur Cell Mater. 2012;23:13–27. doi: 10.22203/ecm.v023a02. [DOI] [PubMed] [Google Scholar]
- 20.Rao TP, Kühl M. An updated overview on Wnt signaling pathways: a prelude for more. Circ Res. 2010;106(12):1798–1806. doi: 10.1161/CIRCRESAHA.110.219840. [DOI] [PubMed] [Google Scholar]
- 21.Krishnan V, Bryant HU, Macdougald OA. Regulation of bone mass by Wnt signaling. J Clin Invest. 2006;116:1202–1209. doi: 10.1172/JCI28551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D'Alimonte I, Nargi E, Lannutti A, et al. Adenosine A1 receptor stimulation enhances osteogenic differentiation of human dental pulp-derived mesenchymal stem cells via WNT signaling. Stem Cell Res. 2013;11(1):611–624. doi: 10.1016/jscr201304002. [DOI] [PubMed] [Google Scholar]
- 23.D'Alimonte I, Lannutti A, Pipino C, et al. Wnt signaling behaves as a “master regulator” in the osteogenic and adipogenic commitment of human amniotic fluid mesenchymal stem cells. Stem Cell Rev. 2013;9(5):642–654. doi: 10.1007/s12015-013-9436-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siddappa R, Doorn J, Liu J, et al. Timing, rather than the concentration of cyclic AMP, correlates to osteogenic differentiation of human mesenchymal stem cells. J Tissue Eng Regen Med. 2010;4(5):356–365. doi: 10.1002/term.246. [DOI] [PubMed] [Google Scholar]
- 25.Siddappa R, Martens A, Doorn J, et al. cAMP/PKA pathway activation in human mesenchymal stem cells in vitro results in robust bone formation in vivo. Proc Natl Acad Sci. 2008;105(20):7281–7286. doi: 10.1073/pnas.0711190105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang FS, Wang CJ, Sheen-Chen SM, et al. Superoxide mediates shock wave induction of ERK-dependent osteogenic transcription factor (Cbfa1) and mesenchymal cell differentiation toward osteoprogenitors. J Biol Chem. 2002;277:10931–10937. doi: 10.1074/jbc.M104587200. [DOI] [PubMed] [Google Scholar]
- 27.Kim SH, Choi YR, Park MS, et al. ERK1/2 activation in enhanced osteogenesis of human mesenchymal stem cells in poly(lactic-glycolic acid) by cyclic hydrostatic pressure. J Biomed Mater Res A. 2007;80:826–836. doi: 10.1002/jbm.a.30945. [DOI] [PubMed] [Google Scholar]
- 28.Jensen ED, Gopalakrishnan R, Westendorf JJ. Regulation of gene expression in osteoblasts. Biofactors. 2010;36:25–32. doi: 10.1002/biof72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou X, Zhang Z, Feng JQ, et al. Multiple functions of Osterix are required for bone growth and homeostasis in postnatal mice. Proc Natl Acad Sci USA. 2010;107:12919–12924. doi: 10.1073/pnas0912855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roybal PG, Wu NL, Sun J, et al. Inactivation of Msx1 and Msx2 in neural crest reveals an unexpected role in suppressing heterotopic bone formation in the head. Dev Biol. 2010;343:28–39. doi: 10.1016/jydbio201004007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Z, Xu Z, Duan C, et al. Role of TCF/LEF transcription factors in bone development and osteogenesis. Int J Med Sci. 2018;15(12):1415–1422. doi: 10.7150/ijms26741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kyllönen L, Haimi S, Mannerström B, et al. Effects of different serum conditions on osteogenic differentiation of human adipose stem cells in vitro. Stem Cell Res Ther. 2013;4(1):17. doi: 10.1186/scrt165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang Z, Nelson ER, Smith RL, Goodman SB. The sequential expression profiles of growth factors from osteoprogenitors [correction of osteroprogenitors] to osteoblasts in vitro. Tissue Eng. 2007;13:2311–2320. doi: 10.1089/ten.2006.0423. [DOI] [PubMed] [Google Scholar]
- 34.Quarles LD, Yohay DA, Lever LW, et al. Distinct proliferative and differentiated stages of murine MC3T3-E1 cells in culture: an in vitro model of osteoblast development. J Bone Miner Res. 1992;7:683–692. doi: 10.1002/jbmr.5650070613. [DOI] [PubMed] [Google Scholar]
- 35.Hoemann CD, El-Gabalawy H, McKee MD. In vitro osteogenesis assays: influence of the primary cell source on alkaline phosphatase activity and mineralization. Pathol Biol. 2009;57:318–323. doi: 10.1016/jpatbio200806004. [DOI] [PubMed] [Google Scholar]
- 36.Bates P, Ramachandran M. Bone injury, healing and grafting. In: Ramachandran M, editor. Basic orthopaedic sciences. The Stanmore Guide. London: Hodder Arnold; 2007. pp. 123–134. [Google Scholar]
- 37.Jones E, English A, Churchman SM, et al. Isolation and characterization of bone marrow multipotential mesenchymal progenitor cells. Arthritis Rheum. 2002;46(12):3349–3360. doi: 10.1002/art.10696. [DOI] [PubMed] [Google Scholar]
- 38.Bragdon BC, Bahney CS. Origin of reparative stem cells in fracture healing. Curr Osteoporos Rep. 2018;6(4):490–503. doi: 10.1007/s11914-018-0458-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Almubarak S, Nethercott H, Freeberg M, et al. Tissue engineering strategies for promoting vascularized bone regeneration. Bone. 2016;83:197–209. doi: 10.1016/jbone201511011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 41.Ben-David U, Benvenisty N. The tumorigenicity of human embryonic and induced pluripotent stem cells. Nat Rev Cancer. 2011;11:268–277. doi: 10.1038/nrc3034. [DOI] [PubMed] [Google Scholar]
- 42.Gutierrez-Aranda I, Ramos-Mejia V, Bueno C, et al. Human induced pluripotent stem cells develop teratoma more efficiently and faster than human embryonic stem cells regardless the site of injection. Stem Cells. 2010;28:1568–1570. doi: 10.1002/stem471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Friedenstein AJ, Chailakhyan RK, Latsinik NV, et al. Stromal cells responsible for transfer-ring the microenvironment of the hemopoietic tissues cloning in vitro and retransplantation in vivo. Transplantation. 1974;17:331–340. doi: 10.1097/00007890-197404000-00001. [DOI] [PubMed] [Google Scholar]
- 44.Kang NH, Hwang KA, Kim SU, et al. Potential antitumor therapeutic strategies of human amniotic membrane and amniotic fluid-derived stem cells. Cancer Gene Ther. 2012;19(8):517–522. doi: 10.1038/cgt201230. [DOI] [PubMed] [Google Scholar]
- 45.Prockop DJ, Kota DJ, Bazhanov N, Reger RL. Evolving paradigms for repair of tissues by adult stem/progenitor cells (MSCs) J Cell Mol Med. 2010;14:2190–2199. doi: 10.3109/146532492010507330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pountos I, Georgouli T, Kontakis G, Giannoudis PV. Efficacy of minimally invasive techniques for enhancement of fracture healing: evidence today. Int Orthop. 2010;34(1):3–12. doi: 10.1007/s00264-009-0892-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Augello A, Kurth TB, De Bari C. Mesenchymal stem cells: a perspective from in vitro cultures to in vivo migration and niches. Eur Cell Mater. 2010;20:121–133. doi: 10.22203/ecm.v020a11. [DOI] [PubMed] [Google Scholar]
- 48.Alvarez-Buylla A, Garcia-Verdugo JM. Neurogenesis in adult subventricular zone. J Neurosci. 2002;22:629–634. doi: 10.1523/jneurosci22-03-006292002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakagomi T, Takagi T, Beppu M, et al. Neural regeneration by regionally induced stem cells within post-stroke brains: novel therapy perspectives for stroke patients. World J Stem Cells. 2019;11(8):452–463. doi: 10.4252/wjscv11i8452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jiang Y, Jahagirdar BN, Reinhardt RL, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 51.Tremain N, Korkko J, Ibberson D, et al. MicroSAGE analysis of 2353 expressed genes in a single cell-derived colony of undifferentiated human mesenchymal stem cells reveals mRNAs of multiple cell lineages. Stem Cells. 2001;19:408–418. doi: 10.1634/stemcells.19-5-408. [DOI] [PubMed] [Google Scholar]
- 52.Schwartz RE, Reyes M, Koodie L, et al. Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte-like cells. J Clin Invest. 2002;109:1291–1302. doi: 10.1172/JCI15182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tropel P, Platet N, Platel JC, et al. Functional neuronal differentiation of bone marrow-derived mesenchymal stem cells. Stem Cells. 2006;24:2868–2876. doi: 10.1634/stemcells.2005-0636. [DOI] [PubMed] [Google Scholar]
- 54.Rose RA, Keating A, Backx PH. Do mesenchymal stromal cells transdifferentiate into functional cardiomyocytes? Circ Res. 2008;103:e120. doi: 10.1161/CIRCRESAHA108186908. [DOI] [PubMed] [Google Scholar]
- 55.Pijnappels DA, Schalij MJ, Ramkisoensing AA, et al. Forced alignment of mesenchymal stem cells undergoing cardiomyogenic differentiation affects functional integration with cardiomyocyte cultures. Circ Res. 2008;103:167–176. doi: 10.1161/CIRCRESAHA108176131. [DOI] [PubMed] [Google Scholar]
- 56.Bhattacharyya S, Kumar A, Lal Khanduja K. The voyage of stem cell toward terminal differentiation: a brief overview. Acta Biochim Biophys Sin (Shanghai) 2012;4(6):463–475. doi: 10.1093/abbs/gms027. [DOI] [PubMed] [Google Scholar]
- 57.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 58.Tokalov SV, Grüner S, Schindler S, et al. Age-related changes in the frequency of mesenchymal stem cells in the bone marrow of rats. Stem Cells Dev. 2007;16(3):439–446. doi: 10.1089/scd.2006.0078. [DOI] [PubMed] [Google Scholar]
- 59.Dalton S, Smith K, Singh K, et al. Accumulation of kynurenine elevates oxidative stress and alters microRNA profile in human bone marrow stromal cells. Exp Gerontol. 2020;130:110800. doi: 10.1016/j.exger.2019.110800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li S, Wang Y, Guan L, Ji M. Characteristics of human umbilical cord mesenchymal stem cells during ex vivo expansion. Mol Med Rep. 2015;12:4320–4325. doi: 10.3892/mmr20153999. [DOI] [PubMed] [Google Scholar]
- 61.Araújoj AB, Salton GD, Furlan JM, et al. Comparison of human mesenchymal stromal cells from four neonatal tissues: amniotic membrane, chorionic membrane, placental decidua and umbilical cord. Cytotherapy. 2017;19(5):577–585. doi: 10.1016/jjcyt201703001. [DOI] [PubMed] [Google Scholar]
- 62.Moraghebi R, Kirkeby A, Chaves P, et al. Term amniotic fluid: an unexploited reserve of mesenchymal stromal cells for reprogramming and potential cell therapy applications. Stem Cell Res Ther. 2017;8(1):190. doi: 10.1186/s13287-017-0582-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bajek A, Olkowska J, Walentowicz-Sadłecka M, et al. High quality independent from a donor: human amniotic fluid derived stem cells-a practical analysis based on 165 clinical cases. J Cell Biochem. 2017;118(1):116–126. doi: 10.1002/jcb25618. [DOI] [PubMed] [Google Scholar]
- 64.Liu J, Yu F, Sun Y. Concise reviews: characteristics and potential applications of human dental tissue-derived mesenchymal stem cells. Stem Cells. 2015;33(3):627–638. doi: 10.1002/stem1909. [DOI] [PubMed] [Google Scholar]
- 65.Botelho J, Cavacas MA, Machado V, Mendes JJ. Dental stem cells: recent progresses in tissue engineering and regenerative medicine. Ann Med. 2017;49(8):644–651. doi: 10.1080/0785389020171347705. [DOI] [PubMed] [Google Scholar]
- 66.Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7(2):211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 67.Zimmerlin L, Donnenberg VS, Pfeifer ME, et al. Stromal vascular progenitors in adult human adipose tissue. Cytometry A. 2010;77(1):22–30. doi: 10.1002/cytoa20813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bourin P, Bunnell BA, Casteilla L, et al. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT) Cytotherapy. 2013;15(6):641–648. doi: 10.1016/jjcyt201302006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dufrane D. Impact of age on human adipose stem cells for bone tissue engineering. Cell Transplant. 2017;26:1496–1504. doi: 10.1177/0963689717721203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.D'Alimonte I, Mastrangelo F, Giuliani P, et al. Osteogenic differentiation of mesenchymal stromal cells: a comparative analysis between human subcutaneous adipose tissue and dental pulp. Stem Cells Dev. 2017;26(11):843–855. doi: 10.1089/scd20160190. [DOI] [PubMed] [Google Scholar]
- 71.Strem BM, Hicok KC, Zhu M, et al. Multipotential differentiation of adipose tissue-derived stem cells. Keio J Med. 2005;54(3):132–141. doi: 10.2302/kjm.54.132. [DOI] [PubMed] [Google Scholar]
- 72.Kern S, Eichler H, Stoeve J, et al. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24(5):1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 73.Banyard DA, Salibian AA, Widgerow AD, Evans GR. Implications for human adipose-derived stem cells in plastic surgery. J Cell Mol Med. 2015;19(1):21–30. doi: 10.1111/jcmm12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Frese L, Dijkman PE, Hoerstrup SP. Adipose tissue-derived stem cells in regenerative medicine. Transfus Med Hemother. 2016;43(4):268–274. doi: 10.1159/000448180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li J, Martin EC, Gimble JM (2016) Adipose-derived stem/stromal cells, in: regenerative medicine–from protocol to patient 2. Stem Cell Science and Technology. G Steinhoff. Springer International Publishing AG Switzerland pp 363-387.
- 76.Virjula S, Zhao F, Leivo J, et al. The effect of equiaxial stretching on the osteogenic differentiation and mechanical properties of human adipose stem cells. J Mech Behav Biomed Mater. 2017;72:38–48. doi: 10.1016/jjmbbm201704016. [DOI] [PubMed] [Google Scholar]
- 77.Li Z, Liu C, Xie Z, et al. Epigenetic dysregulation in mesenchymal stem cell aging and spontaneous differentiation. PLoS One. 2011;6(6):e20526. doi: 10.1371/journalpone0020526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jackson WM, Nesti LJ, Tuan RS. Concise review: clinical translational of wound healing therapies based on mesenchymal stem cells Stem Cells. Translat Med. 2012;1:44–50. doi: 10.5966/sctm2011-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liao HT, Chen CT. Osteogenic potential: Comparison between bone marrow and adipose-derived mesenchymal stem cells. World J Stem Cells. 2014;6(3):288–295. doi: 10.4252/wjscv6i3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Han Y, Li X, Zhang Y, et al. Mesenchymal stem cells for regenerative medicine. Cells. 2019;8(8):pii: E886. doi: 10.3390/cells8080886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yorukoglu AC, Kiter AE, Akkaya S et al (2017, 2017) A concise review on the use of mesenchymal stem cells in cell s-based tissue engineering with special emphasis on bone tissue regeneration. Stem Cells Int:2374161. 10.1155/2017/2374161 [DOI] [PMC free article] [PubMed]
- 82.Soleimanifar F, Hosseini FS, Atabati H, et al. Adipose-derived stem cells-conditioned medium improved osteogenic differentiation of induced pluripotent stem cells when grown on polycaprolactone nanofibers. J Cell Physiol. 2019;234(7):10315–10323. doi: 10.1002/jcp27697. [DOI] [PubMed] [Google Scholar]
- 83.Ishihara A, Bertone AL. Cell-mediated and direct gene therapy for bone regeneration. Expert Opin Biol Ther. 2012;12:411–423. doi: 10.1517/147125982012661709. [DOI] [PubMed] [Google Scholar]
- 84.Evans NR, Davies EM, Dare CJ, Oreffo RO. Tissue engineering strategies in spinal arthrodesis: the clinical imperative and challenges to clinical translation. Regen Med. 2013;8(1):49–64. doi: 10.2217/rme12106. [DOI] [PubMed] [Google Scholar]
- 85.Dhaulakhandi DB, Rohilla S, Rattan KN. Neural tube defects: review of experimental evidence on stem cell therapy and newer treatment options. Fetal Diagn Ther. 2010;28:72–78. doi: 10.1159/000318201. [DOI] [PubMed] [Google Scholar]
- 86.Li H, Gao F, Ma L, et al. Therapeutic potential of in utero mesenchymal stem cell (MSCs) transplantation in rat fetuses with spina bifida aperta. J Cell Mol Med. 2012;16:1606–1617. doi: 10.1111/j1582-4934201101470x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Steigman SA, Ahmed A, Shanti RM, et al. Sternal repair with bone grafts engineered from amniotic mesenchymal stem cells. J Pediatr Surg. 2009;44:1120–1126. doi: 10.1016/jjpedsurg200902038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Duailibi MT, Duailibi SE, Youn CS, et al. Bioengineered teeth from cultured rat tooth bud cells. J Dent Res. 2004;83:523–528. doi: 10.1177/154405910408300703. [DOI] [PubMed] [Google Scholar]
- 89.Young CS, Abukawa H, Asrican R, et al. Tissue-engineered hybrid tooth and bone. Tissue Eng. 2005;11:1599–1610. doi: 10.1089/ten.2005.11.1599. [DOI] [PubMed] [Google Scholar]
- 90.Shanbhag S, Pandis N, Mustafa K, et al. Alveolar bone tissue engineering in critical-size defects of experimental animal models: a systematic review and meta-analysis. J Tissue Eng Regen Med. 2017;11(10):2935–2949. doi: 10.1002/term2198. [DOI] [PubMed] [Google Scholar]
- 91.Monsarrat P, Vergnes JN, Nabet C, et al. Concise review: mesenchymal stromal cells used for periodontal regeneration: a systematic review. Stem Cells Transl Med. 2014;Jun 3(6):768–774. doi: 10.5966/sctm.2013-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Trofin EA, Monsarrat P, Kemoun P. Cell therapy of periodontium: from animal to human? Front Physiol. 2013;4:325. doi: 10.3389/fphys.2013.00325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Volk SW, Theoret C. Translating stem cell therapies: the role of companion animals in regenerative medicine. Wound Rep Reg. 2013;21:382–394. doi: 10.1111/wrr12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Khojasteh A, Behnia H, Dashti SG, Stevens M. Current trends in mesenchymal stem cell application in bone augmentation: a review of the literature. J Oral Maxillofac Surg. 2012;70(4):972–982. doi: 10.1016/j.joms.2011.02.133. [DOI] [PubMed] [Google Scholar]
- 95.Janicki P, Schmidmaier G. What should be the characteristics of the ideal bone graft substitute? Combining scaffolds with growth factors and/or stem cells. Injury Int J. 2011;42:577–581. doi: 10.1016/j.injury.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 96.Lieberman JR, Daluiski A, Einhorn TA. The role of growth factors in the repair of bone. Biology and clinical applications. J Bone Joint Surg Am. 2002;84A(6):1032–1044. doi: 10.2106/00004623-200206000-00022. [DOI] [PubMed] [Google Scholar]
- 97.Quarto R, Mastrogiacomo M, Cancedda R, et al. Repair of large bone defects with the use of autologous bone marrow stromal cells. N Engl J Med. 2001;344(5):385–386. doi: 10.1056/NEJM200102013440516. [DOI] [PubMed] [Google Scholar]
- 98.Hernigou P, Poignard A, Beaujean F, Rouard H. Percutaneous autologous bone-marrow grafting for non-unions. Influence of the number and concentration of progenitor cells. J Bone Joint Surg Am. 2005;87(7):1430–1437. doi: 10.2106/JBJS.D.02215. [DOI] [PubMed] [Google Scholar]
- 99.Marcacci M, Kon E, Moukhachev V, et al. Stem cells associated with macro-porous bioceramics for long bone repair: 6- to 7-year outcome of a pilot clinical study. Tissue Eng. 2007;13(5):947–955. doi: 10.1089/ten.2006.0271. [DOI] [PubMed] [Google Scholar]
- 100.Šponer P, Kučera T, Brtková J, et al. Comparative study on the application of mesenchymal stromal cells combined with tricalcium phosphate scaffold into femoral bone defects. Cell Transplant. 2018;27(10):1459–1468. doi: 10.1177/0963689718794918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hernigou P, Dubory A, Homma Y, et al. Cell therapy versus simultaneous contralateral decompression in symptomatic corticosteroid osteonecrosis: a thirty-year follow-up prospective randomized study of one hundred and twenty-five adult patients. Int Orthop. 2018;42(7):1639–1649. doi: 10.1007/s00264-018-3941-8. [DOI] [PubMed] [Google Scholar]
- 102.Noriega DC, Ardura F, Hernández-Ramajo R, et al. Intervertebral disc repair by allogeneic mesenchymal bone marrow cells: a randomized controlled trial. Transplantation. 2017;101(8):1945–1951. doi: 10.1097/TP.0000000000001484. [DOI] [PubMed] [Google Scholar]
- 103.Gómez-Barrena E, Rosset P, Lozano D, et al. Bone fracture healing: cell therapy in delayed unions and nonunions. Bone. 2015;70:93–101. doi: 10.1016/j.bone.2014.07.033. [DOI] [PubMed] [Google Scholar]
- 104.Cristante AF, Barros-Filho TE, Tatsui N, et al. Stem cells in the treatment of chronic spinal cord injury: evaluation of somato-sensitive evoked potentials in 39 patients. Spinal Cord. 2009;47:733–738. doi: 10.1038/sc.2009.24. [DOI] [PubMed] [Google Scholar]
- 105.Karamouzian S, Nematollahi-Mahani SN, Nakhaee N, Eskandary H. Clinical safety and primary efficacy of bone marrow mesenchymal cell trans-plantation in subacute spinal cord injured patients. Clin Neurol Neurosurg. 2012;114:935–939. doi: 10.1038/cgt.2012.30. [DOI] [PubMed] [Google Scholar]
- 106.Forostyak S, Jendelova P, Sykova E. The role of mesenchymal stromal cells in spinal cord injury, regenerative medicine and possible clinical applications. Biochimie. 2013;95:2257–2270. doi: 10.1016/j.biochi.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 107.Gjerde C, Mustafa K, Hellem S, et al. Cell therapy induced regeneration of severely atrophied mandibular bone in a clinical trial. Stem Cell Res Ther. 2018;9(1):213. doi: 10.1186/s13287-018-0951-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Egusa H, Sonoyama W, Nishimura M, et al. Stem cells in dentistry–part I: clinical applications. J Prosthodont Res. 2012;56:229–248. doi: 10.1016/j.jpor.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 109.Egusa H, Sonoyama W, Nishimura M, et al. Stem cells in dentistry–part II: clinical applications. J Prosthodont Res. 2012;56:229–248. doi: 10.1016/j.jpor.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 110.Norambuena GA, Khoury M, Jorgensen C. Mesenchymal stem cells in osteoarticular pediatric diseases: an update. Pediatric Res. 2012;71(4):452–458. doi: 10.1038/pr.2011.68. [DOI] [PubMed] [Google Scholar]
- 111.Jin SE, Sung JH. Hair regeneration using adipose-derived stem cells. Histol Histopathol. 2016;31(3):249–256. doi: 10.14670/HH-11-686. [DOI] [PubMed] [Google Scholar]
- 112.Di Summa PG, Kingham PJ, Raffoul W, et al. Adipose-derived stem cells enhance peripheral nerve regeneration. J Plast Reconstr Aesthet Surg. 2010;63(9):1544–1552. doi: 10.1016/j.bjps.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 113.Brenner CH, David R, Franz WM. Regenerative Medicine – from Protocol to Patient, 2. Stem Cell Science and Technology. G Steinhoff: Springer International Publishing AG Switzerland; 2016. Stem cells for cardiovascular regeneration; pp. 145–167. [Google Scholar]
- 114.Lin G, Wang G, Liu G, et al. Treatment of type 1 diabetes with adipose tissue-derived stem cells expressing pancreatic duodenal homeobox 1. Stem Cells Dev. 2009;18(10):1399–1406. doi: 10.1089/scd.2009.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Banas A, Teratani T, Yamamoto Y, et al. Rapid hepatic fate specification of adipose-derived stem cells and their therapeutic potential for liver failure. J Gastroenterol Hepatol. 2009;24(1):70–77. doi: 10.1111/j.1440-1746.2008.05496.x. [DOI] [PubMed] [Google Scholar]
- 116.Collawn SS, Banerjee NS, De La Torre J, et al. Adipose-derived-stromal cells accelerate wound healing in an organotypic raft culture model. Ann Plast Surg. 2012;68(5):501–504. doi: 10.1097/SAP.0b013e31823b69fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Strong AL, Bowles AC, Maccrimmon CP, et al. Adipose stromal cells repair pressure ulcers in both young and elderly mice: potential role of adipogenesis in skin repair. Stem Cells Transl Med. 2015;4(6):632–642. doi: 10.5966/sctm.2014-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lendeckel S, Jödicke A, Christophis P, et al. Autologous stem cells (adipose) and fibrin glue used to treat widespread traumatic calvarial defects: case report. J Craniomaxillofac Surg. 2004;32(6):370–373. doi: 10.1016/j.jcms.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 119.Pak J, Lee JH, Park KS, et al. Current use of autologous adipose tissue-derived stromal vascular fraction cells for orthopedic applications. J Biomed Sci. 2017;24(1):9. doi: 10.1186/s12929-017-0318-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Merfeld-Clauss S, Gollahalli N, March KL, Traktuev DO. Adipose tissue progenitor cells directly interact with endothelial cells to induce vascular network formation. Tissue Eng. Part A. 2010;16(9):2953–2966. doi: 10.1089/ten.TEA.2009.0635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Veronesi F, Borsari V, Sartori M, et al. The use of cell conditioned medium for musculoskeletal tissue regeneration. J Cell Physiol. 2018;233(6):4423–4442. doi: 10.1002/jcp.26291. [DOI] [PubMed] [Google Scholar]
- 122.Majidinia M, Sadeghpour A, Yousefi B. The roles of signaling pathways in bone repair and regeneration. J Cell Physiol. 2018;233(4):2937–2948. doi: 10.1002/jcp.26042. [DOI] [PubMed] [Google Scholar]
- 123.Burnstock G. Introduction to Purinergic Signaling. Methods Mol Biol. 2041;2020:1–15. doi: 10.1007/978-1-4939-9717-6_1. [DOI] [PubMed] [Google Scholar]
- 124.Brown W, Foote CS (1998) Organic Chemistry, 2nd Edition.
- 125.Camici M, Garcia-Gil M, Tozzi MG. The inside story of adenosine. Int J Mol Sci. 2018;19(3):pii: E784. doi: 10.3390/ijms19030784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rathbone MP, Middlemiss PJ, Gysbers JW, et al. Trophic effects of purines in neurons and glial cells. Prog. Neurobiol. 1999;59(6):663–690. doi: 10.1016/s0301-0082(99)00017-9. [DOI] [PubMed] [Google Scholar]
- 127.Lazarowski ER, Sesma JI, Seminario-Vidal L, Kreda SM. Molecular mechanisms of purine and pyrimidine nucleotide release. Adv Pharmacol. 2011;61:221–261. doi: 10.1016/B978-0-12-385526-8.00008-4. [DOI] [PubMed] [Google Scholar]
- 128.Burnstock G, Verkhratsky A. Mechanisms of ATP release and inactivation. In: Burnstock G, Verkhratsky A, editors. Purinergic signalling and the nervous system. Berlin: Germany. Springer; 2012. pp. 79–118. [Google Scholar]
- 129.Ferrari D, McNamee EN, Idzko M, Gambari R, et al. Purinergic signaling during immune cell trafficking. Trends in Immunology. 2016;37:399–411. doi: 10.1016/j.it.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 130.Kong W, Engel K, Wang J. Mammalian nucleoside transporters. Curr Drug Metab. 2004;5:63–84. doi: 10.2174/1389200043489162. [DOI] [PubMed] [Google Scholar]
- 131.Yegutkin GG. Nucleotide- and nucleoside-converting ectoenzymes: important modulators of purinergic signalling cascade. Biochim Biophys Acta. 2008;1783:673–694. doi: 10.1016/j.bbamcr.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 132.Di Liberto V, Mudò G, Garozzo R, et al. The guanine-based purinergic system: the tale of an orphan neuromodulation. Front Pharmacol. 2016;7:158. doi: 10.3389/fphar.2016.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yegutkin GG. Enzymes involved in metabolism of extracellular nucleotides and nucleosides: functional implications and measurement of activities. Crit Rev Biochem Mol Biol. 2014;49:473–497. doi: 10.3109/10409238.2014.953627. [DOI] [PubMed] [Google Scholar]
- 134.Inoue K. Molecular basis of nucleobase transport systems in mammals. Biol Pharm Bull. 2017;40(8):1130–1138. doi: 10.1248/bpb.b17-00374. [DOI] [PubMed] [Google Scholar]
- 135.Glaser T, Cappellari AR, Pillat MM, et al. Perspectives of purinergic signaling in stem cell differentiation and tissue regeneration. Purinergic Signal. 2012;8(3):523–537. doi: 10.1007/s11302-011-9282-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Burnstock G. Purinergic signalling: from discovery to current developments. Exp Physiol. 2014;99:16–34. doi: 10.1113/expphysiol.2013.071951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rafehi M, Müller CE. Tools and drugs for uracil nucleotide-activated P2Y receptors. Pharmacol Ther. 2018;190:24–80. doi: 10.1016/j.pharmthera.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 138.Volonté C, D'Ambrosi N. Membrane compartments and purinergic signalling: the purinome, a complex interplay among ligands, degrading enzymes, receptors and transporters. FEBS J. 2009;276(2):318–329. doi: 10.1111/j.1742-4658.2008.06793.x. [DOI] [PubMed] [Google Scholar]
- 139.Di Virgilio F, Adinolfi E. Extracellular purines, purinergic receptors and tumor growth. Oncogene. 2017;36(3):293–303. doi: 10.1038/onc.2016.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bakopoulou A, Apatzidou D, Aggelidou E, et al. Isolation and prolonged expansion of oral mesenchymal stem cells under clinical-grade, GMP-compliant conditions differentially affect “stemness” properties. Stem Cell Res Ther. 2017;8(1):247. doi: 10.1186/s13287-017-0705-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Genetos DC, Geist DJ, Liu D, et al. Fluid shear-induced ATP secretion mediates prostaglandin release in MC3T3-E1 osteoblasts. J Bone Miner Res. 2005;20:41–49. doi: 10.1359/JBMR.041009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Romanello M, Codognotto A, Bicego M, et al. Autocrine/paracrine stimulation of purinergic receptors in osteoblasts: Contribution of vesicular ATP release. Biochem Biophys Res Commun. 2005;331:1429–1438. doi: 10.1016/j.bbrc.2005.03.246. [DOI] [PubMed] [Google Scholar]
- 143.Orriss IR, Knight GE, Utting JC, et al. Hypoxia stimulates vesicular ATP release from rat osteoblasts. J Cell Physiol. 2009;220:155–162. doi: 10.1002/jcp.21745. [DOI] [PubMed] [Google Scholar]
- 144.Sabirov RZ, Okada Y. The maxi-anion channel: a classical channel playing novel roles through an unidentified molecular entity. J Physiol Sci. 2009;59:3–21. doi: 10.1007/s12576-008-0008-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Thi MM, Islam S, Suadicani SO, Spray DC (2012) Connexin43 and pannexin1 channels in osteoblasts: Who is the “hemichannel”? J Membr Biol 245: 401–409. https;//doi.org/ 10.1007/s00232-012-9462-2 [DOI] [PMC free article] [PubMed]
- 146.Brandao-Burch A, Key ML, Patel JJ, et al. The P2X7 Receptor is an important regulator of extracellular ATP levels. Front Endocrinol (Lausanne) 2012;3:41. doi: 10.3389/fendo.2012.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Donahue HJ. Gap junctions and biophysical regulation of bone cell differentiation. Bone. 2000;26:417–422. doi: 10.1016/S8756-3282(00)00245-3. [DOI] [PubMed] [Google Scholar]
- 148.Noronha-Matos JB, Costa MA, Magalhães-Cardoso MT, et al. Role of ecto-NTPDases on UDP-sensitive P2Y receptor activation during osteogenic differentiation of primary bone marrow stromal cells from postmenopausal women. J Cell Physiol. 2012;227:2694–2709. doi: 10.1002/jcp.23014. [DOI] [PubMed] [Google Scholar]
- 149.Hecht E, Liedert A, Ignatius A, et al. Local detection of mechanically induced ATP release from bone cells with ATP microbiosensors. Biosens Bioelectron. 2013;44:27–33. doi: 10.1016/j.bios.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 150.Alvarenga EC, Rodrigues R, Caricati-Neto A, et al. Low-intensity pulsed ultrasound-dependent osteoblast proliferation occurs by via activation of the P2Y receptor: Role of the P2Y1 receptor. Bone. 2010;46:355–362. doi: 10.1016/j.bone.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 151.Burnstock G, Arnett TR, Orriss IR, et al. Purinergic signaling in the musculo-skeletal system. Purinergic Signal. 2013;9:541–572. doi: 10.1007/s11302-013-9381-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Roszek K, Błaszczak A, Wujak M, Komoszyński M. Nucleotides metabolizing ecto-enzymes as possible markers of mesenchymal stem cell osteogenic differentiation. Biochem Cell Biol. 2013;91:176–181. doi: 10.1139/bcb-2012-0093. [DOI] [PubMed] [Google Scholar]
- 153.Roszek K, Bomastek K, Drożdżal M, Komoszyński M. Dramatic differences in activity of purines metabolizing ecto-enzymes between mesenchymal stem cells isolated from human umbilical cord blood and umbilical cord tissue. Biochem Cell Biol. 2013;91:519–525. doi: 10.1139/bcb-2013-0050. [DOI] [PubMed] [Google Scholar]
- 154.Iser IC, Bracco PA, Gonçalves CEI, et al. Mesenchymal stem cells from different murine tissues have differential capacity to metabolize extracellular nucleotides. J. Cell Biochem. 2014;115:1673–1682. doi: 10.1002/jcb.24830. [DOI] [PubMed] [Google Scholar]
- 155.Naasani LIS, Rodrigues C, de Campos RP, et al. Extracellular nucleotide hydrolysis in dermal and limbal mesenchymal stem cells: a source of adenosine production. J. Cell Biochem. 2017;118:2430–2442. doi: 10.1002/jcb.25909. [DOI] [PubMed] [Google Scholar]
- 156.Katebi M, Soleimani M, Cronstein BN. Adenosine A2A receptors play an active role in mouse bone marrow-derived mesenchymal stem cell development. J Leukoc Biol. 2009;85:438–444. doi: 10.1189/jlb.0908520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hoebertz A, Arnett TR, Burnstock G. Regulation of bone resorption and formation by purines and pyrimidines. Trends Pharmacol Sci. 2003;24(6):290–297. doi: 10.1016/S0165-6147(03)00123-8. [DOI] [PubMed] [Google Scholar]
- 158.Zhang B, Hou R, Zou Z, Luo T, Zhang Y, Wang L, Wang B. Mechanically induced autophagy is associated with ATP metabolism and cellular viability in osteocytes in vitro. Redox Biol. 2018;14:492–498. doi: 10.1016/j.redox.2017.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ferrari D, Gulinelli S, Salvestrini V, et al. Purinergic stimulation of human mesenchymal stem cells potentiates their chemotactic response to CXCL12 and increases the homing capacity and production of proinflammatory cytokines. Exp Hematol. 2011;39:360–374. doi: 10.1016/j.exphem.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 160.Peng H, Hao Y, Mousawi F, Roger S, Li J, Sim JA, Jiang LH. Purinergic and store-operated Ca2+ signalling mechanisms in mesenchymal stem cells and their roles in ATP-induced stimulation of cell migration. Stem Cells. 2016;34:2102–2114. doi: 10.1002/stem.2370. [DOI] [PubMed] [Google Scholar]
- 161.Evans BA, Elford C, Pexa A, et al. Human osteoblast precursors produce extracellular adenosine, which modulates their secretion of IL-6 and osteoprotegerin. J Bone Miner Res. 2006;21:228–236. doi: 10.1359/JBMR.051021. [DOI] [PubMed] [Google Scholar]
- 162.Costa MA, Barbosa A, Neto E, et al. On the role of subtype selective adenosine receptor agonists during proliferation and osteogenic differentiation of human primary bone marrow stromal cells. J Cell Physiol. 2011;226(5):1353–1366. doi: 10.1002/jcp.22458. [DOI] [PubMed] [Google Scholar]
- 163.Tuli R, Tuli S, Nandi S, et al. Characterization of multipotential mesenchymal progenitor cells derived from human trabecular bone. Stem Cells. 2003;21:681–693. doi: 10.1634/stemcells.21-6-681. [DOI] [PubMed] [Google Scholar]
- 164.Hau K-L, Ranzoni AM, Vlahova F, et al. TGFβ-induced osteogenic potential of human amniotic fluid stem cells via CD73-generated adenosine production. Scientific Reports. 2017;7:6601. doi: 10.1038/s41598-017-06780-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lenertz LY, Baughman CJ, Waldschmidt NV, et al. Control of bone development by P2X and P2Y receptors expressed in mesenchymal and hematopoietic cells. Gene. 2015;570(1):1–7. doi: 10.1016/j.gene.2015.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Orriss IR, Burnstock G, Arnett TR. Purinergic signalling and bone remodelling. Curr Opin Pharmacol. 2010;10(3):322–330. doi: 10.1016/j.coph.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 167.He W, Cronstein BN. Adenosine A1 receptor regulates osteoclast formation by altering TRAF6/TAK1 signaling. Purinergic Signal. 2012;8(2):327–337. doi: 10.1007/s11302-012-9292-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Mediero A, Perez-Aso M, Cronstein BN. Activation of adenosine A2A receptor reduces osteoclast formation via PKA and ERK1/2-mediated suppression of NFkappaB nuclear translocation. Br J Pharmacol. 2013;169(6):1372–1388. doi: 10.1111/bph.12227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Mediero A, Kara FM, Wilder T, Cronstein BN. Adenosine A 2A receptor ligation inhibits osteoclast formation. Am J Pathol. 2012;180(2):775–786. doi: 10.1016/j.ajpath.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Vaupel P, Mayer A. Hypoxia-driven adenosine accumulation: a crucial microenvironmental factor promoting tumor progression. Adv Exp Med Biol. 2016;876:177–183. doi: 10.1007/978-1-4939-3023-4_22. [DOI] [PubMed] [Google Scholar]
- 171.Borhani S, Corciulo C, Larranaga-Vera A, Cronstein BN. Adenosine A(2A) receptor (A2AR) activation triggers Akt signaling and enhances nuclear localization of β-catenin in osteoblasts. FASEB J. 2019;33(6):7555–7562. doi: 10.1096/fj.201900014R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.He W, Mazumder A, Wilder T, Cronstein BN. Adenosine regulates bone metabolism via A1, A2A, and A2B receptors in bone marrow cells from normal humans and patients with multiple myeloma. FASEB J. 2013;27(9):3446–3454. doi: 10.1096/fj.13-231233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Gharibi B, Abraham AA, Ham J, Evans BA. Adenosine receptor subtype expression and activation influence the differentiation of mesenchymal stem cells to osteoblasts and adipocytes. J Bone Miner Res. 2011;26:2112–2124. doi: 10.1002/jbmr.424. [DOI] [PubMed] [Google Scholar]
- 174.Carroll SH, Wigner NA, Kulkarni N, et al. A2B adenosine receptor promotes mesenchymal stem cell differentiation to osteoblasts and bone formation in vivo. J Biol Chem. 2012;287:15718–15727. doi: 10.1074/jbc.M112.344994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ciciarello M, Zini R, Rossi L, et al. Extracellular purines promote the differentiation of human bone marrow-derived mesenchymal stem cells to the osteogenic and adipogenic lineages. Stem Cells Dev. 2013;22:1097–1111. doi: 10.1089/scd.2012.0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hajjawi MOR, Patel JJ, Corcelli M, et al. Lack of effect of adenosine on the function of rodent osteoblasts and osteoclasts in vitro. Purinergic Signal. 2016;12(2):247–258. doi: 10.1007/s11302-016-9499-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Rath-Wolfson L, Bar-Yehuda S, Madi L, et al. IBMECA, an A3 adenosine receptor agonist prevents bone resorption in rats with adjuvant induced arthritis. Clin Exp Rheumatol. 2006;24(4):400–406. [PubMed] [Google Scholar]
- 178.Orriss IR, Key ML, Brandao-Burch A, et al. The regulation of osteoblast function and bone mineralisation by extracellular nucleotides: the role of p2x receptors. Bone. 2012;51:389–400. doi: 10.1016/j.bone.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 179.Carluccio M, Zuccarini M, Ziberi S, et al. Involvement of P2X7 receptors in the osteogenic differentiation of mesenchymal stromal/stem cells derived from human subcutaneous adipose tissue. Stem Cell Rev. 2019;15(4):574–589. doi: 10.1007/s12015-019-09883-6. [DOI] [PubMed] [Google Scholar]
- 180.Morrison MS, Turin L, King BF, et al. ATP is a potent stimulator of the activation and formation of rodent osteoclasts. J Physiol. 1998;511:495–500. doi: 10.1111/j.1469-7793.1998.495bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kanaya K, Iba K, Dohke T, Okazaki S, Yamashita T. TRPV1, ASICs and P2X2/3 expressed in bone cells simultaneously regulate bone metabolic markers in ovariectomized mice. J Musculoskelet Neuronal Interact. 2016;16(2):145–151. [PMC free article] [PubMed] [Google Scholar]
- 182.Orriss IR, Knight GE, Ranasinghe S, Burnstock G, Arnett TR. Osteoblast responses to nucleotides increase during differentiation. Bone. 2006;39(2):300–309. doi: 10.1016/j.bone.2006.02.063. [DOI] [PubMed] [Google Scholar]
- 183.Nakamura E, Uezono Y, Narusawa K, et al. ATP activates DNA synthesis by acting on P2X receptors in human osteoblast-like MG-63 cells. Am J Physiol Cell Physiol. 2000;279:C510–C519. doi: 10.1152/ajpcell.2000.279.2.C510. [DOI] [PubMed] [Google Scholar]
- 184.Gartland A, Hipskind RA, Gallagher JA, Bowler WB. Expression of a P2X7 receptor by a subpopulation of human osteoblasts. J Bone Miner Res. 2001;16:846–856. doi: 10.1359/jbmr.2001.16.5.846. [DOI] [PubMed] [Google Scholar]
- 185.Gartland A, Orriss IR, Rumney RM, et al. Purinergic signalling in osteoblasts. Front Biosci. 2012;17:16–29. doi: 10.2741/3912. [DOI] [PubMed] [Google Scholar]
- 186.Agrawal A, Henriksen Z, Syberg S, et al. P2X7Rs are involved in cell death, growth and cellular signaling in primary human osteoblasts. Bone. 2017;95:101. doi: 10.1016/j.bone.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 187.Noronha-Matos JB, Coimbra J, Sa-e-Sousa RR, et al. P2X7-induced zeiosis promotes osteogenic differentiation andmineralization of postmenopausal bone marrow-derived mesenchymal stem cells. FASEB J. 2014;28:5208–5222. doi: 10.1096/fj.14-257923. [DOI] [PubMed] [Google Scholar]
- 188.Rodrigues-Ribeiro R, Alvarenga EC, Calio ML, et al. Dual role of P2 receptors during osteoblast differentiation. Cell Biochem Biophys. 2015;71(2):1225–1233. doi: 10.1007/s12013-014-0332-7. [DOI] [PubMed] [Google Scholar]
- 189.Bowler WB, Dixon CJ, Halleux C, et al. Signaling in human osteoblasts by extracellular nucleotides. Their weak induction of the c-fos proto-oncogene via Ca2+ mobilization is strongly potentiated by a parathyroid hormone/cAMP-dependent protein kinase pathway independently of mitogen-activated protein kinase. J Biol Chem. 1999;274:14315–14324. doi: 10.1074/jbc.274.20.14315. [DOI] [PubMed] [Google Scholar]
- 190.Hoebertz A, Mahendran S, Burnstock G, Arnett TR. ATP and UTP at low concentrations strongly inhibit bone formation by osteoblasts: a novel role for the P2Y2 receptor in bone remodeling. J Cell Biochem. 2002;86:413–419. doi: 10.1002/jcb.10236. [DOI] [PubMed] [Google Scholar]
- 191.Li W, Wei S, Liu C, et al. Regulation of the osteogenic and adipogenic differentiation of bone marrow-derived stromal cells by extracellular uridine triphosphate: the role of P2Y2 receptor and ERK1/2 signaling. Int J Mol Med. 2016;37(1):63–73. doi: 10.3892/ijmm.2015.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Liu PS, Chen CY. Butyl benzyl phthalate suppresses the ATP-induced cell proliferation in human osteosarcoma HOS cells. Toxicol Appl Pharmacol. 2010;244:308–314. doi: 10.1016/j.taap.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 193.Syberg S, Brandao-Burch A, Patel JJ, et al. Clopidogrel (Plavix), a P2Y12 receptor antagonist, inhibits bone cell function in vitro and decreases trabecular bone in vivo. J Bone Miner Res. 2012;27:2373–2386. doi: 10.1002/jbmr.1690. [DOI] [PubMed] [Google Scholar]
- 194.Biver G, Wang N, Gartland A, et al. Role of the P2Y13 receptor in the differentiation of bone marrow stromal cells into osteoblasts and adipocytes. Stem Cells. 2013;31:2747–2758. doi: 10.1002/stem.1411. [DOI] [PubMed] [Google Scholar]
- 195.Cho J, Yusuf R, Kook S, et al. Purinergic P2Y14 receptor modulates stress-induced hematopoietic stem/progenitor cell senescence. J Clin Invest. 2014;124(7):3159–3171. doi: 10.1172/JCI61636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zippel N, Limbach CA, Ratajski N, et al. Purinergic receptors influence the differentiation of human mesenchymal stem cells. Stem Cells Dev. 2012;21:884–900. doi: 10.1089/scd.2010.0576. [DOI] [PubMed] [Google Scholar]
- 197.Kotova PD, Bystrova MF, Rogachevskaja OA, et al. Coupling of P2Y receptors to Ca(2+) mobilization in mesenchymal stromal cells from the human adipose tissue. Cell Calcium. 2018;71:1–14. doi: 10.1016/j.ceca.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 198.Forostyak O, Butenko O, Anderova M, et al. Specific profiles of ion channels and ionotropic receptors define adipose- and bone marrow derived stromal cells. Stem Cell Res. 2016;16:622–634. doi: 10.1016/j.scr.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 199.Ali S, Turner J, Fountain SJ. P2Y(2) and P2Y(6) receptor activation elicits intracellular calcium responses in human adipose-derived mesenchymal stromal cells. Purinergic Signal. 2018;14(4):371–384. doi: 10.1007/s11302-018-9618-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Strazzulla LC, Cronstein BN. Regulation of bone and cartilage by adenosine signaling. Purinergic Signal. 2016;12:583–593. doi: 10.1007/s11302-016-9527-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Eijken M, Meijer IMJ, Westbroek I, et al. Wnt signaling acts and is regulated in a human osteoblast differentiation dependent manner. J Cell Biochem. 2008;104(2):568–579. doi: 10.1002/jcb.21651. [DOI] [PubMed] [Google Scholar]
- 202.Van der Horst G, van der Werf SM, Farih-Sips H, et al. Downregulation of Wnt signaling by increased expression of Dickkopf-1 and -2 is a prerequisite for late-stage osteoblast differentiation of KS483 cells. J Bone Miner Res. 2005;20(10):1867–1877. doi: 10.1359/JBMR.050614. [DOI] [PubMed] [Google Scholar]
- 203.Mediero A, Wilder T, Perez-Aso M, Cronstein BN. Direct or indirect stimulation of adenosine A2A receptors enhances bone regeneration as well as bone morphogenetic protein-2. FASEB J. 2015;29:1577–1590. doi: 10.1096/fj.14-265066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Bronwen AJ, Evans BA (2012) Does adenosine play a role in bone formation, resorption and repair? Purinergic Signal 8(2): 177–180. 10.1007/s11302-012-9317-4. [DOI] [PMC free article] [PubMed]
- 205.Shih YV, Liu M, Knon SK, et al. Dysregulation of ectonucleotidase-mediated extracellular adenosine during postmenopausal bone loss. Sci Adv. 2019;5(8):eaax1387. doi: 10.1126/sciadv.aax1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Plotkin LI, Manolagas SC, Bellido T. Transduction of cell survival signals by connexin-43 hemichannels. J. Biol. Chem. 2002;277:8648–8657. doi: 10.1074/jbc.M108625200. [DOI] [PubMed] [Google Scholar]
- 207.Orriss IR, Guneri D, Hajjawi MOR, et al. Activation of the P2Y(2) receptor regulates bone cell function by enhancing ATP release. J Endocrinol. 2017;233:341–356. doi: 10.1530/JOE-17-0042. [DOI] [PubMed] [Google Scholar]
- 208.Du D, Zhou Z, Zhu L, et al. TNF-α suppresses osteogenic differentiation of MSCs by accelerating P2Y(2) receptor in estrogen-deficiency induced osteoporosis. Bone. 2018;117:161–170. doi: 10.1016/j.bone.2018.09.012. [DOI] [PubMed] [Google Scholar]
- 209.Alqallaf SM, Evans BA, Kidd EJ. A typical P2X receptor pharmacology in two human osteoblast-like cell lines. Br J Pharmacol. 2009;156:1124–1135. doi: 10.1111/j.1476-5381.2009.00119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Ihara H, Hirukawa K, Goto S, Togari A. ATP-stimulated interleukin-6 synthesis through P2Y receptors on human osteoblasts. Biochem Biophys Res Commun. 2005;326:329–334. doi: 10.1016/j.bbrc.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 211.Panupinthu N, Rogers JT, Zhao L, et al. P2X7 receptors on osteoblasts couple to production of lysophosphatidic acid: a signaling axis promoting osteogenesis. J Cell Biol. 2008;181:859–871. doi: 10.1083/jcb.200708037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Panupinthu N, Zhao L, Possmayer F, et al. P2X7 nucleotide receptors mediate blebbing in osteoblasts through a pathway involving lysophosphatidic acid. J Biol Chem. 2007;282:3403–3412. doi: 10.1074/jbc.M605620200. [DOI] [PubMed] [Google Scholar]
- 213.Li J, Liu D, Ke HZ, et al. The P2X7 nucleotide receptor mediates skeletal mechanotransduction. J Biol Chem. 2005;280:42952–42959. doi: 10.1074/jbc.M506415200. [DOI] [PubMed] [Google Scholar]
- 214.Ke HZ, Qi H, Weidema AF, et al. Deletion of the P2X7 nucleotide receptor reveals its regulatory roles in bone formation and resorption. Mol Endocrinol. 2003;17:1356–1367. doi: 10.1210/me.2003-0021. [DOI] [PubMed] [Google Scholar]
- 215.Friedman E, Moran DS, Ben-Avraham D, Yanovich R, Atzmon G. Novel candidate genes putatively involved in stress fracture predisposition detected by whole-exome sequencing. Genet Res (Camb) 2014;96:e004. doi: 10.1017/S001667231400007X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Jorgensen NR, Husted LB, Skarratt KK, et al. Single-nucleotide polymorphisms in the P2X7 receptor gene are associated with postmenopausal bone loss and vertebral fractures. Eur J Hum Genet. 2012;20:675–681. doi: 10.1038/ejhg.2011.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Ohlendorff SD, Tofteng CL, Jensen JE, et al. Single nucleotide polymorphisms in the P2X7 gene are associated to fracture risk and to effect of estrogen treatment. Pharmacogenet Genomics. 2007;17:555–567. doi: 10.1097/FPC.0b013e3280951625. [DOI] [PubMed] [Google Scholar]
- 218.Husted LB, Harslof T, Stenkjaer L, et al. Functional polymorphisms in the P2X7 receptor gene are associated with osteoporosis. Osteoporos Int. 2013;24:949–959. doi: 10.1007/s00198-012-2035-5. [DOI] [PubMed] [Google Scholar]
- 219.Varley I, Greeves JP, Sale C, et al. Functional polymorphisms in the P2X7 receptor gene are associated with stress fracture injury. Purinergic Signal. 2016;12(1):103–113. doi: 10.1007/s11302-016-9495-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Gharibi B, Abraham AA, Ham J, Evans BA. Contrasting effects of A1 and A2b adenosine receptors on adipogenesis. Int J Obes. 2012;36:397–406. doi: 10.1038/ijo.2011.129. [DOI] [PubMed] [Google Scholar]
- 221.North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 222.Sperlàgh B, Illes P. P2X7 receptor: an emerging target in central nervous system diseases. Trends Pharmacol Sci. 2014;35(10):537–547. doi: 10.1016/j.tips.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 223.Cheewatrakoolpong B, Gilchrest H, Anthes JC, Greenfeder S. Identification and characterization of splice variants of the human P2X7 ATP channel. Biochm Biophys Res Commun. 2005;332:17–27. doi: 10.1016/j.bbrc.2005.04.087. [DOI] [PubMed] [Google Scholar]
- 224.Rassendren F, Buell GN, Virginio C, et al. The permeabilizing ATP receptor, P2X7. Cloning and expression of a human cDNA. J Biol Chem. 1997;272:5482–5486. doi: 10.1074/jbc.272.9.5482. [DOI] [PubMed] [Google Scholar]
- 225.Sluyter R. The P2X7 receptor. Adv Exp Med Biol. 2017;1051:17–53. doi: 10.1007/5584_2017_59. [DOI] [PubMed] [Google Scholar]
- 226.Feng YH, Li X, Wang L, et al. A truncated P2X7 receptor variant (P2X7-j) endogenously expressed in cervical cancer cells antagonizes the full-length P2X7 receptor through hetero-oligomerization. J Biol Chem. 2006;281:17228–17237. doi: 10.1074/jbc.M602999200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Jiang LH, Baldwin JM, Roger S, Baldwin SA. Insights into the molecular mechanisms underlying mammalian P2X7 receptor functions and contributions in diseases, revealed by structural modeling and single nucleotide polymorphisms. Front Pharmacol. 2013;4:55. doi: 10.3389/fphar.2013.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Adinolfi E, Cirillo M, Woltersdorf R, et al. Trophic activity of a naturally occurring truncated isoform of the P2X7 receptor. FASEB J. 2010;24:3393–3404. doi: 10.1096/fj.09-153601. [DOI] [PubMed] [Google Scholar]
- 229.Gallagher JA, Wiley JS. Polymorphisms in the P2X7 receptor gene are associated with low lumbar spine bone mineral density and accelerated bone oss in postmenopausal women. Eur J Hum Genet. 2012;20:559–564. doi: 10.1038/ejhg.2011.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Xing S, Grol MW, Grutter PH, et al. Modeling interactions among individual P2 receptors to explain complex response patterns over a wide range of ATP concentrations. Front Physiol. 2016;7:294. doi: 10.3389/fphys.2016.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Lenertz LY, Gavala ML, Zhu Y, Bertics PJ. Transcriptional control mechanisms associated with the nucleotide receptor P2X7, a critical regulator of immunologic, osteogenic, and neurologic functions. Immunol Res. 2011;50:22–38. doi: 10.1007/s12026-011-8203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Di Virgilio F, Schmalzing G, Markwardt F. The Elusive P2X7 Macropore. Trends Cell Biol. 2018;28:392–404. doi: 10.1016/j.tcb.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 233.Agrawal A, Gartland A. P2X7 receptors: role in bone cell formation and function. J Mol Endocrinol. 2015;54:75–88. doi: 10.1530/JME-14-0226. [DOI] [PubMed] [Google Scholar]
- 234.Gavala ML, Hill LM, Lenertz LY, et al. Activation of the transcription factor FosB/activating protein-1 (AP-1) is a prominent downstream signal of the extracellular nucleotide receptor P2RX7 in monocytic and osteoblastic cells. J Biol Chem. 2010;285(44):34288–34298. doi: 10.1074/jbc.M110.142091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Wagner EF. Bone development and inflammatory disease is regulated by AP-1 (Fos/Jun) Ann Rheum Dis. 2010;69(Suppl 1):i86–i88. doi: 10.1136/ard.2009.119396. [DOI] [PubMed] [Google Scholar]
- 236.Sindhavajiva PR, Sastravaha P, Arksornnukit M, Pavasant P. Purinergic 2X7 receptor activation regulates WNT signaling in human mandibular-derived osteoblasts. Arch Oral Biol. 2017;81:167–174. doi: 10.1016/j.archoralbio.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 237.Sun D, Junger WG, Yuan C, et al. Shockwaves induce osteogenic differentiation of human mesenchymal stem cells through ATP release and activation of P2X7 receptors. Stem Cells. 2013;31(6):1170–1180. doi: 10.1002/stem.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Gartland A, Di Virgilio F, Adinolfi E. Trophic activity of human P2X7 receptor isoforms A and B in osteosarcoma. PLoS ONE. 2014;9:e107224. doi: 10.1371/journal.pone.0107224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Jiang LH, Mousawi F, Yang X, Roger S. ATP-induced Ca(2+)-signalling mechanisms in the regulation of mesenchymal stem cell migration. Cell Mol Life Sci. 2017;74:3697–3710. doi: 10.1007/s00018-017-2545-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Yu X, Quan J, Long W, et al. LL-37 inhibits LPS-induced inflammation and stimulates the osteogenic differentiation of BMSCs via P2X7 receptor and MAPK signaling pathway. Exp. Cell Res. 2018;372(2):178–187. doi: 10.1016/j.yexcr.2018.09.024. [DOI] [PubMed] [Google Scholar]
- 241.Li W, Li G, Zhang Y, et al. Role of P2X7 receptor in the differentiation of bone marrow stromal cells into osteoblasts and adipocytes. Exp Cell Res. 2015;339(2):367–379. doi: 10.1016/j.yexcr.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 242.Kariya T, Tanabe N, Shionome C, et al. Tension force-induced ATP promotes osteogenesis through P2X7 receptor in osteoblasts. J Cell Biochem. 2015;116:12–21. doi: 10.1002/jcb.24863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Jindrichova M, Bhattacharya A, Rupert M, et al. Functional characterization of mutants in the transmembrane domains of the rat P2X7 receptor that regulate pore conductivity and agonist sensitivity. J Neurochem. 2015;133(6):815–827. doi: 10.1111/jnc.13078. [DOI] [PubMed] [Google Scholar]
- 244.Jørgensen NR. The purinergic P2X7 ion channel receptor-a ‘repair’ receptor in bone. Curr Opin Immunol. 2018;52:32–38. doi: 10.1016/j.coi.2018.03.016. [DOI] [PubMed] [Google Scholar]
- 245.Stoltz JF, de Isla N, Li YP et al (2015) Stem cells and regenerative medicine: myth or reality of the 21th century. Stem Cells Int. 2015: 734731. https://doi.org: 10.1155/2015/734731 [DOI] [PMC free article] [PubMed]
- 246.Eleuteri S, Fierabracci A. Insights into the secretome of mesenchymal stem cells and its potential applications. Int J Mol Sci. 2019;20(18):pii E4597. doi: 10.3390/ijms20184597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Kim S, Kim TM. Generation of mesenchymal stem-like cells for producing extracellular vesicles. World J Stem Cells. 2019;11(5):270–280. doi: 10.4252/wjsc.v11.i5.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Praaven LK, Kandoi S, Misra R, et al. The mesenchymal stem cell secretome: a new paradigm towards cell-free therapeutic mode in regenerative medicine. Cytokine Growth Factor Rev. 2019;46:1–9. doi: 10.1016/j.cytogfr.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 249.Roura S, Bayes-Genis A. Toward standardization of mesenchymal stromal cell-derived extracellular vesicles for therapeutic use: a call for action. Proteomics. 2017;19(1–2):e1800397. doi: 10.1002/pmic.201800397. [DOI] [PubMed] [Google Scholar]
- 250.Vizoso FJ, Eiro N, Cid S, Schneider J, Perez-Fernandez R. Mesenchymal stem cell secretome: toward cell-free therapeutic strategies in regenerative medicine. Int J Mol Sci. 2017;18(9):pii: E1852. doi: 10.3390/ijms18091852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.van Balkom BWM, Gremmels H, Giebel B, Lim SK. Proteomic signature of mesenchymal stromal cell-derived small extracellular vesicles. Proteomics. 2019;19(1–2):e1800163. doi: 10.1002/pmic.201800163. [DOI] [PubMed] [Google Scholar]
- 252.Qiu G, Zheng G, Ge M, et al. Functional proteins of mesenchymal stem cell-derived extracellular vesicles. Stem Cell Res Ther. 2019;10(1):359. doi: 10.1186/s13287-019-1484-6. [DOI] [PMC free article] [PubMed] [Google Scholar]