Abstract
We aim to investigate the role of A2A receptor in peritonitis-related sepsis by injection of a fecal solution (FS) as a model of polymicrobial infection. C57/black J6 wild-type (WT) and A2A-deficient mice (A2AKO) were exposed to sepsis induced by intraperitoneal injection of a FS (FS-induced peritonitis) or instead was injected with saline buffer (Sham). Survival rate and sepsis score were measured up to 48 h. The presence of bacteria in tissue homogenates was analyzed. Telemetry and speckle laser Doppler were used for systemic blood pressure and peripheral blood perfusion analysis, respectively. Histological analysis and identification of active caspase 3 were performed in selected organs, including the liver. The survival rate of A2AKO mice exposed to FS-induced peritonitis was significantly higher, and the sepsis score was lower than their respective WT counterpart. Injection of FS increases (50 to 150 folds) the number of colonies forming units in the liver, kidney, blood, and lung in WT mice, while these effects were significantly attenuated in A2AKO mice exposed to FS-induced peritonitis. A significant reduction in both systolic and diastolic blood pressure, as well as in the peripheral perfusion was observed in WT and A2AKO mice exposed to FS-induced peritonitis. Although, these last effects were significantly attenuated in A2AKO mice. Histological analysis showed a large perivascular infiltration of polymorphonuclear in the liver of WT and A2AKO mice exposed to FS-induced peritonitis, but again, this effect was attenuated in A2AKO mice. Finally, high expression of active caspase 3 was found only in the liver of WT mice exposed to FS-induced peritonitis. The absence of the A2A receptor increases the survival rate in mice exposed to polymicrobial sepsis. This outcome was associated with both hemodynamic compensation and enhanced anti-bacterial response.
Keywords: Sepsis, A2A receptor, Adenosine, Immune response, Bacterial load, Endothelial damage
Introduction
Sepsis has been defined as a life-threatening systemic dysfunction linked with deregulation of the host response to bacteria, fungi, viruses, or parasites infections [1]. Sepsis is the leading cause of mortality in critical care units [2], with a mortality rate between 25 and 77% [3]. Despite this high negative impact in social, epidemiological, and health care [4], the pathophysiology of sepsis is not completely understood.
Current understanding of the pathophysiology of sepsis involves an imbalance between the synthesis of pro-inflammatory cytokines (such as interleukin-6) [5] and anti-inflammatory cytokines (such as interleukin-10) [6] that promotes an immunosuppressive state, which in turn leads to uncontrolled infection, sepsis progression, multisystem fail, and death [7]. The liver plays a central role in metabolic hemostasis and the host defense against pathogens; albeit, liver function can be acutely compromised by shock as in severe sepsis. Indeed, the development of liver failure in sepsis is recognized as a major complication, which contributes to the severity of the sepsis and limits positive outcomes [8]. Also, septic patients exhibit generalized hemodynamic failure and decline in tissue perfusion [9–11] associated with both macro- and microvascular alterations and endothelial dysfunction [7]. All of those alterations involve paracrine mediators such as adenosine [12].
High plasma levels of adenosine (ADO), a nucleotide derived from the metabolism of adenosine triphosphate (ATP), have been described in septic patients [13]. The clinical significance of this elevation is unclear. But, adenosine activates a family of G-coupled protein membrane receptors identified as adenosine receptors type A1, A2A, A2B, and A3, which are involved in the regulation of inflammation, immunity, vascular tone, hemostasis, angiogenesis, among others key homeostatic process [14, 15] altered in septic patients.
In sepsis, the A2A receptor expressed by antigen-presenting cells [16], lymphocytes [17], and neutrophils [12] has been initially associated with anti-inflammatory effects [18–20]. The relevance of A2A adenosine receptors in sepsis has been further confirmed using mice lacking this receptor (A2AKO). However, reports using A2AKO are contradictory. For instance, compared to wild-type (WT) mice, some reports indicate that A2AKO mice have increased mortality when sepsis was generated by monomicrobial infection [21], while other reports indicate that these mice have increased survival rate when sepsis was generated by polymicrobial infection [22, 23]. Among several differences between the models of sepsis in A2AKO mice, appears that in sepsis generated by polymicrobial infection (i.e., cecal ligation and puncture (CLP)), A2AKO mice exhibit longer survival rates, reduced anti-inflammatory response, and limited systemic bacterial progression than their WT counterparts [8, 21, 22, 24], suggesting that participation of A2A in sepsis is more complex than initially thought. Due to the ubiquitous expression of A2A on different cells belong to cardiovascular, immune, or hepatic systems, it is highly probable that lacking A2A also interferes in the normal function of those systems.
In the cardiovascular system, there is no information on how the lack of A2A receptors may interfere in the hemodynamic alterations observed in septic mice. This is relevant since it is well-characterized that WT mice exposed to CLP exhibit an hypodynamic phase 24 h after infection insult. This hypodynamic phase is characterized by a decrease in cardiac output, mean blood pressure, oxygen utilization, and importantly, a reduction in the blood flow perfusion in organs such as the intestine, spleen, and kidneys. All these changes are associated with an increase in vascular resistance [25]. Whether the A2A receptor is participating in these hemodynamic and tissue perfusion changes during sepsis is unknown. Although, activation of the A2A receptor generates vasodilation under physiological conditions [26, 27], while the endotoxin-induced hypotension is not further affected after A2A stimulation [28].
This study aims to investigate the role of A2A receptor in peritonitis-related sepsis by injection of a fecal solution (FS-induced peritonitis) as a model of polymicrobial infection. Our a priori hypothesis was that A2AKO mice exposed to FS-induced peritonitis has a better survival rate associated with reduced bacteremia and vascular alterations than their WT counterpart.
Methodology
Animals and ethics
C57BlackL/6 mice were purchased from the animal facility of the Pontificia Universidad Católica de Chile (PUC). Dr. Jiang-Fan Chen from Boston University, USA, donated A2AKO mice. The generation of A2AKO mice has been described in detail previously (Chen et al., 1999). In brief, an A2A receptor genomic fragment was split by a positive selection marker (neomycin cassette), which replaced the 3′ end of exon 2, the adjacent 5′ splice junction, and intron sequences. Confirmation of A2AKO was performed using the amplification of neomycin cassette using PCR. Mice were housed at the Universidad de Valparaiso, Chile animal facility where they were kept under standard environmental conditions, which included controlled temperature (25 °C) and humidity, exposure to 12/12-h light/darkness cycles, and food and water supply at libitum. Specimens were maintained in a pathogen-free environment [29]. All the experiments were performed in compliance with the “3R” principals for animal experimentation [30, 31], and following the recommendations of the guidelines for the Care and Use of Laboratory Animals published by the US National Institute of Health. The Ethical Committee from the Universidad del Bio Bio (UBB) and FONDECYT (FONDECYT 1140586, Chile) approved the protocol.
As the inflammatory response differs between males and females [32], we decided to include only male mice (average age was 8 ± 0.5 months) that were subdivided into four groups: (1) Sham wild-type mice (WT-sham, n = 4); (2) WT mice exposed to FS-induced peritonitis (n = 11); (3) Sham A2AKO mice (n = 4); and (4) A2AKO mice exposed to FS-induced peritonitis (n = 11). Also, a 48-h time point was chosen to analyze bacterial clearance, hemodynamics, and organ damage to characterize the response of the surviving mice.
Sepsis by injection of a fecal solution
Intraperitoneal injection of a fecal solution (FS) was performed as previously described [33]. Briefly, fresh feces were collected from a donor mouse, which was previously isolated and maintained under similar conditions as the experimental mice. The feces were weighed and subsequently diluted in saline solution (NaCl 0.9% g/v) at a final concentration of 45 mg/ml. To ensure reproducibility, the procedure was standardized using a fresh solution prepared from the same donor mouse. The solution was filtered using a 50-μm nylon polymer membrane.
For the induction of sepsis, a single intraperitoneal injection of FS (1.5 mg/kg) was performed. As a control (sham group), another group of mice received only an intraperitoneal injection of saline solution (NaCl 0.9%, g/v). All procedures were performed in mice anesthetized with isoflurane (2–2.5 μg/g weight). After anesthesia, mice received two doses (every 12 h) of ketoprofen (5 μg/g of body weight).
To avoid day-to-day variations in the bacterial content of the fecal material, stool samples were collected only in the morning, after a fast of at least 12 h. A maximal of four mice per day were injected with the same FS. From them, WT and A2AKO mice were treated side-by-side.
Monitoring of the clinical condition
Mice were clinically evaluated at 0, 6, 12, 24, and 48 h after induction of sepsis using the “murine sepsis score (MSS)” scale, which was previously validated [33]. Mice were evaluated while they were in their cages (with the caps removed for better visualization). The MSS is directly proportional to the severity of the sepsis.
Speckle laser perfusion analysis
Tissue perfusion analysis was performed using the Pericam® PSI-HR system (Perimed Ltd., Stockholm, Sweden). In this equipment, the interaction between the laser and the red blood cells produced a set of points that generate a color map with a chromatic scale ranging from blue (reduced blood flow) to red (high blood flow). The analysis matrix includes an area of 64 × 64 points. Blood flow was recorded in the dorsal area of the anesthetized animal (inhalation isoflurane, a loading dose of 2–2.5 μL/g for 5 min). Both WT and A2AKO mice were analyzed side-by-side. Briefly, mice had their dorsal area shaved 1 day before initiate the tissue perfusion analysis. Shaving was also performed in anesthetized mice (isoflurane 2 μL/g for 30 seg) and using both an electric and manual razor to completely remove the hair. This prior depilation avoids the detection of changes in blood perfusion due to skin irritation. Besides, blood perfusion was measured without any pharmacological or physical stimulation. Two independent observers who did not know the experimental condition carried out the analysis of the images. The observers defined the regions of interest (ROI) considering the stability of recorded signals.
Blood pressure
Blood pressure was measured with the CODA® system (Kent Scientific, Torrington, CT, USA). Blood pressure was measured at baseline and 48 h post sepsis induction. In preparation for the non-invasive blood pressure procedure, all mice were previously anesthetized (inhalation isoflurane, a loading dose of 2–2.5 μL/g). After 5 min of adaptation, pressure measurements were performed on anesthesia-free conditions.
Samples of blood and organs
Blood
Blood samples were obtained after a cardiac puncture at 48 h post sepsis induction. Collected blood samples were split into tubes without and with heparin (Becton Dickinson, Franklin Lakes, New Jersey, USA). For isolation of serum or plasma, respectively, the blood was centrifuged at 2.000 g per 10 min. Serum and plasma were frozen at − 80 °C until further analysis.
Aliquots of heparinized blood samples (1 μL) were sowed on blood agar plates (Valtek, Santiago, Chile) and incubated for 48 h at 37 °C. The number of colony-forming units (CFU) was visually counted and multiplied by 1.000, as previously described [34].
Tissues
Organs were dissected under sterile conditions. Tissue procurement was conducted ex vivo through a medial incision in the thorax and abdomen. The organs collected included kidneys, liver, and lungs. Those were deposited in sterile tubes. After that, tissues (1 g) were homogenized (Homogenizer, Daihan, Korea) in a sterile phosphate buffer solution (PBS; NaCl 138 mM, KCl 3 mM, Na2HPO4 8.1 mM, KH2PO4 1.5 mM solution) (1 ml) and subsequently centrifuged (2500 g per 10 min). An aliquot of the supernatant (1 μL) from each tissue was sowed on blood agar plates (Valtek, Santiago, Chile) and used for CFU counting as indicated above.
Histology
Samples of the spleen, heart, liver, and kidney were fixed in formalin (in PBS, 4%, v/v) for 48 h. Tissue inclusion in paraffin was performed automatically (Leica Biosystem, Wetzlar, Germany) using the protocol previously described [35]. Subsequently, tissues were sectioned (4 μm) and stained with hematoxylin/eosin. Photos were taken with × 400 magnification in an optical microscope (Leica Biosystem, Wetzlar, Germany). A blind histological examination was performed by a pathologist (PS) considering previous publications [36].
Also, images of tissues stained with hematoxylin/eosin were used to estimate the area occupied by polymorphonuclear cells (PMN). Briefly, random histological sections were used to specifically visualize areas with PMN infiltration. Then, areas of PMN infiltration identify by microscopy were photographed. Photos were processed using ImageJ software (NIH, Bethesda, Maryland, USA). Thus, the obtained picture was used to contrast differences (threshold) in 16-bit images to identify representative cellularity of PMN. Subsequently, the number of particles (i.e., PMN) was quantified using a specific plug-in from the Image J software. The particle count is expressed per area analyzed.
Immunohistochemistry
Immunohistochemistry of active caspase 3 was performed according to a previously established protocol [37]. Random sections (4 μm) were deparaffinized and rehydrated. Antigen retrieval was performed by incubating the sections in 10 mM acetate sodium buffer (pH 6.0) at 95 °C for 10 min, aiming to restore epitope-antibody binding. After, the sections were treated with 3% (v/v) hydrogen peroxide (H2O2) in phosphate buffer saline (PBS) for 30 min, to block endogenous peroxidase activity. Each of the succeeding steps was followed by a thorough rinse with PBS. All steps were performed in a humid chamber, and care was taken to avoid the dehydration of the sections. Nonspecific staining was blocked by immersion in Cas-Block solution (Thermo Fisher Scientific, Whaltham, MA, USA) and subsequently by goat serum (Gibco) for 30 min. Sections were incubated with the primary antibody anti-active caspase 3 using a rabbit polyclonal antibody directed to the large fragment (17 kDa) of the active protein (BD Biosciences, Le Pont-de-Claix, France), diluted 1:500 in PBS containing 0.3% (v/v) Tween 20, overnight at 4 °C. After extensive rinsing in PBS, all sections were incubated for 1 h at room temperature, with peroxidase-conjugated goat anti-rabbit IgG (Rockland) diluted 1:1500 in PBS. The peroxidase reaction was visualized using the NovaRED® kit (Vector lab, Burlingame, CA, USA). After immunostaining, sections were lightly stained with Mayer’s hematoxylin (Merck, Darmstadt, Germany).
For each immunohistochemical reaction, controls were performed by incubating the sections with PBS or by omitting the primary antibody. Sections were examined in a Zeiss Axioskop 2 microscope, and the images were captured using a digital camera (Canon) and KS 100 3.0 software (Zeiss, Oberkochen, Germany). The samples were analyzed in a blind manner using images taken at × 40. Estimation of active caspase 3 levels was performed using a scale of crosses, using the Image-Pro-Plus software (Media Cybernetics, Silve Spring, MD, USA).
Statistical analysis
Values are presented as means ± SEM. Variables were analyzed using a nonparametric test considering data distribution. Kaplan-Meier curve and log-rank were used for survival analysis. Besides, since analysis includes two factors, A2AKO and FS-induced peritonitis, the analysis of differences between groups was analyzed using a two-way analysis of variance (ANOVA). When significant differences were found in the ANOVA analysis, the Mann-Whitney test was used to compare two groups. p < 0.05 was considered as statistically significant. GraphPad Prism 5.00 (GraphPad Software, San Diego, CA, USA) was used for statistical analysis.
Results
Effect of A2A receptor knockdown in survival rates after FS-induced peritonitis
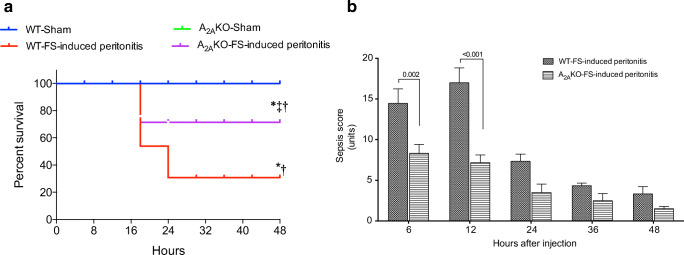
Mice exposed to FS-induced peritonitis were clinically monitored using the MSS [33] up to 48 h post-infection. A significantly higher survival rate in A2AKO than WT mice exposed to FS-induced peritonitis (70% versus 30%, Chi2; 32; p < 0.0001) was found (Fig. 1a). Survival in the sham groups was 100% in both WT and A2AKO mice. Correspondingly, survived mice in the A2AKO group exposed to FS-induced peritonitis were clinically less compromised since the score in the MSS at 6 and 12 h after FS injection was significantly lower than their respective counterpart in the WT group (Fig. 1b). The maximum score of the MSS was reached earlier in the A2AKO than the WT mice (6 h versus 12 h, respectively). After that, a sustained decline in the MSS was found in both groups. The control group (sham) showed minimal score in the MSS during the whole period of analysis in both WT and A2AKO mice. Then, compared to FS-induced peritonitis, sham WT mice showed significantly reduced MSS (p < 0.001) up to 24 h after FS injection, while significant differences (p < 0.05) between FS-induced peritonitis versus sham A2AKO mice were found only up to 12 h post-FS injection.
Fig. 1.
Survival analysis and clinical evaluation of septic mice. Wild-type (WT) and A2A-deficient (A2AKO) mice exposed to FS-induced peritonitis or injection of saline solution (Sham) were included for a survival analysis and b murine sepsis score (MSS) scale assessment up to 48 h post-injection. WT-sham, n = 4; WT-FS-induced peritonitis, n = 11; A2AKO-sham, n = 4; A2AKO-FS-induced peritonitis, n = 11. In a, *p < 0.05 vs. WT-sham. †p < 0.05 vs. A2AKO-sham. ‡p < 0.05 vs. WT-FS-induced peritonitis
Effect of A2A receptor knockdown on bacterial load
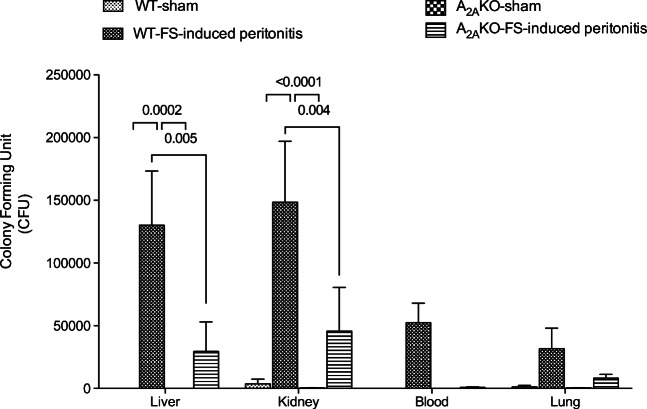
To analyze the spread of bacterial load (i.e., septicemia), we measured the CFU using supernatants of tissue homogenates obtained from the liver, kidney, and lungs, as well as plasma from the bloodstream. Septicemia was evident only in WT exposed to FS-induced peritonitis, since a high number of CFU was found in all analyzed tissues, reaching statistical significance in the liver and kidney compared to sham mice. However, the number of CFU found in A2AKO mice exposed to FS-induced peritonitis was low in all analyzed tissues compared to WT mice exposed to FS-induced peritonitis. In the liver, kidney, and blood of A2AKO mice exposed to FS-induced peritonitis, the number of CFU did not exceed 30% of those present in their respective counterpart in WT mice (Fig. 2).
Fig. 2.
Analysis of bacteriological load in septic mice. Survived wild-type (WT) and A2A-deficient (A2AKO) mice exposed to FS-induced peritonitis or injection of saline solution (Sham) were included for counting the number of colony-forming units (CFU) in blood agar. Tissue extractions of survived mice at 48 h post-injection were included. N = 4 per group
Hemodynamic changes induced by sepsis
Basally, there were no significant differences in systolic (124 ± 28 versus 130 ± 27 mmHg) or diastolic blood pressure (91 ± 27 versus 97 ± 25 mmHg) between sham WT and sham A2AKO mice, respectively (Fig. 3a, b). However, after FS-induced peritonitis (48 h), a significant drop (p < 0.0001) in systolic and diastolic blood pressures was found in both WT and A2AKO mice. Interestingly, the decline in both systolic (− 33 ± 7 versus − 40 ± 5 mmHg) and diastolic blood pressure (− 29 ± 5 versus − 35 ± 4 mmHg) induced by FS-induced peritonitis was lower in A2AKO mice than WT, respectively.
Fig. 3.

Analysis of blood pressure in septic mice. Survived wild-type (WT) and A2A-deficient (A2AKO) mice exposed to FS-induced peritonitis or injection of saline solution (Sham) were included to compare a systolic and b diastolic blood pressure. N = 4–5 per group
Tissue perfusion was analyzed in the skin (dorsal area, Fig. 4a). Tissue perfusion was significantly reduced in sham A2AKO compared to sham WT mice (93 ± 3 vs. 134 ± 5 perfusion units, respectively, p < 0.0001) (Fig. 4b, c). Furthermore, WT mice exposed to FS-induced peritonitis exhibited a substantial drop in the blood perfusion at 48 h post-infection (p < 0.0001), while this effect was absent in A2AKO mice. Indeed, perfusion units in A2AKO exposed to FS-induced peritonitis mice were 2-fold higher than their respective counterpart in WT mice (p < 0.0001) (Fig. 4c).
Fig. 4.
Analysis of tissue perfusion in septic mice. Survived wild-type (WT) and A2A-deficient (A2AKO) mice exposed to FS-induced peritonitis or injection of saline solution (Sham) were included to analyze tissue perfusion using speckle laser Doppler. a Representative image of the dorsal area in the analyzed mice. Dots (red and blue) represent areas of interest. b Representative figures of blood perfusion in the skin (dorsal area) of WT and A2AKO at 48 h post-injection. The pseudocolor scale represents larger perfusion (red) to no perfusion (blue). c Tissue perfusion measured in arbitrary units. N = 4–5 per group
Ex vivo analysis of selected tissues
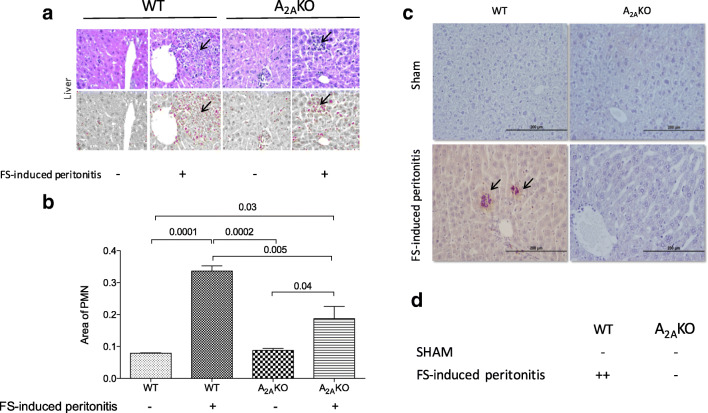
Large inflammatory infiltration was found only in the liver of WT and A2AKO mice exposed to FS-induced peritonitis compared to respective sham controls (Fig. 5a). Microscopic analysis showed that these infiltrations were mainly neutrophils (or polymorphonuclear cells, PMN). This PMN infiltration was particularly located in the perivascular area. Remarkably, the area of PMN infiltration was lower in A2AKO mice than WT mice exposed to FS-induced peritonitis (1.7 ± 0.2 vs. 4.2 ± 0.1-fold, respectively, p = 0.01) (Fig. 5b).
Fig. 5.
Histological analysis and cell apoptosis in the liver of septic mice. The liver from survived wild-type (WT) and A2A-deficient (A2AKO) mice exposed to FS-induced peritonitis or injection of saline solution (Sham) were used for a histological analysis using hematoxylin/eosin staining. Arrow represents polymorphonuclear (PMN) infiltration in the perivascular area. b Quantification of the area occupied by PMN (remarked with arrows) in the perivascular area of the liver. c Representative image of immune detection of active caspase 3 (arrows). d Semiquantitative analysis (in cruses) of active caspase 3 active staining. N = 4 per group
We also look for expression of the apoptosis marker, active caspase 3, in tissues such as the liver, spleen, heart, and kidney. Again, significant differences were found only in the liver. Thus, staining for active caspase 3 was higher in WT mice exposed to FS-induced peritonitis compared to A2AKO mice (Fig. 5c, d). Indeed, the staining for active caspase 3 active was neglected in A2AKO mice.
Histological studies found no pathological changes in the spleen, heart, and kidney of mice of both strains, neither considering sham or sepsis induced by FS-induced peritonitis (data not shown).
Discussion
We found that a lack of A2A receptor resulted in an enhanced survival rate after polymicrobial infection induced by injection of a FS. This effect was associated with an improvement in the clinical and hemodynamic status. But, it also was associated with a decrease in both systemic bacterial spread and liver inflammatory alterations (including reduced liver infiltration of PMN and cellular apoptosis). Therefore, our results suggest that blockage of A2A may be a therapeutic option for reducing mortality associated with sepsis.
The participation of the A2A receptor in sepsis has shown apparent contradictory results when experimentation includes the analysis of A2AKO mice. The reasons for this apparent discrepancy are not clear, but we suggest that the induction of sepsis by a monomicrobial [21] or polymicrobial stimuli [22, 23] provides different outcomes that are not comparable to each other. For example, on one side, activation of A2A using the selective agonist, ATL146e, protected mice from lipopolysaccharide (LPS) challenge [20]. Also, the genetic deletion of the A2A receptor inhibited the protection mediated by ATL146e in this model. Furthermore, activation of the A2A receptor provided substantive hepatoprotective effects in septic mice [8]. Underling mechanisms of the pro-survival role of A2A in those models of sepsis may be associated with its anti-inflammatory effect [38], evidenced as the reduction in the levels of pro-inflammatory interleukins [20, 39], such as including IL-6 [40], tumor necrosis factor-alpha (TNFα) [16], interleukin 12 (IL-12) [41], and interferon-gamma (IFN-γ) [42].
On the other hand, adenosine signaling has been recognized as the mediator of immunosuppressive signals within the inflammatory microenvironment that are safeguarding bacteria by rendering immune cells hyporesponsive. A study [22] showed that A2AKO mice were protected from the lethal effect of CLP-induced polymicrobial sepsis, and had improved bacterial clearance compared with WT mice. Those last findings agree with our results analyzing the survival rate of A2AKO mice exposed to FS-induced peritonitis. Also, in the above study, apoptosis was attenuated in the spleen of septic A2AKO mice, which again agree with our findings in the liver of A2AKO mice exposed to FS-induced peritonitis. Other studies had confirmed that inhibition of the A2A receptor improves the welfare of mice with sepsis [43], as A2A adenosine receptor deficiency dramatically decreased peritoneal, splenic, and blood bacterial levels in a chronic model of CLP-induced sepsis. Those last results are compatible with our findings in which significantly less bacterial load (i.e., CFU) was found in the liver and kidney of septic A2AKO mice, compared to their respective WT counterpart. Also, contrary to those previous publications [22, 43], we did not study the secretion of cytokines, but we have characterized changes in the blood pressure and peripheral blood perfusion (see below). Nevertheless, mechanistically, activation of A2A receptors can upregulate the expression of anti-inflammatory interleukins, such as IL-10 in monocyte [44] or macrophages [45], and not only pro-inflammatory cytokines as described above. Furthermore, A2AKO mice exposed to CLP showed reduced levels of IL-6, TNF srl, TNF srll, without changes in TNF- α, IL-1β, and MCP1 at 6 h after infection [43]. Altogether, these data demonstrate that A2A blockade may be an effective immunotherapy treatment to prevent bacterial overgrowth and reduce mortality in septic patients, in particular, due to polymicrobial infection such as we presented in our results.
Potential mechanisms leading to opposite outcomes after polymicrobial challenge versus single-strand bacteria/LPS studies under A2A-depleted conditions are unclear. In the human context, evidence has demonstrated that mortality in septic patients is associated with secondary or multiple infections [46], while the differential outcome based on the intensity of the inflammation has been previously reported in septic patients [47]. Then, the difference may be explained, at least in part, due to the chosen animal model. For instance, while the LPS-induced sepsis would not be an appropriate model for replicating human sepsis and its immunological response [48], the CLP model may resemble “more closely” human sepsis with its characteristic pro-inflammatory interleukin burst [49, 50]. We used the FS-induced peritonitis, as a sepsis model, which has been compared to CLP model in rats [51]. Among many similarities, the FS-induced peritonitis induces a more severe alterations in coagulation, microcirculation, and multiple organ dysfunction, which was traduced in higher (100% versus 60%, 72 h mortality) and faster mortality (70% versus 40% mortality occurred at 12 h, respectively) than the CLP model. These differences were attributed to the massive release of inflammatory factors in the early stage, characteristic of FS-induced peritonitis. Then, the FS-induced model has been recommended to investigate the acute stage of the sepsis, while the CLP model could be used in the investigation related to the acute inflammatory stage, as well as the immune-inhibition stage [51]. Whether different sepsis models might generate a differential activation of adenosine receptors is largely unknown. Although, we suggest that A2A-dependent inhibition at the early stage of the inflammatory cascade, as using A2AKO mice, might prevent the triggering of the interleukin burst and/or delimitate the dynamic in the synthesis and release of the anti-inflammatory and pro-inflammatory cytokines observed in sepsis.
Another potential issue will be the potential compensatory effect that may generate other adenosine receptors in the A2AKO mice model of FS-induced peritonitis. Particularly, studies have shown that the deletion of either A1 [52] or A2B [23, 53] or A3 [54] adenosine receptors results in impaired survival in response to CLP-induced sepsis. Similarly to A2AKO mice, contradictory findings in A2B receptor–deficient mice (A2BKO) exposed to CLP have been described, showing higher [53] or reduced mortality [23] compared to WT mice. As indicated, previously, differences might be interpreted as methodological variations, as stated by the authors [23] such as variation in the numbers of bacteria in the peritoneal cavity, as well as differentially inflammatory responses. Also, similar to the A2A receptor, activation of the A2B receptor was associated with elevated or decreased levels of inflammatory mediators. Then, the apparent paradox of adenosine receptors to protect or not the host while safeguarding pathogenic bacteria can be explained by the conserved role for adenosine to prevent self-inflicted tissue damage during inflammation. In this regard, despite we did not study the expression of the A2B or any of the other adenosine receptors in our experimental setting, previous evidence in our laboratory have shown no significant changes in the mRNA levels of A1, A2B, or A3 receptor in pulmonary endothelial cells isolated from A2AKO mice compared to cells from WT mice (unpublished results). Future studies should be focused on better understand the crosstalk between the adenosine receptors during sepsis.
The improved clinical condition observed in A2AKO mice at 48 h post-infection might be associated with the high bactericidal capacity of PMN. In particular, neutrophils are an abundant source of adenosine, while activation of A2A receptor reduces the granule release, and oxidative burst, therefore limiting the excessive tissue damage and promotes endothelial barrier function and repair [55]. In this regard, we found reduced PMN infiltration in the liver, associated with less bacterial load (i.e., CFU) in different tissues of A2AKO mice exposed to FS-induced peritonitis. We could speculate that cellular and humoral response might be more effective with less tissue damage in A2AKO mice exposed to FS-induced peritonitis than their WT counterpart. In favor of this last hypothesis, our results indicate increased hepatic cellular apoptosis only in the WT mice exposed to FS-induced peritonitis, suggesting excessive liver compromise in those mice.
Sepsis is also associated with endothelial dysfunction [56], as suggested in our results. In this regard, adenosine and activation of A2A regulate vascular functions including vasorelaxation [26], and angiogenesis [57], which in turn are upregulated in sepsis [58]. Our results showing reduced perfusion levels in sham-treated A2AKO mice might reflect A2A-mediate vasorelaxation [26]. Since high levels of adenosine have been detected in septic patients [13], we can expect that these vascular effects of sepsis could be also upregulated. However, little is known about A2A-mediated vascular effects during sepsis. In our experimental setting, it is not clear, if the drop in peripheral perfusion in septic WT mice is directly caused by A2A receptor activation in the cardiovascular system or whether it is the consequence of hemodynamic failure following a deranged immune response. To better define the role of adenosine, future studies testing how treatment with adenosine (or A2A agonists) affects cutaneous microperfusion in healthy WT mice should be performed.
Using an adenosine-regulating agent, such as acadesine, that increases the bioavailability of adenosine, Zhang et al. [59] showed that treatment with acadesine almost eliminated the deposit of fibrin in the endothelium of venules in the liver of septic mice, confirming that adenosine is an endothelium protective molecule. However, it is unclear whether these protective effects on endothelium are dependent of the A2A receptor. In our experimental setting, we found that A2AKO mice exposed to FS-induced peritonitis have better peripheral perfusion (microcirculation) and less reduction in the blood pressure, suggesting that excessive activation of A2A in the circulatory system would be one of the key participants in the hemodynamic deterioration found in septic mice. Alternatively, less hemodynamic deterioration observed in A2AKO mice exposed to FS-induced peritonitis may be associated with redistribution of blood perfusion to vital organs like the heart, kidneys, and the brain, which is a major adaptive response to increase survival [60].
Our study however has some limitations, due to the combined information gathered from ex vivo and in vivo experiments. For instance, the 48-h time point, which was chosen to analyze bacterial clearance, hemodynamics, and organ damage, is not ideal as it excludes more severely ill mice that die within 24 h. Also, sepsis scores from 24 h up to 48 h after FS-induced peritonitis was not significantly different between WT and A2AKO mice. Then, an earlier time point or serial measurements would have been more informative to describe the response of A2AKO mice to sepsis. Therefore, our results should be interpreted as the response of the surviving animals in front of septic injury. Besides, a trend in reduction of sepsis score was maintained during the whole period of analysis in A2AKO, which suggests that survival advantage may be extended beyond 48 h. Unfortunately, due to experimental design and ethical concerns, we did not analyze survival or other clinical markers beyond 48 h. Despite that, our results present that both kinetics and overall survival was improved in septic A2AKO mice.
In conclusion, the absence of the A2A receptor increases survival in mice exposed to polymicrobial sepsis. This outcome seems to be associated with reduced endothelial dysfunction and liver injury, but the underlying molecular mechanisms remain to be elucidated. Therefore, we encourage further studies to characterize the mechanisms by which adenosine via activation of adenosine receptors such as A2A regulates the immune and hemodynamic response in sepsis, to propose new therapeutic alternatives for the management of this deadly condition.
Acknowledgments
We would like to thank the research staff of the Vascular Physiology Laboratory from the Universidad del Bío-Bío for their technical support. We also thank the researchers from the GRIVAS Health group for the outstanding discussion of the ideas presented in this manuscript.
Abbreviations
- A2A
A2A adenosine receptor
- ADO
Adenosine
- eNOS
Endothelial nitric oxide synthase
- IL
Interleukins
- iNOS
Nitric oxide inducible synthase
- NF- κB
Nuclear factor κB
- NO
Nitric oxide
- TNFα
Tumor necrosis factor α
- VEGF
Endothelial growth factor
Authors’ contributions
This work was carried out as collaboration among all authors. CE defined the research topic. MM and JA performed most of the experiments. SSM and PS performed immunohistochemistry and tissue analysis. KH and FT helped in the animal care and in vivo analysis. MM and CE co-wrote the manuscript. CA, MG, AG, and PT-V critically monitored experimental work and review the manuscript. All authors edited and approved the final version of the manuscript.
Funding information
This study was supported by Fondequip EQM140104, DIUBB 184309 4/R, and GI 171709/VC. CE is currently supported by Fondecyt Regular 1200250.
Data availability
All data is available upon author request.
Compliance with ethical standards
Conflicts of interest
Miguel Meriño declares that he has not conflict of interest.
Sebastián San Martín declares that he has not conflict of interest.
Pedro Sandaña declares that he has not conflict of interest.
Kurt Herlitz declares that he has not conflict of interest.
Claudio Aguayo declares that he has not conflict of interest.
Alejandro Godoy declares that he has not conflict of interest.
Pablo Torres-Vergara declares that he has not conflict of interest.
Marcelo Gonzalez declares that he has not conflict of interest.
Felipe Troncoso declares that he has not conflict of interest.
Jesenia Acurio declares that she has not conflict of interest.
Carlos Escudero declares that he has not conflict of interest.
Ethical approval
All the experiments were performed following the recommendations of the guidelines for the Care and Use of Laboratory Animals published by the US National Institute of Health. The Ethical Committee from the Universidad del Bio Bio (UBB) and FONDECYT (FONDECYT 1140586, Chile) approved the protocol.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Laszlo I, Trasy D, Molnar Z, Fazakas J. Sepsis: from pathophysiology to individualized patient care. J Immunol Res. 2015;2015:510436–510413. doi: 10.1155/2015/510436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The third international consensus definitions for sepsis and septic shock (sepsis-3) Jama. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mayr FB, Yende S, Angus DC. Epidemiology of severe sepsis. Virulence. 2014;5(1):4–11. doi: 10.4161/viru.27372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Ma L, Zhang H, Yin YL, Guo WZ, Ma YQ, Wang YB, Shu C, Dong LQ. Role of interleukin-6 to differentiate sepsis from non-infectious systemic inflammatory response syndrome. Cytokine. 2016;88:126–135. doi: 10.1016/j.cyto.2016.08.033. [DOI] [PubMed] [Google Scholar]
- 6.Steinhauser ML, Hogaboam CM, Kunkel SL, Lukacs NW, Strieter RM, Standiford TJ. IL-10 is a major mediator of sepsis-induced impairment in lung antibacterial host defense. J Immunol. 1999;162(1):392–399. [PubMed] [Google Scholar]
- 7.Hotchkiss RS, Monneret G, Payen D. Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect Dis. 2013;13(3):260–268. doi: 10.1016/s1473-3099(13)70001-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Savio LEB, de Andrade MP, Figliuolo VR, de Avelar Almeida TF, Santana PT, Oliveira SDS, et al. CD39 limits P2X7 receptor inflammatory signaling and attenuates sepsis-induced liver injury. J Hepatol. 2017;67(4):716–726. doi: 10.1016/j.jhep.2017.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsuda N, Hattori Y. Vascular biology in sepsis: pathophysiological and therapeutic significance of vascular dysfunction. J Smooth Muscle Res. 2007;43(4):117–137. doi: 10.1540/jsmr.43.117. [DOI] [PubMed] [Google Scholar]
- 10.Bourgoin A, Leone M, Delmas A, Garnier F, Albanese J, Martin C. Increasing mean arterial pressure in patients with septic shock: effects on oxygen variables and renal function. Crit Care Med. 2005;33(4):780–786. doi: 10.1097/01.CCM.0000157788.20591.23. [DOI] [PubMed] [Google Scholar]
- 11.LeDoux D, Astiz ME, Carpati CM, Rackow EC. Effects of perfusion pressure on tissue perfusion in septic shock. Crit Care Med. 2000;28(8):2729–2732. doi: 10.1097/00003246-200008000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Antonioli L, Fornai M, Blandizzi C, Pacher P, Hasko G. Adenosine signaling and the immune system: when a lot could be too much. Immunol Lett. 2019;205:9–15. doi: 10.1016/j.imlet.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 13.Martin C, Leone M, Viviand X, Ayem ML, Guieu R. High adenosine plasma concentration as a prognostic index for outcome in patients with septic shock. Crit Care Med. 2000;28(9):3198–3202. doi: 10.1097/00003246-200009000-00014. [DOI] [PubMed] [Google Scholar]
- 14.Tawfik HE, Schnermann J, Oldenburg PJ, Mustafa SJ. Role of A1 adenosine receptors in regulation of vascular tone. Am J Physiol Heart Circ Physiol. 2005;288(3):H1411–H1416. doi: 10.1152/ajpheart.00684.2004. [DOI] [PubMed] [Google Scholar]
- 15.Feoktistov I, Goldstein AE, Ryzhov S, Zeng D, Belardinelli L, Voyno-Yasenetskaya T, Biaggioni I. Differential expression of adenosine receptors in human endothelial cells: role of A2B receptors in angiogenic factor regulation. Circ Res. 2002;90(5):531–538. doi: 10.1161/01.RES.0000012203.21416.14. [DOI] [PubMed] [Google Scholar]
- 16.Hasko G, Szabo C, Nemeth ZH, Kvetan V, Pastores SM, Vizi ES. Adenosine receptor agonists differentially regulate IL-10, TNF-alpha, and nitric oxide production in RAW 264.7 macrophages and in endotoxemic mice. J Immunol (Baltimore, Md : 1950) 1996;157(10):4634–4640. [PubMed] [Google Scholar]
- 17.Koshiba M, Rosin DL, Hayashi N, Linden J, Sitkovsky MV. Patterns of A2A extracellular adenosine receptor expression in different functional subsets of human peripheral T cells. Flow cytometry studies with anti-A2A receptor monoclonal antibodies. Mol Pharmacol. 1999;55(3):614–624. [PubMed] [Google Scholar]
- 18.Moore CC, Martin EN, Lee GH, Obrig T, Linden J, Scheld WM. An A2A adenosine receptor agonist, ATL313, reduces inflammation and improves survival in murine sepsis models. BMC Infect Dis. 2008;8:141. doi: 10.1186/1471-2334-8-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sivak KV, Vasin AV, Egorov VV, Tsevtkov VB, Kuzmich NN, Savina VA, et al. Adenosine A2A receptor as a drug target for treatment of sepsis. Mol Biol. 2016;50(2):231–245. doi: 10.7868/s0026898416020233. [DOI] [PubMed] [Google Scholar]
- 20.Sullivan GW, Fang G, Linden J, Scheld WM. A2A adenosine receptor activation improves survival in mouse models of endotoxemia and sepsis. J Infect Dis. 2004;189(10):1897–1904. doi: 10.1086/386311. [DOI] [PubMed] [Google Scholar]
- 21.Li Y, Figler RA, Kolling G, Bracken TC, Rieger J, Stevenson RW, Linden J, Guerrant RL, Warren CA. Adenosine A2A receptor activation reduces recurrence and mortality from Clostridium difficile infection in mice following vancomycin treatment. BMC Infect Dis. 2012;12:342. doi: 10.1186/1471-2334-12-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Németh ZH, Csóka B, Wilmanski J, Xu D, Lu Q, Ledent C, et al. Adenosine A(2A) receptor inactivation increases survival in polymicrobial sepsis. J Immunol (Baltimore, Md : 1950) 2006;176(9):5616–5626. doi: 10.4049/jimmunol.176.9.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Belikoff BG, Hatfield S, Georgiev P, Ohta A, Lukashev D, Buras JA, Remick DG, Sitkovsky M. A2B adenosine receptor blockade enhances macrophage-mediated bacterial phagocytosis and improves polymicrobial sepsis survival in mice. J Immunol. 2011;186(4):2444–2453. doi: 10.4049/jimmunol.1001567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alam MS, Kurtz CC, Wilson JM, Burnette BR, Wiznerowicz EB, Ross WG, Rieger JM, Figler RA, Linden J, Crowe SE, Ernst PB. A2A adenosine receptor (AR) activation inhibits pro-inflammatory cytokine production by human CD4+ helper T cells and regulates Helicobacter-induced gastritis and bacterial persistence. Mucosal Immunol. 2009;2(3):232–242. doi: 10.1038/mi.2009.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang S, Chung CS, Ayala A, Chaudry IH, Wang P. Differential alterations in cardiovascular responses during the progression of polymicrobial sepsis in the mouse. Shock. 2002;17(1):55–60. doi: 10.1097/00024382-200201000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Ponnoth DS, Sanjani MS, Ledent C, Roush K, Krahn T, Mustafa SJ. Absence of adenosine-mediated aortic relaxation in A(2A) adenosine receptor knockout mice. Am J Physiol Heart Circ Physiol. 2009;297(5):H1655–H1660. doi: 10.1152/ajpheart.00192.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li J, Fenton RA, Wheeler HB, Powell CC, Peyton BD, Cutler BS, Dobson JG., Jr Adenosine A2a receptors increase arterial endothelial cell nitric oxide. J Surg Res. 1998;80(2):357–364. doi: 10.1006/jsre.1998.5439. [DOI] [PubMed] [Google Scholar]
- 28.Tofovic SP, Zacharia L, Carcillo JA, Jackson EK. Inhibition of adenosine deaminase attenuates endotoxin-induced release of cytokines in vivo in rats. Shock. 2001;16(3):196–202. doi: 10.1097/00024382-200116030-00005. [DOI] [PubMed] [Google Scholar]
- 29.Fernández Hernández J, Heuze de Icaza YM. El programa interno para el cuidado y uso de los animales de laboratorio en las instituciones biomédicas docentes, de investigación científica e industria farmacéutica. Acta Bioeth. 2007;13:17–24. doi: 10.4067/S1726-569X2007000100003. [DOI] [Google Scholar]
- 30.Naderi MM, Sarvari A, Milanifar A, Boroujeni SB, Akhondi MM. Regulations and ethical considerations in animal experiments: international laws and islamic perspectives. Avicenna J Med Biotechnol. 2012;4(3):114–120. [PMC free article] [PubMed] [Google Scholar]
- 31.Russell WB, RL. The principles of humane experimental technique. London: Methuen; 1959. [Google Scholar]
- 32.van Eijk LT, Dorresteijn MJ, Smits P, van der Hoeven JG, Netea MG, Pickkers P. Gender differences in the innate immune response and vascular reactivity following the administration of endotoxin to human volunteers. Crit Care Med. 2007;35(6):1464–1469. doi: 10.1097/01.ccm.0000266534.14262.e8. [DOI] [PubMed] [Google Scholar]
- 33.Shrum B, Anantha RV, Xu SX, Donnelly M, Haeryfar SM, McCormick JK, et al. A robust scoring system to evaluate sepsis severity in an animal model. BMC Res Notes. 2014;7:233. doi: 10.1186/1756-0500-7-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanders ER. Aseptic laboratory techniques: plating methods. J Vis Exp : JoVE. 2012;63:e3064. doi: 10.3791/3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mena M, Lloveras B, Tous S, Bogers J, Maffini F, Gangane N, Kumar RV, Somanathan T, Lucas E, Anantharaman D, Gheit T, Castellsagué X, Pawlita M, de Sanjosé S, Alemany L, Tommasino M, the HPV-AHEAD study group Development and validation of a protocol for optimizing the use of paraffin blocks in molecular epidemiological studies: the example from the HPV-AHEAD study. PLoS One. 2017;12(10):e0184520. doi: 10.1371/journal.pone.0184520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Masserdotti C. Architectural patterns in cytology: correlation with histology. Vet Clin Pathol. 2006;35(4):388–396. doi: 10.1111/j.1939-165X.2006.tb00154.x. [DOI] [PubMed] [Google Scholar]
- 37.Giachini FR, Carriel V, Capelo LP, Tostes RC, Carvalho MH, Fortes ZB, et al. Maternal diabetes affects specific extracellular matrix components during placentation. J Anat. 2008;212(1):31–41. doi: 10.1111/j.1469-7580.2007.00839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ohta A, Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature. 2001;414(6866):916–920. doi: 10.1038/414916a. [DOI] [PubMed] [Google Scholar]
- 39.Sullivan GW, Linden J, Buster BL, Scheld WM. Neutrophil A2A adenosine receptor inhibits inflammation in a rat model of meningitis: synergy with the type IV phosphodiesterase inhibitor, rolipram. J Infect Dis. 1999;180(5):1550–1560. doi: 10.1086/315084. [DOI] [PubMed] [Google Scholar]
- 40.Varani K, Padovan M, Vincenzi F, Targa M, Trotta F, Govoni M, Borea P. A2A and A3 adenosine receptor expression in rheumatoid arthritis: upregulation, inverse correlation with disease activity score and suppression of inflammatory cytokine and metalloproteinase release. Arthritis Res Ther. 2011;13(6):R197. doi: 10.1186/ar3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Link AA, Kino T, Worth JA, McGuire JL, Crane ML, Chrousos GP, et al. Ligand-activation of the adenosine A2a receptors inhibits IL-12 production by human monocytes. J Immunol (Baltimore, Md : 1950) 2000;164(1):436–442. doi: 10.4049/jimmunol.164.1.436. [DOI] [PubMed] [Google Scholar]
- 42.Lappas CM, Rieger JM, Linden J. A2A adenosine receptor induction inhibits IFN-gamma production in murine CD4+ T cells. J Immunol (Baltimore, Md : 1950) 2005;174(2):1073–1080. doi: 10.4049/jimmunol.174.2.1073. [DOI] [PubMed] [Google Scholar]
- 43.Belikoff B, Hatfield S, Sitkovsky M, Remick DG. Adenosine negative feedback on A2A adenosine receptors mediates hyporesponsiveness in chronically septic mice. Shock (Augusta, Ga) 2011;35(4):382–387. doi: 10.1097/SHK.0b013e3182085f12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khoa ND, Montesinos MC, Reiss AB, Delano D, Awadallah N, Cronstein BN. Inflammatory cytokines regulate function and expression of adenosine A(2A) receptors in human monocytic THP-1 cells. J Immunol (Baltimore, Md : 1950) 2001;167(7):4026–4032. doi: 10.4049/jimmunol.167.7.4026. [DOI] [PubMed] [Google Scholar]
- 45.Csoka B, Nemeth ZH, Virag L, Gergely P, Leibovich SJ, Pacher P, et al. A2A adenosine receptors and C/EBPbeta are crucially required for IL-10 production by macrophages exposed to Escherichia coli. Blood. 2007;110(7):2685–2695. doi: 10.1182/blood-2007-01-065870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Horiguchi H, Loftus TJ, Hawkins RB, Raymond SL, Stortz JA, Hollen MK, et al. Innate immunity in the persistent inflammation, immunosuppression, and catabolism syndrome and its implications for therapy. Front Immunol. 2018;9:595. doi: 10.3389/fimmu.2018.00595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reinhart K, Menges T, Gardlund B, Harm Zwaveling J, Smithes M, Vincent JL, Maria Tellado J, Salgado-Remigio A, Zimlichman R, Withington S, Tschaikowsky K, Brase R, Damas P, Kupper H, Kempeni J, Eiselstein J, Kaul M. Randomized, placebo-controlled trial of the anti-tumor necrosis factor antibody fragment afelimomab in hyperinflammatory response during severe sepsis: the RAMSES Study. Crit Care Med. 2001;29(4):765–769. doi: 10.1097/00003246-200104000-00015. [DOI] [PubMed] [Google Scholar]
- 48.Poli-de-Figueiredo LF, Garrido AG, Nakagawa N, Sannomiya P. Experimental models of sepsis and their clinical relevance. Shock. 2008;30(Suppl 1):53–59. doi: 10.1097/SHK.0b013e318181a343. [DOI] [PubMed] [Google Scholar]
- 49.Starr ME, Steele AM, Saito M, Hacker BJ, Evers BM, Saito H. A new cecal slurry preparation protocol with improved long-term reproducibility for animal models of sepsis. PLoS One. 2014;9(12):e115705. doi: 10.1371/journal.pone.0115705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dejager L, Pinheiro I, Dejonckheere E, Libert C. Cecal ligation and puncture: the gold standard model for polymicrobial sepsis? Trends Microbiol. 2011;19(4):198–208. doi: 10.1016/j.tim.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 51.Fang H, Gong C, Fu J, Liu X, Bi H, Cheng Y, et al. Evaluation of 2 rat models for sepsis developed by improved cecal ligation/puncture or feces intraperitoneal-injection. Med Sci Monit. 2020;26:e919054. doi: 10.12659/MSM.919054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gallos G, Ruyle TD, Emala CW, Lee HT. A1 adenosine receptor knockout mice exhibit increased mortality, renal dysfunction, and hepatic injury in murine septic peritonitis. Am J Physiol Renal Physiol. 2005;289(2):F369–F376. doi: 10.1152/ajprenal.00470.2004. [DOI] [PubMed] [Google Scholar]
- 53.Csoka B, Nemeth ZH, Rosenberger P, Eltzschig HK, Spolarics Z, Pacher P, et al. A2B adenosine receptors protect against sepsis-induced mortality by dampening excessive inflammation. J Immunol. 2010;185(1):542–550. doi: 10.4049/jimmunol.0901295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee HT, Kim M, Joo JD, Gallos G, Chen JF, Emala CW. A3 adenosine receptor activation decreases mortality and renal and hepatic injury in murine septic peritonitis. Am J Physiol Regul Integr Comp Physiol. 2006;291(4):R959–R969. doi: 10.1152/ajpregu.00034.2006. [DOI] [PubMed] [Google Scholar]
- 55.Barletta KE, Ley K, Mehrad B. Regulation of neutrophil function by adenosine. Arterioscler Thromb Vasc Biol. 2012;32(4):856–864. doi: 10.1161/ATVBAHA.111.226845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peters K, Unger RE, Brunner J, Kirkpatrick CJ. Molecular basis of endothelial dysfunction in sepsis. Cardiovasc Res. 2003;60(1):49–57. doi: 10.1016/S0008-6363(03)00397-3. [DOI] [PubMed] [Google Scholar]
- 57.Acurio J, Herlitz K, Troncoso F, Aguayo C, Bertoglia P, Escudero C. Adenosine A(2A) receptor regulates expression of vascular endothelial growth factor in feto-placental endothelium from normal and late-onset pre-eclamptic pregnancies. Purinergic Signal. 2017;13(1):51–60. doi: 10.1007/s11302-016-9538-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Suzuki T, Hashimoto S, Toyoda N, Nagai S, Yamazaki N, Dong HY, Sakai J, Yamashita T, Nukiwa T, Matsushima K. Comprehensive gene expression profile of LPS-stimulated human monocytes by SAGE. Blood. 2000;96(7):2584–2591. doi: 10.1182/blood.V96.7.2584. [DOI] [PubMed] [Google Scholar]
- 59.Zhang W, Wang J, Wang H, Tang R, Belcher JD, Viollet B, Geng JG, Zhang C, Wu C, Slungaard A, Zhu C, Huo Y. Acadesine inhibits tissue factor induction and thrombus formation by activating the phosphoinositide 3-kinase/Akt signaling pathway. Arterioscler Thromb Vasc Biol. 2010;30(5):1000–1006. doi: 10.1161/ATVBAHA.110.203141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.De Backer D, Orbegozo Cortes D, Donadello K, Vincent JL. Pathophysiology of microcirculatory dysfunction and the pathogenesis of septic shock. Virulence. 2014;5(1):73–79. doi: 10.4161/viru.26482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen JF, Huang Z, Ma J, Zhu J, Moratalla R, Standaert D, et al. A(2A) adenosine receptor deficiency attenuates brain injury induced by transient focal ischemia in mice. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1999;19(21):9192–200. doi: 10.1523/JNEUROSCI.19-21-09192.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data is available upon author request.