Abstract
Introduction
The spatial position and dimensions of oral and pharyngeal soft tissues change post-mandibular advancement (MA) surgery which involves changes in position of soft palate, tongue and associated musculature. There is no study which simultaneously evaluates changes in tongue length and height post-MA surgery and correlates these changes with changes in upper airway dimensions and the amount of MA.
Materials and Methods
Treatment records of 18 patients that underwent MA with bilateral sagittal split ramus osteotomy were evaluated at T1 (01 week before surgery) and T2 (06 months post-surgery). Linear airway and tongue measurements were done on lateral cephalogram. Mean volume and mean pharyngeal area values were recorded from the acoustic pharyngometry (AP) records of patients.
Results
A statistically significant increase in tongue length (P value < 0.001) and nonsignificant change in tongue height were observed at T2 (P value > 0.05). A statistically significant increase in airway parameters recorded on both lateral cephalogram and AP was observed at T2 (P value < 0.001). Correlation analysis did not show a statistically significant correlation of change in tongue length and tongue height at T2 with the amount of MA, change in airway parameters on lateral cephalogram and AP (P value > 0.05).
Conclusions
Mandibular advancement surgery is a viable option for improvement in pharyngeal airway in skeletal Class II patients with retrognathic mandible. Changes in tongue length observed in our study may correspond to the stretch of protruders of tongue, especially genioglossus, and may point toward possible relapse on a long-term follow-up.
Keywords: Mandibular advancement (MA), Obstructive sleep apnea (OSA), Airway, BSSRO
Introduction
Orthognathic surgeries involving maxilla and/or mandible are mainly performed for bringing about positive improvement in facial and smile aesthetics of the patient apart from correction of various facial deformities [1, 2]. Maxillomandibular advancement by orthognathic surgery/distraction osteogenesis (DO) is a well-documented treatment modality for the management of obstructive sleep apnea (OSA) secondary to hypoplastic/retruded maxilla and/or mandible. This treatment modality brings positive improvement in airway by causing physical expansion of the pharyngeal hard and soft tissues [3].
The surgeries involving mandibular setback (MS) are well known to cause detrimental effects on upper airway [4, 5]. The literature reveals that the spatial position of oral and pharyngeal soft tissues also changes post-surgery which involves changes in the position of soft palate, tongue and associated musculature [6, 7]. The surgeries involving mandibular advancement (MA) cause forward positioning of the tongue [4, 5]. This increases the retropharyngeal airway space and may be helpful in individuals with compromised airway.
Few studies have been reported in the literature documenting the changes in tongue dimensions and position post-surgery in MA cases [1, 2, 4, 8]. There is no study which concurrently associates these tongue changes with the altered pharyngeal airway dimensions and amount of MA. Keeping this background in mind, the present study was conducted to evaluate the changes in tongue height and length post-MA surgery and simultaneously correlate these changes with changes in upper airway dimensions.
Materials and Methods
Study Design
Retrospective analytical study.
Study Sample
This study was carried out at the Department of Orthodontics and Dentofacial Orthopedics of a tertiary care teaching institution. The study sample included treatment records of 18 patients randomly selected from the institutional archives that underwent MA surgery with bilateral sagital split ramus osteotomy (BSSRO) between Jan 01, 2014, and Dec 31, 2016, and met the inclusion criteria of the study. Lateral cephalogram used in this study was recorded with a standardized technique using the same machine (model: ADVAPX cephalostat machine, company: Panorraitic System, printer: Fujifilms DRY PIX 7000). Acoustic pharyngometry (AP) was used as a noninvasive tool for evaluating and comparing area and volumetric changes in airway post-surgery. AP for all patients was recorded by the same operator with ECCOVISION® Acoustic Pharyngometer™ using the standard protocol.
Inclusion Criteria
Adult patients aged 18–28 years (both the sex).
Complete set of pre- and post-treatment orthodontic records available with minimum 06-month follow-up.
The impacted mandibular third molars removed minimum 06 months prior to surgery.
Cases who underwent only MA with BSSRO surgery without genioplasty.
Skeletal Class II cases with ANB ≥ 4° and overjet ≥ 4 mm.
Nonextraction cases with crowding/spacing ≤ 5 mm.
Exclusion Criteria
Cleft/syndromic/patients with neuromuscular disorders/psychiatric patients.
Patients with history of recurrent pharyngeal infections, enlarged tonsils/adenoids, or any other medical condition compromising airway.
History of previous ortho-surgical treatment or trauma to jaw bones.
Presence of ankyloglossia or any other disorder affecting tongue morphology and/or mobility.
Study Design and Data Collection
The selected cases (18 patients, 10 males and 08 females) underwent MA with BSSRO surgery and were treated with a standardized ortho-surgical treatment protocol at the institute. Pre- and postsurgical orthodontics was carried out using 0.022 MBT pre-adjusted edgewise appliance with standard wire sequence protocol.
The lateral cephalogram and AP were recorded at two time frames:
- T1
01 week before surgery.
- T2
06 months post-surgery.
The lateral cephalograms were manually traced, and pharyngeal airway and tongue measurements were done at T1 and T2 (Table 1; Fig. 1). The pharyngeal airway dimensions (mean volume and mean area) were recorded at T1 and T2 from the AP records of the patients. The amount of MA performed was recorded from the case sheets of the patients. Changes in the above-mentioned parameters were measured at T2 to know the changes in tongue and pharyngeal airway dimensions post-surgery. The data were collected and compiled in MS Excel work sheet and were subjected to statistical analysis.
Table 1.
Airway and tongue parameters studied on lateral cephalogram
| S. no. | Parameter | Measurement |
|---|---|---|
| 1. | Superior pharyngeal airway space (SAS) | Linear distance measured from tip of the soft palate (TSP) to the nearest pharyngeal wall (PW) |
| 2. | Posterior airway space (PAS) | Linear distance from posterior margin of the base of the tongue to the nearest pharyngeal wall on Gonion-Point B (Go-B) line |
| 3. | Minimum/hypopharyngeal airway space (MAS) | Minimum linear distance measured from point V (intersection of epiglottis and base of the tongue) to the nearest pharyngeal wall |
| 4. | Tongue length (TL) | Measured from point V to tip of the tongue (TT) |
| 5. | Tongue height (TH) | Perpendicular distance from the highest point on the superior surface of the tongue (H) to V-TT line |
Fig. 1.
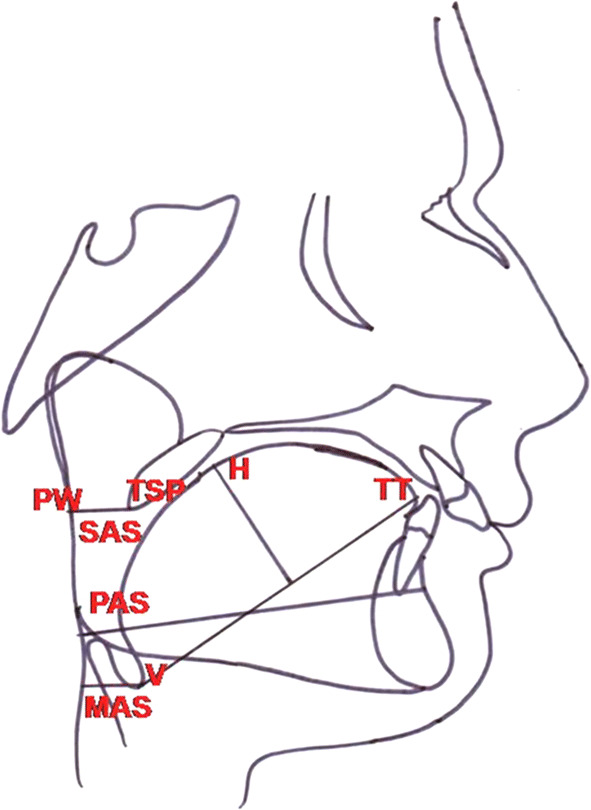
Landmarks used on lateral cephalogram
Statistical Analysis
The entire data were statistically analyzed using Statistical Package for Social Sciences (SPSS ver 21.0, IBM Corporation, USA) for MS Windows. The data on continuous variables were presented as mean and standard deviation (SD). The statistical comparison of continuous variables was done using paired t test. Pearson’s correlation was carried out to study the correlations among the parameters studied. The underlying normality assumption was tested before subjecting each variable to t test and Pearson’s correlation analysis. All the results are shown in tabular as well as graphical format (using Box–Whisker plot) to visualize the statistically significant difference more clearly. In the entire study, the P values < 0.05 were considered to be statistically significant. All the hypotheses were formulated using two-tailed alternatives against each null hypothesis (hypothesis of no difference).
Results
Post-surgical Changes in Parameters Studied (Table 2)
Table 2.
Postsurgical changes in parameters studied
| Parameter | Mean | SD |
|---|---|---|
| Tongue length (mm) | ||
| Pre-op | 69.22 | 1.39 |
| Post-op | 70.56 | 1.88 |
| P value | ||
| Pre versus post | 0.002** | |
| Tongue height (mm) | ||
| Pre-op | 29.33 | 2.06 |
| Post-op | 29.00 | 1.58 |
| P value | ||
| Pre versus post | 0.524NS | |
| SAS (mm) | ||
| Pre-op | 12.22 | 1.39 |
| Post-op | 13.22 | 1.56 |
| P value | ||
| Pre versus post | 0.003** | |
| PAS (mm) | ||
| Pre-op | 11.67 | 1.73 |
| Post-op | 13.56 | 1.81 |
| P value | ||
| Pre versus post | 0.002** | |
| MAS (mm) | ||
| Pre-op | 18.78 | 4.35 |
| Post-op | 20.06 | 4.45 |
| P value | ||
| Pre versus post | 0.013* | |
| Mean vol (mm3) | ||
| Pre-op | 25.11 | 5.04 |
| Post-op | 34.48 | 3.70 |
| P value | ||
| Pre versus post | 0.001*** | |
| Mean area (mm2) | ||
| Pre-op | 2.53 | 0.51 |
| Post-op | 3.45 | 0.37 |
| P value | ||
| Pre versus post | 0.001*** |
Values are mean and SD, P values by paired t test
NS statistically nonsignificant
*P value < 0.05; **P value < 0.01; ***P value < 0.001
The distribution of mean post-op (T2) tongue length (70.56 mm, SD = 1.88 mm) was significantly higher compared to mean pre-op (T1) tongue length (69.22 mm, SD = 1.39 mm). The mean increase in tongue length was 1.33 mm (SD = 0.87 mm), and this was statistically significant (P value < 0.001).
The distribution of mean post-op tongue height (29 mm, SD = 1.58) did not differ significantly compared to mean pre-op tongue height (29.33 mm, SD = 2.06 mm). The mean decrease in tongue height was 0.33 mm with a SD of 1.50 mm, but this was not statistically significant (P value > 0.05).
The distribution of mean post-op airway parameters on lateral cephalogram (SAS, PAS and MAS) was significantly higher compared to mean pre-op airway parameters (P value < 0.001 for all).
The distribution of mean post-op parameters on AP (mean volume and mean area) was significantly higher compared to mean pre-op parameters (P value < 0.001 for all).
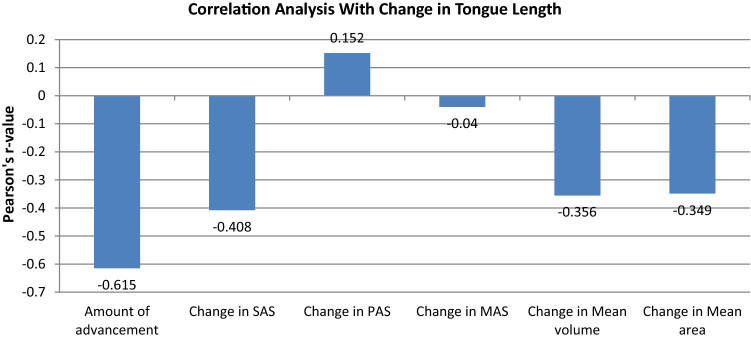
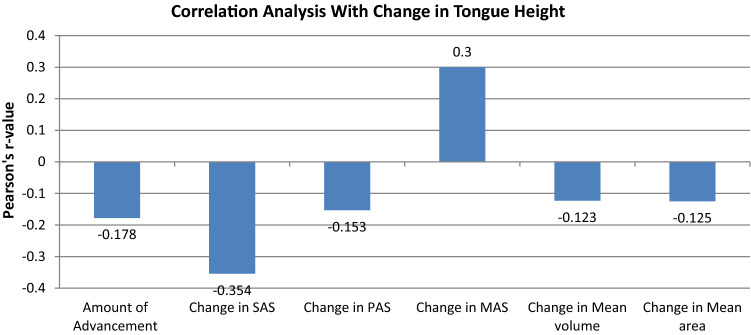
Correlation Analysis of Parameters Studied with Change in Tongue Parameters (Table 3, Figs. 2, 3)
Table 3.
Correlation analysis of parameters studied with change in tongue parameters
| Tongue parameter | Correlation with | r value | P value |
|---|---|---|---|
| Change in tongue length | Amount of advancement | − 0.615 | 0.078NS |
| Change in SAS | − 0.408 | 0.275NS | |
| Change in PAS | 0.152 | 0.697NS | |
| Change in MAS | − 0.040 | 0.919NS | |
| Change in mean volume | − 0.356 | 0.347NS | |
| Change in mean area | − 0.349 | 0.357NS | |
| Change in tongue height | Amount of advancement | − 0.178 | 0.647NS |
| Change in SAS | − 0.354 | 0.351NS | |
| Change in PAS | − 0.153 | 0.694NS | |
| Change in MAS | 0.300 | 0.432NS | |
| Change in mean volume | − 0.123 | 0.752NS | |
| Change in mean area | − 0.125 | 0.749NS |
Correlation analysis by Pearson’s method
NS statistically nonsignificant
P value < 0.05 is considered to be statistically significant correlation
*P value < 0.05; **P value < 0.01; ***P value < 0.001
Fig. 2.
Correlation analysis of parameters studied with change in tongue length
Fig. 3.
Correlation analysis of parameters studied with change in tongue height
The correlation analysis revealed that change in tongue length (Fig. 2) and tongue height (Fig. 3) after surgery (at T2) did not show statistically significant correlation with the amount of MA, change in airway parameters (PAS, SAS, MAS) on lateral cephalogram and change in AP parameters (mean volume and mean area) in the study sample (P value > 0.05 for all).
Discussion
Various studies have demonstrated relationship of oral and pharyngeal soft tissues with the craniofacial and dentofacial structures. Any change in the skeletal tissues due to growth, functional appliance therapy or orthognathic surgery may cause spatial and dimensional changes in the associated pharyngeal soft tissues (e.g., soft palate and tongue) [9–12].
The tongue is an active and functional muscular organ situated in the floor of the mouth. It is attached to the lingual surface (genial tubercles) of the mandible, hyoid bone, epiglottis and soft palate by various muscular attachments [13, 14]. Various studies have shown that the root of the tongue is more posteriorly positioned in skeletally class II subjects as compared to Class I and Class III subjects. This compromises the pharyngeal airway, especially in the PAS and MAS region [14, 15]. MA causes forward positioning of the tongue with a positive impact on airway. This may also lead to changes in tongue dimensions. Genioglossus muscle, which is the primary protruder muscle of the tongue, gets stretched during MA [16]. This stretch may cause changes in tongue length and height, and this may also be a cause of relapse in future. Based on these inputs, this study was designed to evaluate the changes in length and height of the tongue post-MA and also to find correlation between these changes and airway parameters assessed on lateral cephalogram and AP.
The literature reveals that lateral cephalogram is a reliable tool in determining airway dimensions [17, 18] with efficacy comparable to computed tomography (CT) scans [19]. A study [20] revealed that majority of the airway landmarks can be reliably identified on a lateral cephalogram. Also, it is routinely advised to all orthodontic patients; hence, no additional cost and radiation exposure are incurred to the patients. Therefore, lateral cephalogram was used in our study to evaluate tongue and linear airway dimensions.
AP is a noninvasive modality based on acoustic reflection technique for assessment of airway dimensions and also to ascertain changes post-MA. It can be recorded chair side at the orthodontic clinic, and its reliability is comparable to CT scans [21]. Therefore, AP was used to assess changes in mean volume and mean area in our study.
A statistically significant increase in tongue height on a 06-month follow-up after MA has been observed in one study [22]. The change in tongue length was not statistically significant in this study at a 06-month follow-up. These findings are not in agreement with our study wherein a significant increase in tongue length has been observed on a 06-month follow-up. The change in tongue height was, however, not statistically significant in our study. Prospective studies with a larger sample size may throw more light in this regard.
Studies have shown that the tongue area remains unchanged after the orthognathic surgery which provides a more functional space to the tongue making it to assume a more forward position [23]. This anterior positioning of the tongue may be a reason for increased tongue length in our study as the anterior portion of the tongue moves forward during MA with stretching of the genioglossus muscle.
The authors could not find any study which correlates changes in tongue length and height with the amount of MA. There is no literature available which correlates the altered tongue dimensions post-MA with airway parameters. AP was additionally used in our study to evaluate changes in mean airway volume and area, and these changes were also correlated with the altered tongue dimensions. The correlation analysis revealed that change in tongue length and height at T2 did not show statistically significant correlation with the amount of MA, change in airway parameters on lateral cephalogram and changes in mean volume and mean area evaluated by AP. A study [24] observed mean anterior tongue displacement of 5.8 mm by MA with DO and greater tongue displacement when DO was accompanied with genioplasty. The tongue displacement showed a strong correlation with the increase in PAS width and anterior displacement of hyoid bone. A statistically significant increase in PAS and other linear, area and volumetric parameters was also observed in our study at T2. But in our study, the parameters were correlated with tongue height and length rather than tongue displacement.
One study [25] observed no significant correlation between the pattern of tongue deformation on a dynamic MRI and craniofacial structures (changes in tongue volume, changes in airway volume and changes in SNA and SNB angles). These results are similar to our study, except that AP and lateral cephalogram were used to evaluate airway instead of dynamic MRI and these parameters were correlated with changes in tongue height and length instead of tongue deformation. Changes in SNA and SNB angles were not evaluated in our study.
Conclusions
From the findings of this study, it can be concluded that expansion of the skeletal tissues shows corresponding changes in the oropharyngeal soft tissues. Mandibular advancement surgery is a viable option for improvement in pharyngeal airway in skeletal Class II patients with retrognathic mandible. The changes in tongue length observed in our study may correspond to the stretch of protruders of the tongue, especially genioglossus, and may point toward relapse on a long-term follow-up. Prospective studies with a larger sample size and a long-term follow-up are required to validate the findings of this study.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no competing interests.
Ethics Statement
The study design was approved by the institutional ethical committee.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Eggensperger N, Smolka W, Iizuka T. Long term changes of hyoid bone position and pharyngeal airway size following mandibular setback by sagittal split ramus osteotomy. J Cranio Maxillo Fac Surg. 2005;33:111–117. doi: 10.1016/j.jcms.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Sahoo NK, Roy ID, Kulkarni V. Mandibular setback and its effects on speech. Oral Maxillofac Surg Cases. 2018 doi: 10.1016/j.omsc.2018.10.001. [DOI] [Google Scholar]
- 3.Zaghi S, Holty CEJ, Certal V, et al. Maxillomandibular advancement for treatment of obstructive sleep apnea: a meta-analysis. JAMA Otolaryngol Head Neck Surg. 2016;142(1):58–66. doi: 10.1001/jamaoto.2015.2678. [DOI] [PubMed] [Google Scholar]
- 4.Marsan G, Oztas E, Cura N, Kuvat SV, Emekli U. Changes in head posture and hyoid bone position in Turkish Class III patients after mandibular setback surgery. J Cranio Maxillo Fac Surg. 2010;38:113–121. doi: 10.1016/j.jcms.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 5.Chen F, Terada K, Hua Y, Saito I. Effect of bimaxillary surgery and mandibular setback surgery on pharyngeal airway measurements in patients with class III skeletal deformities. Am J Dentofac Orthop. 2007;131:372–377. doi: 10.1016/j.ajodo.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 6.Guven O, Saracoglu U. Changes in pharyngeal airway space and hyoid bone positions after body ostectomies and sagittal split ramus osteotomies. J Craniofac Surg. 2005;16:23–30. doi: 10.1097/00001665-200501000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Hasebe D, Kobayashi T, Hasegawa M, et al. Changes in oropharyngeal airway and respiratory function during sleep after orthognathic surgery in patients with mandibular prognathism. Int J Oral Maxillofac Surg. 2011;40:584–592. doi: 10.1016/j.ijom.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 8.Yoshida K, Rivera GA, Matsuo N, et al. Long-term prognosis of BSSO mandibular relapse and its relation to different facial types. Angle Orthod. 2000;70:220–226. doi: 10.1043/0003-3219(2000)070<0220:LTPOBM>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 9.Tangugsorn V, Skatvedt O, Krogstad O, Lyberg T. Obstructive sleep apnea: a cephalometric study. Part I. Cervico-craniofacial skeletal morphology. Eur J Orthod. 1995;17:45–56. doi: 10.1093/ejo/17.1.45. [DOI] [PubMed] [Google Scholar]
- 10.Tsuchiya M, Lowe AA, Pae EK, Fleetham JA. Obstructive sleep apnea subtypes by cluster analysis. Am J Orthod Dentofac Orthop. 1992;101:533–542. doi: 10.1016/0889-5406(92)70128-W. [DOI] [PubMed] [Google Scholar]
- 11.Kaur S, Rai S, Sinha A, Ranjan V, Mishra D, Panjwani S. A lateral cephalogram study for evaluation of pharyngeal airway space and its relation to neck circumference and body mass index to determine predictors of obstructive sleep apnea. J Indian Acad Oral Med Radiol. 2015;27:2–8. doi: 10.4103/0972-1363.167062. [DOI] [Google Scholar]
- 12.Turvey TA, Hall DJ. Alteration in nasal airway resistance following superior repositioning of the maxilla. Am J Orthod Dentofac Orthop. 1984;45:109–114. doi: 10.1016/0002-9416(84)90002-2. [DOI] [PubMed] [Google Scholar]
- 13.Zhou L, Zhao Z, Lu D. The analysis of the changes of tongue shape and position, hyoid position in Class II, division 1 malocclusion treated with functional appliances (FR-I) Hua Xi Kou Qiang Yi Xue Za Zhi. 2000;18(2):123–125. [PubMed] [Google Scholar]
- 14.Tseng Y, Wu J, Chen C, Hsu K. Correlation between change of tongue area and skeletal stability after correction of mandibular prognathism. Kaohsiung J Med Sci. 2017 doi: 10.1016/j.kjms.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 15.Yamaoka M, Furusawa K, Uematsu T, Okafuji N, Kayamoto D, Kurihara S. Relationship of the hyoid bone and posterior surface of the tongue in prognathism and micrognathia. J Oral Rehabil. 2003;30:914–920. doi: 10.1046/j.1365-2842.2003.01069.x. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi S, Kuribayashi G, Ono T, Ishiwata Y, Kuroda T. Modulation of masticatory muscle activity by tongue position. Angle Orthod. 2005;75:35–39. doi: 10.1043/0003-3219(2005)075<0035:MOMMAB>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 17.Malkoc S, Usumez S, Nur M, Donaghy CE. Reproducibility of airway dimensions and tongue and hyoid positions on lateral cephalograms. Am J Orthod Dentofac Orthop. 2005;128:513–516. doi: 10.1016/j.ajodo.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Aboudara C, Nielsen I, Huang JC, Maki K, Miller AJ, Hatcher D. Comparison of airway space with conventional lateral headfilms and 3 dimensional reconstruction from cone beam computed tomography. Am J Orthod Dentofac Orthop. 2009;135:468–479. doi: 10.1016/j.ajodo.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 19.Riley RW, Powell NB, Guilleminault C. Maxillary, mandibular, and hyoid advancement for treatment of obstructive sleep apnea: a review of 40 patients. J Oral Maxillofac Surg. 1990;48:20–26. doi: 10.1016/0278-2391(90)90174-Z. [DOI] [PubMed] [Google Scholar]
- 20.Miles PG, O’Reilly M, Close J. The reliability of upper airway landmark identification. Aust Orthod J. 1995;14:3–6. [PubMed] [Google Scholar]
- 21.Agarwal SS, Jayan B, Kumar S. Therapeutic efficacy of a hybrid mandibular advancement device in the management of obstructive sleep apnea assessed with acoustic reflection technique. Indian J Dent Res. 2015;26:86–89. doi: 10.4103/0970-9290.156820. [DOI] [PubMed] [Google Scholar]
- 22.Achilleos S, Krogstad O, Lyberg T. Surgical mandibular advancement and changes in uvuloglossopharyngeal morphology and head posture: a short- and long-term cephalometric study in males. Eur J Orthod. 2000;22:367–381. doi: 10.1093/ejo/22.4.367. [DOI] [PubMed] [Google Scholar]
- 23.Turnbull NR, Battagel JM. The effects of orthognathic surgery on pharyngeal airway dimensions and quality of sleep. J Orthod. 2000;27:235–247. doi: 10.1179/ortho.27.3.235. [DOI] [PubMed] [Google Scholar]
- 24.Sriram SG, Andrade NN. Cephalometric evaluation of the pharyngeal airway space after orthognathic surgery and distraction osteogenesis of the jaw bones. Indian J Plast Surg. 2014;47:346–353. doi: 10.4103/0970-0358.146589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lowth A, Juge L, Knapman F, et al. Dynamic MRI tongue deformation patterns during mandibular advancement and associations with craniofacial anatomy in OSA. J Sleep Res. 2018 doi: 10.1111/jsr.169_12766. [DOI] [Google Scholar]