Abstract
CD39 and CD73 are ecto-nucleotidases present on human peripheral blood mononuclear cells (PBMCs) and are emerging biomarkers on these cells in various disorders including cancer. Many factors influence PBMC quality, so it is essential to validate sample processing methods prior to incorporation in clinical studies. This study examined the impact of both PBMC cryopreservation and PBMC isolation using SepMate density gradient centrifugation on CD39 and CD73 expressing subsets. First, PBMCs were isolated from the peripheral blood of 11 healthy donors by routine Ficoll-Paque density gradient centrifugation, cryopreserved and compared with freshly isolated PBMCs by flow cytometry. The proportions of T and B cells expressing combinations of CD39 and CD73 were relatively stable over 6-month cryopreservation, although some T cell combinations revealed small but significant changes. Second, peripheral blood was collected from six healthy donors to compare PBMCs isolated by SepMate or Ficoll-Paque density gradient centrifugation. Compared with Ficoll-Paque, the more rapid SepMate method yielded 9.1% less PBMCs but did not alter cell viability or proportions of T and B cells expressing combinations of CD39 and CD73. The present study reveals that cryopreservation is suitable for studying T and B cells expressing combinations of CD39 and CD73. However, caution should be exercised when observing small differences in these cryopreserved subsets between different cohorts. Further, SepMate and Ficoll-Paque methods of PBMC isolation show similar results for T and B cell subset analysis; however, SepMate is a faster and easier approach.
Keywords: Ecto-enzymes, ENTPD1, NT5E, Purinergic signalling, Purinergic receptor, Liquid biopsy
Introduction
CD39 and CD73 are ectonucleotidases expressed on the surface of immune and other cells types and together mediate the hydrolysis of extracellular adenosine triphosphate (ATP) into adenosine [1, 2]. Specifically, CD39, an ectonucleoside triphosphate diphosphohydrolase, hydrolyses ATP and adenosine diphosphate (ADP) to adenosine monophosphate (AMP) [1]. CD73, an ecto-5′-nucleotidase, then hydrolyses AMP to adenosine on the cell surface [2]. This adenosine-producing CD39/CD73 pathway has roles in many disorders, including cancer [3, 4], myocardial ischemia [5], graft-versus-host disease [6, 7], and acquired immunodeficiency syndrome [8]. Thus, these molecules may be potential biomarkers of disease outcome or treatment response.
Analysis of CD39 and CD73 in the blood is of particular interest, as blood collection is minimally invasive and suitable for incorporation into routine clinical testing and compared with tissue biopsies, is less susceptible to selection biases [9, 10]. Moreover, peripheral blood mononuclear cells (PBMCs), which comprise T and B cells, as well as innate lymphocyte and myeloid subsets, are relatively easy to isolate from whole blood and provide valuable information concerning the immunological status of the host [11]. In particular, there is emerging evidence to support the importance of CD39 and/or CD73 as biomarkers on PBMCs in cancer. For example, the proportion of CD4+CD39+ T cells is increased in chronic lymphocytic leukaemia patients and associated with advanced stage of the disease [12]. Lower baseline percentage of CD8+PD-1+CD73+ T cells is associated with overall survival and clinical benefit to anti-PD-1 immunotherapy (Nivolumab) in metastatic melanoma patients [13]. Furthermore, higher proportions of CD4+CD73+ and CD8+CD73+ T cells represent an exhausted phenotype in head and neck squamous cell carcinoma, whereby, T cells lose effector functions and express inhibitory receptors [14]. Finally, CD39+CD73+ B cells, through adenosine production, may influence the function of effector lymphocytes in head and neck squamous cell carcinoma [15]. Together these finding support the notion that the proportions of immune cells expressing CD39 and CD73 represent potential biomarkers and can be identified using flow cytometric approaches.
The origins of the routine isolation of human PBMCs by Ficoll-Paque density gradient centrifugation (Ficoll-Paque) can be traced back to 1968 [16]. However, density gradient separation requires careful handling and prolonged centrifugation to minimise mixing of layers, so there have been a number of modern refinements to improve ease of isolation and reduce time of processing. One such example is the development of SepMate PBMC isolation tubes, by STEMCELL Technologies (Vancouver, Canada), which includes an insert to allow faster preparation times. STEMCELL Technologies also produces an alternative to Ficoll-Paque density gradient medium, called Lymphoprep, which is routinely used with SepMate PBMC isolation tubes. However, studies comparing PBMCs isolated by SepMate density gradient centrifugation (SepMate) to those isolated by Ficoll-Paque density gradient centrifugation are limited [17], and to the best of our knowledge, none have compared the impact of these two techniques on CD39+ and CD73+ cell types.
Cryopreservation is the mainstay of long-term sample storage and is often necessary in large pre-clinical and clinical studies being conducted at single or multiple sites, where the analysis of fresh PBMCs is not always practical [11]. Cryopreserved PBMCs allow for the batch analyses of samples, reducing intralaboratory and interlaboratory variability, while optimising the use of resources [18, 19]. However, cryopreservation is a potentially destructive process, having the potential to induce phenotypic and/or functional changes to PBMCs [20]. Therefore, it is important to validate that cryopreservation does not influence cell subsets or cell molecules of interest or to at least be aware of any such changes and any potential limitations in analysis of such samples. There have been a small number of studies that have evaluated the effect of cryopreservation on PBMC populations. Studies have shown that proportions of major lymphocyte populations, including CD3+, CD4+ and CD8+ T cells, and CD19+ B cells remain relatively stable during cryopreservation when compared with freshly isolated PBMCs [19–21]. However, other subpopulations, such as naive (CD45RA+CD62L+) and central memory (CD45RO+CD62L+) T cells, have been shown to decrease during cryopreservation [20]. Conversely, proportions of CD39+CD4+CD25+ T cells and CD39+CD4+CD25hi regulatory T cells, but not other subtypes of CD39+ regulatory T cells, increased following cryopreservation [19]. To the best of our knowledge, there are no studies reporting the impact of cryopreservation on other CD39+ cell types or any CD73+ cell types.
The current study aimed to determine the impact of cryopreservation of human PBMCs on T and B cells, including CD39 and CD73 expressing subsets. The study also aimed to determine if PBMCs isolated by SepMate density gradient centrifugation are equivalent to those isolated by Ficoll-Paque density gradient centrifugation, with a focus on T and B cells, including CD39 and CD73 expressing subsets.
Materials and methods
Materials
Dulbecco’s phosphate buffered solution (D-PBS) was from Thermo Fisher Scientific (Waltham, USA). Ficoll-Paque was from GE Healthcare (Uppsala, Sweden). SepMate and Lymphoprep were from STEMCELL Technologies (Vancouver, Canada). RPMI-1640 medium (Life Technologies; Carlsbad, USA) was prepared at the Illawarra Health and Medical Research Institute (Wollongong, Australia). Foetal calf serum (FCS) (heat-inactivated before use) was from Bovogen Biologicals (East Keilor, Australia). Trypan blue solution, dimethyl sulfoxide (DMSO) and isopropanol were from Sigma-Aldrich (St. Louis, USA). The Zombie NIR Fixable Viability Kit was from BioLegend (San Diego, USA). Fluorochrome-conjugated monoclonal antibodies (mAbs) (Table 1) were from BD Bioscience (San Jose, USA).
Table 1.
The mAb for flow cytometric analyses of leukocytes
| CD molecule | Fluorochrome | Clone | Catalogue numbera | Excitation wavelength (nm) | Detection filter (nm) |
|---|---|---|---|---|---|
| CD3 | BV711 | UHCT1 | 563725 | 405 | 710/50 |
| CD19 | PerCP-Cy5.5 | HIB19 | 561295 | 488 | 695/40 |
| CD39 | PE | TU66 | 555464 | 561 | 586/15 |
| CD45 | APC | HI30 | 555485 | 640 | 670/30 |
| CD73 | PE-Cy7 | AD2 | 561258 | 561 | 780/60 |
Abbreviations: BV, Brilliant Violet; PerCP-Cy5.5, peridinin-chlorophyll-protein-cyanine5.5; PE, phycoerythrin; APC, allophycocyanin; PE-Cy7, phycoerythrin-cyanine7
aAll mAb from BD Bioscience
Human blood collection
All experiments with human blood were conducted in accordance with approval by the University of Wollongong Human Research Ethics Committee (Wollongong, Australia) and with informed, signed consent from donors. For experiments investigating the impact of cryopreservation, peripheral blood was collected from 11 healthy human donors (mean age of 28, range 21–49, 36% female) by venepuncture into Vacutainer lithium heparin tubes (BD Bioscience). For experiments comparing SepMate and Ficoll-Paque density gradient centrifugation, peripheral blood was collected from six healthy human donors (mean age of 23, range 21–27, 0% female) as above. All blood was processed within 4 h of collection.
Human PBMC isolation by Ficoll-Paque density gradient centrifugation
Whole blood was diluted with an equal volume of D-PBS (at room temperature), underlaid with Ficoll-Paque (at room temperature), then centrifuged (600×g, 30 min, 21 °C) on a Heraeus Multifuge X3R (Thermo Fisher Scientific) with acceleration set to five and deceleration set to zero (all other centrifugation steps were performed with acceleration set to nine and deceleration set to nine unless stated otherwise). PBMCs were collected from the Ficoll-Paque-plasma interface into a 50 mL tube (Greiner Bio-One; Frickenhausen, Germany) and washed (500×g, 10 min, 4 °C) with RPMI-1640 medium containing 10% FCS (RPMI-FCS) before being resuspended in RPMI-FCS. PBMCs were enumerated and assessed for viability, using trypan blue and a Boeco haemocytometer (Hamburg, Germany).
Human PBMC isolation by SepMate density gradient centrifugation
Lymphoprep (at room temperature) was pipetted into a SepMate tube via the insert hole according to the manufacturer’s instructions. Whole blood was diluted with an equal volume of D-PBS (at room temperature), gently added down the inside of the tube, then centrifuged (1200×g, 10 min, 21 °C) on a Heraeus Multifuge X3R with acceleration and deceleration both set to nine. Plasma and PBMCs were poured into a separate 50 mL tube and washed (500×g, 10 min, 4 °C) with RPMI-FCS before being resuspended in RPMI-FCS. PBMCs were enumerated and assessed for viability as above.
Cryopreservation of PBMCs
For cryopreservation of PBMCs, isolated cells were centrifuged (400×g, 10 min, 4 °C) and resuspended in RPMI-FCS at a concentration of 6–10 × 106 cell/mL. Cells were then diluted with an equal volume of ice-cold freeze down medium (RPMI-1640 medium containing 40% FCS and 20% DMSO) in a dropwise fashion with continuous mixing. Aliquots of 1 mL were transferred to Cryo.S tubes (Greiner Bio-One), placed in a Nalgene Cryo 1 °C Freezing Container (Nalgene Nunc, Rochester, USA) with isopropanol and frozen overnight at − 80 °C, before being transferred to liquid nitrogen. Following 1-, 3- or 6-month storage, PBMCs were thawed in a 37 °C water bath with constant swirling. Thawed samples were then washed with RPMI-FCS (300×g, 5 min, 21 °C) and resuspended in RPMI-FCS and assessed for viability as above.
Immunophenotyping by flow cytometry
Freshly isolated and thawed PBMCs were washed with D-PBS (300×g, 3 min, 21 °C) and incubated with Zombie Dye NIR for 15 min at room temperature in the dark. Cells were then washed with D-PBS containing 2% FCS and centrifuged (300×g, 3 min, 21 °C). Cells were then incubated with fluorochrome-conjugated mAbs (Table 1) for 15 min at room temperature in the dark. Fluorescence minus one (FMO)-stained samples were included for CD39 and CD73, in which mAbs to these respective molecules were omitted from the panels. FMO-stained samples were used to assist with the setting of flow cytometry gates for these markers in combination with gating regions from previous studies of CD39 and CD73 on CD3+ T cells [22, 23] and CD19+ B cells [24]. Cells were then washed with D-PBS (300×g, 3 min, 21 °C) and a total of 5 × 104 live lymphocytes were acquired using a BD LSR Fortessa X-20 flow cytometer and BD FACSDiva software v8.0. Zombie dye NIR was detected using an excitation of 640 nm and a 780/60-nm band pass filter. Excitation and band pass filter wavelengths for each fluorochrome-conjugated mAb are listed in Table 1. The relative percentages of lymphocyte subsets were analysed with FlowJo software v8.7.1 (TreeStar Inc., USA), using consistent gating strategies.
Statistics
Data is represented as mean ± standard deviation (SD). Normality of data was assessed using Shapiro-Wilk test. Normally distributed data was compared using a paired Student’s t test. Non-normally distributed data was compared using a Wilcoxon matched pairs test. All statistical analyses and graphs were generated using GraphPad Prism software version 8.0 (GraphPad Software; La Jolla, USA). For all analyses, differences were considered significant if P < 0.05.
Results
Proportions of CD39+/−CD73+/− T and B cell subsets in freshly isolated PBMCs
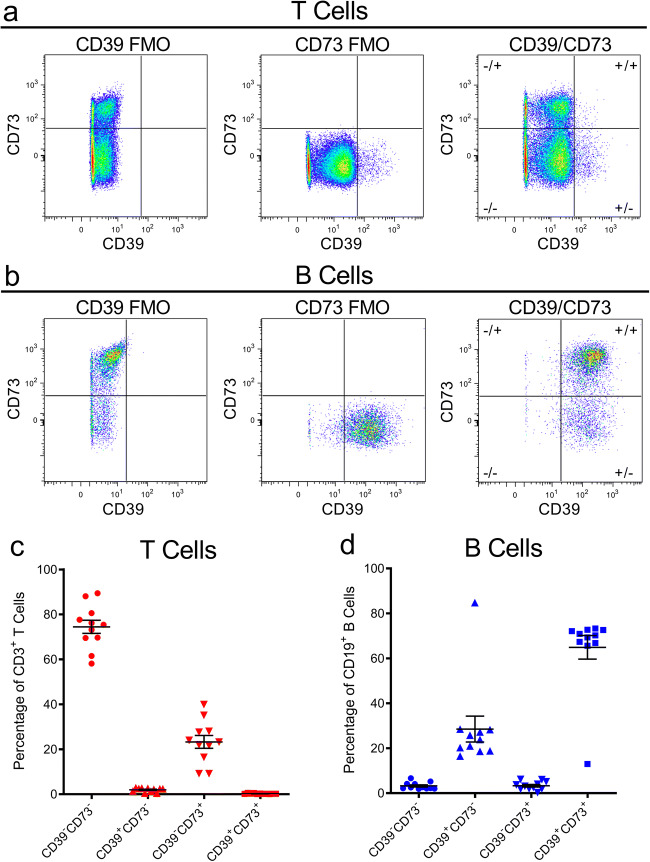
Before comparing the effect of cryopreservation on CD39 and CD73 on T and B cells, the proportion of CD3+ T cells and CD19+ B cells amongst CD45+ cells in freshly isolated PBMCs from 11 healthy donors were assessed by flow cytometry (Fig. 1a), to ensure the expected proportion of these cells were present. In addition, this data provided the basis for comparison with cryopreserved PBMCs. The mean proportion of CD3+ T cells in the freshly isolated PBMCs was 68.4 ± 7.4%, while the mean proportion of CD19+ B cells was 11.4 ± 4.2% (Fig. 1b) Both values are comparable with previous studies [25].
Fig. 1.
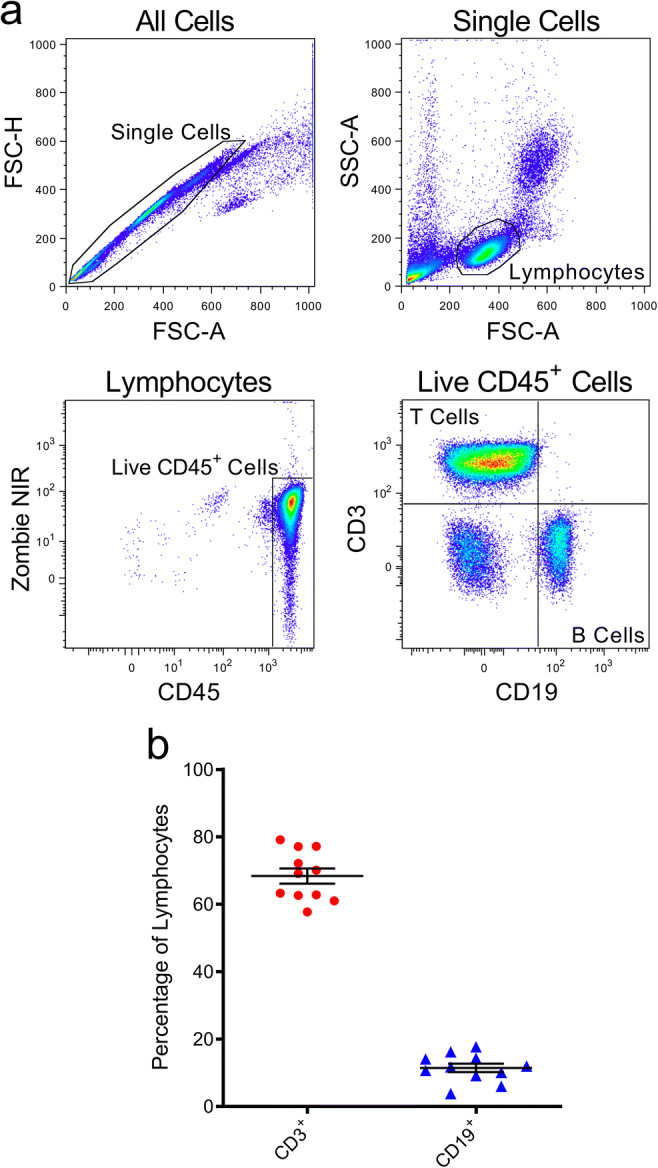
Proportions of T and B cells in freshly isolated PBMCs. a, b PBMCs, freshly isolated by Ficoll-Paque density gradient centrifugation, were labelled with fluorochrome-conjugated mAbs and data acquired by flow cytometry. a Sequential flow cytometric gates were selected left to right (top to bottom), as shown, to gate single cells, lymphocytes, and live (Zombie NIR−) CD45+ lymphocytes, in order to determine b proportions of CD3+CD19− T cells and CD3−CD19+ B cells amongst CD45+ cells. Data represents group means ± SD (n = 11 donors); symbols represent individual donors
Next, before comparing the effects of cryopreservation on CD39 and CD73 on T and B cells, the proportions of T and B cells expressing combinations of these ectonucleotidases in freshly isolated PBMCs were assessed by flow cytometry using the representative gating strategies for T cells (Fig. 2a) and B cells (Fig. 2b). Amongst CD3+ T cells, the mean proportions of CD39−CD73−, CD39+CD73−, CD39−CD73+ and CD39+CD73+ T cells were 74.5 ± 9.7, 2.0 ± 0.9, 23.3 ± 9.5 and 0.3 ± 0.2%, respectively (Fig. 2c). Amongst CD19+ B cells, the mean proportions of CD39−CD73−, CD39+CD73−, CD39−CD73+ and CD39+CD73+ B cells were 3.3 ± 1.6, 28.5 ± 19.1, 3.3 ± 1.9 and 64.9 ± 17.4%, respectively (Fig. 2d).
Fig. 2.
Proportions of CD39+/−CD73+/− T and B cell subsets in freshly isolated PBMCs. a–d PBMCs, freshly isolated by Ficoll-Paque density gradient centrifugation, were labelled with fluorochrome-conjugated mAbs and data acquired by flow cytometry. a, b Cells were gated as shown in Fig. 1, to identify a CD3+CD19− T cells and b CD3−CD19+ B cells, with fluorescence minus one (FMO)-stained samples used to set CD39 and CD73 quadrants, to determine the proportions of c CD39+/−CD73+/− T cells and d CD39+/−CD73+/− B cells. c, d Data represents group means ± SD (n = 11 donors); symbols represent individual donors
One of the donors displayed unusual proportions of CD39+CD73− and CD39+CD73+ B cells compared with the other ten donors. The relative proportion of CD39+CD73− B cells was 3.7-fold higher compared with the mean for all the other donors, whilst the proportion of cells CD39+CD73+ was 81.4% lower than the mean for all the other donors (Fig. 2d), suggesting an abnormal CD73 phenotype. The unusual CD73 phenotype was not observed in the T cells from this or any other donor (Fig. 2d). To ensure this unusual phenotype in the donor was not an artefact or due to experimental error, freshly isolated PBMCs were re-obtained from this donor, with similar results to the initial observations (data not shown).
Cryopreservation causes minor yet significant changes to proportions of CD39+/−CD73+/− T cells, while CD39+/−CD73+/− B cells remain relatively unaltered
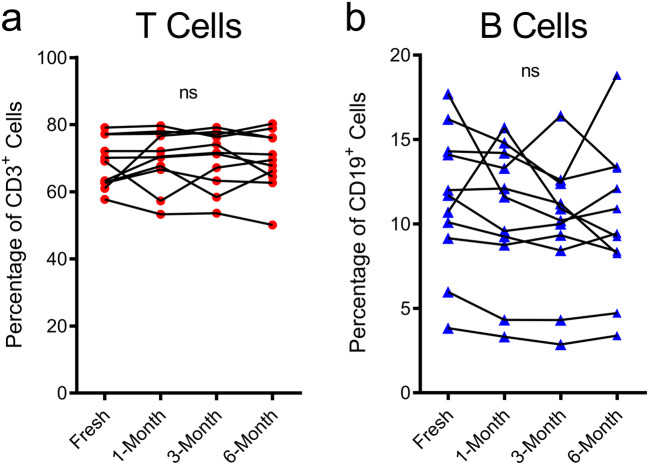
To determine the effect of cryopreservation on CD39 and CD73, the flow cytometric analyses of freshly isolated PBMCs were compared with PBMCs cryopreserved for 1-, 3- and 6 months from the 11 donors above. The proportions of CD3+ T cells and CD19+ B cells were first compared between fresh and cryopreserved samples. The same flow cytometric gating strategies were applied as outlined above (Figs. 1a and 2a, b). The proportions of CD3+ T cells (Fig. 3a) or CD19+ B cells (Fig. 3b) amongst CD45+ cells were not significantly altered by cryopreservation at any of the time points examined.
Fig. 3.
Proportions of CD3+ T cells and CD19+ B cells are not altered by cryopreservation. a, b PBMCs, freshly isolated by Ficoll-Paque density gradient centrifugation and corresponding PBMCs cryopreserved for 1-, 3- and 6 months were labelled with fluorochrome-conjugated mAbs, and the proportions of a CD3+CD19− T cells and b CD3−CD19+ B cells amongst CD45+ cells were determined by flow cytometry. a, b Connected symbols represent individual donors (n = 11); P < 0.05 compared with corresponding freshly isolated PBMCs indicates significance; ns, no significances; paired two-tailed Student’s t test
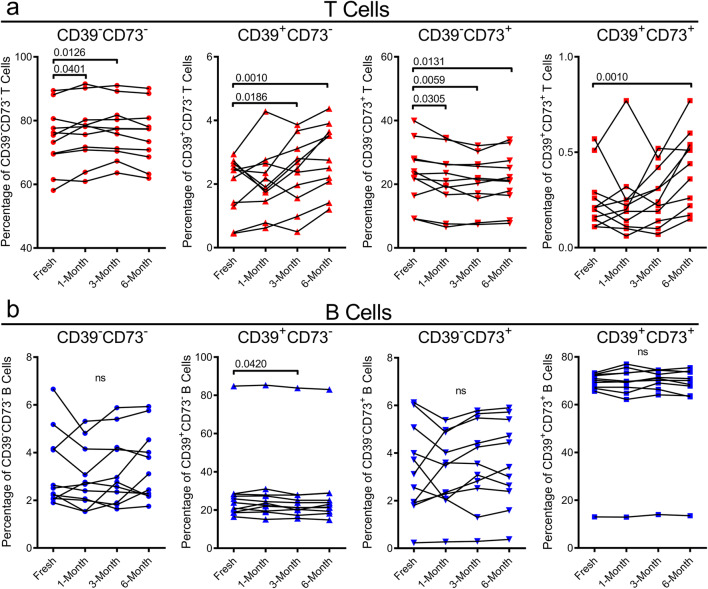
Amongst CD3+ T cells, minor but statistically significant changes to the relative proportions of CD39−CD73−, CD39+CD73−, CD39−CD73+ or CD39+CD73+ T cells were observed at some time points. There was a statistically significant 1.03-fold increase in the proportion of CD39−CD73− T cells at both 1- and 3-month cryopreservation (P = 0.0401 and P = 0.0126, respectively), with no significant change at 6 months (Fig. 4a). In the CD39+CD73− T cells, there were 1.20- and 1.44-fold increases following 3- and 6-month cryopreservation (P = 0.0186 and P = 0.0010, respectively) (Fig. 4a). Conversely, in the CD39−CD73+ T cell population, there were significant reductions at 1-month (8.4%), 3-month (11.9%) and 6-month (8.5%) cryopreservation (P = 0.0305, P = 0.0059 and P = 0.0131, respectively) (Fig. 4a). Proportions of CD39+CD73+ T cells significantly increased 1.63-fold at 6-month cryopreservation (P = 0.0010) (Fig. 4a).
Fig. 4.
Cryopreservation causes minor yet significant changes to proportions of CD39+/−CD73+/− T cells, while CD39+/−CD73+/− B cells remain relatively unaltered. a, b PBMCs, freshly isolated by Ficoll-Paque density gradient centrifugation and corresponding PBMCs cryopreserved for 1-, 3- and 6 months were labelled with fluorochrome-conjugated mAbs, and the proportions of a CD39+/−CD73+/− T cells and b CD39+/−CD73+/− B cells were determined by flow cytometry. Connected symbols represent individual donors (n = 11); P < 0.05 compared with corresponding freshly isolated PBMCs indicates significance; ns, no significances; paired two-tailed Student’s t test or Wilcoxon matched-pairs signed rank test
Amongst CD19+ B cells, proportions of CD39−CD73−, CD39−CD73+ or CD39+CD73+ B cells remained unchanged (Fig. 4b). In the CD39+CD73− B cell population, there was a minor but statistically significant 3.1% reduction following 3-month cryopreservation (P = 0.0420), but no significant change at 1 or 6 months (Fig. 4b).
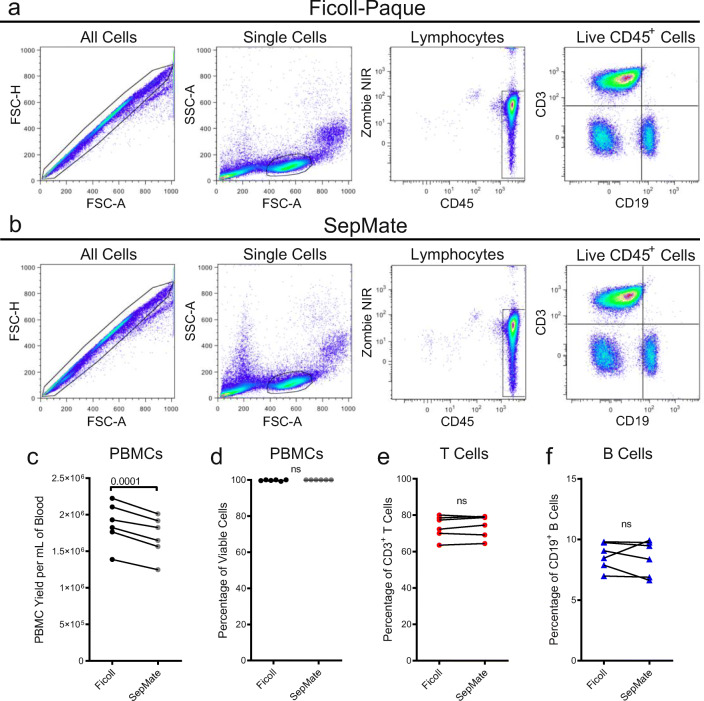
SepMate yields less PBMCs but does not influence cell viability or the proportion of T and B cells compared with Ficoll-Paque
Peripheral blood was then collected from six healthy donors, and equal volumes of blood from each donor were separated by density gradient centrifugation using either Ficoll-Paque or SepMate tubes with Lymphoprep. Representative gating strategies for PBMCs isolated by Ficoll-Paque (Fig. 5a) and SepMate (Fig. 5b) showed no major differences in cell populations in the flow cytometric panels assessed between the two PBMC preparations. Cell counts and viability, assessed using a haemocytometer and trypan blue staining, demonstrated that the SepMate procedure resulted, on average, in a statistically significant 9.1% lower PBMC yield per mL of blood (P = 0.0001) (Fig. 5c), but similar cell viabilities (Fig. 5d) compared with Ficoll-Paque. Next, the proportion of T and B cells amongst CD45+ cells in freshly isolated PBMCs were assessed by flow cytometry. The mean proportion of CD3+ T cells (Fig. 5e) and CD19+ B cells (Fig. 5f) did not significantly differ between the two techniques.
Fig. 5.
SepMate density gradient centrifugation yields less PBMCs but does not influence cell viability or proportion of T and B cells compared with Ficoll-Paque. a–f Peripheral blood was collected from six healthy donors, and equal volumes were used to separate PBMCs by Ficoll-Paque and SepMate density gradient centrifugation. c Cell numbers and d cell viabilities were assessed using trypan blue and a haemocytometer. a, b, e, f Cells were labelled with fluorochrome-conjugated mAbs and data acquired by flow cytometry. a, b Sequential flow cytometric gates were selected left to right, as shown, to gate single cells, lymphocytes, live (Zombie NIR−) CD45+ lymphocytes, in order to determine proportions of e CD3+CD19− T cells and f CD3−CD19+ B cells amongst CD45+ cells. a, b A representative gating strategy of PBMCs isolated by a Ficoll-Paque or b SepMate density gradient centrifugation is shown. c, e, f Connected or d individual symbols represent individual donors (n = 6); P < 0.05 indicates significance; ns, no significances; paired two-tailed Student’s t test
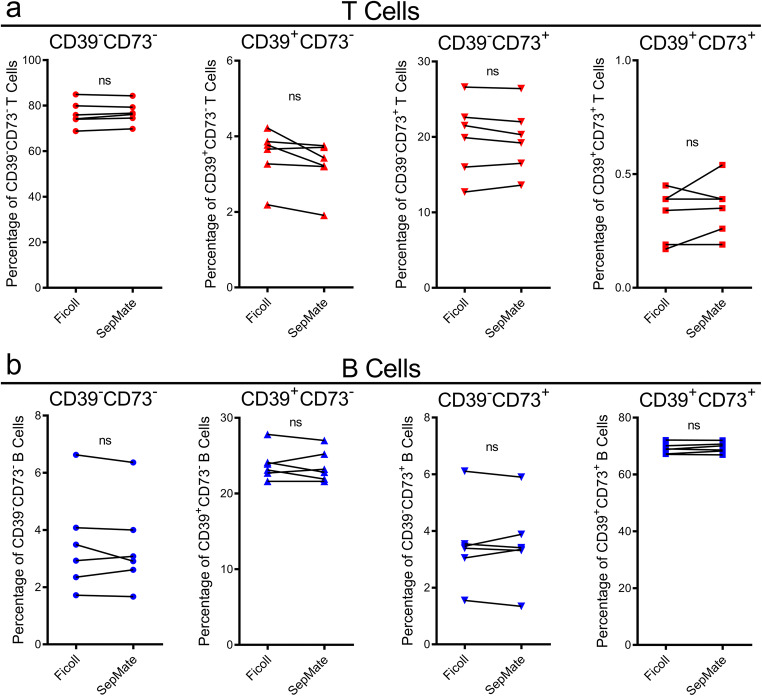
Ficoll-Paque and SepMate yields similar proportions of CD39+/−CD73+/− T and B cells subsets
Next, the proportions of CD39+/−CD73+/− T and B cells were assessed on the samples analysed above (Fig. 5). Amongst CD3+ T cells, the proportions of CD39−CD73−, CD39+CD73−, CD39−CD73+ or CD39+CD73+ cells were similar and not significantly different between Ficoll-Paque- and SepMate-isolated PBMCs (Fig. 6a). The mean proportions of CD39−CD73−, CD39+CD73−, CD39−CD73+ and CD39+CD73+ T cells in PBMCs isolated using Ficoll-Paque were 76.3 ± 5.5, 3.5 ± 0.7, 19.9 ± 4.9 and 0.3 ± 0.1%, respectively (Fig. 6a). While the mean proportions of CD39−CD73−, CD39+CD73−, CD39−CD73+ and CD39+CD73+ T cells in PBMCs isolated using SepMate were 76.8 ± 4.8, 3.2 ± 0.7, 19.7 ± 4.4 and 0.4 ± 0.1%, respectively (Fig. 6a).
Fig. 6.
Ficoll-Paque and SepMate density gradient centrifugation yield similar proportions of CD39+/−CD73+/− T and B cell subsets. a, b PBMCs freshly isolated using Ficoll-Paque and SepMate density gradient centrifugation were incubated with fluorochrome-conjugated mAbs and the proportions of a CD39+/−CD73+/− T cells and b CD39+/−CD73+/− B cells were determined by flow cytometry. Connected symbols represent individual donors (n = 6); P < 0.05 indicates significance; ns, no significances; paired two-tailed Student’s t test
Amongst CD19+ B cells, the mean proportions of CD39−CD73−, CD39+CD73−, CD39−CD73+ and CD39+CD73+ B cells in PBMCs isolated using Ficoll-Paque were 3.5 ± 1.7, 23.9 ± 2.1, 3.5 ± 1.5 and 69.1 ± 1.9%, respectively (Fig. 6b). The use of SepMate did not significantly alter proportions of these subsets, with mean proportions of CD39−CD73−, CD39+CD73−, CD39−CD73+ and CD39+CD73+ B cells being 3.4 ± 1.6, 23.6 ± 2.1, 3.5 ± 1.5 and 69.4 ± 1.9%, respectively (Fig. 6b).
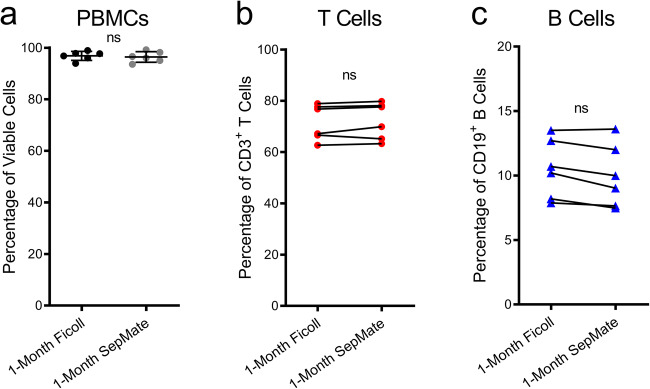
Ficoll-Paque and SepMate yields similar viabilities and proportions of T and B cells in 1-month cryopreserved PBMCs
Aliquots of PBMCs, isolated by either Ficoll-Paque or SepMate density gradient centrifugation above, were cryopreserved for 1 month, and the viability of cells upon thawing were compared. There was no significant difference in cell viabilities between the two techniques, with Ficoll-Paque- and SepMate-isolated PBMCs displaying mean viabilities of 96.9 ± 1.8 and 96.4 ± 2.1%, respectively (Fig. 7a). Next, the proportion of T and B cells amongst CD45+ cells in thawed PBMCs were compared by flow cytometry. Comparison of cryopreserved PBMCs originally isolated by either technique revealed no significant differences in the proportions of CD3+ T cells (Fig. 7b) or CD19+ B cells (Fig. 7c).
Fig. 7.
Ficoll-Paque and SepMate density gradient centrifugation yield similar viabilities and proportions of T and B cells in 1-month cryopreserved PBMCs. a–c At time of isolation, aliquots of PBMCs were cryopreserved for 1 month. Upon thawing, cell viabilities were assessed using trypan blue and a haemocytometer. b, c PBMCs were incubated with fluorochrome-conjugated mAbs and the proportions of b CD3+CD19− T cells and c CD3−CD19+ B cells of CD45+ cells were determined by flow cytometry. a Bars represent group means, and a individual or b, c connected symbols represent individual donors (n = 6); P < 0.05 indicates significance; ns, no significances; paired two-tailed Student’s t test
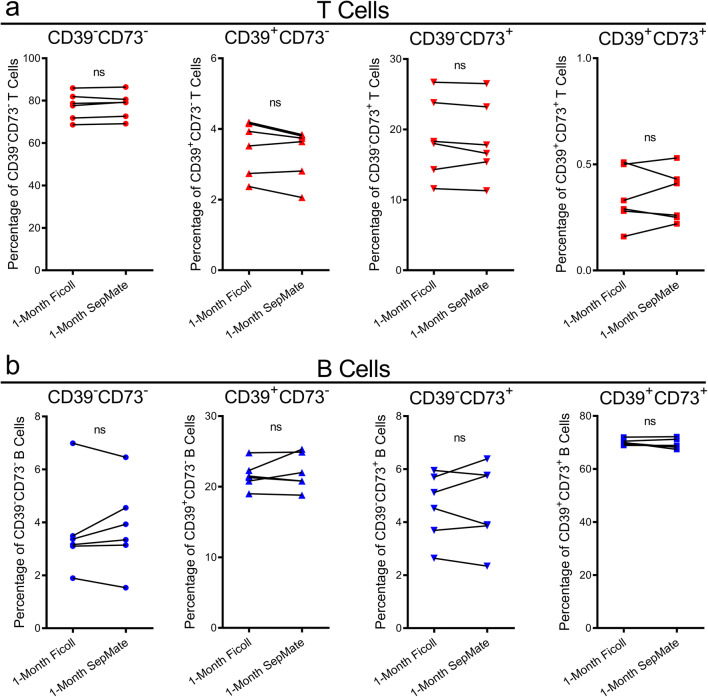
Ficoll-Paque and SepMate yield similar proportions of CD39+/−CD73+/− T and B cell subsets in 1-month cryopreserved PBMCs
Finally, the proportions of CD39+/−CD73+/− T and B cells were assessed on the cryopreserved samples analysed above (Fig. 7). Amongst CD3+ T cells, the proportions of CD39−CD73−, CD39+CD73−, CD39−CD73+ or CD39+CD73+ cells did not differ between cryopreserved PBMCs originally isolated by Ficoll-Paque or SepMate (Fig. 8a). Similarly, amongst CD19+ B cells, the proportions of CD39−CD73−, CD39+CD73−, CD39−CD73+ or CD39+CD73+ cells did not differ between cryopreserved PBMCs originally isolated by the two techniques (Fig. 8b).
Fig. 8.
Ficoll-Paque and SepMate density gradient centrifugation yield similar proportions CD39+/−CD73+/− T and B cells in 1-month cryopreserved PBMCs. a, b At time of isolation, aliquots of PBMCs were cryopreserved for 1 month. Upon thawing, PBMCs were incubated with fluorochrome-conjugated mAbs and the proportions of a CD39+/−CD73+/− T cells and b CD39+/−CD73+/− B cells were determined by flow cytometry. a, b Connected symbols represent individual donors (n = 6); P < 0.05 indicates significance; ns, no significances; paired two-tailed Student’s t test or Wilcoxon matched-pairs signed rank test
Discussion
The first part of this study aimed to determine if the proportion of T or B cells expressing combinations of CD39 and CD73 varies during cryopreservation. This study revealed that the proportions of T and B cell-expressing combinations of CD39 and CD73 were relatively stable over 6-month cryopreservation, although some T cell combinations revealed small but significant changes. Results also showed that proportions of CD3+ T cells and CD19+ B cells were unaltered over 6-month cryopreservation. This observation is consistent with previous findings from two studies, which also demonstrated no changes in the proportions of these cells cryopreserved for up to 12 months [19, 21]. Finally, the proportions of T and B cells in freshly isolated PBMCs were similar to previous reports [25]. Likewise, the proportions of CD3+ T cells (or CD4+ and CD8+ T cells combined) and CD19+ B cells expressing combinations of CD39 and CD73 were similar to previous reports [22, 23, 26–32]. These results validate the isolation and flow cytometric techniques used in the current study to investigate proportions of T and B cells expressing combinations of CD39 and CD73.
This study indicates that cryopreservation over 6 months has a minor yet statistically significant influence on the proportions of T cells expressing CD39 and/or CD73, while proportions of these molecules on B cells remained relatively unaltered. The increase in the relatively small proportion of CD39+ T cells within PBMCs at later time points parallels a previous study that revealed increased proportions of both CD39+CD4+CD25+ T cells and CD39+CD4+CD25hi regulatory T cells following 12 months of cryopreservation [19]. Thus, it remains possible that the increases in CD39+ T cells in the current study represent changes to specific but minor T cell populations rather than changes to all CD3+ T cells. This previous study [19] and one other study [33] examining CD39 on cryopreserved PBMCs did not report any data concerning CD39+CD3+ T cells or CD39+CD19+ B cells, while the stability of CD73+ T cells or CD73+ B cells following cryopreservation is yet to be reported.
Beyond the explanation above, it remains unknown why cryopreservation induces small changes in the proportions of T cells expressing CD39 and/or CD73. Cryopreservation may alter CD39 and/or CD73 expression. The increase in the proportion of CD39+CD73+ and CD39+CD73− T cells, and decrease in CD39−CD73+, may be due to certain minor subpopulations of T cells having enhanced susceptibility to apoptosis by cryopreservation and an impaired ability to recover during subsequent thawing [19, 34]. Apoptosis can be induced by both physical and physiological stimuli, and sensitivity is known to differ between lymphocyte subsets [19]. The observed changes in CD39 and CD73 expression may also be due to conformational changes induced by the presence of DMSO and/or the thawing process, altering binding of the respective mAbs [35]. However, this seems unlikely as changes in T cells were not observed in the B cells within the same PBMC preparation, although structural differences in CD39 or CD73, as a result of differences in glycosylation, for example, between T and B cells cannot be excluded.
The second part of this study aimed to determine if SepMate density gradient centrifugation could isolate PBMCs equivalent to those isolated by Ficoll-Paque density gradient centrifugation. This study demonstrated that SepMate achieves equivalent viabilities, and proportions of T and B cells, including combinations of CD39 and CD73 subsets, in freshly isolated and 1-month cryopreserved PBMCs. Our results also revealed that PBMC yields following SepMate density gradient centrifugation were slightly but significantly lower than the following Ficoll-Paque density gradient centrifugation. This contrasts with prior observations, where a study reported that SepMate achieved 1.3-fold higher PBMC yields compared with Ficoll-Paque [17]. This may be due to variations in the Ficoll-Paque protocols used for PBMC isolation. For example, Ficoll-Paque density gradient centrifugation in the current study was performed at 600×g for 30 min, while this step was performed at 400×g for 40 min by Grievink et al. [17]. Although the temperature of samples, separation media or centrifuges can each affect PBMC yields [36], major differences in the temperature of these elements do not explain the discrepancy in PBMC yields between the two studies as isolations in both studies (including reagents) were performed (and used) at room temperature.
The current study also demonstrates that SepMate density gradient centrifugation achieves statistically equivalent proportions of PBMC populations compared with Ficoll-Paque density gradient centrifugation. Specifically, the current study reports no significant differences in proportion of CD3+ T cells and CD19+ B cells, including combinations of CD39 and CD73 subsets. The aforementioned study [17] also revealed no significant differences in CD3+ T cells and CD20+ B cells between the two isolation techniques. Of note, CD20 was used to identify B cells [17], providing further evidence that the proportions of B cells are not altered by either isolation method. The current study is the first to compare potential differences in the proportion of the T and B cells expressing CD39 and CD73 in PBMCs isolated by either isolation method. Furthermore, it was demonstrated that SepMate density gradient centrifugation does not influence the viability or proportion of PBMCs following 1-month cryopreservation compared with those isolated by Ficoll-Paque density gradient centrifugation. Specifically, no significant differences in proportion of CD3+ T cells or CD19+ B cells, including combinations of CD39 and CD73 subsets were observed.
The current study confirms that SepMate isolation of PBMCs is a much faster isolation procedure than Ficoll-Paque. The time taken to isolate PBMCs using SepMate was routinely 45 min less than Ficoll-Paque. This is largely attributed to the time difference in the centrifugation steps, with SepMate density gradient centrifugation taking 10 min in total and Ficoll-Paque density gradient centrifugation taking 45 min in total. In addition, the SepMate insert afforded the ability to quickly overlay blood without layer mixing and to simply decant PBMCs by pouring into a new tube. Although SepMate proved to be faster and easier than Ficoll-Paque, consumables are approximately twice the cost for the same volume of blood. This price difference however is reversed if labour costs are accounted for.
A major limitation of the present study is that CD39 and CD73 were examined on CD3+ T cells but not T cell subsets. Of value would have been the inclusion of CD4+ and CD8+ T cells, including regulatory and memory T cell subsets within these populations. Moreover, the inclusion of other minor T cell subsets, such as Vδ1+ T cells, would have also been of interest. Recent comparisons revealed significantly higher proportions of CD39+Vδ1+ T cells and significantly lower proportions of CD73+Vδ1+ T cells in PBMCs of people with breast cancer compared with PBMCs from healthy donors [37]. Another limitation of the present study was that it was restricted to PBMCs and did not include mononuclear cells isolated from tissues. Given the central role of adenosinergic signalling pathways in cancer [38], for example, it will be of future importance to examine the stability of tumour-infiltrating lymphocytes, including CD39 and CD73 expression, following different isolation techniques, as well as cryopreservation.
In conclusion, the proportions of T and B cells expressing combinations of CD39 and CD73 are relatively stable over 6-month cryopreservation, although some T cell combinations revealed small but significant changes. The current study also indicated that SepMate density gradient centrifugation can be used as a faster and easier alternative to Ficoll-Paque density gradient centrifugation when studying fresh or cryopreserved T and B cells, including subsets expressing CD39 and/or CD73. The study, however, revealed that SepMate results in slightly lower PBMC yields, which may be a minor limitation if blood sample volumes are small. Collectively, the present study reveals that cryopreservation and Ficoll-Paque or SepMate density gradient isolation of human PBMCs are suitable for studying T and B cells expressing combinations of CD39 and CD73. However, caution should be exercised when observing small differences in these cryopreserved subsets between different cohorts. Results from this study will provide a useful resource for future studies collecting and biobanking PBMCs for the potential identification of biomarkers in diseases with an immune component including cancer.
Acknowledgements
The authors thank all blood donors involved in this study and the Illawarra Health and Medical Research Institute technical officers for technical support.
Funding information
This project was funded by Molecular Horizons (University of Wollongong, Wollongong) and the Radiation Oncology Trust Fund (Illawarra Cancer Care Centre, Wollongong).
Compliance with ethical standards
Conflict of interest
Ross J. Turner declares that he has no conflict of interest.
Nicholas J. Geraghty declares that he has no conflict of interest.
Jonathan G. Williams declares that he has no conflict of interest.
Diane Ly declares that she has no conflict of interest.
Daniel Brungs declares that he has no conflict of interest.
Martin G. Carolan declares that he has no conflict of interest.
Thomas V. Guy declares that he has no conflict of interest.
Debbie Watson declares that she has no conflict of interest.
Jeremiah F. de Leon declares that he has no conflict of interest.
Ronald Sluyter declares that he has no conflict of interest.
Ethical approval
All experiments with human blood were conducted in accordance with approval by the University of Wollongong Human Research Ethics Committee (Wollongong, Australia).
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Robson SC, Sévigny J, Zimmermann H. The E-NTPDase family of ectonucleotidases: structure function relationships and pathophysiological significance. Purinergic Signal. 2006;2(2):409–430. doi: 10.1007/s11302-006-9003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yegutkin GG. Nucleotide- and nucleoside-converting ectoenzymes: important modulators of purinergic signalling cascade. Biochim Biophys Acta. 2008;1783(5):673–694. doi: 10.1016/j.bbamcr.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 3.Stagg J, Divisekera U, McLaughlin N, Sharkey J, Pommey S, Denoyer D, Dwyer KM, Smyth MJ. Anti-CD73 antibody therapy inhibits breast tumor growth and metastasis. Proc Natl Acad Sci U S A. 2010;107(4):1547–1552. doi: 10.1073/pnas.0908801107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perrot I, Michaud HA, Giraudon-Paoli M, Augier S, Docquier A, Gros L, Courtois R, Dejou C, Jecko D, Becquart O, Rispaud-Blanc H, Gauthier L, Rossi B, Chanteux S, Gourdin N, Amigues B, Roussel A, Bensussan A, Eliaou JF, Bastid J, Romagne F, Morel Y, Narni-Mancinelli E, Vivier E, Paturel C, Bonnefoy N. Blocking antibodies targeting the CD39/CD73 immunosuppressive pathway unleash immune responses in combination cancer therapies. Cell Rep. 2019;27(8):2411–2425. doi: 10.1016/j.celrep.2019.04.091. [DOI] [PubMed] [Google Scholar]
- 5.Bonner F, Borg N, Burghoff S, Schrader J. Resident cardiac immune cells and expression of the ectonucleotidase enzymes CD39 and CD73 after ischemic injury. PLoS One. 2012;7(4):e34730. doi: 10.1371/journal.pone.0034730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geraghty NJ, Watson D, Sluyter R. Pharmacological blockade of the CD39/CD73 pathway but not adenosine receptors augments disease in a humanized mouse model of graft-versus-host disease. Immunol Cell Biol. 2019;97(6):597–610. doi: 10.1111/imcb.12251. [DOI] [PubMed] [Google Scholar]
- 7.Adhikary SR, Cuthbertson P, Turner RJ, Sluyter R, Watson D. A single-nucleotide polymorphism in the human ENTPD1 gene encoding CD39 is associated with worsened graft-versus-host disease in a humanized mouse model. Immunol Cell Biol. 2020;98(5):397–410. doi: 10.1111/imcb.12328. [DOI] [PubMed] [Google Scholar]
- 8.Nikolova M, Carriere M, Jenabian MA, Limou S, Younas M, Kok A, Hue S, Seddiki N, Hulin A, Delaneau O, Schuitemaker H, Herbeck JT, Mullins JI, Muhtarova M, Bensussan A, Zagury JF, Lelievre JD, Levy Y. CD39/adenosine pathway is involved in AIDS progression. PLoS Path. 2011;7(7):e1002110. doi: 10.1371/journal.ppat.1002110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pawelec G. Immune signatures predicting responses to immunomodulatory antibody therapy. Curr Opin Immunol. 2018;51:91–96. doi: 10.1016/j.coi.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 10.Gandara DR, Paul SM, Kowanetz M, Schleifman E, Zou W, Li Y, Rittmeyer A, Fehrenbacher L, Otto G, Malboeuf C, Lieber DS, Lipson D, Silterra J, Amler L, Riehl T, Cummings CA, Hegde PS, Sandler A, Ballinger M, Fabrizio D, Mok T, Shames DS. Blood-based tumor mutational burden as a predictor of clinical benefit in non-small-cell lung cancer patients treated with atezolizumab. Nat Med. 2018;24(9):1441–1448. doi: 10.1038/s41591-018-0134-3. [DOI] [PubMed] [Google Scholar]
- 11.Maecker HT, McCoy JP, Nussenblatt R. Standardizing immunophenotyping for the human immunology project. Nat Rev Immunol. 2012;12:191–200. doi: 10.1038/nri3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perry C, Hazan-Halevy I, Kay S, Cipok M, Grisaru D, Deutsch V, Polliack A, Naparstek E, Herishanu Y. Increased CD39 expression on CD4+ T lymphocytes has clinical and prognostic significance in chronic lymphocytic leukemia. Ann Hematol. 2012;91(8):1271–1279. doi: 10.1007/s00277-012-1425-2. [DOI] [PubMed] [Google Scholar]
- 13.Capone M, Fratangelo F, Giannarelli D, Sorrentino C, Turiello R, Zanotta S, Galati D, Madonna G, Tuffanelli M, Scarpato L, Grimaldi AM, Esposito A, Azzaro R, Pinto A, Cavalcanti E, Pinto A, Morello S, Ascierto PA. Frequency of circulating CD8+CD73+T cells is associated with survival in nivolumab-treated melanoma patients. J Transl Med. 2020;18(1):121. doi: 10.1186/s12967-020-02285-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deng W-W, Li Y-C, Ma S-R, Mao L, Yu G-T, Bu L-L, Kulkarni AB, Zhang W-F, Sun Z-J. Specific blockade CD73 alters the “exhausted” phenotype of T cells in head and neck squamous cell carcinoma. Int J Cancer. 2018;143(6):1494–1504. doi: 10.1002/ijc.31534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeske SS, Brand M, Ziebart A, Laban S, Doescher J, Greve J, Jackson EK, Hoffmann TK, Brunner C, Schuler PJ. Adenosine-producing regulatory B cells in head and neck cancer. Cancer Immunol Immunother. 2020;69(7):1205–1216. doi: 10.1007/s00262-020-02535-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Investig Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- 17.Grievink HW, Luisman T, Kluft C, Moerland M, Malone KE. Comparison of three isolation techniques for human peripheral blood mononuclear cells: cell recovery and viability, population composition, and cell functionality. Biopreserv Biobank. 2016;14(5):410–415. doi: 10.1089/bio.2015.0104. [DOI] [PubMed] [Google Scholar]
- 18.Weinberg A, Song L-Y, Wilkening C, Sevin A, Blais B, Louzao R, Stein D, Defechereux P, Durand D, Riedel E, Raftery N, Jesser R, Brown B, Keller MF, Dickover R, McFarland E, Fenton T. Optimization and limitations of use of cryopreserved peripheral blood mononuclear cells for functional and phenotypic T-cell characterization. Clin Vaccine Immunol. 2009;16(8):1176–1186. doi: 10.1128/cvi.00342-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tompa A, Nilsson-Bowers A, Faresjo M. Subsets of CD4+, CD8+, and CD25hi lymphocytes are in general not influenced by isolation and long-term cryopreservation. J Immunol. 2018;201(6):1799–1809. doi: 10.4049/jimmunol.1701409. [DOI] [PubMed] [Google Scholar]
- 20.Costantini A, Mancini S, Giuliodoro S, Butini L, Regnery CM, Silvestri G, Montroni M. Effects of cryopreservation on lymphocyte immunophenotype and function. J Immunol Methods. 2003;278(1):145–155. doi: 10.1016/S0022-1759(03)00202-3. [DOI] [PubMed] [Google Scholar]
- 21.Rasmussen SM, Bilgrau AE, Schmitz A, Falgreen S, Bergkvist KS, Tramm AM, Bæch J, Jacobsen CL, Gaihede M, Kjeldsen MK, Bødker JS, Dybkær K, Bøgsted M, Johnsen HE. Stable phenotype of B-cell subsets following cryopreservation and thawing of normal human lymphocytes stored in a tissue biobank. Cytom Part B-Clin Cy. 2015;88(1):40–49. doi: 10.1002/cyto.b.21192. [DOI] [PubMed] [Google Scholar]
- 22.Hilchey SP, Kobie JJ, Cochran MR, Secor-Socha S, Wang J-CE, Hyrien O, Burack WR, Mosmann TR, Quataert SA, Bernstein SH. Human follicular lymphoma CD39+-infiltrating T cells contribute to adenosine-mediated T cell hyporesponsiveness. J Immunol. 2009;183(10):6157–6166. doi: 10.4049/jimmunol.0900475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moncrieffe H, Nistala K, Kamhieh Y, Evans J, Eddaoudi A, Eaton S, Wedderburn LR. High expression of the ectonucleotidase CD39 on T cells from the inflamed site identifies two distinct populations, one regulatory and one memory T cell population. J Immunol. 2010;185(1):134–143. doi: 10.4049/jimmunol.0803474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saze Z, Schuler PJ, Hong CS, Cheng D, Jackson EK, Whiteside TL. Adenosine production by human B cells and B cell-mediated suppression of activated T cells. Blood. 2013;122(1):9–18. doi: 10.1182/blood-2013-02-482406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bisset LR, Lung TL, Kaelin M, Ludwig E, Dubs RW. Reference values for peripheral blood lymphocyte phenotypes applicable to the healthy adult population in Switzerland. Eur J Haematol. 2004;72(3):203–212. doi: 10.1046/j.0902-4441.2003.00199.x. [DOI] [PubMed] [Google Scholar]
- 26.Dwyer KM, Hanidziar D, Putheti P, Hill PA, Pommey S, McRae JL, Winterhalter A, Doherty G, Deaglio S, Koulmanda M, Gao W, Robson SC, Strom TB. Expression of CD39 by human peripheral blood CD4+ CD25+ T cells denotes a regulatory memory phenotype. Am J Transplant. 2010;10(11):2410–2420. doi: 10.1111/j.1600-6143.2010.03291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pulte D, Furman RR, Broekman MJ, Drosopoulos JHF, Ballard HS, Olson KE, Kizer JR, Marcus AJ. CD39 expression on T lymphocytes correlates with severity of disease in patients with chronic lymphocytic leukemia. Clin Lymphoma Myeloma Leuk. 2011;11(4):367–372. doi: 10.1016/j.clml.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clayton A, Al-Taei S, Webber J, Mason MD, Tabi Z. Cancer exosomes express CD39 and CD73, which suppress T cells through adenosine production. J Immunol. 2011;187(2):676–683. doi: 10.4049/jimmunol.1003884. [DOI] [PubMed] [Google Scholar]
- 29.Bai A, Moss A, Rothweiler S, Serena Longhi M, Wu Y, Junger WG, Robson SC. NADH oxidase-dependent CD39 expression by CD8+ T cells modulates interferon gamma responses via generation of adenosine. Nat Commun. 2015;6(1):8819. doi: 10.1038/ncomms9819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Figueiró F, Muller L, Funk S, Jackson EK, Battastini AMO, Whiteside TL. Phenotypic and functional characteristics of CD39(high) human regulatory B cells (Breg) Oncoimmunology. 2016;5(2):e1082703. doi: 10.1080/2162402X.2015.1082703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raczkowski F, Rissiek A, Ricklefs I, Heiss K, Schumacher V, Wundenberg K, Haag F, Koch-Nolte F, Tolosa E, Mittrücker H-W. CD39 is upregulated during activation of mouse and human T cells and attenuates the immune response to listeria monocytogenes. PLoS One. 2018;13(5):e0197151. doi: 10.1371/journal.pone.0197151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ziebart A, Huber U, Jeske S, Laban S, Doescher J, Hoffmann TK, Brunner C, Jackson EK, Schuler PJ. The influence of chemotherapy on adenosine-producing B cells in patients with head and neck squamous cell carcinoma. Oncotarget. 2018;9(5):5834–5847. doi: 10.18632/oncotarget.23533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang W, Nilles TL, Johnson JR, Margolick JB. The effect of cellular isolation and cryopreservation on the expression of markers identifying subsets of regulatory T cells. J Immunol Methods. 2016;431:31–37. doi: 10.1016/j.jim.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fowke KR, Behnke J, Hanson C, Shea K, Cosentino LM. Apoptosis: a method for evaluating the cryopreservation of whole blood and peripheral blood mononuclear cells. J Immunol Methods. 2000;244(1):139–144. doi: 10.1016/S0022-1759(00)00263-5. [DOI] [PubMed] [Google Scholar]
- 35.Thurgood LA, Lower KM, Macardle C, Kuss BJ. Aberrant determination of phenotypic markers in chronic lymphocytic leukemia (CLL) lymphocytes after cryopreservation. Exp Hematol. 2018;63:28–32. doi: 10.1016/j.exphem.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 36.Tan YS, Lei YL. Isolation of tumor-infiltrating lymphocytes by Ficoll-Paque density gradient centrifugation. Methods Mol Biol. 2019;1960:93–99. doi: 10.1007/978-1-4939-9167-9_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chabab G, Barjon C, Abdellaoui N, Salvador-Prince L, Dejou C, Michaud H-A, Boissière-Michot F, Lopez-Crapez E, Jacot W, Pourquier D, Bonnefoy N, Lafont V. Identification of a regulatory Vδ1 gamma delta T cell subpopulation expressing CD73 in human breast cancer. J Leukoc Biol. 2020;107(6):1057–1067. doi: 10.1002/jlb.3ma0420-278rr. [DOI] [PubMed] [Google Scholar]
- 38.Boison D, Yegutkin GG. Adenosine metabolism: emerging concepts for cancer therapy. Cancer Cell. 2019;36(6):582–596. doi: 10.1016/j.ccell.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]