Abstract
Background:
Mannitol increases blood–brain barrier permeability and can improve the efficiency of systemically administered stem cells by facilitating stem cell entry from the periphery into the injured brain. The aim of this study was to elucidate the neuroprotective effects of a combination of mannitol pretreatment and stem cell transplantation on stroke-induced neural injury.
Methods:
The experimental rats were randomly assigned to three groups 24 h after middle cerebral artery occlusion and reperfusion. One group received intravenous (IV) injections of phosphate-buffered saline (vehicle), another group received IV injections of human adipose-derived stem cells (hADSCs), and the last group received IV injections of hADSCs 10 min after IV mannitol injections. Neurobehavioral functions and infarct volume were compared. Immunohistochemistry (IHC) analyses were performed using antibodies against ionized calcium binding adapter-1 (IBA-1), rat endothelial antigen-1 (RECA-1), and bromodeoxyuridine/doublecortin (BrdU/DCX).
Results:
PKH-26 labeling revealed no difference in the number of stem cells that had migrated into the injured brain, and hADSC transplantation did not improve the infarct volume. However, neurobehavioral functions improved in the mannitol group. IHC showed higher numbers of RECA-1-positive cells in the peri-infarcted brain and BrdU-/DCX-colocalized cells in the subventricular zone in the mannitol group. IBA-1-positive cell number decreased in the hADSC-only and mannitol-pretreatment groups compared with the vehicle group even though there was no difference between the former two groups.
Conclusion:
Combinatorial treatment with mannitol and hADSC transplantation may have better therapeutic potential than hADSC monotherapy for ischemic stroke.
Keywords: Ischemic stroke, Mannitol, Human adipose-derived stem cells, Combination, Pretreatment
Introduction
Acute ischemic stroke is one of the most frequent causes of disability and death worldwide, and enormous social and financial costs result from rehabilitation, long treatment duration, and loss of productivity [1–3]. Current treatment strategies, including intravenous thrombolysis and mechanical thrombectomy, are extremely time-dependent. The mainstay of the current ischemic stroke treatment is a narrow time window of a few hours even though it has widened over time [4–6]. This narrow window eventually results in a high treatment failure rate and neurological sequelae. To overcome this limitation, new therapeutic strategies have been explored to target the restorative stage in place of the existing narrow therapeutic window, including cell-based therapy.
The development of stem cell biology has accelerated considerably in recent decades [7], and stem cell grafting after ischemic stroke has been postulated to significantly extend the period of intervention and target the subacute as well as the chronic phases of stroke. Although the transplantation of stem cells promotes substantial functional recovery from stroke, several limitations may impede its clinical application.
The blood–brain barrier (BBB) protects the central nervous system (CNS) by preventing the entry of immune cells and serum proteins, thereby creating an immune-restricted zone. In addition, it helps in the maintenance of homeostasis in the brain by selectively transporting molecules and cells across the barrier [8]. The BBB poses a major challenge for drugs or stem cell treatments of stroke. Various methods have been developed to increase BBB permeability, but they are associated with adverse side effects and are therefore, not clinically applicable [9]. Mannitol has been used for controlling intracranial pressure (ICP) for many decades, and has been proven to be safe for use. BBB permeabilization via mannitol can thus be utilized as a therapeutic agent (e.g. drugs) delivery system [10] as it facilitates the entry of therapeutic biologics, including stem cells [11], into the brain [8].
Direct administration of stem cells into CNS lesions can cause adverse effects, including immunologic rejection, arrhythmia during injection, vascular occlusion, and seizure [12], and may be dangerous for patients with neurological sequelae from stroke. Furthermore, IV administration is associated with a disadvantage of reduced transplantation efficiency due to stem cell entrapment in the capillary system in the lungs, liver, and spleen [13, 14]. In addition, because of their relatively large size, stem cells cannot cross the BBB as the barrier action is dependent on the molecular size [15]. Thus, various methods were analyzed for enabling the migration of stem cells across the BBB and into the brain, including hyperventilation, hypothermia, and hypoperfusion [15, 16]. One of the methods for increasing the number of stem cells across the BBB is mannitol pretreatment [8, 16, 17]. As described above, it is expected that the administration of mannitol prior to IV administration of stem cells will increase the permeability of the BBB, helping more stem cells migrate from blood vessels into CNS lesions, resulting in increased therapeutic efficacy of stem cell transplantation in stroke patients. This study was conducted to elucidate the neuroprotective effects of a combination of IV mannitol injection and stem cell transplantation on stroke-induced neural injury.
Materials and methods
Animal model of ischemic stroke
All animal experiments were approved by the Institutional Review Board of Korea University (Seoul, Korea; KUIACUC-20140709-3) and performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publication No. 80-23, revised in 1996).
Adult male Sprague–Dawley rats weighing 300–320 g (Orient Bio Inc., Seongnam, Korea) were used in this study. Acute stroke was induced using the standard middle cerebral artery occlusion (MCAO) method as previously described [18]. Animals were anesthetized with 5% isoflurane and maintained under anesthesia with 1.5% isoflurane prepared in a mixture of 70% N2O and 30% O2. After exposure of the common carotid artery, a 4-0 silicone-coated monofilament (Doccol Corp., Sharon, MA, USA) was inserted into the internal carotid artery to occlude the ipsilateral middle cerebral artery. The occlusion was maintained for 90 min, and the monofilament was subsequently removed. Each rat was tested for spontaneous circling, and the tail-lifting test was also performed. In this experiment, the subacute stage cerebral infarction was reached 24 h after the onset of cerebral infarction.
Preparation of hADSCs
Human adipose-derived stem cells (hADSCs) were provided by K-STEMCELL (previously RNL Bio) Co., Ltd. (Seoul, Korea). They were harvested from human subcutaneous fat tissue obtained by liposuction from healthy donors with informed consent. The hADSCs were expanded and characterized as previously described [19]. Live hADSCs were allowed to grow to 90% confluency in 100 mm diameter culture dishes using Keratinocyte-serum-free medium (SFM; Invitrogen, Carlsbad, CA, USA) containing 0.2 mM ascorbic acid, 0.09 mM calcium, 5 ng/mL rEGF, and 5% fetal bovine serum. The hADSCs were harvested from passages 5 and 6. For PKH-26 labeling, the cells were detached using trypsin–EDTA and incubated with PKH-26 dye (Sigma-Aldrich, St. Louis, MO, USA) according to manufacturer’s instructions.
Experimental groups
Twenty-four hours after MCAO reperfusion, the experimental rats were randomly assigned to three groups. The vehicle group (n = 7) received IV injections of phosphate-buffered saline (PBS). The hADSC group received IV injections of hADSCs (2 × 106 in PBS). The mannitol pretreatment group (n = 7) received IV injections of hADSCs (2 × 106 in PBS) 10 min after receiving mannitol (1.5 g/kg IV; JW Pharmaceutical Corp., Seoul, Korea) pretreatment.
Neurobehavioral tests
Neurobehavioral functions were evaluated on days 1, 4, 8, 11, and 15 after MCAO, using the modified Neurological Severity Score (mNSS) [17]. The mNSS is a composite of motor (muscle status and abnormal movement), sensory (visual, tactile, and proprioceptive), and balance tests. Neurological function was graded on a scale of 0–18 (normal to maximum deficit).
Tissue preparation and immunohistochemistry
Fifteen days after MCAO, the rats were anesthetized with Zoletil 50 (30 mg/kg) and xylazine (5 mg/kg) and perfused transcardially with saline, followed by fixing with 4% paraformaldehyde in 0.1 mol/L phosphate buffer. After perfusion, the rat brains were removed and fixed in a 4% paraformaldehyde solution overnight at 4 °C, then placed in a 30% sucrose solution for cryoprotection. The frozen brains were sliced into 20 μm-thick coronal sections using a cryostat vibratome (CM3050S; Leica Microsystems, Wetzlar, Germany) and stored at − 80 °C until further processing.
For immunohistochemistry (IHC), the tissue sections were treated with 2 N HCl for 30 min at 37 °C followed by neutralization with immersion in 0.1 mol/L borate buffer (pH 8.5). Non-specific binding was blocked by incubating the sections with 10% horse serum in PBS. The sections were then incubated with primary antibodies overnight at 4 °C.
The primary antibodies and dilutions used for IHC were as follows: ionized calcium-binding adapter molecule 1 (IBA-1; 1:400; Wako Chemicals USA, Inc., Dallas, TX, USA), rat endothelial cell antigen-1 (RECA-1; 1:400; Bio-Rad Laboratories, Hercules, CA, USA), bromodeoxyuridine (BrdU; 1:50; Roche, Basel, Switzerland), and doublecortin (DCX; 1:100; Santa Cruz Biotechnology Inc., Dallas, TX, USA). The following secondary antibodies were used: Alexa Fluor 488 anti-mouse IgG (1:400; Invitrogen), Alexa Fluor 594 anti-goat IgG (1:800; Invitrogen), Alexa Fluor 488 anti-rabbit IgG (1:400; Invitrogen), and Rhodamine Red-X-conjugated anti-Mouse IgG (1:100; Jackson ImmunoResearch Inc., West Grove, PA, USA). Next, sections were counterstained with DAPI (Thermo Fisher Scientific, Waltham, MA, USA), and staining was visualized using a fluorescence microscope (BX61; Olympus Corp., Tokyo, Japan). Immunofluorescence images were acquired using a Zeiss LSM 700 confocal laser microscope (Carl Zeiss, Oberkochen, Germany).
Measurement of cerebral infarction volume
For infarct volume measurement, the brain sections were stained with 0.1% crystal violet (Nissl method). Images were acquired using a 4 × objective lens under a bright-field microscope (BF53; Olympus). The areas of infarction were quantified using ImageJ software (National Institutes of Health [NIH], Bethesda, MD, USA), and the infarct volume was calculated by multiplying the sum of the infarct area by the distance between sections. After correcting for edema, the infarction volume was calculated as follows:
Quantification and statistics
To count the total number of BrdU- and DCX-positive cells, 6 sections were obtained every 280 µm beginning with a section 1.2 mm rostral to the bregma, and IHC and quantification analyses in the subventricular zone (SVZ) were performed to confirm neurogenesis. IBA-1-positive cells were counted under a fluorescence microscope (BX61; Olympus) in 3 microscopic fields of the peri-infarct area of each section. RECA-1-positive vessel density was evaluated in images captured from ischemic hemispheres using MetaMorph imaging software (version 7.8.1; Molecular Devices, San Jose, CA, USA). Six sections from each animal were averaged, and the data were presented as mean ± standard error of the mean (SEM). Results were analyzed using one-way analysis of variance (ANOVA) followed by Tukey’s honest significant difference post hoc comparison. For behavioral data, one-way analysis of variance was performed, and a Bonferroni post hoc comparison was used to analyze the treatment differences between groups. P values < 0.05 were considered significant.
Results
Effect of mannitol on stem cell migration into the host brain
PKH-26 labeling was performed on all prepared specimens for evaluation of the effect of mannitol on stem cell migration into the host brain. PKH-26 labeling was performed in the penumbra zone of MCAO rats and counted in each brain section as previously described. The number of PKH-26-positive cells observed in both the hADSC-only and mannitol pretreatment groups was below 10 in every section, which was very low compared to the number of stem cells administered to the rats (2 × 106 in PBS). Furthermore, there was no significant difference between the hADSC-only and mannitol pretreatment groups (p > 0.05; Fig. 1).
Fig. 1.

Demonstration of PKH-26 labeling in the hADSC-only and mannitol pretreatment groups. The number of PKH-26-positive cells (red) counted in the MCAO rat SVZs in both groups is below 10 and presents no significant difference (p > 0.05). DAPI staining (blue) indicates stained nucleus of every cell of the section. Scale bar = 20 µm. hADSC human adipose-derived stem cell, Mann mannitol pretreatment, PKH PKH-26-positive cells, MCAO middle-cerebral artery occlusion, SVZ subventricular zone, DAPI 4′,6-diamidino-2-phenylindole
Measured infarction volumes between the hADSC-only and mannitol pretreatment groups
Cerebral infarctions were found in the cerebral hemisphere ipsilateral to the occlusion in all rats (Fig. 2). The average infarct volume was 66.7 ± 2.50% in the vehicle group. Calculated infarct volumes of the hADSC-only and mannitol pretreatment groups decreased, however, and presented no significant difference (p > 0.05, hADSC-only group: 46.5 ± 10.2%; mannitol pretreatment group: 48.8 ± 14.1%).
Fig. 2.
Representative sections of the brain stained with cresyl violet. Scale bar = 1 mm. hADSC human adipose-derived stem cell, Mann mannitol pretreatment
Role of mannitol in stem cell therapeutic effects on neurobehavioral outcomes
Using an MCAO rat model, we evaluated the effects of hADSC transplantation on the recovery of behavioral functions using the mNSS test. Before MCAO, neurological scores were similar among the vehicle, hADSCs-only, and mannitol pretreatment groups. One day after hADSC transplantation, there was no significant difference between the groups; however, as time passed, lower mean mNSS scores were observed in the hADSC-only group (P < 0.001 on the 15th day), and a further decrease were observed in the mannitol pretreatment group (P < 0.001 on the 15th day). Figure 3 demonstrates chronological mNSS score improvements in the vehicle, hADSC-only, and mannitol pretreatment groups.
Fig. 3.
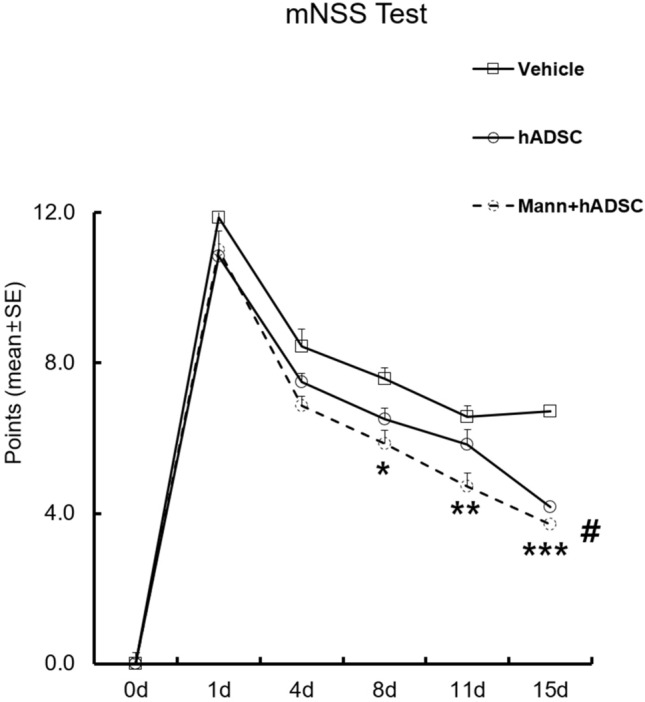
Demonstration of the chronological change in mNSS in the experimental groups. Chronological decrease in the mNSS of the vehicle and hADSC transplantation groups after MCAO was observed. The graph demonstrates improvements in neurobehavioral function after MCAO and hADSC transplantation. *p = 0.048, **p = 0.002, ***p < 0.001, #p < 0.001, mNSS modified neurological severity score, hADSC human adipose-derived stem cell, MCAO middle cerebral artery occlusion, Mann mannitol pretreatment, SE standard error
IHC analyses
Neuronal proliferation in the SVZ
Significantly greater numbers of BrdU- and DCX-positive cells were found in the SVZ in both the hADSC-only and mannitol pretreatment groups compared to the vehicle group (Fig. 4). In addition, significantly increased presentation of BrdU-/DCX-double-positive cells was observed in both the hADSC-only (p = 0.014) and mannitol pretreatment groups (p < 0.001). This finding suggests that hADSC transplantation promotes neuronal proliferation in injured brains after subacute stage of cerebral ischemia. Furthermore, the significance was stronger in the mannitol pretreatment group compared to the hADSC-only group, implying that neuronal proliferation is enhanced by mannitol pretreatment.
Fig. 4.
Immunohistochemistry in ischemic rat brains. Neurogenesis after hADSC transplantation was examined using BrdU (green) and DCX (red) immunostaining. A BrdU- and DCX-positive cells in SVZs on the 15th day after MCAO. White arrows indicate BrdU-/DCX-double-positive cells. Scale bar = 20 µm. B Counts of BrdU-/DCX- double-positive cells in SVZs. The number of BrdU-/DCX-double-positive cells is significantly higher in the hADSC transplantation (p = 0.014) and hADSC transplantation with mannitol pretreatment (p < 0.001) groups. Data are expressed as mean ± SEM. BrdU bromodeoxyuridine, DCX doublecortin, hADSC human adipose-derived stem cell, Mann mannitol pretreatment, SVZ subventricular zone, MCAO middle cerebral artery occlusion, SEM standard error of the mean
Inhibition of microglial activation in the ischemic hemisphere
Based on IHC analyses, the hADSC-only (p = 0.04) and mannitol pretreatment groups (p = 0.04) presented fewer IBA-1-positive cells compared to the vehicle group (Fig. 5), suggesting that MCAO-induced accumulation of microglia-positive cells in ischemic brain regions is attenuated by hADSC transplantation. This observation suggests that hADSC transplantation could suppress the inflammatory reaction that occurs in stroke. However, no additional reduction in IBA-1-positive cells following mannitol pretreatment was observed (p = 0.87), indicating that mannitol provides no additional anti-inflammatory effects.
Fig. 5.
Decreased microglial activation after hADSC transplantation. A Representative images of IBA-1 immunostaining in the ischemic hemisphere on the 15th day after MCAO. A decreased number of IBA-1-positive cells was observed. Scale bar = 20 µm. B Count of IBA-1-positive microglia. The number of IBA-1-positive cells decreased in the hADSC transplantation as well as in the hADSC transplantation with mannitol pretreatment groups. Data are expressed as means ± SEMs. IBA-1 ionized calcium-binding adapter molecule-1, hADSC human adipose-derived stem cell, Mann mannitol pretreatment, MCAO middle cerebral artery occlusion, SEM standard error of the mean
Angiogenesis in the peri-infarcted area of the ischemic hemisphere
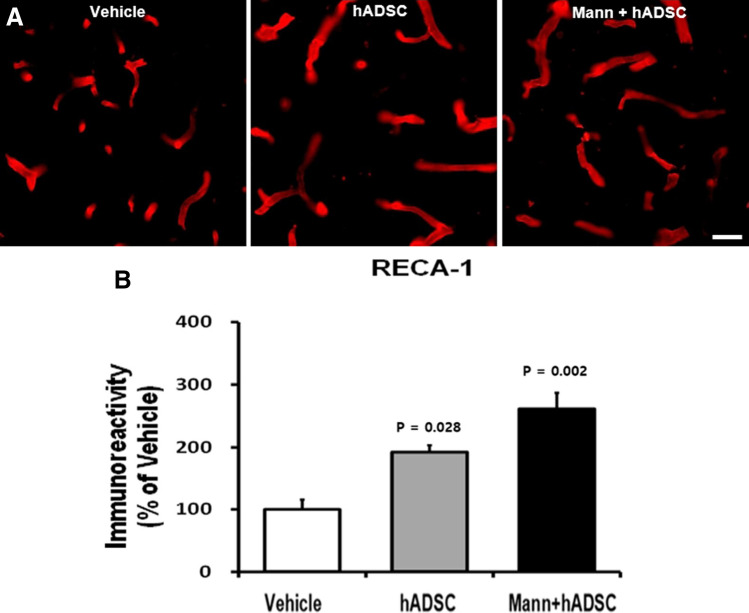
IHC analyses revealed significantly higher numbers of RECA-1-positive cells in the peri-infarcted area of the ischemic hemisphere in both the hADSC-only and mannitol pretreatment groups compared to the vehicle group (Fig. 6). This finding suggests that hADSC transplantation promotes angiogenesis in the peri-infarcted area after the subacute stage of cerebral ischemia. The mannitol pretreatment group (p = 0.02) presented a much lower p value than the hADSC group (p = 0.028). Thus, we presume that mannitol pretreatment could enhance the therapeutic efficacy of hADSC transplantation.
Fig. 6.
Increased angiogenesis after hADSC transplantation. A Representative images of RECA-1-positive vessels in ischemic rat brains on the 15th day after MCAO. Scale bar = 50 µm. B Analysis of RECA-1 immunoreactivity using six cryosections. Immunostaining reveals an increased vessel area in the hADSC transplantation as well as in the hADSC transplantation with mannitol pretreatment groups. Data are expressed as percentages of immunoreactivity in the control groups compared to the vehicle group. RECA-1 rat endothelial cell antigen-1, hADSC human adipose-derived stem cell, Mann mannitol pretreatment, MCAO middle cerebral artery occlusion
Discussion
Mature neural tissue possesses weak potential for intrinsic repair following different types of neural injury [20], therefore, transplantation of stem cells into the injured brain may have a neuroprotective effect on stroke-induced brain injury [21]. The hADSCs are mesenchymal stem cells that can be isolated from a wide variety of sources by minimally invasive procedures [22–24]. The therapeutic efficacy of hADSC transplantation in stroke models has been demonstrated in numerous studies, but the mechanisms underlying this effective therapy are not well known [25]. One such therapeutic mechanism is angiogenesis. When hemorrhagic or ischemic stroke transforms into the chronic type, the injured regions lack blood vessels to supply nutrients and maintain cell survival. Thus, vascular endothelial growth factor (VEGF) may be a possible solution to protect neurons from cell death [26]. Reports also suggest that hADSCs could restore brain function through the secretion of VEGF to promote angiogenesis in the injured region. Another possible mechanism for the therapeutic effect of hADSC transplantation is neurogenesis. Transplantation of hADSCs to injured regions induces neuronal differentiation and stimulates brain repair markers associated with neurogenesis, eventually resulting in the recovery of brain function. It is known that hADSCs differentiate into neuron-like or glia-like cells that express neuronal nuclei (NeuN), nestin, or glial fibrillary acidic protein (GFAP) [27, 28]. Mesenchymal stem cells can modulate local and systemic inflammation [29], and hADSCs have also been reported to reduce post-stroke inflammation following gliosis in the infarct boundary. Activated microglia and macrophages secrete inflammatory cytokines and lead to astrocyte stimulation. Such inflammatory reactions promote neuronal cell death and fibrotic scar formation, subsequently preventing neuronal regeneration during the healing stage after stroke [24, 30, 31]. Our study suggests that transplantation with hADSCs significantly improves neurobehavioral deficits after cerebral ischemia and measured infarct volume, and these improvements are enhanced by mannitol pretreatment. Furthermore, immunofluorescence staining revealed that rats treated with hADSCs presented a significantly lower number of IBA-1-positive cells, higher number of RECA-1 in the peri-infarcted brain region as well as significantly higher numbers of BrdU- and DCX-positive cells in the subventricular zone (SVZ), regardless of mannitol pretreatment. These findings demonstrate that hADSC transplantation reduces inflammation, promotes angiogenesis, and increases neurogenesis in an ischemic rat model.
Mannitol is a hyperosmolar extracellular agent that has been used to increase ICP stemming from various causes for many decades and remains the osmotic agent of choice [8, 32]. It induces osmotic-driven fluid movement into intravascular space from cerebral tissue, which consequently results in the shrinkage of endothelial cells along the BBB and increases BBB permeability [11, 33]. Thus, mannitol enhances the migration of hADSCs across the BBB into the brain, thereby increasing the therapeutic efficacy of hADSC transplantation. In our study, there was no evidence that mannitol pretreatment enhances stem cell migration into the host brain across the BBB. We hypothesized that the number of stem cells migrating into the brain across the BBB was relatively small owing to the IV delivery of stem cells, and no statistically significant difference was found. Further studies with alternative administration routes are needed to further evaluate the optimal stem cell delivery route and subsequent effects of mannitol on stem cell migration across the BBB. Our study also revealed lower mNSS scores and calculated infarct volumes in the hADSC transplantation with mannitol pretreatment group compared to the hADSC-only group. Furthermore, IHC analyses revealed greater numbers of BrdU-/DCX-positive cells and RECA-1-positive cells in the SVZ, and this phenomenon was enhanced by mannitol pretreatment. Although no evidence was found that mannitol increased stem cell migration in our study, we concluded that the mannitol pretreatment group displayed more improvements in neurological deficits and increased neuronal proliferation based on the IHC analyses. We hypothesized that this is due to the increased migration of trophic factors secreted by stem cells from the blood to the brain when permeability increases. Mesenchymal stem cells themselves do not usually differentiate into neural cells [34]. Stem cells themselves may have therapeutic effects, but the trophic factors (various cytokines and growth factors with both paracrine and autocrine effects) secreted by them suppress local immune responses to reduce fibrosis, inhibit apoptosis, promote angiogenesis, and stimulate mitosis and differentiation of tissue-intrinsic repair or stem cells. However, in our study, no difference in microglial activity was observed between the hADSC-only and mannitol pretreatment groups. Some reports suggest that increased BBB permeability mediates the exacerbation of injury caused by increasing inflammation at the injury site. When stroke occurs, inflammation plays a major role in secondary damage to the brain tissue. While mannitol increases BBB permeability and passage of hADSCs, it also promotes passage of systemic inflammatory factors, resulting in increased neural injury caused by inflammation. Borlongan et al. [17] provide a possible solution, which is to inhibit the nuclear factor κ-light chain enhancer of B-cell (NF-κB) activation. It is possible to limit NF-κB expression by using a similar oligonucleotide to compete with and limit the binding of NF-κB and thus prevent additional inflammation.
As far as we know, there was no determined optimal timing of mannitol-facilitated transient opening of BBB. For example, Yasuhara et al. [35] used mannitol immediately after transplantation of stem cells and Cosolo et al. [36] used an intra-arterial mannitol injection and investigated the intracerebral methotrexate level. However, an IV mannitol injection is more practical in the clinical field, and we used mesenchymal stem cells, which are distinct from chemical drugs such as methotrexate and Evans blue. Thus, we conducted a preliminary study to deduce the optimal time interval between IV mannitol injection and stem cell transplantation. Although there was no statistical significance, a stem cell injection 10 min after mannitol pretreatment tended to show better efficacy (data are not shown). Based on these preliminary data, we conducted the main experiment with a 10-min time interval between mannitol pretreatment and hADSC transplantation.
There are some limitations in this study. The sample size was relatively small, and we failed to show that mannitol can increase migration of the IV-injected stem cells into the brain. We could not investigate whether mannitol can increase the permeability of trophic factors from the injected hADSCs. Additionally, more experimental group categories could have been used. In our study, mannitol was administered 10 min before hADSC transplantation, but further detailed studies may be needed to confirm the optimal time interval between the mannitol injection and stem cell transplantation.
In conclusion, our study demonstrates that IV transplantation of hADSCs improves neurobehavioral status after stroke, promotes neurogenesis and angiogenesis, and inhibits inflammatory reactions, suggesting that hADSC transplantation could be used as a promising therapeutic modality for ischemic stroke. The addition of mannitol injection could enhance the therapeutic effects of systemically transplanted hADSCs through increased vascular permeability, although the anti-inflammatory activity of mesenchymal stem cells may be neutralized by increased pro-inflammatory factors. We hypothesized that this is due to increased trophic factors migrating into the brain through mannitol pretreatment rather than the increased number of stem cells. Further studies with larger and more delicately categorized experimental groups are needed.
Acknowledgements
This study was supported in part by grants from Korea University (K1913911, K2008071) and the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (2013R1A1A2057994). The funding bodies did not play any role in the design, collection, analysis, or interpretation of the data; in the writing of the manuscript; or in the decision to submit the manuscript for publication.
Compliance with ethical standards
Conflict of interest
Authors declare there is no conflict of interest with the manuscript.
Ethical statement
Animal studies were performed after receiving approval of the Institutional Animal Care and Use Committee (IACUC) in Korea University (IACUC approval No. KUIACUC-20140709-3).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Benneth DA, et al. Global and regional burden of stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet. 2014;383:245–254. doi: 10.1016/S0140-6736(13)61953-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Norrving B, Kissela B. The global burden of stroke and need for a continuum of care. Neurology. 2013;80:S5–S12. doi: 10.1212/WNL.0b013e3182762397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ovbiagele B, Goldstein LB, Higashida RT, Howard VJ, Johnston SC, Khavjou OA, et al. Forecasting the future of stroke in the United States: a policy statement from the American Heart Association and American Stroke Association. Stroke. 2013;44:2361–2375. doi: 10.1161/STR.0b013e31829734f2. [DOI] [PubMed] [Google Scholar]
- 4.Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378:11–21. doi: 10.1056/NEJMoa1706442. [DOI] [PubMed] [Google Scholar]
- 5.Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50:e344–e418. doi: 10.1161/STROKEAHA.118.022606. [DOI] [PubMed] [Google Scholar]
- 6.Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49:e46–e110. doi: 10.1161/STR.0000000000000158. [DOI] [PubMed] [Google Scholar]
- 7.Minassian A, Green C, Diedenhofen M, Vogel S, Hess S, Stoeber M, et al. Human neural stem cell induced functional network stabilization after cortical stroke: a longitudinal resting-state FMRI study in mice. Front Cell Neurosci. 2020;14:86. doi: 10.3389/fncel.2020.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gonzales-Portillo GS, Sanberg PR, Franzblau M, Gonzales-Portillo C, Diamandis T, Staples M, et al. Mannitol-enhanced delivery of stem cells and their growth factors across the blood-brain barrier. Cell Transplant. 2014;23:531–539. doi: 10.3727/096368914X678337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi C, Kim HM, Shon J, Park J, Kim HT, Kang SH, et al. The combination of mannitol and temozolomide increases the effectiveness of stem cell treatment in a chronic stroke model. Cytotherapy. 2018;20:820–829. doi: 10.1016/j.jcyt.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Kalemci O, Aydin HE, Kizmazoglu C, Kaya I, Yilmaz H, Arda NM. Effects of quercetin and mannitol on erythropoietin levels in rats following acute severe traumatic brain injury. J Korean Neurosurg Soc. 2017;60:355–361. doi: 10.3340/jkns.2016.0505.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ikeda M, Bhattacharjee AK, Kondoh T, Nagashima T, Tamaki N. Synergistic effect of cold mannitol and Na(+)/Ca(2+) exchange blocker on blood-brain barrier opening. Biochem Biophys Res Commun. 2002;291:669–674. doi: 10.1006/bbrc.2002.6495. [DOI] [PubMed] [Google Scholar]
- 12.Bhasin A, Kumaran SS, Bhatia R, Mohanty S, Padma Srivastava MVP. Safety and feasibility of autologous mesenchymal stem cell transplantation in chronic stroke in Indian patients. A four-year follow up. J Stem Cells Regen Med. 2017;13:14–19. doi: 10.46582/jsrm.1301003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schrepfer S, Deuse T, Reichenspurner H, Fischbein MP, Robbins RC, Pelletier MP. Stem cell transplantation: the lung barrier. Transplant Proc. 2007;39:573–576. doi: 10.1016/j.transproceed.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 14.Youn SW, Jung KH, Chu K, Lee JY, Lee ST, Bahn JJ, et al. Feasibility and safety of intra-arterial pericyte progenitor cell delivery following mannitol-induced transient blood-brain barrier opening in a canine model. Cell Transplant. 2015;24:1469–1479. doi: 10.3727/096368914X682413. [DOI] [PubMed] [Google Scholar]
- 15.Nau R, Sörgel F, Eiffert H. Penetration of drugs through the blood-cerebrospinal fluid/blood-brain barrier for treatment of central nervous system infections. Clin Microbiol Rev. 2010;23:858–883. doi: 10.1128/CMR.00007-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang M, Etu J, Joshi S. Enhanced disruption of the blood brain barrier by intracarotid mannitol injection during transient cerebral hypoperfusion in rabbits. J Neurosurg Anesthesiol. 2007;19:249–256. doi: 10.1097/ANA.0b013e3181453851. [DOI] [PubMed] [Google Scholar]
- 17.Borlongan CV, Hadman M, Sanberg CD, Sanberg PR. Central nervous system entry of peripherally injected umbilical cord blood cells is not required for neuroprotection in stroke. Stroke. 2004;35:2385–2389. doi: 10.1161/01.STR.0000141680.49960.d7. [DOI] [PubMed] [Google Scholar]
- 18.Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, et al. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32:1005–1011. doi: 10.1161/01.STR.32.4.1005. [DOI] [PubMed] [Google Scholar]
- 19.Hur JW, Cho TH, Park DH, Lee JB, Park JY, Chung YG. Intrathecal transplantation of autologous adipose-derived mesenchymal stem cells for treating spinal cord injury: a human trial. J Spinal Cord Med. 2016;39:655–664. doi: 10.1179/2045772315Y.0000000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lim CY, Keun KI, Choi JJ, Lee HJ, Park SH, Lee WS, et al. BDNF enhancement of neuronal differentiation with adipose-derived stem cells (ADSCs) Tissue Eng Regen Med. 2008;5:764–771. [Google Scholar]
- 21.Lee JY, Kim HS, Kim SH, Kim HS, Cho BP. Combination of human mesenchymal stem cells and repetitive transcranial magnetic stimulation enhances neurological recovery of 6-hydroxydopamine model of Parkinsonian’s disease. Tissue Eng Regen Med. 2020;17:67–80. doi: 10.1007/s13770-019-00233-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharath SS, Ramu J, Nair SV, Iyer S, Mony U, Rangasamy J. Human adipose tissue derivatives as a potent native biomaterial for tissue regenerative therapies. Tissue Eng Regen Med. 2020;17:123–140. doi: 10.1007/s13770-019-00230-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwon SK, Song JJ, Cho CG, Park SW. Regeneration of facial nerve using mesenchymal stem cells in facial nerve palsy animal model. Tissue Eng Regen Med. 2009;6:300–306. [Google Scholar]
- 24.Chan TM, Harn HJ, Lin HP, Chiu SC, Lin PC, Wang HI, et al. The use of ADSCs as a treatment for chronic stroke. Cell Transplant. 2014;23:541–547. doi: 10.3727/096368914X678409. [DOI] [PubMed] [Google Scholar]
- 25.Ryu S, Lee JM, Bae CA, Moon CE, Cho KO. Therapeutic efficacy of neuregulin 1-expressing human adipose-derived mesenchymal stem cells for ischemic stroke. PLoS One. 2019;14:e0222587. doi: 10.1371/journal.pone.0222587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fang Z, Yang Q, Xiong W, Li G, Xiao J, Guo F, et al. Neurogenic differentiation of murine adipose derived stem cells transfected with EGFP in vitro. J Huazhong Univ Sci Technolog Med Sci. 2010;30:75–80. doi: 10.1007/s11596-010-0113-5. [DOI] [PubMed] [Google Scholar]
- 27.Gutiérrez-Fernandez M, Rodríguez-Frutos B, Ramos-Cejudo J, Vallejo-Cremades MT, Fuentes B, Cerdán S, et al. Effects of intravenous administration of allogenic bone marrow- and adipose tissue-derived mesenchymal stem cells on functional recovery and brain repair markers in experimental ischemic stroke. Stem Cell Res Ther. 2013;4:11. doi: 10.1186/scrt159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park D, Yang G, Bae DK, Lee SH, Yang YH, Kyung J, et al. Human adipose tissue-derived mesenchymal stem cells improve cognitive function and physical activity in ageing mice. J Neurosci Res. 2013;91:660–670. doi: 10.1002/jnr.23182. [DOI] [PubMed] [Google Scholar]
- 29.Karaoz E, Tepekoy F, Yilmaz I, Subasi C, Kabatas S. Reduction of inflammation and enhancement of motility after pancreatic islet derived stem cell transplantation following spinal cord injury. J Korean Neurosurg Soc. 2019;62:153–165. doi: 10.3340/jkns.2018.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oh SH, Jeong YW, Choi W, Noh JE, Lee S, Kim HS, et al. Multimodal therapeutic effects of neural precursor cells derived from human-induced pluripotent stem cells through episomal plasmid-based reprogramming in a rodent model of ischemic stroke. Stem Cells Int. 2020;2020:4061516. doi: 10.1155/2020/4061516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stoll G, Jander S, Schroeter M. Inflammation and glial responses in ischemic brain lesions. Prog Neurobiol. 1998;56:149–171. doi: 10.1016/S0301-0082(98)00034-3. [DOI] [PubMed] [Google Scholar]
- 32.Yilmaz N, Dulgar H, Kiymaz N, Yilmaz C, Gudu BO, Demir I. Activity of mannitol and hypertonic saline therapy on the oxidant and antioxidant system during the acute term after traumatic brain injury in the rats. Brain Res. 2007;1164:132–135. doi: 10.1016/j.brainres.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 33.Tajiri N, Lee JY, Acosta S, Sanberg PR, Borlongan CV. Breaking the blood-brain barrier with mannitol to aid stem cell therapeutics in the chronic stroke brain. Cell Transplant. 2016;25:1453–1460. doi: 10.3727/096368916X690971. [DOI] [PubMed] [Google Scholar]
- 34.Jeong SK, Choi I, Jeon SR. Current status and future strategies to treat spinal cord injury with adult stem cells. J Korean Neurosurg Soc. 2020;63:153–162. doi: 10.3340/jkns.2019.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yasuhara T, Hara K, Maki M, Xu L, Yu G, Ali MM, et al. Mannitol facilitates neurotrophic factor up-regulation and behavioural recovery in neonatal hypoxic-ischaemic rats with human umbilical cord blood grafts. J Cell Mol Med. 2010;14:914–921. doi: 10.1111/j.1582-4934.2008.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cosolo WC, Martinello P, Louis WJ, Christophidis N. Blood-brain barrier disruption using mannitol: time course and electron microscopy studies. Am J Physiol. 1989;256:R443–R447. doi: 10.1152/ajpregu.1989.256.2.R443. [DOI] [PubMed] [Google Scholar]