Abstract
Aberrant expression of glycosphingolipids has been implicated in a myriad of diseases, but our understanding of the strucural diversity, spatial distribution, and biological function of this class of biomolecules remains limited. These challenges partly stem from a lack of sensitive tools that can detect, identify, and quantify glycosphingolipids at the molecular level. Mass spectrometry has emerged as a powerful tool poised to address most of these challenges. Here, we review the recent developments in analytical glycosphingolipidomics with an emphasis on sample preparation, mass spectrometry and tandem mass spectrometry-based structural characterization, label-free and labeling-based quantification. We also discuss the nomenclature of glycosphingolipids, and emerging technologies like ion mobility spectrometry in differentiation of glycosphingolipid isomers. The intrinsic advantages and shortcomings of each method are carefully critiqued in line with an individual’s research goals. Finally, future perspectives on analytical sphingolipidomics are stated, including a need for novel and more sensive methods in isomer separation, low abundance species detection, and profiling the spatial distribution of glycosphingolipid molecular species in cells and tissues using imaging mass spectrometry.
Graphical Abstract
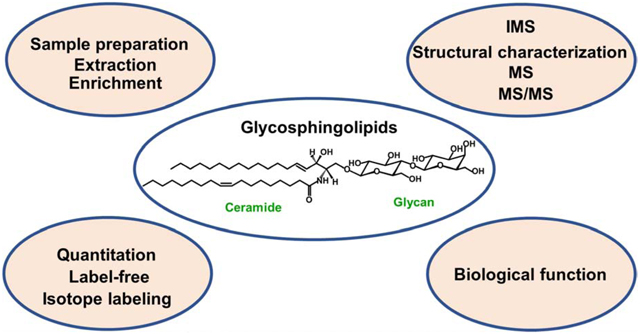
1. Introduction
Glycosphingolipids are ubiquitous component of the cell membranes and membrane-bound subcellular organelles [1–3]. Large differences in physicochemical properties between the glyan head and the lipid tail — two substructures within glycosphingolipids make it challengening to analyze this class of lipids, as the methods developed for either the glycan or the lipid portion oftentimes do not work well at the whole molecular level. However, the biological functions of glycospingolipids are determined by the lipid and the glycan collectively [4–6], which requires these molecules to be analyzed in their intact form.
Mass spectrometry has been instrumental in structural elucidation and quantitation of glycosphingolipids. However, prior to the appearance of current mass spectrometric techniques, glycosphingolipids are typically being analyzed by mass spectrometry with the glycan and lipid portion characterized separately [2, 7–11]. Innovations in mass spectrometry and chromatography have fueled analysis of glycosphingolipid into development of glycospingolipidomics [1, 2, 12–15], where the glycosphingolipids can be analyzed intact while providing the sequence of the glycan and the composition of the lipid portion simutanouly.
This paper reviews the methods used in the contemporary mass spectrometric analysis of glycosphingolipids. We discuss the principles, advantages, and limitations of each analytical approach. Current challenges associated with the analysis of intact glycosphingolipids are also emphasized.
1.1. Structure
Glycosphingolipids are amphiphilic molecules composed of a ceramide backbone (hydrophobic) and a glycan head (hydrophilic) (Fig. 1A). The ceramide contains amino alcohol that is amide-linked to a fatty acyl chain, which can be very different from the fatty acyl compositions in glycerophospholipids. The fatty acyls in glycosphingolipids generally are long (C16 – C26) monounsaturated or saturated, while those in phospholipids often have varied lengths, and either saturated or polyunsaturated. The amino alcohol is also called long-chain base or sphingoid base, a common one is d18:1, where the d stands for ‘di’ (two) hydroxyl groups (one used in glycosidic linkage and the other as free hydroxyl group), 18 for the number of carbons, and 1 for the number of carbon-carbon double bond. Other sphingoid bases like d18:0, d20:0, d16:1, and d20:1 are also present in eukaryotes [1, 12, 13]. Lyso-glycosphingolipids are characterized by a lack of the N-fatty acyl chain [16], oftentimes as a result of degradation of glycosphingolipids by glycosidases [17].
Figure 1. The general structure of glycosphingolipids and sialic acid.
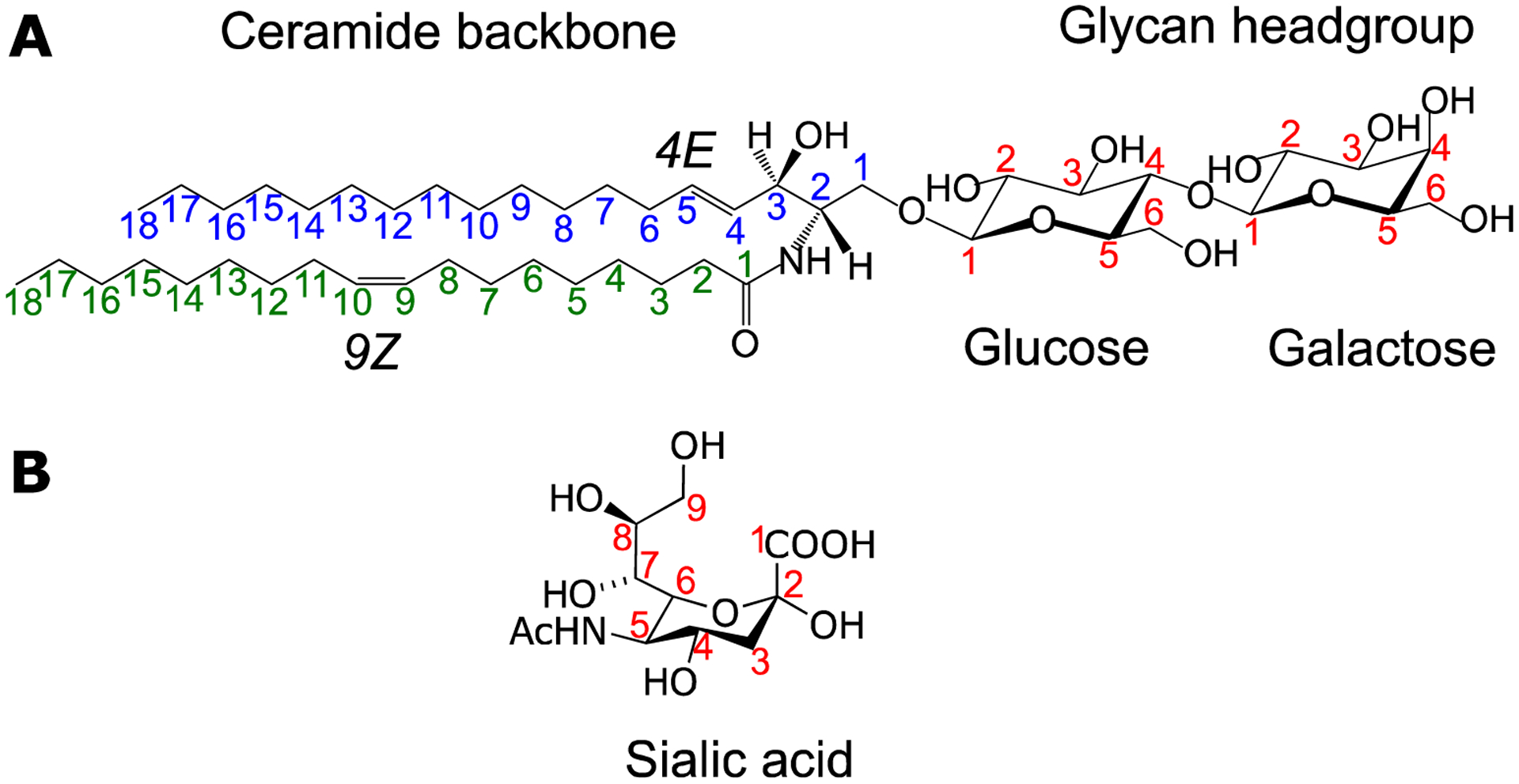
A) Shown here is LacCer d18:1 (4E)/18:1(9Z), composed of a ceramide backbone with a long-chain sphingosine base (d18:1) and oleic acid connected via an amide bond. The double bonds are numbered x and the stereochemistry assigned using the E/Z system. B) The structure of N-acetylneuraminic (Neu5Ac) sialic acid (α-anomer). The numbering shown here will be used throughout this manuscript to address these positions in the structure of glycosphingolipids.
Glycosphingolipids are classified as neutral (cerebrosides and globosides) or acidic (gangliosides and sulfatides) based on the glycan composition. Cerebrosides are composed of a hexose sugar, which can be either glucose (Glc) or galactose (Gal); globosides may have more than one neutral, monosaccharide units; gangliosides contain sialic acid, and sulfatides may have one or more carbohydrate-sulfate ester groups [18]. Two forms of sialic acid exist in humans, namely, N-acetylneuraminic (Neu5Ac) and N-glycolylneuraminic (Neu5Gc)[19]. The latter is present only in trace amounts and may originate from the diet [20].
Chemical modifications can also occur in the glycan and lipid moieties to further increase the structural diversity of glycosphingolipids. Acetylation and lactonization may occur on the glycans, and hydroxylation and unsaturation (carbon-carbon double bonds) on the N-fatty acyls [2, 21, 22]. Considering the high propensity of glycans to form different linkage isomers, along with the variation of the position and stereochemistry of double bonds in the lipid backbone, it is likely that the > 3,000 unique glycosphingolipids listed in the Lipid MAPS database [23] are an underestimate, as many glycosphingolipids remain undetected or uncharacterized [5].
1.2. Nomenclature
Symbols and abbreviations are traditionally being used for naming the most commonly occurring glycosphingolipids. For example, the ganglioside nomenclature proposed by Svennerholm in the 1960s remains widely used till today [24]. Here, gangliosides are abbreviated by two letters followed by a number, e.g., in GM3 the G stands for gangliosides; M stands for ‘mono’ which means 1 sialic acid; and the number 3 referring to the elution sequence in thin layer chromatography (TLC)[24]. Each abbreviation represents a clearly defined glycan structure, i.e. GM3 stands for the structure of Neu5Acα3Galβ4GlcCer. However, the discovery of many more novel glycosphingolipids quickly overwhelms this system. To this end, IUPAC-IUB Joint Commission on Biochemical Nomenclature recommended in 1997 to use a name that includes linkage information of sugars and any modification present in the glycan headgroup. This nomenclature uses a set of pre-defined core structures – which detail the glycan composition and linkage – to serve as a base name [25]. For example, for neutral glycosphingolipids, one of the core structures is GalNAcβ1–4Galβ1–4Glcβ1–1’, also called Gg3 (base name). A glycosphingolipid with this composition: GalNAcβ1–4Galβ1–4Glcβ1–1’Cer d18:1/18:0, is named Gg3Cer d18:1/18:0. If the glycosphingolipid contains sugars that extend beyond this core structure, they are put as a prefix to this name. The prefix contains: 1) Roman numeral to indicate the sugar where modification occurs; 2) a superscript on the Roman numeral to denote the linkage position; 3) the configuration of the linkage; and 4) the abbreviated name of the attached monosaccharide unit. In the same example, if sialic acid (Neu5Ac) is connected to the galactose position 3 with an α linkage configuration, the new sequence is: GalNAcβ1–4(Neu5Acα1–3)Galβ1–4Glcβ1–1’Cer d18:1/18:0. The systematic name of this sequence becomes: II3-α-Neu5Ac-Gg3Cer d18:1/18:0. In this notation, the Roman numeral indicates which glycan the Neu5Ac is connected to (second monosaccharide, i.e. Roman numeral II), the superscript and the Greek letter α indicate what type of linkage was used, in this case, α(1–3) linkage (i.e., 3 and α, respectively, in II3-α-). The use of this system eliminates ambiguity in the chemical literature, especially when dealing with many glycosphingolipid isomers. However, this system does not provide a guideline on how to name the lipid part.
In 2005, the Lipid MAPS consortium proposed a comprehensive classification system and developed a structural database for biologically relevant lipids [23, 26, 27]. Both the system name and the common name are listed for each glycosphingolipid species, including the known long-chain bases and N-linked fatty acyls. As different mass spectrometric approaches provide different levels of molecular structural details, Liebisch et al. [28] in 2013 proposed a shorthand notation to practically annotate lipid structures derived from mass spectrometry data. Notably, for glycosphingolipids, the number of hydroxyl groups in the long-chain base should be listed, and if it is unknown, the sum of the long-chain base and N-linked fatty acyl should be given as the total number of carbons: total number of double bonds.
To demonstrate Liebisch et al.’s proposed nomenclature [28], consider the glycosphingolipid shown in Fig. 1A. This glycosphingolipid can be annotated in different ways (Note: Hex2Cer should be used instead of LacCer if the monosacchride identity is not known): 1) LacCer 36:2 (if double bond positions and stereochemistry are unknown and assuming a sphingoid base with two hydroxyl groups); 2) LacCer d36:2 (if long-chain base hydroxyl is known but double bond position, whether in the long-chain base or fatty acyl chain is unknown; 3) LacCer d18:1/18:1 (if the double bond can be ascertained to be in the long-chain base or fatty acyl chain); 4) LacCer d18:1(4E)/18:1(9Z) (if both position and stereochemistry or double bonds are known). This widely accepted proposal only covers the oligosaccharide headgroups of up to two monosaccharide units, the more complex glycosphingolipids were not covered. Given the simplicity of the Svennerholm system and the abbreviations in naming glycan headgroups and the practicality of shorthand notation to describe the lipid portion, a combination of these two would be convenient to annotate structures of complex glycosphingolipids derived from mass spectrometry data.
1.3. Biological significance
Glycosphingolipids can form high molecular weight micelles, segregate within the plasma membrane, and form lipid rafts and microdomains with cholesterol, sphingomyelin, and proteins [29]. In these domains, glycosphingolipids interact with receptors and modulate signal transduction. Their hydrophilic heads form a cluster on the cell surface called “glycosynapse” [29].
Table 1 summarizes some of the known functions of the headgroup and the lipid moiety. The glycan headgroup assumes many functions in biology [30, 31]. For example, it can serve as a receptor of viruses and bacteria [4, 32], or as an antigen in some autoimmune diseases [33]. The structure and composition of the glycan also play vital roles in protein-protein interactions, receptor regulation, adhesion, initiation/modulation of signal transduction, trafficking, cell recognition and differentiation [4, 29, 32, 34, 35]. To name a few: a change in glycan structure perturbs antigenicity [36] and embryonic development [4, 32]; a high degree of sialylation of glycosphingolipids is also crucial in cancer and other diseases[37]; the negatively charged sulfate group in sulfatides is vital in the kidneys, the central nervous system [38], and in cardiovascular processes, as reviewed elsewhere [18].
Table 1.
Key biological functions of the two components of glycosphingolipids.
| Glycan headgroup | Ceramide backbone |
|---|---|
|
|
The ceramide serves as an anchor to the cell membrane and modulates glycan traits [39, 40]. Desaturases introduce double bonds at specific positions in the fatty acyl chains [41]. The presence of double bonds and hydroxyls in ceramide can impact biological processes. For example, in the study of verotoxins (toxin from E. coli), the presence or absence of a carbon-carbon double bond affected the toxin binding to the Gb3 receptor, with the one containing the double bond having higher affinity than the one without [4, 42]. Lipid double bonds can also influence membrane order and signal transduction [4]. Both double bonds and length of the fatty acyl chain may affect spermatogenesis [43, 44] and retinal function [45]. The hydroxylation of fatty acyls enhances hydrogen bonding, which makes more rigid lipid rafts [46]. Interestingly, recent metabolic tracer studies showed that turnover rates of glycosphingolipids are related to the lipid backbone composition [47]. Taken together, this body of literature emphasizes that intact structure analysis can help advance our understanding of the role of glycosphingolipids in biology.
1.4. Glycosphingolipids as disease biomarkers and therapeutic targets
Glycosphingolipids play a role in many pathological conditions [3–6, 30, 48]. The composition or quantity of glycans may fluctuate in the course of disease progression and serve as potential disease biomarkers [49]. Many brain diseases have dysregulated metabolism of gangliosides and sulfatides [38, 50–52]. In lysosomal storage diseases, glycosphingolipids accumulate in cells and tissues because enzymes needed to metabolize them are either absent or weak [6]. Cancerous cells could be potentially differentiated from the healthy ones by their different glycosphingolipid profiles [53]. Dysregulation of glycosphingolipids have also been implicated in autoimmune, metabolic, and other complex diseases [53, 54].
Glycosphingolipids are also promising targets for immunotherapy because they can induce therapeutic cytokines and cell-mediated responses [55]. One such types of cells, invariant natural killer T cells (iNKT cells) recognize glycosphingolipids and release cytokines that kill cancer or virally-infected cells, making this a potential modality to treat cancer [56, 57] and infectious diseases [55]. Knowing the exact molecular structures and the involved pathways would further advance therapeutic appliations of glycosphingolipids.
2. Analytical challenges
Within biological samples, glycosphingolipids co-present with phospholipids and are less abundant [58]. Higher levels of phospholipids interference with sample extraction and compete for ionization in mass spectrometric detection [59], which poses notable challenges for analysis of glycosphingolipids. For example in exraction, the Folch method uses methanol (CH3OH) and chloroform (CHCl3) (1:3, vol/vol) as the extraction solvent, which is effective in extracting the less complex global lipidome, but it is not effective for the more complex glycosphingolipids like gangliosides, as the latter requires higher aqueous proportion. In general, no single extraction method can recover all glycosphingolipid classes completely. As such, the current global lipidomics pipeline that relies on a single extraction method (e.g., Folch’s) may fail to detect and interrogate these molecules.
Glycosphingolipids are also problematic for detection using either optical or mass spectrometric techniques. The lack of chromophones makes them difficult to detect using optical methods. Such chromophores could be introduced by derivatization (e.g., 2-Aminobenzamide derivatization) to enable glycosphingolipid analysis using visible and fluorescence spectrophotometry. In mass spectrometry, heavily glycosylated glycosphingolipids suffer from low ionization efficiency, partly because of the decreased hydrophobicity and volatility [60, 61]. If present, labile groups such as sialic acid or fucose, further exacerbate this issue. These structures may be cleaved off during ionization and may be overlooked if the method is not tailored to these labile groups.
The presence of many structural variants often yields ambiguous results if not considered early in the method development pipeline. It is important to distinguish isomers because they may have distinct biological functions. Structural variants may result from the headgroup and the lipid tail. Headgroup variants involve molecules that differ in connectivity (positional isomers) or orientation in space (stereoisomers). In gangliosides, positional isomers may result from varied placement of sialic acid. Examples include: disialogangliosides (GD1a, GD1b, and GD1c) (Fig. 2A); monosialogangliosides (GM1a and GM1b); and trisialogangliosides (GT1a, GT1b, and GT1c) [21]. Positional isomers can also result from varying connectivity in the hexosyl ring. The following are some notable examples: 1) Gb3 and iGb3 isomers differ in the connectivity of terminal Gal to the second hexosyl [62] (Fig. 2B); 2) the sialic acid linkage position on the hexosyl ring can also be either α2–3 or α2–6 (Fig. 2C)[63]. These positional isomers are very important in virology, mounting evidence suggested that different sialic linkages in protein-linked glycans could dictate the degree of viral entry to host [64, 65]. A method dubbed as sialic acid linkage specific alkylamidation (SALSA) derivatization has been recently developed to distinguish different sialic linkages in glycosphingolipids [66]. Stereoisomerism in the glycan can result from a difference in the configuration of bonds in the hexosyl ring. The best example of stereoisomers is GlcCer and GalCer, which only differ in the orientation of the hydroxyl group at the C-4 position (Fig. 1A).
Figure 2. Representative structures that show the structural diversity of glycosphingolipids.
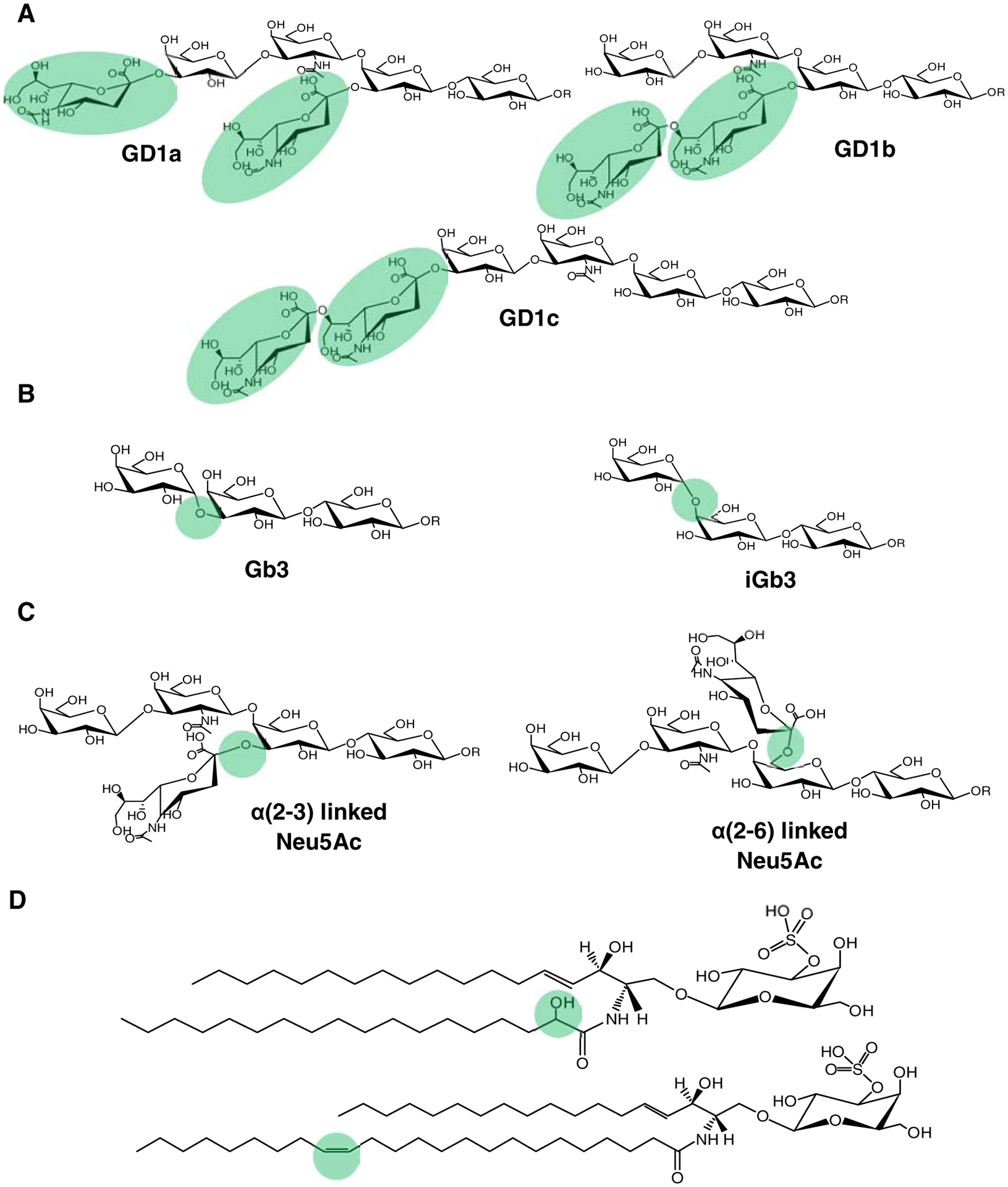
A) Disialogangliosides as examples of positional isomers with varying connectivity of sialic acid. B) Globotriaosylceramide positional isomers where the terminal galactose is linked differently. C) Positional isomers that differ in the sialic acid linkage. D) Structural variants in the lipid tail that often result in isomeric and isobaric species.
On the other hand, the presence of a hydroxyl group and carbon-carbon double bonds in N-fatty acyls yields isomeric and isobaric species (Fig. 2D). For example, the following species are both isobaric and isomeric: GalCer d18:1(n-14)/23:1(n-9)(OH), GalCer d18:1(n-14)/23:1(n-7)(OH), and GalCer d18:1/24:0, i.e., all have a nominal mass of 811 Da; two of them only differ in the double bond position of n-7 vs. n-9 [67]. The identification of these variants is difficult using traditional mass spectrometry techniques. Newer methods attempted to address these issues [66, 68–72], but their wide adoption remains limited.
Finally, a lack of commercially available standards obstructs the analysis of glycosphingolipids. To date, a few standards derived from natural sources (bovine or porcine brain) are available for gangliosides (GM1, GM2, GM3, GD1a, GD1b, GD2, GT1, and GQ1b), and neutral glycosphingolipids like cerebrosides and globosides (up to four sugar units). Most isotopically labeled standards are deuterated. However, the 13C-labeled standards [73] are more desirable for LC-MS based analysis because it ensures coelution of internal standards and target analytes in reversed-phase chromatography. No standards are available for the O-acetylated, lactonized, and sulfated complex glycosphingolipids that could greatly facilitate studies of these rare molecules.
3. Sample preparation
Various types of samples have been used for analyses of glycosphingolipids depending on the analytical goal and sample availability. Because of the uniqueness of each tissue or biofluid and the different sample matrixes associated with it, various methods have been developed to extract glycosphingolipids using either liquid-liquid or solid phase extraction (SPE) approaches or to enrich from complex sample matrix utilizing chemical or enzymatic modifications.
3.1. Type of samples
Glycosphingolipids exist in all tissue, organ and biofluid [3]. The tissues obtained from biopsy or post-mortem samples are widely used for immunohistochemical staining to identify the distribution of glycosphingolipids [74]. The rat brain tissue [20, 22, 71, 75–81] is often used as a model to demonstrate new analytical methods [20, 82, 83]. An appreciable amount of glycosphingolipids have been found in the following: lung [84], heart [85], intestines [86–88], kidneys [74, 89, 90], retina [91], ovarian cells [92] [93], breast cancer stem cells [94–97], fetal tissues [98, 99], and cerebrospinal fluids [100–102]. Blood and urine are suitable for biomarker studies because 1) they can be obtained quite easily in a less invasive procedure, and 2) during pathologic states, some of the glycosphingolipids could be shed-off from the surface of diseased tissues or cells and end up in blood circulation [103]. Urine could also reflect the pathologic state of specific organs such as the kidneys [104]. Recently, dried blood spots have also been used, especially in the screening of metabolic errors in infants [105].
3.2. Extraction
In biological matrix, glycosphingolipids co-exist with major lipids and biopolymers (proteins and nucleic acids). These molecules can suppress ionization of glycosphingolipids in mass spectrometric analysis and therefore must be removed during sample preparation [106]. Extraction is a common procedure to remove the interfering matrix molecules. Prior to extraction, homogenization is performed for tissues or cells; in the case of biological fluids, this step is not necessary, but the removal of suspended particles is needed (Fig. 3). Sample amounts are study dependent, and the following starting quantities are commonly used: 1.0 to 500 mg for tissues, at least 106 counts for cells, and 1.0 to 100 μL for biological fluids.
Figure 3. General workflow to extract and isolate glycosphingolipids.
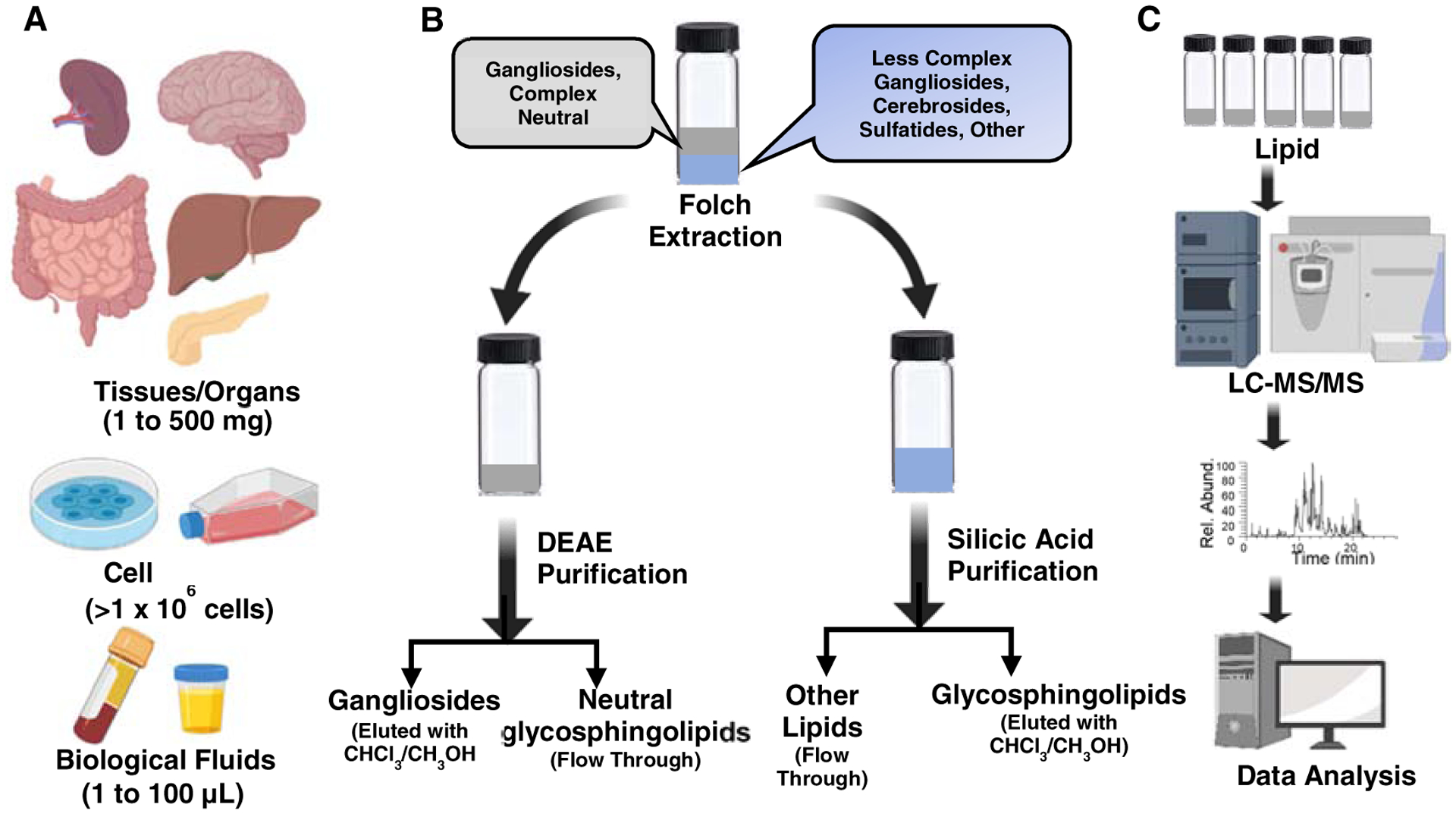
A) The tissue, cell culture or biological fluid is homogenized with CHCl3/CH3OH and partitioned with water. B) The top aqueous layer contains complex hydrophilic lipids such as gangliosides and complex neutral glycosphingolipids. Hydrophobic lipids including cerebrosides and sulfatides are in the lower layer. Further purification uses DEAE cellulose chromatography to recover neutral and acidic glycosphingolipids. Silicic acid chromatography is used to separate most lipids from glycosphingolipids. C) Lipid extracts (crude, or enriched for a specific class) can be directly analyzed by MS via direct infusion, or LC-MS/MS.
In lipidomics studies, total lipids are typically extracted using CHCl3/CH3OH (2:1) in the Folch method [107], CHCl3/CH3OH (1:2) in the Bligh and Dyer method [108], or 1-butanol/CH3OH (3:1) in the butanol-methanol (BUME) method [109]. Addition of CH3OH disrupts the interactions between lipids and biopolymers so the lipids can be dissolved in the organic phase more effectively. However, these methods are not very effective in recovery of the amphiphilic glycosphingolipids. Alternatively, the lower phase of the two-layer isopropanol: hexane: H2O (55:25:20, v/v) solvent system is used before and after CHCl3/CH3OH (1:1, v/v) extractions to further increase the recovery of complex glycosphingolipids [110]. The crude extract is dried and partitioned with CHCl3/CH3OH/H2O (30:60:8). The resultant CHCl3 lower phase contains phospholipids, cholesterol, and the more hydrophobic glycosphingolipids, i.e., cerebrosides and sulfatides. The upper phase contains complex neutral glycosphingolipids and gangliosides. Proteins and other debris remain in the interface. Cerebrosides and sulfatides can be recovered from the lower layer via optional peracetylation, followed by silicic acid column chromatography and deacetylation (Fig. 3b) [111, 112]. The impurities can be removed efficiently using acetylation/deacetylation, but this step destroys O-acetyl groups [111]. In silicic acid chromatography, phospholipids are retained on the column, while acetylated glycosphingolipids are eluted out using CHCl3/CH3OH gradient. Acidic and neutral glycosphingolipids can be separated using a weak anion exchange column, diethylaminoethane (DEAE)-Sephadex A25. Resultant glycosphingolipids fractions are purified by C18 or C8 SPE or dialysis using a membrane with 3 kDa molecular weight cutoff [112]. SPE cartridges of aminopropyl and silica gel packing materials can also be used to fractionate crude lipid extracts. Aminopropyl SPE cartridge has been used to isolate cerebrosides and globosides using acetone – methanol (9:1.35 v/v) solvent system [113]. However, this approach cannot separate sulfatides from sphingosine-1-phosphate and ceramide-1-phosphate. Silica gel SPE cartridge has been used to enrich sulfatides from crude extracts [114]. Titanium dioxide (TiO2) is also useful for glycosphingolipids [115]. More recently, selective phospholipid removal during ganglioside extraction was accomplished using Phree™ columns [116].
Gangliosides and sulfatides can be selectively extracted using simple procedures. Svennerholm and Fredman’s method [117] can be used for gangliosides. This method uses a higher proportion of polar solvents (methanol and water) to increase extraction efficiency since complex gangliosides are relatively more soluble in water. Svennerholm and Thorin’s [118] and Hara and Radin’s [119] methods are useful for sulfatides; the latter is CHCl3-free, which makes it a safer alternative. For dried blood spot samples, sulfatides can be efficiently extracted using methanol or isopropanol after being incubated with water [120]. Methods involving non-ionic detergent called hexaethyleneglycol mono-n-tetradecyl ether [121] and monophasic extraction [106] have also been described. Alternatively, preparative thin-layer chromatography (TLC) can be used to isolate specific classes of glycosphingolipids [122, 123]. Isolation is done by developing a TLC plate in an appropriate solvent system, followed by detection using reversible stains such as primuline, and then scraping the spots of interest prior to extraction using CHCl3/CH3OH.
On the other hand, glycosphingolipids are relatively resistant to alkaline hydrolysis compared to other ester-linked lipids. As such, alkaline hydrolysis can be used to eliminate major lipids such as phospholipids, and glycerides using dilute sodium hydroxide or potassium hydroxide in the presence of methanol [12, 124]. The resultant lower analytical background significantly improved the sensitivity of LC-MS/MS analysis of permethylated glycosphingolipids [125].
3.3. Novel enrichment strategies
The common enrichment strategies generally require biomeclues having functional groups/reactive sites, such as aldehyde, ketone, or free amine. These groups are lacking in glycosphingolipids but could be selectively generated by chemical or enzymatic modifications (Fig. 4). For chemical-based oxidation, solution-phase ozonolysis can be used to cleave the carbon-carbon double bond on the sphingosine backbone to introduce an aldehyde in the lipid. This method also cleaves any carbon-carbon double bonds in the N-fatty acyl, if present, providing more than one reactive site [126]. Another very selective way to introduce an aldehyde is through oxidation of the trihydroxy functional group in sialic acids using dilute sodium meta periodate (NaIO4) [127]. Other chemical oxidation approaches include oxidation of the sphingosine hydroxy group to allyl ketone using 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ)[128]. More recently, the oxidative release of the natural glycans (ORNG) method was used to oxidize the ceramide backbone of glycosphingolipids using sodium hypochlorite (NaOCl) [129]. The resultant nitrile can be reduced to an amine and subsequently used for other chemistries.
Figure 4. Strategies to introduce reactive functional groups in glycosphingolipids.
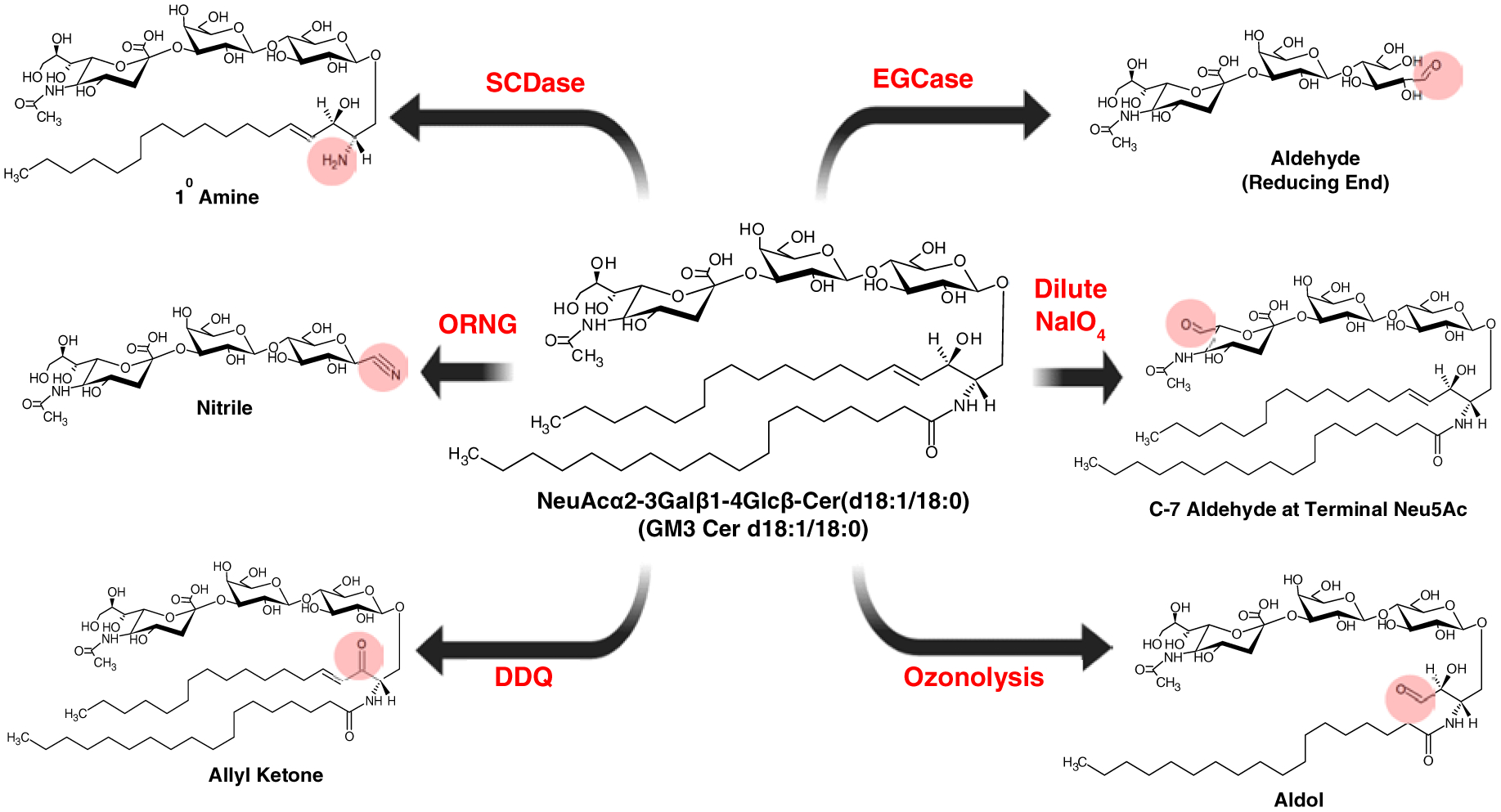
Sphingoid N-deacylase (SCDase) is used to remove the fatty acyl chain leaving behind the sphingosine backbone. Endoglycoceraminidase (EGcase) is used to remove the lipid part leaving the reducing end of the glycan intact. Oxidative release of natural glycans (ORNG) uses bleach (NaOCl) to convert the rest of the lipid tail to a nitrile group, which could be further reduced to form a primary amine group and used for other chemistries. Sodium meta periodate (NaIO4) can selectively oxidize the trihydroxyfunctional group of sialic acid to aldehyde. Selective oxidation of the sphingosine double bond can be accomplished using 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ). Ozonolysis cleaves the C=C on the sphingosine but could also cleave other C=C bonds if present.
Enzymes such as endoglycoceramidase (EGcase) [103, 130, 131], or sphingolipid ceramide N-deacylase (SCDase) [132, 133] can also be used to add reactive sites to glycosphingolipids. EGcase cleaves the glycosidic bond between the ceramide and the glycan moiety to form the oligosaccharide with a free reducing end (aldehyde form). SCDase cleaves the amide bond releasing the fatty acyl chain while leaving behind a free amino group.
Glycoblotting can be used to enrich glycans released from glycoproteins [134] and glycosphingolipids [103, 135] to improve sensitivity in mass spectrometry [126]. Here, an appropriate glycan release strategy introduces an aldehyde group (Fig. 4). The aldehyde forms a covalent bond via reduction with the amino groups on the beads, typically hydrazide. The excess hydrazide groups are blocked by esterification, and the glycans are then recovered by transamination, and collected as an aqueous solution (Fig. 5). Another approach is to immobilize the glycosphingolipids on the polyvinylidene difluoride (PVDF) membrane, followed by EGcase treatment [130]. The hydrophobic PVDF facilitates immobilization of glycosphingolipids through interaction between the PVDF surface and the ceramide backbone, so that the glycan portion is adequately exposed to EGcase. This technique was successfully applied to analysis of glycosphingolipids in ovarian cancer cells and tissues.
Figure 5. Glycoblotting technique to enrich glycosphingolipids.
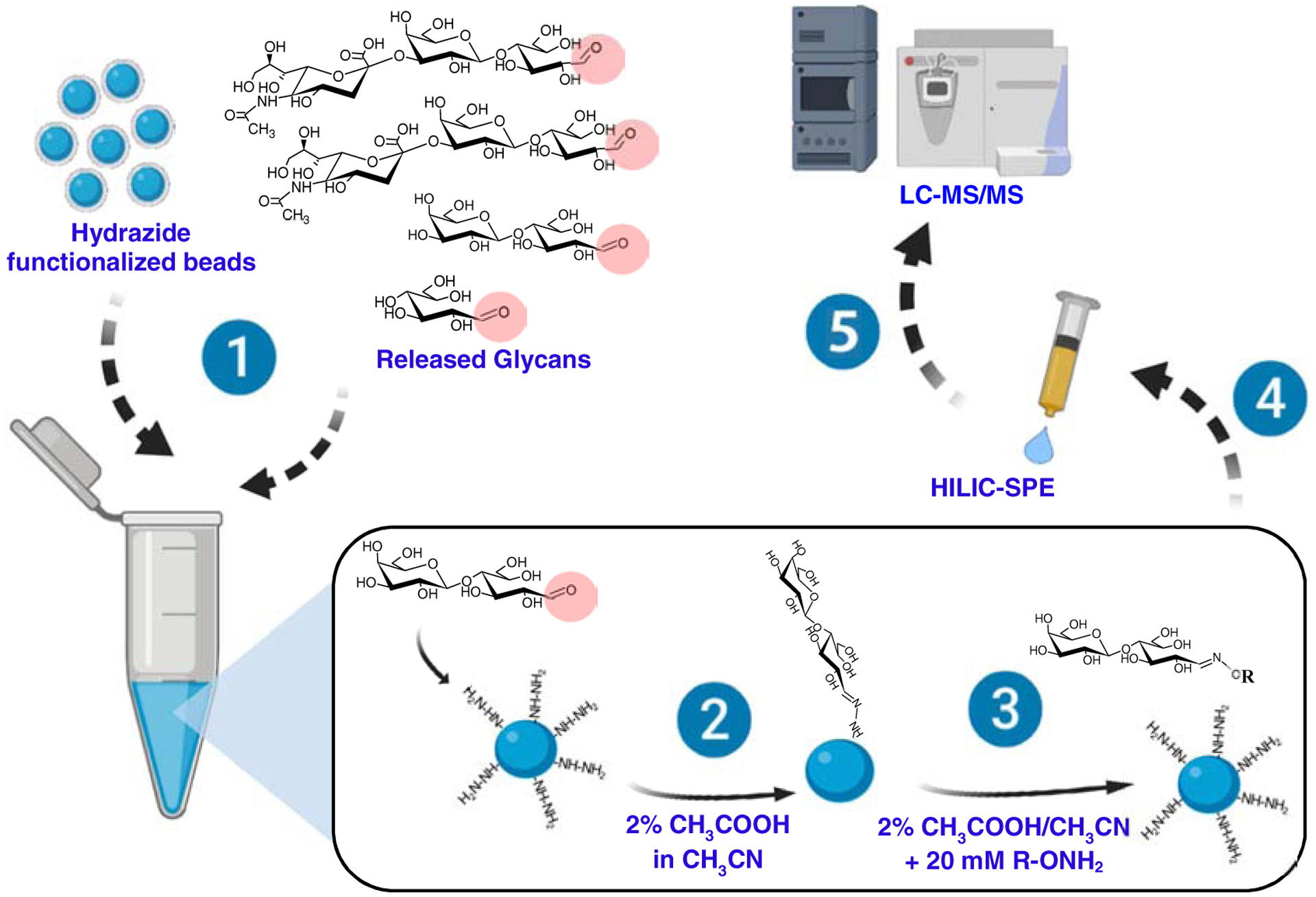
Glycoblotting involves the following steps: 1) Glycans with a free reducing end (e.g., aldehyde) are mixed with amine-functionalized beads; 2) Reductive amination takes place to conjugate the glycans on the beads; 3) After washing, the glycans are released by transamination, typically using an aminooxy-functionalized compound, R-ONH2; 4) The released glycans are purified by HILIC-SPE; and 5) Analyzed by LC-MS/MS.
4. Common ionization methods
Mass spectrometry has emerged as an essential tool to structurally characterize and quantitavely profile glycosphingolipids [7, 10, 11, 136–138]. One of the keys to a successful mass spectrometric analysis is the choice of the appropriate ionization method, which is both sample and study dependent. In this section, we discuss the soft ionization techniques commonly used for glycosphingolipid analysis.
4.1. Electrospray ionization (ESI)
ESI is the most commonly used method for ionization of biomolecules, particulary when front end chromatographic separation is coupled online to mass spectrometric detection. In ESI, glycosphingolipids are ionized by electrospray (typically, 1–3 kV), and form [M+alkaline]+, [M+H]+, or [M+NH4]+ adducts in the positive mode, and [M-H]−, [M+HCOOH-H]− or [M+halide]− in the negative mode, depending on the composition of the solvent used to dissolve the sample. The most common form is [M+Na]+ because sodium ion is ubiquitous, and glycosylated molecules have a high affinity toward sodium ion. This is especially the case when sodium formate is used to calibrate mass spectrometers, and no lengthy flushing of the ESI source is made before infusing samples. When added in excess, acetic acid or formic acid overwhelms sodium ions and favors [M+H]+ ions [139, 140]. In most cases, neutral glycosphingolipids are detected as [M+NH4]+, [M+H]+, [M+Na]+ or [M+Li]+ (when a source of lithium ion, such as lithium chloride is introduced) while acidic glycosphingolipids are detected mostly as [M-H]−, [M+HCOOH-H]− or [M+halide]− ions. Gangliosides with more than one sialic acids form multiply charged species as [M-2H]2− and [M-3H]3− [141]. Besides spiking the LC mobile phase with ammonium formate, sodium ion or lithium ions can be infused post-LC using a T-connector [142]. The use of different adducts could impact fragmentation behaviors. For example, fragmentation of [M+Na]+ but not [M+H]+, can be used to differentiate GlcCer and GalCer [72]. In ozone-induced dissociation (discussed in Section 5.6), [M+H]+, [M+Na]+, and [M+Li]+ can reveal distinct structural information. These suggest that early on in an analytical method development, it is important to consider appropriate solvent and select appropriate adducts for downstream analysis.
4.2. Matrix-assisted laser desorption ionization (MALDI)
Besides ESI, MALDI is another commonly used soft ionization technique in biomolecular analysis, which unlikes ESI, typically generates singly charged molecular ions. This ionization method starts with sample co-crystallized with an Ultraviolet (UV)-absorbing organic acid matrix on a metallic plate, and then irradiated by laser to generate ions [143]. MALDI was first applied to glycosphingolipids by Costello and colleagues (for a comprehensive review, see Ref. [144–147]). Common matrices used for glycosphingolipids include 2,5-Dihydroxybenzoic acid (DHB), 1,5-Diaminonapthalene, 4-Hydrazinobenzoic acid, and 6-Aza-2-thiothymine [144]. Selective ionization of sulfatides can be achieved using 9-Aminoacridine (9-AA) [148]. MALDI can operate in positive and negative ionization modes to yield [M+Na]+ and [M-H]− ions, respectively. The negative ions are more sensitive and provide richer fragmentation for gangliosides [144]. In contrast to ESI, MALDI is more tolerant to salts and can ionize even the higher mass glycosylated lipids, but they generally have lower ionization efficiency.
Poor choice of a matrix can result in potential post-source decay of glycosphingolipids, which results in prominent decarboxylation reactions and loss of sialic acid moiety, especially for gangliosides. Infrared lasers and collisional cooling have been introduced to address this issue [149, 150]. Alternatively, gangliosides are often permethylated [151] to increase the stability of sialic acids while improving their overall hydrophobicity, volatility, and ionization efficiency. MALDI-MS suffers from high background noise due to matrix clusters that preclude the detection of low molecular weight glycosphingolipids [152]. Newer methods, although matrix-free, have not yet been used for glycosphingolipids [147]. Without prior off-line chromatographic fractionation, MALDI-MS cannot resolve isomers and is prone to ion suppression effects, especially in biological samples. To resolve this, MALDI-MS has been coupled to ion mobility spectrometry but not yet used for glycosphingolipids [153].
Sample spotting on the MALDI plate can be tricky, which may result in uneven sample deposits and variable ion intensities. This issue has been addressed using a normal phase nano-HPLC coupled with an automated robotic spotter; the developed method has been used to analyze a mixture of acidic and neutral glycosphingolipids [154]. Briefly, the samples were separated using normal phase nano-HPLC, and the eluting compounds were precisely collected on the MALDI plates and co-crystallized with matrix [154]. This method gave homogeneous spots; however, it is quite tedious and may be impractical for routine, high throughput applications.
The use of MALDI-MS for quantitation of glycosphingolipids remains challenging, until recently, when quantitative MALDI-TOF MS/MS was demonstrated [103]. After endoglycanase digestion and glycoblotting to prepare the sample, a total of 42 distinct glycosphingolipids were detected in human serum at a concentration of 12.1 to 21.4 μM; this is lower than those of N-linked glycans which were 700 to 850 μM [103]. However, the distributions, both spatial and anatomical, of glycosphingolipids in tissues, are mostly unknown. MALDI-MS is an emerging tool for imaging (also called MALDI imaging mass spectrometry) that can provide both molecular and spatial distributions of analytes [75, 155–160]. The use of MALDI imaging mass spectrometry may unearth previously unknown biology of glycosphingolipids. The reader is referred to a comprehensive review on this subject [161] and we will not elaborate here.
5. MS/MS fragmentation
Tandem mass spectrometry (MS/MS) is essential in structural elucidation of glycosphingolipids [7], which is relying on multiple ion dissociation (fragmentation) techniques that can be broadly classified into four categories: 1) collision-induced; 2) electron-aided; 3) photoinduced; and 4) chemical-induced dissociations. Each dissociation method cleaves bonds at different locations of the molecule and the combined use of these methods can provide complementary and comprehensive structural details of glycosphingolipids. Based on the predictability of fragmentation pathways, structural databases could be constructed in silico to identify both the known and unknown glycosphingolipids by matching experimental MS/MS spectra with the database.
5.1. Nomenclature of fragments
The systematic naming of MS/MS fragments was proposed by Domon and Costello [162] and later modified by Ann and Adams to include more detailed lipid backbone fragments [163]. The fragments are labeled based on whether the fragment contains the non-reducing end (A, B, and C) or the reducing end (X, Y, and Z) (Fig. 6). Fragments B, C, Y, and Z correspond to glycosidic bond cleavages that determine carbohydrate sequence, while A and X are cross-ring cleavages that mark linkage positions. The superscripts in cross-ring cleavages denote which bond is fragmented, for example, cleavage of the bonds between C1-C2 and C5-O forms the 1,5X ion. The ceramide fragment ions include E, S, T, U, and O” which result from the cleavage of glycosidic bond between the ceramide and the glycan. Among them, the major fragment ions are the O” and E. The O” fragment is diagnostic of the long-chain base composition (Fig. 6B).
Figure 6. Nomenclature of glycosphingolipid fragments generated in MS/MS.
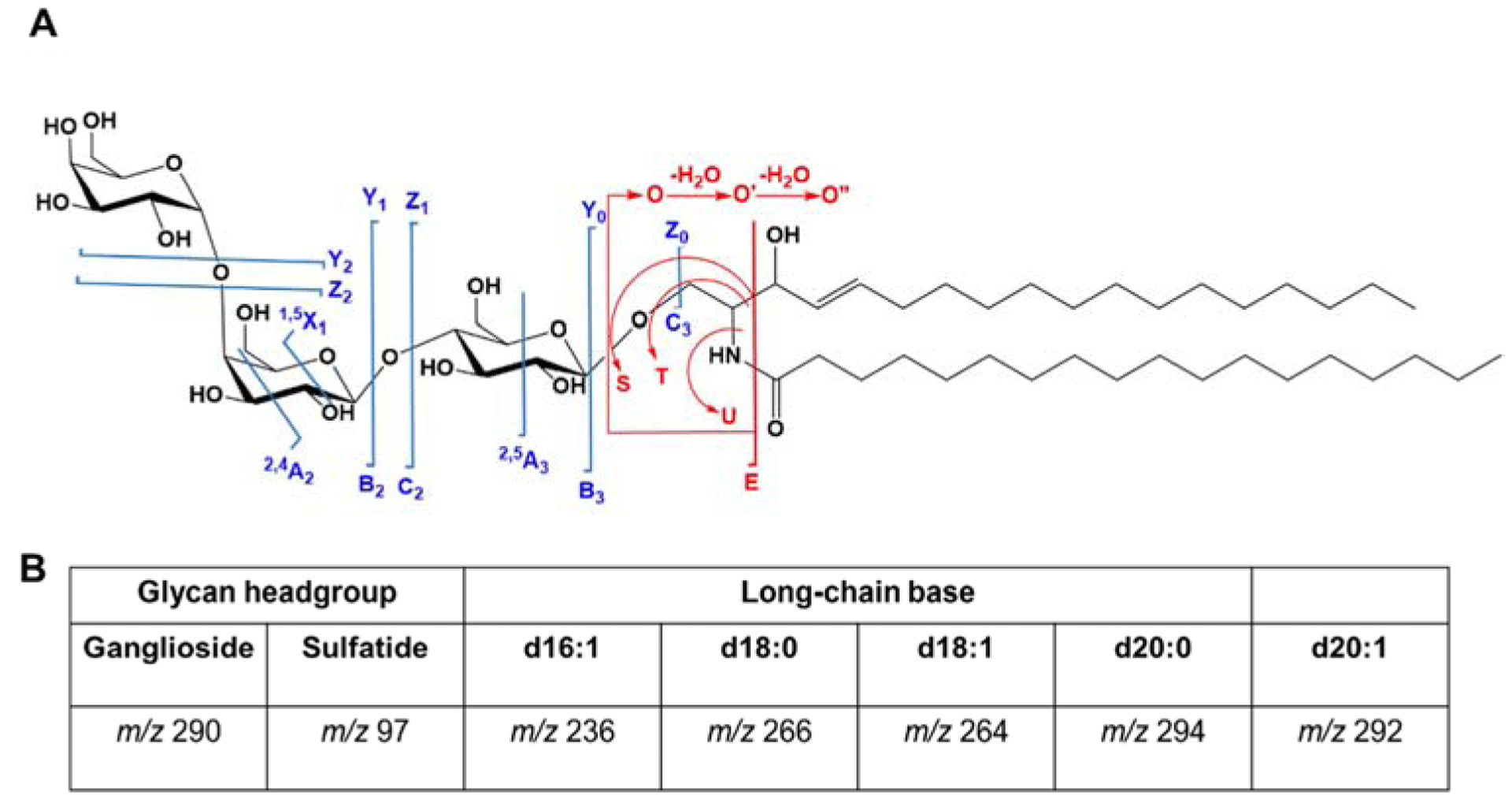
A) The common fragments observed involving ceramide backbone (in red) and glycan linkage (in blue) are shown. B) The diagnostic fragment ions in MS/MS spectra. The m/z 290 and m/z 97 are observed in negative mode, while m/z 236, m/z 266, m/z 264, m/z 294, and 292 (O” fragment ions) are in positive mode.
5.2. Collision-induced dissociation
Collision-induced dissociation is the bread and butter of all dissociation techniques and the default fragmentation technique for MS/MS in modern mass spectrometers. It includes low energy collision-induced dissociation (CID) and higher-energy collisional dissociation (HCD), with the latter uniquely on orbitrap instruments. The CID fragmentation of neutral and acidic glycosphingolipids has been extensively reviewed [7].
Both CID and HCD provide glycan and lipid structural information. The B and C type ions aid in elucidating the glycan sequence. Incremental mass differences of 162 Da and 204 Da indicate the loss of a hexose and N-acetylhexosamine, respectively. Sialic acid and sulfate groups can be confirmed using m/z 290 and m/z 97, respectively, in the negative mode. The lipid composition can be identified from the following in positive mode: m/z 266 (d18:0), m/z 264 (d18:1), m/z 236 (d16:1), and m/z 292 (d20:1) [9]. These ions are characteristic in targeted tandem mass spectrometric methods for the known and are also very useful in structural elucidation of novel glycosphingolipids when combined with accurate mass measurement [140, 164, 165].
One caveat of CID-MS/MS is that it cannot distinguish isomers with subtle structural differences even having chromatographic separation in the front end. Multi-stage collisional dissociation is more capable in this respect. For example, globo- and isoglobo-glycosphingolipids can be differentiated in cell extracts using MS5 experiments [62].
5.3. Electron-aided dissociation
Electron-aided dissociation involves two general steps: 1) precursor ions are converted to radical ions; and 2) the metastable radical ions dissociate. Thorough discussion on electron-aided dissociation is provided in a recent review [166]. While sharing similar strategy in ion dissication, electron capture dissociation (ECD), electron detachment dissociation (EDD), and electron transfer dissociation (ETD) differ in how radical ions are formed. In ECD, multiply charged positive ions pass through a beam of low energy electrons, and capture the electrons to form radical cations. EDD operates just like ECD but applies to multiply charged negative precursors instead. Both have been implemented mainly in FTICR instruments, but attempts have been made to integrate them into the more accessble instruments such as orbitrap, which would allow greater access to the scientific community [167]. EDD is relatively inefficient for glycosphingolipids, while ECD provides extensive fragmentation that could aid structural elucidation.
In ETD, the precursor ions react with radical anions generated in situ, e.g., fluoranthene [166]. The reaction generates odd and even electron fragments, and ions with a lower charge state. This was used initially in peptide sequencing [168] but was shown to work equally well for oligosaccharides. ETD yields ceramide backbone fragments and identifies acetylated glycans [169]. To our knowledge, ETD has not been applied to glycosphingolipids, probably because most of these molecules seldom form multiply charged positive ions, a prerequisite for using this technique.
5.4. Ultraviolet photodissociation
Photoinduced dissociation uses energetic photons to activate and fragment chemical bonds. Ultraviolet photodissociation (UVPD) uses UV light to irradiate precursor ions in a collision cell [170]. The typical wavelengths used are 266 nm, 213 nm, 193 nm, and 156 nm, depending on the application. The 193 nm has been shown to provide extensive fragmentation of both the glycan headgroup and the lipid backbone in gangliosides and neutral glycosphingolipids [171]. While CID and HCD produce only B/Y type and C/Z type ions (Fig. 7), UVPD provides more informative cross-ring fragments (A/X types) to differentiate isomeric species [171]. This approach can also provide some level of fragmentation adjacent to the carbon-carbon double bonds to pinpoint their location, albeit at lower efficiency [172]. Being the dissociation technique generated the most comprehensive, structurally-informative fragmentation ions for glycosphingolipid anlaysis, this method, however, lacks wide adoption by the community partly due to the lack of software for automated spectral annotation.
Figure 7. Fragmentation of ganglioside GD1 using different techniques.
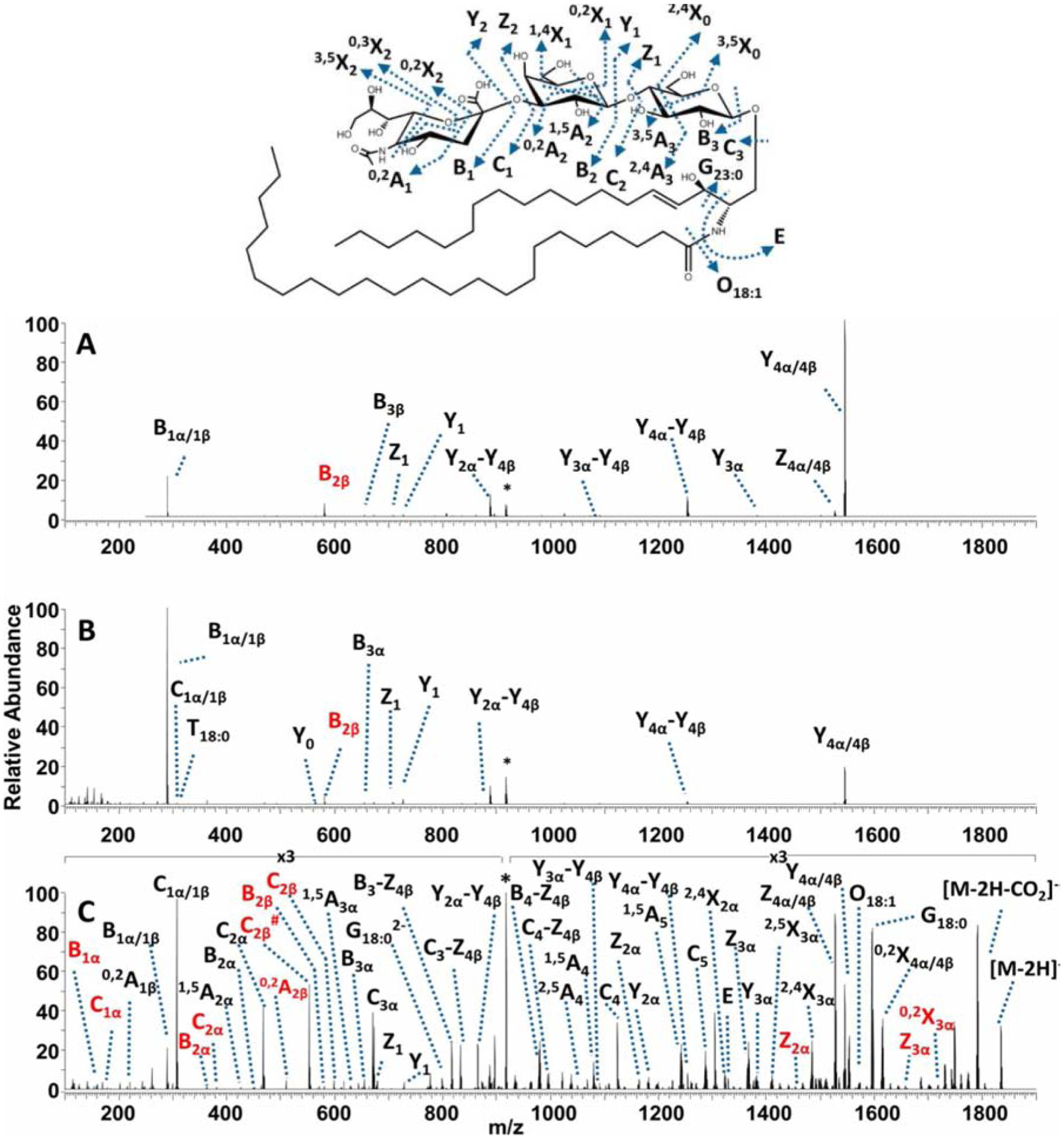
(A) CID, (B) HCD, and (C) UVPD mass spectra of the doubly deprotonated porcine brain ganglioside GD1 18:1/18:0. The precursor ion in each spectrum is labeled with an asterisk. Fragment ions corresponding to GD1a 18:1/18:0 are labeled in black. Fragment ions corresponding to GD1b 18:1/18:0 are labeled in red. Reprinted with permission from O’Brien, J.P. & Brodbelt, J. Anal. Chem., 85, 10399–10407 (2013)[171] © 2013 American Chemical Society.
5.5. Radical directed dissociation
Radical directed dissociation (RDD) is unique in that it combines the principles of electron-aided activation, collisional dissociation, and photoinduced dissociation. RDD involves three steps: 1) derivatization to introduce chromophores in the molecule; 2) generation of radical ion using UV, and 3) fragmentation of the radical ion using CID or HCD. RDD requires attachment of chromophores with labile bonds, e.g., carbon-iodine bonds that break via homolytic cleavage to form radicals. These radicals abstract protons from the analyte ions to form a radical cation before being fragmented using CID.
RDD was used by Pham and Julian to differentiate the glycosphingolipid epimers (GlcCer and GalCer) [69]. Briefly, lyso-glycosphingolipids were prepared from intact glycosphingolipids by removal of the N-fatty acyl chain using alkaline hydrolysis in aqeous solution. The product was non-covalently complexed with a molecule that contains a labile carbon-iodine bond [4-iodobenzoyl 18-crown-6 (IB18C6)], where the free amino group in the lyso- species served as an anchor for IB18C6. Photodissociation (PD) using 266 nm UV laser cleaved the carbon-iodine bond to form a radical ion. The radical ion was mass-selected for CID fragmentation to give the RDD (PD followed by CID) spectra (Fig. 8). The two epimers gave the same major fragment ions (i.e., m/z 443 and m/z 250) but inverted abundance of the fragments. The difference in abundance was attributed to the differential ability of the epimers to lose water during fragmentation (neutral loss of water is easier in galactose than in glucose) which in turn depends on the configuration of the -OH group at the C-4 position. This approach could potentially be used to distinguish GalCer and GlcCer, but wide application to analysis of other glysosphingolipids would likely be limited due to the complicated sample preparation and ion dissociation processes involved.
Figure 8. RDD spectrum of lyso-GalCer and lyso-GlcCer.
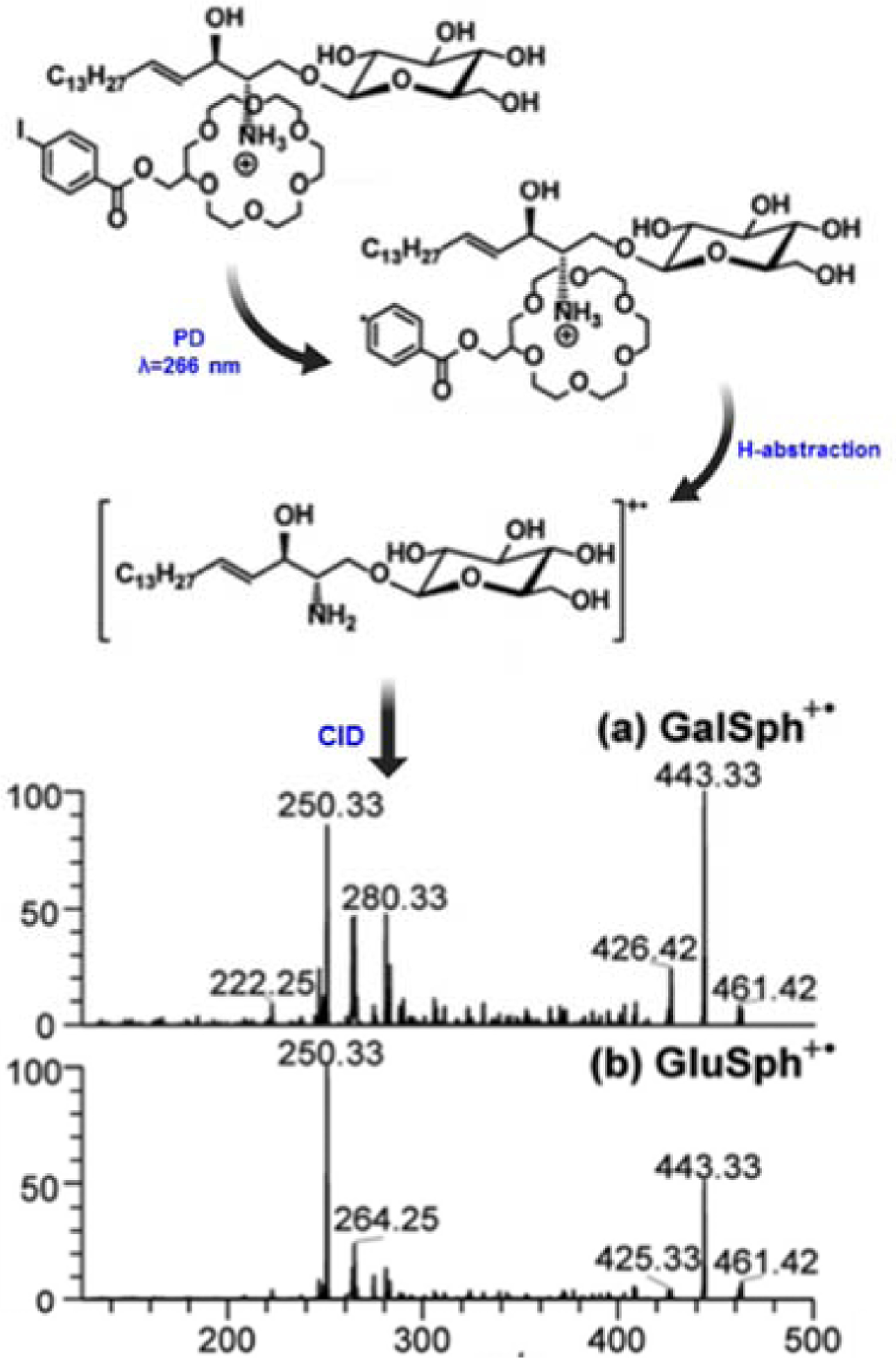
In RDD, lyso-glycosphingolipids were prepared from intact glycosphingolipids by alkaline hydrolysis, and then complexed via non-covalent bond with 4-iodobenzoyl 18-crown-6 (IB18C6). The complex was photoactivated using UV at λ=266 nm to cleave C-I bond and form a radical cation. The resultant radical cation was subjected to CID fragmentation to yield the RDD (PD followed by CID) spectra shown. Reprinted and modified with permission from Pham, H., and Julian, R. Analyst, 141, 1273–1278 (2016) [69] © The Royal Society of Chemistry.
5.6. Ozone-induced dissociation
Ozone induced dissociation (OzID) is based on alkene and ozone (O3) reaction chemistry [173]. Historically, this approach was used to elucidate double bond locations in lipids using gas chromatography. In 2008, Blanksby’s group implemented this reaction inside a mass spectrometer by replacing collision gas with O3 and O2 mixture [174]. In this reaction, O3 forms ozonide through a cycloaddition reaction; the metastable ozonide spontaneously decays to the more stable Criegee and aldehyde product ions, which can indicate position of the double bond [173].
Recently, we implemented OzID in a high-resolution mass spectrometer with enhanced ozonolysis efficiency [175]. We studied unsaturated glycosphingolipids, for example, LacCer d18:1(4E)/18:1(9Z) (Fig. 9). OzID fragmentation of the [M+Na]+ adduct gave two pairs of products (Criegee and aldehyde) corresponding to each double bond [67]. The mass difference between OzID products and the precursor ion was used to pinpoint the double bond position [173]. We were able to differentiate isobars and isomers in porcine brain galactocerebrosides mixture, which were non-distinguishable by CID alone [67]. The differential reactivity of the long-chain base double bond and the fatty acyl double bond was very useful in assigning which acyl chain contains the double bond. We also found that the OzID fragmentation behavior of glycosphingolipids could be dictated by the adduct type: [M+Li]+ and [M+Na]+ fragment mainly at the double bond position; [M+H]+ undergoes dehydration followed by CID-like loss of the glycan head group [176].
Figure 9. Gas-phase ozonolysis of LacCer d18:1/18:1(9Z) in a modified mass spectrometer.
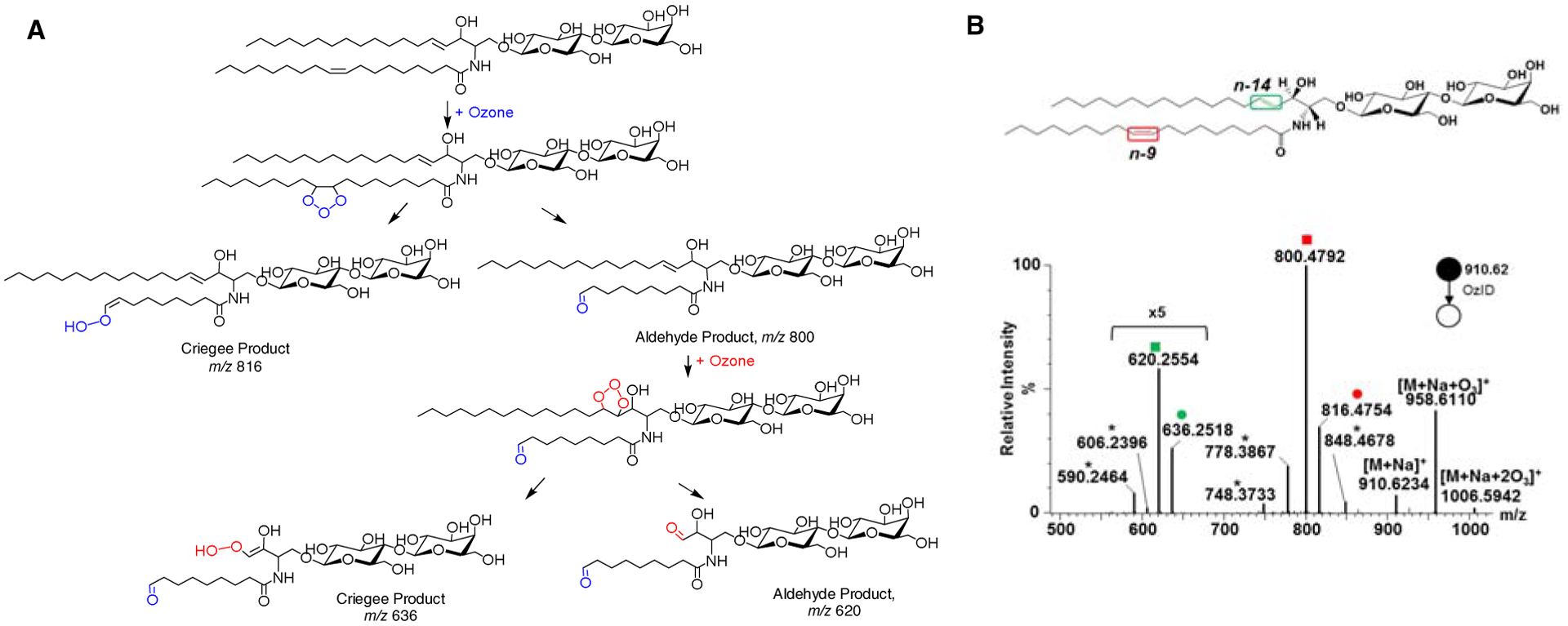
A) Proposed reaction pathway showing selective cleavage of carbon-carbon double bonds producing Criegee and aldehyde ions. B) OzID-MS spectrum showing the Criegee and aldehyde product ions are depicted as (●) and (■), respectively. Open square (□) and circle (○) indicate ions generated through the elimination of H2C=O from the aldehyde ion and Criegee ion, respectively. Mass differences (neutral losses) between the precursor ion and Criegee/aldehyde ions are used to pinpoint the location of double bonds. Asterisk (*) indicates ions generated through secondary oxidation from [M+Na+O3]+. Colors represent different double bond locations. Reprinted with permission from Barrientos R.C. et al. J. Am. Soc. Mass Spectrom., 28, 2330–2343 (2017)[67] © American Society for Mass Spectrometry.
Taken together, the various fragmentation techniques discussed here require different instrumental setup and provide distinct levels of structural information. Depending on the study aims, necessary information like glycan sequence, lipid composition can be obtained from CID and HCD. More detailed structural features like branching and linkage in the glycan and double bond position in the lipid can be determined using more specialized techniques (ECD, EDD, UVPD, RDD, and OzID).
6. Common operating modes of tandem mass spectrometry
CID-MS/MS generates fragment ions diagnostic of the glycan composition, long-chain base, or N-fatty acyls (see Section 5.2). For instance, m/z 264 is characteristic of the long-chain base d18:1 (sphingosine backbone) (Fig. 6b). Sulfatides and gangliosides yield m/z 97 and m/z 290 attributed to the sulfate and sialic acid moieties, respectively. When measured with high resolution mass spectrometers such as Fourier transform ion cyclotron resonance (FTICR), time-of-flight (TOF), or orbitrap that are capable of distinguishing isobaric species and allowing the assignment of elemental compositions [177], these characteristic fragment ions and the corresponding precursor ions permit identification of glycosphingolipids in the untargetd workflows and differentiation of ganglioside positional isomers. In this respect, high resolution untargeted MS/MS, when combined with HILIC-based separation, raises the total number of ganglioside species identified to 145 in a diverse set of tissues [178].
The characteristic fragment ions, however, are more oftenly used in the low resolution triple quadrupole mass spectrometers for targeted analysis of glycosphingolipids. This can be achieved through different operating modes of MS/MS, namely, precursor ion scan and multiple reaction monitoring (MRM) (Fig. 10). In the precursor ion scan, all mass-selected ions from the source are fragmented, and a diagnostic ion is monitored. For example, the precursor ion scan has been used in ganglioside analysis, where all ions were scanned for the presence of m/z 290 fragment [179]. In MRM, specific precursor-product ion pairs are monitored for improved sensitivity and specificity. A sensitive MRM method has also been developed for comprehensive quantification of various classes of gangliosides based on the sialic acid-specific ion of m/z 290 and for further differentiation of isomeric glycosphingolipids based on diagnostic ions unique to the terminal regions of the sugar chains [141, 180]. Similar approach has been applied to measure Gb3 in plasma and urine of Fabry disease patients by monitoring the loss of m/z 162 [181].
Figure 10. MS/MS scan modes used in the analysis of glycosphingolipids.
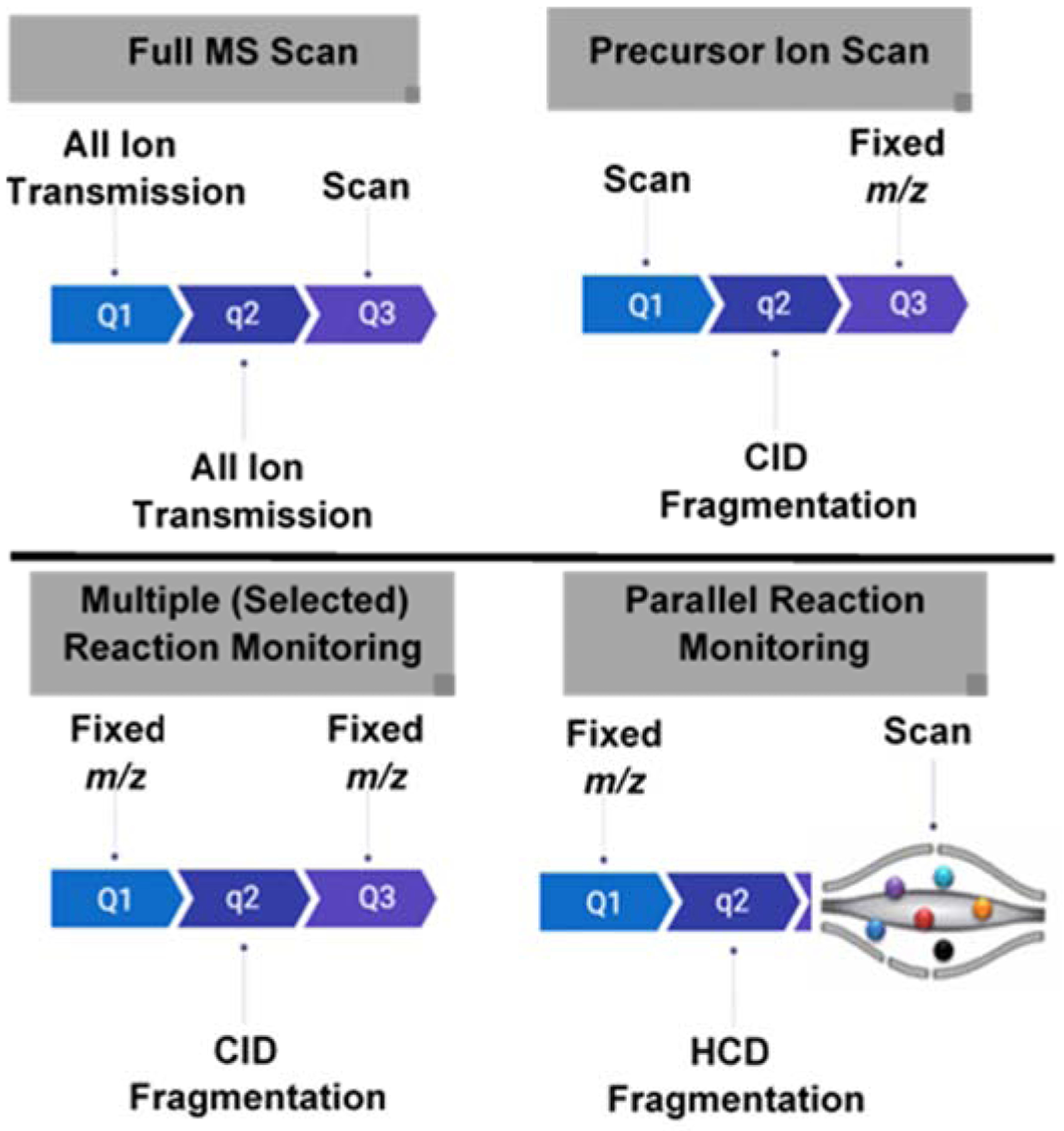
In full scan, all ions generated from the source are detected without fragmentation. In precursor ion scan, all ions generated from the source are isolated and fragmented while scaning to detect which precursor ion generating a predefined fragment ion. In multiple reaction monitoring (MRM), a fixed predefined precursor-product ion pair is monitored. In parallel reaction monitoring (PRM), a predefined ion is mass-selected and fragmented; all product ions are detected.
One feature of MS/MS in orbitrap-based instruments is parallel reaction monitoring (PRM). Unlike MRM that uses only predefined MS/MS transitions without preserving information for all fragment ions in the MS2 scan, PRM provides both precursor ion information (MS1) and all generated ions during MS/MS, thus providing a wealth of data for identification and quantitation. This was applied for in-depth profiling of sphingolipids [165], and for quantitative analysis of gangliosides after isobaric labeling [140], which will be detailed below (section 8.3).
7. Absolute quantification
Omic level quantification of biomolecules is often conducted relatively, i.e. comparing the changes of a molecules in one group of samples (e.g. disease) relative to another group (e.g. controls). While this serves the purpose of molecular profiling or biomarker discovery studies, it is not adequate in the aspect of biological modeling or clinical diagnosis, where knowing the absolute amount of the anlyate is critically important. Considering the complex biological matrices the glycosphingolipids typically reside in, internal standardization and standard addition are commonly used in absolute quantification by mass spectrometry, both requires standard calibration curves to interpolate the unknown concentration of analytes in a sample.
Internal standardization has the advantage of normalizing any variation introduced during sample preparation, chromatography, and mass spectrometry analysis, which requires compounds spiked into the sample mimic the physical and chemical traits of the analytes. Deuterated, 13C-, or 15N-labeled versions of the analyte are frequently used for such a purpose. The 13C and 15N-labeled standards are more desirable because unlike deuterium (2H), these isotopes do not perturb the overall physical and chemical properties of the analyte, for example, they coelute perfectly with endogenous anlaytes and have the same ionization efficiency [12]. Briefly, a known amount of internal standards is spiked into the samples prior to extraction. After extraction, samples, along with calibration standards are analyzed by LC-MS/MS [12]. Because glycosphingolipids are less abundant compared to other molecules in a biological sample, they are often measured by the more sensitive targeted mass spectrometry methods, typically using MRM scan mode on a triple quadrupole instrument. The ratios of the peak areas of analyte to the internal standards are plotted against the concentration of analytes to construct a linear calibration curve. The concentration of glycosphingolipids in the unknown sample is interpolated using this curve. Internal standardization technique has been applied to quantify glycosphingolipids in samples derived from patients with lysosomal storage diseases in GM3 deficiency [182], determination of Gb3 and lyso-Gb3 in Fabry disease [183] and of sulfatides in dried-blood spots [105] as well as in tissues and biological fluids [114]. The same approach has also been applied to measure glucosylceramide, glucosylsphingosine and galactosylsphingosine in biological samples [184–186].
In the case where isotopically-labeled standards are not available or unaffordable, standard addition method may be a practical alternative. Here, unlabeled standards (i.e. analytes) of known amounts are spiked to the sample at increasing concentration and analyzed by LC-MS/MS. The peak areas are plotted against concentration of the spiked standards to create a linear calibration curve for each analyte. The value of x-intercept, i.e., x at y = 0, is the concentration of glycosphingolipids in the unknown sample. This approach has been used to quantify gangliosides in milk samples [187, 188].
8. Labeling approaches for qualitative and quantitative analysis
Glycosphingolipids are low abundant compared to other lipids and have low ionization efficiency, especially the heavily glycosylated ones. Chemical derivatization strategies are often used to improve the detectability and enable a more accurate and multiplexed quantification. In this section, we focus on the most common chemical labeling strategies in mass spectrometric analysis of glycosphingolipids.
8.1. Permethylation
The active protons (-OH and -NH) in glycans react with methyl iodide in the presence of sodium hydroxide and dimethylsulfoxide [189][190] (Fig. 11). This permethylation reaction enhances the volatility and sensitivity of the analytes. It has been used for qualitative analysis of glycans released from glycoproteins and some quantitative analysis using direct infusion ESI-MS or MALDI-MS [60, 191–193].
Figure 11. Labeling of glycosphingolipids by isotopic permethylation.
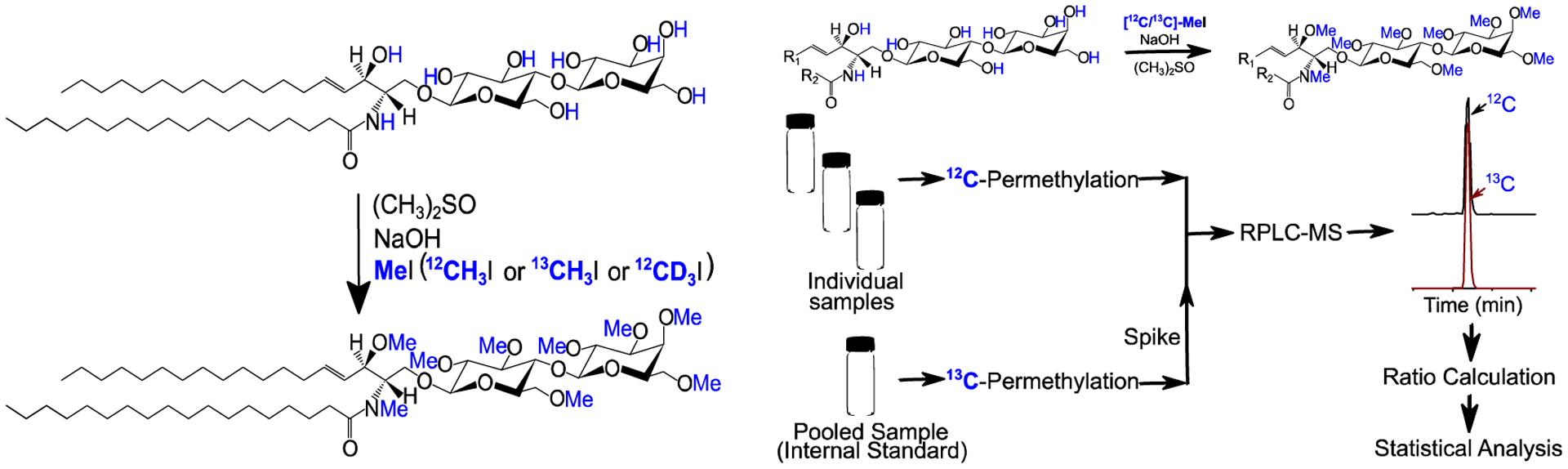
Shown here are the conversions of all active protons (−OH and −NH) using isotopically labeled methyl iodide (MeI) in the form of 12C, 13C, or 2H in the presence of alkali hydroxide (commonly, NaOH) and an aprotic solvent (dimethyl sulfoxide) under anhydrous conditions.
Permethylation can be performed using both the natural and isotope-labeled methyl iodide (13C or 2H atoms). When combined, the so-called differential isotope labeling technique is very useful in quantitative analysis [192, 193]. Here, individual samples are labeled with one version of the isotope and a reference sample is labeled with another version, (Fig. 11). The pooled mixture is then measured as a single sample for MS analysis, where the ratio of intensities serves as a surrogate of analyte concentrations. We recently implemented differential isotope labeling by permethylation and reversed-phase LC-MS/MS to quantify intact glycosphingolipids [125]. This method addressed the lack of commercially available 13C-labeled internal standards and the low sensitivity of glycosphingolipids in the presence of total lipids extract. Aliquots from individual samples were pooled to serve as a reference sample and permethylated with 13CH3I; the individual samples were permethylated using 12CH3I. After mixing, final samples (13CH3I and 12CH3I labeled) were analyzed by reversed-phase LC-MS/MS (Fig. 11). We observed up to 20-fold signal enhancement in the permethylated samples. We also found that highly alkaline conditions of permethylation depleted those ion-suppressing ester-linked lipids. The MS/MS fragments and retention time provided different levels but complementary information for compound identification. These results were encouraging and illustrated the usefulness of permethylation for quantitative analysis of low-abundant glycosphingolipids in complex mixtures.
8.2. PAEA labeling
To improve the ionization efficiency of gangliosides, Huang and co-workers developed a labeling approach for monosialogangliosides (GM) using 2-(2-Pyridilamino)-ethylamine (PAEA) [194]. In this approach, GM species were derivatized using 4-(4,6-Dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride (DMTMM) and PAEA, with DMTMM as amidation reagent (Fig. 12) [194]. The PAEA was conjugated to the carboxylic acid group of sialic acid. The signal intensity was enhanced by 15-fold in positive mode compared to the underivatized analog. Coupled with MRM-based LC-MS/MS analysis, this derivatization method was successfully applied to determine the monosialoganglioside levels in plasma of patients with GM3 synthase deficiency [194].
Figure 12. Labeling of intact, monosialoganglioside using PAEA.
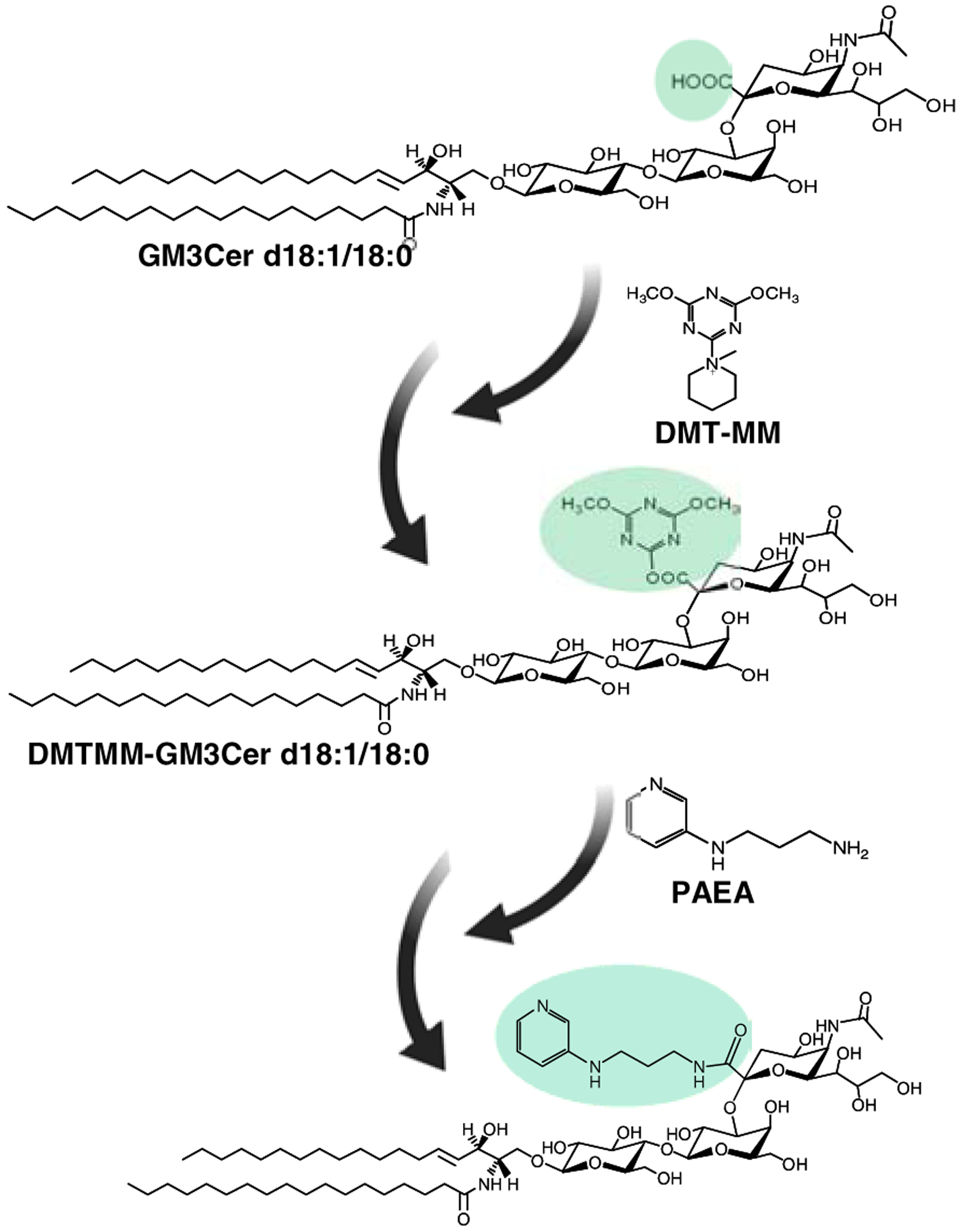
The carboxylic acid group in Neu5Ac is activated by DMT-MM, followed by amidation using PAEA.
8.3. Isobaric labeling
Isobaric labeling is a relatively new technique to glycosphingolipid analysis but has been used extensively in proteomics and glycomics [191, 195]. It provides quantitative information in a multiplexed fashion, where samples are labeled with isobaric tags that can fragment under CID or HCD to yield reporter ions as surrogate for the original sample (Fig. 13). The tag consists of the same molecule with a varying placement of heavy isotopes (e.g. 13C and 15N). Two versions of commercial isobaric tags have been applied to glycosphingolipids: iTRAQ™ (isobaric tags for relative and absolute quantitation) and aminoxyTMT™ (aminoxy tandem mass tag) (Fig. 13)[133, 140, 196]. The iTRAQ™ and aminoxyTMT™ differ in terms of reaction chemistry: iTRAQ™ reacts with free amines; aminoxyTMT™ reacts with carbonyls (aldehyde and ketone). In terms of multiplexing, iTRAQ™ can be used for up to eight samples while aminoxyTMT™, up to six samples, currently.
Figure 13. Principle of isobaric labeling and the chemical structures of mass tags used for glycosphingolipids.
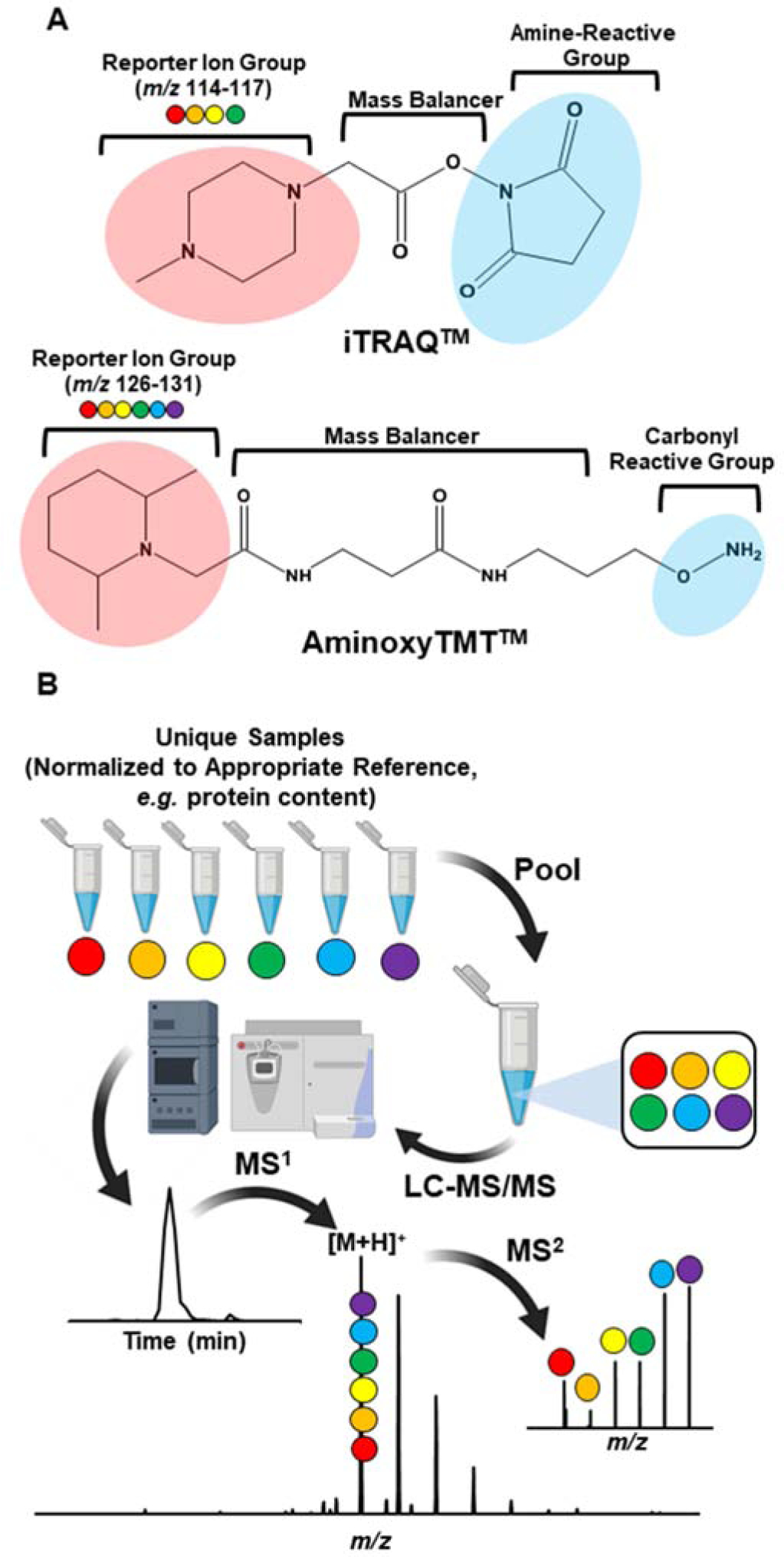
A) Amine-reactive iTRAQ™, and carbonyl-reactive aminoxyTMT™. Both isobaric tags are commercially available and differ in their reaction chemistries. B) Workflow in isobaric labeling-based quantification of gangliosides. Briefly, samples are individually labeled with an isobaric tag. The labeled-samples are pooled and analyzed by LC-MS/MS. Sample clean-up maybe necessary in most cases, which can be accomplished using C18 SPE or other appropriate method. The isobaric tag fragments to yield the reporter ions, and the relative intensities of these ions serve as surrogates of analyte concentrations in the sample.
Nabetani and co-workers used iTRAQ™ to measure sphingolipids, which includes hexosylceramides (HexCer) in cells [133]. These analytes contain no free amines required by iTRAQ™. The N-fatty acyls were cleaved off from the HexCer using sphingolipid ceramide N-deacylase (SCDase), then, the resultant free amine was reacted with iTRAQ™. Improved sensitivity was observed after iTRAQ™ labeling. One limitation of this method, however, is the complete disregard of the N-fatty acyl chain, which are structurally importand and may have critical biological functions.
To understand the distribution of glycosphingolipids at the subcellular level and the roles these lipids played in the biological processes, it is necessary to track and quantify the glycosphingolipid metabolism in cells simultaneously. In this respect, BODIPY-labelled LacCer – where the BODIPY dye is attached in place of N-fatty acyl – has been developed as a mimic to the endogenous LacCer and in this way its metabolism in vivo can be traced by fluorescence technique [197, 198]. To facilitate quantification, Son and co-workers [199] developed a strategy to add isobaric tags to the fluorophore using click chemistry. Briefly, an azide functional group was first introduced to BODIPY, which serves as an achor for successful attachment of iTRAQ™. This novel bifunctional BODIPY-iTRAQ-LacCer was used for metabolic tracing experiments in cells and facilitated quantitative multiplexed analysis of LacCer metabolism. This approach, similarly as the previous iTRAQ labeling method on sphingolipids [133], is not capable of provding information of endogenous N-fatty acyls.
Recently, we used aminoxyTMT™ for multiplexed analysis of gangliosides [140]. To keep the ganglioside molecules structurally intact, we introduced a reactive aldehyde in the terminal sialic acids by selective oxidation of the glycerol hydroxyls with dilute solution of sodium meta periodate (NaIO4). The newly formed aldehydes were then labeled with aminoxyTMT™, six individual samples can be pooled and analyzed together by reversed phase LC-MS/MS (Fig. 14). Sensitivity was greatly improved in positive ionization mode (~40-fold). Characteristic fragment ions helped elucidate structure for both the glycan and the lipid components. For its sensitivity and specificity, this method is promising in analysis of gangliosides.
Figure 14. Isobaric labeling-based multiplexed quantification of gangliosides.
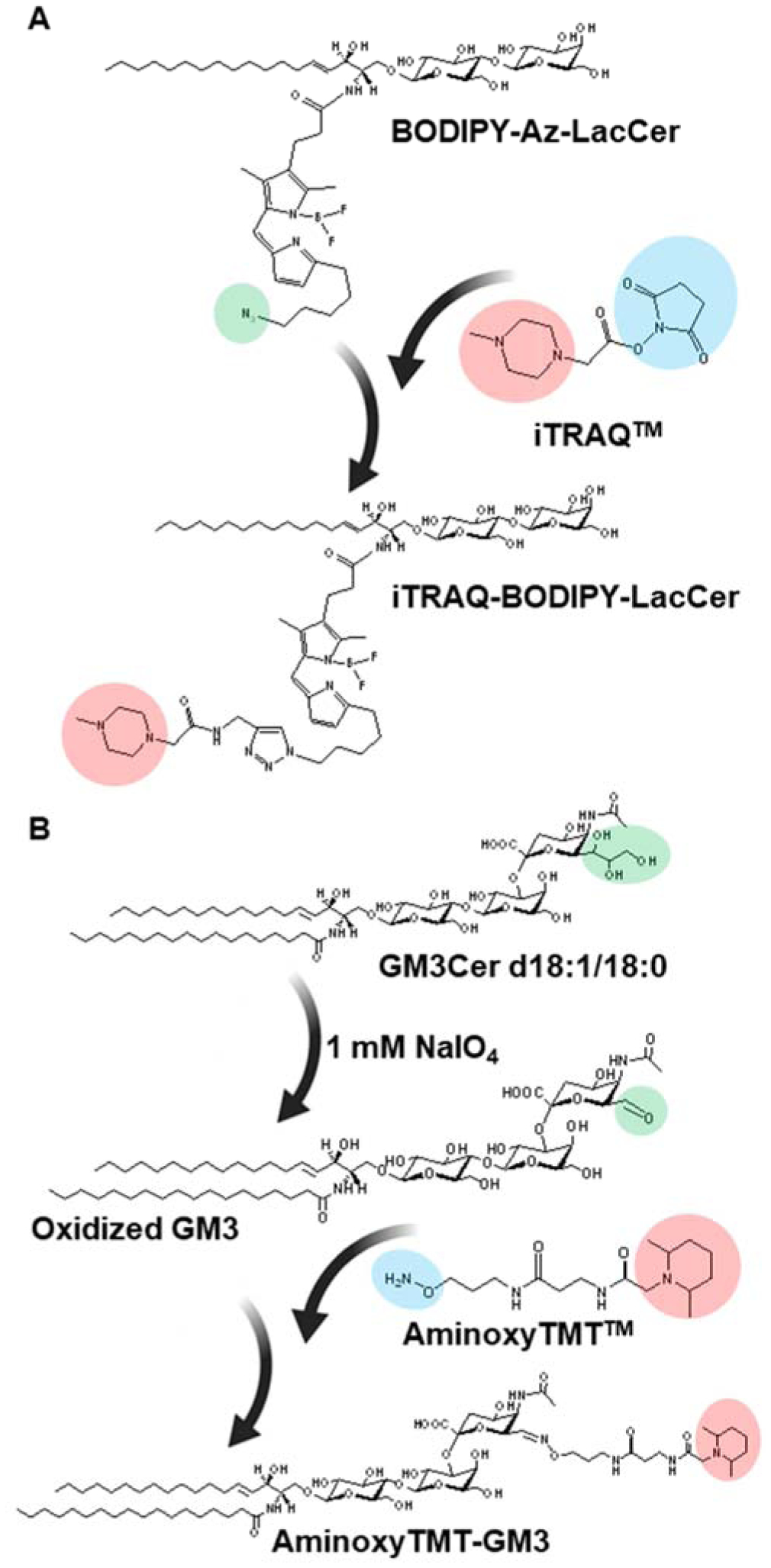
A) Labeling of BODIPY-modified LacCer with iTRAQ™. The BODIPY was functionalized with azide (−N3) which reacts iTRAQ™ via click chemistry. B) Labeling of intact gangliosides using aminoxyTMT™. Gangliosides were chemoselectively oxidized at the trihydroxy functional group of sialic acid using NaIO4 and the resulting aldehyde is labeled with aminoxyTMT™. Reprinted with permission from Barrientos, R.C. & Zhang, Q. Anal. Chem. 90, 2578–2586 (2018)[140], © 2018 American Chemical Society.
Taken together, the novel labeling approaches presented here greatly facilitated the LC-MS/MS based quantification of glycosphingolipids by enhancing the ionization efficiency and enabling multiplexed quantification. Their integration with fluorescence labeling also enabled multiplexed quantification and live cell imaging. It is expected that more specific labeling strategies will be developed to enrich and quantify specific type of glycosphingolipids.
9. Ion Mobility-MS
Ion mobility mass spectrometry is a gas-phase separation technique that is based on ions with different size, shape and charge having different mobility through a buffer gas under an applied electric field. Ion mobility has emerged as a useful tool for analysis of carbohydrates [200, 201]. This technique provides the collision cross-section (CCS), a measure of ion conformation in the gas phase. CCS is independent of instrument type and experimental measurement conditions; it can be used as another dimension for compound identification. In this respect, the ion mobility database for glycans and glycoproteins was developed (GlycoMob) [202].
The currently available ion mobility platforms are: drift tube ion mobility (DTIMS), traveling wave ion mobility (TWIMS), high-field asymmetric waveform ion mobility (FAIMS), and serpentine multi-pass structures for lossless ion manipulations (SLIMS) [203]. Except for SLIMS, these platforms are commercially available and are suitable for use in conjunction with most mass spectrometers. The simplest, DTIMS, uses a uniform weak electric field in a drift tube filled with buffer gas (e.g., helium or nitrogen). Due to the uniform field strength, CCS values can be directly calculated using the Mason–Schamp equation. TWIMS uses alternating radiofrequency (rf) potentials on adjacent stacked ring ion guides (SRIGs) to confine the ions. When a transient direct current (DC) voltage from one end of the device to the other is applied, a ‘traveling wave’ is created, which carries the ions through based on their mobility differences. In contrast to DTIMS, CCS cannot be calculated directly in TWIMS, but can be obtained after a suitable calibration with ions having similar charge state and CCS. FAIMS, also called differential mobility spectrometry (DMS), uses an asymmetric high electric field and a compensation voltage to effectively “filter” ions, so only ions with certain compensation voltage can exit the device without being neutralized on the electrode plates. FAIMS devices cannot be used to measure CCS values but they can achieve very high resolving power and thus typically being used for targeted analysis of specific ions. SLIMS operates on a similar principle as TWIMS, but instead of using SRIGs, it uses arrays of electrodes patterned on two parallel surfaces, thus greatly increases the flight path for very high resolving power ion mobility separation, and aslo functions as a versatile ion manipulation device. These techniques have been thoroughly reviewed [201, 203].
The application of ion mobility-MS in glycosphingolipids remains limited. The first use of ion mobility in gangliosides was reported by Jackson and colleagues [205]. Briefly, gangliosides were ionized in the positive mode as cesium adducts and analyzed by MALDI-TOF MS. The drift times varied with the number of sialic acid residues, but it failed to resolve GD1a and GD1b isomers. More recently, Sarbu and co-workers applied TWIMS for the first time in human fetal brain gangliosides using Q-TOF MS [98]. Ion mobility reduced chemical background noise and separated gangliosides based on glycan length and degree of sialylation. This resulted in at least 143 distinct gangliosides species identified, three times higher than the number previously reported. Separation of glycosylsphingosine epimers (Glc and Gal), and gangliosides (GD1a and GD1b) with the same lipid backbones was achieved using SLIMS [204] (Fig. 15). DMS, coupled with HILIC-MS, effectively resolved GlcCer and GalCer species from human plasma and cerebrospinal fluids [206]. Very recently, CCS databases for lipids, including a few glycosphingolipid species, were compiled from experimental measurement and computational prediction, which are expected to facilitate differentiation of lipid isomers in comprehensive lipidomics research [207, 208].
Figure 15. Baseline resolution of isomeric GD1a and GD1b gangliosides.
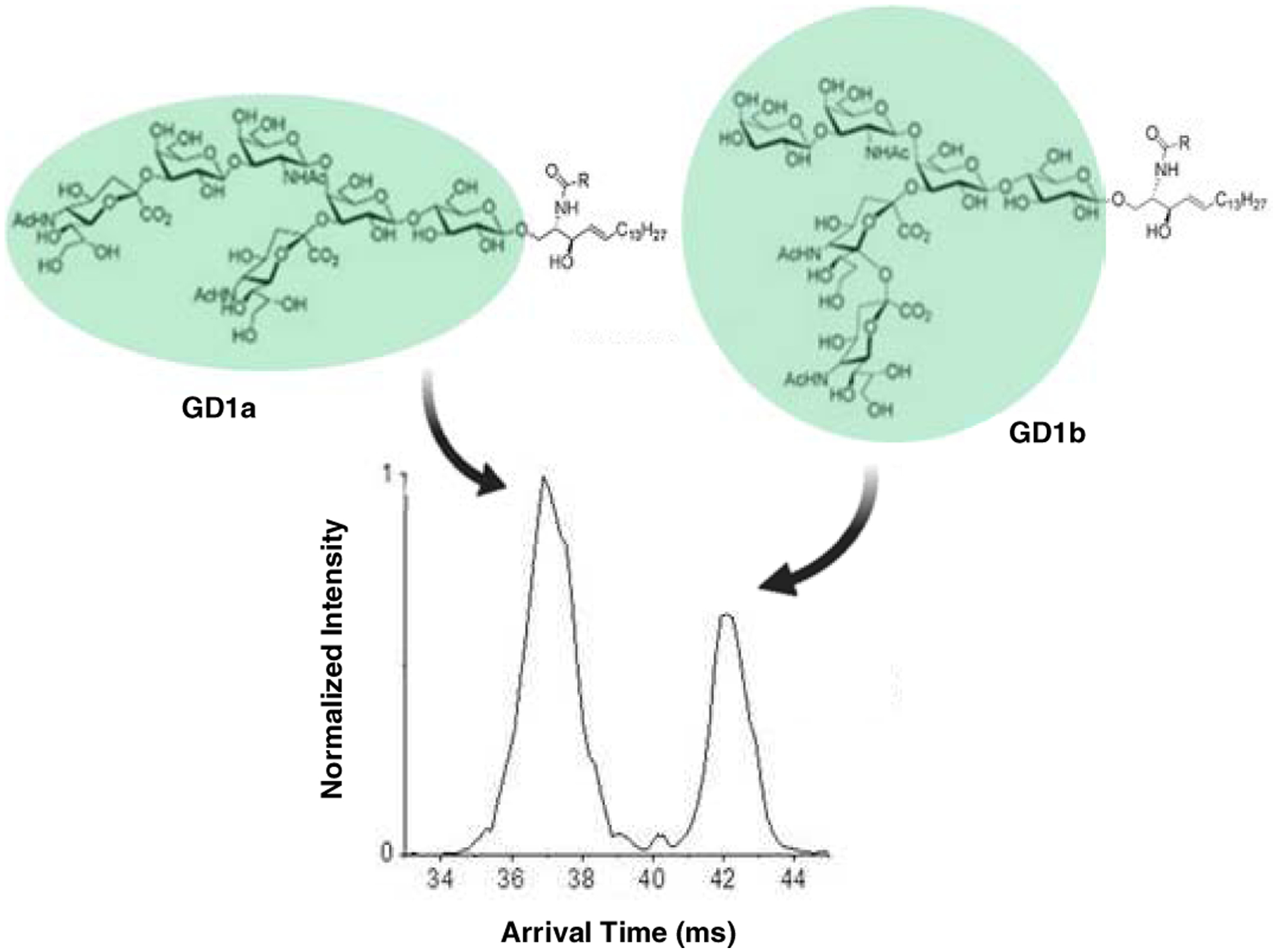
Samples were analyzed as [M+2Na]2+ adduct using a serpentine multi-pass SLIM-IMS. Reprinted from Wojcik et al. Int. J. Mol. Sci., 18, 183 (2017)[204] © The Authors.
10. Conclusion and future perspectives
Our current understanding of glycosphingolipid biology remains scant. The presence of a vast repertoire of structural variants serves both as an analytical challenge and a research opportunity. Multiple experimental evidence suggest that the lipid backbone can influence the function of the glycan moiety, which underscores the importance of analyzing glycosphingolipids in their intact form for a holistic understanding of their biological functions. We reviewed the current state of the mass spectrometric analysis of glycosphingolipids, including sample preparation, structural characterization, and quantification. Differentiation of isomers that differ only on the position of carbon-carbon double bonds, linkage between sugars, and stereochemistry of those linkages in the headgroup are fertile areas for future investigation. In this respect, chemoselective derivatization, modern fragmentation methods (electron-aided fragmentation, photodissociation, and OzID-MS), and ion mobility all serve as promising tools. On the other hand, disease-relevant glycosphingolipids are extremely low abundant, which requires the development of more sensitive strategies to detect them. These include powerful separation methods before mass spectrometry, derivatization strategies to improve ionization efficiency or reduction of analytical background noise via the use of enrichment strategies or ion mobility spectrometry. Finally, while it is imperative to elucidate the structure of glycosphingolipids, it is equally important to know their spatial distribution. In this respect, the use of imaging mass spectrometry tools can provide novel biological insights reflected by the differential distribution of glycosphingolipids in tissues, cells and subcellular structures. Coupling mass spectrometry imaging with novel structural analysis tools presented here, in our opinion, can greatly facilitate uncovering the roles of glycosphingolipids in biology and pathology.
Highlights.
A critical review of recent advances in MS analysis of glycosphingolipidome
Nomenclature and biological significance of glycosphingolipids are covered
Comprehensive coverage of novel enrichment strategies for glycosphingolipids
Extensive coverage of fragmentation techniques in glycosphingolipid analysis
Labeling approaches for quantitation and perspectives on future directions
Acknowledgment
This work was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK 123499) of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Merrill AH, Sphingolipid and Glycosphingolipid Metabolic Pathways in the Era of Sphingolipidomics, Chem Rev, 111 (2011) 6387–6422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Farwanah H, Kolter T, Lipidomics of glycosphingolipids, Metabolites, 2 (2012) 134–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Schnaar RL, Kinoshita T, Glycosphingolipids, in: Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, Darvill AG, Kinoshita T, Packer NH, Prestegard JH, Schnaar RL, Seeberger PH (Eds.) Essentials of Glycobiology, Cold Spring Harbor Laboratory Press, Cold Spring Harbor (NY), 2015, pp. 125–135. [PubMed] [Google Scholar]
- [4].Lingwood CA, Glycosphingolipid Functions, Cold Spring Harb Perspect Biol, 3 (2011) a004788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].D’Angelo G, Capasso S, Sticco L, Russo D, Glycosphingolipids: synthesis and functions, FEBS J, 280 (2013) 6338–6353. [DOI] [PubMed] [Google Scholar]
- [6].Wennekes T, van den Berg RJBHN, Boot RG, van der Marel GA, Overkleeft HS, Aerts JMFG, Glycosphingolipids—Nature, Function, and Pharmacological Modulation, Angew Chem Int Ed Engl, 48 (2009) 8848–8869. [DOI] [PubMed] [Google Scholar]
- [7].Levery SB, Glycosphingolipid structural analysis and glycosphingolipidomics, Methods Enzymol, 405 (2005) 300–369. [DOI] [PubMed] [Google Scholar]
- [8].Blanksby SJ, Mitchell TW, Advances in Mass Spectrometry for Lipidomics, Annu Rev Anal Chem, 3 (2010) 433–465. [DOI] [PubMed] [Google Scholar]
- [9].Haynes CA, Allegood JC, Park H, Sullards MC, Sphingolipidomics: Methods for the comprehensive analysis of sphingolipids, J Chromatogr B, 877 (2009) 2696–2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Farwanah H, Kolter T, Sandhoff K, Mass spectrometric analysis of neutral sphingolipids: Methods, applications, and limitations, Biochim Biophys Acta Mol Cell Biol Lipids, 1811 (2011) 854–860. [DOI] [PubMed] [Google Scholar]
- [11].Sarbu M, Zamfir AD, Modern separation techniques coupled to high performance mass spectrometry for glycolipid analysis, Electrophoresis, 39 (2018) 1155–1170. [DOI] [PubMed] [Google Scholar]
- [12].Merrill AH, Sullards MC, Allegood JC, Kelly S, Wang E, Sphingolipidomics: High-throughput, structure-specific, and quantitative analysis of sphingolipids by liquid chromatography tandem mass spectrometry, Methods, 36 (2005) 207–224. [DOI] [PubMed] [Google Scholar]
- [13].Han X, Lipidomics for studying metabolism, Nat Rev Endocrinol, 12 (2016) 668–679. [DOI] [PubMed] [Google Scholar]
- [14].Han X, Jiang X, A review of lipidomic technologies applicable to sphingolipidomics and their relevant applications, Eur J Lipid Sci Technol, 111 (2009) 39–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Rustam YH, Reid GE, Analytical Challenges and Recent Advances in Mass Spectrometry Based Lipidomics, Anal Chem, 90 (2018) 374–397. [DOI] [PubMed] [Google Scholar]
- [16].Spassieva S, Bieberich E, Lysosphingolipids and sphingolipidoses: Psychosine in Krabbe’s disease, J Neurosci Res, 94 (2016) 974–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ferraz MJ, Marques AR, Gaspar P, Mirzaian M, van Roomen C, Ottenhoff R, Alfonso P, Irun P, Giraldo P, Wisse P, Sa Miranda C, Overkleeft HS, Aerts JM, Lyso-glycosphingolipid abnormalities in different murine models of lysosomal storage disorders, Mol Genet Metab, 117 (2016) 186–193. [DOI] [PubMed] [Google Scholar]
- [18].Takahashi T, Suzuki T, Role of sulfatide in normal and pathological cells and tissues, J Lipid Res, 53 (2012) 1437–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].de Haan N, Yang S, Cipollo J, Wuhrer M, Glycomics studies using sialic acid derivatization and mass spectrometry, Nat Rev Chem, (2020). [DOI] [PubMed] [Google Scholar]
- [20].Schnaar RL, Gerardy-Schahn R, Hildebrandt H, Sialic acids in the brain: gangliosides and polysialic acid in nervous system development, stability, disease, and regeneration., Physiol Rev, 94 (2014) 461–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kolter T, Ganglioside Biochemistry, ISRN Biochem, 2012 (2012) 506160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Mlinac K, Fabris D, Vukelic Z, Rozman M, Heffer M, Bognar SK, Structural analysis of brain ganglioside acetylation patterns in mice with altered ganglioside biosynthesis, Carbohydr Res, 382 (2013) 1–8. [DOI] [PubMed] [Google Scholar]
- [23].Fahy E, Subramaniam S, Murphy RC, Nishijima M, Raetz CR, Shimizu T, Spener F, van Meer G, Wakelam MJ, Dennis EA, Update of the LIPID MAPS comprehensive classification system for lipids, J Lipid Res, 50 Suppl (2009) S9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Svennerholm L, The Gangliosides J Lipid Res, 5 (1964) 145–155. [PubMed] [Google Scholar]
- [25].IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN) Nomenclature of glycolipids, Eur J Biochem, 257 (1998) 293–298. [DOI] [PubMed] [Google Scholar]
- [26].Fahy E, Subramaniam S, Brown HA, Glass CK, Merrill AH Jr., Murphy RC, Raetz CR, Russell DW, Seyama Y, Shaw W, Shimizu T, Spener F, van Meer G, VanNieuwenhze MS, White SH, Witztum JL, Dennis EA, A comprehensive classification system for lipids, J Lipid Res, 46 (2005) 839–861. [DOI] [PubMed] [Google Scholar]
- [27].Sud M, Fahy E, Cotter D, Brown A, Dennis EA, Glass CK, Merrill AH Jr., Murphy RC, Raetz CR, Russell DW, Subramaniam S, LMSD: LIPID MAPS structure database, Nucleic Acids Res, 35 (2007) D527–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Liebisch G, Vizcaino JA, Kofeler H, Trotzmuller M, Griffiths WJ, Schmitz G, Spener F, Wakelam MJ, Shorthand notation for lipid structures derived from mass spectrometry, J Lipid Res, 54 (2013) 1523–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hakomori S, The glycosynapse, Proc Nat Acad Sci USA, 99 (2002) 225–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zhang T, de Waard AA, Wuhrer M, Spaapen RM, The Role of Glycosphingolipids in Immune Cell Functions, Front Immunol, 10 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Russo D, Capolupo L, Loomba JS, Sticco L, D’Angelo G, Glycosphingolipid metabolism in cell fate specification, J Cell Sci, 131 (2018) jcs219204. [DOI] [PubMed] [Google Scholar]
- [32].Hakomori S, Traveling for the glycosphingolipid path, Glycoconj J, 17 (2000) 627–647. [DOI] [PubMed] [Google Scholar]
- [33].De Libero G, Mori L, How T lymphocytes recognize lipid antigens, FEBS Lett, 580 (2006) 5580–5587. [DOI] [PubMed] [Google Scholar]
- [34].Frey SL, Lee KY, Number of sialic acid residues in ganglioside headgroup affects interactions with neighboring lipids, Biophys J, 105 (2013) 1421–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ravindran MS, Tanner LB, Wenk MR, Sialic acid linkage in glycosphingolipids is a molecular correlate for trafficking and delivery of extracellular cargo, Traffic, 14 (2013) 1182–1191. [DOI] [PubMed] [Google Scholar]
- [36].Porubsky S, Speak AO, Salio M, Jennemann R, Bonrouhi M, Zafarulla R, Singh Y, Dyson J, Luckow B, Lehuen A, Malle E, Müthing J, Platt FM, Cerundolo V, Gröne H-J, Globosides but not isoglobosides can impact the development of invariant natural killer T cells and their interaction with dendritic cells, J Immunol, 189 (2012) 3007–3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Park DD, Xu G, Wong M, Phoomak C, Liu M, Haigh NE, Wongkham S, Yang P, Maverakis E, Lebrilla CB, Membrane glycomics reveal heterogeneity and quantitative distribution of cell surface sialylation, Chemical science, 9 (2018) 6271–6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Han X, Lipid alterations in the earliest clinically recognizable stage of Alzheimer’s disease: implication of the role of lipids in the pathogenesis of Alzheimer’s disease, Curr Alzheimer Res, 2 (2005) 65–77. [DOI] [PubMed] [Google Scholar]
- [39].Garcia-Ruiz C, Morales A, Fernández-Checa JC, Glycosphingolipids and cell death: one aim, many ways, Apoptosis, 20 (2015) 607–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Iwabuchi K, Nakayama H, Oizumi A, Suga Y, Ogawa H, Takamori K, Role of Ceramide from Glycosphingolipids and Its Metabolites in Immunological and Inflammatory Responses in Humans, Mediators Inflamm, 2015 (2015) 120748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Hashimoto K, Yoshizawa AC, Okuda S, Kuma K, Goto S, Kanehisa M, The repertoire of desaturases and elongases reveals fatty acid variations in 56 eukaryotic genomes, J Lipid Res, 49 (2008) 183–191. [DOI] [PubMed] [Google Scholar]
- [42].Kiarash A, Boyd B, Lingwood CA, Glycosphingolipid receptor function is modified by fatty acid content. Verotoxin 1 and verotoxin 2c preferentially recognize different globotriaosyl ceramide fatty acid homologues, J Biol Chem, 269 (1994) 11138–11146. [PubMed] [Google Scholar]
- [43].Sandhoff R, Geyer R, Jennemann R, Paret C, Kiss E, Yamashita T, Gorgas K, Sijmonsma TP, Iwamori M, Finaz C, Proia RL, Wiegandt H, Gröne H-J, Novel Class of Glycosphingolipids Involved in Male Fertility, J Biol Chem, 280 (2005) 27310–27318. [DOI] [PubMed] [Google Scholar]
- [44].Kihara A, Very long-chain fatty acids: elongation, physiology and related disorders, J Biochem, 152 (2012) 387–395. [DOI] [PubMed] [Google Scholar]
- [45].Harkewicz R, Du H, Tong Z, Alkuraya H, Bedell M, Sun W, Wang X, Hsu Y-H, Esteve-Rudd J, Hughes G, Su Z, Zhang M, Lopes VS, Molday RS, Williams DS, Dennis EA, Zhang K, Essential role of ELOVL4 protein in very long chain fatty acid synthesis and retinal function, J Biol Chem, 287 (2012) 11469–11480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Hama H, Fatty acid 2-Hydroxylation in mammalian sphingolipid biology, Biochim Biophys Acta, 1801 (2010) 405–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Skotland T, Ekroos K, Kavaliauskiene S, Bergan J, Kauhanen D, Lintonen T, Sandvig K, Determining the Turnover of Glycosphingolipid Species by Stable-Isotope Tracer Lipidomics, J Mol Biol, 428 (2016) 4856–4866. [DOI] [PubMed] [Google Scholar]
- [48].Wong M, Xu G, Park D, Barboza M, Lebrilla CB, Intact glycosphingolipidomic analysis of the cell membrane during differentiation yields extensive glycan and lipid changes, Sci Rep, 8 (2018) 10993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Zamfir AD, Neurological Analyses: Focus on Gangliosides and Mass Spectrometry, in: Woods AG, Darie CC (Eds.) Advancements of Mass Spectrometry in Biomedical Research, Springer International Publishing, Cham, 2014, pp. 153–204. [DOI] [PubMed] [Google Scholar]
- [50].Sandhoff K, Harzer K, Gangliosides and Gangliosidoses: Principles of Molecular and Metabolic Pathogenesis, J Neurosci, 33 (2013) 10195–10208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Cheng H, Wang M, Li J-L, Cairns NJ, Han X, Specific changes of sulfatide levels in individuals with pre-clinical Alzheimer’s disease: an early event in disease pathogenesis, J Neurochem, 127 (2013) 733–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Han X, Holtzman DM, McKeel DW Jr, Kelley J, Morris JC, Substantial sulfatide deficiency and ceramide elevation in very early Alzheimer’s disease: potential role in disease pathogenesis, J Neurochem, 82 (2002) 809–818. [DOI] [PubMed] [Google Scholar]
- [53].Zhuo D, Li X, Guan F, Biological Roles of Aberrantly Expressed Glycosphingolipids and Related Enzymes in Human Cancer Development and Progression, Front Physiol, 9 (2018) 466–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Xu YH, Barnes S, Sun Y, Grabowski GA, Multi-system disorders of glycosphingolipid and ganglioside metabolism, J Lipid Res, 51 (2010) 1643–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Carreno LJ, Saavedra-Avila NA, Porcelli SA, Synthetic glycolipid activators of natural killer T cells as immunotherapeutic agents, Clin Trans Immunol, 5 (2016) e69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Durrant LG, Noble P, Spendlove I, Immunology in the clinic review series; focus on cancer: glycolipids as targets for tumour immunotherapy, Clin Exp Immunol, 167 (2012) 206–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Tyler A, Johansson A, Karlsson T, Gudey SK, Brännström T, Grankvist K, Behnam-Motlagh P, Targeting glucosylceramide synthase induction of cell surface globotriaosylceramide (Gb3) in acquired cisplatin-resistance of lung cancer and malignant pleural mesothelioma cells, Exp Cell Res, 336 (2015) 23–32. [DOI] [PubMed] [Google Scholar]
- [58].Quehenberger O, Dennis EA, The Human Plasma Lipidome, New Engl J Med, 365 (2011) 1812–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Guo X, Lankmayr E, Phospholipid-based matrix effects in LC–MS bioanalysis, Bioanalysis, 3 (2011) 349–352. [DOI] [PubMed] [Google Scholar]
- [60].Zaia J, Mass Spectrometry of Oligosaccharides, Mass Spectrom Rev, 23 (2004) 161–227. [DOI] [PubMed] [Google Scholar]
- [61].Zaia J, Mass Spectrometry and Glycomics, Omics, 14 (2010) 401–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Li Y, Zhou D, Xia C, Wang PG, Levery SB, Sensitive quantitation of isoglobotriaosylceramide in the presence of isobaric components using electrospray ionization-ion trap mass spectrometry, Glycobiology, 18 (2008) 166–176. [DOI] [PubMed] [Google Scholar]
- [63].Ravindran MS, Tanner LB, Wenk MR, Sialic Acid Linkage in Glycosphingolipids Is a Molecular Correlate for Trafficking and Delivery of Extracellular Cargo, Traffic, 14 (2013) 1182–1191. [DOI] [PubMed] [Google Scholar]
- [64].Stencel-Baerenwald JE, Reiss K, Reiter DM, Stehle T, Dermody TS, The sweet spot: defining virus-sialic acid interactions, Nat Rev Microbiol, 12 (2014) 739–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Oshansky CM, Pickens JA, Bradley KC, Jones LP, Saavedra-Ebner GM, Barber JP, Crabtree JM, Steinhauer DA, Tompkins SM, Tripp RA, Avian Influenza Viruses Infect Primary Human Bronchial Epithelial Cells Unconstrained by Sialic Acid α2,3 Residues, PLoS One, 6 (2011) e21183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Hanamatsu H, Nishikaze T, Miura N, Piao J, Okada K, Sekiya S, Iwamoto S, Sakamoto N, Tanaka K, J.-i. Furukawa, Sialic Acid Linkage Specific Derivatization of Glycosphingolipid Glycans by Ring-Opening Aminolysis of Lactones, Anal Chem, 90 (2018) 13193–13199. [DOI] [PubMed] [Google Scholar]
- [67].Barrientos RC, Vu N, Zhang Q, Structural Analysis of Unsaturated Glycosphingolipids Using Shotgun Ozone-Induced Dissociation Mass Spectrometry, J Am Soc Mass Spectrom, 28 (2017) 2330–2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Nakajima K, Akiyama H, Tanaka K, Kohyama-Koganeya A, Greimel P, Hirabayashi Y, Separation and analysis of mono-glucosylated lipids in brain and skin by hydrophilic interaction chromatography based on carbohydrate and lipid moiety, J Chromatogr B, 1031 (2016) 146–153. [DOI] [PubMed] [Google Scholar]
- [69].Pham HT, Julian RR, Characterization of glycosphingolipid epimers by radical-directed dissociation mass spectrometry, Analyst, 141 (2016) 1273–1278. [DOI] [PubMed] [Google Scholar]
- [70].Kirsch S, Muthing J, Peter-Katalinic J, Bindila L, On-line nano-HPLC/ESI QTOF MS monitoring of alpha2–3 and alpha2–6 sialylation in granulocyte glycosphingolipidome, Biol Chem, 390 (2009) 657–672. [DOI] [PubMed] [Google Scholar]
- [71].Boutin M, Sun Y, Shacka JJ, Auray-Blais C, Tandem Mass Spectrometry Multiplex Analysis of Glucosylceramide and Galactosylceramide Isoforms in Brain Tissues at Different Stages of Parkinson Disease, Anal Chem, 88 (2016) 1856–1863. [DOI] [PubMed] [Google Scholar]
- [72].Brennan PJ, Cheng T-Y, Pellicci DG, Watts GFM, Veerapen N, Young DC, Rossjohn J, Besra GS, Godfrey DI, Brenner MB, Moody DB, Structural determination of lipid antigens captured at the CD1d–T-cell receptor interface, Proc Natl Acad Sci U S A, 114 (2017) 8348–8353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Mirzaian M, Wisse P, Ferraz MJ, Marques ARA, Gaspar P, Oussoren SV, Kytidou K, Codée JDC, van der Marel G, Overkleeft HS, Aerts JM, Simultaneous quantitation of sphingoid bases by UPLC-ESI-MS/MS with identical 13C-encoded internal standards, Clin Chim Acta, 466 (2017) 178–184. [DOI] [PubMed] [Google Scholar]
- [74].Kaneko T, Tsubakihara Y, Fushimi H, Yamaguchi S, Takabatake Y, Rakugi H, Kawakami H, Isaka Y, Histochemical and immunoelectron microscopic analysis of ganglioside GM3 in human kidney, Clin Exp Nephrol, 19 (2015) 403–410. [DOI] [PubMed] [Google Scholar]
- [75].Zhang Y, Wang J, Liu J.a., Han J, Xiong S, Yong W, Zhao Z, Combination of ESI and MALDI mass spectrometry for qualitative, semi-quantitative and in situ analysis of gangliosides in brain, Sci Rep, 6 (2016) 25289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Kanda T, Ariga T, Kubodera H, Jin HL, Owada K, Kasama T, Yamawaki M, Mizusawa H, Glycosphingolipid composition of primary cultured human brain microvascular endothelial cells, J Neurosci Res, 78 (2004) 141–150. [DOI] [PubMed] [Google Scholar]
- [77].Lam SM, Wang Y, Duan X, Wenk MR, Kalaria RN, Chen CP, Lai MK, Shui G, Brain lipidomes of subcortical ischemic vascular dementia and mixed dementia, Neurobiol Aging, 35 (2014) 2369–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Valdes-Gonzalez T, Goto-Inoue N, Hirano W, Ishiyama H, Hayasaka T, Setou M, Taki T, New approach for glyco- and lipidomics--molecular scanning of human brain gangliosides by TLC-Blot and MALDI-QIT-TOF MS, J Neurochem, 116 (2011) 678–683. [DOI] [PubMed] [Google Scholar]
- [79].Bian L, Yang J, Sun Y, Isolation and purification of monosialotetrahexosylgangliosides from pig brain by extraction and liquid chromatography, Biomed Chromatogr, 29 (2015) 1604–1611. [DOI] [PubMed] [Google Scholar]
- [80].Chan RB, Oliveira TG, Cortes EP, Honig LS, Duff KE, Small SA, Wenk MR, Shui G, Paolo GD, Comparative Lipidomic Analysis of Mouse and Human Brain with Alzheimer Disease, J Biol Chem, 287 (2012) 2678–2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Sato Y, Bernier F, Suzuki I, Kotani S, Nakagawa M, Oda Y, Comparative lipidomics of mouse brain exposed to enriched environment, J Lipid Res, 54 (2013) 2687–2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Moyano AL, Li G, Lopez-Rosas A, Mansson JE, van Breemen RB, Givogri MI, Distribution of C16:0, C18:0, C24:1, and C24:0 sulfatides in central nervous system lipid rafts by quantitative ultra-high-pressure liquid chromatography tandem mass spectrometry, Anal Biochem, 467 (2014) 31–39. [DOI] [PubMed] [Google Scholar]
- [83].Schnaar RL, Gangliosides of the Vertebrate Nervous System, J Mol Biol, 428 (2016) 3325–3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Zamfir AD, Serb A, Vukeli Z, Flangea C, Schiopu C, Fabris D, Kalanj-Bognar S, Capitan F, Sisu E, Assessment of the molecular expression and structure of gangliosides in brain metastasis of lung adenocarcinoma by an advanced approach based on fully automated chip-nanoelectrospray mass spectrometry, J Am Soc Mass Spectrom, 22 (2011) 2145–2159. [DOI] [PubMed] [Google Scholar]
- [85].Barone A, Benktander J, Teneberg S, Breimer ME, Characterization of acid and non-acid glycosphingolipids of porcine heart valve cusps as potential immune targets in biological heart valve grafts, Xenotransplantation, 21 (2014) 510–522. [DOI] [PubMed] [Google Scholar]
- [86].Beausoleil HE, Lepine F, Dubreuil JD, LC-MS analysis of pig intestine sulfatides: interaction with Escherichia coli STb enterotoxin and characterization of molecular species present, FEMS Microbiol Lett, 209 (2002) 183–188. [DOI] [PubMed] [Google Scholar]
- [87].Joo EJ, Weyers A, Li G, Gasimli L, Li L, Choi WJ, Lee KB, Linhardt RJ, Carbohydrate-containing molecules as potential biomarkers in colon cancer, Omics, 18 (2014) 231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Holst S, Stavenhagen K, Balog CI, Koeleman CA, McDonnell LM, Mayboroda OA, Verhoeven A, Mesker WE, Tollenaar RA, Deelder AM, Wuhrer M, Investigations on aberrant glycosylation of glycosphingolipids in colorectal cancer tissues using liquid chromatography and matrix-assisted laser desorption time-of-flight mass spectrometry (MALDI-TOF-MS), Mol Cell Proteomics, 12 (2013) 3081–3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Ruh H, Sandhoff R, Meyer B, Gretz N, Hopf C, Quantitative characterization of tissue globotetraosylceramides in a rat model of polycystic kidney disease by PrimaDrop sample preparation and indirect high-performance thin layer chromatography-matrix-assisted laser desorption/ionization-time-of-flight-mass spectrometry with automated data acquisition, Anal Chem, 85 (2013) 6233–6240. [DOI] [PubMed] [Google Scholar]
- [90].Marsching C, Jennemann R, Heilig R, Grone HJ, Hopf C, Sandhoff R, Quantitative imaging mass spectrometry of renal sulfatides: validation by classical mass spectrometric methods, J Lipid Res, 55 (2014) 2343–2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Brush RS, Tran J-TA, Henry KR, McCellan ME, Elliott MH, Mandal MNA, Retinal Sphingolipids and Their Very-Long-Chain Fatty Acid–Containing Species, Invest Ophthalmol Vis Sci Data, 51 (2010) 4422–4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Liu Y, Chen Y, Momin A, Shaner R, Wang E, Bowen NJ, Matyunina LV, Walker LD, McDonald JF, Sullards MC, Merrill AH Jr., Elevation of sulfatides in ovarian cancer: an integrated transcriptomic and lipidomic analysis including tissue-imaging mass spectrometry, Mol Cancer, 9 (2010) 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Rajanayake KK, Taylor WR, Isailovic D, The comparison of glycosphingolipids isolated from an epithelial ovarian cancer cell line and a nontumorigenic epithelial ovarian cell line using MALDI-MS and MALDI-MS/MS, Carbohydr Res, 431 (2016) 6–14. [DOI] [PubMed] [Google Scholar]
- [94].Yang L, Cui X, Zhang N, Li M, Bai Y, Han X, Shi Y, Liu H, Comprehensive lipid profiling of plasma in patients with benign breast tumor and breast cancer reveals novel biomarkers, Anal Bioanal Chem, 407 (2015) 5065–5077. [DOI] [PubMed] [Google Scholar]
- [95].Stimmer L, Dehay S, Nemati F, Massonnet G, Richon S, Decaudin D, Klijanienko J, Johannes L, Human breast cancer and lymph node metastases express Gb3 and can be targeted by STxB-vectorized chemotherapeutic compounds, BMC Cancer, 14 (2014) 916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Liang YJ, Ding Y, Levery SB, Lobaton M, Handa K, Hakomori SI, Differential expression profiles of glycosphingolipids in human breast cancer stem cells vs. cancer non-stem cells, Proc Natl Acad Sci U S A, 110 (2013) 4968–4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Zhu T, Xu L, Xu X, Wang Z, Zhu J, Xie Q, Zhang B, Wang Y, Ju L, He Y, Ye X, Zhou D, Li Y, Analysis of breast cancer-associated glycosphingolipids using electrospray ionization-linear ion trap quadrupole mass spectrometry, Carbohydr Res, 402 (2015) 189–199. [DOI] [PubMed] [Google Scholar]
- [98].Sarbu M, Robu AC, Ghiulai RM, Vukelić Ž, Clemmer DE, Zamfir AD, Electrospray Ionization Ion Mobility Mass Spectrometry of Human Brain Gangliosides, Anal Chem, 88 (2016) 5166–5178. [DOI] [PubMed] [Google Scholar]
- [99].Ghiulai RM, Sarbu M, Vukelić Ž, Ilie C, Zamfir AD, Early stage fetal neocortex exhibits a complex ganglioside profile as revealed by high resolution tandem mass spectrometry, Glycoconj J, 31 (2014) 231–245. [DOI] [PubMed] [Google Scholar]
- [100].Moyano AL, Pituch K, Li G, van Breemen R, Mansson JE, Givogri MI, Levels of plasma sulfatides C18 : 0 and C24 : 1 correlate with disease status in relapsing-remitting multiple sclerosis, J Neurochem, 127 (2013) 600–604. [DOI] [PubMed] [Google Scholar]
- [101].Moyano AL, Li G, Boullerne AI, Feinstein DL, Sulfatides in extracellular vesicles isolated from plasma of multiple sclerosis patients, J Neurosci Res, 94 (2016) 1579–1587. [DOI] [PubMed] [Google Scholar]
- [102].Podbielska M, Dasgupta S, Levery SB, Tourtellotte WW, Annuk H, Moran AP, Hogan EL, Novel myelin penta- and hexa-acetyl-galactosyl-ceramides: structural characterization and immunoreactivity in cerebrospinal fluid, J Lipid Res, 51 (2010) 1394–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Furukawa J, Sakai S, Yokota I, Okada K, Hanamatsu H, Kobayashi T, Yoshida Y, Higashino K, Tamura T, Igarashi Y, Shinohara Y, Quantitative GSL-glycome analysis of human whole serum based on an EGCase digestion and glycoblotting method, J Lipid Res, 56 (2015) 2399–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Tipthara P, Thongboonkerd V, Urinary Lipidomics, in: Wang X, Wu D, Shen H (Eds.) Lipidomics in Health & Disease: Methods & Application, Springer Singapore, Singapore, 2018, pp. 97–111. [Google Scholar]
- [105].Barcenas M, Suhr TR, Scott CR, Turecek F, Gelb MH, Quantification of sulfatides in dried blood and urine spots from metachromatic leukodystrophy patients by liquid chromatography/electrospray tandem mass spectrometry, Clin Chim Acta, 433 (2014) 39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Lydic TA, Busik JV, Reid GE, A monophasic extraction strategy for the simultaneous lipidome analysis of polar and nonpolar retina lipids, J Lipid Res, 55 (2014) 1797–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Folch J, Lees M, Sloane Stanley GH, A simple method for the isolation and purification of total lipides from animal tissues, J Biol Chem, 226 (1957) 497–509. [PubMed] [Google Scholar]
- [108].Bligh EG, Dyer WJ, A rapid method of total lipid extraction and purification, Can J Biochem Physiol, 37 (1959) 911–917. [DOI] [PubMed] [Google Scholar]
- [109].Löfgren L, Forsberg G-B, Ståhlman M, The BUME method: a new rapid and simple chloroform-free method for total lipid extraction of animal tissue, Sci Rep, 6 (2016) 27688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Yin B, Hawke D, Zhou D, Mass spectrometric analysis of glycosphingolipid antigens for NKT cells, J Vis Exp, (2013) 10.3791/4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Gregson NA, The Extraction and Analysis of Glycosphingolipids, in: Graham JM, Higgins JA (Eds.) Biomembrane Protocols: I. Isolation and Analysis, Humana Press, Totowa, NJ, 1993, pp. 287–301. [DOI] [PubMed] [Google Scholar]
- [112].Schnaar RL, [22] Isolation of glycosphingolipids, Methods Enzymol, Academic Press; 1994, pp. 348–370. [DOI] [PubMed] [Google Scholar]
- [113].Bodennec J, Koul O, Aguado I, Brichon G, Zwingelstein G, Portoukalian J, A procedure for fractionation of sphingolipid classes by solid-phase extraction on aminopropyl cartridges, J Lipid Res, 41 (2000) 1524–1531. [PubMed] [Google Scholar]
- [114].Mirzaian M, Kramer G, Poorthuis BJHM, Quantification of sulfatides and lysosulfatides in tissues and body fluids by liquid chromatography-tandem mass spectrometry, J Lipid Res, 56 (2015) 936–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Noda A, Kato M, Miyazaki S, Kyogashima M, Separation of glycosphingolipids with titanium dioxide, Glycoconj J, 35 (2018) 493–498. [DOI] [PubMed] [Google Scholar]
- [116].Khoury S, Masson E, Sibille E, Cabaret S, Berdeaux O, Rapid sample preparation for ganglioside analysis by liquid chromatography mass spectrometry, J Chromatogr B, 1137 (2020) 121956. [DOI] [PubMed] [Google Scholar]
- [117].Svennerholm L, Fredman P, A Procedure for Quantitative Isolation of Brain Gangliosides, Biochim Biophys Acta, 617 (1980) 97–109. [DOI] [PubMed] [Google Scholar]
- [118].Svennerholm L, Thorin H, Quantitative isolation of brain sulfatides, J Lipid Res, 3 (1962) 483–485. [Google Scholar]
- [119].Hara A, Radin NS, Simple procedures for the rapid cleavage of ester lipids and for the large-scale isolation from brain of cerebroside sulfate, Anal Biochem, 100 (1979) 364–370. [DOI] [PubMed] [Google Scholar]
- [120].Spacil Z, Babu Kumar A, Liao HC, Auray-Blais C, Stark S, Suhr TR, Scott CR, Turecek F, Gelb MH, Sulfatide Analysis by Mass Spectrometry for Screening of Metachromatic Leukodystrophy in Dried Blood and Urine Samples, Clin Chem, 62 (2016) 279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Schwarz A, Terstappen GC, Futerman AH, Isolation of gangliosides by cloud-point extraction with a nonionic detergent, Anal Biochem, 254 (1997) 221–225. [DOI] [PubMed] [Google Scholar]
- [122].Radin NS, Preparative isolation of cerebrosides (galactosyl and glucosyl ceramide), J Lipid Res, 17 (1976) 290–293. [PubMed] [Google Scholar]
- [123].Schnaar RL, Needham LK, [23] Thin-layer chromatography of glycosphingolipids, Methods Enzymol, Academic Press; 1994, pp. 371–389. [DOI] [PubMed] [Google Scholar]
- [124].Jiang X, Cheng H, Yang K, Gross RW, Han X, Alkaline methanolysis of lipid extracts extends shotgun lipidomics analyses to the low-abundance regime of cellular sphingolipids, Anal Biochem, 371 (2007) 135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Barrientos RC, Zhang Q, Differential Isotope Labeling by Permethylation and Reversed-Phase Liquid Chromatography-Mass Spectrometry for Relative Quantification of Intact Neutral Glycolipids in Mammalian Cells, Anal Chem, 91 (2019) 9673–9681. [DOI] [PubMed] [Google Scholar]
- [126].Nagahori N, Abe M, Nishimura S-I, Structural and Functional Glycosphingolipidomics by Glycoblotting with an Aminooxy-Functionalized Gold Nanoparticle, Biochemistry, 48 (2009) 583–594. [DOI] [PubMed] [Google Scholar]
- [127].Hermanson GT, Bioconjugate Techniques (3), Academic Press, San Diego, US, 2013. [Google Scholar]
- [128].Ghidoni R, Sonnino S, Masserini M, Orlando P, Tettamanti G, Specific tritium labeling of gangliosides at the 3-position of sphingosines, J Lipid Res, 22 (1981) 1286–1295. [PubMed] [Google Scholar]
- [129].Song X, Ju H, Lasanajak Y, Kudelka MR, Smith DF, Cummings RD, Oxidative release of natural glycans for functional glycomics, Nat Meth, 13 (2016) 528–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Anugraham M, Everest-Dass AV, Jacob F, Packer NH, A platform for the structural characterization of glycans enzymatically released from glycosphingolipids extracted from tissue and cells, Rapid Commun Mass Spectrom, 29 (2015) 545–561. [DOI] [PubMed] [Google Scholar]
- [131].Albrecht S, Vainauskas S, Stöckmann H, McManus C, Taron CH, Rudd PM, Comprehensive Profiling of Glycosphingolipid Glycans Using a Novel Broad Specificity Endoglycoceramidase in a High-Throughput Workflow, Anal Chem, 88 (2016) 4795–4802. [DOI] [PubMed] [Google Scholar]
- [132].Arigi E, Blixt O, Buschard K, Clausen H, Levery SB, Design of a covalently bonded glycosphingolipid microarray, Glycoconj J, 29 (2012) 1–12. [DOI] [PubMed] [Google Scholar]
- [133].Nabetani T, Makino A, Hullin-Matsuda F, Hirakawa T.-a., Takeoka S, Okino N, Ito M, Kobayashi T, Hirabayashi Y, Multiplex analysis of sphingolipids using amine-reactive tags (iTRAQ), J Lipid Res, 52 (2011) 1294–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Furukawa J.-i., Shinohara Y, Kuramoto H, Miura Y, Shimaoka H, Kurogochi M, Nakano M, Nishimura S-I, Comprehensive Approach to Structural and Functional Glycomics Based on Chemoselective Glycoblotting and Sequential Tag Conversion, Anal Chem, 80 (2008) 1094–1101. [DOI] [PubMed] [Google Scholar]
- [135].Fujitani N, Takegawa Y, Ishibashi Y, Araki K, Furukawa J.-i., Mitsutake S, Igarashi Y, Ito M, Shinohara Y, Qualitative and Quantitative Cellular Glycomics of Glycosphingolipids Based on Rhodococcal Endoglycosylceramidase-assisted Glycan Cleavage, Glycoblotting-assisted Sample Preparation, and Matrix-assisted Laser Desorption Ionization Tandem Time-of-flight Mass Spectrometry Analysis, J Biol Chem, 286 (2011) 41669–41679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Zaia J, Mass Spectrometry and the Emerging Field of Glycomics, Chem Biol, 15 (2008) 881–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Wuhrer M, Glycomics using mass spectrometry, Glycoconj J, 30 (2013) 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Kailemia MJ, Ruhaak LR, Lebrilla CB, Amster IJ, Oligosaccharide Analysis by Mass Spectrometry: A Review of Recent Developments, Anal Chem, 86 (2014) 196–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Narvaez-Rivas M, Zhang Q, Comprehensive untargeted lipidomic analysis using core–shell C30particle column and high field orbitrap mass spectrometer, J Chromatogr A, 1440 (2016) 123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Barrientos RC, Zhang Q, Isobaric Labeling of Intact Gangliosides toward Multiplexed LC-MS/MS-Based Quantitative Analysis, Anal Chem, 90 (2018) 2578–2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Ikeda K, Shimizu T, Taguchi R, Targeted analysis of ganglioside and sulfatide molecular species by LC/ESI-MS/MS with theoretically expanded multiple reaction monitoring, J Lipid Res, 49 (2008) 2678–2689. [DOI] [PubMed] [Google Scholar]
- [142].Wang Q, Shimizu K, Maehata K, Pan Y, Sakurai K, Hikida T, Fukada Y, Takao T, Lithium ion adduction enables UPLC-MS/MS-based analysis of multi-class 3-hydroxyl group-containing keto-steroids, J Lipid Res, 61 (2020) 570–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Hillenkamp F, Karas M, The MALDI Process and Method, MALDI MS, Wiley-VCH Verlag GmbH & Co. KGaA2007, pp. 1–28. [Google Scholar]
- [144].Harvey DJ, Matrix-assisted laser desorption/ionization mass spectrometry of carbohydrates, Mass Spectrom Rev, 18 (1999) 349–451. [DOI] [PubMed] [Google Scholar]
- [145].Harvey DJ, Analysis of carbohydrates and glycoconjugates by matrix-assisted laser desorption/ionization mass spectrometry: an update covering the period 1999–2000, Mass Spectrom Rev, 25 (2006) 595–662. [DOI] [PubMed] [Google Scholar]
- [146].Harvey DJ, Analysis of carbohydrates and glycoconjugates by matrix-assisted laser desorption/ionization mass spectrometry: an update for 2007–2008, Mass Spectrom Rev, 31 (2012) 183–311. [DOI] [PubMed] [Google Scholar]
- [147].Harvey DJ, Analysis of carbohydrates and glycoconjugates by matrix-assisted laser desorption/ionization mass spectrometry: an update for 2009–2010, Mass Spectrom Rev, 34 (2015) 268–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Cheng H, Sun G, Yang K, Gross RW, Han X, Selective desorption/ionization of sulfatides by MALDI-MS facilitated using 9-aminoacridine as matrix, J Lipid Res, 51 (2010) 1599–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].O’Connor PB, Mirgorodskaya E, Costello CE, High pressure matrix-assisted laser desorption/ionization Fourier transform mass spectrometry for minimization of ganglioside fragmentation, J Am Soc Mass Spectrom, 13 (2002) 402–407. [DOI] [PubMed] [Google Scholar]
- [150].Zhang J, LaMotte L, Dodds ED, Lebrilla CB, Atmospheric Pressure MALDI Fourier Transform Mass Spectrometry of Labile Oligosaccharides, Anal Chem, 77 (2005) 4429–4438. [DOI] [PubMed] [Google Scholar]
- [151].Harvey DJ, Derivatization of carbohydrates for analysis by chromatography: electrophoresis and mass spectrometry, J Chromatogr B, 879 (2011) 1196–1225. [DOI] [PubMed] [Google Scholar]
- [152].Torretta E, Fania C, Vasso M, Gelfi C, HPTLC-MALDI MS for (glyco)sphingolipid multiplexing in tissues and blood: A promising strategy for biomarker discovery and clinical applications, Electrophoresis, 37 (2016) 2036–2049. [DOI] [PubMed] [Google Scholar]
- [153].Harvey DJ, Scarff CA, Crispin M, Scanlan CN, Bonomelli C, Scrivens JH, MALDI-MS/MS with Traveling Wave Ion Mobility for the Structural Analysis of N-Linked Glycans, J Am Soc Mass Spectrom, 23 (2012) 1955–1966. [DOI] [PubMed] [Google Scholar]
- [154].Zarei M, Kirsch S, Muthing J, Bindila L, Peter-Katalinic J, Automated normal phase nano high performance liquid chromatography/matrix assisted laser desorption/ionization mass spectrometry for analysis of neutral and acidic glycosphingolipids, Anal Bioanal Chem, 391 (2008) 289–297. [DOI] [PubMed] [Google Scholar]
- [155].Caughlin S, Hepburn JD, Park DH, Jurcic K, Yeung KKC, Cechetto DF, Whitehead SN, Increased Expression of Simple Ganglioside Species GM2 and GM3 Detected by MALDI Imaging Mass Spectrometry in a Combined Rat Model of Aβ Toxicity and Stroke, PLoS One, 10 (2015) e0130364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Chen Y, Allegood J, Liu Y, Wang E, Cachón-González B, Cox TM, Merrill AH, Sullards MC, Imaging MALDI Mass Spectrometry Using an Oscillating Capillary Nebulizer Matrix Coating System and Its Application to Analysis of Lipids in Brain from a Mouse Model of Tay–Sachs/Sandhoff Disease, Anal Chem, 80 (2008) 2780–2788. [DOI] [PubMed] [Google Scholar]
- [157].Karlsson O, Michno W, Ransome Y, Hanrieder J, MALDI Imaging Delineates Hippocampal Glycosphingolipid Changes Associated With Neurotoxin Induced Proteopathy Following Neonatal BMAA Exposure, Biochim Biophys Acta Proteins Proteomics, 1865 (2017) 740–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Kouzel IU, Soltwisch J, Pohlentz G, Schmitz JS, Karch H, Dreisewerd K, Müthing J, Infrared MALDI mass spectrometry imaging of TLC-separated glycosphingolipids with emphasis on Shiga toxin receptors isolated from human colon epithelial cells, Int J Mass Spectrom, 416 (2017) 53–60. [Google Scholar]
- [159].Ruh H, Salonikios T, Fuchser J, Schwartz M, Sticht C, Hochheim C, Wirnitzer B, Gretz N, Hopf C, MALDI imaging MS reveals candidate lipid markers of polycystic kidney disease, J Lipid Res, 54 (2013) 2785–2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Vens-Cappell S, Kouzel IU, Kettling H, Soltwisch J, Bauwens A, Porubsky S, Müthing J, Dreisewerd K, On-Tissue Phospholipase C Digestion for Enhanced MALDI-MS Imaging of Neutral Glycosphingolipids, Anal Chem, 88 (2016) 5595–5599. [DOI] [PubMed] [Google Scholar]
- [161].Norris JL, Caprioli RM, Analysis of Tissue Specimens by Matrix-Assisted Laser Desorption/Ionization Imaging Mass Spectrometry in Biological and Clinical Research, Chem Rev, 113 (2013) 2309–2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Domon B, Costello C, A Systematic Nomenclature for Carbohydrate Fragmentations in FAB-MS/MS Spectra of Glycoconjugates, Glycoconj J, 5 (1988) 397–409. [Google Scholar]
- [163].Ann Q, Adams J, Structure-specific collision-induced fragmentations of ceramides cationized with alkali-metal ions, Anal Chem, 65 (1993) 7–13. [Google Scholar]
- [164].Masson EA, Sibille E, Martine L, Chaux-Picquet F, Bretillon L, Berdeaux O, Apprehending ganglioside diversity: a comprehensive methodological approach, J Lipid Res, 56 (2015) 1821–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Peng B, Weintraub ST, Coman C, Ponnaiyan S, Sharma R, Tews B, Winter D, Ahrends R, A Comprehensive High-Resolution Targeted Workflow for the Deep Profiling of Sphingolipids, Anal Chem, 89 (2017) 12480–12487. [DOI] [PubMed] [Google Scholar]
- [166].Qi Y, Volmer DA, Electron-based fragmentation methods in mass spectrometry: An overview, Mass Spectrom Rev, 36 (2017) 4–15. [DOI] [PubMed] [Google Scholar]
- [167].Fort KL, Cramer CN, Voinov VG, Vasil’ev YV, Lopez NI, Beckman JS, Heck AJR, Exploring ECD on a Benchtop Q Exactive Orbitrap Mass Spectrometer, J Proteome Res, 17 (2018) 926–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Kim M-S, Pandey A, Electron transfer dissociation mass spectrometry in proteomics, Proteomics, 12 (2012) 530–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].McFarland MA, Marshall AG, Hendrickson CL, Nilsson CL, Fredman P, Månsson J-E, Structural Characterization of the GM1 Ganglioside by Infrared Multiphoton Dissociation, Electron Capture Dissociation, and Electron Detachment Dissociation Electrospray Ionization FT-ICR MS/MS, J Am Soc Mass Spectrom, 16 (2005) 752–762. [DOI] [PubMed] [Google Scholar]
- [170].Reilly JP, Ultraviolet photofragmentation of biomolecular ions, Mass Spectrom Rev, 28 (2009) 425–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].O’Brien JP, Brodbelt JS, Structural characterization of gangliosides and glycolipids via ultraviolet photodissociation mass spectrometry, Anal Chem, 85 (2013) 10399–10407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Ryan E, Nguyen CQN, Shiea C, Reid GE, Detailed Structural Characterization of Sphingolipids via 193 nm Ultraviolet Photodissociation and Ultra High Resolution Tandem Mass Spectrometry, J Am Soc Mass Spectrom, 28 (2017) 1406–1419. [DOI] [PubMed] [Google Scholar]
- [173].Brown SHJ, Mitchell TW, Blanksby SJ, Analysis of unsaturated lipids by ozone-induced dissociation, Biochim Biophys Acta Mol Cell Biol Lipids, 1811 (2011) 807–817. [DOI] [PubMed] [Google Scholar]
- [174].Thomas MC, Mitchell TW, Harman DG, Deeley JM, Nealon JR, Blanksby SJ, Ozone-Induced Dissociation: Elucidation of Double Bond Position within Mass-Selected Lipid Ions, Anal Chem, 80 (2008) 303–311. [DOI] [PubMed] [Google Scholar]
- [175].Vu N, Brown J, Giles K, Zhang Q, Ozone-induced dissociation on a traveling wave high-resolution mass spectrometer for determination of double-bond position in lipids, Rapid Commun Mass Spectrom, 31 (2017) 1415–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Barrientos RC, Zhang Q, Fragmentation Behavior and Gas-Phase Structures of Cationized Glycosphingolipids in Ozone-Induced Dissociation Mass Spectrometry, J Am Soc Mass Spectrom, 30 (2019) 1609–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].He H, Emmett MR, Nilsson CL, Conrad CA, Marshall AG, High mass accuracy and resolution facilitate identification of glycosphingolipids and phospholipids, Int J Mass Spectrom, 305 (2011) 116–119. [Google Scholar]
- [178].Hajek R, Jirasko R, Lisa M, Cifkova E, Holcapek M, Hydrophilic Interaction Liquid Chromatography-Mass Spectrometry Characterization of Gangliosides in Biological Samples, Anal Chem, 89 (2017) 12425–12432. [DOI] [PubMed] [Google Scholar]
- [179].Lee H, Lerno LA Jr., Choe Y, Chu CS, Gillies LA, Grimm R, Lebrilla CB, German JB, Multiple precursor ion scanning of gangliosides and sulfatides with a reversed-phase microfluidic chip and quadrupole time-of-flight mass spectrometry, Anal Chem, 84 (2012) 5905–5912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Ikeda K, Taguchi R, Highly sensitive localization analysis of gangliosides and sulfatides including structural isomers in mouse cerebellum sections by combination of laser microdissection and hydrophilic interaction liquid chromatography/electrospray ionization mass spectrometry with theoretically expanded multiple reaction monitoring, Rapid Commun Mass Spectrom, 24 (2010) 2957–2965. [DOI] [PubMed] [Google Scholar]
- [181].Kruger R, Bruns K, Grunhage S, Rossmann H, Reinke J, Beck M, Lackner KJ, Determination of globotriaosylceramide in plasma and urine by mass spectrometry, Clin Chem Lab Med, 48 (2010) 189–198. [DOI] [PubMed] [Google Scholar]
- [182].Aoki K, Heaps AD, Strauss KA, Tiemeyer M, Mass spectrometric quantification of plasma glycosphingolipids in human GM3 ganglioside deficiency, Clin Mass Spectrom, 14 (2019) 106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Sueoka H, Aoki M, Tsukimura T, Togawa T, Sakuraba H, Distributions of Globotriaosylceramide Isoforms, and Globotriaosylsphingosine and Its Analogues in an α-Galactosidase A Knockout Mouse, a Model of Fabry Disease, PLoS One, 10 (2015) e0144958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Sidhu R, Mikulka CR, Fujiwara H, Sands MS, Schaffer JE, Ory DS, Jiang X, A HILIC-MS/MS method for simultaneous quantification of the lysosomal disease markers galactosylsphingosine and glucosylsphingosine in mouse serum, Biomed Chromatogr, 32 (2018) e4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Zheng K, Ji A, Chung LL, Culm-Merdek K, Liu H, Richards S, Sung C, Enhancement of human plasma glucosylceramide assay sensitivity using delipidized plasma, Mol Genet Metab Rep, 8 (2016) 77–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Ji AJ, Wang H, Ziso-Qejvanaj E, Zheng K, Chung LL, Foley T, Chuang W-L, Richards S, Sung C, A novel approach for quantitation of glucosylceramide in human dried blood spot using LC–MS/MS, Bioanalysis, 7 (2015) 1483–1496. [DOI] [PubMed] [Google Scholar]
- [187].Lee H, German JB, Kjelden R, Lebrilla CB, Barile D, Quantitative analysis of gangliosides in bovine milk and colostrum-based dairy products by ultrahigh performance liquid chromatography-tandem mass spectrometry, J Agric Food Chem, 61 (2013) 9689–9696. [DOI] [PubMed] [Google Scholar]
- [188].Sorensen LK, A liquid chromatography/tandem mass spectrometric approach for the determination of gangliosides GD3 and GM3 in bovine milk and infant formulae, Rapid Commun Mass Spectrom, 20 (2006) 3625–3633. [DOI] [PubMed] [Google Scholar]
- [189].Ciucanu I, Kerek F, A simple and rapid method for the permethylation of carbohydrates, Carbohydr Res, 131 (1984) 209–217. [Google Scholar]
- [190].Gunnarsson A, N-andO-alkylation of glycoconjugates and polysaccharides by solid base in dimethyl sulphoxide/alkyl lodide, Glycoconj J, 4 (1987) 239–245. [Google Scholar]
- [191].Atwood JA, Cheng L, Alvarez-Manilla G, Warren NL, York WS, Orlando R, Quantitation by Isobaric Labeling: Applications to Glycomics, J Proteome Res, 7 (2008) 367–374. [DOI] [PubMed] [Google Scholar]
- [192].Alvarez-Manilla G, Warren NL, Abney T, Atwood J 3rd, Azadi P, York WS, Pierce M, Orlando R, Tools for glycomics: relative quantitation of glycans by isotopic permethylation using 13CH3I, Glycobiology, 17 (2007) 677–687. [DOI] [PubMed] [Google Scholar]
- [193].Kang P, Mechref Y, Kyselova Z, Goetz JA, Novotny MV, Comparative Glycomic Mapping through Quantitative Permethylation and Stable-Isotope Labeling, Anal Chem, 79 (2007) 6064–6073. [DOI] [PubMed] [Google Scholar]
- [194].Huang Q, Liu D, Xin B, Cechner K, Zhou X, Wang H, Zhou A, Quantification of monosialogangliosides in human plasma through chemical derivatization for signal enhancement in LC–ESI-MS, Anal Chim Acta, 929 (2016) 31–38. [DOI] [PubMed] [Google Scholar]
- [195].Rauniyar N, Yates JR, Isobaric Labeling-Based Relative Quantification in Shotgun Proteomics, J Proteome Res, 13 (2014) 5293–5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Ross PL, Huang YN, Marchese JN, Williamson B, Parker K, Hattan S, Khainovski N, Pillai S, Dey S, Daniels S, Purkayastha S, Juhasz P, Martin S, Bartlet-Jones M, He F, Jacobson A, Pappin DJ, Multiplexed protein quantitation in Saccharomyces cerevisiae using amine-reactive isobaric tagging reagents, Mol Cell Proteomics, 3 (2004) 1154–1169. [DOI] [PubMed] [Google Scholar]
- [197].Whitmore CD, Hindsgaul O, Palcic MM, Schnaar RL, Dovichi NJ, Metabolic Cytometry. Glycosphingolipid Metabolism in Single Cells, Anal Chem, 79 (2007) 5139–5142. [DOI] [PubMed] [Google Scholar]
- [198].Keithley RB, Rosenthal AS, Essaka DC, Tanaka H, Yoshimura Y, Palcic MM, Hindsgaul O, Dovichi NJ, Capillary electrophoresis with three-color fluorescence detection for the analysis of glycosphingolipid metabolism, Analyst, 138 (2013) 164–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Son S-H, Daikoku S, Ohtake A, Suzuki K, Kabayama K, Ito Y, Kanie O, Syntheses of lactosyl ceramide analogues carrying novel bifunctional BODIPY dyes directed towards the differential analysis of multiplexed glycosphingolipids by MS/MS using iTRAQ, Chem Commun, 50 (2014) 3010–3013. [DOI] [PubMed] [Google Scholar]
- [200].Hofmann J, Pagel K, Glycan Analysis by Ion Mobility–Mass Spectrometry, Angew Chem Int Ed Engl, 56 (2017) 8342–8349. [DOI] [PubMed] [Google Scholar]
- [201].Gray CJ, Thomas B, Upton R, Migas LG, Eyers CE, Barran PE, Flitsch SL, Applications of ion mobility mass spectrometry for high throughput, high resolution glycan analysis, Biochim Biophys Acta Gen Subj, 1860 (2016) 1688–1709. [DOI] [PubMed] [Google Scholar]
- [202].Struwe WB, Pagel K, Benesch JLP, Harvey DJ, Campbell MP, GlycoMob: an ion mobility-mass spectrometry collision cross section database for glycomics, Glycoconj J, 33 (2016) 399–404. [DOI] [PubMed] [Google Scholar]
- [203].Lanucara F, Holman SW, Gray CJ, Eyers CE, The power of ion mobility-mass spectrometry for structural characterization and the study of conformational dynamics, Nat Chem, 6 (2014) 281–294. [DOI] [PubMed] [Google Scholar]
- [204].Wojcik R, Webb I, Deng L, Garimella S, Prost S, Ibrahim Y, Baker E, Smith R, Lipid and Glycolipid Isomer Analyses Using Ultra-High Resolution Ion Mobility Spectrometry Separations, Int J Mol Sci, 18 (2017) 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Jackson SN, Colsch B, Egan T, Lewis EK, Schultz JA, Woods AS, Gangliosides’ analysis by MALDI-ion mobility MS, Analyst, 136 (2011) 463–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Xu H, Boucher FR, Nguyen TT, Taylor GP, Tomlinson JJ, Ortega RA, Simons B, Schlossmacher MG, Saunders-Pullman R, Shaw W, Bennett SAL, DMS as an orthogonal separation to LC/ESI/MS/MS for quantifying isomeric cerebrosides in plasma and cerebrospinal fluid, J Lipid Res, 60 (2019) 200–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Leaptrot KL, May JC, Dodds JN, McLean JA, Ion mobility conformational lipid atlas for high confidence lipidomics, Nat Commun, 10 (2019) 985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Zhou Z, Tu J, Xiong X, Shen X, Zhu ZJ, LipidCCS: Prediction of Collision Cross-Section Values for Lipids with High Precision To Support Ion Mobility-Mass Spectrometry-Based Lipidomics, Anal Chem, 89 (2017) 9559–9566. [DOI] [PubMed] [Google Scholar]


