Abstract
Introduction
Invasive meningococcal disease (IMD) has a high rate of fatality and may cause severe clinical sequelae. Over the years, the epidemiology of IMD has changed significantly in various regions of the world, and laboratory surveillance of this disease is important for mapping epidemiologic changes.
Aim
To perform phenotypic characterization of Neisseria meningitidis strains isolated from invasive disease in Brazil from 2002 to 2017, as a complementation of the data obtained in the period of 1990–2001.
Methodology
In total, 8,689 isolates sent to Adolfo Lutz Institute confirmed as N. meningitidis by conventional methods were serogrouped by slide agglutination against MenA, MenB, MenC, MenE, MenW, MenX, MenY and MenZ; serotyped and serosubtyped by a whole-cell dot-blotting assay with monoclonal antibodies.
Results
The isolates were sent from all regions of Brazil, and the southeast region was responsible for the largest number of isolates (57.2 %). Overall, the total sample (n=8,689) was represented by serogroups C (n=4,729; 54.4 %), B (n=3,313; 38.1 %), W (n=423; 4.9 %), Y (n=203; 2.3 %), X (n=5; 0.1 %) and others (n=16; 0.2 %). A shift in the prevalence of serogroups was observed in 2006, when serogroup C became the most prevalent (65.5 %), surpassing the serogroup B (21.9 %). The main isolated phenotypes were C:23:P1.14–6; B:4,7:P1.19,15; W:2a:P1.5 and W:2a:P1.5,2.
Conclusion
The data show an important change in the distribution of meningococcal serogroups, serotypes and subtypes occurring during 2002–2017. A continuous laboratory-based surveillance provides robust information to implement appropriate strategies to IMD control.
Keywords: meningococcal surveillance, Neisseria meningitidis, laboratory meningococcal surveillance
Introduction
Invasive meningococcal disease (IMD) is one of the most feared diseases due to its rate of fatality of 5–10 %, and causes severe clinical sequelae such as deafness, amputation and mental impairment [1]. Twelve serogroups of Neisseria meningitidis have been characterized based on their capsular polysaccharides, although serogroups A, B, C, W, Y and X are the most clinically significant [2, 3]. Most cases of IMD occur sporadically and vary according to the age, socioeconomic conditions, geographic regions and serogroups involved; outbreaks and epidemics may occur at irregular intervals [4–6]. Since 1975, IMD is a notifiable disease in Brazil and the National Surveillance System (SNV) monitors the epidemiological characteristics of the disease.
From 1990 to 2001, 68, 332 IMD cases were reported to the Brazilian National Notifiable Disease Surveillance System (SINAN), and the annual incidence rate was estimated to be around 1–3 cases per 100, 000 persons [7]; in contrast, in the period from 2002 to 2017, 41, 192 IMD cases were notified and the annual incidence rate was estimated at around 0.55–2.12 cases per 100,000 persons, presenting a clear reduction trend in the period, from 2.12 cases in 2002 to 0.55 cases per 100, 000 in 2017 [8]. Thirty-one percent (n=12, 621) of the cases notified between 2002 and 2017 were confirmed by isolation of the causative strain, from which 68.8 % (n=8,689) were referred to the National Reference Laboratory for Meningitis at Adolfo Lutz Institute, São Paulo, herein named IAL, for species confirmation and further characterization.
Over the years, the epidemiology of IMD has changed significantly in various regions of the world. The aim of this study was to analyse the phenotypic characteristics of N. meningitidis strains causing IMD in Brazil, thus complementing the previously described results [7] of the period from 1990 to 2001.
Methods
Meningococcal isolate collection
The IAL receives invasive meningococcal isolates from public health laboratories and hospitals from all Brazilian regions for full phenotypic characterization.
Of the 12, 621 cases of IMD laboratory-confirmed by culture between 2002 and 2017 in Brazil, 8,689 isolates (68.8 %) were sent to IAL.
Identification and serogrouping of N. meningitidis
All the 8,689 IMD isolates were confirmed as N. meningitidis by conventional methods (oxidase test and carbohydrate utilization testing for glucose, maltose, sucrose and lactose). Serogrouping was performed by slide agglutination with polyclonal goat or horse antisera, prepared at the IAL against MenA, MenB, MenC, MenE, MenW, MenX, MenY and MenZ, as described previously [9, 10].
Serotype and serosubtype determination
In order to determine the PorB and PorA types, all the isolates were serotyped and serosubtyped by a whole-cell dot-blotting assay with monoclonal antibodies (mAbs) [11]. The full sets consisted of mAbs for serotypes 1, 2a, 2b, 2 c, 4, 5, 7, 9, 10, 11, 14, 15, 17, 19, 21, 22 and 23, and for serosubtypes P1.1, P1.2, P1.3, P1.4, P1.5, P1.7, P1.9, P1.10, P1.12, P1.14, P1.14–6, P1.15, P1.16, P1.19 and P1.22–1. A set of reference strains of each serotype and serosubtype was used in each reaction as positive and negative controls.
Results and discussion
Of the 8,689 N . meningitidis isolates (68.8 %) sent to IAL, 4,969 (57.2 %) were from the Southeast, 1,612 (18.6 %) from the North West, 1,222 (14.1 %) from the South, 596 (6.8 %) from the Central West and 290 (3.3 %) from the North region of Brazil (Table 1). This distribution follows the pattern found in the previous period [7], and is in agreement with the 2010 demographic census, which shows that 42.1 % of the Brazilian population is concentrated in the Southeast region while 8.3 % is in the Northern region (https://censo2010.ibge.gov.br/sinopse/index.php?dados=5&uf=00).
Table 1.
Distribution of Neisseria meningitidis serogroups by geographical regions and by age group, from 2002 to 2017, in Brazil
|
2002–2006 |
2007–2010 |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
B n (%) |
C n (%) |
W n (%) |
Y n (%) |
Others* n (%) |
Total |
B n (%) |
C n (%) |
W n (%) |
Y n (%) |
Others* n (%) |
Total |
|||
|
Geographic region |
||||||||||||||
|
North |
168 (82.0) |
37 (18.0) |
– |
– |
– |
205 |
23 (45.1) |
27 (52.9) |
1 (2.0) |
– |
– |
51 |
||
|
North west |
607 (75.6) |
186 (23.2) |
5 (0.6) |
5 (0.6) |
– |
803 |
128 (25.8) |
362 (72.8) |
4 (0.8) |
3 (0.6) |
– |
497 |
||
|
Central west |
132 (59.7) |
81 (36.7) |
4 (1.8) |
4 (1.8) |
– |
221 |
33 (15.3) |
166 (76.9) |
12 (5.5) |
5 (2.3) |
– |
216 |
||
|
South east |
871 (45.8) |
929 (48.8) |
70 (3.7) |
30 (1.6) |
3 (0.1) |
1903 |
280 (17.8) |
1149 (73.0) |
101 (6.4) |
39 (2.5) |
5 (0.3) |
1574 |
||
|
South |
387 (74.4) |
111 (21.3) |
20 (3.9) |
2 (0.4) |
– |
520 |
152 (56.5) |
90 (33.5) |
25 (9.3) |
2 (0.7) |
– |
269 |
||
|
All regions |
2165 (59.3) |
1344 (36.8) |
99 (2.7) |
41 (1.1) |
3 (0.1) |
3652 |
616 (23.6) |
1794 (68.8) |
143 (5.5) |
49 (1.9) |
5 (0.2) |
2607 |
||
|
Age group |
||||||||||||||
|
< 12 mo |
309 (59.2) |
192 (36.8) |
15 (2.9) |
6 (1.1) |
– |
522 |
100 (30.9) |
189 (58.3) |
28 (8.6) |
7 (2.2) |
– |
324 |
||
|
12–23 mo |
140 (53.8) |
110 (42.3) |
8 (3.1) |
1 (0.4) |
1 (0.4) |
260 |
51 (28.8) |
111 (62.7) |
9 (5.1) |
6 (3.4) |
– |
177 |
||
|
24–59 mo |
397 (61.2) |
228 (35.1) |
16 (2.5) |
7 (1.1) |
1 (0.1) |
649 |
109 (28.4) |
260 (67.7) |
11 (2.9) |
4 (1.0) |
– |
384 |
||
|
5–14 y |
565 (61.7) |
328 (35.8) |
17 (1.9) |
5 (0.6) |
– |
915 |
131 (21.5) |
444 (72.8) |
25 (4.1) |
10 (1.6) |
– |
610 |
||
|
15–29 y |
347 (61.2) |
187 (33.0) |
23 (4.0) |
10 (1.8) |
– |
567 |
87 (18.0) |
360 (74.5) |
26 (5.4) |
9 (1.9) |
1 (0.2) |
483 |
||
|
30–49 y |
118 (48.6) |
114 (46.9) |
5 (2.0) |
6 (2.5) |
– |
243 |
43 (16.1) |
206 (77.1) |
13 (4.9) |
5 (1.9) |
– |
267 |
||
|
>= 50 y |
64 (55.2) |
45 (38.8) |
6 (5.1) |
1 (0.9) |
– |
116 |
32 (20.3) |
95 (60.1) |
21 (13.3) |
7 (4.4) |
3 (1.9) |
158 |
||
|
Unknown |
225 (59.2) |
140 (36.8) |
9 (2.4) |
5 (1.3) |
1 (0.3) |
380 |
63 (30.9) |
129 (63.2) |
10 (4.9) |
1 (0.5) |
1 (0.5) |
204 |
||
|
Total |
2165 (59.3) |
1344 (36.8) |
99 (2.7) |
41 (1.1) |
3 (0.1) |
3652 |
616 (23.6) |
1794 (68.8) |
143 (5.5) |
49 (1.9) |
5 (0.2) |
2607 |
||
|
2011–2017 |
Entire period, 2002–2017 |
|||||||||||||
|
B n (%) |
C n (%) |
W n (%) |
Y n (%) |
X n (%) |
Others* n (%) |
Total |
B n (%) |
C n (%) |
W n (%) |
Y n (%) |
X n (%) |
Others* n (%) |
Total |
|
|
Geographic region |
||||||||||||||
|
North |
10 (29.4) |
23 (67.7) |
1 (2.9) |
– |
– |
– |
34 |
201 (69.3) |
87 (30.0) |
2 (0.7) |
– |
– |
– |
290 |
|
North west |
54 (17.3) |
223 (71.5) |
24 (7.7) |
10 (3.2) |
– |
1 (0.3) |
312 |
789 (48.9) |
771 (47.8) |
33 (2.1) |
18 (1.1) |
– |
1 (0.1) |
1612 |
|
Central west |
29 (18.2) |
112 (70.5) |
13 (8.2) |
4 (2.5) |
– |
1 (0.6) |
159 |
194 (32.5) |
359 (60.2) |
29 (4.9) |
13 (2.2) |
– |
1 (0.2) |
596 |
|
South east |
306 (20.5) |
1027 (68.8) |
71 (4.8) |
81 (5.4) |
1 (0.1) |
6 (0.4) |
1492 |
1457 (29.3) |
3105 (62.5) |
242 (4.9) |
150 (3.0) |
1 (**) |
14 (0.3) |
4969 |
|
South |
133 (30.7) |
206 (47.6) |
72 (16.6) |
18 (4.2) |
4 (0.9) |
– |
433 |
672 (55.0) |
407 (33.3) |
117 (9.6) |
22 (1.8) |
4 (0.3) |
– |
1222 |
|
All regions |
532 (21.9) |
1591 (65.5) |
181 (7.4) |
113 (4.7) |
5 (0.2) |
8 (0.3) |
2430 |
3313 (38.1) |
4729 (54.4) |
423 (4.9) |
203 (2.3) |
5 (**) |
16 (0.2) |
8689 |
|
Age group |
||||||||||||||
|
< 12 mo |
133 (51.8) |
80 (31.1) |
35 (13.6) |
9 (3.5) |
– |
– |
257 |
542 (49.1) |
461 (41.8) |
78 (7.1) |
22 (2.0) |
– |
– |
1103 |
|
12–23 mo |
51 (65.4) |
7 (9.0) |
15 (19.2) |
5 (6.4) |
– |
– |
78 |
242 (47.0) |
228 (44.3) |
32 (6.2) |
12 (2.3) |
– |
1 (0.2) |
515 |
|
24–59 mo |
80 (35.4) |
110 (48.6) |
25 (11.1) |
9 (4.0) |
2 (0.9) |
– |
226 |
586 (46.5) |
598 (47.5) |
52 (4.1) |
20 (1.6) |
2 (0.2) |
1 (0.1) |
1259 |
|
5–14 y |
98 (17.6) |
423 (75.1) |
16 (2.8) |
23 (4.0) |
1 (0.2) |
2 (0.3) |
563 |
794 (38.0) |
1195 (57.2) |
58 (2.8) |
38 (1.9) |
1 (**) |
2 (0.1) |
2088 |
|
15–29 y |
79 (14.6) |
414 (76.4) |
29 (5.3) |
19 (3.5) |
– |
1 (0.2) |
542 |
513 (32.2) |
961 (60.4) |
78 (4.9) |
38 (2.4) |
– |
2 (0.1) |
1592 |
|
30–49 y |
46 (11.1) |
325 (78.3) |
27 (6.5) |
15 (3.6) |
– |
2 (0.5) |
415 |
207 (22.4) |
645 (69.7) |
45 (4.9) |
26 (2.8) |
– |
2 (0.2) |
925 |
|
>= 50 y |
33 (11.3) |
192 (66.0) |
31 (10.7) |
30 (10.3) |
2 (0.7) |
3 (1.0) |
291 |
129 (22.8) |
332 (58.8) |
58 (10.3) |
38 (6.7) |
2 (0.3) |
6 (1.1) |
565 |
|
Unknown |
12 (20.7) |
40 (68.9) |
3 (5.2) |
3 (5.2) |
– |
– |
58 |
300 (46.7) |
309 (48.2) |
22 (3.4) |
9 (1.4) |
– |
2 (0.3) |
642 |
|
Total |
532 (21.9) |
1591 (65.5) |
181 (7.4) |
113 (4.7) |
5 (0.2) |
8 (0.3) |
2430 |
3313 (38.1) |
4729 (54.4) |
423 (4.9) |
203 (2.3) |
5 (**) |
16 (0.2) |
8689 |
*Serogroup E, not serogrouped or polyagglutinable.
**Number of isolates too small to calculate a percentage.
The isolates were recovered from normally sterile sites, i.e. cerebrospinal fluid (n=6,493; 74.7 %), blood (n=2,186; 25.2 %), and others (n=10; 0.1 %) (data not shown).
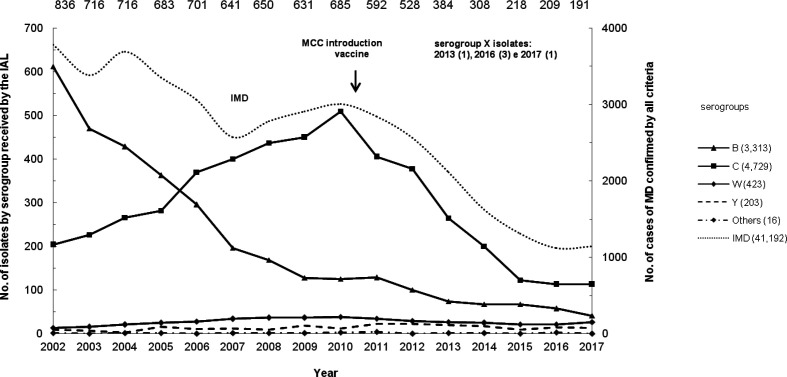
The distribution of isolates by serogroup is shown in Fig. 1, and is similar to the distribution curve of IMD cases reported over this period. Overall, the total sample (n=8,689) was represented by serogroups C (n=4,729; 54.4 %), B (n=3,313; 38.1 %), W (n=423; 4.9 %), Y (n=203; 2.3 %), X (n=5; 0.1 %) and others (n=16; 0.2 %).
Fig. 1.
Number of Neisseria meningitidis isolates from invasive meningococcal disease in Brazil by serogroup per year, 2002–2017. The total number of isolates for each year is shown above the respective data points.
Serogroup B has been the main cause of IMD in Brazil since 1980, with B:4,7:P1.19,15-ST-32 clonal complex (CC) as the most frequent phenotype isolated at all ages until 2002. Serogroup B remains present in all regions of Brazil, with the clone B:4,7:P1.19,15 still being the most prevalent (56 %) among the serogroup B strains typed [7, 12]. However, from 2002 the incidence of serogroup B began to decline, and, in 2006, it was surpassed by a new emerging meningococcal serogroup C clone, C:23:P1.14–6. This serogroup C belonging to ST-103 CC, was described for the first time associated with an epidemic situation in Brazil [13]. Isolates of MenC-ST-103 CC, belonging exclusively to ST-5133, were the most common amongst MenC in Poland in 2005 and constituted 42 % of all MenC in the period of 2009–2011, but the outbreaks notified there, between 2002 and 2011, were all caused by MenC of ST-11 CC [14]. The ST-103 CC has been also described among sporadic cases of IMD by serogroup C in the Czech Republic accounting for less than 1 % of serogroup C isolates [15]. In Brazil, this new clone MenC-ST-103, represented by the main STs: ST-3779, ST-3780, ST-5122 and ST-5123, has been responsible for several IMD outbreaks in the period 2003 to 2010, reaching its highest incidence rate in 2010 as 0.62 per 100,000, significantly higher than the 0.10 per 100,000 for serogroup B in this same year [16–20]. The shift from serogroup B to serogroup C was first detected in the Southeast region (2002–2006) followed by the North West and Central West regions (2007–2010), and finally by the North and South regions (2011–2017) (Table 1). This new MenC clone remains currently the main cause of IMD in Brazil, although with a lower incidence rate of 0.17 per 100,000 in 2017, due to the introduction in 2010 of a Meningococcal C Conjugate Vaccine (MCC vaccine) into the national immunization program. Initially, the strategy was to vaccinate children at 3 and 5 months, with a booster dose at 12 months of age, and no catch-up campaigns. After implementation of this vaccine, the IMD overall rate has decreased from 1.54 per 100,000 in 2010 to 0.55 per 100,000 in 2017 [8]. As shown in Table 1, there has been a substantial reduction in the frequency of serogroup C isolates from infants and older children, but the prevalence of serogroup C isolates remained relatively high in adolescents and young adults. As suggested by Andrade et al. [21], the reduction of IMD cases in children was mostly due to the direct effect of the vaccination. To provide protection for the older groups, serogroup C vaccination for adolescents aged 12 to 13 years was implemented in March 2017, and by 2020 the age range will be gradually increased, starting at 9 years of age [21]; http://www.aids.gov.br/pt-br/legislacao/nota-informativa-no-3112016cgpnidevitsvsms.
Although serogroup C remains prevalent in all regions of Brazil, the increased incidence of serogroup W in the Southern states from 2014 is of concern because of its potential to cause epidemics, severe disease and its high case fatality rate as has been seen in other countries [22, 23] (Table 1). The clone W:2a:P1.5,2-ST-11 CC, called ‘South American strain’, emerged in 2003 in Southern Brazil [24]. Although it is characterized as ST11/ET37 with similar phenotypic characteristics as to the isolates associated with Hajj 2000, a whole genome sequencing (WGS) analysis demonstrated that the MenW:CC11 ‘South American strain’ is distinct from the Hajj outbreak strain [25]. The ‘South American strain’ has spread to Argentina, Chile, England and Wales, where they became endemic after undergoing diversification in a sublineage named the 2013-strain [26].
Serogroup Y has been most commonly related to carriage isolates over time [27, 28] but this situation has changed in many countries like the USA, for example, where it has become (during the latter half of the 1990s) an important cause of IMD. The increase in the prevalence of serogroup Y in the USA is attributed to the emergence of a ST-23 clone and, while the incidence of all serogroups has fallen in the USA in recent years, serogroup Y continues to be responsible for approximately a third of endemic disease cases [29]. The proportion of MenY disease has also increased during the last years in some European countries such as Sweden, Finland, England and Wales, which may be in part due to the use of MenC conjugate vaccine as opposed to the quadrivalent serogroup ACWY vaccine [3]. The most frequent genotype that has been described is Y:P1.5–2,10-1 CC-23 [30, 31].
Many countries in Central and South America have experienced an increasing incidence of IMD caused by serogroup Y, such as Costa Rica, Colombia, Venezuela, Argentina, Chile and Uruguay. These strains belong mainly to ST-23 CC, similar to those prevalent in the USA [32–34].
In Brazil, the incidence of serogroup Y presented a slight increase in the Southeast region, in the period 2011–2017, but in cumulative terms, from 2002 to 2017, it was recovered from about 2.3 % of all IMD isolates (Table 1). A study carried out by Abad et al. [33] with 45 serogroup Y strains from Brazil, isolated in the period of 2000–2006, showed that two main clones, ST-23 and ST-5770, accounted for 35.6 and 48.9 % of the isolates, respectively.
In contrast to the sub-Saharan Africa, where MenX has caused outbreaks in several countries since 2006 [35–37], developed countries have reported only sporadic cases of serogroup X IMD. In Brazil, serogroup X was first recovered from an IMD case in 2013 in the Southeast region, at the São Paulo state. Since then, another three cases occurred in the Southern states Rio Grande do Sul and Santa Catarina in 2016, and one more in the São Paulo state in 2017, isolated from bronchial washing fluid; all of them belonging to the clone X:4,7:P1.19,15-ST-2888 (unpublished data, personal communication, Lemos AP), the same clone that has been described in Italy [38]. Interestingly, the MenX clone X:NT:P1.5–1-CC-181, the most prevalent in recent IMD epidemic outbreaks in many African regions [37], was not recovered in Brazil despite the significant movement of migrants from Africa to southern Brazil (Rio Grande do Sul state) since 2013 (https://gauchazh.clicrbs.com.br/geral/noticia/2014/08/Novos-imigrantes-mudam-o-cenario-do-Rio-Grande-do-Sul-4576728.html).
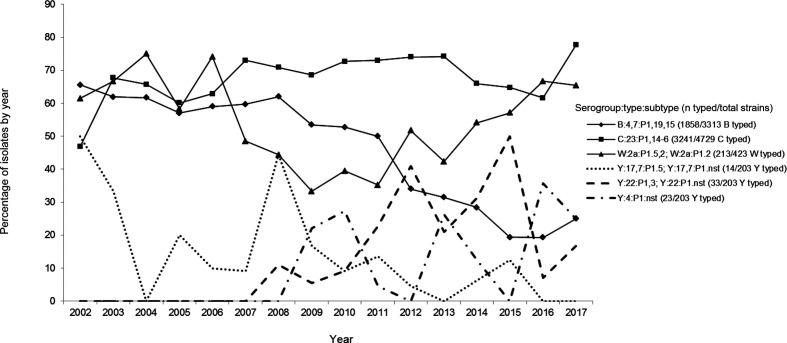
Various phenotypes were recovered over the period of 2002–2017, and the prevalent ones are shown in Fig. 2. The serotype and serosubtype distributions of meningococcal isolates are shown in Tables 2 and 3, respectively. Serogroup B isolates displayed the highest diversity of phenotypes (202 different serotype:serosubtype compositions, data not shown), but B:4,7:P1.19,15 remains the most prevalent among serogroup B isolates (1,858/3,313; 56 %) ever since 1980. Serotype 19 was the second most prevalent serotype linked to serogroup B strains, increasing slightly from 2007, contributing in the whole period with 18 % of the B serotypes. In a previous study on antimicrobial resistance of meningococcal strains from the period of 2009–2016, we identified that the vast majority of serotype 19 isolates linked to serogroup B showed intermediate resistance to penicillin [39].
Fig. 2.
Annual proportion of the main phenotypes of N. meningitidis from invasive meningococcal disease in Brazil, 2002–2017. The percentage refers to the number of strains in a given serogroup that were further typed and subtyped.
Table 2.
Serotype distribution of N meningitidis isolates by the main serogroups (B, C, W, Y, X), Brazil 2002–2017
|
2002–2006 |
2007–2010 |
2011–2017 |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Serotype |
B |
C |
w |
Y |
B |
C |
w |
Y |
B |
C |
w |
Y |
X |
|
|
n (%) |
n (%) |
n (%) |
n (%) |
n (%) |
n (%) |
n (%) |
n (%) |
n (%) |
n (%) |
n (%) |
n (%) |
n (%) |
|
2a |
16 (0.7) |
68 (5.0) |
78 (78.9) |
2 (4.9) |
1 (0.2) |
135 (7.5) |
82 (57.3) |
1 (2.0) |
3 (0.6) |
87 (5.5) |
112 (61.9) |
4 (3.5) |
– |
|
2b |
2 (0.1) |
43 (3.2) |
11 (11.1) |
– |
– |
19 (1.1) |
52 (36.4) |
– |
– |
9 (0.6) |
52 (28.7) |
3 (2.7) |
– |
|
2c |
– |
– |
– |
– |
– |
2 (0.1) |
1 (0.7) |
– |
– |
– |
– |
2 (1.8) |
– |
|
4 |
30 (1.4) |
14 (1.0) |
– |
– |
12 (2.0) |
– |
– |
7 (14.3) |
18 (3.4) |
– |
– |
18 (16.0) |
– |
|
4,7 |
1698 (78.5) |
87 (6.5) |
3 (3.0) |
3 (7.3) |
433 (70.3) |
30 (1.7) |
1 (0.7) |
2 (4.1) |
255 (47.9) |
18 (1.1) |
– |
4 (3.5) |
5 (100) |
|
4,10 |
54 (2.5) |
4 (0.3) |
1 (1.0) |
– |
3 (0.5) |
– |
1 (0.7) |
– |
10 (1.9) |
– |
– |
4 (3.5) |
– |
|
4,14 |
– |
– |
– |
1 (2.4) |
6 (0.9) |
– |
– |
4 (8.2) |
– |
– |
– |
– |
– |
|
14 |
16 (0.7) |
1 (0.1) |
– |
2 (4.9) |
– |
1 (0.05) |
– |
1 (2.0) |
– |
– |
– |
– |
– |
|
15 |
86 (4.0) |
– |
– |
1 (2.4) |
18 (2.9) |
– |
– |
1 (2.0) |
6 (1.1) |
– |
– |
1 (0.9) |
– |
|
17,7 |
44 (2.0) |
4 (0.3) |
– |
14 (34.2) |
9 (1.5) |
1 (0.05) |
– |
9 (18.4) |
9 (1.7) |
5 (0.3) |
– |
6 (5.3) |
– |
|
19 |
30 (1.4) |
6 (0.5) |
1 (1.0) |
– |
21 (3.4) |
– |
– |
3 (6.1) |
96 (18.0) |
3 (0.2) |
– |
8 (7.1) |
– |
|
19,1 |
41 (1.9) |
1 (0.1) |
– |
2 (4.9) |
36 (5.8) |
2 (0.1) |
– |
– |
44 (8.3) |
1 (0.05) |
– |
8 (7.1) |
– |
|
19,7 |
29 (1.3) |
3 (0.2) |
– |
3 (7.3) |
14 (2.3) |
1 (0.05) |
– |
– |
27 (5.1) |
3 (0.2) |
– |
1 (0.9) |
– |
|
19,10 |
26 (1.2) |
1 (0.1) |
1 (1.0) |
2 (4.9) |
4 (0.7) |
1 (0.05) |
2 (1.4) |
2 (4.1) |
2 (0.4) |
– |
1 (0.5) |
7 (6.2) |
– |
|
19,14 |
23 (1.1) |
– |
– |
11 (26.8) |
13 (2.1) |
3 (0.2) |
– |
5 (10.2) |
– |
– |
– |
– |
– |
|
22 |
– |
1 (0.1) |
– |
– |
– |
2 (0.1) |
1 (0.7) |
3 (6.1) |
1 (0.2) |
1 (0.05) |
– |
31 (27.4) |
– |
|
23 |
7 (0.3) |
1057 (78.6) |
1 (1.0) |
– |
7 (1.1) |
1524 (84.9) |
1 (0.7) |
– |
14 (2.6) |
1397 (87.8) |
5 (2.8) |
– |
– |
|
nt |
63 (2.9) |
54 (4.0) |
3 (3.0) |
– |
39 (6.3) |
73 (4.1) |
2 (1.4) |
11 (22.5) |
47 (8.8) |
67 (4.2) |
11 (6.1) |
16 (14.1) |
– |
|
Total |
2165 |
1344 |
99 |
41 |
616 |
1794 |
143 |
49 |
532 |
1591 |
181 |
113 |
5 |
Table 3.
Serosubtype distribution of N meningitidis isolates by the main serogroups (B, C, W, Y, X), Brazil 2002–2017
|
2002–2006 |
2007–2010 |
2011–2017 |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Serosubtype |
B |
C |
w |
Y |
B |
C |
w |
Y |
B |
C |
w |
Y |
X |
|
|
n (%) |
n (%) |
n (%) |
n (%) |
n (%) |
n (%) |
n (%) |
n (%) |
n (%) |
n (%) |
n (%) |
n (%) |
n (%) |
|
P1.1 |
1 (0.05) |
– |
– |
– |
2 (0.3) |
5 (0.3) |
– |
– |
4 (0.8) |
– |
– |
– |
– |
|
P1.2 |
12 (0.6) |
10 (0.7) |
61 (61.6) |
2 (4.9) |
2 (0.3) |
13 (0.7) |
59 (41.2) |
6 (12.3) |
1 (0.2) |
2 (0.1) |
41 (22.6) |
– |
– |
|
P1.3 |
52 (2.4) |
15 (1.2) |
– |
– |
17 (2.8) |
5 (0.3) |
3 (2.1) |
4 (8.2) |
5 (0.9) |
2 (0.1) |
7 (3.9) |
19 (16.8) |
– |
|
P1.4 |
6 (0.3) |
3 (0.2) |
– |
– |
5 (0.8) |
2 (0.1) |
– |
– |
16 (3.0) |
10 (0.6) |
– |
1 (0.9) |
– |
|
P1.5 |
25 (1.2) |
55 (4.1) |
2 (2.0) |
18 (43.9) |
7 (1.1) |
126 (7.0) |
9 (6.3) |
7 (14.3) |
7 (1.3) |
81 (5.1) |
4 (2.2) |
11 (9.7) |
– |
|
P1.5,2 |
4 (0.2) |
49 (3.7) |
17 (17.2) |
3 (7.3) |
2 (0.3) |
17 (1.0) |
37 (25.9) |
5 (10.2) |
4 (0.8) |
12 (0.8) |
104 (57.5) |
13 (11.5) |
– |
|
P1.7 |
19 (0.9) |
3 (0.2) |
– |
– |
9 (1.5) |
1 (0.05) |
– |
1 (2.0) |
7 (1.3) |
6 (0.4) |
– |
– |
– |
|
P1.7,1 |
224 (10.3) |
30 (2.2) |
– |
1 (2.4) |
28 (4.5) |
11 (0.6) |
– |
– |
7 (1.3) |
3 (0.2) |
– |
– |
– |
|
P1.7,16 |
68 (3.1) |
– |
– |
– |
9 (1.5) |
– |
– |
– |
5 (0.9) |
– |
– |
– |
– |
|
P1.9 |
57 (2.6) |
10 (0.7) |
– |
– |
12 (2.0) |
1 (0.05) |
– |
– |
12 (2.3) |
3 (0.2) |
– |
10 (8.8) |
– |
|
P1.10 |
1 (0.05) |
12 (0.9) |
– |
2 (4.9) |
– |
2 (0.1) |
– |
– |
– |
– |
– |
2 (1.8) |
– |
|
P1.14 |
24 (1.1) |
– |
– |
– |
5 (0.8) |
– |
– |
– |
35 (6.6) |
– |
– |
– |
– |
|
P1.14–6 |
10 (0.5) |
870 (64.7) |
– |
– |
11 (1.8) |
1,365 (76.1) |
– |
– |
21 (3.9) |
1,198 (75.3) |
– |
– |
– |
|
P1.16 |
15 (0.7) |
– |
1 (1.0) |
– |
6 (1.0) |
3 (0.2) |
7 (4.9) |
1 (2.0) |
9 (1.7) |
– |
– |
2 (1.8) |
– |
|
P1.19,15 |
1,468 (67.8) |
63 (4.7) |
5 (5.1) |
– |
397 (64.5) |
18 (1.0) |
– |
1 (2.0) |
224 (42.1) |
17 (1.1) |
– |
1 (0.9) |
5 (100) |
|
P1.22–1,14 |
35 (1.6) |
4 (0.3) |
– |
– |
15 (2.4) |
– |
– |
– |
9 (1.7) |
– |
– |
– |
– |
|
nt |
144 (6.6) |
220 (16.4) |
13 (13.1) |
15 (36.6) |
89 (14.4) |
225 (12.5) |
28 (19.6) |
24 (49.0) |
166 (31.2) |
257 (16.1) |
25 (13.8) |
54 (47.8) |
– |
|
Total |
2,165 |
1,344 |
99 |
41 |
616 |
1,794 |
143 |
49 |
532 |
1,591 |
181 |
113 |
5 |
Serogroup C isolates displayed 98 phenotypes, and the most prevalent were 23:P1.14–6 (3,241/4,729; 68.5 %) followed by 23:P1.nst (569/4,729; 12 %) and 2a:P1.5 plus 2a:P1.5,2 (201/4,729; 4.2 %). The clone C:23:P1.14–6 emerged in 2002, and remains prevalent today.
Serogroup W showed 34 phenotypes, of which 2a:P1.5 plus 2a:P1.5,2 was the most prevalent (213/423; 50.2 %), followed by 2b:P1.5 plus 2b:P1.5,2 (92/423; 21.7 %). Although the isolates were not typed molecularly, the prevalence of W:2a, a surrogate marker, suggests the prevalence of the hyper virulent W:2a ST-11 CC causing IMD in this period in Brazil [40]. As reported by other authors, the fatality rate for group W IMD has been high also in Brazil, ranging from 17 to 37 % between 2010 and 2017 [8, 22, 23, 40, 41].
Serogroup Y displayed 55 phenotypes, and the following three were the most prevalent: 22:P1.3 plus 22:P1.nst (33/203; 16.3 %), 4:P1.nst (23/203; 11.3 %) and 17,7:P1.5 plus 17,7:P1.nst (14/203; 6.9 %).
As presented in Tables 2 and 3, serogroup X showed a single phenotype, 4,7:P1.19,15 (5/5; 100 %).
Conclusion
The data presented herein show an important change in the IMD epidemiological scenario due to a significant shift from serogroup B to serogroup C observed from 2006 onwards, when serogroup C became the most frequent capsular type.
Performing a continuous laboratory-based surveillance highlighting the changes over time in serogroups and serotypes:serosubtypes distribution of invasive N. meningitidis contributes to the knowledge of the IMD epidemiology in Brazil, providing robust information to implement appropriate strategies to the IMD control.
Funding information
The authors received no specific grant from any funding agency.
Acknowledgements
We thank Maria Vaneide de Paiva Batista, Rosemeire Capoani Almendros and Ueslei José Dias for assistance in microbiological tests. We thank the Central Laboratories of the Brazilian states for providing the meningococcal isolates. The authors would also like to extend their particular thanks to the Fleury Group for providing the Neisseria meningitidis serogroup X strain in 2017.
Author contributions
M.C.G. and A.P.L – performed the experiments, study conception and design, acquisition of data and original draft preparation.
M.C.G., A.P.L., C.M., G.P., J.M.W.P., A.P.B – performed critical review and editing of the manuscript.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Footnotes
Abbreviations: CC, clonal complex; ET, electrophoretic type; IAL, Adolfo Lutz Institute; IMD, invasive meningococcal disease; mAbs, monoclonal antibodies; MCC vaccine, meningococcal C conjugate vaccine; MenA, MenB, MenC, MenE, MenW, MenX, MenY, and MenZ, meningococcal serogroups A, B, C, E, W, X, Y and Z, respectively; N. meningitidis, Neisseria meningitidis; nst, non serosubtypeable; NT, non typeable; SINAN, Brazilian National Notifiable Disease Surveillance System; SNV, National Surveillance System; ST, sequence type; WGS, whole genome sequencing.
References
- 1.Abio A, Neal KR, Beck CR. An epidemiological review of changes in meningococcal biology during the last 100 years. Pathog Glob Health. 2013;107:373–380. doi: 10.1179/2047773213Y.0000000119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trotter CL, Maiden MCJ. Meningococcal vaccines and herd immunity: lessons learned from serogroup C conjugate vaccination programs. Expert Rev Vaccines. 2009;8:851–861. doi: 10.1586/erv.09.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pelton SI. The global evolution of meningococcal epidemiology following the introduction of meningococcal vaccines. J Adolesc Health. 2016;59:S3–S11. doi: 10.1016/j.jadohealth.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 4.Presa JV, de Almeida RS, Spinardi JR, Cane A. Epidemiological burden of meningococcal disease in Brazil: a systematic literature review and database analysis. Int J Infect Dis. 2019;80:137–146. doi: 10.1016/j.ijid.2019.01.009. [DOI] [PubMed] [Google Scholar]
- 5.Safadi MAP, Berezin EN, Arlant LHF. Meningococcal disease: epidemiology and early effects of immunization programs. J Pediatric Infect Dis Soc. 2014:1–3. doi: 10.1093/jpids/piu027. [DOI] [PubMed] [Google Scholar]
- 6.Tzeng YL, Stephens DS. Epidemiology and pathogenesis of Neisseria meningitidis . Microbes Infect. 2000;2:687–700. doi: 10.1016/S1286-4579(00)00356-7. [DOI] [PubMed] [Google Scholar]
- 7.Lemos APS, Brandão AP, Gorla MCO, Paiva MV, Simonsen V, et al. Phenotypic characterization of Neisseria meningitidis strains isolated from invasive disease in Brazil from 1990 to 2001. J Med Microbiol. 2006;55:751–757. doi: 10.1099/jmm.0.46451-0. [DOI] [PubMed] [Google Scholar]
- 8.Ministry of Health Meningococcal disease in Brazil. 2019.
- 9.Popovic T, Ajello GW, Facklam RR. Laboratory Methods for the Diagnosis of Meningitis Caused by Neisseria Meningitidis, Streptococcus Pneumoniae, and Haemophilus Influenzae. Atlanta, GA: Centers for Disease Control, World Health Organization; 1998. [Google Scholar]
- 10.Alkmin MGA, Shimizu SH, Landgraf IM, Gaspari EN, Melles CEA. Production and immunochemical characterization of Neisseria meningitidis group B antiserum for the diagnosis of purulent meningitis. Braz J Med Biol Res. 1994;27:1627–1634. [PubMed] [Google Scholar]
- 11.Wedege E, H iby EA, Rosenqvist E, Fr holm LO. Serotyping and subtyping of Neisseria meningitidis isolates by co-agglutination, dot-blotting and ELISA. J Med Microbiol. 1990;31:195–201. doi: 10.1099/00222615-31-3-195. [DOI] [PubMed] [Google Scholar]
- 12.Sacchi CT, Pessoa LL, Ramos SR, Milagres LG, Camargo MC, et al. Ongoing group B Neisseria meningitidis epidemic in São Paulo, Brazil, due to increased prevalence of a single clone of the ET-5 complex. J Clin Microbiol. 1992b;30:1734–1738. doi: 10.1128/jcm.30.7.1734-1738.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Lemos APS, Yara TY, Gorla MCO, de Paiva MV, de Souza AL, et al. Clonal distribution of invasive Neisseria meningitidis serogroup C strains circulating from 1976 to 2005 in greater Sao Paulo, Brazil. J Clin Microbiol. 2007;45:1266–1273. doi: 10.1128/JCM.02510-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skoczynska A, Wasko I, Kuch A, Kadłubowski M, Gołebiewska A, et al. And participants of a laboratory-based surveillance of community acquired invasive bacterial infections (BINet). A decade of invasive meningococcal disease surveillance in Poland. Plos One. 2013;8:1–11.:e71943. doi: 10.1371/journal.pone.0071943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jandova Z, Musilek M, Vackova Z, Kozakova J, Krizova P. Serogroup and Clonal Characterization of Czech Invasive Neisseria meningitidis Strains Isolated from 1971 to 2015. PLoS One. 2016;11:1–13.:e0167762. doi: 10.1371/journal.pone.0167762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boletim Epidemiológico Paulista Campanha De Vacinas Contra Doença Meningocócica Sorogrupo C-Itapeva/sp/brasil. Luiz Jacintho da Silva: 2004. Ano I – Número 11. ISSN 1806-4272, Novembro. [Google Scholar]
- 17.Informe Técnico dA Secretaria municipal de Saúde de São Paulo- Secretaria de estado de Saúde de São Paulo. Investigação de surto comunitário de Doença Meningocócica no município de São Paulo, julho 2007. Rev Saúde Públ. 2007;41:873–878. doi: 10.1590/s0034-89102007000500026. [DOI] [PubMed] [Google Scholar]
- 18.Iser BPM, Lima H, Moraes C, Almeida RPA, Watanabe LT, et al. Outbreak of Neisseria meningitidis C in workers at a large food-processing plant in Brazil: challenges of controlling disease spread to the larger community. Epidemiol Infect. 2011:1–10. doi: 10.1017/S0950268811001610. [DOI] [PubMed] [Google Scholar]
- 19.Liphaus BL, Cappeletti-Gonçalves-Okai MI, Silva-Delemos AP, Gorla MC, Rodriguez-Fernandes M, et al. Outbreak of Neisseria meningitidis C in a Brazilian oil refinery involving an adjacent community. Enferm Infecc Microbiol Clin. 2013;31:88–92. doi: 10.1016/j.eimc.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 20.Gorla MCO, de Lemos APS, Quaresma M, Vilasboas R, Marques O, et al. Phenotypic and molecular characterization of serogroup C Neisseria meningitidis associated with an outbreak in Bahia, Brazil. Enferm Infecc Microbiol Clin. 2012;30:56–59. doi: 10.1016/j.eimc.2011.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andrade AL, Minamisava R, Tomich LM, Lemos AP, Gorla MC, et al. Impact of meningococcal C conjugate vaccination four years after introduction of routine childhood immunization in Brazil. Vaccine. 2017;35:2025–2033. doi: 10.1016/j.vaccine.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 22.Gottberg A, du Plessis M, Cohen C, Prentice E, Schrag S, et al. For the group for enteric, respiratory and meningeal disease surveillance in South Africa. emergence of endemic serogroup W135 meningococcal disease associated with a high mortality rate in South Africa. Clin Infect Dis. 2008;46:377–386. doi: 10.1086/525260. [DOI] [PubMed] [Google Scholar]
- 23.Moreno G, Lopez D, Vergara N, Advis MF, Loayza S. Clinical characterization of cases with meningococcal disease by W135 group in Chile, 2012. Rev Chil Infectol. 2013;30:350–360. doi: 10.4067/S0716-10182013000400002. [DOI] [PubMed] [Google Scholar]
- 24.Weidlich L, Baethgen LF, Mayer LW, Moraes C, Klein CC, et al. High prevalence of Neisseria meningitidis hypervirulent lineages and emergence of W135:P1.5,2:ST-11 clone in Southern Brazil. J Infect. 2008;57:324–331. doi: 10.1016/j.jinf.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 25.Lucidarme J, Hill DMC, Bratcher HB, Gray SJ, du Plessis M, et al. Genomic resolution of an aggressive, widespread, diverse and expanding meningococcal serogroup B, C and W lineage. J Infect. 2015;71:544–552. doi: 10.1016/j.jinf.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lucidarme J, Scott KJ, Ure R, Smith A, Lindsay D, et al. An international invasive meningococcal disease outbreak due to a novel and rapidly expanding serogroup W strain, Scotland and Sweden, July to August 2015. Euro Surveill. 2016;21:30395. doi: 10.2807/1560-7917.ES.2016.21.45.30395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dolan‐Livengood JM, Miller YK, Martin LE, Urwin R, Stephens DS. Genetic Basis for Nongroupable Neisseria meningitidis . J Infect Dis. 2003;187:1616–1628. doi: 10.1086/374740. [DOI] [PubMed] [Google Scholar]
- 28.Leimkugel J, Hodgson A, Forgor AA, Pfluger V, Dangy JP, et al. Clonal Waves of Neisseria Colonization and Disease in African Meningitis Belt:Eight-Year Longitudinal Study in Northern Ghana. Plos Med. 2007;4:535–544. doi: 10.1371/journal.pmed.0040101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baccarini C, Ternouth A, Wieffer H, Vyse A. The changing epidemiology of meningococcal disease in North America 1945–2010. Hum Vaccin Immunother. 2013;9:162–171. doi: 10.4161/hv.22302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bröker M, Bukovski S, Culic D, Jacobsson S, Koliou M, et al. Meningococcal serogroup Y emergence in Europe. Hum Vaccin Immunother. 2014;10:1725–1728. doi: 10.4161/hv.28206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bröker M, Emonet S, Fazio C, Jacobsson S, Koliou M, et al. Meningococcal serogroup Y disease in Europe: continuation of high importance in some European regions in 2013. Hum Vaccin Immunother. 2015;11:2281–2286. doi: 10.1080/21645515.2015.1051276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Agudelo CI, Sanabria OM, Ovalle MV. Serogroup Y meningococcal disease, Colombia. Emerg Infect Dis. 2008;14:990–991. doi: 10.3201/eid1406.071357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abad R, Agudelo CI, Brandileone MC, Chanto G, Gabastou JM, et al. Molecular characterization of invasive serogroup Y Neisseria meningitidis strains isolated in the Latin America region. J Infect. 2009;1:e11. doi: 10.1016/j.jinf.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 34.Pavon ABI, Lemos AP, Gorla MC. Regueira M, and SIREVA II Working Group", Gabastou JM. Laboratory-Based Surveillance of Neisseria meningitidis Isolates from Disease Cases in Latin American and Caribbean Countries, SIREVA II 2006–2010. PLoS One. 2012;7:e44102. doi: 10.1371/journal.pone.0044102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boisier P, Nicolas P, Djibo S, Taha M-K, Jeanne I, et al. Meningococcal meningitis: unprecedented incidence of serogroup X-related cases in 2006 in niger. Clin Infect Dis. 2007;44:657–663. doi: 10.1086/511646. [DOI] [PubMed] [Google Scholar]
- 36.Xie O, Pollard AJ, Mueller JE, Norheim G. Emergence of serogroup X meningococcal disease in Africa: need for a vaccine. Vaccine. 2013;31:2852–2861. doi: 10.1016/j.vaccine.2013.04.036. [DOI] [PubMed] [Google Scholar]
- 37.Agnememel A, Hong E, Giorgini D, Nuñez-Samudio V, Deghmane A-E, et al. Neisseria meningitidis Serogroup X in Sub-Saharan Africa. Emerg Infect Dis. 2016;22:698–702. doi: 10.3201/eid2204.150653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fazio C, Starnino S, Solda MD, Sofia T, Neri A, et al. Neisseria meningitidis serogroup X sequence type 2888, Italy. Emerg Infect Dis. 2010;16:359–360. doi: 10.3201/eid1602.091553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gorla MC, Pinhata JMW, Dias UJ, Moraes C, Lemos AP. Surveillance of antimicrobial resistance in Neisseria meningitidis strains isolated from invasive cases in Brazil from 2009 to 2016. J Med Microbiol. 2018:1–7. doi: 10.1099/jmm.0.000743. [DOI] [PubMed] [Google Scholar]
- 40.Campbell H, Parikh SR, Borrow R, Kaczmarski E, Ramsay ME, et al. Presentation with gastrointestinal symptoms and high case fatality associated with group W meningococcal disease (MenW) in teenagers, England, July 2015 to January 2016. Euro Surveill. 2016;21:pii=30175. doi: 10.2807/1560-7917.ES.2016.21.12.30175. [DOI] [PubMed] [Google Scholar]
- 41.Honskus M, Okonji Z, Musilek M, Kozakova J, Krizova P. Whole genome sequencing of Neisseria meningitidis W isolates from the Czech Republic recovered in 1984–2017. PLoS One. 2018;13:e0199652. doi: 10.1371/journal.pone.0199652. [DOI] [PMC free article] [PubMed] [Google Scholar]