Abstract
Hand, foot, and mouth disease (HFMD) recently emerged as a global public threat. The licensure of inactivated enterovirus A71 (EV-A71) vaccine was the first step in using a vaccine to control HFMD. New challenges arise from changes in the pathogen spectrum while vaccines directed against other common serotypes are in the preclinical stage. The mission of a broad-spectrum prevention strategy clearly favors multivalent vaccines. The development of multivalent vaccines was attempted via the simple combination of potent monovalent vaccines or the construction of chimeric vaccines comprised of epitopes derived from different virus serotypes. The present review summarizes recent advances in HFMD vaccine development and discusses the next steps toward a safe and effective HFMD vaccine that is capable of establishing a cross-protective antibody response.
Keywords: Hand, foot, and mouth disease (HFMD); Inactivated whole virus vaccine; Virus-like particles; Multivalent vaccines; Chimeric vaccines
Introduction
Human hand-foot-and-mouth disease (HFMD) caused several large outbreaks across the Asian-Pacific region, and it represents a global public health issue. Several viruses were identified as the primary HFMD-related pathogens, and this list includes enterovirus A71 (EV-A71), coxsackievirus A16 (CV-A16), CV-A6 and CV-A10, which all belong to the genus Enterovirus within the Picornaviridae family (Fang and Liu 2018). HFMD frequently occurs in children under five years old, and it is generally characterized by vesicular exanthema with self-limitation. There appears to be a link between the range of clinical manifestations and serotype differences, with some EV-A71 infections resulting in severe complications, including brainstem encephalitis, aseptic meningitis, acute flaccid paralysis, cardiopulmonary failure, or death, but other serotypes generally showing mild symptoms (Lin et al. 2019). Historically, EV-A71 and CV-A16 primarily accounted for the global HFMD outbreaks; however, other serotypes are gradually gaining dominance due to the broad inoculation of and protection by inactivated EV-A71 vaccines. Indeed, CV-A6 displaced EV-A71 and CV-A16 as the predominant serotype in 2013 in Shanghai, and CV-A10 has gradually become the dominating HFMD-related enterovirus (Song et al. 2017; Wang J et al. 2018; Bian et al. 2019).
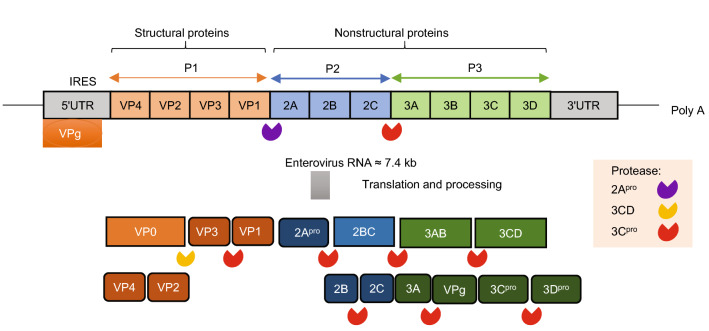
Enteroviruses are positive-stranded RNA viruses with a genome size of approximately 7.4 kb, which encodes a single polyprotein of ~ 2100 amino acids. The polyprotein is divided into three subregions, namely, P1, P2, and P3. The P1 region encodes four structural proteins (VP4–VP2–VP3–VP1), and the P2 and P3 regions encode seven nonstructural proteins (P2–2A, 2B, 2C; P3–3A, 3B, 3C, 3D) (Fig. 1). The four structural proteins assemble to form the basic building block of the virion capsid, namely, a protomer. Five protomers come together to form a pentamer, and 12 pentamers plus the viral genome form an icosahedral virion of ~ 30 nm diameter (Yi et al. 2017).
Fig. 1.
The structure of enterovirus 71 genome and virion organization. The RNA genome of EV-A71 is approximately 7.4 kb, with an untranslated region (UTR) at the 5′ and 3′ ends of the genome. The 5′UTR contains an internal ribosomal entry site (IRES) for cap-independent translation. The 5′UTR is bound covalently to VPg (3B), and the 3′UTR includes a poly-A tail. The RNA is translated to a polyprotein that is sequentially cleaved by the viral 2A protease (2Apro), 3CD protease, and 3C protease (3Cpro).
Host Immune Responses to Natural Infection of HFMD-Related Viruses
Humoral immune responses against HFMD-related viruses produce virus-specific neutralizing antibodies, which are generally sufficient to curb virus spreading and makes HFMD a self-limiting disease. However, there were reported cases in infants and young children where severe complications developed despite normal or nearly normal antibody titers compared to patients with mild HFMD, which indicates that other factors contribute to the disease severity (Lim and Poh 2019). The following factors may influence severity: (1) Variation in the IgG composition. The different IgG subclasses that elicited by the viral infection behave differently in virus control. For instance, the IgG1 subclass, and to a lesser extent the IgG2 subclass, primarily mediate the virus-neutralizing activity, while the IgG3 subclass does not (Cao et al. 2013). (2) Variation in cellular immunity. The circulating virus-specific CD8+ T cells and CD4+ T cells must be effectively engaged for the timely clearance of virus-infected cells and helping the antibody production (Aw-Yong et al. 2019). (3) Genetic variations. There may be intrinsic differences between individuals in countering a viral invasion due to inherited variations in host factors that determine viral susceptibility at the cellular and organismal levels (Yee and Poh 2018). Among the three abovementioned factors, the cellular immune response is the most feasible target for vaccine-based prevention.
Critical Epitopes Recognized by Neutralizing Antibodies
As components of the virion capsid, VP1, VP2 and VP3 are the main targets of human neutralizing antibodies. VP1 contributes to the majority of neutralizing epitopes, and its binding may be used as a valuable assay to assess vaccine potency. VP2 and VP3 proteins harbor fewer neutralizing epitopes compared to VP1, despite structural similarity. Among the common HFMD-related enteroviruses, EV-A71 and CV-A16 show high conservation in capsid proteins, with approximately 80% sequence identity, and their neutralizing epitopes are largely overlapped (Anasir and Poh 2019).
The neutralizing epitopes are classified into linear epitopes and conformational epitopes. Most of the linear epitopes are located in the B–C, E–F, and G–H loops, and the C-terminus of VP1, the E–F loops of VP2, and N-terminal regions of VP3. CV-A16 also has linear epitopes in the canyon floor of VP1 and the G-H loop of VP3 (Xu et al. 2015; Fang and Liu 2018). Linear neutralizing antigenic sites were also reported in the E–F loop of CV-A10 VP2, in the G-H loop as well as the C-terminus of CV-A6 VP1 (Chen et al. 2018; Dai et al. 2019).The identification of conserved neutralizing linear epitopes provides important targets for the development of multivalent vaccines (Xu et al. 2015). For example, MAB979 is an antibody raised by EV-A71 immunization, and it recognized residues 136–150 of VP2 on extensive synthetic peptide screening. It is cross-reactive with CV-A16, but with low neutralizing titers, which indicates a potential epitope for multivalent targeting (Liu C et al. 2011).
The conformational epitopes, which are comprised of amino acids that are discontinuous in sequence but are brought to proximity with three-dimensional protein folding, are more difficult to define compared to linear epitopes, and its ultimate validation may require structural analyses. A number of conformational neutralizing epitopes were identified, and most of these sites were derived from an EV-A71-related study (Fang and Liu 2018). A remarkable example is provided by mAb E18/19, which are two antibodies generated by immunization with an immature EV-A71 virus. Both antibodies neutralize EV-A71, but via different mechanisms. E18 binds to the virus and causes a conformational change of the bound virus that promotes the release of the viral genome and renders the virus inactivated E19 does not induce genome injection. Structural analyses subsequently revealed that E18 and E19 recognize conformation epitopes on the EV-A71 capsid, but their targets are different. The binding sites of E18 are located between the VP4–VP2–VP3–VP1 protomers, and the E19 sites are exclusively within a single promoter (Plevka et al. 2014). Additional conformational epitopes were identified in other structural features of the capsids of EV-A71, including the five-fold axis, “knob” and G-H loop, canyon northern rim, canyon floor, canyon southern rim, three-fold plateau, and two-fold plateau (Lee et al. 2013; Kiener et al. 2014; Jiang et al. 2015; Arthur Huang et al. 2017; Jia et al. 2017). Studies on CV-A6 indicated that there are also conformational epitopes located in the B–C, E–F, H–I loops of VP1 (Chen et al. 2018; Fang and Liu 2018).
Monovalent Candidates of HFMD Vaccines
Inactivated Whole Virus Vaccine
In comparison to other vaccine candidates, inactivated EV-A71 vaccines are the only vaccines entering the market. China’s Food and Drug Administration (FDA) has issued drug certificates and production licenses for EV-A71 inactivated vaccines from 3 companies, namely, Sinovac, Vigoo, and the Chinese Academy of Medical Sciences (CAMS), all of which are based on C4 subgenotype—the most common genotype in China, although with different virus strain and variation in manufacturing process (Mao et al. 2016) (Table 1). Following successful phase I–III clinical trials, a recent large-scale cohort phase IV study of licensed inactivated EV-A71 vaccine revealed an overall protection effectiveness of 89.7% against EV-A71 infection along with a 4.58% rate of reported adverse reaction (Guan et al. 2019). However, both the Sinovac and CAMS EV-A71 vaccines were ineffective for CVA16-associated HFMD, unraveling their genotype specificity (Li et al. 2016; Li R et al. 2014). Besides Chinese mainland, inactivated EV-A71 vaccines were also developed in Taiwan region and Singapore, targeting B3 and B4 subgenotypes respectively. The EV-A71 developed in the Taiwan region has been evaluated in a phase II clinical trials involving a total of 365 infants or children aging from 2 months to 11 years, achieving a seroprotection (neutralization titer ≥ 1:32) lasting for 2 years in most participants without reported serious adverse events (SAEs) (ClinicalTrials.gov number, NCT02200237). In addition, a cross-reaction was observed against other EV-A71 strain genotypes, including B5, C4a, C4b, and C5 (Huang et al. 2019). A phase III clinical trial has initiated in 2019 and is expected to be completed in 2022 (ClinicalTrials.gov number, NCT03865238) (Lin et al. 2019). Only a small-scale phase I clinical trial has been conducted for EV-A71 vaccine developed in Singapore, and the study claimed that the vaccine induced a high immune response against HFMD caused by EV-A71, although the data has not been publicly disclosed (ClinicalTrials.gov number, NCT01376479). Immunizations with inactivated virions derived from CV-A16, CV-A10 and CV-A6 have been only studied in animal models, and the results provided immunological and functional evidence supporting their efficacy. The generated serum contained high levels of virus-specific neutralizing antibodies, and the serum from immunized mother mice afforded protection against lethal challenges with virulent HFMD-related viruses when it was passively transferred to neonatal mice (Qi An et al. 2014; Zhang et al. 2017a). Therefore, inactivated whole virus vaccine represents the most attainable monovalent HFMD-related vaccine.
Table 1.
Official licensed inactivated EV-A71 vaccines by the Chinese Food and Drug Administration.
| Organizations | Sinovac Biotech Co., Ltd | Beijing Vigoo Biological Co., Ltd | Chinese Academy of Medical Sciences |
|---|---|---|---|
| EV-A71 Strain | H07 (C4) | FY (C4) | M01 (C4) |
| Inactivation technique | Formalin | Formalin | Formalin |
| Cell substrate | Vero cells | Vero cells | Human diploid KMB-17 cell |
| Dosages | 400 U, two-dose | 320 U, two-dose | 100 U, two-dose |
| Adjuvant | Aluminum hydroxide | Aluminum hydroxide | Aluminum hydroxide |
| Population target | Children (6–35 month) | Children (6–35 month) | Children (6–71 month) |
| Enrollment | 10,077 | 10,245 | 12,000 |
| References | NCT01507857 | NCT01508247 | NCT01569581 |
Synthetic Peptide and Protein Vaccines
Synthetic peptide vaccines are usually related to the selected neutralizing epitopes, and the two peptides located in VP1, called SP55 (E–F loop; aa 163–177) and SP70 (G-H loop; aa 208–222) have been shown to elicit EV-A71 specific neutralizing antibodies. SP70 raised a relatively higher titer of neutralizing antibody against EV-A71 than that of SP55. However, the neutralizing antibody titer elicited from the peptide SP70 was just one-fourth of that observed in mice immunized with heat-inactivated EV-A71 (Foo et al. 2007). Thus, given that EV-A71 VP1 peptide or whole protein was only able to raise a neutralizing antibody response generally inferior to that of inactivated EV-A71 vaccines and consequently showed a protective effect in animal models limited to a low-dose virus challenge (Premanand et al. 2012), synthetic peptide and protein vaccines have only been tried in the research stage without progression to more commercial development.
Recombinant Subunit Vaccines
Virus-like particles (VLPs) are a special form of recombinant subunit vaccines for non-enveloped viruses, and can be generated by a number of biosystems. The principle of EV VLPs is to coexpress the genes encoding capsid protein precursor P1 and protease 3CD, which results in the cleavage of P1 into three capsid subunit proteins VP0, VP1, and VP3 through the action of the 3CD protease. VP0, VP1 and VP3 are subsequently self-assembled into VLPs, which adopt the natural structure of virus capsid and can serve as potential vaccine candidates after purification. VLPs derived from EV-A71, CV-A16, CV-A6 and CV-A10 species were reported to be successfully produced in baculovirus-insect cell (Somasundaram et al. 2016), Pichia pastoris yeast (Zhang et al. 2016) and saccharomyces cerevisiae yeast (Zhao et al. 2013; Zhou et al. 2016; Zhang W et al. 2018). Immunization study in mice showed that VLPs were able to elicit high titers of neutralizing antibodies and afford effective protection against lethal viral challenge (Wang X et al. 2018; Zhou et al. 2018). In addition, a recent study reported that VLP vaccines for HFMD induced a high antigen-specific B cell response that is comparable to inactivated vaccines (Yang et al. 2019) (Table 2). This study produced EV71-VLPs in Pichia pastoris, attaining a high expression level of EV71-VLPs greater than 250 mg/L (Yang et al. 2019). With the higher yield capacity to be more cost effective, EV71-VLPs produced in Pichia pastoris are in clinical trial (CXSL1900022), representing a good start toward future commercialization.
Table 2.
The producing systems of enterovirus-related virus-like particle (VLP).
| VLP-producing systems | Yield capacity | Properties | Status | Ref. |
|---|---|---|---|---|
| Baculovirus-insect cell | Moderate (64.3 mg/L) | Moderate-yield; Relatively high cost; Large stocks (cell & viruses); Contamination risk of virus | Lab | (Chung et al. 2010) |
| Saccharomyces cerevisiae yeast | Low (0.25 mg/L) | Low-yield; Low cost; Ease in manipulation | Lab | (Li et al. 2013) |
| Pichia pastoris yeast | High (270 mg/L) | High-yield; Low cost; Easy manipulation | Clinical trial (CXSL1900022) | (Yang et al. 2019) |
| Recombinant vesicular stomatitis virus (rVSV) | – |
Attenuated(ΔM51); Replication-competent and may have adverse effects |
Lab | (Yan et al. 2016) |
| Recombinant adenovirus 5 (Ad-EVVLP) | – |
Replication-incompetent(ΔE1/ΔE3); 3C-specific cellular immunity Ad-EVVLPs from EV71 genes can protect against CVA16 infection |
Lab | (Tsou et al. 2015) |
Recombinant Virus-Vector Vaccines
Researchers inserted P1 and 3CD genes of EV-A71 into one vesicular stomatitis virus (VSV) backbone to generate a recombinant VSV to produce VLPs, which protected neonatal mice against lethal viral challenge (Yan et al. 2016). A novel recombinant adenovirus vaccine, Ad-EVVLP, with P1 and 3CD genes of EV-A71 inserted into the adenoviral genome to express VLPs, induced EV-A71-specific neutralizing antibodies and Th1/Th2-balanced cellular responses in immunized mice, whereas inactivated EV-A71 vaccine activated only Th2-mediated neutralizing antibody responses to protect against virus challenge (Tsou et al. 2015) (Table 3). The immunogenicity of 71-6 epitope (aa 176–190 of VP3) was tested using the norovirus P particle as the vaccine carrier, and serum from mice immunized with the resulting chimeric P particle could protect suckling mice from a lethal dose of EV-A71 infection (Jiang et al. 2015).
Table 3.
The characteristics of the primary experimental enterovirus vaccine formats.
| Vaccine format | Conformation | Immunogenicity | mAb responses | Limitation | Advantages |
|---|---|---|---|---|---|
| Inactivated whole virus | Natural virion with genome | Strong (+++) | High; Cross-genotype protection | Low cross-serotypic protection | Mature technology |
| VLP | Natural virion without genome | Moderate (++) | High; Cross-genotype protection | Low cross-serotypic protection | Safe; Low cost; Explicit composition; Easy large-scale production and quality control |
| Synthetic peptide or recombinant subunit | Linear epitope or antigen | Relatively weak (+) | Low; Cross-genotype protection | Low cross-serotypic protection; Strong adjuvant requirement | Safe; Inexpensive; Explicit composition; Easy large-scale production and quality control |
| Novel chimeric vaccines | Natural virion without genome or linear epitopes of antigens | Relatively high (++/+++) | High; Cross-genotype protection | Required to know key neutralization domain and need to design the optimal chimeric strategy | May induce cross-protection of serotypes |
| Recombinant virus-vector vaccines | Natural virion without genome of target viruses but vectors | Relatively high (++/+++) | High; Cross genotype protection | Risk of vector replication | May induce cross-protection of serotypes; Comprehensive T-cell immune response |
Recent Development of Multivalent Vaccines
Patients with recurrent HFMD are a clear indicator of lack of efficient cross-reactivity among serotypes, informing the need for development of multivalent vaccine. Accordingly, several approaches have been attempted to develop vaccine covering multiple serotypes. The most straightforward approach is simply combining the existing monovalent vaccines into one formulation. There were studies showing that immunization of combined multiserotypic formulations, in the form of either activated virus or VLP, led to effective protection against corresponding viruses without interference, suggesting no cross-serotypic effect. Though simple “mixing” approach does show promise in providing a solution to the issue of multi-protection, it faces the problems of relatively high cost and reliability issue. Consequently, an alternative approach has been also explored utilizing chimeric vaccines generated by engineering vector to co-express viral proteins or peptides from multiple serotypes, generally achieved via partial antigenic substitution and insertion.
Inactivated Multivalent Vaccines
Bivalent vaccine approach was first tested on the two major causative agents, EV-A71 and CV-A16. A vaccine formulated by combining inactivated EV-A71 and CV-A16 viruses induced a balanced protective immunity in mice model against EV-A71 and CV-A16 infection without detectable immune interference (Cai et al. 2014). Furthermore, in rhesus macaques model, intradermal immunization of two doses of bivalent EV-A71/CV-A16 inactivated vaccine showed excellent virus containment and protection without immunopathological effect against a subsequent viral challenged with EV-A71 or CV-A16 (Fan et al. 2020). CV-A6 and CV-A10 of the inactivated whole-virus combination vaccines also induce antigen-specific systemic immune responses, which elicit active immunization to achieve a protection rate of > 80% in controlling homotypic and heterotypic CV-A6 and CV-A10 infections (Zhang Z et al. 2018). A trivalent vaccine candidate containing inactivated EV-A71, CV-A16, and CV-A6 delivered full protection from lethal challenge against EV-A71 and CV-A16, and protection from CV-A6 challenge was accomplished in a passive transfer study involving serum raised against the trivalent vaccine (Caine et al. 2015). Another inactivated-CV-A6, CV-A10, and CV-A16 trivalent vaccine induced sufficient neutralizing antibodies and cell-mediated immune responses, and there was no sufficient cross-protectivity against heterologous strains (Lim et al. 2018). Collectively, these results indicate that there is no immunological interference between the antigens in their ability to induce virus-specific immune responses, which provides proof-of-concept for multivalent vaccines for broad protection against HFMD.
Multivalent VLPs Vaccines
A bivalent EV-A71/CV-A16-VLPs vaccine induced a balanced neutralizing antibody response and passively protected mice against EV-A71 and CV-A16 infections (Ku et al. 2014). A tetravalent vaccine, including CV-A10-VLP, EV-A71-VLP, CV-A16-VLP, and CV-A6-VLP, elicited antigen-specific and long-lasting serum antibody responses and neutralization titers against EV-A71, CV-A16, CV-A10, and CV-A6 strains similar to the monovalent vaccines, which indicates good compatibility among the four antigens in the combination vaccine (Zhang W et al. 2018).
Novel Chimeric Vaccines
A chimeric EV-A71 virus, in which the VP1 (aa 210–225) epitope was replaced with the epitope of CV-A16, was constructed using a reverse genetics technique to produce an EV-A71/CV-A16 bivalent vaccine candidate (Yang et al. 2016). The other attempt was to replace the EV-A71-neutralizing epitope SP70 with the epitope of CV-A16 to form chimeric EV-A71 virus-like particles (ChiEV-A71 VLPs), and immunization with ChiEV-A71 VLPs in mice elicited robust Th1/Th2-dependent immune responses against EV-A71 and CV-A16. Passive immunization with sera raised against ChiEV-A71 VLPs conferred full protection against lethal challenge with EV-A71 and CV-A16 in neonatal mice (Zhao et al. 2015). Structural studies revealed that SP70 epitope replacement converted the surface charge potential of VLP, coupled with variations in amino acid sequences, which most likely accounted for the additional neutralization capability of the ChiEV-A71 VLP. A newly published patent showed that EV-A71 VLP displaying CV-A16 VP1 polypeptides maintained the important neutralizing antibody epitopes of EV-A71 itself, and a CV-A16 VLP displaying EV-A71 VP1 polypeptides elicited a protective neutralizing antibody response directed against EV-A71 and CV-A16 viruses (PCT/MY2017/050059-US2019/0224304 A1). Generally, the results above indicate that the substitution and incorporation of key peptides/proteins between serotypes into one construction will be a reasonable method to construct a multivalent HFMD vaccine.
Recombinant Virus-Vector Vaccines
Bivalent chimeric VLPs presenting SP70 of VP1 and VP2 E–F loop epitopes (aa 141–155) of EV-A71 used the hepatitis B virus core protein (HBc) as a carrier (HBc-E1/2) and induced higher IgG and neutralization titers against EV-A71 and CV-A16 than immunization with only one epitope incorporated into HBc. More importantly, passive immunization with recombinant HBc-E2 particles protected neonatal mice from lethal EV-A71 and CV-A16 infections, and therefore, the VP2 epitope is immunodominant between the two serotypes (Xu et al. 2015). Another bivalent chimeric VLP using the core carrier of a truncated hepatitis B virus (tHBc) displayed conserved epitopes of EV-A71 in SP90 (aa 208–222) of VP1, VP2 (aa 248–263) and CV-A16 in PEP91 (aa 271–285) of VP1, which induced humoral and cellular immune responses and protected neonatal mice born to dams from lethal EV-A71 and partially from CV-A16 infection (Huo et al. 2017). Researchers described a hexon-modified chimpanzee adenovirus serotype 68 (AdC68) bivalent vaccine that incorporated the neutralizing epitope of CV-A16, PEP71, and a shortened neutralizing epitope of EV-A71, sSP70, into the AdC68 hexon, and EV-A71-VP1 was cloned into the E1-region of the AdC68 vectors. The candidate elicited neutralizing antibodies against CV-A16 and EV-A71 and conferred protection to suckling mice against a lethal challenge of both viruses, which indicates a potential carrier and epitope-displaying platform (Zhang et al. 2015). Accordingly, the integration of chimeric VLPs into novel virus vectors induced the effect of multivalence, characterized by an enhanced broad systemic immune response.
Experimental Animal Model and Regimen
Murine and non-primate models are the two major animal models for evaluating HFMD-related vaccines. In mouse model, adult BALB/c or ICR mice of 6–8 weeks were routinely selected for immunogenicity assessment. Prime-boost represents the most common vaccination strategy with vaccine(s) applied two or three times with an interval of 2–4 weeks via intraperitoneal (i.p.) or intramuscular (i.m.) route (Wang and Yu 2014). Given the HFMD-related viruses only cause minor phenotypes in adult mice, the protective efficacy of experimental vaccines has to be indirectly examined using neonatal mice, which are more susceptible to virus infection than adult mice possibly due to their immature immune systems (Yu et al. 2000; Fang and Liu 2018). One-day-old ICR mice infected with EV-A71 at a lethal dose of greater than 108 PFU can reach a mortality rate of 100% following i.p. inoculation (Yu et al. 2000). Neonatal mice infected with the highest dose of CV-A16 at 106.5 CCID50 had a 100% mortality by day 6, and a 100% mortality by day 13 with the lowest dose at 102.5 CCID50 (Li J et al. 2014). CV-A6 and CV-A10 infection murine model have been developed using 5-day-old neonatal mice with 105.5 TCID50 viruses via i.m. inoculation (Zhang et al. 2017a, b). Moreover, researchers have generated a transgenic (Tg) mouse expressing hSCARB2, the cellular receptor of EV-A71, which can infect EV-A71 within 1- to 14-day-old at a dose of 3 × 104 to 106 PFU via subcutaneous (s.c.) injection and exhibited neurological disease and pathology very similar to that observed in humans (Yang et al. 2009; Fujii et al. 2013). The vaccine protectiveness is measured by transfusing sera from immunized adult mice to young mice and examining their effect on subsequent viral challenge. An alternative version of this two-step protocol was recently developed by first combining immunized serum with virus in vitro and then applying the neutralization mixture to suckling mice, whereby the time required for the process is greatly shortened (Wang et al. 2016). Gerbil has recently emerged as a new model animal for studying HFMD-related virus as research showed that gerbils up to 21-day-old were fully susceptible to CV-A16 of 105.5 TCID50 and this susceptibility, marked by eventual death from neurological disorders, could be achieved on 60-day-old gerbils once the infection dose increased to 108 TCID50. Moreover, gerbils up to the age of 14-day-old were also susceptible to CV-A10 of 108.5 TCID50, with all animals succumbed five days after infection (Sun et al. 2016; Yao et al. 2019; Chen et al. 2020).
The research exploring the non-human primate model of HFMD-related viruses is limited, but the results are promising. In one report, the neonatal rhesus monkeys were challenged with EV-A71 (104.5 CCID50/monkey) via intratracheal infection, and HFMD-liked vesicular lesions were found in the mouth and foot, demonstrating the suitability of neonatal non-human primate for dissecting the complete process of EV-A71 infection (Liu L et al. 2011). In another report, upon CV-A16 infection via nasal insufflation, rhesus macaques developed oral mucosa and limb vesicles, a major classical clinical manifestation of HFMD infection. Strikingly, the infected macaques did not elicit CV-A16-specific neutralizing antibodies and functional memory T-cells. Furthermore, transfusion of sera from macaques immunized with inactivated CV-A16 vaccine failed to mount protection against a viral challenge in young macaque recipient. These surprising revelations suggest that the immunological mechanism of CV-A16 infection need to be further investigated (Wang et al. 2017).
Conclusions
The inactivated EV-A71 vaccines show high efficacy, good immunogenicity persistence and acceptable safety profiles in the vaccination population and efficiently reduce the incidence of HFMD, especially severe cases. However, concerns have risen on changes in dominant HFMD-causing virus strains and emerging new disease-causing serotypes. Therefore, it is imperative to explore multivalent vaccine formulation with broad-spectrum protection and sufficient safety. This exploration would be facilitated by a combinatorial effort involving improved vaccine design and strategy, better utilization of old vaccine vector along with development of new vaccine platforms. Lastly, it will be also helpful to gain a better understanding of how immunological memories develop upon infection with different serotypes, which could serve as an instructive guide for vaccine development.
Acknowledgements
This work was sponsored by the National Natural Science Foundation of China (81672018), the National 13th Five-Year Grand Program on Key Infectious Disease Control (2017ZX10202102), the 13th Five-Year National Science and Technology Major Project for infectious Diseases (2017ZX10305501-002), Shanghai Pujiang Program (19PJ1409100), the Technology Service Platform for Detecting High level Biological Safety Pathogenic Microorganism Supported by Shanghai Science and Technology Commission (18DZ2293000).
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Animal and Human Rights Statement
This article does not contain any studies with human or animal subjects performed by any of the authors.
Contributor Information
Xiaoyan Zhang, Email: zhangxiaoyan@shphc.org.cn.
Jianqing Xu, Email: xujianqing@shphc.org.cn.
References
- Anasir MI, Poh CL. Advances in antigenic peptide-based vaccine and neutralizing antibodies against viruses causing hand, foot, and mouth disease. Int J Mol Sci. 2019;20:1256. doi: 10.3390/ijms20061256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur Huang KY, Chen MF, Huang YC, Shih SR, Chiu CH, Lin JJ, Wang JR, Tsao KC, Lin TY. Epitope-associated and specificity-focused features of EV71-neutralizing antibody repertoires from plasmablasts of infected children. Nat Commun. 2017;8:762. doi: 10.1038/s41467-017-00736-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aw-Yong KL, NikNadia NMN, Tan CW, Sam IC, Chan YF. Immune responses against enterovirus A71 infection: implications for vaccine success. Rev Med Virol. 2019;29:e2073. doi: 10.1002/rmv.2073. [DOI] [PubMed] [Google Scholar]
- Bian L, Gao F, Mao Q, Sun S, Wu X, Liu S, Yang X, Liang Z. Hand, foot, and mouth disease associated with coxsackievirus A10: more serious than it seems. Expert Rev Anti Infect Ther. 2019;17:233–242. doi: 10.1080/14787210.2019.1585242. [DOI] [PubMed] [Google Scholar]
- Cai Y, Ku Z, Liu Q, Leng Q, Huang Z. A combination vaccine comprising of inactivated enterovirus 71 and coxsackievirus A16 elicits balanced protective immunity against both viruses. Vaccine. 2014;32:2406–2412. doi: 10.1016/j.vaccine.2014.03.012. [DOI] [PubMed] [Google Scholar]
- Caine EA, Fuchs J, Das SC, Partidos CD, Osorio JE. Efficacy of a trivalent hand, foot, and mouth disease vaccine against enterovirus 71 and coxsackieviruses A16 and A6 in mice. Viruses. 2015;7:5919–5932. doi: 10.3390/v7112916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao RY, Dong DY, Liu RJ, Han JF, Wang GC, Zhao H, Li XF, Deng YQ, Zhu SY, Wang XY, Lin F, Zhang FJ, Chen W, Qin ED, Qin CF. Human IgG Subclasses against Enterovirus Type 71: neutralization versus Antibody Dependent Enhancement of Infection. PLoS ONE. 2013;8:e64024. doi: 10.1371/journal.pone.0064024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Zhang C, Zhou Y, Zhang X, Shen C, Ye X, Jiang W, Huang Z, Cong Y. A 3.0-angstrom resolution cryo-electron microscopy structure and antigenic sites of coxsackievirus A6-like particles. J Virol. 2018;92:e01257-17. doi: 10.1128/JVI.01257-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Xia Y, Zhu S, Xu F, Sun Y, Lu H, Gao M, Yang Z, Mao Z, Ge Q. Muscle destruction caused by coxsackievirus A10 in gerbils: construction of a novel animal model for antiviral evaluation. Virus Res. 2020;286:198067. doi: 10.1016/j.virusres.2020.198067. [DOI] [PubMed] [Google Scholar]
- Chung CY, Chen CY, Lin SY, Chung YC, Chiu HY, Chi WK, Lin YL, Chiang BL, Chen WJ, Hu YC. Enterovirus 71 virus-like particle vaccine: improved production conditions for enhanced yield. Vaccine. 2010;28:6951–6957. doi: 10.1016/j.vaccine.2010.08.052. [DOI] [PubMed] [Google Scholar]
- Dai W, Xiong P, Zhang X, Liu Z, Chen J, Zhou Y, Ye X, Zhang C. Recombinant virus-like particle presenting a newly identified coxsackievirus A10 neutralization epitope induces protective immunity in mice. Antiviral Res. 2019;164:139–146. doi: 10.1016/j.antiviral.2019.02.016. [DOI] [PubMed] [Google Scholar]
- Fan S, Liao Y, Jiang G, Jiang L, Wang L, Xu X, Feng M, Yang E, Zhang Y, Cui W, Li Q. Study of integrated protective immunity induced in rhesus macaques by the intradermal administration of a bivalent EV71-CA16 inactivated vaccine. Vaccine. 2020;38:2034–2044. doi: 10.1016/j.vaccine.2019.12.057. [DOI] [PubMed] [Google Scholar]
- Fang CY, Liu CC. Recent development of enterovirus A vaccine candidates for the prevention of hand, foot, and mouth disease. Expert Rev Vaccines. 2018;17:819–831. doi: 10.1080/14760584.2018.1510326. [DOI] [PubMed] [Google Scholar]
- Foo DGW, Alonso S, Phoon MC, Ramachandran N, Chow VTK, Poh CL. Identification of neutralizing linear epitopes from the VP1 capsid protein of Enterovirus 71 using synthetic peptides. Virus Res. 2007;125:61–68. doi: 10.1016/j.virusres.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Fujii K, Nagata N, Sato Y, Ong KC, Wong KT, Yamayoshi S, Shimanuki M, Shitara H, Taya C, Koike S. Transgenic mouse model for the study of enterovirus 71 neuropathogenesis. PNAS. 2013;110:14753–14758. doi: 10.1073/pnas.1217563110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan X, Che Y, Wei S, Li S, Zhao Z, Tong Y, Wang L, Gong W, Zhang Y, Zhao Y. Effectiveness and safety of an inactivated enterovirus 71 vaccine in children aged 6–71 months in a phase IV study. Clin Infect Dis. 2019 doi: 10.1093/cid/ciz1114. [DOI] [PubMed] [Google Scholar]
- Huang LM, Chiu CH, Chiu NC, Lin CY, Li MT, Kuo TY, Weng YJ, Hsieh EF, Tai IC. Immunogenicity, safety, cross-reaction, and immune persistence of an inactivated enterovirus A71 vaccine in children aged from two months to 11 years in Taiwan. Vaccine. 2019;37:1827–1835. doi: 10.1016/j.vaccine.2019.02.023. [DOI] [PubMed] [Google Scholar]
- Huo C, Yang J, Lei L, Qiao L, Xin J, Pan Z. Hepatitis B virus core particles containing multiple epitopes confer protection against enterovirus 71 and coxsackievirus A16 infection in mice. Vaccine. 2017;35:7322–7330. doi: 10.1016/j.vaccine.2017.10.101. [DOI] [PubMed] [Google Scholar]
- Jia Q, Ng Q, Chin W, Meng T, Chow VTK, Wang C-I, Kwang J, He F. Effective in vivo therapeutic IgG antibody against VP3 of enterovirus 71 with receptor-competing activity. Sci Rep. 2017;7:46402. doi: 10.1038/srep46402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Fan R, Sun S, Fan P, Su W, Zhou Y, Gao F, Xu F, Kong W, Jiang C. A new EV71 VP3 epitope in norovirus P particle vector displays neutralizing activity and protection in vivo in mice. Vaccine. 2015;33:6596–6603. doi: 10.1016/j.vaccine.2015.10.104. [DOI] [PubMed] [Google Scholar]
- Kiener TK, Jia Q, Meng T, Chow VTK, Kwang J. A novel universal neutralizing monoclonal antibody against enterovirus 71 that targets the highly conserved “knob” region of VP3 protein. PLoS Negl Trop Dis. 2014;8:e2895. doi: 10.1371/journal.pntd.0002895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku Z, Liu Q, Ye X, Cai Y, Wang X, Shi J, Li D, Jin X, An W, Huang Z. A virus-like particle based bivalent vaccine confers dual protection against enterovirus 71 and coxsackievirus A16 infections in mice. Vaccine. 2014;32:4296–4303. doi: 10.1016/j.vaccine.2014.06.025. [DOI] [PubMed] [Google Scholar]
- Lee H, Cifuente JO, Ashley RE, Conway JF, Makhov AM, Tano Y, Shimizu H, Nishimura Y, Hafenstein S. A strain-specific epitope of enterovirus 71 identified by cryo-electron microscopy of the complex with fab from neutralizing antibody. J Virol. 2013;87:11363–11370. doi: 10.1128/JVI.01926-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HY, Han JF, Qin CF, Chen R. Virus-like particles for enterovirus 71 produced from Saccharomyces cerevisiae potently elicits protective immune responses in mice. Vaccine. 2013;31:3281–3287. doi: 10.1016/j.vaccine.2013.05.019. [DOI] [PubMed] [Google Scholar]
- Li J, Chang J, Liu X, Yang J, Guo H, Wei W, Zhang W, Yu XF. Protection from lethal challenge in a neonatal mouse model by circulating recombinant form coxsackievirus A16 vaccine candidates. J Gen Virol. 2014;95:1083. doi: 10.1099/vir.0.063560-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Liu L, Mo Z, Wang X, Xia J, Liang Z, Zhang Y, Li Y, Mao Q, Wang J, Jiang L, Dong C, Che Y, Huang T, Jiang Z, Xie Z, Wang L, Liao Y, Liang Y, Nong Y, Liu J, Zhao H, Na R, Guo L, Pu J, Yang E, Sun L, Cui P, Shi H, Wang J, Li Q. An inactivated enterovirus 71 vaccine in healthy children. N Engl J Med. 2014;370:829–837. doi: 10.1056/NEJMoa1303224. [DOI] [PubMed] [Google Scholar]
- Li JX, Song YF, Wang L, Zhang XF, Hu YS, Hu YM, Xia JL, Li J, Zhu FC. Two-year efficacy and immunogenicity of Sinovac Enterovirus 71 vaccine against hand, foot and mouth disease in children. Expert Rev Vaccines. 2016;15:129–137. doi: 10.1586/14760584.2016.1096782. [DOI] [PubMed] [Google Scholar]
- Lim HX, Poh CL. Insights into innate and adaptive immune responses in vaccine development against EV-A71. Ther Adv Vaccines Immunother. 2019;7:2515135519888998. doi: 10.1177/2515135519888998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim H, In HJ, Lee JA, Sik Yoo J, Lee SW, Chung GT, Choi YK, Chung JK, Cho SJ, Lee JW. The immunogenicity and protection effect of an inactivated coxsackievirus A6, A10, and A16 vaccine against hand, foot, and mouth disease. Vaccine. 2018;36:3445–3452. doi: 10.1016/j.vaccine.2018.05.005. [DOI] [PubMed] [Google Scholar]
- Lin JY, Kung YA, Shih SR. Antivirals and vaccines for Enterovirus A71. J Biomed Sci. 2019;26:65. doi: 10.1186/s12929-019-0560-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CC, Chou AH, Lien SP, Lin HY, Liu SJ, Chang JY, Guo MS, Chow YH, Yang WS, Chang KHW, Sia C, Chong P. Identification and characterization of a cross-neutralization epitope of Enterovirus 71. Vaccine. 2011;29:4362–4372. doi: 10.1016/j.vaccine.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Liu L, Zhao H, Zhang Y, Wang J, Che Y, Dong C, Zhang X, Na R, Shi H, Jiang L. Neonatal rhesus monkey is a potential animal model for studying pathogenesis of EV71 infection. Virology. 2011;412:91–100. doi: 10.1016/j.virol.2010.12.058. [DOI] [PubMed] [Google Scholar]
- Mao Q, Wang Y, Bian L, Xu M, Liang Z. EV-A71 vaccine licensure: a first step for multivalent enterovirus vaccine to control HFMD and other severe diseases. Emerg Microbes Infect. 2016;5:1–7. doi: 10.1038/emi.2016.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plevka P, Lim P-Y, Perera R, Cardosa J, Suksatu A, Kuhn RJ, Rossmann MG. Neutralizing antibodies can initiate genome release from human enterovirus 71. PNAS. 2014;111:2134–2139. doi: 10.1073/pnas.1320624111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premanand B, Kiener TK, Meng T, Tan YR, Jia Q, Chow VT, Kwang J. Induction of protective immune responses against EV71 in mice by baculovirus encoding a novel expression cassette for capsid protein VP1. Antiviral Res. 2012;95:311–315. doi: 10.1016/j.antiviral.2012.05.017. [DOI] [PubMed] [Google Scholar]
- Qi An W, Guo SuZ, Wen Pan R, Ping Yang B, Chao Zhang Y, Shi L, Li Q. The immunogenicity and protection effect of the BPL-inactivated CA16 vaccine in different animal systems. Hum Vaccin Immunother. 2014;10:628–639. doi: 10.4161/hv.27295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somasundaram B, Chang C, Fan YY, Lim PY, Cardosa J, Lua L. Characterizing Enterovirus 71 and Coxsackievirus A16 virus-like particles production in insect cells. Methods. 2016;95:38–45. doi: 10.1016/j.ymeth.2015.09.023. [DOI] [PubMed] [Google Scholar]
- Song Y, Zhang Y, Ji T, Gu X, Yang Q, Zhu S, Xu W, Xu Y, Shi Y, Huang X, Li Q, Deng H, Wang X, Yan D, Yu W, Wang S, Yu D, Xu W. Persistent circulation of Coxsackievirus A6 of genotype D3 in mainland of China between 2008 and 2015. Sci Rep. 2017;7:5491. doi: 10.1038/s41598-017-05618-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun YS, Li YJ, Xia Y, Xu F, Wang WW, Yang ZN, Lu HJ, Chen ZP, Miao ZP, Liang WF, Xu ZY, Dong HJ, Qiu DH, Zhu ZY, van der Veen S, Qian J, Zhou B, Yao PP, Zhu HP. Coxsackievirus A16 induced neurological disorders in young gerbils which could serve as a new animal model for vaccine evaluation. Sci Rep. 2016;6:1–11. doi: 10.1038/srep34299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsou YL, Lin YW, Shao HY, Yu SL, Wu SR, Lin HY, Liu CC, Huang C, Chong P, Chow YH. Recombinant adeno-vaccine expressing enterovirus 71-like particles against hand, foot, and mouth disease. PLoS NTD. 2015;9:e0003692. doi: 10.1371/journal.pntd.0003692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YF, Yu CK. Animal models of enterovirus 71 infection: applications and limitations. J Biomed Sci. 2014;21:31. doi: 10.1186/1423-0127-21-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KT, Lin SJ, Wang HC, Chen PC, Lin JJ, Chiang JR, Chang CL, Shih DY, Lo CF, Wang DY. Establishment of an animal challenge model as a potency assay for an inactivated Enterovirus Type 71 vaccine. Biologicals. 2016;44:183–190. doi: 10.1016/j.biologicals.2016.01.003. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhang Y, Zhang X, Hu Y, Dong C, Liu L, Yang E, Che Y, Pu J, Wang X. Pathologic and immunologic characteristics of coxsackievirus A16 infection in rhesus macaques. Virology. 2017;500:198–208. doi: 10.1016/j.virol.2016.10.031. [DOI] [PubMed] [Google Scholar]
- Wang J, Teng Z, Cui X, Li C, Pan H, Zheng Y, Mao S, Yang Y, Wu L, Guo X, Zhang X, Zhu Y. Epidemiological and serological surveillance of hand-foot-and-mouth disease in Shanghai, China, 2012–2016. Emerg Microbes Infect. 2018;7:8. doi: 10.1038/s41426-017-0011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Ku Z, Zhang X, Ye X, Chen J, Liu Q, Zhang W, Zhang C, Fu Z, Jin X, Cong Y, Huang Z. Structure, immunogenicity, and protective mechanism of an Engineered Enterovirus 71-like particle vaccine mimicking 80S empty capsid. J Virol. 2018;92:e01330–17. doi: 10.1128/JVI.01330-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, He D, Yang L, Li Z, Ye X, Yu H, Zhao H, Li S, Yuan L, Qian H, Que Y, Shih JW, Zhu H, Li Y, Cheng T, Xia N. A broadly cross-protective vaccine presenting the neighboring epitopes within the VP1 GH loop and VP2 EF loop of enterovirus 71. Sci Rep. 2015;5:12973. doi: 10.1038/srep12973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Q, Wu L, Chen L, Qin Y, Pan Z, Chen M. Vesicular stomatitis virus-based vaccines expressing EV71 virus-like particles elicit strong immune responses and protect newborn mice from lethal challenges. Vaccine. 2016;34:4196–4204. doi: 10.1016/j.vaccine.2016.06.058. [DOI] [PubMed] [Google Scholar]
- Yang B, Chuang H, Yang KD. Sialylated glycans as receptor and inhibitor of enterovirus 71 infection to DLD-1 intestinal cells. Virol J. 2009;6:141. doi: 10.1186/1743-422X-6-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Liu Y, Li S, Zhao H, Lin Q, Yu H, Huang X, Zheng Q, Cheng T, Xia N. A novel inactivated enterovirus 71 vaccine can elicit cross-protective immunity against coxsackievirus A16 in mice. Vaccine. 2016;34:5938–5945. doi: 10.1016/j.vaccine.2016.10.018. [DOI] [PubMed] [Google Scholar]
- Yang Z, Gao F, Wang X, Shi L, Zhou Z, Jiang Y, Ma X, Zhang C, Zhou C, Zeng X, Liu G, Fan J, Mao Q, Shi L. Development and characterization of an enterovirus 71 (EV71) virus-like particles (VLPs) vaccine produced in Pichia pastoris. Hum Vaccin Immunother. 2019 doi: 10.1080/21645515.2019.1649554:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao PP, Miao ZP, Xu F, Lu HJ, Sun YS, Xia Y, Chen C, Yang ZN, Xia SC, Jm Jiang. An adult gerbil model for evaluating potential coxsackievirus A16 vaccine candidates. Vaccine. 2019;37:5341–5349. doi: 10.1016/j.vaccine.2019.07.046. [DOI] [PubMed] [Google Scholar]
- Yee PTI, Poh CL. T Cell immunity to enterovirus 71 infection in humans and implications for vaccine development. Int J Med Sci. 2018;15:1143. doi: 10.7150/ijms.26450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi EJ, Shin YJ, Kim JH, Kim TG, Chang SY. Enterovirus 71 infection and vaccines. Clin Exp Vaccine Res. 2017;6:4–14. doi: 10.7774/cevr.2017.6.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu CK, Chen CC, Chen CL, Wang JR, Liu CC, Yan JJ, Su IJ. Neutralizing antibody provided protection against enterovirus type 71 lethal challenge in neonatal mice. J Biomed Sci. 2000;7:523–528. doi: 10.1007/BF02253368. [DOI] [PubMed] [Google Scholar]
- Zhang C, Yang Y, Chi Y, Yin J, Yan L, Ku Z, Liu Q, Huang Z, Zhou D. Hexon-modified recombinant E1-deleted adenoviral vectors as bivalent vaccine carriers for Coxsackievirus A16 and Enterovirus 71. Vaccine. 2015;33:5087–5094. doi: 10.1016/j.vaccine.2015.08.016. [DOI] [PubMed] [Google Scholar]
- Zhang C, Liu Q, Ku Z, Hu Y, Ye X, Zhang Y, Huang Z. Coxsackievirus A16-like particles produced in Pichia pastoris elicit high-titer neutralizing antibodies and confer protection against lethal viral challenge in mice. Antiviral Res. 2016;129:47–51. doi: 10.1016/j.antiviral.2016.02.011. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Dong Z, Li J, Carr MJ, Zhuang D, Wang J, Zhang Y, Ding S, Tong Y, Li D. Protective efficacies of formaldehyde-inactivated whole-virus vaccine and antivirals in a murine model of coxsackievirus A10 infection. J Virol. 2017;91:e00333-17. doi: 10.1128/JVI.00333-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Dong Z, Wei Q, Carr MJ, Li J, Ding S, Tong Y, Li D, Shi W. A neonatal murine model of coxsackievirus A6 infection for evaluation of antiviral and vaccine efficacy. J Virol. 2017;91:e02450–16. doi: 10.1128/JVI.02450-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Dai W, Zhang C, Zhou Y, Xiong P, Wang S, Ye X, Liu Q, Zhou D, Huang Z. A virus-like particle-based tetravalent vaccine for hand, foot, and mouth disease elicits broad and balanced protective immunity. Emerg Microbes Infect. 2018;7:94. doi: 10.1038/s41426-018-0094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Dong Z, Wang Q, Carr MJ, Li J, Liu T, Li D, Shi W. Characterization of an inactivated whole-virus bivalent vaccine that induces balanced protective immunity against coxsackievirus A6 and A10 in mice. Vaccine. 2018;36:7095–7104. doi: 10.1016/j.vaccine.2018.09.069. [DOI] [PubMed] [Google Scholar]
- Zhao H, Li HY, Han JF, Deng YQ, Li YX, Zhu SY, He YL, Qin ED, Chen R, Qin CF. Virus-like particles produced in Saccharomyces cerevisiae elicit protective immunity against Coxsackievirus A16 in mice. Appl Microbiol Biotechnol. 2013;97:10445–10452. doi: 10.1007/s00253-013-5257-3. [DOI] [PubMed] [Google Scholar]
- Zhao H, Li HY, Han JF, Deng YQ, Zhu SY, Li XF, Yang HQ, Li YX, Zhang Y, Qin ED, Chen R, Qin CF. Novel recombinant chimeric virus-like particle is immunogenic and protective against both enterovirus 71 and coxsackievirus A16 in mice. Sci Rep. 2015;5:7878. doi: 10.1038/srep07878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Shen C, Zhang C, Zhang W, Wang L, Lan K, Liu Q, Huang Z. Yeast-produced recombinant virus-like particles of coxsackievirus A6 elicited protective antibodies in mice. Antiviral Res. 2016;132:165–169. doi: 10.1016/j.antiviral.2016.06.004. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Zhang C, Liu Q, Gong S, Geng L, Huang Z. A virus-like particle vaccine protects mice against coxsackievirus A10 lethal infection. Antiviral Res. 2018;152:124–130. doi: 10.1016/j.antiviral.2018.02.016. [DOI] [PubMed] [Google Scholar]