Abstract
Background
Patients with coronavirus disaese 2019 (COVID-19) can develop a cytokine release syndrome that eventually leads to acute respiratory distress syndrome requiring invasive mechanical ventilation (IMV). Because IL-6 is a relevant cytokine in acute respiratory distress syndrome, the blockade of its receptor with tocilizumab (TCZ) could reduce mortality and/or morbidity in severe COVID-19.
Objective
We sought to determine whether baseline IL-6 serum levels can predict the need for IMV and the response to TCZ.
Methods
A retrospective observational study was performed in hospitalized patients diagnosed with COVID-19. Clinical information and laboratory findings, including IL-6 levels, were collected approximately 3 and 9 days after admission to be matched with preadministration and postadministration of TCZ. Multivariable logistic and linear regressions and survival analysis were performed depending on outcomes: need for IMV, evolution of arterial oxygen tension/fraction of inspired oxygen ratio, or mortality.
Results
One hundred forty-six patients were studied, predominantly males (66%); median age was 63 years. Forty-four patients (30%) required IMV, and 58 patients (40%) received treatment with TCZ. IL-6 levels greater than 30 pg/mL was the best predictor for IMV (odds ratio, 7.1; P < .001). Early administration of TCZ was associated with improvement in oxygenation (arterial oxygen tension/fraction of inspired oxygen ratio) in patients with high IL-6 (P = .048). Patients with high IL-6 not treated with TCZ showed high mortality (hazard ratio, 4.6; P = .003), as well as those with low IL-6 treated with TCZ (hazard ratio, 3.6; P = .016). No relevant serious adverse events were observed in TCZ-treated patients.
Conclusions
Baseline IL-6 greater than 30 pg/mL predicts IMV requirement in patients with COVID-19 and contributes to establish an adequate indication for TCZ administration.
Key words: COVID-19, IL-6, tocilizumab, invasive mechanical ventilation
Abbreviations used: ARDS, Acute respiratory distress syndrome; CAR, Chimeric antigen receptor; COPD, Chronic obstructive pulmonary disease; COVID-19, Coronavirus disease 2019; CRP, C-reactive protein; FiO2, Fraction of inspired oxygen; IL-6R, IL-6 receptor; IMV, Invasive mechanical ventilation; IQR, Interquartile range; LDH, Lactate dehydrogenase; PaO2, Arterial oxygen tension; ROC, Receiver-operating characteristic; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; TCZ, Tocilizumab
The recent exponential increase in cases of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has led the World Health Organization to declare a pandemic. The disease, known as coronavirus disease 2019 (COVID-19), has pushed national health systems to the brink of collapse, prompting national governments to impose complete population lockdowns in an attempt to slow down the dynamics of infection.1 , 2
The spectrum of COVID-19 clinical manifestations varies widely, from mild to severe cases of atypical pneumonia, some of them developing acute respiratory distress syndrome (ARDS), which often requires invasive mechanical ventilation (IMV) and is the leading cause of death. It is suggested that the severity of the respiratory disease caused by SARS-CoV-2 is largely due to an exacerbated immune response against the virus.3 , 4 This response has been observed in previous respiratory virus outbreaks and can be also seen in patients treated with chimeric antigen receptor (CAR) T-cell therapy.5, 6, 7 Proinflammatory cytokines such as IL-1 and IL-6 are crucial mediators of this process.3 , 6 In this regard, recent studies have indicated the usefulness of IL-6 serum levels, lymphocyte count, fibrinogen, or D-dimer to evaluate the development of ARDS and its mortality.8, 9, 10, 11 However, further evidence is required to support these observations.12
Tocilizumab (TCZ), an anti–IL-6 receptor (IL-6R) antibody, is the only drug currently licensed for the treatment of the cytokine release syndrome associated with CAR T-cell therapy.13 Because of the virulence of the current outbreak, its use has been advised in severe cases of COVID-19.14, 15, 16 The rationale is to curb the deleterious effects of inflammation, thereby limiting lung damage. Early promising results have prompted ongoing randomized clinical trials.17 We aimed to explore the ability of IL-6 serum levels at baseline to predict the need for IMV and the response to TCZ in patients with severe COVID-19.
Methods
Study design and population
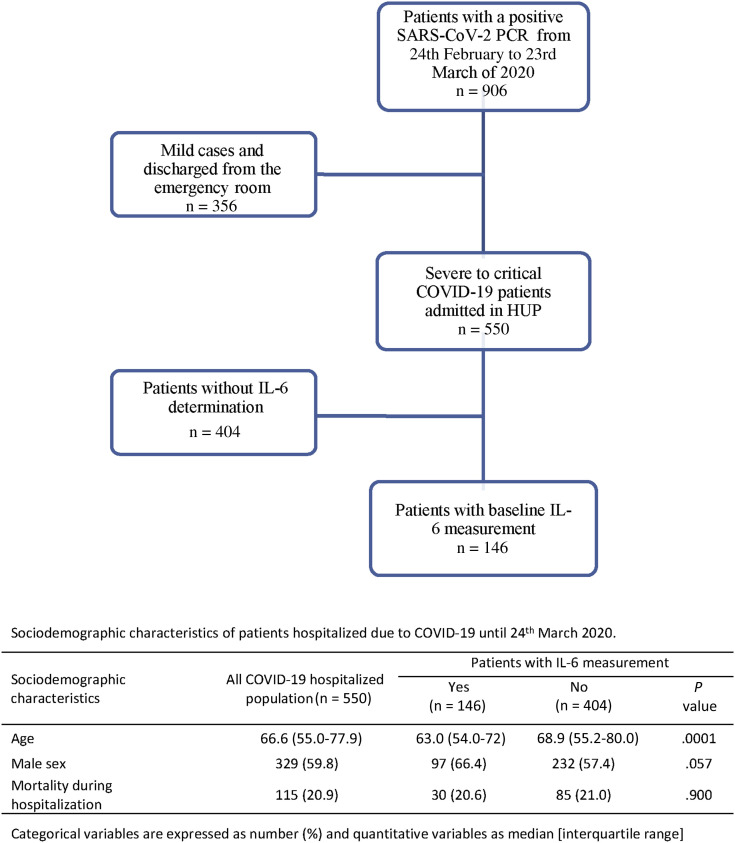
This is a retrospective observational study including 146 consecutive patients with confirmed detection of SARS-CoV-2 RNA, baseline IL-6 serum level measurement, and admitted to the Hospital Universitario La Princesa with severe to critical COVID-19,18 from February 24 to March 23, 2020 (see flowchart in Fig E1 in this article’s Online Repository at www.jacionline.org).
Fig E1.
Flowchart of patients included in the study.
This time frame was decided on the basis of the fact that after March 23, clinicians involved in the treatment of these patients were aware of the possibility of measuring IL-6 serum levels to decide treatment initiation with TCZ (RoActemra, Roche, Basel, Switzerland). Furthermore, around that date Spanish facilities capable of providing IMV were overrun (see Fig E2 in this article’s Online Repository at www.jacionline.org); thus, the decision for IMV application was based on availability rather than the patients’ needs. Notwithstanding, during the study period, patients were prompted to IMV when they presented a marked worsening of oxygenation (arterial oxygen tension/fraction of inspired oxygen [PaO2/FiO2] ratio <200), provided that their baseline conditions did not contraindicate it.
Fig E2.
Histogram showing the daily evolution of COVID-19 cases in Spain according to their clinical situation.E1
Data collection
Clinical, laboratory, and therapeutic data were collected from electronic clinical records and included in an anonymized database. Baseline evaluation was performed around the third day of admission (median, 3 days; interquartile range [IQR], 2-5). The second evaluation was obtained around the ninth day of admission (median, 9 days; IQR, 7-12). No significant differences at both evaluation time points were observed between patients treated and not treated with TCZ.
In addition, 23 serum samples from healthy donors obtained before the pandemic onset were used to determine the variability in IL-6 serum levels in baseline conditions.
SARS-CoV-2 RNA detection
Samples from nasopharyngeal and throat exudates were obtained with specific swabs as previously described.19 Then, we performed real-time RT-PCR assay targeting the E gene of SARS-CoV-2 as the first-line screening tool, with Real Time ready RNA Virus Master (Roche), followed by confirmatory testing with the assay TaqPath COVID-19 CE-IVD Kit RT-PCR (Applied Biosystems, Foster City, Calif), including 3 assays that target SARS-CoV-2 genes (Orf1ab, S gene, N gene) and 1 positive control assay that targets the human RNase P RPPH1 gene.20 All the procedures were performed on Applied Biosystems TM Quant Studio-5 Real-Time PCR System.
IL-6 serum level measurement
Surplus sera from laboratory routine determinations were used to assess IL-6 levels, which were retrospectively quantified in duplicate with the Human IL-6 Quantikine high sensitivity enzyme-immune assay from R&D Systems Europe Ltd (Abingdon, UK). The intraassay and interassay variability rates were 3% and 5%, respectively.
TCZ treatment
The rationale to treat patients with severe COVID-19 with TCZ was based on its previously approved indication for treating the cytokine release syndrome associated with CAR T-cell therapy, as well as unpublished experience in patients with COVID-19 from China and Italy. Treatment required approval by the Hospital Universitario La Princesa COVID-19 Committee, following the recommendations of the Spanish Agency for Drugs and Health Devices (Agencia Española de Medicamentos y Productos Sanitarios)21:
-
•
Interstitial pneumonia with severe respiratory failure (score = 2);
-
•
Rapid respiratory worsening requiring noninvasive or invasive ventilation (score ≥ 3 on the COVID respiratory severity scale);
-
•
Presence of extrapulmonary organ failure (shock or score ≥ 3 on the Sequential Organ Failure Assessment [SOFA] scale);
-
•
Criteria for severe systemic inflammatory response. In adults: elevated levels of IL-6 (>40 pg/mL); alternatively, increased levels of D-dimer (>1500 ng/mL) or progressively increasing D-dimer; and
-
•
Patients who, according to their baseline clinical condition, would be IMV subsidiary.
Therefore, the decision to treat with TCZ was based on the Agencia Española de Medicamentos y Productos Sanitarios criteria, excluding IL-6 greater than 40 pg/mL, because no information about IL-6 results was available to physicians during the study period. After a preliminary analysis of data by the end of March, IL-6 measurement was included in the baseline assessment of patients with COVID-19.
The administration schedule of TCZ at the time of the study was a first intravenous infusion of 8 mg/kg (maximum 800 mg) followed by a second one after 12 hours.
Variables
To analyze whether IL-6 levels can predict disease severity, 2 main outcomes were considered: need for IMV and all-cause mortality.
To determine the effect of TCZ, we analyzed the evolution of PaO2/FiO2 between both evaluation times. In 160 of 267 evaluations, PaO2 was unavailable. So, to avoid missing data in these relevant outcomes, this parameter was estimated from mean oxygen saturation as proposed elsewhere.22
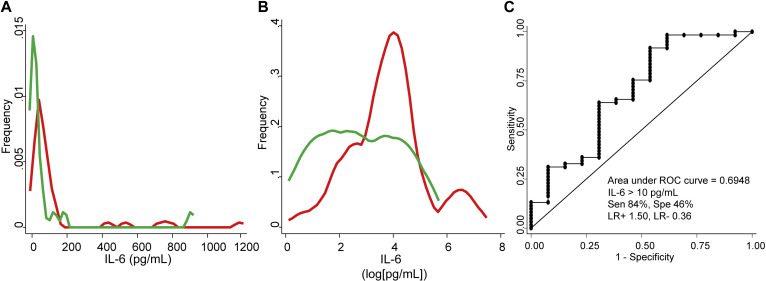
IL-6 serum levels showed a heterogeneous distribution in patients and in healthy donors (see Fig E3, A, in this article’s Online Repository at www.jacionline.org). To improve its representation in figures, this variable was normalized through natural logarithmic transformation (Fig E3, B). The procedure to determine the cutoff for high baseline IL-6 is described below.
Fig E3.
A, Plots showing the distribution of raw IL-6 serum levels in patients (red) vs healthy donors (green). B, Distribution of log-transformed serum IL-6 levels in the former groups. C, ROC curve showing the ability of IL-6 serum levels to classify patients with COVID-19 vs healthy donors. LR+, Positive likelihood ratio; LR−, negative likelihood ratio; Sen, sensitivity; Spe, specificity.
Statistical analysis
Statistical analyses were performed using Stata 14 for Windows (Stata Corp LP, College Station, Tex). Quantitative variables following a nonnormal distribution were represented as median and IQR, and the Mann-Whitney test was used to assess significant differences. Variables with a normal distribution were described by mean ± SD, and differences between groups were assessed with Student t test. Qualitative variables were described as counts and proportions, and chi-square or Fisher exact test was used for comparisons. Correlation between quantitative variables was analyzed using the Pearson correlation test. To estimate the 95% CI of correlation coefficients, we used the ci2 command of Stata.
To determine whether IL-6 serum levels were able to discriminate between (1) patients with COVID-19 versus healthy donors, (2) patients requiring IMV versus those who did not, or (3) patients treated with TCZ versus not treated, receiver-operating characteristic (ROC) analysis was performed using the roctab command. Each cutoff point was selected on the basis of best trade-off values between sensitivity, specificity, and the percentage of patients correctly classified. Positive and negative likelihood ratios and ROC curves were also obtained.
To determine the variables associated with the need for IMV, we performed a multivariable logistic regression analysis that was first modeled by adding all the variables with a P value lower than .15 in the bivariable analysis, namely total lymphocyte count, D-dimer, lactate dehydrogenase (LDH), PaO2/FiO2, chronic obstructive pulmonary disease (COPD), obesity, hypertension, C-reactive protein (CRP), and IL-6 (high vs low). The final model was reached with backward stepwise removal of variables with P value higher than .15, and using Wald tests to demonstrate that each model was better than its previous iteration.
Next, we performed a multivariable analysis using generalized linear models nested by patient and visit (xtgee command) in which the dependent variable was PaO2/FiO2. This approach allowed us to identify which variables influenced the evolution of PaO2/FiO2. The first model included all variables with a P value less than .15 in the bivariable analysis, namely hypertension, baseline radiological pattern, LDH, total lymphocyte count, baseline CRP, and IMV. After that, through backward stepwise approach, we obtained the best model as described above. Then, to assess the role of IL-6 as predictor of TCZ effect on PaO2/FiO2, the composite variable IL-6/TCZ (low IL-6/no TCZ, low IL-6/early TCZ, low IL-6/late TCZ, high IL-6/no TCZ, high IL-6/early TCZ, and high IL-6/late TCZ) was forced in the model.
Survival time was analyzed by Kaplan-Meier method with the sts command of Stata. Date of admission was considered the date of entry and for exit date we considered the exitus date. For those patients without the event, the last revision of the database (electronic chart or telephone call) on May 21 was used to censor their follow-up. Differences in time to death by different variables were analyzed by log-rank test.
Ethics
This study was approved by the local Research Ethics Committee (register number 4070), and it was carried out following the ethical principles established in the Declaration of Helsinki. All included patients (or their representatives) were informed about the study and gave an oral informed consent as proposed by Agencia Española de Medicamentos y Productos Sanitarios due to COVID-19 emergency.
This article was written following the Strengthening the Reporting of Observational Studies in Epidemiology guidelines taking into consideration the difficulties to obtain all the needed information in the setting of the COVID-19 pandemic.
Results
Demographic and clinical characteristics of the study population
One hundred forty-six patients were included; their main demographic and clinical characteristics are presented in Table I . Median age was 63 years (IQR, 54-71; range, 30-86), 97 (66%) were men, and 100 (69%) presented comorbidities. The most frequent were hypertension, 55 (38%); obesity, 23 (16%); diabetes mellitus, 26 (18%); and COPD, 9 (6%); 19 (13%) patients had a history of malignancy. Median duration of symptoms before admission was 6 days (IQR, 4-7); 36 (25%) arrived at the emergency room presenting fever (≥38°C), with a SatO2 of 91% ± 5%. Most individuals (121 [83%]) were admitted to the internal medicine or pneumology wards; however, 16 (11%) patients were admitted directly to the intensive care unit because of IMV requirement, and 9 (6%) to the hematology ward because of preexisting conditions. Additional details of patient baseline features can be found in Table E1 in this article’s Online Repository at www.jacionline.org.
Table I.
Baseline clinical characteristics and laboratory findings of the study population
| Characteristic | Study population (n = 146) |
|---|---|
| Age (y) | 63 (54-71) |
| Sex: male | 97 (66) |
| Comorbidities | 100 (69) |
| Duration of symptoms at admission (d) | 6 (4-7) |
| Baseline PaO2/FiO2 | 215 (112-310) |
| Treatment during hospitalization | |
| Hydroxychloroquine | 137 (96) |
| Lopinavir/Ritonavir | 119 (83) |
| Azithromycin | 82 (57) |
| IFN-β | 7 (5) |
| Glucocorticoids | 85 (59) |
| Methylprednisolone bolus | 61 (42) |
| Laboratory findings | |
| White blood cell count (103/mm3) | 7.64 (5.25-10.68) |
| Lymphocyte count (103/mm3) | 0.83 (0.60-11.7) |
| Creatinine (mg/dL) | 0.86 (0.70-1.10) |
| LDH (U/L) | 341 (256-461) |
| CK (U/L) | 72 (48-155) |
| Serum IL-6 (pg/mL) | 21.36 (7.53-54.21) |
| Ferritin (ng/mL) | 1598 (830-2305) |
| CRP (mg/dL) | 11.55 (5.16-22.53) |
| PCT (ng/mL) | 0.15 (0.10-0.35) |
| D-dimer (mg/mL) | 0.75 (0.48-1.48) |
CK, Creatine kinase; PCT, procalcitonin.
All categorical variables are expressed as number (%) and quantitative variables as median (p25-p75).
IL-6 serum levels and disease severity
IL-6 serum levels above 10 pg/mL discriminated patients with COVID-19 from healthy donors with low accuracy (area under the ROC curve, 0.695; sensitivity, 84%; specificity, 46%; positive likelihood ratio, 1.5; negative likelihood ratio, 0.4; Fig E3, C), probably due to their intrinsic heterogeneity (Fig E3, A and B).
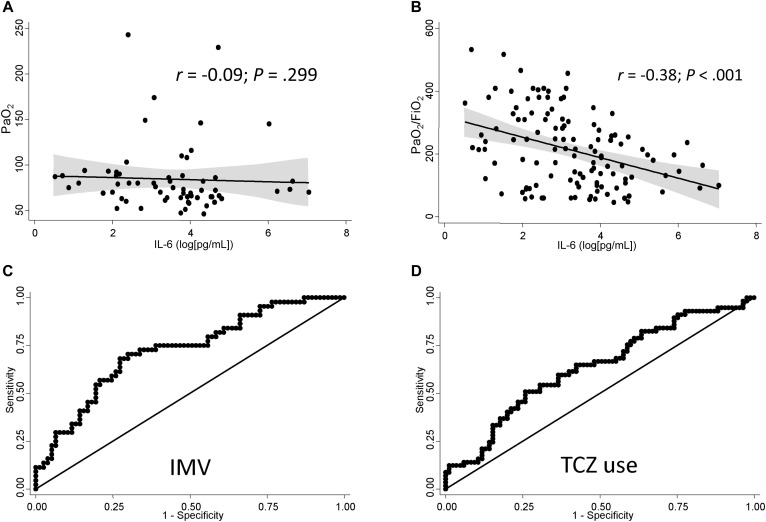
No significant correlation was found between PaO2 and IL-6 serum levels at baseline (r = −0.09; 95% CI, −0.270 to 0.085; P = .299; Fig 1 , A), likely due to a higher oxygen supply in the most severe cases; in fact, serum IL-6 levels showed a significant negative correlation with PaO2/FiO2 (Fig 1, B; r = −0.38; 95% CI, −0.526 to −0.218; P < .001), meaning that higher levels of IL-6 at baseline were associated with lower PaO2/FiO2.
Fig 1.
IL-6 serum levels predict disease severity and TCZ use. A, Correlation between log-transformed IL-6 serum levels and PaO2. B, Correlation between log-transformed IL-6 serum levels and PaO2/FiO2. Data in panels A and B are shown as dot-plot and their fitted linear prediction with 95% CI (transparent gray shadow) estimated using the 2-way command of Stata with the lfitci option. C, ROC curve showing the ability of log-transformed IL-6 serum levels to discriminate between patients requiring and not requiring IMV. D, ROC curve for the ability of log-transformed IL-6 serum levels to discriminate between TCZ-treated and nontreated patients. The best cutoff for discrimination of patients requiring IMV (Fig 1, C) or TCZ treatment (D) was 30 pg/mL.
In this regard, 44 (30%) patients required IMV at some point during their hospitalization. As expected, these patients showed significantly worse PaO2/FiO2 levels than those not requiring IMV (P < .001; Table II ). In addition, they showed increased leukocytes, total lymphocytes, IL-6, CRP, and procalcitonin, showing a higher inflammatory status than those not requiring IMV (P ≤ .001 except P = .003 for CRP and P = .029 for lymphocyte count; Table II).
Table II.
Baseline clinical characteristics of groups requiring vs not requiring IMV
| Characteristic | IMV |
||
|---|---|---|---|
| Required (n = 44) | Not required (n = 102) | P value | |
| Age (y) | 63.5 (56.5-72) | 62 (54-71) | .517 |
| Sex: male | 32 (73) | 65 (64) | .291 |
| Comorbidities | 30 (68) | 70 (69) | .893 |
| Duration of symptoms at admission (d) | 5 (5-7) | 7 (4-8) | .265 |
| Baseline PaO2/FiO2 | 125.5 (75-207) | 247 (172-348) | <.001 |
| Treatment during hospitalization | |||
| Hydroxychloroquine | 38 (86) | 99 (100) | <.001 |
| Lopinavir/Ritonavir | 38 (86) | 81 (82) | .502 |
| Azithromycin | 24 (55) | 58 (59) | .652 |
| IFN-β | 3 (7) | 4 (4) | .676 |
| Glucocorticoids | 27 (61) | 58 (59) | .755 |
| Methylprednisolone bolus | 21 (48) | 40 (40) | .414 |
| Laboratory findings | |||
| White blood cell count (103/mm3) | 9.39 (6.59-13.31) | 6.93 (5.13-8.78) | <.001 |
| Lymphocyte count (103/mm3) | 0.74 (0.58-1.08) | 0.87 (0.62-1.26) | .029 |
| Creatinine (mg/dL) | 0.99 (0.71-1.20) | 0.85 (0.72-1.1) | .398 |
| LDH (U/L) | 413 (315-496) | 302 (224-443) | .001 |
| CK (U/L) | 67 (39.50-167.50) | 94 (59-140) | .617 |
| Serum IL-6 (pg/mL) | 49.20 (17.28-103.57) | 16.08 (6.09-42.03) | <.001 |
| Ferritin (ng/mL) | 1665 (602-2765) | 1573 (1012-2300) | .832 |
| CRP (mg/dL) | 17.09 (7.69-28.98) | 10.13 (4.83-18.48) | .003 |
| PCT (ng/mL) | 0.29 (0.14-0.46) | 0.13 (0.08-0.26) | .001 |
| D-dimer (mg/mL) | 0.92 (0.56-2.31) | 0.71 (0.48-1.19) | .058 |
CK, Creatine kinase; PCT, procalcitonin.
All categorical variables are expressed as number (%) and quantitative variables as median (p25-p75).
Furthermore, a baseline IL-6 above 30 pg/mL (henceforth high IL-6) discriminated patients requiring IMV with 68% sensitivity and 73% specificity. Area under the ROC curve was 0.725 (Fig 1, C; positive likelihood ratio, 2.5; negative likelihood ratio, 0.4). A logistic regression model, adjusted for COPD and baseline white blood cell count, also showed that high baseline IL-6 was a predictive biomarker for IMV (odds ratio, 7.1; 95% CI, 3.0-16.6; see Table E2 in this article’s Online Repository at www.jacionline.org).
Response to TCZ
Fifty-eight (40%) patients received treatment with TCZ. No significant differences between groups were observed in most sociodemographic and therapeutic variables, except for patients not treated with TCZ, which were more often obese and COPD (P = .023 and P = .071, respectively; see Table E3 in this article’s Online Repository at www.jacionline.org). Importantly, patients in the TCZ-treated group presented several baseline findings indicating that they suffered more severe COVID-19, such as lower PaO2/FiO2 (P < .001; Table III ), higher levels of serum LDH (P < .001), CRP (P = .005), IL-6 levels at baseline (P = .007), and total lymphocyte count (P = .001). Other elevated markers in this group included aspartate aminotransferase, ferritin, and procalcitonin (P < .05 for all comparisons; Table III).
Table III.
Baseline clinical characteristics of groups treated vs not treated with TCZ
| Characteristic | TCZ |
||
|---|---|---|---|
| Treated (n = 58) | Not treated (n = 88) | P value | |
| Age (y) | 61 (54-70) | 64 (54-72) | .288 |
| Sex: male | 40 (69) | 57 (65) | .600 |
| Comorbidities | 35 (61) | 64 (73) | .124 |
| Duration of symptoms at admission (d) | 6 (5-7) | 7 (4-8) | .612 |
| Baseline PaO2/FiO2 | 137 (88-232) | 248 (183-348) | <.001 |
| Treatment during hospitalization | |||
| Hydroxychloroquine | 53 (93) | 84 (98) | .171 |
| Lopinavir/Ritonavir | 51 (89) | 68 (79) | .103 |
| Azithromycin | 33 (58) | 49 (57) | .913 |
| IFN-β | 2 (4) | 5 (6) | .532 |
| Glucocorticoids | 38 (67) | 47 (55) | .152 |
| Methylprednisolone bolus | 31 (54) | 30 (35) | .018 |
| Laboratory findings | |||
| White blood cell count (103/mm3) | 7.99 (5.17-11.85) | 7.52 (5.4-10.36) | .527 |
| Lymphocyte count (103/mm3) | 0.74 (0.52-0.997) | 0.93 (0.66-1.47) | .001 |
| Creatinine (mg/dL) | 0.83 (0.70-1.05) | 0.90 (0.72-1.14) | .177 |
| LDH (U/L) | 425 (302-510) | 293.5 (221-388) | <.001 |
| CK (U/L) | 69 (38-270) | 75.5 (49-125) | .785 |
| Serum IL-6 (pg/mL) | 41.85 (12.37-71.95) | 16.25 (6.27-44.95) | .007 |
| Ferritin (ng/mL) | 1888 (1152-2844) | 1461 (471-1861) | .038 |
| CRP (mg/dL) | 13.73 (8.75-27.08) | 9.09 (4.78-19.31) | .005 |
| PCT (ng/mL) | 0.25 (0.13-0.36) | 0.14 (0.1-0.3) | .045 |
| D-dimer (mg/mL) | 0.75 (0.48-1.48) | 0.71 (0.53-1.22) | .491 |
CK, Creatine kinase; PCT, procalcitonin.
All categorical variables are expressed as number (%) and quantitative variables as median (p25-p75).
Even before physicians were aware of the potential value of IL-6 serum levels as a predictor of severe disease, those patients with high IL-6 were more frequently treated with TCZ (Fig 1, D; area under the ROC curve, 0.634; 30 pg/mL as cutoff showed sensitivity 57%, specificity 69%, positive likelihood ratio 1.9, and negative likelihood ratio 0.7), although with less accuracy than for IMV. The median time from the beginning of symptoms to TCZ treatment was 11 days (IQR, 8-12.5). Therefore, we considered early TCZ when the treatment was applied before 11 days of disease duration and late TCZ after this cutoff.
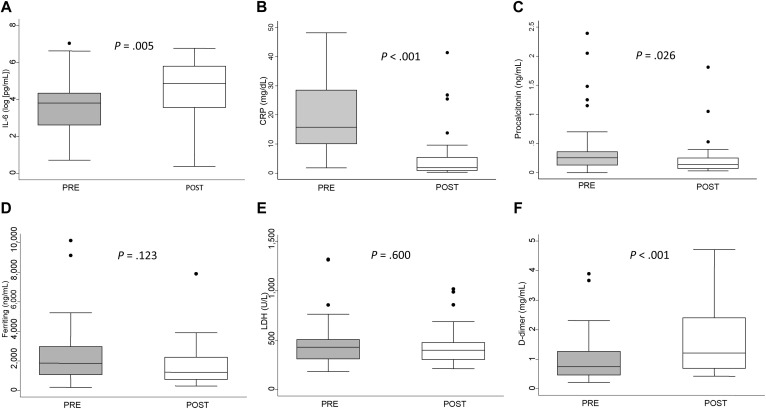
As a consequence of IL-6R blockade with TCZ, a significant trend toward higher IL-6 serum levels after administration of the drug was observed (Fig 2 , A; P = .005). However, IL-6R blockade induced a fast and significant downregulation of CRP (Fig 2, B; P < .001) and procalcitonin (Fig 2, C; P = .026) and a nonsignificant decrease in ferritin (Fig 2, D) and LDH (Fig 2, E). Conversely, it was associated with a significant increase in D-dimer levels (Fig 2, F; P < .001).
Fig 2.
Response of laboratory parameters to TCZ treatment. Differences in (A) log-transformed IL-6 serum levels, (B) C-reactive protein, and (C) procalcitonin, (D) ferritin, (E) LDH, and (F) D-dimer. Data are presented as the IQR (p75 upper edge, p25 lower edge, p50 midline), p95 (line above the box), and p5 (line below the box) of levels for each parameter before (gray boxes) and after (white boxes) treatment with TCZ. PRE and POST mean first and second evaluation, respectively.
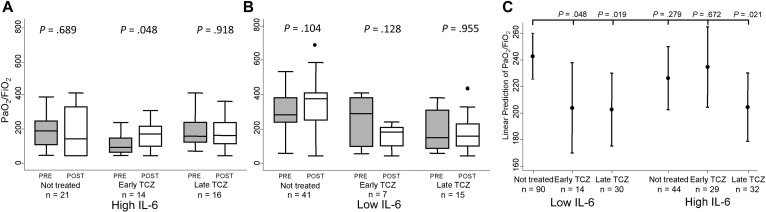
After an average 6 days of TCZ treatment, despite this improvement in inflammatory parameters, PaO2/FiO2 did not show a significant improvement in the whole population (data not shown). Only patients with high IL-6 who underwent early TCZ treatment showed a significant PaO2/FiO2 increase (Fig 3 , A, mid boxes; P = .048). Patients with low IL-6 did not improve their PaO2/FiO2 after treatment with TCZ (Fig 3, B). Those patients not treated with TCZ showed a heterogeneous behavior in their PaO2/FiO2, with a trend to improve in patients with low IL-6 (Fig 3, A and B, 2 left boxes of each panel).
Fig 3.
Change in PaO2/FiO2 in patients with COVID-19 treated early (before 11 days of symptoms onset) or late with TCZ and not treated. A, Patients with high baseline IL-6 (cutoff 30 pg/mL). B, Subjects with low baseline IL-6 serum levels. Data in A and B are shown as the IQR (p75 upper edge, p25 lower edge, p50 midline), p95 (line above the box), and p5 (line below the box) before (gray boxes) and after (white boxes) treatment with TCZ. In nontreated patients, PRE and POST mean first and second evaluation, respectively. Statistical significance was determined with the Mann-Whitney test. C, The graph represents the predicted mean (dots) with 95% CI (bars) of PaO2/FiO2 according to baseline IL-6 levels and early or late TCZ treatment. Data were obtained with the command marginsplot of Stata, after adjustment by baseline PaO2/FiO2 and radiological pattern, hypertension, LDH and CRP levels, lymphocyte blood count, and need for IMV, according to the multivariable analysis displayed in Table E2 (see the Methods section for further information).
Because relevant differences were observed between patients treated and not treated with TCZ, we used a multivariable analysis to determine which variables influenced the evolution of PaO2/FiO2 at the short-term. Baseline PaO2/FiO2 and radiological pattern, hypertension, LDH, and CRP levels, and total lymphocyte blood count significantly explained variation in PaO2/FiO2 (see Table E4 in this article’s Online Repository at www.jacionline.org). Adjusted by these confounders, the best PaO2/FiO2 evolution was achieved in patients with low IL-6 not requiring TCZ treatment (Fig 3, C, first dot on the left; reference group in analysis showed in Table E4), likely because they were the less severe patients. Patients with low IL-6 who because of their bad evolution were prescribed TCZ showed a significant worsening of PaO2/FiO2 (Fig 3, C, second and third dots; Table E4). A similar evolution was observed in patients with high IL-6 and late TCZ treatment, whereas those with high IL-6 not treated or treated early with TCZ showed no significant differences compared with the reference group (Fig 3, C; Table E4).
Mortality
Next, we were interested in the long-term evolution of patients depending on baseline IL-6 and treatment with TCZ. After a median follow-up of 61 days (IQR, 58-64), we observed 30 deaths in our sample (21%). The survival curves according to the baseline level of IL-6 and TCZ treatment are shown in Fig 4 . Six of 59 (10%) patients died in the reference group (low IL-6/no TCZ), 9 of 28 (32%) in the low IL-6/TCZ-treated group (hazard ratio, 3.6; 95% CI, 1.3-10.0; P = .016), 10 of 28 (36%) in the high IL-6/no TCZ group (hazard ratio, 4.6; 95% CI, 1.7-12.7; P = .003), and 5 of 31 (16%) in the high IL-6/TCZ-treated group (hazard ratio, 1.6; 95% CI, 0.5-5.4; P = .411).
Fig 4.
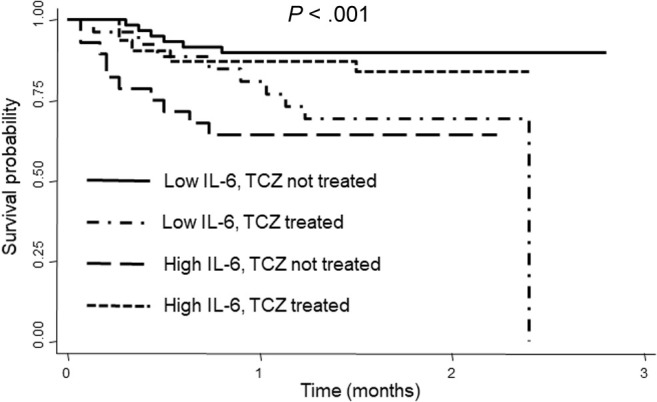
Survival curves of patients with COVID-19 grouped according to baseline IL-6 levels and TCZ treatment. Statistical significance was established with log-rank test.
Safety of TCZ treatment
Regarding safety, no relevant cytopenia, hypertransaminasemia, bowel perforation, or secondary bacterial infections were observed during or after treatment with TCZ for the time of the study. Ten (7%) patients had positive blood cultures; most of them in the non–TCZ-treated group (7 vs 3; P = .03).
Discussion
To our knowledge, this is the first study showing that high baseline IL-6 levels predict both the need for IMV and the response to TCZ in severe patients hospitalized with COVID-19. Our results confirm the hypothesis that respiratory failure in the advanced phase of severe COVID-19 is mainly due to an exacerbated inflammatory response. These findings are in accordance with cytokine storms described in previous experiences with H5N1 influenzae virus5 and previous coronaviruses severe acute respiratory syndrome coronavirus and Middle East respiratory syndrome,7 as well as CAR T-cell therapy.23 , 24 Together, these data suggest a key role for inflammation of the small distal airways in the severity of this condition.25 Hence, approximately one-third of patients with ARDS display elevated levels of inflammatory mediators (IL-6, IL-8, and soluble TNF receptor 1, among others), increasing the prevalence of shock and mortality.26 Accordingly, our data show that IL-6 levels higher than 30 pg/mL predict the need for IMV, and correlated with other severity data. A similar threshold has been described to discriminate between mild and severe COVID-19 in Chinese patients.27
The association of high IL-6 with a more severe disease in our population supports the use of TCZ to treat patients with COVID-19. TCZ is a humanized antibody that blocks both soluble and membrane-bound forms of IL-6R. Thus, TCZ prevents ligand binding, which likely explains why IL-6 serum levels significantly increased after treatment.28 However, the TCZ-mediated blockade of IL-6R signaling led to the observed relevant decrease in circulating inflammatory mediators. Severe high IL-6 patients with COVID-19 treated with TCZ showed an early respiratory improvement, represented by a moderate but significantly increased PaO2/FiO2 when TCZ was prescribed before the 11th day of symptoms and lower overall mortality, independently of other treatments or clinical factors. Furthermore, patients with severe COVID-19 and high IL-6 levels who were not treated with TCZ displayed a higher mortality.
On the other hand and of outstanding interest, patients with low IL-6 who were treated with TCZ because of a severe or critical COVID-19 did not improve and showed significantly higher mortality. These observations pose the question whether it is possible that severe patients with low IL-6 included in phase 3 trials with TCZ and sarilumab account for the failure to meet their primary end points.29 , 30 Therefore, our results support the measurement of baseline IL-6 levels in hospitalized patients with COVID-19, because in those severe or critical patients with low IL-6 levels, other cytokines such as IL-1 or TNF could be driving the exacerbated inflammatory response in lungs.25 Probably, this specific group could benefit from receiving other anti-inflammatory agents such as IL-1 or TNF blockers. In this regard, the need for biomarkers of response to TCZ has been recently highlighted,31 and neither COVACTA study (TCZ trial; NCT04320615) nor ex-US sarilumab clinical trial (NCT04327388) listed increased baseline IL-6 serum levels within the inclusion criteria. Nevertheless, both clinical trials reported a decrease in duration of hospital stay in the active arm, which was statistically significant in the COVACTA study.29 , 30
In agreement with our data, other case series with few patients have shown that TCZ can improve the outcomes of patients with COVID-19 with ARDS6 , 8 , 31 , 32; some of these reports described the use of lower doses of TCZ. In this regard, the protocol in our hospital, after these preliminary data, has evolved toward lower doses of TCZ (a single dose of TCZ 400 mg if <80 kg and 600 mg if >80 kg) administered earlier with similar efficacy (unpublished observation).
In addition, during our study, the use of TCZ was safe and it did not increase the number of serious bacterial infections. These findings are consistent with the results of both the COVACTA and sarilumab trials.29 , 30 The only unexpected observation was the rise in D-dimer levels at the second evaluation. Possible explanations are either (1) IL-6 does not play a role in the regulation of D-dimer production or (2) more likely, D-dimer production has a slower kinetics than CRP or other acute-phase reactants, because in patients not treated with TCZ, a similar rise in D-dimer levels was observed (data not shown).
Apart from the novelty and the immediate clinical utility of these findings, a drawback of our study is its retrospective and observational nature involving mainly very severe cases in the group of treatment with TCZ. A stricter selection of a control group through a propensity score strategy was unfeasible, because once the physicians were aware of IL-6 measurement, they focused their efforts on treating with TCZ those patients with the highest IL-6 levels. Therefore, prospective studies should be carried out to confirm our observations.
In addition, there is some controversy about the reliability of PaO2/FiO2 as an outcome for improvement of lung damage, especially in patients with IMV. However, our population was a mix of patients with IMV and non-IMV, so despite the many factors that could interfere with PaO2/FiO2, we considered it the most objective outcome for patients’ assessment.
Finally, our findings are of relevance for clinical decision making in the ongoing COVID-19 pandemic. Increased levels of circulating IL-6 predict IMV requirement in patients with severe disease, and can contribute to establish the indication for timely TCZ administration. Furthermore, the improvement in respiratory parameters achieved on treatment with TCZ may reduce IMV demand in these patients. However, in our population, there was a small group of patients with severe COVID-19 and low IL-6 serum levels, which probably should have been treated with blockade of IL-1 or TNF-α because their evolution with TCZ was inadequate.
Clinical implications.
Elevated levels of circulating IL-6 predict the need for IMV in patients with severe COVID-19 and may contribute to establish the indication for timely administration of TCZ, possibly reducing intensive care unit demand.
Acknowledgments
Special thanks to Dr Miguel Vicente Manzanares and Dr Manuel Gomez Gutierrez for their excellent editing assistance. The REINMUN-COVID Group includes the following:
-
•
Cardiology: Teresa Alvarado, Pablo Martínez, and Francisco Javier de la Cuerda Llorente
-
•
Emergency Service: Carmen del Arco, Juan Mariano Aguilar, Natalia Villalba, Mónica Negro, Elvira Contreras, Ana del Rey, Cristina Santiago, Manuel Junquera, Raquel Caminero, Francisco Javier Val, Sonia González, Marta Caño, Isabel López, Andrés von Wernitz, Bárbara Retana, Iñigo Guerra, Jorge Sorando, Lydia Chao, María José Cárdenas, Verónica Espiga, Pablo Chicharro, and Pedro Rodríguez
-
•
Endocrinology: Iñigo Hernando Alday and Miguel Sampedro
-
•
ENT: Jorge Prada
-
•
Gastroenterology: Eukene Rojo Aldama, Yolanda Real, María Caldas, Sergio Casabona, and Aitor Lanas-Gimeno
-
•
Hematology Service: Rafael de la Camara, Angela Figuera Alvárez, and Beatriz Aguado
-
•
Hospital Pharmacy: Alberto Morell, Esther Ramírez, Amparo Ibáñez Zurriaga, María Pérez Abanades, Silvia Ruiz García, Tomás Gallego Aranda, María Ruiz, Concepción Martínez Nieto, and José María Serra
-
•
Immunology: Francisco Sánchez-Madrid, Cecilia Muñoz-Calleja, Arantzazu Alfranca, Javier Aspa, Ana Marcos-Jiménez, Santiago Sánchez-Alonso, Ana Alcaraz-Serna, Tamara Mateu-Albero, Ildefonso Sánchez-Cerrillo, Laura Esparcia, Pedro Martínez-Fleta, Celia López-Sanz, Ligia Gabrie, Luciana del Campo Guerola, Elena Fernández, Ma José Calzada, and Reyes Tejedor
-
•
Intensive Care Unit: Alfonso Canabal, Patricia Albert, Diego A. Rodríguez-Serrano, Judit Iglesias, Fernando Suarez, Juan Antonio Sánchez, and Beatriz Abad
-
•
Internal Medicine-Infectious Diseases: Carmen Suarez, Ignacio de los Santos, José María Galván-Román, Emilia Roy, Pablo Rodríguez-Cortes, Lucio García-Fraile, Jesus Sanz, Eduardo Sanchez, Fernando Moldenhauer, Pedro Casado, Jose Curbelo, Angela Gutierrez, Azucena Bautista, Nuria Ruiz Giménez, Angelica Fernandez, Pedro Parra, Berta Moyano, Ana Barrios, Diego Real de Asua, Beatriz Sanchez, Carmen Saez, and Marianela Ciudad
-
•
Medical Biology: Desiré Navas
-
•
Microbiology: Laura Cardeñoso Domingo, María del Carmen Cuevas Torresano, Diego Domingo García, Teresa Alarcón Cavero, Alicia García Blanco, Alexandra Martín Ramírez, María Auxiliadora Semiglia Chong, Ainhoa Gutiérrez Cobos, Nelly Daniela Zurita Cruz, and Arturo Manuel Fraile Torres
-
•
Nephrology: Carmen Sanchez-Gonzalez and Antonio Fernádez Perpén
-
•
Neurology: Carolina Díaz Pérez
-
•
Pneumology: Julio Ancochea, Tamara Alonso, Pedro Landete, Joan Soriano, Carolina Cisneros, Elena García Castillo, Francisco Javier García Pérez, Rosa María Girón, Celeste Marcos, and Enrique Zamora
-
•
Radiology: Patricia García García
-
•
Rheumatology: Santos Castañeda, Rosario García-Vicuña, Isidoro González-Álvaro, Sebastián Rodríguez-García, Carlos Fernández-Díaz, Irene Llorente Cubas, Eva G. Tomero, Noelia García Castañeda, Ana Ma Ortiz, Cristina Valero, Miren Uriarte, and Nuria Montes
Footnotes
This study was funded by Spanish Ministry of Economy, Industry and Competitiveness (MINECO) and Instituto de Salud Carlos III (grant nos. RD16/0011/0012 and PI18/0371 to I.G.A., grant no. PI19/00549 to A.A., and grant no. SAF2017-82886-R to F.S.-M.) and co-funded by the European Regional Development Fund. The study was also funded by “La Caixa Banking Foundation” (grant no. HR17-00016 to F.S.-M.) and “Fondos Supera COVID19” by Banco de Santander and CRUE. None of these sponsors have had any role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Disclosure of potential conflict of interest: S. de la C. Rodríguez-García reports grants from Spanish Rheumatology Foundation, during the conduct of the study; nonfinancial support from Roche, Lilly, Pfizer, and Abbvie; personal fees and nonfinancial support from Novartis, Sanofi, and MSD and from UCB-Pharma, outside the submitted work. C. Fernández-Díaz reports personal fees from BMS and nonfinancial support from Novartis, outside the submitted work. J. Ancochea reports grants and personal fees from GlaxoSmithKline and Boehringer Ingelheim; grants from Linde Healthcare; and grants, personal fees, and nonfinancial support from Roche and from Chiesi, outside the submitted work. D. A. Rodríguez-Serrano reports personal fees from MSD, outside the submitted work. R. de la Camara reports personal fees from MSD, ASTELLAS, Clinigen, Janssen, Roche, and IQONE Health Care outside the submitted work. R. García-Vicuña reports grants, personal fees, and nonfinancial support from Abbvie, BMS, Lilly, Novartis, Sanofi, Sandoz, and MSD; personal fees from Biogen and Celltrion and from Mylan, outside the submitted work; personal fees and nonfinancial support from Pfizer; grants from Roche; and grants and personal fees from Janssen. C. Suarez-Fernández reports personal fees from Bayer, BMS, Daichi Sankyo, MSD, and Pfizer, outside the submitted work. C. Muñoz-Calleja reports competitive grants from Instituto de Salud Carlos III during the conduct of the study. I. González-Álvaro reports grants from Instituto de Salud Carlos III, during the course of the study; personal fees from Lilly and Sanofi; personal fees and nonfinancial support from BMS and Abbvie; research support, personal fees, and nonfinancial support from Roche Laboratories; and nonfinancial support from MSD, Pfizer, and Novartis, not related to the submitted work. The rest of the authors declare that they have no relevant conflicts of interests.
Contributor Information
REINMUN-COVID Group:
Teresa Alvarado, Pablo Martínez, Francisco Javier de la Cuerda Llorente, Carmen del Arco, Juan Mariano Aguilar, Natalia Villalba, Mónica Negro, Elvira Contreras, Ana del Rey, Cristina Santiago, Manuel Junquera, Raquel Caminero, Francisco Javier Val, Sonia González, Marta Caño, Isabel López, Andrés von Wernitz, Bárbara Retana, Iñigo Guerra, Jorge Sorando, Lydia Chao, María José Cárdenas, Verónica Espiga, Pablo Chicharro, Pedro Rodríguez, Iñigo Hernando Alday, Miguel Sampedro, Jorge Prada, Eukene Rojo Aldama, Yolanda Real, María Caldas, Sergio Casabona, Aitor Lanas-Gimeno, Rafael de la Camara, Angela Figuera Alvárez, Beatriz Aguadol, Alberto Morell, Esther Ramírez, Amparo Ibáñez Zurriaga, María Pérez Abanades, Silvia Ruiz García, Tomás Gallego Aranda, María Ruiz, Concepción Martínez Nieto, José María Serra, Francisco Sánchez-Madrid, Cecilia Muñoz-Calleja, Arantzazu Alfranca, Javier Aspa, Ana Marcos-Jiménez, Santiago Sánchez-Alonso, Ana Alcaraz-Serna, Tamara Mateu-Albero, Ildefonso Sánchez-Cerrillo, Laura Esparcia, Pedro Martínez-Fleta, Celia López-Sanz, Ligia Gabrie, Luciana del Campo Guerola, Elena Fernández, Ma José Calzada, Reyes Tejedor, Alfonso Canabal, Patricia Albert, Diego A. Rodríguez-Serrano, Judit Iglesias, Fernando Suarez, Juan Antonio Sánchez, Beatriz Abad, Carmen Suarez, Ignacio de los Santos, José María Galván-Román, Emilia Roy, Pablo Rodríguez-Cortes, Lucio García-Fraile, Jesus Sanz, Eduardo Sanchez, Fernando Moldenhauer, Pedro Casado, Jose Curbelo, Angela Gutierrez, Azucena Bautista, Nuria Ruiz Giménez, Angelica Fernandez, Pedro Parra, Berta Moyano, Ana Barrios, Diego Real de Asua, Beatriz Sanchez, Carmen Saez, Marianela Ciudad, Desiré Navas, Laura Cardeñoso Domingo, María del Carmen Cuevas Torresano, Diego Domingo García, Teresa Alarcón Cavero, Alicia García Blanco, Alexandra Martín Ramírez, María Auxiliadora Semiglia Chong, Ainhoa Gutiérrez Cobos, Nelly Daniela Zurita Cruz, Arturo Manuel Fraile Torres, Carmen Sanchez-Gonzalez, Antonio Fernádez Perpén, Carolina Díaz Pérez, Julio Ancochea, Tamara Alonso, Pedro Landete, Joan Soriano, Carolina Cisneros, Elena García Castillo, Francisco Javier García Pérez, Rosa María Girón, Celeste Marcos, Enrique Zamora, Patricia García García, Santos Castañeda, Rosario García-Vicuña, Isidoro González-Álvaro, Sebastián Rodríguez-García, Carlos Fernández-Díaz, Irene Llorente Cubas, Eva G. Tomero, Noelia García Castañeda, Ana Ma Ortiz, Cristina Valero, Miren Uriarte, and Nuria Montes
Appendix
Table E1.
Baseline clinical characteristics of the study population and groups requiring vs not requiring IMV
| Characteristic | Study population (n = 146) | IMV |
||
|---|---|---|---|---|
| Required (n = 44) | Not required (n = 102) | P value | ||
| Age (y) | 63 (54-71) | 63.5 (56.5-72) | 62 (54-71) | .517 |
| Sex: male | 97 (66) | 32 (73) | 65 (64) | .291 |
| Comorbidities (1 [0.68%]) | 100 (69) | 30 (68) | 70 (69) | .893 |
| Hypertension (1 [0.68%]) | 55 (38) | 20 (45) | 35 (35) | .218 |
| Obesity (1 [0.68%]) | 23 (16) | 11 (25) | 12 (12) | .047 |
| Diabetes mellitus (1 [0.68%]) | 26 (18) | 8 (18) | 18 (18) | .883 |
| COPD (1 [0.68%]) | 9 (6) | 5 (11) | 4 (4) | .089 |
| Immune-mediated disease∗ (1 [0.68%]) | 8 (6) | 3 (7) | 5 (5) | .702 |
| History of malignancy† (1 [0.68%]) | 19 (13) | 5 (11) | 14 (14) | .792 |
| Others‡ (1 [0.68%]) | 76 (52) | 25 (57) | 51 (52) | .558 |
| Duration of symptoms at admission (d) (1 [0.68%]) | 6 (4-7) | 5 (5-7) | 7 (4-8) | .265 |
| Fever at admission (≥38ºC) (15 [10%]) | 36 (27) | 14 (32) | 21 (25) | .389 |
| Baseline PaO2/FiO2 (5 [3%]) | 215 (112-310) | 125.5 (75-207) | 247 (172-348) | <.001 |
| Treatment during hospitalization | ||||
| Hydroxychloroquine (2 [1.35%]) | 137 (96) | 38 (86) | 99 (100) | <.001 |
| Lopinavir/Ritonavir (2 [1.35%]) | 119 (83) | 38 (86) | 81 (82) | .502 |
| Azithromycin (2 [1.35%]) | 82 (57) | 24 (55) | 58 (59) | .652 |
| IFN-β (2 [1.35%]) | 7 (5) | 3 (7) | 4 (4) | .676 |
| Glucocorticoids (2 [1.35%]) | 85 (59) | 27 (61) | 58 (59) | .755 |
| Methylprednisolone bolus (1 [0.68%]) | 61 (42) | 21 (48) | 40 (40) | .414 |
| Laboratory findings | ||||
| White blood cell count (103/mm3) (9 [6%]) | 7.64 (5.25-10.68) | 9.39 (6.59-13.31) | 6.93 (5.13-8.78) | <.001 |
| Lymphocyte count (103/mm3) (10 [6.76%]) | 0.83 (0.60-11.7) | 0.74 (0.58-1.08) | 0.87 (0.62-1.26) | .029 |
| Creatinine (mg/dL) (7 [4.73%]) | 0.86 (0.70-1.10) | 0.99 (0.71-1.20) | 0.85 (0.72-1.1) | .398 |
| Bilirubin (mg/dL) (9 [6.08%]) | 0.55 (0.41-0.87) | 0.62 (0.44-1.15) | 0.53 (0.39-0.78) | .057 |
| AST (U/L) (9 [6.08%]) | 41 (28-64) | 43 (30-60) | 39 (27-70) | .526 |
| ALT (U/L) (9 [6.08%]) | 37 (24-68) | 30 (22-63) | 39 (24-71) | .287 |
| GGT (U/L) (26 [17.57%]) | 73 (36-159) | 81.5 (41-152) | 67 (36-159) | .623 |
| LDH (U/L) (14 [9.46%]) | 341 (256-461) | 413 (315-496) | 302 (224-443) | .001 |
| CK (U/L) (102 [69%]) | 72 (48-155) | 67 (39.50-167.50) | 94 (59-140) | .617 |
| Serum IL-6 (pg/mL) (7 [4.73%]) | 21.36 (7.53-54.21) | 49.20 (17.28-103.57) | 16.08 (6.09-42.03) | <.001 |
| Ferritin (ng/mL) (96 [64.86%]) | 1598 (830-2305) | 1665 (602-2765) | 1573 (1012-2300) | .832 |
| CRP (mg/dL) (23 [15.54%]) | 11.55 (5.16-22.53) | 17.09 (7.69-28.98) | 10.13 (4.83-18.48) | .003 |
| PCT (ng/mL) (52 [35.14%]) | 0.15 (0.10-0.35) | 0.29 (0.14-0.46) | 0.13 (0.08-0.26) | .001 |
| D-dimer (mg/mL) (25 [16.89%]) | 0.75 (0.48-1.48) | 0.92 (0.56-2.31) | 0.71 (0.48-1.19) | .058 |
| Radiologic findings (1 [0.68%]) | .209 | |||
| Clear | 11 (8) | 4 (9) | 8 (7) | |
| Diffuse ground glass opacities | 50 (35) | 13 (30) | 37 (37) | |
| Unilateral alveolar pattern | 20 (14) | 3 (7) | 17 (17) | |
| Bilateral alveolar pattern | 63 (43) | 24 (55) | 37 (37) | |
ALT, Alanine aminotransferase; AST, aspartate aminotransferase; CK, creatine kinase; GGT, gamma-glutamyl transferase; PCT, procalcitonin.
All categorical variables are expressed as number (%) and quantitative variables as median (p25-p75). Missing data in each clinical characteristic are expressed as (number [%]). Variables not disclosing it do not present any missing values.
Includes rheumatoid arthritis, systemic lupus erythematosus, ulcerative colitis, etc.
Includes solid organ and hematologic malignancies.
Includes obstructive sleep apnea syndrome, asthma, hypothyroidism, ischemic cardiomyopathy, etc.
Table E2.
Logistic regression model for IVM
| Variable | OR | P value | 95% CI |
|---|---|---|---|
| COPD | 5.407 | .030 | 1.172-24.938 |
| White blood cell count (103) | 1.050 | .116 | 0.998-1.115 |
| High IL-6 baseline serum levels | 7.087 | <.001 | 3.022-16.617 |
OR, Odds ratio.
High IL-6 was considered if >30 pg/mL.
Table E3.
Baseline clinical characteristics of groups treated vs not treated with TCZ
| Characteristic | TCZ |
||
|---|---|---|---|
| Treated (n = 58) | Not treated (n = 88) | P value | |
| Age (y) | 61 (54-70) | 64 (54-72) | .288 |
| Sex: male | 40 (69) | 57 (65) | .600 |
| Comorbidities (1 [0.68%]) | 35 (61) | 64 (73) | .124 |
| Hypertension (1 [0.68%]) | 17 (30) | 37 (42) | .124 |
| Obesity (1 [0.68%]) | 14 (25) | 9 (10) | .023 |
| Diabetes mellitus (1 [0.68%]) | 9 (16) | 16 (18) | .687 |
| COPD (1 [0.68%]) | 1 (2) | 8 (9) | .071 |
| Immune-mediated disease∗ (1 [0.68%]) | 5 (9) | 3 (3) | .181 |
| History of malignancy† (1 [0.68%]) | 7 (12) | 12 (14) | .763 |
| Others‡ (1 [0.68%]) | 30 (52) | 46 (52) | .892 |
| Duration of symptoms at admission (d) (1 [0.68%]) | 6 (5-7) | 7 (4-8) | .612 |
| Fever at admission (≥38ºC) (15 [10%]) | 17 (31) | 19 (25) | .483 |
| Baseline PaO2/FiO2 (5 [3%]) | 137 (88-232) | 248 (183-348) | <.001 |
| Treatment during hospitalization | |||
| Hydroxychloroquine (2 [1.35%]) | 53 (93) | 84 (98) | .171 |
| Lopinavir/Ritonavir (2 [1.35%]) | 51 (89) | 68 (79) | .103 |
| Azithromycin (2 [1.35%]) | 33 (58) | 49 (57) | .913 |
| IFN-β (2 [1.35%]) | 2 (4) | 5 (6) | .532 |
| Glucocorticoids (2 [1.35%]) | 38 (67) | 47 (55) | .152 |
| Methylprednisolone bolus (1 [0.68%]) | 31 (54) | 30 (35) | .018 |
| Laboratory findings | |||
| White blood cell count (103/mm3) (9 [6%]) | 7.99 (5.17-11.85) | 7.52 (5.4-10.36) | .527 |
| Lymphocyte count (103/mm3) (10 [6.76%]) | 0.74 (0.52-0.997) | 0.93 (0.66-1.47) | .001 |
| Creatinine (mg/dL) (7 [4.73%]) | 0.83 (0.70-1.05) | 0.90 (0.72-1.14) | .177 |
| Bilirubin (mg/dL) (9 [6.08%]) | 0.62 (0.46-1.04) | 0.52 (0.38-0.78) | .070 |
| AST (U/L) (9 [6.08%]) | 47 (29-77.50) | 34.5 (28-53) | .047 |
| ALT (U/L) (9 [6.08%]) | 37.5 (25-77) | 36 (22.5-64.5) | .588 |
| GGT (U/L) (26 [17.57%]) | 73 (41-186) | 71 (35-145) | .374 |
| LDH (U/L) (14 [9.46%]) | 425 (302-510) | 293.50 (221.50-388.50) | <.001 |
| CK (U/L) (102 [69%]) | 69 (38-270) | 75.5 (49-125) | .785 |
| Serum IL-6 (pg/mL) (7 [4.73%]) | 41.85 (12.37-71.95) | 16.25 (6.27-44.95) | .007 |
| Ferritin (ng/mL) (96 [64.86%]) | 1888 (1152-2844) | 1461 (471-1861) | .038 |
| CRP (mg/dL) (23 [15.54%]) | 13.73 (8.75-27.08) | 9.09 (4.78-19.31) | .005 |
| PCT (ng/mL) (52 [35.14%]) | 0.25 (0.13-0.36) | 0.14 (0.1-0.3) | .045 |
| D-dimer (mg/mL) (25 [16.89%]) | 0.75 (0.48-1.48) | 0.71 (0.53-1.22) | .491 |
| Radiologic findings (1 [0.68%]) | .818 | ||
| Clear | 4 (7) | 8 (8) | |
| Diffuse ground glass opacities | 18 (32) | 32 (37) | |
| Unilateral alveolar pattern | 10 (18) | 10 (11) | |
| Bilateral alveolar pattern | 25 (44) | 37 (43) | |
ALT, Alanine aminotransferase; AST, aspartate aminotransferase; CK, creatine kinase; GGT, gamma-glutamyl transferase; PCT, procalcitonin.
All categorical variables are expressed as number (%) and quantitative variables as median (p25-p75). Missing data are expressed as (number [%]). Variables not disclosing it do not present any missing values.
Includes rheumatoid arthritis, systemic lupus erythematosus, ulcerative colitis, etc.
Includes solid organ and hematologic malignancies
Includes obstructive sleep apnea syndrome, asthma, hypothyroidism, ischemic cardiomyopathy, etc.
Table E4.
Variables explaining evolution of PaO2/FiO2
| Variable | β coefficient | P value | 95% CI |
|---|---|---|---|
| Baseline PaO2/FiO2 | 0.713 | <.001 | 0.567 to 0.753 |
| Lymphocyte count | 0.033 | <.001 | 0.015 to 0.052 |
| Hypertension | −19.059 | .067 | −39.432 to 1.314 |
| LDH | −0.101 | .001 | −0.163 to −0.039 |
| CRP | −1.188 | .038 | −2.311 to 0.065 |
| Radiologic findings | |||
| Clear | Reference | ||
| Diffuse ground glass opacities | 49.256 | .006 | 13.785 to 84.727 |
| Unilateral alveolar pattern | 31.638 | .131 | −9.378 to 72.654 |
| Bilateral alveolar pattern | 60.708 | .001 | 25.986 to 95.431 |
| Others | −123.818 | .113 | −276.853 to 29.217 |
| IL-6 serum/TCZ∗ | |||
| Low IL-6, no TCZ | Reference | ||
| Low IL-6, early TCZ | −38.854 | .048 | −77.390 to −0.319 |
| Low IL-6, late TCZ | −40.150 | .019 | −73.765 to −6.535 |
| High IL-6, no TCZ | −16.513 | .279 | −46.440 to 13.413 |
| High IL-6, early TCZ | −8.134 | .672 | −45.831 to 29.562 |
| High IL-6, late TCZ | −38.359 | .021 | −70.837 to −5.881 |
IL-6 serum/TCZ: refers to the interaction between both high or low IL-6 (cutoff 30 pg/mL) and TCZ (no treatment, early or late treatment; cutoff 11 d since symptom onset) as an independent predictor within the model.
References
- 1.Bhatraju P.K., Ghassemieh B.J., Nichols M., Kim R., Jerome K.R., Nalla A.K., et al. Covid-19 in critically ill patients in the Seattle region - case series. N Engl J Med. 2020;382:2021–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lescure F.X., Bouadma L., Nguyen D., Parisey M., Wicky P.H., Behillil S., et al. Clinical and virological data of the first cases of COVID-19 in Europe: a case series. Lancet Infect Dis. 2020;20:697–706. doi: 10.1016/S1473-3099(20)30200-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siddiqi H.K., Mehra M.R. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant. 2020;39:405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Jong M.D., Simmons C.P., Thanh T.T., Hien V.M., Smith G.J., Chau T.N., et al. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat Med. 2006;12:1203–1207. doi: 10.1038/nm1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le R.Q., Li L., Yuan W., Shord S.S., Nie L., Habtemariam B.A., et al. FDA approval summary: tocilizumab for treatment of chimeric antigen receptor T cell-induced severe or life-threatening cytokine release syndrome. Oncologist. 2018;23:943–947. doi: 10.1634/theoncologist.2018-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang W., Ye L., Ye L., Li B., Gao B., Zeng Y., et al. Up-regulation of IL-6 and TNF-alpha induced by SARS-coronavirus spike protein in murine macrophages via NF-kappaB pathway. Virus Res. 2007;128:1–8. doi: 10.1016/j.virusres.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu X., Han M., Li T., Sun W., Wang D., Fu B., et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A. 2020;117:10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:1–11. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waterer G.W., Rello J., Wunderink R.G. COVID-19: first do no harm. Am J Respir Crit Care Med. 2020;201:1324–1325. doi: 10.1164/rccm.202004-1153ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.European Medicines Agency RoActemra, INN-tocilizumab: summary of product characteristics. 2013. https://www.ema.europa.eu/en/documents/product-information/roactemra-epar-product-information_en.pdf Available at:
- 14.De Luna G., Habibi A., Deux J.F., Colard M., d’Alexandry d’Orengiani A., Schlemmer F., et al. Rapid and severe Covid-19 pneumonia with severe acute chest syndrome in a sickle cell patient successfully treated with tocilizumab. Am J Hematol. 2020;95:876–887. doi: 10.1002/ajh.25833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang X., Song K., Tong F., Fei M., Guo H., Lu Z., et al. First case of COVID-19 in a patient with multiple myeloma successfully treated with tocilizumab. Blood Adv. 2020;4:1307–1310. doi: 10.1182/bloodadvances.2020001907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang C., Wu Z., Li J.W., Zhao H., Wang G.Q. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor (IL-6R) antagonist tocilizumab may be the key to reduce the mortality. Int J Antimicrob Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.ClinicalTrials.gov Search: COVID19 AND tocilizumab. 2020. https://clinicaltrials.gov/ct2/results?cond=covid19+tocilizumab&term=&cntry=&st%20ate=&city=&dist= Available at:
- 18.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention [published online ahead of print 2020]. JAMA. https://doi.org/10.1001/jama.2020.2648. [DOI] [PubMed]
- 19.Wang W., Xu Y., Gao R., Lu R., Han K., Wu G., et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323:1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The Spanish Agency for Medicine and Health Products [Agencia Española de Medicamentos y Productos Sanitarios (AEMPS)] Treatments available for the management of respiratory infection by SARS-CoV-2 [Tratamientos disponibles para el manejo de la infección respiratoria por SARS-CoV-2] 2020. https://www.aemps.gob.es/laAEMPS/docs/medicamentos-disponibles-SARS-CoV-2-16-4-2020.pdf Available at:
- 22.Severinghaus J.W. Simple, accurate equations for human blood O2 dissociation computations. J Appl Physiol Respir Environ Exerc Physiol. 1979;46:599–602. doi: 10.1152/jappl.1979.46.3.599. [DOI] [PubMed] [Google Scholar]
- 23.Garcia Borrega J., Gödel P., Rüger M.A., Onur Ö.A., Shimabukuro-Vornhagen A., Kochanek M., et al. In the eye of the storm: immune-mediated toxicities associated with CAR-T cell therapy. Hemasphere. 2019;3:e191. doi: 10.1097/HS9.0000000000000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Norelli M., Camisa B., Barbiera G., Falcone L., Purevdorj A., Genua M., et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat Med. 2018;24:739–748. doi: 10.1038/s41591-018-0036-4. [DOI] [PubMed] [Google Scholar]
- 25.Pires-Neto R.C., Morales M.M., Lancas T., Inforsato N., Duarte M.I., Amato M.B., et al. Expression of acute-phase cytokines, surfactant proteins, and epithelial apoptosis in small airways of human acute respiratory distress syndrome. J Crit Care. 2013;28:111.e9–111.e15. doi: 10.1016/j.jcrc.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 26.Matthay M.A., Zemans R.L., Zimmerman G.A., Arabi Y.M., Beitler J.R., Mercat A., et al. Acute respiratory distress syndrome. Nat Rev Dis Primers. 2019;5:18. doi: 10.1038/s41572-019-0069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao Y., Li T., Han M., Li X., Wu D., Xu Y., et al. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J Med Virol. 2020;92:791–796. doi: 10.1002/jmv.25770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishimoto N., Terao K., Mima T., Nakahara H., Takagi N., Kakehi T. Mechanisms and pathologic significances in increase in serum interleukin-6 (IL-6) and soluble IL-6 receptor after administration of an anti-IL-6 receptor antibody, tocilizumab, in patients with rheumatoid arthritis and Castleman disease. Blood. 2008;112:3959–3964. doi: 10.1182/blood-2008-05-155846. [DOI] [PubMed] [Google Scholar]
- 29.Roche Roche provides an update on the phase III COVACTA trial of Actemra/RoActemra in hospitalised patients with severe COVID-19 associated pneumonia. 2020. https://www.roche.com/investors/updates/inv-update-2020-07-29.htm Available at:
- 30.Sanofi-Genzyme Sanofi provides update on Kevzara® (sarilumab) phase 3 trial in severe and critically ill COVID-19 patients outside the U.S. 2020. https://www.sanofigenzyme.com/en/about-us/newsroom/2020/2020-09-01-00-00-00 Available at:
- 31.Sciascia S., Apra F., Baffa A., Baldovino S., Boaro D., Boero R., et al. Pilot prospective open, single-arm multicentre study on off-label use of tocilizumab in patients with severe COVID-19. Clin Exp Rheumatol. 2020;38:529–532. [PubMed] [Google Scholar]
- 32.Luo P., Liu Y., Qiu L., Liu X., Liu D., Li J. Tocilizumab treatment in COVID-19: a single center experience. J Med Virol. 2020;92:814–818. doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reference
- https://covid19.isciii.es/resources/CURVASTATUS.png Ministry of Health of Spain. Daily evolution of COVID-19 in Spain 2020. Available at: Accessed April 9, 2020.