Abstract
This study is aimed at determining the relationship of flavonoid structures to their chemical and intracellular antioxidant activities. The antioxidant activities of 60 flavonoids were investigated by three different antioxidant assays, including 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity, oxygen radical absorption capacity (ORAC), and cellular antioxidant activity (CAA) assays. The result showed 6 flavonoids as good cellular antioxidants evaluated for the first time. The cellular antioxidant activities of compounds 7-methoxy-quercetin, 3-O-methylquercetin, 8-hydroxy-kaempferol, quercetin-3-O-α-arabinofuranose, kaempferol-7-O-glucopyranoside, and luteolin6-C-glucoside were linked with the upregulation of antioxidant enzyme activities (superoxide dismutase, catalase, and glutathione peroxidase). A structure-activity relationship suggested that 2,3-double bond, 4-keto groups, 3′,4′-catechol structure, and 3-hydroxyl in the flavonoid skeleton played important roles in the antioxidant behavior. Furthermore, the cell proliferative assay revealed a low cytotoxicity for 3-O-methylquercetin. The present results provide valuable information for the dietary application of flavonoids with different structures for high antioxidant.
1. Introduction
The reactive oxygen species (ROS) are known to damage the tissues of the body, which leads to disturb the established order on the body system. The ROS attacked biomolecules like DNA, lipids, and proteins to free radical damage, which stimulated the development of many diseases, such senility, angiocardiopathy, and cancer [1]. At present, researchers have found that flavonoid consumption can improve cancer and cardiovascular diseases [2]. There are inverse relationship between dietary flavonoids and chronic diseases, which displayed the importance of studying flavonoids [3].
Flavonoids is one of the most abundant phenolic compounds in various fruits, vegetables, grains, spices, beverages, and medicinal plants, which are structured by a C6-C3-C6 skeleton labeled with the rings A, B, and C (Table 1). The subclasses included flavones, flavonols, flavanones, flavanols, anthocyanidins, and isoflavonoids [4]. Many researchers have discovered a wide range of biological activities of the flavonoids in prevention and relieve various diseases such as obesity, diabetes, cancer, angiocardiopathy, and heart diseases [5–7]. Therefore, the flavonoids were considered to be candidates for these disease management due to the ROS and iNOS caused [8]. The capacity of flavonoids depends on their substituent groups, the number of hydroxyl groups, other substitutions, and conjugations. In addition, quercetin, kaempferol, rutin, hesperidin, naringin, genistein, phloretin, isoquercitrin, taxifolin, epicatechin, cyanidin chloride, and their derivatives were widely distributed in apples, blueberries, cherries, grapes, tea, citrus, peppers, red wine, chocolate, etc., which has extensive biological activity [9–11]. However, to our knowledge, systematic studies on differences in the antioxidant ability of various flavonoids and the structure-activity relationships are still scarce. In particular, the influence between different structural flavonoids and the antioxidant enzyme activities (superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px)) has rarely been studied.
Table 1.
The chemical structures of 60 flavonoids.
| No | Flavonoids | Core structure | Substructure |
|---|---|---|---|
| Flavone |
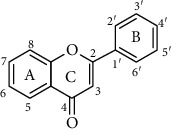
|
R=H | |
| 1 | Isorhamnetin | R3, R5, R7, R4′=OH, R5′=OCH3 | |
| 2 | Rhamnetin | R3, R5, R4′, R5′=OH, R7=OCH3 | |
| 3 | Kaempferide | R3, R5, R7=OH, R4′=OCH3 | |
| 4 | Morin | R3, R5, R7, R4′, R6′=OH | |
| 5 | 3-O-methylquercetin | R3= OCH3, R5, R7, R4′, R6′=OH | |
| 6 | Kaempferol | R3, R5, R7, R4′=OH | |
| 7 | Quercetin | R3, R5, R7, R4′, R5′=OH | |
| 8 | Herbacetin | R3, R5, R7, R8, R4′=OH | |
| 9 | Myricitrin | R3=Orha, R5, R7, R3′, R4′, R5′=OH | |
| 10 | Avicularin | R3=Oara, R5, R7, R3′, R4′=OH | |
| 11 | Trifolin | R3=Oglc, R5, R7, R3′=OH | |
| 12 | Kaempferol-4′-O-glucopyranoside | R3, R5, R7=OH, R4′=Oglc | |
| 13 | Kaempferol-7-O-glucopyranoside | R3, R5=OH, R7=Oglc, R4′=OH | |
| 14 | Kaempferol-3-O-arabinoside | R3=Oara, R5, R7, R3′=OH | |
| 15 | Isorhamnetin-3-O-glucopyranoside | R3=Oglc, R5, R7, R3′=OH, R4′=OCH3 | |
| 16 | Rutin | R3=Orha, R5, R7, R4′, R5′=OH | |
| 17 | Spiraeoside | R3, R5, R7, R5′=OH, R4=Oglc | |
| 18 | Myricetin | R3, R5, R7, R3′, R4′, R5′=OH | |
| 19 | Tangeretin | R5, R6, R7, R8, R4′=OCH3 | |
| 20 | Chrysin | R5, R7=OH | |
| 21 | Baicalein | R5, R6, R7=OH | |
| 22 | Apigenin | R5, R7, R4′=OH | |
| 23 | Luteolin | R5, R7, R3′, R4′=OH | |
| 24 | Cynaroside | R7=Oglc, R3′, R4′=OH | |
| 25 | Myricetin-3-O-galactoside | R3=Ogal, R5, R7, R3′, R4′, R5′=OH | |
| 26 | Quercetin-3-O-galactoside | R3=Ogal, R5, R7, R3′, R4′′=OH | |
| 27 | Quercetin-3-O-rhamnoside | R3, R5=OH, R7=Orha, R3′, R4′=OH | |
| 28 | Quercitrin | R3=Orha, R5, R7, R3′, R4′=OH | |
| 29 | Isoquercitrin | R3=Oglc, R5, R7, R3′, R4′=OH | |
| 30 | Vitexin | R5=Cglc, R6, R8, R4′=OH | |
| 31 | Orientin | R8=Cglc, R5, R7, R3′, R4′=OH | |
| 32 | Isoorientin | R4=Cglc, R5, R7, R3′, R4′=OH | |
| 33 | Isovitexin | R5, R7, R4′=OH, R6=Cglc | |
| 34 | Galangin | R3, R5, R7=OH | |
| 35 | Fisetin | R3, R7, R3′, R4′=OH | |
| 36 | Diosmetin | R5, R7, R3′=OH, R4′=OCH3 | |
| 37 | Genkwanin flavanones |
R5, R4′=OH, R7=OCH3 R=H |
|
|
| |||
| 38 | Dihydromyricetin |
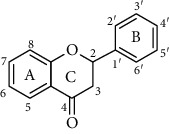
|
R3, R5, R7, R3′, R4′, R5′=OH |
| 39 | Taxifolin | R3, R5, R7, R4′, R5′=OH | |
| 40 | Dihydromorin | R3, R5, R7, R4′=OH | |
| 41 | Neohesperidin | R5, R3′=OH, R7=Oglcgla, R5′=OCH3 | |
| 42 | Narirutin | R7=Oglcgla, R4′=OH | |
| 43 | Hesperetin | R5, R7, R4′=OH, R5′=OCH3 | |
| 44 | Hesperidin | R5, R5′=OH, R7=Oglcgla, R4′=OCH3 | |
| 45 | Naringenin | R5, R7, R4′=OH | |
| 46 | Liquiritigenin | R7, R4′=OH | |
|
| |||
| Chalcone |
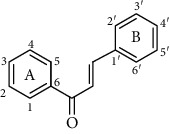
|
R=H | |
| 47 | Neohesperidin dihydrochalcone | R3, R3′, R6′=OH, R4′=Oglcgla | |
| 48 | Phloretin | R1, R3, R5, R4′=OH | |
| 49 | Phlorizin | R1, R3, R4′=OH, R5=Oglc | |
| 50 | Isoliquiritigenin | R1, R3, R4′=OH | |
|
| |||
| Anthocyanidin |
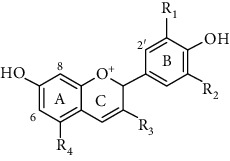
|
R=H | |
| 51 | Cyanidin chloride | R1=OH | |
| 52 | Delphinidin chloride | R2, R3=OH | |
| 53 | Cyanin chloride | R1=OH, R2=H, R3, R4=Oglc | |
| 54 | Cyanidin-3-O-glucoside chloride | R1=OH, R2=H, R3=Oglc | |
| 55 | Pelargonin chloride | R1, R2=H, R3, R4=Oglc | |
| 56 | Oenin chloride | R1, R2=OCH3, R3=Oglc | |
| 57 | Malvin | R1, R2=OCH3, R3, R4=Oglc | |
|
| |||
| Flavans |
|
R=H | |
| 58 | Epicatechin | R3, R5, R7, R4′, R5′=OH | |
| 59 | Catechin | R3, R5, R7, R4′, R5′=OH | |
| 60 | Epigallocatechin gallate | R3=gallic acid, R5, R7, R3′, R4′, R5′=OH | |
Orha: -O-α-L-rhamnopyranoside; Oara: -O-α-L-arabinofuranoside; Oglc: -O-glucopyranoside; Ogal: -O-β-L-galactopyranoside; Cglc: -C-glucopyranoside; Oglcgla: -O-(6-deoxy-α-L-mannopyranosyl)-β-D-glucopyranoside. The values having no letters in common are significantly different (P < 0.05). R is the number in core structure.
Therefore, we have chosen 60 flavonoids, which have the diversity of their core structures and substitution patterns, which contribute to systematic studies on the differences in chemical and cell-based antioxidant assays in this work. The antioxidant activities of a series of flavonoids (Table 1) which are commonly found in diet, including flavones, flavonols, flavanones, flavanols, flavanes, chalcones, and anthocyanidins, were examined by 2,2-diphenyl-1-picrylhydrazyl radical scavenging activity, oxygen radical absorption capacity, and cellular antioxidant activity assays. The structure-activity relationship of different structures of dietary flavonoids was analyzed for obtaining the substructures with high antioxidant activity. The cellular antioxidant activity assay was closer to physiological conditions for giving an extensive evaluation of the antioxidant. Moreover, the cytotoxicity and antiproliferative activity assays were also measured. This study has provided the theoretical foundation for the structural modification of flavonoids as effective antioxidant.
2. Material and Methods
2.1. Chemical and Reagents
Dimethyl sulfoxide (DMSO), 2,2-diphenyl-1-picrylhydrazyl (DPPH), Trolox, fluorescein sodium salt, 2′,7′-dichlorfluorescin diacetate (DCFH–DA), and 2,2-azobis (2-amidinopropane) dihydrochloride solution (ABAP) were purchased from Sigma Chemical Co. (Sigma-Aldrich, St. Louis, MO, USA). Flavonoid standards were purchased from Solarbio Science & Technology Co., Ltd. (Beijing, China). Phosphate buffer (PBS), MEM/EBSS, foetal bovine serum (FBS), penicillin, and streptomycin were purchased from HyClone (Logan, UT, USA). Cell Counting Kit-8 was obtained from Dojindo China Co., Ltd. (Shanghai, China). Kits for the determination of superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and catalase (CAT) were purchased from Beyotime Biotechnology (Shanghai, China).
2.2. Oxygen Radical Antioxidant Capacity (ORAC) Assay
The ORAC assay was evaluated as previously described by Cao et al. with some modifications [12, 13]. 50 μL of samples or Trolox with different concentrations and the fluorescein solution was added to a 96-well microplate, which was incubated at 37°C for 10 min. Then, 50 μL of 119 mM AAPH (freshly prepared) was added to each well. The fluorescence generation was measured using a microplate reader at excitation of 485 nm and emission of 520 nm for 60 cycles every 2 min. The ORAC values were calculated by the regression equation between the Trolox concentration and the net area under the curve (expressed as μmol Trolox eq/μmol sample).
2.3. DPPH Radical Scavenging Activity
This assay was conducted as previously described by Wen et al. with some modifications [14]. DPPH was freshly prepared in methanol at a concentration of 0.1 mM. The solution (20 μL) containing the tested compounds with different concentrations was added into the DPPH solution (180 μL) in the 96-well plates. The plates were incubated at 37°C for 30 min in the dark, and the absorbance value was recorded at 515 nm. The IC50 value was calculated on the scavenging activity against DPPH radical.
2.4. Cellular Antioxidant Activity
2.4.1. Determination of Cellular Antioxidant Activity (CAA)
The CAA assay was tested as described previously [15]. 6 × 104 cells/well of HepG2 cells were seeded at a 96-well microplate with 100 μL of growth medium/well. The cells were primarily treated with 100 μL of medium containing the tested compounds and DCFH-DA (25 μM) for 1 h at 37°C. Then, the cells were washed with PBS and treated with 100 μL of 600 μM ABAP (dissolved in HBSS), and the 96-well microplate was immediately placed into an Infinite SpectraMax i3x Multi-Mode Detection plate-reader at 37°C. The fluorescence reading was measured at an emission of 535 nm and excitation of 485 nm every 5 min for 1 h. Quercetin was used as positive control; the EC50 values were expressed in micromoles of quercetin equivalents per 100 μmol of tested compounds (μmol QE/100 μmol of sample).
2.4.2. Activity Determinations of Cellular Antioxidant Enzymes
HepG2 cells were seeded (1 × 106 cells/well) in six-well plates. After incubation for 24 h, the cells were pretreated with different concentration samples. Medium was washed by PBS and treated with 600 μM ABAP. The cells were collected and treated with cell lysis buffer (20 mM Tris at pH 7.5, 150 mM NaCl and 1% Triton X-100) at 4°C. The lysed cells were used to measure the intracellular activities of SOD, CAT, and GSH-Px by kits according to the manufacturer instructions (Wen 2015). Cells without sample and ABAP treatment were used as positive control (PC), while cells treated with ABAP but not sample were used as negative control (NC).
2.4.3. Cytotoxicity and Antiproliferative Activity Assays
The cytotoxicity and antiproliferative activity assays were performed by using the CCK-8 assay kit [16]. Briefly, HepG2 cells were cultured at a density of 4 × 104 cells/well or 2.5 × 104 cells/well in a 96-well microplate with growth medium. After incubation at 37°C, the growth medium is treated with 100 μL of growth medium containing different concentrations of tested compounds for 24 h or 72 h. The wells having growth medium without the tested compound served as control. Then, the cells were incubated with 10 μL/well CCK-8 solutions for 2 h at 37°C. The absorbance values of each well were measured at 450 nm using a microplate reader (SpectraMax i3x, ForteBio Analytics Co., Ltd., USA). The cytotoxic activity and antiproliferative effects of the tested compound was calculated as
| (1) |
| (2) |
where As is the absorbance of the well with compound; Ac is the absorbance of control.
2.5. Statistical Analysis
All data were presented as mean ± standard deviation for triplicate analyses (n = 3). One-way analysis of variance (ANOVA) was used to compare the means. Differences were considered significant at P < 0.05. All statistical analysis was performed using IBM SPSS statistical software 21.0 (IBM Corporation, NY, USA).
3. Results and Discussion
3.1. Antioxidant Capacity
3.1.1. Chemical Antioxidant Activity
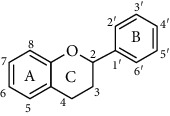
The antioxidant activities of flavonoids were assessed by ORAC and DPPH assays. Quercetin, a well-known antioxidant, was used as positive control. The ORAC assay is based on the oxidation of a fluorescent probe (fluorescein) by radicals coming from the spontaneous decomposition of AAPH. The ORAC process is a classical oxidation process for hydrogen atom transfer [17]. As shown in Figure 1(a), strong oxygen radical absorbance capabilities were observed in compounds 2, 4-8, 16, 18, 22-23, 26, 30, 35-36, 38-40, 44-45, 47, 49, 51-52, 54, and 57-60, with their ORAC values ranging from 4.07 to 12.85 μmol TE/μmol. Among the compounds, compound 16 (12.85 ± 0.42 μmol TE/μmol) was found to possess the highest peroxyl radical scavenging activity, followed by compounds 30, 18, 44, 49, and 60 (6.80 ± 0.42, 6.64 ± 0.03, 6.52 ± 0.15, 6.43 ± 0.14, 6.02 ± 0.14 μmol TE/μmol, respectively). Compounds 2, 4-8, 22-23, 26, 35-36, 38-40, 45, 47, 51, 52, 54, and 56-59 were not significantly different from compound 60. The ORAC values of compounds 1, 3, 9-15, 17, 19-21, 24-25, 27-29, 31-34, 37, 41-43, 46, 48, 50, 53, and 55 ranged from 0.21 to 3.97 μmol TE/μmol (Figure 1(b)). However, compound 19 (0.21 ± 0.01 μmol TE/μmol) had the lowest antioxidant activities in the ORAC assay.
Figure 1.
The antioxidant activities of flavonoids determined by ORAC (a, b), DPPH (c), and the cellular antioxidant (d) assays. The IC50 and EC50 of compounds that were not in the Figure were >200 μM. The data are presented as the mean with standard deviation (SD) bar of three replicates. The values having no letters in common are significantly different (P < 0.05). The data was listed in Table S1.
DPPH assay is based on the reduction of DPPH• in the presence of a hydrogen-donating antioxidant, leading to form DPPHH. The DPPH radical scavenging activities of tested flavonoids are shown in Figure 1(c). Compounds 2, 7, 9-11, 16, 18, 23, 25-28, 35, 39, 51-52, 58, and 60 exhibited a strong DPPH radical scavenging activity with their IC50 value ranging from 19.13 to 96.03 μM, while compounds 1 and 59 (126.48 ± 4.26, 129.99 ± 5.55 μM, respectively) had a much lower radical scavenging activity. The others had no antioxidant activity. Among the tested flavonoids, compounds 2, 7, 18, 35, 52, and 60 were found to possess the highest DPPH radical scavenging activity (34.03 ± 0.61, 21.52 ± 1.90, 21.26 ± 1.33, 25.25 ± 0.62, 36.83 ± 4.26, 19.13 ± 0.62 μM, respectively), followed by compounds 9-11, 16, 23, 25-28, 39, 51, and 58, which were 50.87 ± 2.14, 71.68 ± 0.06, 45.07 ± 2.12, 69.97 ± 1.44, 73.23 ± 0.75, 82.41 ± 2.88, 53.34 ± 2.64, 47.68 ± 1.60, 68.26 ± 1.37, 59.55 ± 3.12, 70.80 ± 2.31, 96.03 ± 0.13 μM, respectively.
3.1.2. Cellular Antioxidant Activity
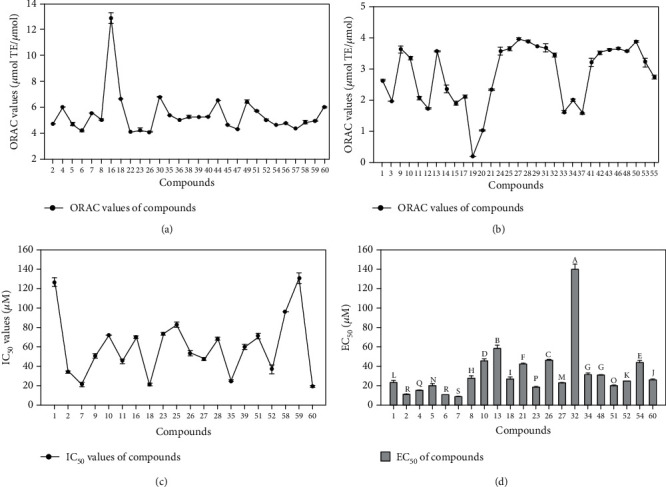
Chemical antioxidant assays are difficult to exactly reflect the antioxidant activity in vivo. Comparatively, the advantage of CAA assay was to simulate cellular biological processes which include uptake, distribution, and metabolism. CAA assay was conducted to quantify the capacity of the analyte to prevent the formation of DCF by AAPH-induced peroxyl free radical in HepG2 cells. The level of cellular fluorescence in CAA assay was relevant to the degree of the DCFH oxidation, which demonstrated that a decrease in fluorescence caused by the analyte shows a cellular antioxidant capacity [18]. The cellular antioxidant activities of compounds 2, 5, 8, 10, 13, and 32 were identified for the first time in this work. The kinetics of DCFH oxidation in HepG2 cells induced by peroxyl radicals are displayed in Figure 2. The results illustrated that the increase in fluorescence due to DCF formation was inhibited by tested flavonoids in a dose-dependent manner.
Figure 2.
Peroxyl radical-induced oxidation of DCFH to DCF in HepG2 cells and the inhibition of oxidation by compounds 2 (a), 5 (b), 8 (c), 10 (d), 13 (e), and 32 (f) over time, using the protocol having no PBS wash.
The EC50 of the compounds are listed in Figure 1(d). In this study, the antioxidant activity of compound 2 was as good as the positive reference, quercetin, which EC50 was 9.84 ± 0.34 μM. The EC50 values of compound 5, 8, 10, 13, and 32 were 19.53 ± 1.48, 27.12 ± 2.47, 45.12 ± 2.12, 57.78 ± 3.12, and 139.21 ± 5.21 μM, respectively.
Compound 2 showed an unexpected effect on the inhibition of DCF formation. Compounds 1 2, 4, 5, 6, 8, 10, 13, 21, 23, 26, 27, 32, and 34 have a similar structure to quercetin had and no hydroxyls exist on C-3, C-3′, and C-5′. The structure difference led to apparent changes in the cellular antioxidant assay. Quercetin (7) and compound 2 had a strong cellular antioxidant activity. The loss of C-3′ or C-5′ hydroxyls influenced the cellular antioxidant activity [19]. The loss of C-3′ or C-5′ hydroxyls destroys the ortho-dihydroxyl structure and thereby decreases the antioxidant activity because ortho-dihydroxyl contributes much to the radical scavenging effect of flavonoid [20]. Therefore, compound 1 [21], compound 4 [22], compound 6 [18], compounds 8, 13, 21 [23], and 34 [24] showed a significant difference from compounds 2 and 7. The loss of 3-hydroxyl moiety also decreased the cellular antioxidant activity, as indicated by compounds 5, 10, 21 [23], 23 [18], compounds 26 [25] and 32. Compounds 48 [18], 51, 52 [26], and 60 [18] had strong activities on account of the number of hydroxyl group. Moreover, an additional 5′-hydroxyl group in the B-ring, as seen compound 18 [18], has been revealed to decrease antioxidant activity. The presence of O-glycoside decreased the antioxidant activity, as indicated by compounds 7 and 27 [25].
A significant cellular antioxidant effect was observed for compounds 2, 5, 8, 10, and 13 which showed a consistent dose-dependent antioxidant effect. Unlike other methods commonly used for measuring chemical antioxidant activity, this assay has been developed a more biologically representative protocol. Antioxidants can act at the cell membrane to break peroxyl radical chain reactions at the cell surface or can be uptaken by the cell and react with ROS intracellularly [19]. The efficiencies of membrane binding and cell uptake are two important factors influencing the antioxidant activity of the tested chemical.
It is noteworthy that although the CAA assay represents a reliable and cost-effective approach to evaluate the potential biological activity of dietary flavonoids on cellular level and conveys important reference value to the functional food development, it does not fully reflect the in vivo metabolism of these compounds. The metabolic process of food-derived polyphenols in the human body could be complicated because they might be extensively degraded and metabolized by various gut enzymes and microflora. The resulting metabolic products of dietary flavonoids would also contribute to biological activities once they are released into the systemic circulation [27].
3.2. Structure-Antioxidant Activity Relationship
3.2.1. Hydroxyl Groups
The spatial arrangement of substituents is more important than the flavan backbone alone in the antioxidant activity. Consistent with most polyphenolic antioxidants, both the number and positioning of the B-ring hydroxyl groups in flavonoids substantially influence the mechanisms of antioxidant activity. Especially, a 3′,4′-catechol structure in the B-ring strongly enhances the antioxidant activity [28]. In the CAA assay, compound 7 (quercetin), which has a 3′,4′-O-dihydroxyl group, had the highest activity with an EC50 of 8.77 ± 0.09 μM. Compounds 2, 5, and 23 had the same skeleton with small moiety differences, which had only slightly lower activities than quercetin. Compound 60 had strong activities on account of the number of hydroxyl group. Compounds 4 and 40, which have two hydroxyl groups in the B-ring, had much lower activity (15.23 ± 0.32, >200 μM) than quercetin. The presence of an m-diphenolic moiety reduced activity compared to the ortho configuration in the previous study [19]. The presence of the ortho-dihydroxyl group in the B-ring has stabilized the antioxidant performance owing to participating electron delocalization and hydrogen bonds between 3′- and 4′-hydroxyls [29]. Compared to quercetin, the 5′-hydroxyl group of compound 18 decreased the cellular antioxidant activity; however, the DPPH radical scavenging activity and ORAC activity were little changed. Compounds 58-59 had lower antioxidant activity than compound 60. In the DPPH and ORAC assays, compounds 2, 23, and 60 showed good activity, which owned hydroxyls but not be affected by other groups. The compounds 4, 5, 40, 58, and 59 have good activity in ORAC assay and lower DPPH radical scavenging activity, but compounds 4 and 5 gained good cellular antioxidant activity, which illustrated the other groups, membrane association, and uptake in cell also played important roles in different antioxidant assays. The presence of a galloyl group in the compound 60 imparted it with high activity in all assays. These results indicate that 3′,4′-O-dihydroxyl group is an important structure feature of substantial antioxidant activity for flavonoids in the CAA assay. This finding was in consistent with the results of DPPH and ORAC assay. Previous researches also suggested that a B-ring catechol group is essential for high antioxidant activity [30, 31].
3.2.2. C/O-Glycoside and O-Methylation
Moreover, an additional C/O-glycoside or O-methylation, as seen in compounds 1, 3, 9-17, 19, 25-33, 36, 41-44, 48-49, and 56-57, has been revealed to decrease antioxidant activity on account of a prooxidant counteracting their antioxidant effect [32]. Compounds 9-17, 25-33, 41-44, 48-49, and 56-57 showed lower cellular antioxidant activity than their aglycones, which indicated the C/O-glycoside decreased the antioxidant activity [6]. This finding was in consistent with the results of DPPH and ORAC assay. Owing to the O-methylation group, compounds 1, 3, 19, and 36 had lower antioxidant activities in three assays. Compounds 9-11, 25, 28-33, 41-44, 48-49, and 56-57 have good ORAC activity and lower cellular antioxidant activity, which revealed the degree of membrane association and uptake in cell, owing to the structure of flavonoids, polarity, and solubility.
3.2.3. The 2,3-Double Bond, 4-Keto Group, and 3-Hydroxyl Moiety
For flavonoids with a B-ring catechol group, the loss of any of the C-ring functional group, the 2,3-double bond, 4-keto group, or 3-hydroxyl moiety lead to decrease antioxidant activity [14]. In the CAA assay, the antioxidant activity of compounds 5, 9-10, 16, 23-26, 28-29, 31-32, 38-40, and 53-54 with 2,3-double bond and 4-keto groups decreased due to the loss of 3-hydroxyl moiety. This finding was in consistent with the results of the DPPH assay. However, the 2,3-double bond of C-ring did not influence the activity in the ORAC assay. Meanwhile, the 2,3-double bond of compounds 7, 23, and 39 would be further impacted than 3-hydroxyl moiety in the CAA assay. The big difference of flavonoids in ORAC, DPPH, and the cell assay suggested some compounds were not so effective in the model of CAA, and this different phenomenon provides information on the degree of membrane association and uptake in cell, owing to their structure, polarity, and solubility.
3.3. Effect on Intracellular Antioxidant Enzymes
The overproduction of ROS caused the imbalance of the intracellular oxidation stress, which may result in damage to cell. It is a leading factor contributing to chronic diseases, which include aging, angiocardiopathy, hypertension, and neurodegenerative diseases [33]. ABAP-induced ROS generation can cause an imbalance of intracellular antioxidant defense system, and SOD, CAT, and GSH-Px were the major radical-scavenging enzymes. In order to further measure the intracellular antioxidant mechanisms of flavonoids, the activities of SOD, CAT, and GSH-Px were determined. Cells without sample and ABAP treatment were used as positive control (PC), while cells treated with ABAP but not sample were used as negative control (NC). The data are shown in Figure 3. The SOD, CAT, and GSH-Px activities of NC cells were 43.47 ± 3.12%, 42.24 ± 3.45%, and 43.21 ± 4.21% of the PC cells, respectively. This suggested that ABAP caused oxidative stress in HepG2 cells. However, pretreating cells with compounds 2, 5, 8, 10, 13, and 32 before ABAP treatment prevented the activity decrease of antioxidant enzyme activities. The cells pretreated with 5 μM compound 2, 15 μM compound 5 and 8, 10 μM compound 10, 20 μM compound 13, or 40 μM compound 32 showed an insignificant increase in SOD activity, while a significant increase activity was found at a higher concentration compared to NC cells. Similarly, compounds 2, 5, 8, 10, 13, and 32 increased the CAT and GSH-Px activities in a dose-dependent manner. The CAT activities were 56.58 ± 3.25%, 74.98 ± 4.25%, and 83.10 ± 4.54%, and the GSH-Px activities were increased by 65.09 ± 3.21%, 71.88 ± 4.23%, and 81.24 ± 5.65% of PC value in cells pretreated with 5, 10, and 15 μM compound 2. The CAT activities were 49.55 ± 3.21%, 69.34 ± 5.15%, and 80.67 ± 7.13%, and the GSH-Px activities were 59.74 ± 3.23%, 69.08 ± 4.27%, and 83.44 ± 4.18% of PC value in cells pretreated with 15, 30, and 45 μM compound 5. The CAT and GSH-Px activities of compound 8 were 50.86 ± 2.23%, 67.01 ± 5.32%, and 80.86 ± 5.21%, 54.02 ± 3.02%, 63.46 ± 2.51%, and 82.02 ± 5.35% of PC value, respectively. Meanwhile, The CAT activities were 51.90 ± 3.21%, 68.95 ± 4.22%, and 81.82 ± 4.25%, and the GSH-Px activities were 54.37 ± 3.05%, 68.21 ± 4.25%, and 81.82 ± 5.52% of PC value in cells pretreated with 10, 20, and 30 μM compound 10. The percentage value of compound 13 was similar to compound 10. However, The CAT and GSH-Px activities of compound 32 were 44.93 ± 2.23%, 52.68 ± 3.42%, and 68.35 ± 3.72%, 47.79 ± 3.28%, 55.99 ± 3.57%, and 71.20 ± 4.28% of PC value, respectively. The results were consistent with the CAA assay, and the compounds have the better cellular activity; the enzyme activities were higher. Therefore, the structure-activity relationship of intracellular antioxidant enzymes was the same as the CAA assay.
Figure 3.
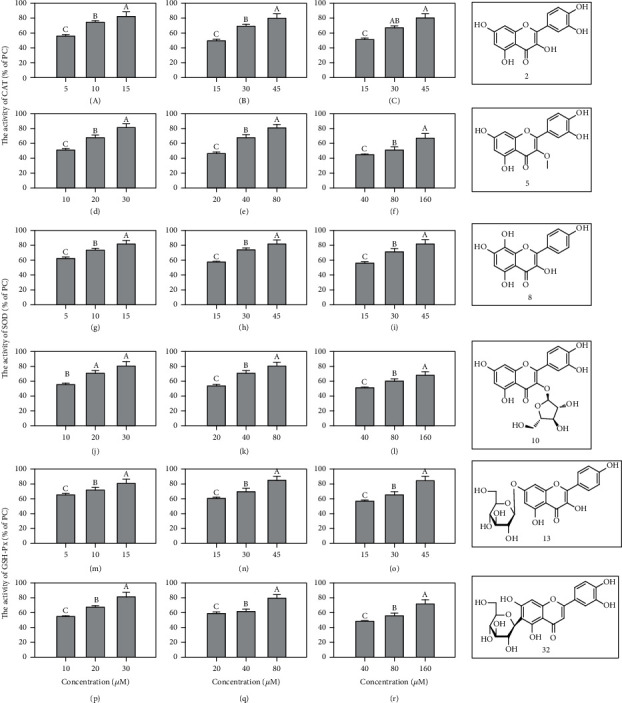
The rate and structures of the compounds 2 (a, g, p), 5 (b, h, q), 8 (c, i, r), 10 (d, j, s), 13 (e, k, t), and 32 (f, o, u) of PC value on the activities of antioxidant enzymes. The activities of CAT, SOD, and GSH-Px of the PC were 106.82 ± 5.32, 4.86 ± 0.84, and 33.3746 ± 2.25 U/mg protein, respectively. The activities of CAT, SOD, and GSH-Px of the NC were 45.11 ± 2.21, 2.12 ± 0.21, and 14.4231 ± 1.25 U/mg protein, respectively. The data are presented as the mean with standard deviation (SD) bar of three replicates. The values having no letters in common are significantly different (P < 0.05).
A previous study indicated that flavonoids can modulate intracellular antioxidant enzyme activities. Diosmetin is a bioflavonoid found in citrus fruits that has strong cellular antioxidant activity and can regulate the intracellular antioxidant enzyme activities to prevent the generation of intracellular ROS, thus effectively attenuate AAPH-induced oxidative stress in erythrocytes [34]. Butin was isolated from several medicinal herbs and reported to protect the cell against H2O2-induced DNA damage through restoring the activity and expressions of cellular antioxidant enzymes [35]. In this work, compounds 2, 5, 8, 10, 13, and 32 could significantly improve the activities of SOD, CAT, and GSH-Px. This could be one of the antioxidant mechanisms for compounds.
3.4. Cytotoxicity and Antiproliferative Activity
The HepG2 cells were selected to determine the antiproliferative activities and cytotoxicities of compounds 2, 5, 8, 10, 13, and 32. As shown in Figure 4, compounds 2, 5, 8, 10, 13, and 32 had no significant effects in the range of 10-160 μΜ, while the compound 13 showed slight cytotoxicity at higher concentration. The results indicated that the reduced fluorescence in the CAA assay was not from cytotoxicity. The compound 5 showed potent antiproliferative activities against HepG2 cells. The IC50 values were 90.72 ± 2.45 μΜ to HepG2 cells, while others were more than 400 μΜ.
Figure 4.
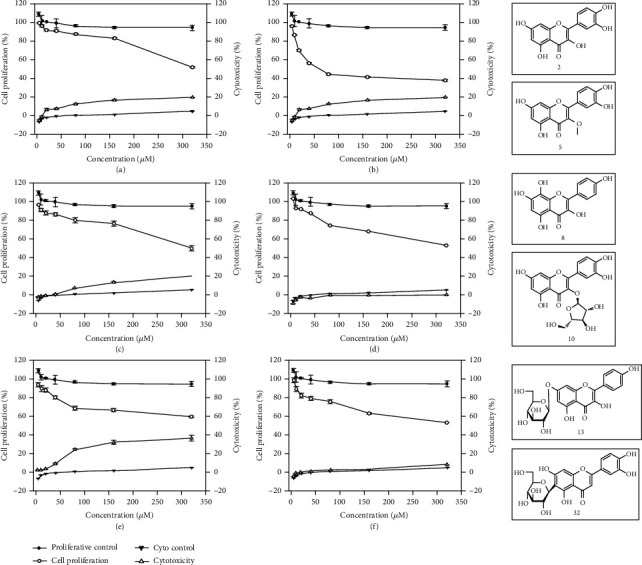
The antiproliferative activities, cytotoxicities, and structures of compounds 2 (a), 5 (b), 8 (c), 10 (d), 13 (e), and 32 (f) against HepG2 cells. The data are presented as the mean with standard deviation (SD) bar of three replicates. Bars with no letters in common were significantly different (P < 0.05).
In the assay of cellular antioxidant, compound 5 has been recognized as a good antioxidant. Meanwhile, in the cancer cell proliferation assay, compound 5 could inhibit the proliferation of cancer cells. This result suggested that the 3-methoxyl group in the tested compounds play an important role in the antiproliferative activity compared to compounds 2 and 5 [36]. It could decrease the cellular antioxidant activity, but improve the antiproliferative activity against cancer cell. As confirmed by literature [37, 38], O-glycosidation usually decreases the antiproliferative activity of flavonoids compared to compounds 2, 10, 13, and 32. Compared to compounds 2, 5, and 8, the addition of the hydroxyl group at C-3, C-3′, and C-8 decreased the antiproliferative activity [39]. And the previous study reported that the C2-C3 double bond and the lack of C-6 hydroxyl group were the structural features needed for the antiproliferative activity of flavonoids [40]. However, the antiproliferative activity also has been influenced by other groups. Compound 5, as reported, could protected normal lung cells from H2O2-induced ROS formation, membrane damage, and DNA damage. Meanwhile, it also increased the expression of p-p38, Nrf2, and SOD [41]. All these results suggested a potential application of flavonoids in anticancer drugs and cosmetic products.
4. Conclusions
A series of flavonoids with different structures were used to determine their chemical and intracellular antioxidant activities, among which the cellular antioxidant activities of compounds 2, 5, 8, 10, 13, and 32 were identified and characterized for the first time in this work. Compounds 2 and 5 potent presented an unexpected cellular antioxidation behavior, which has an order of magnitude as the quercetin. Their intracellular antioxidant properties were related to the upregulation of endogenous antioxidant enzyme activities and inhibition of ROS generation. The 2,3-double bond, 4-keto groups, 3′,4′-catechol structure, and 3-hydroxyl in the flavonoid skeleton play important roles in the antioxidant behavior. Furthermore, the cell proliferative assay revealed a slightly cytotoxicity for compound 5. Therefore, compound 5 would be appropriate for the use of nutraceutical in the future.
Acknowledgments
The author would like to thank Bao Yang for the proofreading of the manuscript. This work was supported by the Agricultural Science and Technology Innovation Program of Chinese Academy of Agricultural Sciences (CAAS-ASTIP-ZFRI) and the Key science and Technology program of Henan Province (No. 202102110209).
Data Availability
All data generated or analyzed during this study are included in this article.
Conflicts of Interest
The authors declare no conflict of interest.
Authors' Contributions
Q.Z. and Z.J. did the conceptualization; Q.Z. and W.Y. did the methodology; Q.Z. and J.L. was assigned on the software; Q.Z. and H.L. did the validation; Q.Z. did the formal analysis; Q.Z. did the investigation; Q.Z., Z.L., C.Z., and D.C. was assigned on the resources; Q.Z. and W.Y. did the data curation; Q.Z. wrote the original draft preparation; Z.J. did the writing, review, and editing; Q.Z. did the visualization; Z.J. did the supervision; Z.J. did the project administration; Q.Z. and Z.J. did the funding acquisition.
Supplementary Materials
Table S1: the antioxidant activities of 60 flavonoids determined by DPPH, ORAC, and CAA assays.
References
- 1.Chen C., Wang L., Wang R., et al. Phenolic contents, cellular antioxidant activity and antiproliferative capacity of different varieties of oats. Food Chemistry. 2018;239:260–267. doi: 10.1016/j.foodchem.2017.06.104. [DOI] [PubMed] [Google Scholar]
- 2.Ahn-Jarvis J. H., Parihar A., Doseff A. I. Dietary flavonoids for immunoregulation and cancer: food design for targeting disease. Antioxidants. 2019;8(7) doi: 10.3390/antiox8070202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hertog M. G. L., Feskens E. J. M., Kromhout D., et al. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet. 1993;342(8878):1007–1011. doi: 10.1016/0140-6736(93)92876-U. [DOI] [PubMed] [Google Scholar]
- 4.Fan M., Ding H., Zhang G., Hu X., Gong D. Relationships of dietary flavonoid structure with its tyrosinase inhibitory activity and affinity. LWT. 2019;107:25–34. doi: 10.1016/j.lwt.2019.02.076. [DOI] [Google Scholar]
- 5.Del Caro A., Piga A., Vacca V., Agabbio M. Changes of flavonoids, vitamin C and antioxidant capacity in minimally processed citrus segments and juices during storage. Food Chemistry. 2004;84(1):99–105. doi: 10.1016/S0308-8146(03)00180-8. [DOI] [Google Scholar]
- 6.Masuoka N., Matsuda M., Kubo I. Characterisation of the antioxidant activity of flavonoids. Food Chemistry. 2012;131(2):541–545. doi: 10.1016/j.foodchem.2011.09.020. [DOI] [Google Scholar]
- 7.Ebegboni V. J., Dickenson J. M., Sivasubramaniam S. D. Antioxidative effects of flavonoids and their metabolites against hypoxia/reoxygenation-induced oxidative stress in a human first trimester trophoblast cell line. Food Chemistry. 2019;272:117–125. doi: 10.1016/j.foodchem.2018.08.036. [DOI] [PubMed] [Google Scholar]
- 8.Varshney R., Mishra R., Das N., Sircar D., Roy P. A comparative analysis of various flavonoids in the regulation of obesity and diabetes: an in vitro and in vivo study. Journal of Functional Foods. 2019;59:194–205. doi: 10.1016/j.jff.2019.05.004. [DOI] [Google Scholar]
- 9.Ontiveros M., Rinaldi D., Marder M., et al. Natural flavonoids inhibit the plasma membrane Ca2+-ATPase. Biochemical Pharmacology. 2019;166:1–11. doi: 10.1016/j.bcp.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 10.Maleki S. J., Crespo J. F., Cabanillas B. Anti-inflammatory effects of flavonoids. Food Chemistry. 2019;299, article 125124 doi: 10.1016/j.foodchem.2019.125124. [DOI] [PubMed] [Google Scholar]
- 11.Fang Y., Cao W., Liang F., Xia M., Pan S., Xu X. Structure affinity relationship and docking studies of flavonoids as substrates of multidrug-resistant associated protein 2 (MRP2) in MDCK/MRP2 cells. Food Chemistry. 2019;291:101–109. doi: 10.1016/j.foodchem.2019.03.111. [DOI] [PubMed] [Google Scholar]
- 12.Cao G., Giovanoni M., Prior R. L. Antioxidant capacity decreases during growth but not aging in rat serum and brain. Archives of gerontology and geriatrics. 1996;22(1):27–37. doi: 10.1016/0167-4943(95)00674-5. [DOI] [PubMed] [Google Scholar]
- 13.Cao G., Alessio H. M., Cutler R. G. Oxygen-radical absorbance capacity assay for antioxidants. Free Radical Biology & Medicine. 1993;14(3):303–311. doi: 10.1016/0891-5849(93)90027-R. [DOI] [PubMed] [Google Scholar]
- 14.Wen L. R., Zhao Y. P., Jiang Y. M., et al. Identification of a flavonoid C-glycoside as potent antioxidant. Free Radical Biology & Medicine. 2017;110:92–101. doi: 10.1016/j.freeradbiomed.2017.05.027. [DOI] [PubMed] [Google Scholar]
- 15.Wolfe K. L., Kang X. M., He X. J., Dong M., Zhang Q. Y., Liu R. H. Cellular antioxidant activity of common fruits. Journal of Agricultural and Food Chemistry. 2008;56(18):8418–8426. doi: 10.1021/jf801381y. [DOI] [PubMed] [Google Scholar]
- 16.Li Q., Liu X., Wang X., et al. Antiproliferative ability and fluorescence tracking of α-linolenic acid-loaded microemulsion as label-free delivery carriers in MDA-MB-231 cells. Journal of Agricultural and Food Chemistry. 2019;67(41):11518–11526. doi: 10.1021/acs.jafc.9b04972. [DOI] [PubMed] [Google Scholar]
- 17.Thaipong K., Boonprakob U., Crosby K., Cisneros-Zevallos L., Hawkins Byrne D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. Journal of food composition and analysis. 2006;19(6-7):669–675. doi: 10.1016/j.jfca.2006.01.003. [DOI] [Google Scholar]
- 18.Wolfe K. L., Liu R. H. Cellular antioxidant activity (CAA) assay for assessing antioxidants, foods, and dietary supplements. Journal of Agricultural and Food Chemistry. 2007;55(22):8896–8907. doi: 10.1021/jf0715166. [DOI] [PubMed] [Google Scholar]
- 19.Rice-Evans C. A., Miller N. J., Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radical Biology & Medicine. 1996;20(7):933–956. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- 20.Nanjo F., Goto K., Seto R., Suzuki M., Sakai M., Hara Y. Scavenging effects of tea catechins and their derivatives on 1,1-diphenyl-2-picrylhydrazyl radical. Free Radical Biology & Medicine. 1996;21(6):895–902. doi: 10.1016/0891-5849(96)00237-7. [DOI] [PubMed] [Google Scholar]
- 21.Blasa M., Angelino D., Gennari L., Ninfali P. The cellular antioxidant activity in red blood cells (CAA-RBC): a new approach to bioavailability and synergy of phytochemicals and botanical extracts. Food Chemistry. 2011;125(2):685–691. doi: 10.1016/j.foodchem.2010.09.065. [DOI] [Google Scholar]
- 22.Wolfe K. L., Liu R. H. Structure-activity relationships of flavonoids in the cellular antioxidant activity assay. Journal of Agricultural and Food Chemistry. 2008;56(18):8404–8411. doi: 10.1021/jf8013074. [DOI] [PubMed] [Google Scholar]
- 23.Li K., Fan H., Yin P., et al. Structure-activity relationship of eight high content flavonoids analyzed with a preliminary assign-score method and their contribution to antioxidant ability of flavonoids-rich extract from Scutellaria baicalensis shoots. Arabian Journal of Chemistry. 2018;11(2):159–170. doi: 10.1016/j.arabjc.2017.08.002. [DOI] [Google Scholar]
- 24.Zhang H., Zheng W., Feng X., et al. Nrf2-ARE signaling acts as master pathway for the cellular antioxidant activity of fisetin. Molecules. 2019;24(4) doi: 10.3390/molecules24040708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi S.-J., Tai B. H., Cuong N. M., Kim Y.-H., Jang H.-D. Antioxidative and anti-inflammatory effect of quercetin and its glycosides isolated from mampat (Cratoxylum formosum) Food Science and Biotechnology. 2012;21(2):587–595. doi: 10.1007/s10068-012-0075-4. [DOI] [Google Scholar]
- 26.Cvorovic J., Tramer F., Granzotto M., Candussio L., Decorti G., Passamonti S. Oxidative stress-based cytotoxicity of delphinidin and cyanidin in colon cancer cells. Archives of Biochemistry and Biophysics. 2010;501(1):151–157. doi: 10.1016/j.abb.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 27.Bellion P., Hofmann T., Pool-Zobel B. L., et al. Antioxidant effectiveness of phenolic apple juice extracts and their gut fermentation products in the human colon carcinoma cell line Caco-2. Journal of Agricultural and Food Chemistry. 2008;56(15):6310–6317. doi: 10.1021/jf8005068. [DOI] [PubMed] [Google Scholar]
- 28.Heim K. E., Tagliaferro A. R., Bobilya D. J. Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. The Journal of Nutritional Biochemistry. 2002;13(10):572–584. doi: 10.1016/S0955-2863(02)00208-5. [DOI] [PubMed] [Google Scholar]
- 29.Bors W., Heller W., Michel C., Saran M. [36] Flavonoids as antioxidants: determination of radical-scavenging efficiencies. Methods in Enzymology. 1990;186:343–355. doi: 10.1016/0076-6879(90)86128-I. [DOI] [PubMed] [Google Scholar]
- 30.Silva M. M., Santos M. R., Caroco G., Rocha R., Justino G., Mira L. Structure-antioxidant activity relationships of flavonoids: a re-examination. Free Radical Research. 2002;36(11):1219–1227. doi: 10.1080/198-1071576021000016472. [DOI] [PubMed] [Google Scholar]
- 31.Hidalgo M., Sánchez-Moreno C., de Pascual-Teresa S. Flavonoid–flavonoid interaction and its effect on their antioxidant activity. Food Chemistry. 2010;121(3):691–696. doi: 10.1016/j.foodchem.2009.12.097. [DOI] [Google Scholar]
- 32.van Acker S. A. B. E., van den Berg D. J., Tromp M. N., et al. Structural aspects of antioxidant activity of flavonoids. Free Radical Biology & Medicine. 1996;20(3):331–342. doi: 10.1016/0891-5849(95)02047-0. [DOI] [PubMed] [Google Scholar]
- 33.Valko M., Leibfritz D., Moncol J., Cronin M. T., Mazur M., Telser J. Free radicals and antioxidants in normal physiological functions and human disease. The International Journal of Biochemistry & Cell Biology. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 34.Liao W. Z., Ning Z. X., Chen L. Y., et al. Intracellular antioxidant detoxifying effects of diosmetin on 2,2-azobis(2-amidinopropane) dihydrochloride (AAPH)-induced oxidative stress through inhibition of reactive oxygen species generation. Journal of Agricultural and Food Chemistry. 2014;62(34):8648–8654. doi: 10.1021/jf502359x. [DOI] [PubMed] [Google Scholar]
- 35.Zhang R., Chae S. W., Kang K. A., et al. Protective effect of butin against hydrogen peroxide-induced apoptosis by scavenging reactive oxygen species and activating antioxidant enzymes. Molecular and Cellular Biochemistry. 2008;318(1-2):33–42. doi: 10.1007/s11010-008-9854-x. [DOI] [PubMed] [Google Scholar]
- 36.Yamauchi K., Mitsunaga T., Afroze S. H., Uddin M. N. Structure-activity relationships of methylquercetin on anti-migration and anti-proliferation activity in B16 melanoma cells. Anticancer Research. 2017;37(4):1575–1579. doi: 10.21873/anticanres.11487. [DOI] [PubMed] [Google Scholar]
- 37.Manthey J., Guthrie N. Antiproliferative activities of citrus flavonoids against six human cancer cell lines. Journal of Agricultural and Food Chemistry. 2002;50(21):5837–5843. doi: 10.1021/jf020121d. [DOI] [PubMed] [Google Scholar]
- 38.Kuntz S., Wenzel U., Daniel H. Comparative analysis of the effects of flavonoids on proliferation, cytotoxicity, and apoptosis in human colon cancer cell lines. European Journal of Nutrition. 1999;38(3):133–142. doi: 10.1007/s003940050054. [DOI] [PubMed] [Google Scholar]
- 39.Chidambara Murthy K. N., Kim J., Vikram A., Patil B. S. Differential inhibition of human colon cancer cells by structurally similar flavonoids of citrus. Food Chemistry. 2012;132(1):27–34. doi: 10.1016/j.foodchem.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 40.Rusak G., Gutzeit H. O., Müller J. L. Structurally related flavonoids with antioxidative properties differentially affect cell cycle progression and apoptosis of human acute leukemia cells. Nutrition Research. 2005;25(2):143–155. doi: 10.1016/j.nutres.2004.12.003. [DOI] [Google Scholar]
- 41.Kumar A. D., Bevara G. B., Kaja L. K., Badana A. K., Malla R. R. Protective effect of 3-O-methyl quercetin and kaempferol from Semecarpus anacardium against H2O2 induced cytotoxicity in lung and liver cells. BMC complementary and alternative medicine. 2016;16(1) doi: 10.1186/s12906-016-1354-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: the antioxidant activities of 60 flavonoids determined by DPPH, ORAC, and CAA assays.
Data Availability Statement
All data generated or analyzed during this study are included in this article.


