Recent literature has reported that patients with prostate cancer treated with androgen deprivation therapy (ADT) have a lower incidence of severe acute respiratory syndrome coronavirus 2 (SARS-COV-2), an observation which has been widely reported by media outlets, together with speculation that ADT may be a potential treatment for coronavirus disease 2019 (COVID-19). The study by Montopoli et al.1 was conducted at a population level in Italy, a region experiencing a high level of COVID-19 cases. They observed in a cohort of men with prostate cancer that those prescribed ADT were less likely to report COVID-19 (4/5273 cases versus 114/37 161, odds ratio 4.05, 95% confidence interval 1.55–10.59, P = 0.00043). Benefits were also observed for classification of mild and severe disease and were used as a basis of the conclusion that ‘ADT, based on luteinizing hormone-releasing hormone (LHRH) agonist/antagonists or AR inhibitors, may be considered to reduce SARS-CoV-2 infections or complications in high-risk male populations.’
A useful metric to consider in this context is the number needed to treat, which is calculable from the data reported, but was not included by Montopoli et al.1 We calculate that the number needed to treat with ADT for the prevention of one COVID-19 case is 434. Treatment of these cases is not without risk and this is shown in Figure 1 with data extracted from Bagrodia et al.2 In addition, diabetes mellitus, decreased libido, hot flashes and erectile dysfunction would be expected in a high proportion of men. These adverse events are calculated per person-year, a time frame which may be relevant to prevention of COVID-19. Montopoli et al.1 state that ADT could be administered transiently to minimise adverse events, though this hypothesis has not been tested in the data they present. Transient ADT treatment may be appropriate to reduce complications in those already infected with SARS-COV-2, although ADT adverse events are typically most common immediately after commencement.3
Figure 1.
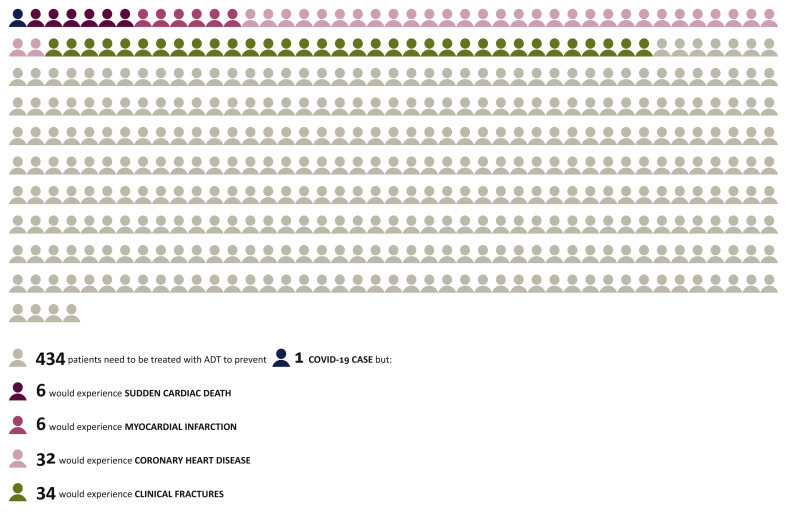
ADT treatment for COVID 19 in context.
ADT, androgen deprivation therapy; COVID-19, coronavirus disease 2019.
The mechanism of action which Montopoli et al.1 describe may provide a novel target for COVID-19 treatments, but it seems unlikely that existing ADTs will provide a viable treatment option.
Acknowledgments
Funding
None declared.
Disclosure
The authors have declared no conflicts of interest.
References
- 1.Montopoli M., Zumerle S., Vettor R. Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a population-based study (N = 4532) Ann Oncol. 2020;31:1040–1045. doi: 10.1016/j.annonc.2020.04.479. 1040-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bagrodia A., Diblasio C.J., Wake R.W., Derweesh I.H. Adverse effects of androgen deprivation therapy in prostate cancer: current management issues. Indian J Urol. 2009;25:169–176. doi: 10.4103/0970-1591.52907. 169-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Farrell S., Garmo H., Holmberg L., Adolfsson J., Stattin P., Van Hemelrijck M. Risk and timing of cardiovascular disease after androgen-deprivation therapy in men with prostate cancer. J Clin Oncol. 2015;33:1243–1251. doi: 10.1200/JCO.2014.59.1792. 1243-1251. [DOI] [PubMed] [Google Scholar]


