Abstract
Background
Bioprosthetic valves are increasingly used for surgical mitral valve replacement (MVR). The long-term outcomes of bovine (BoMVR) vs porcine (PoMVR) remain an enigma regarding the durability. This study aims to examine the outcomes of BoMVR vs PoMVR.
Methods
A retrospective analysis of all bioprosthetic MVRs, with concomitant procedures, at a single tertiary referral institution from January 2005 to December 2008 was conducted. Procedures were classified as BoMVR or PoMVR. The age group was from 40 to 70 years.
Results
We identified 154 BoMVR patients and 120 PoMVR patients after matching the two groups with respect to age, sex, valve size and concomitant procedures. Kaplan-Meier survival analysis model was used for corresponding statistical analysis. Freedom from reoperation (all cause), freedom from non-structural valve deterioration, freedom from structural valve deterioration, freedom from heart failure and freedom from infective endocarditis were 96.4 ± 0.08, 97.1 ± 0.07, 96.4 ± 0.08%, 98.2 ± 0.07, and 98.6 ± 0.06% in PoMVR, respectively, and 92.6 ± 0.09, 91.6 ± 0.08, 90.6 ± 0.09, 94 ± 0.08, and 92.8 ± 0.08% in BoMVR groups, respectively, at the end of 10-year follow-up (mean follow up of 6.2 ± 2.3 years). Overall, 20 (12.9%) patients were lost to follow-up in the BoMVR and 15(12.5%) patients in the PoMVR groups for a global follow-up of 87.1%.
Conclusions
For patients undergoing MVR with a bioprosthetic valve, the choice of PoMVR vs BoMVR favours more in favour of PoMVR as evidenced by the outcome results. Probably long-term follow-up with more patients might throw further light on the debatable topic.
Keywords: Porcine, Bovine, Bioprosthetic valve
Introduction
There is an increasing trend towards the usage of bioprosthetic valves for patients with valvular heart disease even in younger age in order to avoid the complication of lifelong anticoagulation. Regarding the choice of bioprosthetic valves, it always remains an enigma as there are umpteen valve types and valve choices available in the market with each claiming its own merits and demerits.
The problems of valve degeneration and its effect on the outcomes and survival of the patient need to be considered while choosing the valve. There is no long-term follow-up of data comparing the outcomes of two of the popular valve choices, the bovine pericardial material and the porcine aortic valve material, in the mitral position in this geographical locality. This study aims to compare the outcomes of bovine vs porcine bioprosthetic valves in mitral position used in a single tertiary cardiac surgical centre.
Methods
A retrospective analysis of all bioprosthetic MVR, with or without coronary artery bypass grafting (CABG) procedures, at a single tertiary referral institution from January 2005 to December 2008 was conducted using a prospectively maintained database. Procedures were classified as BoMVR or PoMVR according to the type of bioprosthetic valve used. The type of bioprosthetic valve was decided preoperatively by the heart valve team consisting of surgeon, cardiologist and patient preference and in no case was there a necessity to change the valve based on intraoperative finding from that which was decided preoperatively. The outcomes were defined based on Society of Thoracic Surgery (STS) guidelines for valve-related mortality and morbidity. The patients were followed up for 10 years (mean 6.2 years). The age group was from 40 to 70 years. We identified 166 BoMVR patients and 138 PoMVR patients who were matched based on propensity random sampling matching according to age, sex and valve size. The final sample came down to 154 BoMVR and 120 PoMVR patients. Stented bovine pericardial valves included in the analysis were Carpentier-Edwards Perimount (CEP) (Edwards Lifesciences, Irvine, CA) (n = 154). Stented porcine xenograft valves included were St. Jude Biocor (St. Jude Medical Inc) (n = 56) and Medtronic Hancock II (Medtronic Inc., Minneapolis, MN) (n = 64).
Aims and objectives
The outcomes of the study were long-term survival, freedom from reoperation (all cause), freedom from non-structural valve deterioration (NSVD), freedom from structural valve deterioration (SVD), freedom from heart failure and freedom from infective endocarditis (IE) all at the end of 10 years.
The inclusion criteria are as follows:
All adult patients > 18 years who underwent isolated MVR using the any of the above said valve types.
Patients who underwent concomitant procedures along with the MVR other than double valve replacements.
Redo surgeries at the mitral position using the above said valve types.
The exclusion criteria are as follows:
Double valve replacements using bioprosthetic valves (combined aortic and mitral).
MVR using mechanical valves.
There were 70 male and 84 female patients in the BoMVR group vs 65 male and 55 female patients in the PoMVR group. The male to female ratio was 0.83 in the BoMVR group compared with 1.18 in the PoMVR group. The mean age (54 ± 12 years [mean ± standard deviation]) of the PoMVR was significantly higher than that of patients in the BoMVR (52 ± 8 years).
Preoperatively, in the BoMVR group, 80 (51%) patients were in New York Heart Association functional class II, 30 (19%) were in class III, and 44 (30%) were in class IV. In the porcine valve group, 90 (75%) patients were in functional class II, 15 (12.5%) were in functional class III, and 15 (12.5%) were in functional class IV. Mitral stenosis was the predominant valvular lesion (65% in BoMVR and 68% in PoMVR) in both the groups. The preoperative characteristics of both the groups are described in Table 1.
Table 1.
Preoperative characteristics of the patients
| S no. | Preop criteria | BoMVR | PoMVR | p value |
|---|---|---|---|---|
| 1 | No of patients (n) | 154 | 120 | < 0.05 |
| 2 | Age years | |||
| Range | 42–73 | 41–74 | < 0.04 | |
| Mean ± SD | 52 ± 8 | 54 ± 12 | < 0.05 | |
| 3 | Gender | |||
| Male (n) | 70 (45.5%) | 65 (54.1%) | < 0.08 | |
| Female (n) | 84(54.5%) | 55 (45.9%) | < 0.06 | |
| 4 | Aetiology (n) | |||
| Rheumatic | 78 | 67 | < 0.05 | |
| Re operative | 31 | 20 | < 0.03 | |
| Ischemic | 27 | 18 | < 0.08 | |
| Endocarditis | 10 | 10 | < 0.07 | |
| Degenerative | 8 | 5 | < 0.08 | |
| 5 | Lesion (n) | |||
| Stenosis | 102 | 75 | < 0.05 | |
| Regurgitant | 45 | 33 | < 0.08 | |
| Mixed | 7 | 12 | < 0.06 | |
| 6 | Pre op NYHA class (n) | |||
| I | – | – | ||
| II | 80 | 90 | < 0.05 | |
| III | 30 | 15 | < 0.06 | |
| IV | 44 | 15 | < 0.06 | |
| 7 | Rhythm (n) | |||
| Sinus | 97 | 86 | < 0.05 | |
| Atrial fibrillation | 41 | 27 | < 0.05 | |
| Other than the above two | 16 | 7 | < 0.08 | |
| 8 | Pre op LVEF (n) | |||
| > 50% | 57 | 84 | < 0.05 | |
| 35–50% | 32 | 24 | < 0.02 | |
| 20–35% | 65 | 12 | < 0.05 |
The intraoperative variables are listed in Table 2. In the BoMVR group, the valve sizes were 25 mm (n = 27), 27 mm (n = 88), 29 mm (n = 13) and 31 mm (n = 26), and in the PoMVR group, the valve sizes were 25 mm (n = 33), 27 mm (n = 58), 29 mm (n = 10) and 31 mm (n = 19).
Table 2.
Intraoperative details of the patients
| Sl no. | Intraop variable | BoMVR | PoMVR | p value |
|---|---|---|---|---|
| 1 | Valve size (n) | |||
| 25 mm | 27 | 33 | < 0.07 | |
| 27 mm | 88 | 58 | < 0.08 | |
| 29 mm | 13 | 10 | < 0.07 | |
| 31 mm | 26 | 19 | < 0.08 | |
| 2 | Associated procedures (n) | |||
| CABG | 45 | 35 | < 0.08 | |
| Tricuspid valve repair | 24 | 20 | < 0.07 | |
| Others | 13 | 15 | < 0.08 | |
| 3 | Cardiopulmonary bypass time (min) | 120 ± 67 | 145 ± 28 | < 0.05 |
| 4 | Aortic cross clamp time (min) | 67 ± 12 | 87 ± 14 | < 0.05 |
Surgical technique
Ours is a multi-surgeon centre and in both the groups; surgery was conducted through median sternotomy with aorto bicaval cannulation. Majority of the surgeries were through left atriotomy dissecting the Waterston’s groove (85% in BoMVR and 88% in PoMVR). The rest was through septo superior approach. The choice of cardioplegia, the suturing technique and the type of suture was all left to the surgeon preference. Where ever necessary, the associated procedures like CABG and others were carried out in the standard manner as described in standard surgical text books.
Patients were followed on a yearly basis either at the outpatient clinic or through their own physician and follow-up was updated through telephonic call or through the records available in the institute. Overall, 20 (12.9%) patients were lost to follow-up in the BoMVR and 15 (12.5%) patients in the PoMVR groups for a global follow-up of 87.1%. The total duration of follow-up of patients was 10 years (mean follow-up of 6.2 ± 2.3 years) with 1100 patient-years in the BoMVR group vs 987 patient-years in the PoMVR group.
The anticoagulation protocol was to maintain a target international normalized ratio (INR) of 2.5–3.5 with either warfarin sodium or acitrom for 3 months unless there is another indication for continuing anticoagulation. All these patients were also kept on tablet acetyl salicylic acid 75 mg once daily lifelong.
Statistical analysis
Categorical variables are expressed as counts and percentages. Normally distributed continuous variables are expressed as mean ± standard deviation. Comparison between two groups was performed using unpaired two-tailed t test for normally distributed variables and Pearson’s chi square test for categorical variables. Long-term survival, freedom from reoperation (all cause), freedom from NSVD, freedom from SVD, freedom from heart failure and freedom from IE were examined using Kaplan-Meier methods with the log-rank test and p value < 0.05 was set as significant level.
Results
Outcomes
Survival and all cause mortality
There were 8 early deaths among the PoMVR group, an early mortality of 6.6%, and 15 early deaths in the BoMVR group, an early mortality of 9.7%. In the PoMVR group, there were 12 late deaths compared with 20 in the BoMVR group, giving linearized late mortality rates of 2.0%/patient-year and 3.1%/patient-year, respectively. On follow-up in the PoMVR group, at 10 years the overall actuarial survival rate was 96.4 ± 0.08% (83–98%) vs 94.6 ± 0.09% (86–99%) (p < 0.06) in the BoMVR group. Causes of death are shown in Table 3. The most frequent cause was congestive heart failure (not valve related) in both the groups (60 and 57%, in PoMVR and BoMVR, respectively). The valve function was normal in both the groups in the mortality patients as evidenced by the peak and mean gradients. The peak and the mean gradients in the PoMVR group was 14 ± 8 and 5 ± 3 mmHg, respectively, and for the BoMVR group it was 12 ± 6 and 4 ± 2 mmHg, respectively, for such patients.
Table 3.
Causes of death
| Sl no. | Cause of death | PoMVR (n) | BoMVR (n) | p value |
|---|---|---|---|---|
| 1 | Congestive heart failure (not valve related) | 12 | 20 | 0.04 |
| 2 | Perioperative haemorrhage | 5 | 8 | 0.03 |
| 3 | Arrhythmia | 2 | 4 | 0.06 |
| 4 | Cerebrovascular accident | 1 | 3 | 0.07 |
Valve-related complications
SVD
Clinically significant SVD was reported in two patients in the PoMVR group vs nine patients in the BoMVR group. The peak and the mean gradients in the PoMVR group was 43 ± 8 and 24 ± 10 mmHg, respectively, and for the BoMVR group it was 46 ± 10 and 27 ± 4 mmHg, respectively, for the SVD patients. All these patients underwent reoperation for the same. The cause of SVD was calcification in 55% of cases, leaflet tear in 33% and mixed aetiology in 12% in the BoMVR group. The linearized incidence rate was 0.02%/patient-year in the PoMVR vs 0.08%/patient-year in the BoMVR group. Mean time to SVD was 7.1 ± 0.4 years in the BoMVR group vs 8.2 ± 1.1 years in the PoMVR group. At 10 years, actuarial freedom from SVD was 96.4 ± 0.08% (94–96%) in the PoMVR group vs 90.6 ± 0.09% (p < 0.05, 96–98%) in the BoMVR group as shown in Fig. 1.
Fig. 1.
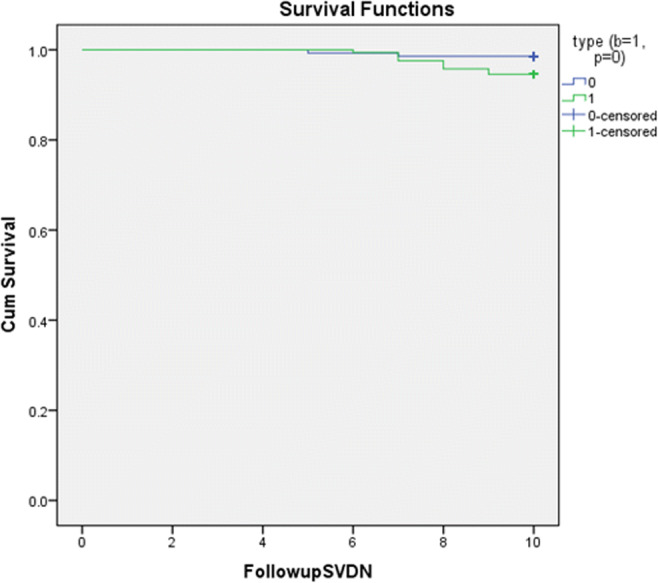
Freedom from SVD between PoMVR (blue) vs BoMVR (green)
Competing risk analysis including three distinct failures (non-valve-related death, valve-related-death and explantation attributable to SVD) in the BOMVR group and PoMVR groups were performed. At 10 years, the cumulative risk of valve explantation secondary to SVD was 90 ± 2%, lower than the corresponding actuarial estimate 96.4 ± 0.08% in the PoMVR, and 82 ± 5%, lower than the corresponding actuarial estimate 90.6 ± 0.09% in the BOMVR groups.
NSVD
Clinically significant NSVD was reported in 2 cases in the PoMVR and 10 cases in BoMVR patients group. The peak and the mean gradients in the PoMVR group was 40 ± 5 and 20 ± 10 mmHg, respectively, and for the BoMVR group it was 38 ± 4 and 21 ± 7 mmHg, respectively, for the NSVD patients. All these patients underwent reoperation for the same. The cause of NSVD was paravalvular leaks in 60% (n = 6) and entrapment by pannus 40% (n = 4) in the rest. The linearized incidence rate was 0.01%/patient-year and 0.09%/patient-year and in PoMVR and BoMVR groups, respectively. At 10 years, the actuarial freedom from NSVD was 97.1 ± 0.07% (96–99%) and 91.6 ± 0.08% (95–98%) (p < 0.04) in the PoMVR and BoMVR groups respectively as shown in Fig. 2.
Fig. 2.
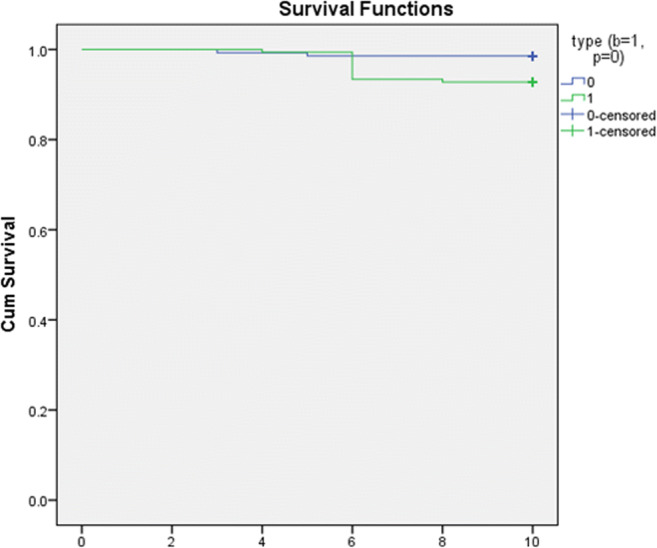
Freedom from NSVD between PoMVR (blue) vs BoMVR (green)
Infective endocarditis
IE was reported in 2 patients in PoMVR and 13 cases in BoMVR group with a linearized incidence rate of 0.2%/patient-year and 1.1%/patient-year, respectively. Of these patients, two died without reoperation, four underwent reoperation and seven were successfully treated using antibiotics alone in the BoMVR group and two patients in the PoMVR group were successfully treated with antibiotics. The 10-year actuarial freedom from endocarditis was 98.6 ± 0.06% (87–96%) and 92.8 ± 0.08% (92.3–98%) (p < 0.07) in the PoMVR and BoMVR groups, respectively.
Reoperation (all cause)
A total of 4 and 23 reoperations were reported in the PoMVR and BoMVR groups; none in operative period and all were valve related (linearized rate, 0.4%/patient-year and 2.0%/patient-year). In the PoMVR group, 2 were due to SVD and 2 were due to NSVD, while in the BoMVR group, 9 were due to SVD and 10 due to NSVD and 4 due to IE. The 10-year actuarial freedom from valve explantation for all causes was 96.4 ± 0.08% (86–95.4%) vs 92.6 ± 0.09% (91.2–95.7%) (p < 0.05) in the PoMVR and BoMVR groups, respectively. There was no mortality due to this complication in PoMVR group while three patients died in the BoMVR group due to bleeding.
Thromboembolic events
No case of valve thrombosis was reported. A total of four thromboembolic events were reported in the PoMVR group and six in the BoMVR group, for a linearized rate of 0.3%/patient-year and 0.6%/patient-year, respectively. All the events in the BoMVR group were minor with patient achieving full recovery, and in the PoMVR group, four events were minor with full recovery and two events resulted in permanent neurological deficit. The 10-year freedom from thromboembolism rate was 95.2 ± 0.08% (94–97.2%) and 98.2 ± 0.04% (92.3–99%) (p < 0.04) in the PoMVR and BoMVR groups, respectively.
Bleeding
A total of 11 bleeding events in the PoMVR and 15 in the BoMVR group were reported (linearized rate, 1%/patient-year and 1.5%/patient–year, respectively). None occurred in the postoperative period, and all were related to the anticoagulant use needing blood transfusion. The mean HASBLED score was 2 in both the groups and similar. During the follow-up period, 73% of the patients in the PoMVR group were anticoagulant free and 27% were on anticoagulation, while in the BoMVR group these numbers were 67 and 33% respectively with atrial fibrillation, the primary reason for continuing anticoagulation. The 10-year actuarial freedom from anticoagulant-related haemorrhage was 95.5 ± 0.02% (92.3–96.4%) and 97.5 ± 0.08% (96–98.2%) (p < 0.04) in the PoMVR and BoMVR groups.
Admissions from heart failure (non-valve related)
During the follow-up, there were 6 admissions in the PoMVR group and 15 admissions in the BoMVR group for non-valve-related heart failure with a linearized incidence rate of 0.6%/patient-year and 1.3%/patient-year, respectively. Myocardial infarction was the major cause of heart failure (62 and 68% in PoMVR and BoMVR) in both the groups although there was a trend of more associated cardiac procedures in the BoMVR group. At 10 years, the actuarial freedom from heart failure was 98.2 ± 0.07% (91.2–97.6%) and 94 ± 0.08% (89.6–96.7%) (p < 0.03) in the PoMVR and BoMVR, respectively.
The linearized incidence rates for all the events are shown in Table 4. The freedom from event rates are shown in Table 5.
Table 4.
Linearized incidence rates of events in both the groups during follow-up
| Sl no. | Event (%/patient-year) | BoMVR | PoMVR |
|---|---|---|---|
| 1 | Late mortality | 3% | 2.1% |
| 2 | SVD | 0.08% | 0.02% |
| 3 | NSVD | 0.09% | 0.01% |
| 4 | IE | 1.1% | 0.2% |
| 5 | Re operation (all cause) | 2.0% | 0.4% |
| 6 | Thromboembolic events | 0.6% | 0.3% |
| 7 | Bleeding | 1.5% | 1.0% |
| 8 | Heart failure (not valve related) | 1.3% | 0.6% |
Table 5.
Freedom from event rates at the end of 10-year follow-up in both the groups
| Sl no. | Event (96% CI) | BoMVR | PoMVR | p value |
|---|---|---|---|---|
| 1 | SVD | 90.6 ± 0.09% (96–98%) | 96.4 ± 0.08% (94–96%) | < 0.05 |
| 2 | NSVD | 91.6 ± 0.08% (95–98%) | 97.1 ± 0.07% (96–99%) | < 0.04 |
| 3 | IE | 92.8 ± 0.08% (92.3–98%) | 98.6 ± 0.06% (87–96%) | < 0.07 |
| 4 | Re operation (all cause) | 92.6 ± 0.09% (91.2–95.7%) | 96.4 ± 0.08% (86–95.4%) | < 0.05 |
| 5 | Thromboembolic event | 95.2 ± 0.08% (92.3–99%) | 98.2 ± 0.04% (94–97.2%) | < 0.04 |
| 6 | Bleeding event | 95.5 ± 0.02% (96–98.2%) | 97.5 ± 0.08% (92.3–96.4%) | < 0.04 |
| 7 | Heart failure (not valve related) | 94 ± 0.08% (89.6–96.7%) | 98.2 ± 0.07% (91.2–97.6%) | < 0.03 |
Discussion
In the present study, we evaluated the long-term durability of the BoMVR and PoMVR and we report 10-year outcomes of these two groups. Very little data is available about the performance of BoMVR and PoMVR valves in literature, and to our knowledge, we feel this is the first paper of this kind to exclusively compare both the groups with a long-term total follow-up data of > 2000 patient-years in the mitral position altogether.
A brief introduction about the design and types of the valves that were used in this study is given below. The CEP valve is a second generation bovine pericardial valve introduced in 1982 [1]. The stress problems of the previous first generation Ionescu-Shiley valve are avoided in this valve. It undergoes “neutralogic stress free fixation” with glutaraldehyde and treatment with XenoLogix to reduce phospholipid content and prevent calcification [2]. The cusps are designed to align like the native human aortic valve in order to achieve better hemodynamics [3]. The pericardial valves are mounted on a lightweight flexible Elgiloy (memory metal) stent covered with polytetrafluoroethylene (PTFE) cloth. As the pericardium of the adjacent cusp does not pass over the stent and passes in between the stent, it allows more flexibility of the valve. Bioprosthetic heart valves (BHV) are fixed in glutaraldehyde (0.2 v/v) to reduce immunogenicity. This causes collagen cross linking resulting in chemical stabilization but at the cost of membrane damage and calcium influx [4]. This calcium influx at the sites of stress forms phospholipid complex leading to tears and stenosis [5]. This calcium influx is reduced further by the anti-mineralization treatment as it binds covalently and prevents calcium influx. The anti-mineralization treatment of Hancock II valve is sodium tetradecyl sulphate and Biocor does not have any. Broom and Thomson [6] reported that high pressure (80 mmHg) fixation leads to more damage seen in the first generation valve and hence currently the second generation valves are fixed at low pressure (0–4 mmHg) to avoid the stent damage seen in high pressure fixation.
Although there are more collagen fibres in bovine pericardial bioprosthetic valves which might translate to better stability [7] at least in theory, and hence one would expect BoMVR to last longer than PoMVR. But our study shows different results and this might be attributed to the fact that tissue damage and calcification are independent mechanisms of bioprosthetic valve failure [8] and only experimental models post explant are available and further insight as to why there is a clinical and theoretical discrepancy in the observed and expected results needs more studies and elaborate research in future.
Outcomes
Survival and all cause mortality
For the group treated from 2005 to 2008, who had follow-up data in 2018 for 10 years, the average life expectancy of a patient at 60 years old in India was 16.5 years for men and 18.5 years for women. From our analysed cohort, 50% of our patients were still alive at 15.5 years, and the area under curve (AUC) was 16.2 years. Therefore, the life expectancy after MVR was similar to the age- and gender-adjusted life expectancy for the general population.
In a study by Wang et al. from China, the actuarial survival rate at 10 years for MVR was 61.7 ± 3.3%. The 10-year survival rate in patients younger than 60 years old was 80.1 ± 5.4%. However, in patients older than 60 years old, it was 55.4 ± 3.9% for MVR groups. The valve they had used was Hancock II [9]. In a study by Bourguignon et al., the 10-year actuarial survival rate was 86.3 ± 0.8%. They had used CEP valve at mitral position [10]. The Rizzoli et al. study showed a 10-year actuarial survival rate of 51.6 ± 5.6% with Biocor valve at the mitral position [11]. Compared with all these studies, our results showed better survival rates both in the PoMVR and BoMVR with almost equal survival rates in both the groups (96.4 ± 0.08 vs 94.6 ± 0.09%), a reason being probably lesser number of patients in our study and sicker substrate in the other studies. Also we had no valve-related deaths as our follow-up mortality events were all related to non-valvular causes, while the other studies had a significant number of valve-related mortality too. Also, there were more patients in NYHA class II rather than class III which we believe is because of the early presentation of patients immediately after symptom onset instead of waiting for the full-blown disease to occur.
Valve-related complications
SVD
David and colleagues [12] reported a 12-year freedom from SVD of 82 ± 5% with the Hancock II for patients with a mean age of 65 years. Jamieson et al. [13] compared the CEP vs the CE porcine valve and reported that at 10 years, the freedom from SVD was lower for the porcine valve (64.7 ± 3.3 vs 84.0 ± 3.7% than the CEP in patients aged < 60 years and it was 75.2 ± 3.7 vs 95.2 ± 2.1% for the CEP in patients aged 61–70 years. The performance of Biocor was even better as evidenced by the reports of Myken et al. [14] who demonstrated a 91% SVD freedom in the mitral position at 15 years in patients older than 61 years. The Chinese group of Wang et al. showed a 10-year freedom from reoperations due to SVD around 94.6%, which was similar to the results of other publications from America, Canada, and Italy [9]. Their documentation was that race and ethnicity do not seem to play a significant major role in determining SVD. The Pelletier et al. [15] study showed that after 6 years, freedom from primary tissue failure of mitral valves was 92 ± 2% with porcine and 70 ± 11% with pericardial bioprostheses (p < 0.0001). However in their study their failure rates of the bovine group was attributed to the low performance of Ionescu-Shiley valve which is a well-known fact. Tables 6 and 7 represent the number of years a patient could expect to be free from reoperation for SVD depending on age at implantation in the BoMVR and PoMVR groups, respectively, which clearly shows the superior performance of PoMVR over BoMVR. In our study in BoMVR and PoMVR groups, the freedom from SVD rates are significantly better than the other study groups with PoMVR faring much better than BoMVR (96.4 ± 0.08% (9.4–10.1) vs 94.6 ± 0.09% (9.6–10.01) (p < 0.05)). Hence overall, we need more conclusive evidence and long-term follow-up data as to decide the effect of valve type on SVD.
Table 6.
Explant due to SVD by age group—BoMVR competing risk (actual) estimates
| Probability (%)/age (years) | 40 years | 50 years | 60 years |
|---|---|---|---|
| 5% | 6.0 | 7.9 | 11.2 |
| 20% | 7.9 | 8.4 | 12.5 |
| 50% | 8.4 | 12.6 | 14.2 |
For example, a 60-year-old patient has a probability of 20% of needing a reoperation due to SVD after 12.5 years; the probability increases to 50% after 14.2 years
Table 7.
Explant due to SVD by age group—PoMVR competing risk (actual) estimates
| Probability (%)/age (years) | 40 years | 50 years | 60 years |
|---|---|---|---|
| 5% | 7.8 | 11.2 | 13.4 |
| 20% | 11.2 | 13.5 | 15.6 |
| 50% | 13.5 | 15.7 | 18.4 |
For example, a 60-year-old patient has a probability of 20% of needing a reoperation due to SVD after 15.6 years; the probability increases to 50% after 18.4 years
NSVD
Not many data is available for NSVD as in most of the studies there are very few incidence of NSVD. However in our study, at 10 years, the actuarial freedom from NSVD was 97.1 ± 0.07% (9.6–9.9) and 91.6 ± 0.08% (9.5–9.8) (p < 0.04) in the PoMVR and BoMVR groups, respectively. One finding which can be attributed for this variation is the significant number of continuous suture technique (67%) which was followed in the BoMVR group (probably contributing to the paravalvular leaks) as against the interrupted suturing technique in the PoMVR (70%) group. It was the individual surgeon’s choice on the suturing technique.
IE
Different studies show different results with the occurrence of IE in BoMVR and PoMVR groups. In a study by Pelletier et al., they found no difference at the 10-year freedom from IE rates in both the groups (97 ± 0.2%). The Chinese group [9] reported a 97.5% freedom from IE at 10 years using the Hancock II prosthesis at the mitral position. Bourguignon et al. [10] reported good results of 94 ± 0.4% 20-year freedom from IE rate using CEP valve at the mitral position. Our study showed lower freedom from IE for the BoMVR group but was not statistically significant. All the IE events were late IE with mean time to IE being 5.2 ± 2.3 and 6.4 ± 1.2 years for the BoMVR and PoMVR groups, respectively.
Reoperation (all cause)
In our study, all the reoperation events were due to valve dysfunction and hence as the SVD and NSVD in BoMVR were higher, obviously we had a better freedom from reoperation in the PoMVR group than the BoMVR group. The Pelletier et al. [15] group showed similar results with better freedom rates for porcine valves (92 ± 2%) at 6 years against 68 ± 11% for bovine valves (p < 0.001), once again SVD being the predominant risk factor for reoperation the bovine group.
Thromboembolic events
The freedom from morbidities at 10 years was 90.3% for thromboembolism according to Yin Wang et al. [9] using the Hancock II valve. Rizzoli et al. [11] reported a 10-year freedom from thromboembolism events of 85.3 ± 2.2% in MVR groups using the same valve. The Bourguignon et al. [10] showed a higher 10-year freedom from thromboembolism of 97 ± 0.2% using the CEP valve. Our study showed better freedom from thromboembolism in the PoMVR group, probably the higher (85 vs 62%) drug compliance in the PoMVR group.
Bleeding
In general, the bleeding complications are far less for bioprosthetic valves than the mechanical valves. Our results also prove the same when compared with other studies. As there are many extrinsic factors like inherent bleeding tendency of the patient, need for prolonged anticoagulation and drug compliance with regular prothrombin time (PT) monitoring which control the overall bleeding rate, it is quite difficult to maintain a bleeding-free subset. Our study showed less bleeding events in the PoMVR group once again warfarin compliance and PT monitoring being better in that group.
Admissions for heart failure (not valve related)
There were different associated concomitant procedures in both the groups and our study showed lesser heart failure events in the PoMVR group which can be explained by the fact that the mean EUROSCORE II was lower in PoMVR (1.1 ± 0.7 vs 2.5 ± 0.8) (p < 0.04) when compared with BoMVR group. Not much studies are available in the literature to support the superiority of one valve type over the other as regards to heart failure.
Modifiable risk factors like statin therapy and metabolic syndrome are associated with faster degeneration of bioprosthetic valves in aortic position. In a study by Briand et al., metabolic syndrome, diabetes, renal insufficiency and higher mean gradient at baseline were independent risk factors for aortic bioprosthetic valve deterioration [16]. No published data exists for proving association between the above said risk factors and mitral bioprosthetic valve dysfunction. There is no data analysis for proving these risk factors and the SVD in this present study and we are planning to do it as a separate study in the future.
Conclusion
This study is one of its kind as to compare PoMVR and BoMVR on a reasonably long follow-up of 10 years from this geographical locality with significant sample size. Our study clearly shows the superiority of porcine valves over bovine valves with regard to SVD, NSVD and reoperation. Although the overall survival rate is almost same in both the groups, porcine valves fare better than the bovine valves in terms of valve-related events. Larger population with long-term follow-up is necessary to see whether the curve diverges after 10 years between the groups considering valve-related events.
Limitations
As the study period included patients from 2005 to 2008, in the BoMVR group, CEP valve was used at that time in the institute. Unfortunately, this valve has been stopped from production since 2017 and is no longer available in the local market. This is the biggest limitation.
Being a retrospective study, matching of both the groups could not be performed as effectively as one would expect in a randomized control trial.
The follow-up period was only up to 10 years while ideally 20 or if not at least 15 years duration would have been more effective in looking at the trend of the valve outcomes. This is especially true in the context of SVD and NSVD which are time-bound events.
In our study, it was observed that there was no valve-related mortality as the mortality was due to causes not related to valve.
The follow-up was not 100% complete and the statistical data analysis has been done to account for the loss of follow-up. The loss to follow-up was close to 12% which when assuming the worst case scenario probability concept might change the results. The actual risk of explantation due to SVD has been calculated taking into account the dropout rate. In general the rule of thumb is if the dropout rate is > 20% then there might be serious concern about the validity of the results, while ideally, if it is < 5% the study has more validity [17].
The valve choice, valve size, suturing technique and choice of cardioplegia were all surgeon dependent, and being a multi-surgeon centre, there was no uniformity in the conduct of surgery which might affect the outcomes, e.g., paravalvular leak due to continuous suturing technique in BoMVR resulting in NSVD.
The compliance to anticoagulation and PT monitoring was not uniform in both the groups.
Sub-group analysis within the PoMVR groups between the two different porcine valves was not done and neither was this group individually compared with the BoMVR which might have added more light towards the performance of these valves.
Acknowledgements
We acknowledge the statistical analysis and support provided by Dr. Balaji, Assistant Professor, Department of Community Medicine, The Madras Medical College.
Discussant
Abha Chandra
Professor and HOD
Department of Cardiovascular and Thoracic Surgery
Sri Venkateswara Institute of Medical Sciences
Tirupati, 517507 Andhra Pradesh
On the offset I would like to congratulate the authors for this study on the performance of both the Bovine and Porcine bio-prosthetic valves in the mitral position in the Indian population. This paper lacks the comprehensive Echocardiographic data which would have put some highlight on the hemodynamic performance of both the bovine and the porcine valves. The authors have tried to incorporate the maximum information to explain their data, but still some questions remain unanswered :
Question 1: The majority of patients undergoing mitral valve replacement in your series were in NYHA II functional class in both the groups, whereas in real world practice, most of the time, patients undergoing mitral valve replacement are in NYHA III – IV status. What do you think could be the reason for this?
Answer: The patients that we operated were referred mostly from the cardiology side and were managed medically well, with all the failure symptoms controlled, before they underwent surgery. The socio-economic status of our patients too was slightly on the higher side and these patients presented to us early, at the onset of slightest symptoms.
Question 2: Some patients underwent concomitant procedures along with the mitral valve replacement. What were these procedures? Did they affect your outcome ?
Answer: The concomitant procedures are elaborately described in table 2 and were mostly coronary artery bypass grafting surgery and tricuspid valve repair. They did not affect the outcomes and they did not reach significant statistical difference when compared between the two groups.
Question 3: In majority of cases, the size of the valve used has been 25 M (n- 27 + 33) and 27 M (n- 88+58), a total of 60 + 146 = 206. What do you think could be the reason for high use of small size of valve in your study?
Answer: The mitral annulus of our patients allowed the usage of only small size valves (the average BSA of such patients being 1.3m2). Further there was no evidence of PPM and in such scenarios we thought it was better not to be over zealous in putting a bigger size valve and creating more complications when the given size does not cause any PPM.
Question 4: There has been a mention of the maximum follow-up only, the mean follow-up should also have been considered.
Answer: The mean follow up (6.2 years) has been mentioned in the study.
Question5. The results show the freedom from non structural valve deterioration in the porcine group to be 97.1 + 0.07% and in the bovine group 91.6 + 0.08% (p<0.04). The freedom from structural valve deterioration has been 96.4 + 0.08% for the porcine group and 90.6 + 0.09% (p<0.05) in the bovine group at the end of the ten years. My question to you is:
-
How was the structural and the non structural deterioration assessed?
Answer: Based on Trans-thoracic and intra-operative trans-esophageal echoes and the corresponding echocardiographic details of such patients have been mentioned in the results analysis.
There has been a 10 years follow-up and apparently a large dropout rate which would have confounded the outcome results. How was this part assessed statistically.
Answer: The loss to follow-up was close to 12% and KM curves have been analysed after censoring for all such events. With any study,loss to follow-up is un avoidable and in general, if the follow-up loss is more than 20%, then there is a possibility of skewness of results, which has been mentioned in our limitations.
Question 6: There has been a very high incidence of mortality in both the groups: There were 8 early deaths among the Po MVR group, a 30-day mortality of 6.6%, and 15 early deaths in the Bo MVR group, a 30-day mortality of 9.7%. In the PoMVR group, there were 12 late deaths compared with 20 in the BoMVR group. What were the causes of death in this cohort?
Answer: the causes of early and late deaths in both the cohorts have been described in table 3.
Question 7: How many of these patients in both groups had valve related death.
Answer: None of the patients had valve related death.
Question 8: There has been no mention as to how many valves actually showed features of degeneration in both the groups. Do you have any figures to show the number of the degenerated valves in both the group?
Answer: The number of SVD and NSVD in both the groups are shown in the results part and in the PoMVR group it came to 4 and in the BoMVR group it was 19.
Funding
Nil Informed consent: Informed consent was obtained from all individual participants included in the study.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical committee clearance
All ethical committee clearances are obtained.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Butany J, Fayet C, Ahluwalia MS, et al. Biological replacement heart valves:identification and evaluation. Cardiovasc Pathol. 2003;12:119–139. doi: 10.1016/S1054-8807(03)00002-4. [DOI] [PubMed] [Google Scholar]
- 2.Jamieson WR, Burr LH, Munro AI, Miyagishima RT. Carpentier- Edwards standard porcine bioprosthesis: a 21-year experience. Ann Thorac Surg. 1998;66:S40–S43. doi: 10.1016/S0003-4975(98)01124-2. [DOI] [PubMed] [Google Scholar]
- 3.Frater RWM, Salomon NW, Rainer WG, Cosgrove DM, Wickham E. The Carpentier-Edwards pericardial aortic valve: intermediate results. Ann Thorac Surg. 1992;53:764–771. doi: 10.1016/0003-4975(92)91432-9. [DOI] [PubMed] [Google Scholar]
- 4.Pibarot P, Dumesnil JG. Prosthetic heart valves: selection of the optimal prosthesis and long-term management. Circulation. 2009;119:1034–1048. doi: 10.1161/CIRCULATIONAHA.108.778886. [DOI] [PubMed] [Google Scholar]
- 5.Cunanan CM, Cabiling CM, Dinh TT, et al. Tissue characterization and calcification potential of commercial bioprosthetic heart valves. Ann Thorac Surg. 2001;71:S417–S421. doi: 10.1016/S0003-4975(01)02493-6. [DOI] [PubMed] [Google Scholar]
- 6.Broom ND, Thomson FJ. Influence of fixation conditions on the performance of glutaraldehyde-treated porcine aortic valves: towards a more scientific basis. Thorax. 1979;34:166–176. doi: 10.1136/thx.34.2.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alavi SH, Ruiz V, Krasieva T, Botvinick EL, Kheradvar A. Characterizing the collagen Fiber orientation in pericardial leaflets under mechanical loading conditions. Ann Biomed Eng. 2013;41:547–561. doi: 10.1007/s10439-012-0696-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vesely I, Barber JE, Ratliff NB. Tissue damage and calcification may be independent mechanisms of bioprosthetic heart valve failure. J Heart Valve Dis. 2001;10:471–477. [PubMed] [Google Scholar]
- 9.Wang Y, Chen S, Hu XJ, Shi JW, Dong NG. Mid- to long-term clinical outcomes of Hancock II bioprosthesis in Chinese population. Chin Med J. 2015;128:3317–3323. doi: 10.4103/0366-6999.171424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bourguignon T, Espitalier F, Pantaleon C, et al. Bioprosthetic mitral valve replacement in patients aged 65 years or younger: long-term outcomes with the Carpentier–Edwards PERIMOUNT pericardial valve. Eur J Cardiothorac Surg. 2018;54:302–309. doi: 10.1093/ejcts/ezy029. [DOI] [PubMed] [Google Scholar]
- 11.Rizzoli G, Mirone S, Ius P, et al. Fifteen-year results with the Hancock II valve: a multicenter experience. J Thorac Cardiovasc Surg. 2006;132:602–609. doi: 10.1016/j.jtcvs.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 12.David TE, Armstrong S, Maganti M. Hancock II bioprosthesis for aortic valve replacement: the gold standard of bioprosthetic valves durability? Ann Thorac Surg. 2010;90:775–781. doi: 10.1016/j.athoracsur.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 13.Jamieson WRE, Burr LH, Miyagishima RT, et al. Reoperation for bioprosthetic mitral structural failure: risk assessment. Circulation. 2003;108:II-98–II–102. doi: 10.1161/01.cir.0000089184.46999.f4. [DOI] [PubMed] [Google Scholar]
- 14.Myken P, Bech Hanssen O, Phipps B, Caidahl K. Fifteen years follow up with the St. Jude Medical Biocor porcine bioprosthesis. J Heart Valve Dis. 2000;9:415–422. [PubMed] [Google Scholar]
- 15.Pelletier LC. Carrier M, Leclerc Y, Lepage G, deGuise P, Dyrda I. porcine versus pericardial bioprostheses: a comparison of late results in 1,593 patients. Ann Thorac Surg. 1989;47:352–361. doi: 10.1016/0003-4975(89)90373-1. [DOI] [PubMed] [Google Scholar]
- 16.Briand M, Pibarot P, Després JP, et al. Metabolic syndrome is associated with faster degeneration of bioprosthetic valves. Circulation. 2006;114:I-512–I-517. doi: 10.1161/CIRCULATIONAHA.105.000422. [DOI] [PubMed] [Google Scholar]
- 17.Sacket DL, Richardson WS, Rosenberg W, et al. Evidence-based medicine: how to practice and teach EBM. New York: Churchill Livingstone; 1997. pp. 121–123. [Google Scholar]


