Abstract
Objectives
The Fontan operation is usually followed by significant pleural effusion. We aimed to study the factors associated with persistent pleural effusion with special reference to serum cortisol levels.
Patients and methods
Thirty-eight patients undergoing the Fontan operation between September 2015 and November 2016 were prospectively studied. Parameters studied included age, weight, symptoms, atrio- ventricular valve regurgitation/stenosis/atresia, ventricular function, pulmonary artery pressures, oxygen saturation, aorto-pulmonary, and veno-venous collaterals, type of Fontan, duration of cardiopulmonary bypass, need for inotropes, duration of mechanical ventilation, conduit size, presence or absence of fenestration, and serum cortisol levels. The latter were measured before and after the Fontan operation and the co-relation between pleural effusion and change in serum cortisol levels was studied.
Results
Mean age at operation was 13.1 ± 5.6 years (median 13 years). Mean duration and amount of pleural drainage was 15.76 ± 13.2 days (median 11.5 days) and 9.15 ± 4.6 mL/kg/day (median 9 mL/kg/day) respectively. Statistically significant risk factors for prolonged pleural effusion were higher pulmonary artery (PA) pressures (r = 0.328, p = 0.003, odds ratio 1.30), higher inotropic score (r = 0.4, p = 0.01), lower rise in serum cortisol (p = 0.03),elevated superior caval venous pressure (CVP) at 6 h (r = 0.44, p = 0.005) and 12 h (r = 0.4, p = 0.01) and higher duration of mechanical ventilation (r = 0.45, p = 0.005).
Conclusions
PA pressures > 15 mmHg, higher inotropic score, higher CVP and lower rise in serum cortisol levels following the Fontan operation were associated with persistent pleural effusion.
Keywords: Fontan operation, Pleural effusion, Cortisol
Introduction
Significant fluid retention is common following the Fontan operation and usually manifests as pleural effusion [1–3]. The etiology of this is probably related to lymphatic failure after the Fontan. It has been proposed earlier that an increase in central venous pressure (CVP) favors the movement of fluid into the interstitial space resulting in prolonged pleural effusion; however other factors may also play an important role in increasing the amount and duration of pleural effusion [4].
A large number of other pre- operative and intra- operative factors have been studied to understand the causes of persistent pleural effusion following the Fontan operation. In a previous study from our institute, Airan et al. [5] found that aortic cross-clamp time more than 60 min and absence of fenestration were risk factors for persistent post-operative pleural effusion. Gupta et al. [6] demonstrated that lower preoperative oxygen saturation, post-operative infection, smaller conduit size, and longer duration of cardiopulmonary bypass were associated with increased pleural effusion following the extra cardiac Fontan operation (ECF).
In 2014, Saiki et al. [7], made an interesting observation that following the Fontan operation, low post-operative Cortisol levels were correlated with low cardiac and urine outputs, whereas high aldosterone levels were correlated with low cardiac output (LCOS) and increased blood pressure and CVP. They presumed that this variation in the postoperative levels of corticosteroids may induce imbalance in the fluid homeostasis that may lead to persistent pleural effusion. We therefore decided to investigate all factors leading to persistent pleural effusion following the Fontan operation. To test the hypothesis proposed by Saiki et al. [7], we studied the pre-and post-operative serum cortisol levels with the hypothesis that a lower rise in serum cortisol increases the risk of persistent pleural effusion following the Fontan operation.
Patients and methods
Between September 2015 and November 2016, thirty-eight patients underwent the Fontan operation at the Department of Cardiothoracic and Vascular Surgery, All India Institute of Medical Sciences, New Delhi, India. All these patients were included in this prospective, cohort study and no exclusions were made. Written informed consent was obtained from all patients or their parents and the study protocol was approved by the institutional ethics committee (IEC/NP-267/10.7.2015.RP-05/2015).
Pre-operative evaluation included detailed clinical history and examination, electrocardiogram, trans-thoracic echocardiography, and complete cardiac catheterization and cineangiography. The following details were collected pre-operatively: age (years), body weight (kg) and height (cm), symptoms (dyspnea/angina/palpitations/cyanosis/spells/syncope), signs (cyanosis/clubbing/pedal edema), and primary diagnosis (Table 1).
Table 1.
Echocardiographic findings in the study population
| Atrioventricular Valves | N (Percentage) |
|---|---|
| Normal | 20 (52.6) |
| Atresia | 11 (28.9) |
| Regurgitant | 07 (18.4) |
| Aortic Valve | |
| Normal | 35 (92.1) |
| Atresia | 02 (5.3) |
| Regurgitant | 01 (2.6) |
| Pulmonary valve | |
| Normal | 01 (2.6) |
| Atresia | 02 (5.3) |
| Regurgitant | 35 (92.1) |
| Dominant chamber | |
| Right | 12 (31.6) |
| Left | 19 (50) |
| Dual | 07 (18.4) |
| Anatomical diagnosis | |
| Double inlet right ventricle | 1 (2.6) |
| Unbalanced AVSD | 5 (13.2) |
| Single ventricle | 3 (7.9) |
| Criss-cross heart, VSD, PS | 1 (2.6) |
| Common atrium, mitral atresia | 1 (2.6) |
| Double inlet left ventricle, VSD, PS | 1 (2.6) |
| Tricuspid atresia, VSD, ASD, PS | 13 (34.2) |
| TGA, non-routable VSD, PS | 5 (13.2) |
| DORV, non-routable VSD, PS | 5 (13.2) |
| cc-TGA, non-routable VSD, PS | 3 (7.9) |
AVSD atrioventricularseptal defect, VSD ventricular septal defect, ASD atrial septal defect, PS pulmonary stenosis, TGA transposition of great arteries, DORV double outlet right ventricle
2D trans-thoracic echocardiography was performed to assess the primary anatomy, any atrio-ventricular valve lesion (regurgitation/stenosis/atresia), aortic valve and pulmonary valve lesions (regurgitation/atresia/stenosis), dominant ventricle, ventricular function, main pulmonary artery (PA) and branch PAs (confluence, size), and the status of a previously performed Glenn/systemic to PA shunt/PA band.
During cardiac catheterization specific attention was directed to measurement of mean PA pressure and systemic arterial saturation. Important parameters detailed on angiography were assessment of PA confluence and sizes of branch PAs, presence of significant aorto-pulmonary and veno-venous collaterals (moderate and large veno-venous collaterals), and evidence of any atrio-ventricular valve (AVV) regurgitation/stenosis or atresia. As published previously by others [8], aortopulmonary collateral arteries (APC) were considered to be significant if they supplied a significant segment of the lungs or if they were ≥ 3 mm in size. To assess the significance of veno-venous collaterals, the size of the innominate vein was measured. If these collaterals were less than 25% of the diameter of the innominate vein, they were considered small; those between 25 and 50% were graded as moderate-sized and those greater than this were graded as large. Inpatients with bilateral superior vena cava (SVC) where the innominate vein was absent, the size of the smaller SVC was used as a guide in a similar fashion [9].
All major APC (n = 24) were occluded by coil embolization in the cardiac catheterization laboratory just prior to the surgical procedure. Because we did not encounter any veno-venous collaterals, coil embolization of these was not required in any patient.
Surgical technique
All surgical procedures were performed by a single surgical team in a standardized manner.
In patients in whom an intracardiac procedure was not needed, extracardiac Fontan (ECF) was performed after establishing cardiopulmonary bypass (CPB) at normothermia, and the heart was kept beating. In seven patients, the ECF was performed without CPB using the technique described by us earlier [10]. In patients undergoing intracardiac procedures or those undergoing the lateral tunnel Fontan or the intra-extracardiac Fontan, CPB with core cooling up to 32 °C was used and a period of aortic cross clamping (ACC) was needed. The technique of intra-extracardiac Fontan has been described in detail by Jonas et al. [11].
Fenestration
Fenestration was performed in 14 patients in the cohort; these included patients undergoing simultaneous AVV replacements (n = 2), AVV repair (n = 2) and patients who had borderline higher PA pressures (n = 10). In all patients undergoing the lateral tunnel Fontan and the intra-extracardiac Fontan, the polytetra fluoroethylene patch/intracardiac conduit was fenestrated close to the opening of the inferior vena cava (IVC)using a 4-mm aortic punch. In patients undergoing extracardiac Fontan, a 4-mm aortic punch was used to create an opening in the conduit and this was then anastomosed to a corresponding opening in the adjacent RA.
Various intra- operative variables were recorded as follows: on/off pump procedure, extracardiac/lateral tunnel/intra-extracardiac Fontan, primary/completion Fontan, presence of previously performed systemic- PA shunt/ PA band/BDG, conduit size in patients undergoing ECF, presence or absence of fenestration, size of fenestration, CPB and ACC times, AVV regurgitation/stenosis/atresia, SVC pressure, and the systolic blood pressure just after induction of anesthesia and post-Fontan SVC pressure (CVP).
After completion of the Fontan operation, chest tubes were placed in the pericardial cavity and both pleural cavities in all patients. All patients were shifted to the intensive care unit (ICU) where a uniform protocol was followed for their post- operative management with an aim of extubation soon after arrival into the ICU. Until extubation, these patients were maintained on mechanical ventilator support in the Fowler’s position at a low PEEP (peak end expiratory pressure, < 5) with a lower tidal volume and a higher respiratory rate to promote flow in the Fontan circuit. Intravascular volume was maintained by administration of colloids (5% albumin). Fresh frozen plasma was avoided and blood was transfused only if the hemoglobin was below 12 g%. CVP and systolic blood pressure were measured continuously and the values at 6, 12, and 24 h were recorded.
Routine inotropic support in these patients consisted of Dobutamine (5 mcg/Kg/min) and sodium nitroprusside (0.5 mcg/Kg/min). Additional inotropes were used as indicated, and the total inotropic requirement was expressed as the Inotropic score [12], that of which was calculated by the formula:
The pericardial chest tube was removed once the drainage was below 50 ml in the preceding 24 h.
Measurement of serum cortisol
Serum cortisol was measured at 8 AM on the day of surgery and 24 h following surgery using the Chemiluminescent Immunoassay (CLIA) kits (Roche Healthcare, Basel, Switzerland). The Cortisone CLIA kit is designed to quantitatively measure Cortisone present in urine, saliva, and serum samples. A cortisone standard is provided to generate a standard curve for the assay and all samples are read off the standard curve. Standards or diluted samples are pipetted into a white micro-titer plate coated with an antibody to capture rabbit antibodies. A cortisone-peroxidase conjugate is added to the standard and sample in the wells. The binding reaction is initiated by the addition of a polyclonal antibody against cortisone to each well. After incubation for 2 h, the plate is washed and the chemiluminescent substrate is added. The substrate reacts with the bound cortisone-peroxidase conjugate to produce light. The generated light is detected in a micro-titer plate reader capable of reading luminescence. After making suitable correction for the dilution of the sample, the concentration of cortisone in the sample is calculated, using software available with most plate readers.
Pleural effusion
Significant pleural effusion was defined as drainage more than 20 ml/Kg/day or if it persisted for more than 2 weeks [6]. The type of pleural effusion (serous/chylous) was also recorded. The pleural tubes were removed once the drainage was less than 3 mL/kg in a 24-h period [6] and the chest X-Ray showed no pleural effusion. A chest tube was reinserted only if a symptomatic pleural effusion was diagnosed. Fontan failure was defined as all deaths within 30 days of operation, need for Fontan takedown, refractory ventricular arrhythmias, systemic venous, arterial or pulmonary thrombosis, refractory pleural effusion, ascites, peripheral edema, declining serum albumin, thrombocytopenia, hyperbilirubinemia, coagulopathy, and systemic desaturation [13].
Statistical analysis
Statistical analysis was performed using the SPSS software version 20 (IBM Corp., Armonk, N.Y. USA). The chi-square test was used for categorical variables (Table 2) and the unpaired Student t test was used for continuous variables which were normally distributed. Non-parametric continuous variables were analyzed using Wilcoxon rank-sum test/Mann Whitney U test. Univariate analysis was performed for identifying variables suspected to predict persistent pleural effusion. Pearson’s correlation coefficient (r) was calculated for continuous variables. Multivariate analysis with logistic regression was used to identify risk factors predictive of persistent pleural effusions with analysis of odds ratio and 95% confidence intervals (CI). A P value of < 0.05 was considered significant.
Table 2.
Categorical variables analyzed for persistent pleural effusion after the Fontan procedures
| Variables | Non-persistent pleural effusion (n = 20) | Persistent pleural effusion (n = 18) | P value |
|---|---|---|---|
| Pre-operative Hematocrit ≥ 70% | 2 | 8 | 0.016 |
| Use of CPB | 17 | 14 | 0.566 |
| AVVR | 1 | 3 | 0.242 |
| Fenestration | 7 | 7 | 0.804 |
| Primary/staged Fontan | 4 Primary/16 completion | 5 Primary/13 completion | 0.573 |
| Aortopulmonary collaterals | 11 | 13 | 0.272 |
Data are expressed as mean ± SD. Figures in bold indicate significant observations. CPB cardiopulmonary bypass, AVVR atrioventricular valve regurgitation
We also determined whether the sample size of 38 patients was sufficient to draw meaningful conclusions. The formula used for this purpose was:
Sample size (number) = Z × Z p(1-p)/ d × d, where Z = 1.96 corresponding to 95% confidence intervals and p represents the incidence of pleural effusion following the Fontan and has previously been derived to be 37% [6]. d represents the degree of precision. Using this equation, the degree of precision of our study involving 38 patients has been calculated to be 0.15, with a statistical power of 80%; thus, the sample size of 38 patients is sufficient to draw statistically meaningful conclusions.
Results
Out of the 38 patients, 27 were male. Mean age was 13.1 ± 5.6 years (range 4–26 years, median 13 years) and mean weight was 32.5 ± 11.9 kg (range 15–60 kg, median 33 kg). Dyspnea and cyanosis were the predominant symptoms (n = 36, 94.7%). The mean PA pressure on cardiac catheterization was 14.9 ± 3.9 mmHg (range 7–23 mmHg). Twenty-nine (76.3%) patients (76.3%) had undergone a bidirectional Glenn (BDG) prior to the Fontan. The remaining patients underwent a single stage Fontan. ECF was performed in 35 (92.1%) patients, two patients underwent the lateral tunnel Fontan and one underwent Fontan using a tube graft within the right atrium (intra-extracardiac Fontan). In 31 patients, the Fontan was performed on CPB, while in seven patients, it was performed without CPB.
There was one early death. This 13-year-old patient developed unstable haemodynamics, arrhythmias and ascites following a lateral tunnel Fontan that was taken down on the fourth post-operative day; however, she died of multi-organ failure. The mean pleural drainage was 9.16 ± 4.72 mL/kg/day (range 1–21 mL/kg/day, median = 9 mL/kg/day), and the mean duration of pleural effusion was 15.7 ± 13.4 days (range 2–68 days, median 11.5 days). The mean ICU stay was 56 ± 34 h (median 48 h, range 4–180 h) and the median duration of hospital stay was 20.1 ± 13.6 days (median 15 days, range 8–72 days).
Table 3 lists the variables that were analyzed to assess their impact on the development of pleural effusion in a univariate model.
Table 3.
Continuous variables analyzed for persistent pleural effusion after the Fontan procedure
| Variables | Univariate analysis | Multivariate analysis with Logistic regression | ||||
|---|---|---|---|---|---|---|
| Total (n = 38) | Non-persistent pleural effusion (n = 20) | Persistent pleural effusion (n = 18) | P value | Odds ratio (95%CI) | P value | |
| Age (years) | 13.11 ± 5.62 | 12.7 ± 6.2 | 13.58 ± 5.1 | 0.64 | 1.032 (0.919–1.159) | 0.594 |
| Mean PAP (mmHg) | 14.89 ± 3.93 | 13.26 ± 3.63 | 16.61 ± 3.57 | 0.007 | 1.306 (1.05–1.624) | 0.017 |
| SpO2 (%) | 80.05 ± 7.85 | 79.28 ± 7.89 | 80.94 ± 7.99 | 0.54 | 1.0280 (.942–1.123) | 0.534 |
| CPB duration (min) | 85.83 ± 28.59 | 79.41 ± 36.73 | 76.93 ± 33.46 | 0.98 | 0.998 (0.977–1.019) | 0.841 |
| Aortic cross clamp duration (min) | 43.44 ± 15.7 (n = 9) | 57.33 ± 14.50 (n = 3) | 36.50 ± 11.73 (n = 6) | 0.06 | 0.846 (0.679–1.054) | 0.137 |
| Conduit size (median) n = 36 | 19 | 17 (n = 20) | 15 (n = 16) | 0.29 | 0.9460.853–1.049 | 0.290 |
| Pre-operative SVC Pressure (mmHg) | 13.5 ± 2.1 | 13.4 ± 1.9 | 13.7 ± 2.2 | 0.63 | 1.083 (0.772–1.520) | 0.643 |
| Pre-operative SBP (mmHg) | 112.6 ± 12.04 | 113.7 ± 10.6 | 111.4 ± 13.7 | 0.56 | 0.972 (0.915–1.033) | 0.362 |
| Inotropic score | 8.1 ± 5.4 | 5.7 ± 3.3 | 10.9 ± 5.9 | 0.001 | 1.367 (1.081–1.729) | 0.009 |
| CVP 6 h (mmHg) | 14.4 ± 2.5 | 13.7 ± 2.6 | 15.3 ± 2.2 | 0.04 | 1.314 (0.983–1.756) | 0.065 |
| CVP 12 h (mmHg) | 13.7 ± 3.1 | 12.8 ± 2.8 | 14.7 ± 3.0 | 0.04 | 1.264 (0.991–1.612) | 0.059 |
| CVP 24 h (mmHg) | 14.1 ± 2.6 | 13.1 ± 2.3 | 15.1 ± 2.5 | 0.02 | 1.427 (1.015–2.006) | 0.041 |
| SBP 6 h (mmHg) | 95.7 ± 12.7 | 99.2 ± 11.5 | 91.9 ± 13.3 | 0.08 | 0.9520.899–1.008 | 0.90 |
| SBP 12 h (mmHg) | 101.1 ± 13.5 | 107.2 ± 12.9 | 94.4 ± 10.9 | 0.002 | 0.919 (0.865–0.977) | 0.006 |
| SBP 24 h (mmHg) | 103.2 ± 12.4 | 107.9 ± 10.9 | 98.8 ± 12.4 | 0.01 | 0.923 (0.862–0.989) | 0.022 |
| Pre-operative Serum cortisol (mg/dL) | 12.24 ± 7.02 | 11.20 ± 5.14 | 13.39 ± 8.66 | 0.34 | 1.099 (0.966–1.250) | 0.151 |
| Post-operative serum cortisol (mg/dL) | 31.21 ± 19.51 | 36 ± 21.38 | 25.89 ± 16.20 | 0.11 | 0.958 (0.913–1.004) | 0.076 |
| Rise in Serum cortisol (mg/dL) | 19.03 ± 17.97 | 24.95 ± 20.56 | 12.44 ± 11.98 | 0.03 | 0.954 (0.911–0.998) | 0.042 |
| Mechanical ventilation (h) | 18.5 ± 65 | 7 ± 2.1 | 31.4 ± 94.1 | 0.25 | 1.170 (0.919–1.489) | 0.204 |
| ICU stay (h) | 56 ± 34 | 49 ± 21 | 64 ± 45 | 0.18 | 1.014 (0.993–1.014) | 0.193 |
| Hospital Stay (days) | 20.1 ± 13.6 | 13.3 ± 2.8 | 27.7 ± 16.6 | 0.0005 | 1.441 (1.0861.911) | 0.011 |
Data are expressed as mean ± SD. Figures in bold indicate significant observations = cardiopulmonary bypass, SVC = superior vena cava, SBP = systolic blood pressure, PAP = pulmonary artery pressure
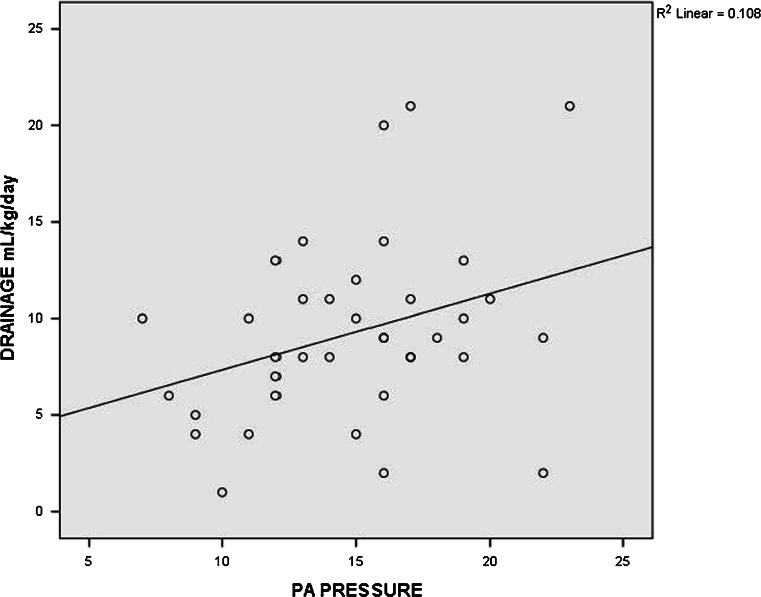
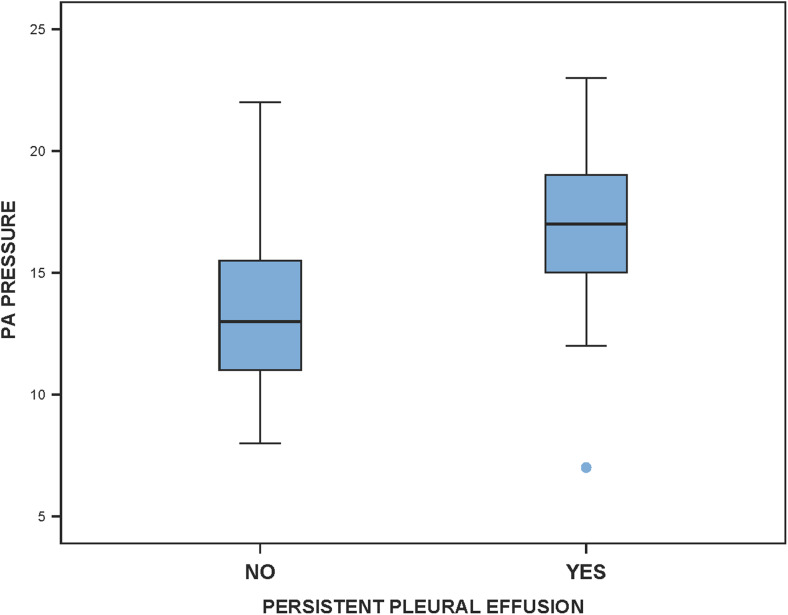
On univariate analysis, a mean PA pressure greater than 15 mmHg was found to be a risk factor for persistent pleural effusion (P = 0.005, odds ratio = 1.30, 95% CI 1.05–1.62). This means that patients with a mean PA pressure more than 15 mmHg had a 1.3 times higher chance of developing persistent pleural effusion when compared to those with a mean PA pressure below 15 mmHg. In addition, an important finding was that the mean PA pressures showed a statistically significant positive correlation with the amount of pleural drainage (r = 0.328, P = 0.04) and its duration (r = 0.4, P = 0.01). These findings are depicted in (Figs. 1 and 2).
Fig. 1.
Box plot showing significantly higher PA pressures (mm Hg) in patients with persistent pleural effusion
Fig. 2.
Two-way scatter diagram showing a statistically significant positive correlation between PA pressure (mm Hg) and the amount of pleural drainage
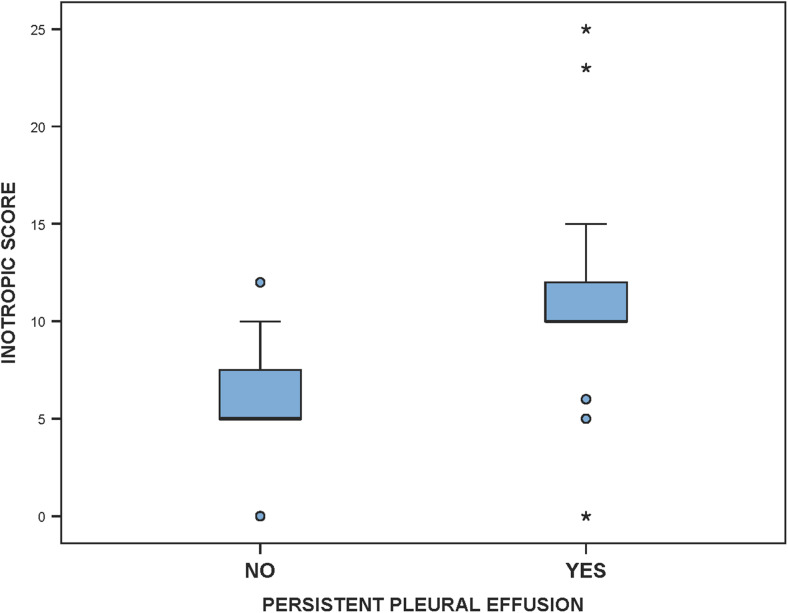
A higher inotropic score at 24 h was associated with persistent pleural effusion (P = 0.001, Fig. 3). Higher inotropic scores were correlated with a higher amount and duration of pleural drainage, which was statistically significant (r = 0.367, P = 0.02 and r = 0.395, P = 0.01, respectively).
Fig. 3.
Box plot depicting statistically significant higher inotropic scores in patients with persistent pleural effusion (ml)
Pre-operative systemic oxygen saturation, use of CPB and its duration, type of Fontan, conduit size, and presence of fenestration and its size had no significant impact on the amount and duration of pleural effusion. Pre-operative SVC pressure, IVC pressure or systolic blood pressure also had no impact.
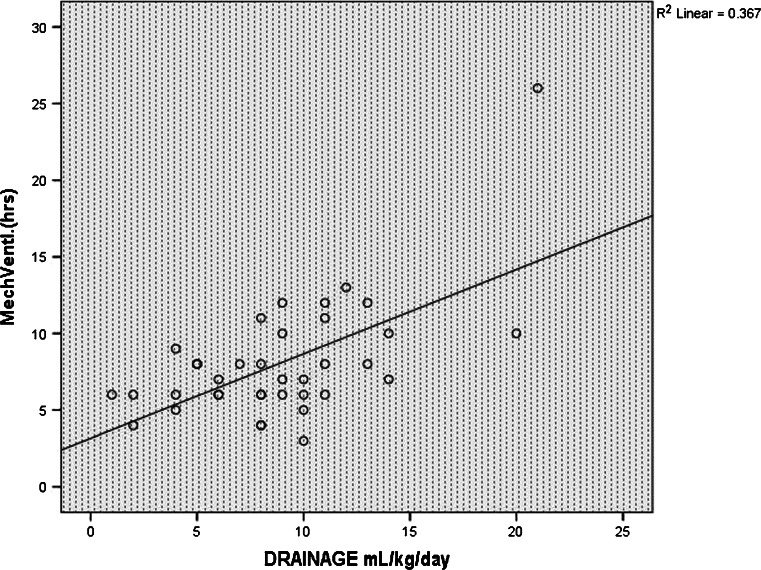
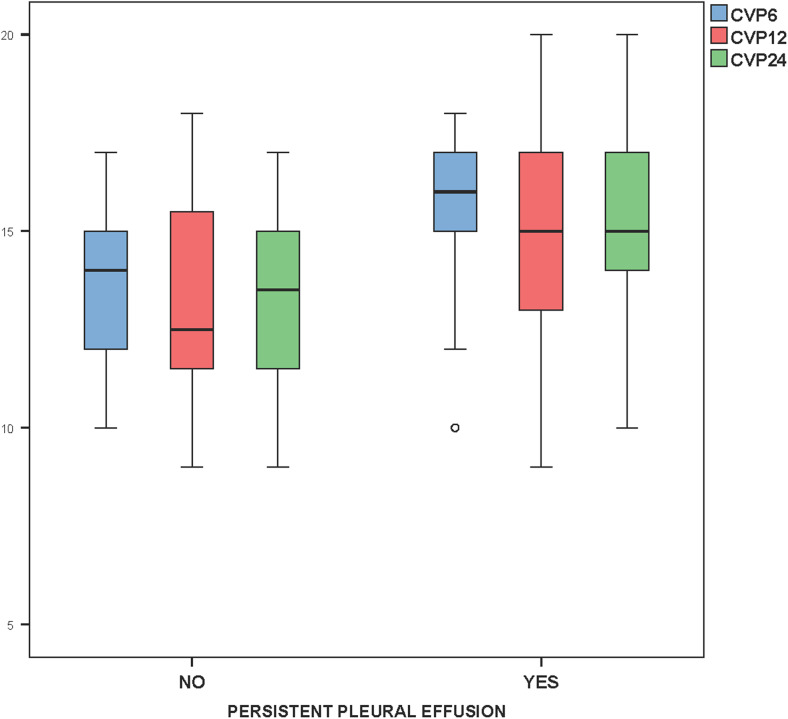
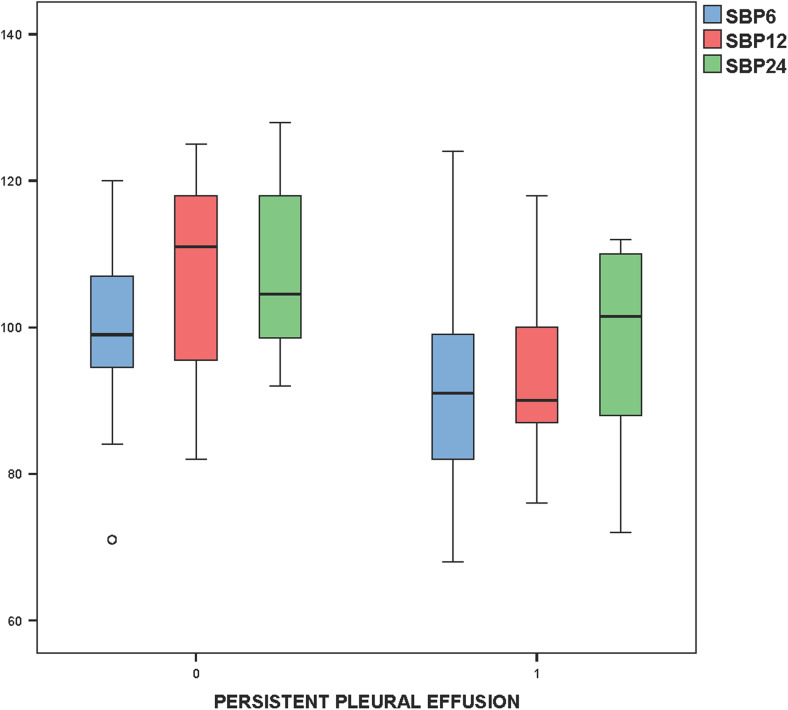
The duration of mechanical ventilation was positively correlated with the amount of pleural drainage (r = 0.606, P = 0.001, Fig. 4). The CVP measured (using the SVC pressure line) at 6, 12, and 24 h in the post-operative period was also statistically related to persistent pleural effusion (P = 0.04, 0.04 and 0.02, respectively (Fig. 5). Post-operative systolic blood pressure was also found to be statistically related to persistent pleural effusion at 12 and 24 h after surgery (P = 0.002 and 0.01, respectively, Fig. 6).
Fig. 4.
Scatter diagram showing positive correlation of the duration of mechanical ventilation with the amount of pleural drainage (n = 37)
Fig. 5.
Box plot showing higher central venous pressures at 6, 12, and 24 h in the post-operative period related to persistence of pleural effusion
Fig. 6.
Box plot indicating a statistically significant relationship of systolic blood pressures at 12 and 24 h post operatively with persistent pleural effusion
Correlation with serum cortisol levels
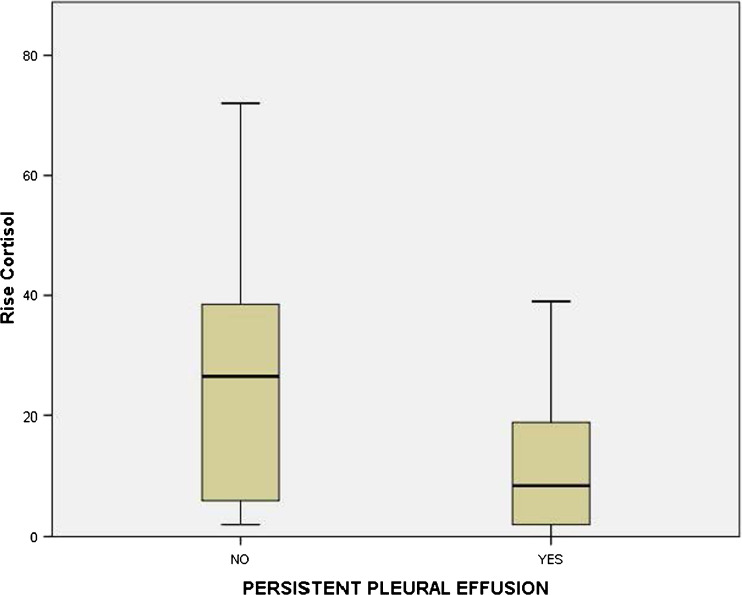
The mean serum cortisol level in the pre-operative period was 12.24 ± 10.5 mg/dL; while in the post-operative period, it was 31.21 ± 23 mg/dL. Mean rise from the baseline serum cortisol values in patients with non-persistent pleural effusion was 24.95 ± 20.56 mg/dL whereas in those with persistent effusion, it was 12.44 ± 11.98 mg/dL. A lower rise was found to be an independent risk factor for persistent pleural effusion on univariate analysis using unpaired Student’s t test (P = 0.03, Fig. 7).
Fig. 7.
Box plot depicting significantly lower rise in serum cortisol (mg/dL) in patients with persistent pleural effusion
Discussion
Since Fontan and Kreutzer described the rerouting of the systemic venous return to the pulmonary arteries [14, 15], the procedure has undergone many technical modifications. However, the incidence of prolonged pleural effusion remains high and is a source of considerable morbidity and prolongs the hospital stay [16].Rogers et al. [16], in their retrospective review of 771 patients, found longer operative times and higher pulmonary artery pressures were correlated with a higher incidence of prolonged pleural drainage and longer hospital stay. There were 27 deaths in their experience (3.5%), whereas our prospective cohort study, we encountered only one early death.
Previous publications also indicate that lower pre-operative oxygen saturation and polycythemia [17] are related to poorer outcomes after the Fontan operation. However, we found no statistically significant correlation between the pre- operative oxygen saturation and the amount and duration of pleural drainage. However, a higher hematocrit was correlated with a higher incidence of significant pleural effusions. Our results for duration of chest tube drainage, prevalence of different anatomical diagnosis and in-hospital deaths were similar to that found by Mendoza et al. [18].
Earlier studies have indicated Fontan at a younger age to be a major risk factor for prolonged morbidity and high mortality [18]. However, we did not observe this. Previous experience [19, 20] has shown that staging of the Fontan after a prior BDG is associated with a reduction in the incidence, amount, and duration of pleural effusion. The results of our study do not seem to indicate this and no differences in these outcome parameters were observed between a primary and staged Fontan. However, higher pre- operative PA pressures, particularly a mean PA pressure above 15 mmHg and post-operative CVP were found to be statistically significant factors that impacted the development of persistent pleural effusion, a finding that has been observed by Fedderly et al. and others [16, 21–23]. A higher inotropic score was also found to be associated with persistent pleural drainage.
Contrary to other studies [6, 24, 25], fenestration of the conduit had no influence on the amount and duration of pleural effusion. We also observed that lower systolic blood pressure measurements at 12 and 24 h following the Fontan operation were related to prolonged pleural effusion and higher need for inotropes. This may be explained by the fact that transient post-cardiotomy myocardial depression is known, and inotropic support can improve the impaired ventricular function [26]. Because of inadequate flow through the Fontan circuit, some of these patients may have required increased pre-load for optimal functioning of the Fontan circuit. This necessitated administration of a higher amount of intravenous fluids to maintain the pre-load. As a consequence, higher CVP levels were observed. These higher CVP levels could have resulted in fluid extravasation and enhanced the risk of development of prolonged pleural effusions [26]. This confirms previous observations that the driving force of a higher systemic venous pressure is a necessity for optimal functioning of the Fontan circuit; this higher systemic venous pressure maintains forward flow into the pulmonary vasculature. However, paradoxically, it also elevates the lower body systemic venous pressure. Hence, acute elevation of the systemic venous pressure is both a necessity and effect of the adaptation to the Fontan physiology and an acutely elevated systemic venous pressure is an integral component of the Fontan circulation. This therefore increases the risk of capillary transudation of fluid and decreased reabsorption of interstitial fluid [27].
We did not observe any significant difference in the incidence of persistent pleural effusion in the on-pump versus off-pump Fontan group. This is contrary to our previous experience [10, 17] and those of others [28] where the patients who underwent an off-pump Fontan had a lower incidence of pleural effusion.
A longer duration of positive pressure ventilation was associated with a statistically higher amount but not the duration of pleural effusion. Yun et al. [23] have previously shown that a low pulmonary vascular compliance is associated with a higher incidence of prolonged pleural effusion. Whether the higher effusion is a cause or effect of prolonged ventilatory support is not clear. We attempted to derive a correlation between the variation in the levels of serum cortisol and pleural effusion and this was one of our primary end-points. On doing this analysis, we observed that a higher rise in cortisol levels after the Fontan operation lowered the risk of pleural effusion. On the other hand, patients who demonstrated a lower rise in serum cortisol levels after the Fontan operation had higher odds of developing persistent pleural effusions (odds ratio 0.954, P = 0.04, 95% CI 0.911–0.998), meaning thereby that patients with a higher rise in serum cortisol levels had 0.9 times the risk of developing significant pleural effusion when compared to those patients who demonstrated a lower rise in serum cortisol levels.
Hence our results indicate that a rise in serum cortisol exhibited an independent negative correlation with the duration and amount of pleural drainage. It is well known that abnormal water retention following the Fontan operation increases the risk of pleural effusion [5]. In response to stress and hemodynamic changes that occur following the Fontan operation, serum levels of cortisol and aldosterone show variable physiologic action and help in preserving the cardiovascular functional integrity. Stewart et al. [29] first proposed the concept that humoral substances may play an etiological role in the effusion process and that in response to stress and hemodynamic alterations, cortisol, and aldosterone levels may act in varied ways to maintain the cardiovascular functional integrity. Mainwairing et al. [30] also showed that patients undergoing a Fontan had increased anti-diuretic hormone (ADH), cortisol, aldosterone, renin, and angiotensin I levels. The increased renin and angiotensin I levels persisted on the fifth postoperative day. Saiki et al. [7] found that after the Fontan operation, the cortisol excretion in the urine was significantly lower as compared to the non-Fontan control group indicating a state of adrenal insufficiency after the Fontan circulation. These hormonal changes may account in part for the fluid retention that typically persists for many days after the Fontan operation.
Cortisol is known to have important physiological actions on the cardiovascular system including enhancement of vascular sensitivity to catecholamines and angiotensin II and preserves ventricular function and reduces vascular permeability and thus may prevent the extravasation of fluid due to systemic venous hypertension after the Fontan operation.
Study limitations
Our study is limited by a small number of patients with limited follow-up information. However, this study carried a statistical power of 80% and a precision of 0.15 and in the absence of larger previous studies (that help to calculate the sample size), this small sample size was adequate to draw meaningful conclusions.
In addition, pleural effusion may recur after hospital discharge in a significant number of patients in follow-up and this issue has not been addressed in this study. Also, the other inflammatory markers that are associated with prolonged effusion were not studied by us. In addition, we measured the serum cortisol levels at two temporal points in time; this could have been improved by serial measurements of serum cortisol levels and correlating the same with the urinary cortisol levels. We do not have any experience with the technique of lymphatic redirection that has been proposed by Hraska [31] to minimize the duration and amount of pleural drainage and are therefore unable to comment on the efficacy of this procedure. In those who do develop significant effusions, it has been our policy to perform a diaphragmatic fenestration after ruling out any residual surgical problems [8].
Excellent results with a low incidence of pleural effusion have been reported earlier in a large experience utilizing a short period of deep hypothermia and circulatory arrest [32]. This is, however, debatable [16] and we have not used the latter in any of our patients.
Conclusions
Following the Fontan operation, the circulatory hemodynamics change at multiple levels and multiple factors are responsible for persistent pleural effusion in these patients. Our results indicate that preoperative mean PA pressures >15 mmHg, higher pre-operative hematocrit, higher inotropic score, higher CVP and lower rise in serum cortisol levels following the Fontan operation were associated with persistent pleural effusion. Modifications in some of these risk factors may reduce the morbidity caused by prolonged pleural effusions in patients undergoing the Fontan operation.
Acknowledgements
The authors thank Dr Vishnubhatla Sreenivas, Professor of Biostatistics, All India Institute of Medical Sciences, New Delhi, for statistical analysis.
Compliance with ethical standards
Conflict of interest
All Authors declare that they have no conflict of interest and do not receive any research grants from any company, have not received a speaker honorarium from any company, do not own any stock in any company, and are not members of a committee.
Statement of human rights/ethical approval
All procedures performed in this study were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this study, formal consent was obtained.
Informed consent
Informed consent was obtained from all individual participants included in the study.
References
- 1.de Vivie ER, Rupprath G. Long-term results after Fontan procedure and its modifications. J Thorac Cardiovasc Surg. 1986;91:690–7. [PubMed]
- 2.Kiziltepe U, Eyileten ZB, Uysalel A, Akalın H. Prolonged pleural effusion following Fontan operation: effective pleurodesis with talc slurry. Int J Cardiol. 2002;85:297–9. [DOI] [PubMed]
- 3.Mascio CE, Wayment M, Colaizy TT, Mahoney LT, Bukhart HM. The modified Fontan procedure and prolonged pleural effusions. Am Surg. 2009;75:175–7. [DOI] [PubMed]
- 4.Mainwaring RD, Lamberti JJ, Uzark K. The bidirectional Glenn procedure: palliation of the univentricular heart. Adv Card Surg. 1994;5:115–140. [PubMed] [Google Scholar]
- 5.Airan B, Sharma R, Choudhary SK, et al. Univentricular repair: is routine fenestration justified? Ann Thorac Surg. 2000;69:1900–6. [DOI] [PubMed]
- 6.Gupta A, Daggett C, Behera S. Risk factors for persistent pleural effusions after the extracardiac Fontan procedure. J Thorac Cardiovasc Surg. 2004;127:1664–9. [DOI] [PubMed]
- 7.Saiki H, Kuwata S, Kurishima C, Iwamoto Y, Ishido H, Masutani S, et al. Aldosterone-cortisol imbalance immediately after Fontan operation with implications for abnormal fluid homeostasis. Am J Cardiol. 2014;114:1578–83. [DOI] [PubMed]
- 8.Talwar S, Das A, Choudhary SK, Airan B. Diaphragmatic fenestration for resistant pleural effusions after univentricular palliation. World J Pediatr Congenit Heart Surg. 2016;7:146–51. [DOI] [PubMed]
- 9.McElhinney DB, Reddy VM, Hanley FL, Moore P. Systemic venous collateral channels after bidirectional cavopulmonary anastomosis: Evolution and management. J Am Coll Cardiol. 1997;30:817–824. doi: 10.1016/S0735-1097(97)00223-4. [DOI] [PubMed] [Google Scholar]
- 10.Talwar S, Muthukkumaran S, Makhija N, Hasija S, Choudhary SK, Airan B. Extra cardiac Fontan without cardiopulmonary bypass: techniques and results. Indian J Thorac Cardiovasc Surg. 2013;29:174–83.
- 11.Jonas RA. The intra/extracardiac conduit fenestrated Fontan. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2011;14:11–8. [DOI] [PubMed]
- 12.Gaies MG, Gurney JG, Yen AH, et al. Vasoactive-inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass. Pediatr Crit Care Med. 2010;11:234–8. [DOI] [PubMed]
- 13.Deal BJ, Jacobs ML. Management of the failing Fontan circulation. Heart. 2012;98:1098–104. [DOI] [PMC free article] [PubMed]
- 14.Fontan F, Baudet E. Surgical repair of tricuspid atresia. Thorax. 1971;26:240–8. [DOI] [PMC free article] [PubMed]
- 15.Kreutzer G, Galíndez E, Bono H, De Palma C, Laura JP. An operation for the correction of tricuspid atresia. J Thorac Cardiovasc Surg. 1973;66:613–21. [PubMed]
- 16.Rogers LS, Glatz AC, Ravishankar C, et al. 18 years of the Fontan operation at a single institution: results from 771 consecutive patients. J Am Coll Cardiol. 2012;60:1018–25. [DOI] [PubMed]
- 17.Talwar S, Agarwala S, Mittal CM, Choudhary SK, Airan B. Pleural effusions in children undergoing cardiac surgery. Ann Pediatr Cardiol. 2010;3:58–64. [DOI] [PMC free article] [PubMed]
- 18.Mendoza A, Albert L, Ruiz E, et al. Fontan operation. Hemodynamic factors associated with postoperative outcomes. Rev Espanola Cardiol Engl Ed. 2012;65:356–62. [DOI] [PubMed]
- 19.Hirsch JC, Goldberg C, Bove EL, et al. Fontan operation in the current era: a 15-year single institution experience. Ann Surg. 2008;248:402–10. [DOI] [PubMed]
- 20.Uemura H, Yagihara T, Kawashima Y, et al. What factors affect ventricular performance after a Fontan-type operation? J Thorac Cardiovasc Surg. 1995;110:405–15. [DOI] [PubMed]
- 21.Fedderly RT, Whitstone BN, Frisbee SJ, Tweddel JS, Litwin SB. Factors related to pleural effusions after fontan procedure in the era of fenestration. Circulation. 2001;104:I-148–51. [DOI] [PubMed]
- 22.Mascio CE, Austin EH. Pleural effusions following the Fontan procedure. Curr Opin Pulm Med. 2010;16:362–6. [DOI] [PubMed]
- 23.Yun T-J, Im Y-M, Jung SH, et al. Pulmonary vascular compliance and pleural effusion duration after the Fontan procedure. Int J Cardiol. 2009;133:55–61. [DOI] [PubMed]
- 24.Bridges ND, Mayer JE, Lock JE, et al. Effect of baffle fenestration on outcome of the modified Fontan operation. Circulation. 1992;86:1762–9. [DOI] [PubMed]
- 25.Lemler MS, Scott WA, Leonard SR, Stromberg D, Ramaciotti C. Fenestration improves clinical outcome of the Fontan procedure: a prospective, randomized study. Circulation. 2002;105:207–12. [DOI] [PubMed]
- 26.Garofalo CA, Cabreriza SE, Quinn TA, et al. Ventricular diastolic stiffness predicts perioperative morbidity and duration of pleural effusions after the Fontan operation. Circulation. 2006;114:I56–61. [DOI] [PubMed]
- 27.Menon S, Chennapragada M, Ugaki S, Scholler GF, Ayer J, Winlaw DS. The lymphatic circulation in adaptations to the Fontan circulation. Pediatr Cardiol. 2017;38:886–892. doi: 10.1007/s00246-017-1576-y. [DOI] [PubMed] [Google Scholar]
- 28.Shikata F, Yagihara T, Kagisaki K, et al. Does the off-pump Fontan procedure ameliorate the volume and duration of pleural and peritoneal effusions? Eur J Cardiothorac Surg. 2008;34:570–75. [DOI] [PubMed]
- 29.Stewart JM, Gewitz MH, Clark BJ, et al. The role of vasopressin and atrial natriuretic factor in postoperative fluid retention after the Fontan procedure. J Thorac Cardiovasc Surg. 1991;102:821–9. [PubMed]
- 30.Mainwaring RD, Lamberti JJ, Moore JW, Billman GF, Nelson JC. Comparison of the hormonal response after bidirectional Glenn and Fontan procedures. Ann Thorac Surg. 1994;57:59–63. [DOI] [PubMed]
- 31.Hraska V. Decompression of thoracic duct: new approach for the treatment of failing Fontan. Ann Thorac Surg. 2013;96:709–711. doi: 10.1016/j.athoracsur.2013.02.046. [DOI] [PubMed] [Google Scholar]
- 32.Meyer DB, Zamora G, Wernovsky G, Ittenbach RF, Gallagher PR, Tabbutt S, et al. Outcomes of the Fontan procedure using cardiopulmonary bypass with aortic cross clamping. Ann Thorac Surg. 2006;82:1611–1620. doi: 10.1016/j.athoracsur.2006.05.106. [DOI] [PubMed] [Google Scholar]