Abstract
Diabetes mellitus (DM) is a leading risk factor for aging-related dementia; however, the underlying mechanisms are not well understood. The present study, utilizing a non-obese T2DN diabetic model, demonstrates that the myogenic response of the middle cerebral artery (MCA) and parenchymal arteriole (PA) and autoregulation of cerebral blood flow (CBF) in the surface and deep cortex were impaired at both young and old ages. The impaired CBF autoregulation was more severe in old than young DM rats, and in the deep than the surface cortex. The myogenic tone of the MCA was enhanced at perfusion pressure in the range of 40–100 mmHg in young DM rats but was reduced at 140–180 mmHg in old DM rats. No change of the myogenic tone of the PA was observed in young DM rats, whereas it was significantly reduced at 30–60 mmHg in old DM rats. Old DM rats had enhanced blood-brain barrier (BBB) leakage and neurodegeneration, reduced vascular density, tight junction, and pericyte coverage on cerebral capillaries in the CA3 region in the hippocampus. Additionally, DM rats displayed impaired functional hyperemia and spatial learning and short- and long-term memory at both young and old ages. Old DM rats had impaired non-spatial short-term memory. These results revealed that impaired CBF autoregulation and enhanced BBB leakage plays an essential role in the pathogenesis of age- and diabetes-related dementia. These findings will lay the foundations for the discovery of anti-diabetic therapies targeting restoring CBF autoregulation to prevent the onset and progression of dementia in elderly DM.
Keywords: Diabetes mellitus, Myogenic response, Autoregulation, Blood-brain barrier, Cognitive impairments
Introduction
Age-related cognitive impairments or dementia is an emerging global health care crisis. The most common form of dementia is a mixed pathology of cerebrovascular disease and Alzheimer’s disease (AD) (Gaugler et al. 2019). Aging is the most significant risk factor for late-onset dementia (Hebert et al. 2010; Hebert et al. 2013), and diabetes mellitus (DM) is one of the leading cardiovascular causes for cognitive deficits (Samaras et al. 2014). Twenty to 40% of type 2 DM patients suffer from cerebrovascular diseases, which consists of 45% of cases of dementia (Mayeda et al. 2015). The incidence of dementia is up to 2.5 times greater in long-standing DM patients (Biessels et al. 2014; Gaugler et al. 2019; Gudala et al. 2013; Mayeda et al. 2015). Given the growing prevalence of DM, and an increasing average life span, the relationships between DM and dementia are of great importance; however, these intriguing epidemiologic findings have not been translated into successful treatments. Additionally, whether long-standing DM induces cerebrovascular dysfunction and if and how it is related to the development of dementia have not been well understood.
The brain accounts for 2% of total body weight and consumes 20% of total body energy (Attwell et al. 2010). Since it lacks fuel storage, the brain needs glucose and oxygen continuously supplied by cerebral blood flow (CBF) (Verweij 2007). It is well accepted that DM promotes endothelial dysfunction and enhances reactive oxygen species (ROS) production (Lagaud et al. 2001; McDaniel 1999; Tabit et al. 2010), which may increase the susceptibility to ischemic brain injury. However, there is not enough evidence to support the view that endothelial dysfunction and presumed intermittent localized hypoxia is sufficient to induce dementia. Previous studies indicated that DM rats exhibited enhanced cerebral vascular permeability at a young age (Li et al. 2010) that is often in association with increased myogenic tone in various vascular beds (Jarajapu et al. 2008; Lagaud et al. 2001; Sachidanandam et al. 2009; Zimmermann et al. 1997). Notably, myogenic tone and vascular density were reduced in the DM rats when they were aging (Abd-Elrahman et al. 2017; Abd-Elrahman et al. 2015; Abdelsaid et al. 2014; Kelly-Cobbs et al. 2011; Kold-Petersen et al. 2012; Murakami et al. 2012). Moreover, CBF was reduced after induction of ischemic injury with middle cerebral artery occlusion (MCAO) in young DM rats even when they had enhanced cerebral vascular density (Li et al. 2010). These controversial observations raise a scientific gap, namely, whether the inverse relationship between age and myogenic tone in different vascular beds of DM also occurs in the cerebral circulation, and whether it causes cerebral vascular dysfunction that contributes to the development of cognitive deficits.
Autoregulation of CBF maintains constant blood flow in the brain to protect against increased transmission pressure to fragile capillaries that can result in blood-brain barrier (BBB) leakage, ischemic injury, and neurodegeneration possibly under aging or other pathological conditions (Fan et al. 2015a, 2016a; Shekhar et al. 2017; Tarantini et al. 2018; Ungvari et al. 2018a, b). CBF autoregulation is mediated by an interplay between the myogenic response of vascular smooth muscle cells (VSMCs) and constrictive pericytes in concert with the release of vasodilatory metabolic mediators from endothelial cells (ECs) and astrocytes (Cipolla et al. 2002; Fan et al. 2015b, 2017; Faraci et al. 1990; Harder et al. 2011; Hartmann et al. 2015; Toth et al. 2017). The myogenic response is defined more than a century ago by Sir William Bayliss (Bayliss 1902) as an intrinsic property of VSMCs that allows blood vessels to constrict in response to elevations in blood pressure. Previous studies demonstrated that a higher incidence of dementia in DM participants in the Atherosclerosis Risk in Communities Neurocognitive Study (Mayeda et al. 2014) was associated with inactivating mutations in genes involved in CBF autoregulation (Fan et al. 2016b). We recently reported that the myogenic response of the middle cerebral artery (MCA) was impaired in diabetic T2DN rats (Shekhar et al. 2017; Wang et al. 2017), possibly due to DM-induced cerebral VSMC dysfunction as we found that high glucose-treated cerebral VSMCs and pericytes exhibited loss of contractile ability in association with imbalanced mitochondrial dynamics and ATP depletion (Guo et al. 2020; Liu et al. 2020; Wang et al. 2019). However, it is unclear whether DM induces impaired CBF autoregulation that is exacerbated with aging and if it contributes to cognitive deficits.
The present study compared the myogenic response of the MCAs and parenchymal arterioles (PAs), which are the lenticulostriate branches of the MCA (Navarro-Orozco and Sanchez-Manso 2020), autoregulation of CBF in the surface and deep cortex in young (3 months) and old (18 months) non-diabetic Sprague Dawley (SD) and diabetic T2DN rats. BBB permeability, cerebral vascular density, tight junction, and pericyte coverage on the cerebral vasculature, neurodegeneration, and cognitive function were also compared in these rats.
Materials and methods
Animals
Age-matched young (3 months) and old (18 months) non-diabetic SD and diabetic T2DN rats were used. The rats were bred and maintained at the University of Mississippi Medical Center (UMMC) housing under standard laboratory animal conditions with access to diet and water ad libitum. Animals were given a low-salt chow (0.1% sodium, Teklad traditional diet 7034, Envigo, Indianapolis, IN) to minimize the development of hypertension and renal injury (Muroya et al. 2018). All animal protocols were approved by the Institutional Animal Care and Use Committees of the UMMC following the NIH guidelines. Every effort was made to minimize the number of animals that were used, consistent with obtaining reliable statistical results.
Baseline biophysical parameters measurements
Body weights were compared in young and old control and DM rats. Blood samples were collected from the lateral tail vein (Lee and Goosens 2015) in conscious rats with ad libitum feeding and drinking 3 h after the light-on cycle for measurements of the baselines of blood glucose, glycosylated hemoglobin (HbA1c), and insulin levels. Plasma glucose and HbA1c levels were measured using a Contour next glucometer (Ascensia Diabetes Care, Parsippany, NJ) and an A1CNow+ (Polymer Technology System, Indianapolis, IN) HbA1c device. The insulin levels were measured using an Ultrasensitive Rat Insulin ELISA kit (Mercodia Inc., Winston Salem, NC).
Intraperitoneal glucose tolerance test
Food was deprived 18 h prior to the experiment. Fasting glucose levels were measured using Contour next glucometer (Ascensia Diabetes Care) as a baseline (time 0). The rats were placed in a restrainer, and glucose (2 g/kg, i.p.) was administrated. Plasma glucose levels were measured 5, 15, 30, 60, 90, and 120 min after the administration of glucose, as we described previously (Muroya et al. 2018).
Insulin tolerance test
ITT was performed by intraperitoneal administration of insulin solution at the dose of 0.3 U/kg body weight. Rats fasted 18 h before the test, and baseline plasma glucose levels (time 0) were measured before the administration of insulin. Plasma glucose levels were measured at the time of 5, 15, 30, 60, 90, 120, and 150 min after the administration of insulin.
Pressure Myography
Isolation of MCA and PA
MCA and PA were isolated following the methods we described previously (Fan et al. 2017; Zhang et al. 2020). The rats were euthanized with 4% isoflurane and weighed. The brains were collected, weighed, and placed in an ice-cold calcium-free physiological salt solution (PSS0Ca), as we have reported (Fan et al. 2013). A 5 × 3 mm section of the brain containing the MCA was dissected and placed in an ice-cold PSS0Ca supplemented with 1% bovine serum albumin (BSA). The pia mater connected to the MCA was carefully separated from brain tissue. A branch-free M2 segment of MCA with an inner diameter (ID) from 150 to 200 μm was dissected and kept in ice-cold PSS0Ca for further study. The lenticulostriate arterioles branching from the MCA penetrating the brain parenchyma (Cipolla et al. 2014a, b; Navarro-Orozco and Sanchez-Manso 2020; Pires et al. 2016) were carefully identified, dissected, and placed in ice-cold PSS0Ca.
Myogenic response
Intact MCA and PA were mounted in a pressure myography chamber (Living System Instrumentation, Burlington, VT), and cannulated with glass pipettes. The chamber was warmed (37 °C), bathed with oxygenated (21% O2, 5% CO2, 74% N2) PSS solution containing CaCl2 (1.6 mM), and connected with an IMT-2 inverted microscope equipped with a camera system (Olympus, Center Valley, PA). The MCA and PA were pressurized to 40 mmHg and 10 mmHg, respectively, during a 30-min equilibration phase to develop a spontaneous tone (Faraci and Heistad 1990; Harper et al. 1984; Mayhan and Heistad 1986). The myogenic response of the MCA and PA were evaluated by recording the changes in IDs in response to increased transmural pressure from 40 to 180 mmHg in steps of 20 mmHg, and from 10 to 60 mmHg at the steps of 10 mmHg, respectively. The IDs were measured using a digital camera (MU 1000, AmScope) under × 10 objective for the MCA or × 20 objective for PA. Intraluminal pressures were reset to 40 mmHg and 10 mmHg for MCA and PA, respectively, and the vessels were washed with PSS0Ca for 5–6 times. Passive ID (ID0Ca) under calcium-free conditions was determined at the pressures described above.
Myogenic tone
Myogenic tone was determined using equations described previously (Pabbidi et al. 2014; Wang et al. 2020; Zhang et al. 2020): Myogenic tone (%) = [(ID0Ca − ID)/ID0Ca] × 100.
Autoregulation of CBF
The rats were anesthetized using inactin (50 mg/kg) and ketamine (30 mg/kg). This combination has minimal effects on the CBF autoregulation and maintains the baseline arterial pressure at 110–120 mmHg (Fan et al. 2014, 2015a; Wang et al. 2020). The tracheal cannulated and connected to a ventilator (SAR-830, CWE Inc.). CO2 levels were monitored utilizing an end-tidal CO2 analyzer (CAPSTAR-100, CWE Inc., Ardmore, PA), and respiratory rate was adjusted to maintain end-tidal CO2 at 35 mmHg after drugs were delivered via the cannulated femoral vein. Mean arterial pressure (MAP) was determined by a pressure transducer connected with the cannulated femoral artery. A thin translucent cranial window was prepared at 2 mm posterior and 6 mm lateral to the bregma using a low-speed air drill. A small hole (1 mm in diameter) was carefully drilled through the bone. A fiber-optic probe (91-00124, Perimed Inc., Las Vegas, NV) coupled to a laser-Doppler flowmeter (LDF, PF5010, Perimed Inc.) probe was implanted into the brain at a depth of 1.5–2 mm for recording CBF in the deep brain cortex (Korbo et al. 1990). The hole was then sealed using bone wax. Another fiber-optic probe was placed above the surface of the cranial windows in the field with no visible vessels for recording regional CBF on the surface cortex. Baselines CBF on the surface and deep cortex were recorded at 100 mmHg. MAP was then elevated to 180 mmHg in steps of 20 mmHg by infusing phenylephrine (0.5–5 μg/min, P6126, Sigma-Aldrich, St. Louis, MO) via the femoral vein. CBF was recorded after achieving a steady state at each stage for 5 min. After recording the response to elevations in pressure, a new baseline LDF was obtained at the pressure of 100 mmHg by withdrawing phenylephrine. CBF was recorded at each level of MAP to 40 mmHg by graded hemorrhage in steps of 20 mmHg.
A laser speckle imaging system (RWD Life Science Co., Ltd., Shenzhen, China) was placed 10–15 cm above the closed cranial window. Saline solution was dropped on the closed cranial window, and the focus was adjusted to achieve a clear CBF image. The surface CBF images were captured at different MAPs. All of the photos were taken under the same zoom, gain, and pseudo-color threshold settings.
BBB permeability
BBB permeability was assessed via infusion of Evans blue (EB), as we described previously (Fan et al. 2015a; Wang et al. 2017; Warrington et al. 2014). Briefly, 2% EB in 3% BSA was injected intravenously through the femoral vein at the dose of 3 ml/kg body weight. MAP was maintained at 180 mmHg for 1 h by infusing phenylephrine. The rat was intracardially flushed with 100 ml saline containing 10 U/ml heparin after collecting blood samples for baseline plasma EB concentration. The brain was homogenized with PSS after weighed and photographed. Fluorescence intensities of the plasma and supernatant of the brain homogenate were measured using a microplate reader (BioTek, Winooski, VT) at the excitation wavelength of 620 nm and an emission of 680 nm.
Functional hyperemia responses
CBF response to whisker stimulation was measured in young and old control and DM rats for comparison of functional hyperemia using LDF, as previously described (Liu et al. 2008; Wang et al. 2020). Briefly, the right whiskers were stimulated for 1 min at 10 Hz, and the left somatosensory cortical CBF was recorded during this period. Three trials were performed every 5 min, and results are presented as the average values ± SEM.
Histology and immunohistochemistry
The rats were anesthetized with 2% isoflurane, weighed, and perfused with 4% paraformaldehyde (PFA. Electron Microscopy Sciences, Hatfield, PA) at the perfusion pressure of 100 mmHg after flushed with 100 ml saline intracardially. The brains were collected, weighed, and post-fixed with 4% PFA at 4 °C overnight, followed with immersion in a 10% cytoprotective solution (consisting of 16.28 g NaH2PO4, 4.28 g NaOH, 100 g sucrose, and 0.1 g thimerosal in 1 L distilled water) for 24 h and 30% cytoprotective solution (increased sucrose concentration to 300 g/L) before sectioning.
Neurodegeneration
Post-fixed brains were embedded with paraffin, and three micrometer-thick serial coronal sections were prepared. The sections containing the CA3 region of the hippocampus were stained with Cresyl violet (Nissl staining), or co-stained with Fluoro-Jade C (FJC), and 4′,6-diamidino-2-phenylindole (DAPI) using a Fluoro-Jade C Staining Kit (Biosensis, Thebarton, South Australia) following the manufactory protocol. The sections posterior from bregma were systematically randomly sampled for stereotactic localization of the hippocampus, according to The Paxinos and Watson Rat Brain Atlas, and the CA3 regions of the hippocampus in these stained sections were imaged. Images of the Nissl and FJC/DAPI-stained sections were captured using the Nikon Eclipse 55i microscope and a DS-FiL1 color camera (Nikon) at the magnification of × 850 and × 1600, respectively. FJC- and DAPI-labeled neurons were excited with blue light excitation and ultra-violet illumination, respectively. Four rats of each group were studied, and 3–4 sections were obtained per animal for quantification. Data are expressed as the number of cells per high-power field for Nissl staining, and the percentage of FJC-positive neurons normalizing with neuron numbers identified with DAPI staining.
Cerebral vascular density, tight junction, and pericyte coverage
Thick coronal brain sections (50 μm) were prepared using a vibrating microtome (VF-300-0Z, Precisionary Instruments LLC, Greenville, NC). Free-floating brain sections were incubated with first antibodies against collagen IV (1:300, ab6586, Abcam, Cambridge, UK) and occludin (E-5) (1:500, sc-133256, Santa Cruz Biotechnology, Dallas, Texas) in a staining solution containing 10% BSA, 2% Triton X-100, and 0.02% sodium azide (Watson et al. 2020) at 4 °C overnight, followed by goat anti-mouse Alexa Fluor 555 (1:1000, A21424, Thermo Fisher Scientific, Waltham, MA) and goat anti-rabbit Alexa Fluor 488 (1:1000, A11070, Thermo Fisher Scientific) secondary antibodies for 2 h at room temperature. The sections were washed three times and mounted on slides with a drop of an anti-fade mounting medium with DAPI (H-1200, Vector Laboratories, Burlingame, CA) and coverslipped.
Another group of free-floating brain sections was incubated with 0.1% trypsin in PBS at 37 °C for 1-h for antigen retrieval. The sections were then incubated with the primary anti-desmin antibody (1:300, sc23879, Santa Cruz Biotechnology) diluted in staining solution overnight, followed by donkey anti-mouse Alexa Fluor 568 (1:500, A10037, Thermo Fisher Scientific). The sections were then incubated with an anti-collagen IV antibody (1:300, ab6586, Abcam) diluted in PBS at 4 °C overnight, followed by incubation with a goat anti-rabbit Alexa Fluor 488 (1:1000, A11070, Thermo Fisher Scientific) for 2 h at room temperature.
Images acquired for comparison of capillary density, length, and tight junction were obtained at a magnification of × 880 and × 2640 using a Nikon C2 laser scanning confocal head mounted on an Eclipse Ti2 inverted microscope (Nikon, Melville, NY). Capillary lengths were measured using the AngioTool software (Zudaire et al. 2011). The scale was calibrated to 0.81 pixel/μm according to the parameter of the microscope used to acquire images of collagen IV staining. Four rats of each group were studied, and 3–4 sections were obtained per animal were compared. The area of collagen IV, percentage of capillary area per image, occludin, and desmin were measured using NIS-Elements Imaging Software 4.6 (Nikon). The percentage area of tight junction in the capillary was calculated as the area of occludin divided by the area of collagen IV. Percentage pericyte coverage on the capillary was determined by the area of desmin divided by the area of collagen IV staining.
Neurobehavioral tests
Open field test
The open field test is widely used to assess locomotor activity and exploratory behaviors (Seibenhener and Wooten 2015; Tatem et al. 2014). Locomotor activity was monitored during a 20-min period using the Opto-Varimex ATM3 Auto-Track System (Columbus Instruments, Columbus, OH). The rats were allowed to acclimate in the behavior test room for 2 h before the test. Each of the rats was then placed in a clear plastic chamber surrounded by frames with photobeams. The Auto-Track System was monitored the beam breaks caused by the rat movements and to calculate the distance the rat traveled, resting time, and the location of the rat. A total distance of the movement and the resting time of the tested rat in this period were compared with determine the general motor activity.
Novel object recognition
Novel object recognition test evaluates hippocampus based non-spatial short-term memory (Broadbent et al. 2004; Camarasa et al. 2010). This is a simple method that does not need external motivation to reward or punishment (Ennaceur 2010). The rats were removed from the chambers after the open field study and returned to their home cages for 1 h. The entire apparatus was thoroughly cleaned and disinfected using 70% ethanol and distilled water. During the training phase of 5 min, the rat was trained to memorize the details of two identical objects placed on the two opposite corners of the chamber, and then, the rat was removed from the testing chamber for 5 min. One of the objects was replaced by a novel object, and the rat was returned to the testing chamber, and exploration was monitored for 5 min.
Eight-arm water maze
This is a procedure for testing spatial learning and short- and long-term memory that does not require deprivation or extensive training (Penley et al. 2013; Wang et al. 2020). The rats were trained to identify a platform marked with a visual cue in one of the eight arms of the pool to escape. There were four memory trials administered 2 and 24 h after the training that consisted of 4 consecutive swims. Time to reach the platform (escape) was recorded.
Statistics
Data are presented as mean values ± SEM. A two-way ANOVA for repeated measures followed by a Holm-Sidak post hoc test was used to compare the significant differences between groups in corresponding values in intraperitoneal glucose tolerance test (IPGTT), insulin tolerance test (ITT), pressure myograph, CBF autoregulation, and swim test. The significance of differences between groups in corresponding values of baseline biophysical parameters, AUC of IPGTT, AUC of ITT, BBB leakage, immunohistochemistry, functional hyperemia, and novel object recognition test was compared using one-way ANOVA. All statistical analyses were performed using GraphPad Prism 8 (GraphPad Software, Inc., La Jolla, CA). A P value < 0.05 was considered to be significant.
Results
Baseline biophysical parameters
As presented in Fig. 1a, body weight was similar between young control and DM rats. Old control and DM rats were both heavier than their young age, but old DM rats displayed significantly lower body weight compared with old control rats. Brain weight was similar between young controls vs. DM and old controls vs. DM rats. T2DN rats exhibited higher plasma glucose and HbA1c at young and old ages compared with age-matched non-diabetic rats. Basal insulin levels were similar between young control, young DM, and old control rats. Insulin levels were significantly higher in old DM rats than young T2DN and age-matched control rats. MAP was similar between age-matched controls vs. DM rats. Old control and DM rats exhibited higher MAP compared with young control and DM rats.
Fig. 1.
Baseline biophysical parameters, intraperitoneal glucose tolerance test (IPGTT), and insulin tolerance test (ITT). All experiments were compared in young (3 M) and old (18 M) non-diabetic (ctrl) and diabetic (DM) rats. a Comparison of body weight, brain weight, plasma glucose, glycosylated hemoglobin (HbA1c), mean arterial pressure (MAP), and insulin levels. N = 6–38 rats per group. b Glycemic profiles after intraperitoneal administration of glucose (2 g/kg). Time 0 indicates baseline glucose levels after 18 h fasting prior to the injection of glucose. Plasma glucose levels were measured at the time of 5, 15, 30, 60, 90, and 120 min then after. c The area under the curve (AUC) of IPGTT curves. d Glycemic profiles after intraperitoneal administration of insulin (0.3 U/kg). Time 0 indicates baseline glucose levels after 18 h fasting prior to the injection of insulin. Plasma glucose levels were measured at the time of 5, 15, 30, 60, 90, 120, and 150 min then after. e The area under the curve (AUC) of ITT curves. Mean values ± SEM are presented. N = 5–9 rats per group (B-E). The asterisk indicates P < 0.05 from the corresponding values in age-matched SD rats. The dagger indicates P < 0.05 from the corresponding values in young rats within a strain
IPGTT
As presented in Fig. 1b, c, plasma glucose increased to the highest levels 15 min following administration of glucose consistently in young (226.3 ± 29.2 mg/dL) and old (381.6 ± 43.6 mg/dL) controls, and young (340.3 ± 16.0 mg/dL) and old (473.0 ± 50.1 mg/dL) DM rats. Both young and old DM rats exhibited impaired IPGTT identified as an increased area under the curve (AUC) of the IPGTT relative to the values age-matched non-diabetic controls. We also found that aging caused impairment of glucose tolerance in both control and DM rats. The degree of glucose intolerance was significantly greater in old T2DN than young DM and old non-diabetic rats.
ITT
Figure 1d, e present insulin sensitivity demonstrated as glycemic profiles after intraperitoneal administration of insulin (0.3 U/kg). Plasma glucose increased to the highest levels 5 min following administration of insulin in young (110.6 ± 1.1 mg/dL) and old (115.3 ± 2.5 mg/dL) controls, and young (111.2 ± 4.1 mg/dL) DM rats. However, peak glucose levels were at 30 min after insulin injection in old DM (124.7 ± 12.0 mg/dL) rats, indicating insulin sensitivity in response to glucose was reduced.
Myogenic response
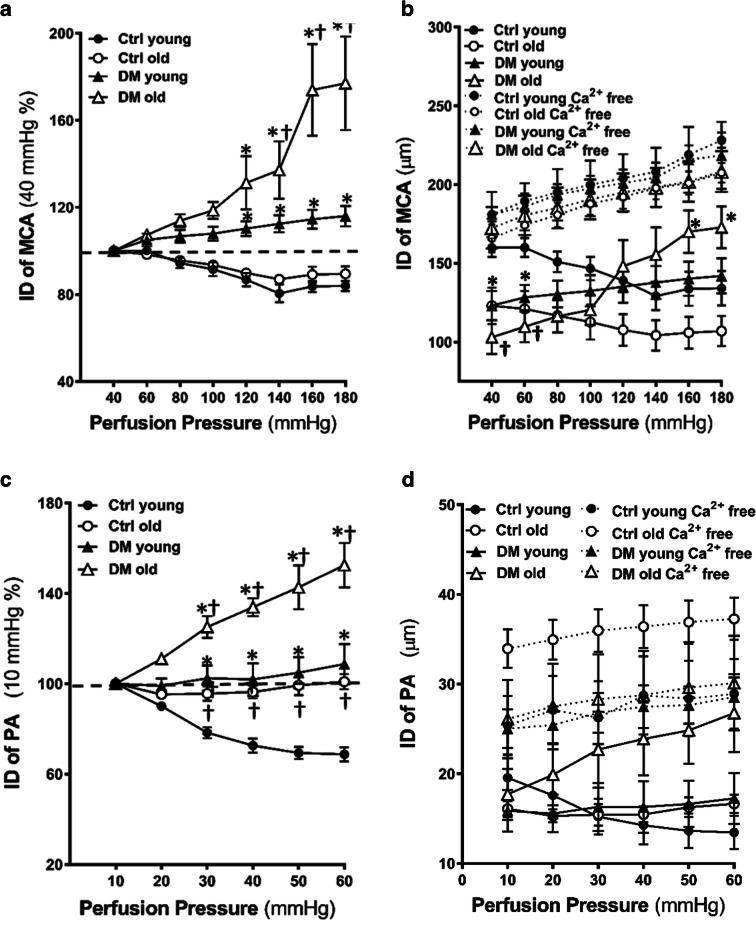
Myogenic response of the MCA in young and old control and DM rats were compared. The MCA constricted by 8.4 ± 3.3% and 19.6 ± 4.4% when perfusion pressure was increased from 40 to 100, and 140 mmHg, respectively, in young control rats indicating an intact myogenic response. The MCA was slightly dilated by 16.1 ± 2.8% at 180 mmHg in this rat. DM rats started to lose the myogenic response at 3 months of age as showing a dilation by 8.0 ± 3.4% and 12.5 ± 4.2% when perfusion pressure was increased from 40 to 100, and 140 mmHg, respectively. Old DM rats exhibited significantly impaired myogenic response with dilation by 18.6 ± 4.2%, 37.2 ± 15.3%, and 77.0 ± 20.0% when perfusion pressure was increased from 40 to 100, 140, and 180 mmHg, respectively. Forced dilatation only occurred at pressures > 140 mmHg in the MCA of elderly DM rats (Fig. 2a). Absolute values of the ID of the MCA in response to pressure changes at PSS and PSS0Ca solutions are presented in Fig. 2b.
Fig. 2.
Myogenic response, myogenic tone, and autoregulation of CBF. Comparison of the myogenic response, myogenic tone of the MCA and PA, and surface and deep cortical CBF autoregulation in young and old non-diabetic control (Ctrl) and DM rats. a Comparison of the myogenic response of the MCA as of % constriction to 40 mmHg. b Absolute values of IDs of the MCA in PSS and PSS0Ca solutions. c Comparison of the myogenic response of the PA as of % constriction to 10 mmHg. d Absolute values of IDs of the PA in PSS and PSS0Ca solutions. e Comparison of the % of the myogenic tone of the MCA. f Comparison of the % of the myogenic tone of the PA. g Comparison of surface cortical CBF autoregulation as of % to 100 mmHg. h Comparison of deep cortical CBF autoregulation as of % to 100 mmHg. i Representative laser speckle contrast images of surface cortical CBF at 60, 100, and 160 mmHg. Mean values ± SEM are presented. N = 4–9 rats per group. The asterisk indicates P < 0.05 from the corresponding values in age-matched SD rats. The dagger indicates P < 0.05 from the corresponding values in young rats within a strain
Myogenic response of the PA in young and old control and DM rats were also compared. The PA constricted by 21.7 ± 2.8% and 31.3 ± 3.5% when perfusion pressure was increased from 10 to 30 and 60 mmHg, respectively, in young control rats. The PA slightly constricted by 4.4 ± 3.5% but dilated by 3.7 ± 4.3% when perfusion pressure was increased to the same extent in old control rats. In contrast, young DM rats lost the myogenic response, and the ID of PA was increased by 2.42 ± 6.4% when transmural pressure was increased from 10 to 30 mmHg. Old DM rats exhibited significantly impaired myogenic response with dilation by 25.1 ± 5.3% and up to 52.5 ± 10.7% when perfusion pressure was increased from 10 to 30, and 60 mmHg, respectively (Fig. 2c). Absolute values of the ID of the PA in response to pressure changes at PSS and PSS0Ca solutions are presented in Fig. 2d.
Myogenic tone
Consistent with previous reports, we found that the myogenic tone of the MCA was elevated in young DM vs. non-diabetic control rats at perfusion pressures over the range from 40 to 100 mmHg. Interestingly, the myogenic tone was enhanced in old vs. young non-diabetic rats at the pressures range from 40 to 120 mmHg but was reduced in old DM vs. young DM and age-matched non-diabetic control rats at higher pressures at 120–180 mmHg (Fig. 2e). Similarly, as we observed in MCA, the myogenic tone of the PA was not changed in young control vs. DM rats, enhanced in old vs. young non-diabetic rats at low pressures (10–30 mmHg). However, the myogenic tone of the PA was reduced in old DM vs. age-matched non-diabetic control but not young DM rats at higher pressures at 30–60 mmHg (Fig. 2f).
Autoregulation of CBF
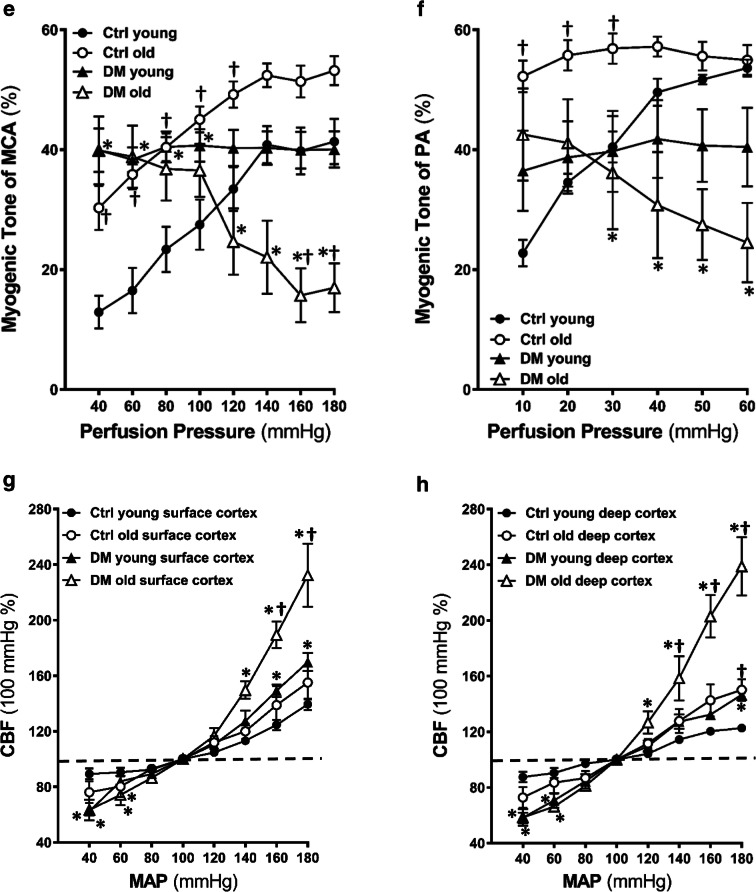
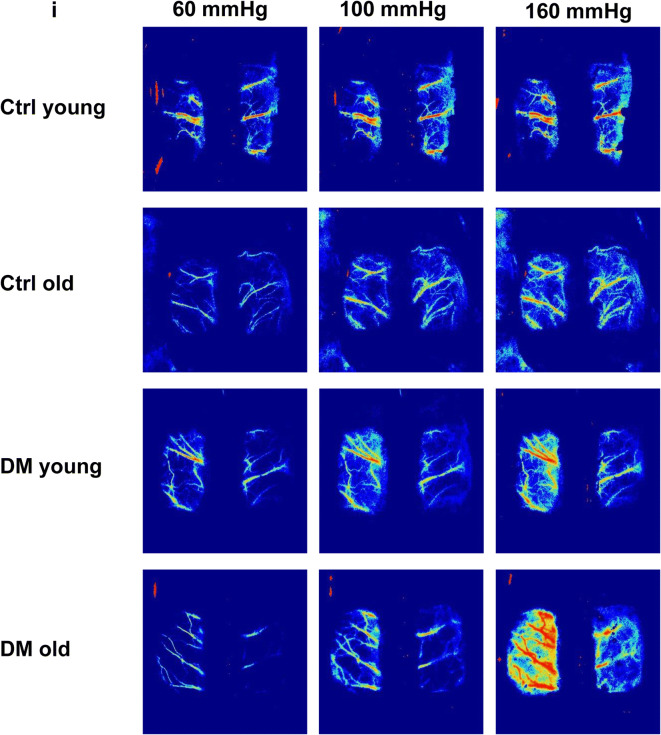
A comparison of surface and deep cortical CBF autoregulation was presented in Fig. 2g, h. There was no significant difference in CBF autoregulation in young DM vs. non-diabetic control rats when blood pressures at 80–140 mmHg (surface cortex, Fig. 2g) and 80 to 160 mmHg (deep cortex, Fig. 2h). However, young DM exhibited autoregulatory breakthrough at higher blood pressures, detected both in the surface and deep cortex. DM rats failed to provide CBF at 40 to 60 mmHg at both young and old ages compared with age-matched controls. The impaired CBF autoregulation was more severe in old DM vs. age-matched non-diabetic rats. CBF increased by 132.36 ± 25.33% (surface) and 138.90 ± 23.40% (deep) in old DM vs. 55.15 ± 13.60% (surface) and 50.12 ± 8.95% (deep) in old control rats, respectively, at a perfusion pressure of 180 mmHg. Additionally, the autoregulatory breakthrough points shifted to the left in old DM rats in both the surface and deep cortex. CBF decreased by 36.60 ± 8.61% (surface) and 41.41 ± 6.92% (deep) in old DM vs. 23.85 ± 9.02% (surface) and 27.20 ± 8.73% (deep) in old control rats, respectively, at a perfusion pressure of 40 mmHg. These results were confirmed by more blood flow observed at high pressure in DM vs. age-matched control rats in laser speckle contrast images (Fig. 2i).
BBB permeability
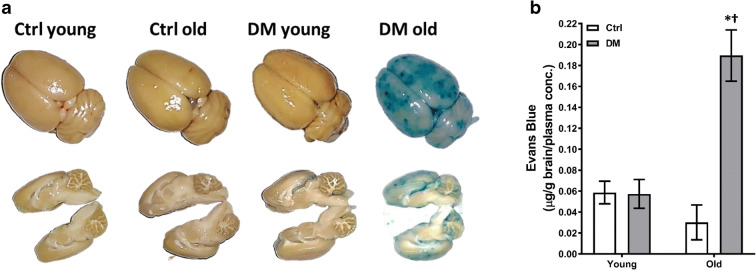
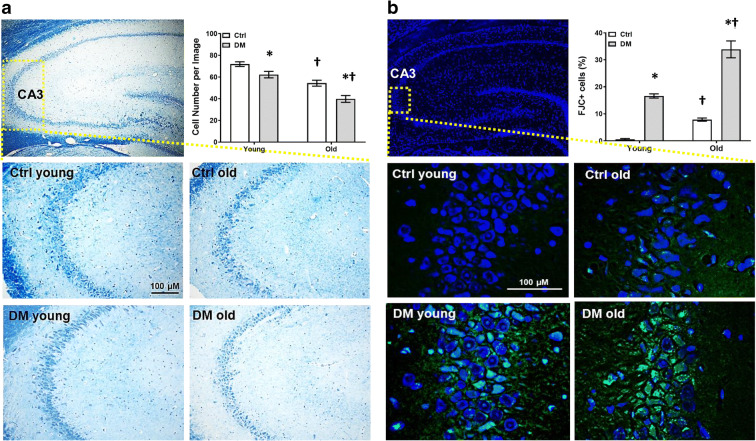
Baseline MAP of young control vs. DM rats and old control (143.0 ± 1.9 mmHg) vs. DM (143.8 ± 1.1 mmHg) rats were similar in this study. BBB leakage was significantly enhanced in the neocortex and hippocampus of old DM rats (Fig. 3a). EB brain concentration was 6-fold higher in the brain of old DM rats compared with the levels seen in old control rats, but not observed in young rats in both strains (Fig. 3b).
Fig. 3.
Blood-brain barrier (BBB) permeability. Comparison of BBB permeability in young and old non-diabetic control (Ctrl) and DM rats. a Representative images of the extravasation of Evans blue after mean blood pressure (MAP) was elevated to 180 mmHg for 1 h. b Tissue concentration of Evans blue in the brains. Mean values ± SEM are presented. N = 4–6 rats per group. The asterisk indicates P < 0.05 from the corresponding values in age-matched SD rats. The dagger indicates P < 0.05 from the corresponding values in young rats within a strain
Functional hyperemia responses
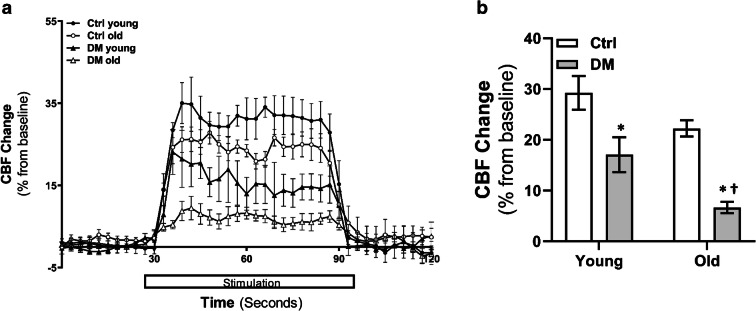
Comparison of CBF response with whisker stimulation in young and old non-diabetic control and DM rats is presented in Fig. 4. The increase in CBF was significantly reduced in DM rats at both young and old ages compared with age-matched non-diabetic control rats during the 60-s whisker stimulation period.
Fig. 4.
Functional hyperemia. Comparison of CBF response to whisker stimulation in young and old non-diabetic control (Ctrl) and DM rats. a Time course of percentage changes of cortical CBF in response to whisker stimulation. b Averaged percentage changes in CBF during 60 s of stimulation. Mean values ± SEM are presented. N = 4 rats per group. The asterisk indicates P < 0.05 from the corresponding values in age-matched SD rats. The dagger indicates P < 0.05 from the corresponding values in young rats within a strain
Neurodegeneration
Comparison of neurodegeneration in the CA3 region in the hippocampus in young and old non-diabetic control and DM rats is presented in Fig. 5. Neurons were distinguished from glial cells by having a larger cell body, visible cytoplasm around nuclei, different appearance of the chromatin within the nucleus, and the presence of nucleus (Garcia-Cabezas et al. 2016). Old DM rats displayed significantly fewer neurons compared with age-matched non-diabetic controls, and both DM and control rats had a neuronal loss when they were at 18 vs. 3 months of age (Fig. 5a). On the other hand, Old DM rats exhibited significantly more FJC-positive neurons compared with age-matched non-diabetic controls, and both DM and control rats had more neurodegeneration when they were at 18 vs. 3 months of ages (Fig. 5b).
Fig. 5.
Neurodegeneration. Comparison of neurodegeneration in the CA3 region of the hippocampus in young and old non-diabetic control (Ctrl) and DM rats. a Representative images and quantitation (right upper panel) of Nissl staining. b Representative images and quantitation (right upper panel) of FJC (green) and DAPI (blue) staining. Data are expressed as the number of cells per high-power field for Nissl staining, and the percentage of FJC-positive neurons normalizing with neuron numbers identified with DAPI staining. Mean values ± SEM are presented. N = 4 rats per group. The asterisk indicates P < 0.05 from the corresponding values in age-matched SD rats. The dagger indicates P < 0.05 from the corresponding values in young rats within a strain
Cerebral vascular density, tight junction, and pericyte coverage
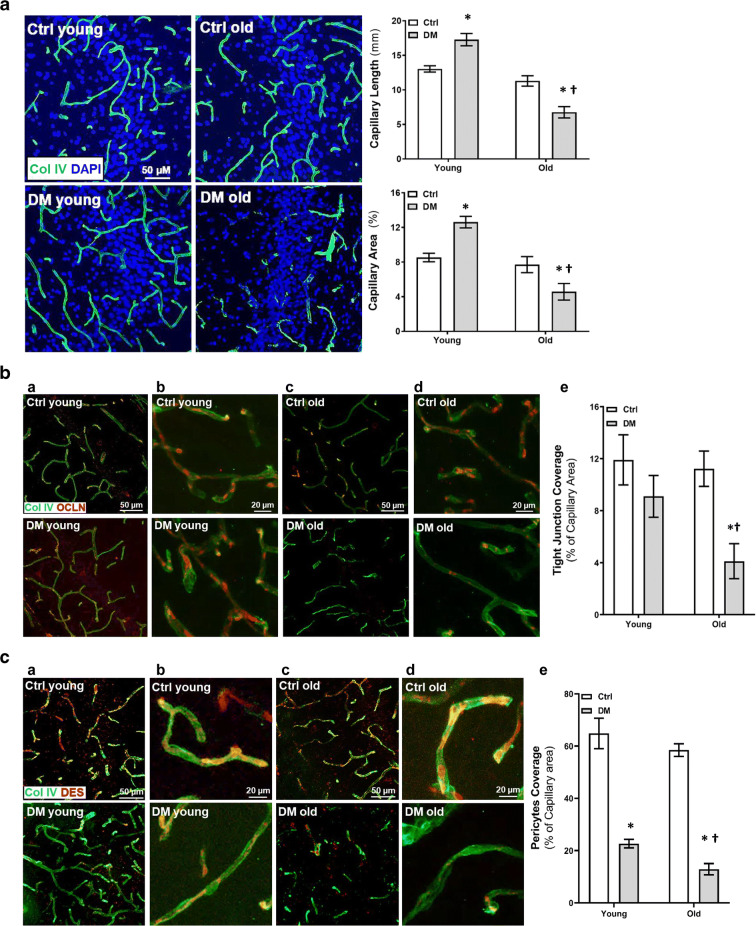
Comparison of vascular density, tight junction, and pericyte coverage on microvessels in the CA3 region of the hippocampus in young and old non-diabetic control and DM rats is presented in Fig. 6. Old DM rats had reduced cerebral capillary density, expressed as capillary length and area, compared with age-matched non-diabetic controls. In contrast, there was increased cerebral capillary density in young DM compared with age-matched control rats (Fig. 6a). Old DM rats also had less tight junction (Fig. 6b) and pericyte (Fig. 6c) coverage on cerebral capillaries compared with age-matched control and young DM rats. We found there were significantly reduced pericyte numbers on cerebral capillaries in young DM vs. control rats; however, the reduction of tight junction coverage in young DM rats was not significant.
Fig. 6.
Vascular density, tight junction, and pericyte coverage.Comparison of cerebral vascular density, tight junction, and pericyte coverage in the CA3 region of the hippocampus in young and old non-diabetic control (Ctrl) and DM rats. a Representative images and quantitation of capillary density by comparison of the length (right upper panel) and percentage area (right lower panel) per field of collagen IV (Col IV, green)-stained capillaries. b Representative images (a–d) and quantitation (e) of tight junction coverage by comparison of the percentage of occludin (OCLN, red) on collagen IV (Col IV, green)-stained capillaries. Magnifications: columns a, c: × 880; columns b, d: × 2640. c Representative images (a–d) and quantitation (e) of pericyte coverage by comparison of the percentage of desmin (DES, red) on collagen IV (Col IV, green)-stained capillaries. Magnifications: columns a, c: × 880; columns b, d: × 2640. Mean values ± SEM are presented. N = 4 rats per group. The asterisk indicates P < 0.05 from the corresponding values in age-matched SD rats. The dagger indicates P < 0.05 from the corresponding values in young rats within a strain
Neurobehavioral tests
Open field test
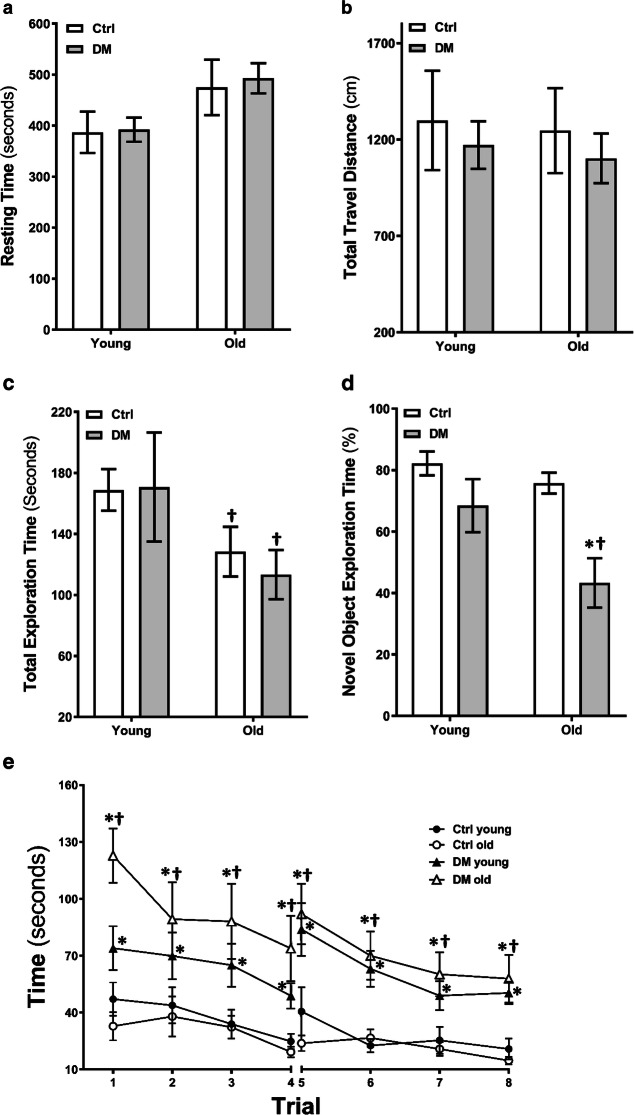
Comparison of locomotor activities by open field tests is presented in Fig. 7a, b. There were no significant differences in resting time (Fig. 7a) and travel distance (Fig. 7b) between young and old non-diabetic control and DM rats.
Fig. 7.
Neurobehavioral tests. Resting time (a) and total travel distance (b) in the open field test in young and old non-diabetic control (Ctrl) and DM rats. N = 6–11 rats per group. Total exploration time (c) and percentage time spent in the vicinity of the novel object (d) in the novel object recognition test. N = 6–11 rats per group. Time to reach the platform per trial in a 2-day eight-arm water maze test (e). N = 9–19 rats per group. Mean values ± SEM are presented. The asterisk indicates P < 0.05 from the corresponding values in age-matched SD rats. The dagger indicates P < 0.05 from the corresponding values in young rats within a strain
Novel object recognition
As shown in Fig. 7 c and d, there were no significant differences in total exploration time between age-matched control and DM rats. However, old control and DM rats spent significantly less exploring time compared with young rats within the strain (Fig. 7c). There were no significant differences in the percentage of time spent in the vicinity of the novel object between young control (82.2 ± 3.9%) and DM (69.0 ± 8.1%) rats. Elderly DM rats spent significantly less time (43.3 ± 8.0%) in the vicinity of the novel object compared with old control rats (75.7 ± 3.3%) and young DM rats (69.0 ± 8.1%. Figure 7d).
Eight-arm water maze
As depicted in Fig. 7e, young and old DM rats took a longer time to escape from the eight-arm water maze compared with age-matched control rats. Old DM rats exhibited longer escape compared with young DM rats in the eight-arm water maze test.
Discussion
The present study demonstrates that (1) The T2DN rat developed hyperglycemia and elevated HbA1C at 3 months and sustained to 18 months of age. Both young and old DM rats exhibited glucose intolerance; however, only elderly DM rats exhibited impaired ITT indicating that insulin insensitivity was developed with aging in this non-obese DM model. (2) The myogenic response of the MCA and PA was impaired in both young and old DM rats. Forced dilatation only occurred at pressures > 140 mmHg in the MCA of elderly DM rats. (3) The myogenic tone of the MCA was enhanced at 40–100 mmHg in young DM rats but was reduced at 140–180 mmHg in old DM rats. The myogenic tone of the PA was no change in young DM rats and was reduced at 30–60 mmHg in old DM rats. (4) CBF autoregulatory breakthrough points in the surface and deep cortex both were shifted to the left in DM rats. The impaired CBF autoregulation was more severe in old vs. young DM rats, and the deep vs. surface cortex. (5) Old DM rats exhibited significantly enhanced BBB leakage in the cortex and hippocampus. (6) There was impaired CBF response to whisker stimulation in DM rats at both young and old ages. (7) Old DM rats had significantly reduced neuron numbers, vascular density, tight junction, and pericyte coverage on cerebral capillaries in the CA3 region in the hippocampus. (8) Old DM rats displayed impaired non-spatial short-term memory and spatial learning and short- and long-term memory without damaged locomotor activity and exploratory behaviors.
These results suggest that impaired CBF autoregulation and enhanced BBB leakage are associated with a serial of downstream pathological consequences, which may play an essential role in the pathogenesis of age- and diabetes-related dementia. CBF autoregulation continuously provides the brain with glucose and oxygen by supplying consistent CBF to protect cerebral microcirculation and prevent the brain from ischemic or hemorrhagic injury despite fluctuations in cerebral perfusion pressure (Cipolla et al. 2014b; Verweij 2007). Autoregulation of CBF in response to elevations in pressure is mediated by the myogenic response of pial arteries and to a lesser extent by pial arterioles (Faraci et al. 1990; Harder et al. 2011; Harper et al. 1984; Kontos et al. 1978; Paulson et al. 1990). About 50% of increases in systemic pressure are transmitted to the pial arteries, including the MCAs, and 10% reaches the terminal pial arterioles and PAs (Harper et al. 1984; Kontos et al. 1978; Lammie 2002). PAs contribute to the CBF autoregulation in the deep cortex by providing additional vascular resistance in response to changes in CBF and pressure transmitted from the surface cortex when the MAP was altered (Harper et al. 1984). Segmental vascular resistance determines CBF autoregulated differentially on the surface and in the brain deep cortex (Faraci et al. 1987). Unlike most of the other vascular beds, both of the pial artery and PA contribute significant resistance in the cerebral circulation. CBF autoregulation on the brain surface cortex is mainly mediated by pial arteries. However, the resistance of pial arteries becomes inadequate when blood pressure exceeds the autoregulatory range, leading to increases in CBF and pressure that will be transmitted to downstream arterioles and capillaries. Our simultaneous measurements of CBF changes on the surface and in the deep brain cortex in response to the increases or decreases in blood pressure provided a powerful approach to determine whether PA contributes to CBF autoregulation, especially when pial arteries and arterioles exceed their capability to regulate CBF at high pressures or under pathological conditions, such as DM and aging in the present study. We found that autoregulation of CBF was impaired at the surface cortex in young DM rats at pressures > 140 mmHg and > 120 mmHg in old DM animals. We also found that DM rats exhibited an inability to dilate the cerebral circulation and maintain CBF when pressures were lowered in the ischemic range < 60 mmHg at both ages. CBF in the deep cortex was failed to respond to high (> 100 mmHg in old and > 160 mmHg in young ages) and low pressures (at 40–60 mmHg in both ages) in DM rats. Our results also indicated that the CBF autoregulation was impaired to a greater extent in old vs. young DM rats, and in the deep vs. surface cortex.
The myogenic mechanism is the predominant factor in CBF autoregulation (Fan et al. 2016a; Pabbidi et al. 2014). The myogenic tone regulates blood flow in an essential organ by providing peripheral resistance and pre-constricted state within the vasculature. In cerebral pial arteries, the myogenic tone was generated at approximately 40 mmHg (Wang et al. 2020; Zhang et al. 2020). Thus, we normalized the diameters of the MCA in response to pressure to diameters at this pressure to specifically compare the myogenic response in control and DM rats. The myogenic response constricts blood vessels in response to elevations in perfusion pressure to maintain constant CBF, which is an intrinsic property of VSMCs and attributed to stretch-activated ion channels (Roman 2002; Roman and Fan 2018). In young individuals, elevations in myogenic reactivity in response to elevated BP, such as during exercise, increase cerebral vascular resistance sufficiently to keep capillary pressure, CBF, and oxygenation in the normal range (Ogoh et al. 2010; Osol and Halpern 1985). This protective effect also exists in the early-stage of normotensive diabetics (Jarajapu et al. 2008; Lagaud et al. 2001; Zimmermann et al. 1997). The increased myogenic tone was observed in cerebral arteries of young diabetic rodents (Jarajapu et al. 2008; Lagaud et al. 2001; Zimmermann et al. 1997). This is attributed to elevated levels of tumor necrosis factor/sphingosine-1-phosphate (Sauve et al. 2016) and endothelin (Abdelsaid et al. 2014), decreased activities of large-conductance Ca2+-activated potassium (BK) channel (Rueda et al. 2013), and elevated activity of rho-kinase (Abd-Elrahman et al. 2017) that contributed to enhanced PKC-dependent vasoconstrictor responses in cerebral arteries. Hyperglycemia induced ROS formation was capable of damaging DNA and proteins and inducing lipid peroxidation (Orasanu and Plutzky 2009). It inhibited the sarcoplasmic reticulum Ca2+-ATPase activity (El-Najjar et al. 2017) resulting in an increase in intracellular Ca2+. DM-mediated PKC activation via the PLC-DAG pathway (Inoguchi et al. 2003) promoted vasoconstriction by activation of NADPH oxidase and ROS production (Du et al. 2000; Yarchoan and Arnold 2014). However, the myogenic response was progressively impaired in rats with long-term DM (Abd-Elrahman et al. 2017; Abd-Elrahman et al. 2015; Abdelsaid et al. 2014; Kelly-Cobbs et al. 2011; Kold-Petersen et al. 2012). This may be related to impaired calcium sensitization and myosin light chain phosphatase activity due to a defect in rho-kinase (Abd-Elrahman et al. 2017; Abd-Elrahman et al. 2015). This inverse relationship between age and myogenic tone suggested that cerebral vascular dysfunction in DM is dependent on the duration and severity of DM (Gros et al. 2002; Nyborg and Nielsen 1990).
Mechanistically, pial arteries and PAs regulate the myogenic reactivity in different ways. PAs have a greater myogenic tone at low pressures compared with pial arteries due to the increased L-type voltage-dependent calcium channels activity and the lack of BK channels (Cipolla et al. 2014b). The PA acts as the “bottleneck” to limit CBF delivery to capillaries in the deep cortex (Nishimura et al. 2007). Under ischemic stroke, myogenic tone of the MCA was reduced, but the myogenic tone of the PA was increased that restricted CBF delivery to the ischemic region (Cipolla et al. 2014b). Hypertension led to ischemic stroke by increasing the myogenic tone of both the MCA and PA (Pires et al. 2015). We extended the age and perfusion pressure ranges in the present study and compared the myogenic reactivity in the MCA and PA in young (3-month) and elderly (18-month) non-diabetic control vs. age-matched DM rats. Consistent with previous reports, we found that the myogenic tone of the MCA was elevated in young DM rats at 40–100 mmHg. However, the myogenic tone of the MCA was reduced in old DM rats at 120–180 mmHg. The myogenic tone of the PA was also decreased in old DM rats at high pressures (30–60 mmHg), although no changes were observed in young DM rats. Additionally, the MCA and PA failed to constrict in response to high pressures in DM rats at both young and old ages. Forced dilation of the MCA and PA only occurred in old DM rats. These ex vivo results are consistent with the in vivo data that CBF autoregulatory breakthrough points in both the surface and deep cortex were shifted to the left and occurred in old DM rats.
The myogenic response was originally defined to constrict blood vessels by VSMCs (Bayliss 1902). More recently, several groups reported that pericytes on the cerebral vasculature, not only in the pre-capillary arterioles but also along the capillaries themselves, can constrict and regulate CBF (Hall et al. 2014; Hartmann et al. 2015; Nortley et al. 2019; Watson et al. 2020), which presented a bigger and broader picture in better understanding CBF autoregulation. We have reported that high glucose-treated cerebral VSMCs and pericytes lost their contractile capabilities in association with enhanced mitochondrial fission protein expression and ATP depletion (Guo et al. 2020; Liu et al. 2020; Wang et al. 2019) suggesting that long-standing hyperglycemia in DM individuals may contribute to impaired cerebral vascular hemodynamics as we observed in this study.
Autoregulation of CBF is often impaired in aging populations which may contribute to the increased incidence of cerebral vascular disease, stroke, vascular dementia (Bidani et al. 2009; Faraco and Iadecola 2013; Gorelick et al. 2011; Lammie 2002) and AD (Brickman et al. 2015; de la Torre 1997; Gorelick et al. 2011), especially in aging and DM (Mayeda et al. 2014). Mild impairment of CBF autoregulation could induce cognitive deficit in the elderly, as reported that CBF dysregulation induced cerebral ischemic injury and cognitive impairment at transmission pressures < 100 mmHg (Toth et al. 2017). On the other hand, at high pressures, the impaired CBF autoregulation and myogenic response led to excessive blood flow and elevated pressure delivered to the downstream capillaries, resulting in capillary rupture and BBB damage (Fan et al. 2014, 2015a). The BBB is structurally unique in maintaining brain homeostasis by regulating molecules transported into and out of the brain. Cerebral ECs are essential in maintaining the BBB integrity and regulating neurovascular coupling (NVC) function (Guerra et al. 2018). These cells are particularly vulnerable to hyperglycemia, which induces overproduction of ROS and increases oxidative stress. These changes damage ECs by decreasing nitric oxide bioavailability, which increases the susceptibility to ischemic brain injury and cognitive deficits in DM individuals (Chrissobolis et al. 2011; Daulatzai 2017; Jansen et al. 2016; Tiehuis et al. 2008). The BBB properties of these specialized ECs depend on tight junctions between adjacent cells (Luissint et al. 2012). Pericytes also regulate BBB and play an essential role in maintaining BBB integrity (Armulik et al. 2010). BBB damage enhances the influx of neurotoxic blood-derived debris and microbial pathogens, triggering neurodegeneration, which was closely associated with neurodegenerative diseases (Sweeney et al. 2018). Other downstream pathological consequences of BBB leakage include NVC dysfunction, which was identified in stroke (Hu et al. 2017) and Alzheimer’s disease (Bell and Zlokovic 2009).
In the present study, we found that aging significantly exacerbated pericyte detachment in DM rats. On the other hand, tight junction coverage was not affected in young DM rats. This could be due to the fact of myogenic tone was maintained and the myogenic response of cerebral vasculature in young DM rats only exhibited a mild impairment without displaying a force dilatation at high pressures. Thus, the MCAs and PAs in young DM rats still provided vascular resistance to protect BBB from damaging by transmission pressure even at high levels. We found that old DM rats exhibited massive BBB leakage in the neocortex and hippocampus, which was associated with neurodegeneration. Moreover, there was impaired functional hyperemia in elderly DM rats detected by less CBF response to whisker stimulation. However, BBB leakage was not detected in young DM rats, although these rats also displayed neurodegeneration and failed to respond to whisker stimulation. Functional hyperemia mediated by NVC mechanisms is essential in regulating CBF independent of changes in blood pressure and is not directly related to the structure and function of MCA. NVC is triggered by local mediators in astrocytes, pericytes, and endothelial cells at the level of capillaries. (Toth et al. 2014, 2015). The signal is propagated in a retrograde manner via gap junctions in endothelial cells to VSMCs in penetrating arterioles. Disruption of pericyte coverage or gap junctions in the endothelium should impair functional hyperemia. The underlying mechanisms involve crosstalk between these cells in sensitivity and activity of capillary endothelial cell inward-rectifier potassium channels, astrocytic end food calcium and potassium channels, and pericyte potassium channels (Dunn and Nelson 2010; Girouard et al. 2010; Hamilton et al. 2010; Hosford et al. 2019; Longden et al. 2017). Neurovascular coupling response also could be affected by the changes in the myogenic reactivity and CBF autoregulation (Wang et al. 2020). The compromised CBF autoregulation at low pressures we found in diabetic rats, starting from a young age, indicated endothelial dysfunction. These results suggest that maintaining BBB integrity is a comprehensive effect of intact myogenic reactivity and pericyte and endothelial function. Many investigators have focused on the role of impaired NVC and transient ischemia as one factor that can lead to neurodegeneration in diabetes and hypertension. We found that functional hyperemia is reduced by 50% in young diabetic animals. Other non-vascular factors, such as mitochondrial dysfunction in neurons, as we observed in HG-treated VSMCs and pericytes (Guo et al. 2020; Liu et al. 2020), all could cause neuronal loss or dysfunction. Neurodegeneration without BBB disruption that we observed in young diabetic animals in the present study thus might be a downstream pathological consequence primarily from impaired functional hyperemia in combination with cerebrovascular pericyte loss, endothelial dysfunction. More studies are needed indeed in this regard. The elderly DM rats displayed impaired non-spatial short-term memory and spatial learning and memory with no changes in locomotor activity and exploratory behaviors. However, unlike the elderly DM rats that exhibited cognitive deficits in both eight-arm water maze and novel object recognition tests, young DM rats only had hippocampal-based spatial learning and working memory disability in an eight-arm water maze study but did not display deficits in hippocampal-based non-spatial memory in the novel object recognition test (Broadbent et al. 2004; Vorhees and Williams 2014). These findings are in line with a previous report that non-spatial memory deficits occurred with lesions larger than 75% of total hippocampal volume. In comparison, 30% of hippocampal damage was sufficient to induce impaired learning and memory in the water maze study (Broadbent et al. 2004). Indeed, there are limitations to the present study. DM individuals also present anxiety- and depression-like behaviors detected by forced swimming and elevated plus maze tests in a recent report using STZ-DM rats, which are associated with elevated levels of pro-inflammatory IL-6 and TNF-α (Rajabi et al. 2018). These results suggest that changes in other behaviors and cytokines should be considered in our future studies.
The T2DN DM model used in the present study was derived by the introgression of the mitochondria genome of the Fawn hooded hypertensive (FHH) rat onto the Goto-Kakizaki (GK) genetic background (Nobrega et al. 2004). T2DN rat closely mimics changes in diabetic patients with hyperglycemia, hyperinsulinemia, and hyperlipidemia with regular diet (Kojima et al. 2013; Muroya et al. 2018; Nobrega et al. 2004). T2DN rat model is a lean T2D model without obesity, as seen in other obesity T2D rat models, such as Zucker fatty or BBZDR/Wor rats (Oana et al. 2005; Yokoi et al. 2013). Thus, it is an ideal model for studying the effects of DM on dementia with less influence on other metabolic factors, as seen in the other T2D animal models. Baseline fasting glucose and HbA1c levels in T2DN rats were higher compared with the GK rat model and high-fat diet/streptozotocin-induced type 1 diabetic animal model, which were widely used as the DM model for investigation of cerebrovascular myogenic reactivity (Abd-Elrahman et al. 2017; Kelly-Cobbs et al. 2012; Sauve et al. 2016). We also used a 0.1% sodium low-salt diet to minimize the development of and influence of superimposed hypertension and renal injury when these rats are aged (Muroya et al. 2018) in this study. We found this rat developed mild insulin resistance only at old age under this condition. However, the elevation in plasma insulin was much less than the levels seen in other obese DM models (Amitani et al. 2013; Maeda et al. 2003). We also performed pilot studies and compared cerebral vascular function in vivo and ex vivo using SD and Wistar rat, which is the genetic background strain of GK and FHH rats (Fan et al. 2020; Liu et al. 2015), and found that there were no differences in all the results, consistent with previous reports (Fan et al. 2020; Muroya et al. 2018). Therefore, we used age-matched SD rats as non-diabetic controls in the present study. In addition, aging exacerbated the impairment of the myogenic response of the MCA and PA in the elderly DM rats, resulting in further impairment of CBF autoregulation in both the surface and deep cortex, which was associated with BBB leakage contributing to impaired functional hyperemia, neurodegeneration, and cognitive deficits.
In conclusion, the present study demonstrated that the myogenic response of both the MCA and PA and CBF autoregulation in both the surface and deep cortex were impaired in elderly DM. The cerebral hemodynamics dysfunction was associated with BBB leakage and promoted downstream pathological consequences that may contribute to cognitive deficits. Although we did not have direct mechanistic evidence regarding whether and how impaired CBF autoregulation contributes to the enhanced BBB permeability observed in old DM rats, our findings provide essential novel information to better understand the vascular contribution to the accelerated cognitive deficits in DM individuals, especially with aging.
Author contributions
S.W., R.J.R, and F.F. conceived and designed research; S.W., W.L., H.Z., Y.G., J.R.J., and M.L. performed experiments; S.W., Y.L., and F.F. analyzed data; S.W., I.A.P., G.R., J.P.S., H.Y., R.J.R., and F.F interpreted results of experiments; S.W. and F.F. prepared figures; S.W. and F.F. drafted the manuscript; S.W., Y.L., L.L., W.G., X.F., X.H., Y.W., T.H.M., R.J.R., and F.F. edited and revised the manuscript; S.W., W.L., H.Z., Y.L., L.L., J.R.J., Y.G., M.L., W.G., X.F., I.A.P., G.R., J.P.S., T.H.M., X.H., Y.W., H.Y., R.J.R., and F.F. approved the final version of the manuscript.
Funding information
This study was supported by grants AG050049, AG057842, P20GM104357, and HL138685 from the National Institutes of Health and by grants 16GRNT31200036 and 20PRE35210043 from the American Heart Association.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abd-Elrahman KS, Walsh MP, Cole WC. Abnormal rho-associated kinase activity contributes to the dysfunctional myogenic response of cerebral arteries in type 2 diabetes. Can J Physiol Pharmacol. 2015;93:177–184. doi: 10.1139/cjpp-2014-0437. [DOI] [PubMed] [Google Scholar]
- Abd-Elrahman KS, Colinas O, Walsh EJ, Zhu HL, Campbell CM, Walsh MP, Cole WC. Abnormal myosin phosphatase targeting subunit 1 phosphorylation and actin polymerization contribute to impaired myogenic regulation of cerebral arterial diameter in the type 2 diabetic Goto-Kakizaki rat. J Cereb Blood Flow Metab. 2017;37:227–240. doi: 10.1177/0271678X15622463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelsaid M, Ma H, Coucha M, Ergul A. Late dual endothelin receptor blockade with bosentan restores impaired cerebrovascular function in diabetes. Life Sci. 2014;118:263–267. doi: 10.1016/j.lfs.2013.12.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amitani M, Asakawa A, Amitani H, Inui A. The role of leptin in the control of insulin-glucose axis. Front Neurosci. 2013;7:51. doi: 10.3389/fnins.2013.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armulik A, et al. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–243. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss WM. On the local reactions of the arterial wall to changes of internal pressure. J Physiol. 1902;28:220–231. doi: 10.1113/jphysiol.1902.sp000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RD, Zlokovic BV. Neurovascular mechanisms and blood-brain barrier disorder in Alzheimer’s disease. Acta Neuropathol. 2009;118:103–113. doi: 10.1007/s00401-009-0522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidani AK, Griffin KA, Williamson G, Wang X, Loutzenhiser R. Protective importance of the myogenic response in the renal circulation. Hypertension. 2009;54:393–398. doi: 10.1161/HYPERTENSIONAHA.109.133777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biessels GJ, Strachan MW, Visseren FL, Kappelle LJ, Whitmer RA. Dementia and cognitive decline in type 2 diabetes and prediabetic stages: towards targeted interventions. Lancet Diabetes Endocrinol. 2014;2:246–255. doi: 10.1016/S2213-8587(13)70088-3. [DOI] [PubMed] [Google Scholar]
- Brickman AM, et al. Cerebral autoregulation, beta amyloid, and white matter hyperintensities are interrelated. Neurosci Lett. 2015. 10.1016/j.neulet.2015.03.005. [DOI] [PMC free article] [PubMed]
- Broadbent NJ, Squire LR, Clark RE. Spatial memory, recognition memory, and the hippocampus. Proc Natl Acad Sci U S A. 2004;101:14515–14520. doi: 10.1073/pnas.0406344101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camarasa J, Rodrigo T, Pubill D, Escubedo E. Memantine is a useful drug to prevent the spatial and non-spatial memory deficits induced by methamphetamine in rats. Pharmacol Res. 2010;62:450–456. doi: 10.1016/j.phrs.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Chrissobolis S, Miller AA, Drummond GR, Kemp-Harper BK, Sobey CG. Oxidative stress and endothelial dysfunction in cerebrovascular disease. Front Biosci (Landmark Ed) 2011;16:1733–1745. doi: 10.2741/3816. [DOI] [PubMed] [Google Scholar]
- Cipolla MJ, Gokina NI, Osol G. Pressure-induced actin polymerization in vascular smooth muscle as a mechanism underlying myogenic behavior. FASEB J. 2002;16:72–76. doi: 10.1096/cj.01-0104hyp. [DOI] [PubMed] [Google Scholar]
- Cipolla MJ, Chan SL, Sweet J, Tavares MJ, Gokina N, Brayden JE. Postischemic reperfusion causes smooth muscle calcium sensitization and vasoconstriction of parenchymal arterioles. Stroke. 2014;45:2425–2430. doi: 10.1161/STROKEAHA.114.005888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolla MJ, Sweet J, Chan SL, Tavares MJ, Gokina N, Brayden JE. Increased pressure-induced tone in rat parenchymal arterioles vs. middle cerebral arteries: role of ion channels and calcium sensitivity. J Appl Physiol (1985) 2014;117:53–59. doi: 10.1152/japplphysiol.00253.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daulatzai MA. Cerebral hypoperfusion and glucose hypometabolism: key pathophysiological modulators promote neurodegeneration, cognitive impairment, and Alzheimer’s disease. J Neurosci Res. 2017;95:943–972. doi: 10.1002/jnr.23777. [DOI] [PubMed] [Google Scholar]
- de la Torre JC. Cerebromicrovascular pathology in Alzheimer’s disease compared to normal aging. Gerontology. 1997;43:26–43. doi: 10.1159/000213834. [DOI] [PubMed] [Google Scholar]
- Du XL, et al. Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc Natl Acad Sci U S A. 2000;97:12222–12226. doi: 10.1073/pnas.97.22.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn KM, Nelson MT. Potassium channels and neurovascular coupling. Circ J. 2010;74:608–616. doi: 10.1253/circj.cj-10-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Najjar N, Kulkarni RP, Nader N, Hodeify R, Machaca K. Effects of hyperglycemia on vascular smooth muscle Ca2+ signaling. Biomed Res Int. 2017;2017:3691349. doi: 10.1155/2017/3691349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennaceur A. One-trial object recognition in rats and mice: methodological and theoretical issues. Behav Brain Res. 2010;215:244–254. doi: 10.1016/j.bbr.2009.12.036. [DOI] [PubMed] [Google Scholar]
- Fan F, et al. 20-Hydroxyeicosatetraenoic acid contributes to the inhibition of K+ channel activity and vasoconstrictor response to angiotensin II in rat renal microvessels. PLoS One. 2013;8:e82482. doi: 10.1371/journal.pone.0082482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan F, Geurts AM, Pabbidi MR, Smith SV, Harder DR, Jacob H, Roman RJ. Zinc-finger nuclease knockout of dual-specificity protein phosphatase-5 enhances the myogenic response and autoregulation of cerebral blood flow in FHH.1BN rats. PLoS One. 2014;9:e112878. doi: 10.1371/journal.pone.0112878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan F, Geurts AM, Murphy SR, Pabbidi MR, Jacob HJ, Roman RJ. Impaired myogenic response and autoregulation of cerebral blood flow is rescued in CYP4A1 transgenic Dahl salt-sensitive rat. Am J Physiol Regul Integr Comp Physiol. 2015;308:R379–R390. doi: 10.1152/ajpregu.00256.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan F, Muroya Y, Roman RJ. Cytochrome P450 eicosanoids in hypertension and renal disease. Curr Opin Nephrol Hypertens. 2015;24:37–46. doi: 10.1097/MNH.0000000000000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan F, et al. Molecular mechanisms and cell signaling of 20-hydroxyeicosatetraenoic acid in vascular pathophysiology. Front Biosci (Landmark Ed) 2016;21:1427–1463. doi: 10.2741/4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan F et al. Functional variants in CYP4A11 and CYP4F2 are associated with cognitive impairment and related dementia endophenotypes in the elderly. Paper presented at the 16th International Winter Eicosanoid Conference, Baltimore, 03/13/2016. 2016b.
- Fan F, Pabbidi MR, Ge Y, Li L, Wang S, Mims PN, Roman RJ. Knockdown of Add3 impairs the myogenic response of renal afferent arterioles and middle cerebral arteries. Am J Physiol Renal Physiol. 2017;312:F971–f981. doi: 10.1152/ajprenal.00529.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan F, et al. A mutation in gamma-adducin impairs autoregulation of renal blood flow and promotes the development of kidney disease. J Am Soc Nephrol In press. 2020. 10.1681/ASN.2019080784. [DOI] [PMC free article] [PubMed]
- Faraci FM, Heistad DD. Regulation of large cerebral arteries and cerebral microvascular pressure. Circ Res. 1990;66:8–17. doi: 10.1161/01.res.66.1.8. [DOI] [PubMed] [Google Scholar]
- Faraci FM, Mayhan WG, Heistad DD. Segmental vascular responses to acute hypertension in cerebrum and brain stem. Am J Physiol. 1987;252:H738–H742. doi: 10.1152/ajpheart.1987.252.4.H738. [DOI] [PubMed] [Google Scholar]
- Faraci FM, Baumbach GL, Heistad DD. Cerebral circulation: humoral regulation and effects of chronic hypertension. J Am Soc Nephrol. 1990;1:53–57. doi: 10.1681/ASN.V1153. [DOI] [PubMed] [Google Scholar]
- Faraco G, Iadecola C. Hypertension: a harbinger of stroke and dementia. Hypertension. 2013;62:810–817. doi: 10.1161/HYPERTENSIONAHA.113.01063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Cabezas MA, John YJ, Barbas H, Zikopoulos B. Distinction of neurons, glia and endothelial cells in the cerebral cortex: an algorithm based on cytological features. Front Neuroanat. 2016;10:107. doi: 10.3389/fnana.2016.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaugler J, James B, Johnson T, Marin A, Weuve J, Assoc As 2019 Alzheimer’s disease facts and figures. Alzheimers Dement. 2019;15:321–387. doi: 10.1016/j.jalz.2019.01.010. [DOI] [Google Scholar]
- Girouard H, Bonev AD, Hannah RM, Meredith A, Aldrich RW, Nelson MT. Astrocytic endfoot Ca2+ and BK channels determine both arteriolar dilation and constriction. Proc Natl Acad Sci U S A. 2010;107:3811–3816. doi: 10.1073/pnas.0914722107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick PB, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros R, Van Wert R, You X, Thorin E, Husain M. Effects of age, gender, and blood pressure on myogenic responses of mesenteric arteries from C57BL/6 mice. Am J Physiol Heart Circ Physiol. 2002;282:H380–H388. doi: 10.1152/ajpheart.2002.282.1.H380. [DOI] [PubMed] [Google Scholar]
- Gudala K, Bansal D, Schifano F, Bhansali A. Diabetes mellitus and risk of dementia: a meta-analysis of prospective observational studies. J Diabetes Investig. 2013;4:640–650. doi: 10.1111/jdi.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra G, Lucariello A, Perna A, Botta L, De Luca A, Moccia F. The role of endothelial Ca(2+) signaling in neurovascular coupling: a view from the lumen. Int J Mol Sci. 2018;19. 10.3390/ijms19040938. [DOI] [PMC free article] [PubMed]
- Guo Y, et al. Accelerated cerebral vascular injury in diabetes is associated with vascular smooth muscle cell dysfunction. Geroscience. 2020. 10.1007/s11357-020-00179-z. [DOI] [PMC free article] [PubMed]
- Hall CN, et al. Capillary pericytes regulate cerebral blood flow in health and disease. Nature. 2014;508:55–60. doi: 10.1038/nature13165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton NB, Attwell D, Hall CN. Pericyte-mediated regulation of capillary diameter: a component of neurovascular coupling in health and disease. Front Neuroenerg. 2010;2. 10.3389/fnene.2010.00005. [DOI] [PMC free article] [PubMed]
- Harder DR, Narayanan J, Gebremedhin D. Pressure-induced myogenic tone and role of 20-HETE in mediating autoregulation of cerebral blood flow. Am J Physiol Heart Circ Physiol. 2011;300:H1557–H1565. doi: 10.1152/ajpheart.01097.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper SL, Bohlen HG, Rubin MJ. Arterial and microvascular contributions to cerebral cortical autoregulation in rats. Am J Physiol. 1984;246:H17–H24. doi: 10.1152/ajpheart.1984.246.1.H17. [DOI] [PubMed] [Google Scholar]
- Hartmann DA, Underly RG, Grant RI, Watson AN, Lindner V, Shih AY. Pericyte structure and distribution in the cerebral cortex revealed by high-resolution imaging of transgenic mice. Neurophotonics. 2015;2:041402. doi: 10.1117/1.NPh.2.4.041402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert LE, Bienias JL, Aggarwal NT, Wilson RS, Bennett DA, Shah RC, Evans DA (2010) Change in risk of Alzheimer disease over time Neurology 75:786–791 10.1212/WNL.0b013e3181f0754f. [DOI] [PMC free article] [PubMed]
- Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010-2050) estimated using the 2010 census. Neurology. 2013;80:1778–1783. doi: 10.1212/WNL.0b013e31828726f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosford PS, Christie IN, Niranjan A, Aziz Q, Anderson N, Ang R, Lythgoe MF, Wells JA, Tinker A, Gourine AV. A critical role for the ATP-sensitive potassium channel subunit KIR6.1 in the control of cerebral blood flow. J Cereb Blood Flow Metab. 2019;39:2089–2095. doi: 10.1177/0271678X18780602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, De Silva TM, Chen J, Faraci FM. Cerebral vascular disease and neurovascular injury in ischemic stroke. Circ Res. 2017;120:449–471. doi: 10.1161/CIRCRESAHA.116.308427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoguchi T, Tsubouchi H, Etoh T, Kakimoto M, Sonta T, Utsumi H, Sumimoto H, Yu H, Sonoda N, Inuo M, N. Sato BSP, N. Sekiguchi BSP, K. Kobayashi BSP, H. Nawata BSP. A possible target of antioxidative therapy for diabetic vascular complications-vascular NAD(P)H oxidase. Curr Med Chem. 2003;10:1759–1764. doi: 10.2174/0929867033457133. [DOI] [PubMed] [Google Scholar]
- Jansen JF, et al. Cerebral blood flow, blood supply, and cognition in type 2 diabetes mellitus. Sci Rep. 2016;6:10. doi: 10.1038/s41598-016-0003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarajapu YP, Guberski DL, Grant MB, Knot HJ. Myogenic tone and reactivity of cerebral arteries in type II diabetic BBZDR/Wor rat. Eur J Pharmacol. 2008;579:298–307. doi: 10.1016/j.ejphar.2007.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly-Cobbs A, Elgebaly MM, Li W, Ergul A. Pressure-independent cerebrovascular remodelling and changes in myogenic reactivity in diabetic Goto-Kakizaki rat in response to glycaemic control. Acta Physiol (Oxf) 2011;203:245–251. doi: 10.1111/j.1748-1716.2010.02230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly-Cobbs AI, Prakash R, Coucha M, Knight RA, Li W, Ogbi SN, Johnson M, Ergul A. Cerebral myogenic reactivity and blood flow in type 2 diabetic rats: role of peroxynitrite in hypoxia-mediated loss of myogenic tone. J Pharmacol Exp Ther. 2012;342:407–415. doi: 10.1124/jpet.111.191296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima N, Williams JM, Takahashi T, Miyata N, Roman RJ. Effects of a new SGLT2 inhibitor, luseogliflozin, on diabetic nephropathy in T2DN rats. J Pharmacol Exp Ther. 2013;345:464–472. doi: 10.1124/jpet.113.203869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kold-Petersen H, Brondum E, Nilsson H, Flyvbjerg A, Aalkjaer C. Impaired myogenic tone in isolated cerebral and coronary resistance arteries from the goto-kakizaki rat model of type 2 diabetes. J Vasc Res. 2012;49:267–278. doi: 10.1159/000335487. [DOI] [PubMed] [Google Scholar]
- Kontos HA, Wei EP, Navari RM, Levasseur JE, Rosenblum WI, Patterson JL., Jr Responses of cerebral arteries and arterioles to acute hypotension and hypertension. Am J Physiol. 1978;234:H371–H383. doi: 10.1152/ajpheart.1978.234.4.H371. [DOI] [PubMed] [Google Scholar]
- Korbo L, Pakkenberg B, Ladefoged O, Gundersen HJ, Arlien-Soborg P, Pakkenberg H. An efficient method for estimating the total number of neurons in rat brain cortex. J Neurosci Methods. 1990;31:93–100. doi: 10.1016/0165-0270(90)90153-7. [DOI] [PubMed] [Google Scholar]
- Lagaud GJ, Masih-Khan E, Kai S, van Breemen C, Dube GP. Influence of type II diabetes on arterial tone and endothelial function in murine mesenteric resistance arteries. J Vasc Res. 2001;38:578–589. doi: 10.1159/000051094. [DOI] [PubMed] [Google Scholar]
- Lammie GA. Hypertensive cerebral small vessel disease and stroke. Brain Pathol. 2002;12:358–370. doi: 10.1111/j.1750-3639.2002.tb00450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G, Goosens KA. Sampling blood from the lateral tail vein of the rat. J Vis Exp. 2015:e52766. 10.3791/52766. [DOI] [PMC free article] [PubMed]
- Li W, et al. Adaptive cerebral neovascularization in a model of type 2 diabetes: relevance to focal cerebral ischemia. Diabetes. 2010;59:228–235. doi: 10.2337/db09-0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Li C, Falck JR, Roman RJ, Harder DR, Koehler RC. Interaction of nitric oxide, 20-HETE, and EETs during functional hyperemia in whisker barrel cortex. Am J Physiol Heart Circ Physiol. 2008;295:H619–H631. doi: 10.1152/ajpheart.01211.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, et al. Comparative genome of GK and Wistar rats reveals genetic basis of type 2 diabetes. PLoS One. 2015;10:e0141859. doi: 10.1371/journal.pone.0141859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wang S, Guo Y, Zhang H, Roman RJ, Fan F. Impaired pericyte constriction and cerebral blood flow autoregulation in diabetes. Stroke. 2020;51:AWP498. doi: 10.1161/STROKEAHA.119.027255. [DOI] [Google Scholar]
- Longden TA, et al. Capillary K(+)-sensing initiates retrograde hyperpolarization to increase local cerebral blood flow. Nat Neurosci. 2017;20:717–726. doi: 10.1038/nn.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luissint AC, Artus C, Glacial F, Ganeshamoorthy K, Couraud PO. Tight junctions at the blood brain barrier: physiological architecture and disease-associated dysregulation. Fluids Barriers CNS. 2012;9:23. doi: 10.1186/2045-8118-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda K, et al. Role of the fatty acid binding protein mal1 in obesity and insulin resistance. Diabetes. 2003;52:300–307. doi: 10.2337/diabetes.52.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayeda ER, et al. Type 2 diabetes and cognitive decline over 14 years in middle-aged African Americans and whites: the ARIC brain MRI study. Neuroepidemiology. 2014;43:220–227. doi: 10.1159/000366506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayeda ER, Whitmer RA, Yaffe K. Diabetes and cognition. Clin Geriatr Med. 2015;31:101–115. doi: 10.1016/j.cger.2014.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayhan WG, Heistad DD. Role of veins and cerebral venous pressure in disruption of the blood-brain barrier. Circ Res. 1986;59:216–220. doi: 10.1161/01.res.59.2.216. [DOI] [PubMed] [Google Scholar]
- McDaniel CF. Diabetes: a model of oxidative accelerated aging. Age (Omaha) 1999;22:145–148. doi: 10.1007/s11357-999-0016-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami S, Fujita N, Kondo H, Takeda I, Momota R, Ohtsuka A, et al. Abnormalities in the fiber composition and capillary architecture in the soleus muscle of type 2 diabetic Goto-Kakizaki rats. Sci World J. 2012, 2012:680189. 10.1100/2012/680189. [DOI] [PMC free article] [PubMed]
- Muroya Y, He X, Fan L, Wang S, Xu R, Fan F, Roman RJ. Enhanced renal ischemia-reperfusion injury in aging and diabetes. Am J Physiol Renal Physiol. 2018;315:F1843–F1854. doi: 10.1152/ajprenal.00184.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro-Orozco D, Sanchez-Manso JC. StatPearls. Treasure Island: StatPearls Publishing; 2020. Neuroanatomy, middle cerebral artery. [PubMed] [Google Scholar]
- Nishimura N, Schaffer CB, Friedman B, Lyden PD, Kleinfeld D. Penetrating arterioles are a bottleneck in the perfusion of neocortex. Proc Natl Acad Sci. 2007;104:365–370. doi: 10.1073/pnas.0609551104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobrega MA, Fleming S, Roman RJ, Shiozawa M, Schlick N, Lazar J, Jacob HJ. Initial characterization of a rat model of diabetic nephropathy. Diabetes. 2004;53:735–742. doi: 10.2337/diabetes.53.3.735. [DOI] [PubMed] [Google Scholar]
- Nortley R, Korte N, Izquierdo P, Hirunpattarasilp C, Mishra A, Jaunmuktane Z, et al. Amyloid β oligomers constrict human capillaries in Alzheimer’s disease via signaling to pericytes. Science. 2019;365. 10.1126/science.aav9518. [DOI] [PMC free article] [PubMed]
- Nyborg NC, Nielsen PJ. The level of spontaneous myogenic tone in isolated human posterior ciliary arteries decreases with age. Exp Eye Res. 1990;51:711–715. doi: 10.1016/0014-4835(90)90056-Z. [DOI] [PubMed] [Google Scholar]
- Oana F, Takeda H, Hayakawa K, Matsuzawa A, Akahane S, Isaji M, Akahane M. Physiological difference between obese (fa/fa) Zucker rats and lean Zucker rats concerning adiponectin. Metabolism. 2005;54:995–1001. doi: 10.1016/j.metabol.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Ogoh S, Brothers RM, Jeschke M, Secher NH, Raven PB. Estimation of cerebral vascular tone during exercise; evaluation by critical closing pressure in humans. Exp Physiol. 2010;95:678–685. doi: 10.1113/expphysiol.2010.052340. [DOI] [PubMed] [Google Scholar]
- Orasanu G, Plutzky J. The pathologic continuum of diabetic vascular disease. J Am Coll Cardiol. 2009;53:S35–S42. doi: 10.1016/j.jacc.2008.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osol G, Halpern W. Myogenic properties of cerebral blood vessels from normotensive and hypertensive rats. Am J Physiol. 1985;249:H914–H921. doi: 10.1152/ajpheart.1985.249.5.H914. [DOI] [PubMed] [Google Scholar]
- Pabbidi MR, Mazur O, Fan F, Farley JM, Gebremedhinm D, Harder DR, Roman RJ. Enhanced large conductance K+ channel (BK) activity contributes to the impaired myogenic response in the cerebral vasculature of fawn hooded hypertensive rats. Am J Physiol Heart Circ Physiol. 2014;306(7):H989–H1000. doi: 10.1152/ajpheart.00636.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson OB, Strandgaard S, Edvinsson L. Cerebral autoregulation Cerebrovascular and brain metabolism reviews. 1990;2:161–92. [PubMed]
- Penley SC, Gaudet CM, Threlkeld SW. Use of an eight-arm radial water maze to assess working and reference memory following neonatal brain injury. J Vis Exp. 2013:50940. 10.3791/50940. [DOI] [PMC free article] [PubMed]
- Pires PW, Jackson WF, Dorrance AM (2015) Regulation of myogenic tone and structure of parenchymal arterioles by hypertension and the mineralocorticoid receptor Am J Physiol Heart Circ Physiol 309:H127–H136 doi:10.1152/ajpheart.00168.2015. [DOI] [PMC free article] [PubMed]
- Pires PW, Dabertrand F, Earley S. Isolation and cannulation of cerebral parenchymal arterioles. J Vis Exp. 2016. 10.3791/53835. [DOI] [PMC free article] [PubMed]
- Rajabi M, Mohaddes G, Farajdokht F, Nayebi Rad S, Mesgari M, Babri S. Impact of loganin on pro-inflammatory cytokines and depression- and anxiety-like behaviors in male diabetic rats. Physiol Int. 2018;105:199–209. doi: 10.1556/2060.105.2018.1.8. [DOI] [PubMed] [Google Scholar]
- Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2002;82:131–185. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- Roman RJ, Fan F. 20-HETE: hypertension and beyond. Hypertension. 2018;72:12–18. doi: 10.1161/HYPERTENSIONAHA.118.10269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueda A, Fernandez-Velasco M, Benitah JP, Gomez AM. Abnormal Ca2+ spark/STOC coupling in cerebral artery smooth muscle cells of obese type 2 diabetic mice. PLoS One. 2013;8:e53321. doi: 10.1371/journal.pone.0053321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachidanandam K, Hutchinson JR, Elgebaly MM, Mezzetti EM, Dorrance AM, Motamed K, Ergul A. Glycemic control prevents microvascular remodeling and increased tone in type 2 diabetes: link to endothelin-1. Am J Physiol Regul Integr Comp Physiol. 2009;296:R952–R959. doi: 10.1152/ajpregu.90537.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaras K, et al. The impact of glucose disorders on cognition and brain volumes in the elderly: the Sydney Memory and Ageing Study. Age (Dordr) 2014;36:977–993. doi: 10.1007/s11357-013-9613-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauve M, et al. Tumor necrosis factor/Sphingosine-1-phosphate signaling augments resistance artery myogenic tone in diabetes. Diabetes. 2016;65:1916–1928. doi: 10.2337/db15-1450. [DOI] [PubMed] [Google Scholar]
- Seibenhener ML, Wooten MC. Use of the Open Field Maze to measure locomotor and anxiety-like behavior in mice. J Vis Exp. 2015:e52434. 10.3791/52434. [DOI] [PMC free article] [PubMed]
- Shekhar S, et al. Impaired cerebral autoregulation-a common neurovascular pathway in diabetes may play a critical role in diabetes-related Alzheimer’s disease. Curr Res Diabetes Obes J. 2017;2:555587. [PMC free article] [PubMed] [Google Scholar]
- Sweeney MD, Sagare AP, Zlokovic BV. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurosci. 2018;14:133–150. doi: 10.1038/nrneurol.2017.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabit CE, Chung WB, Hamburg NM, Vita JA. Endothelial dysfunction in diabetes mellitus: molecular mechanisms and clinical implications. Rev Endocr Metab Disord. 2010;11:61–74. doi: 10.1007/s11154-010-9134-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Valcarcel-Ares MN, Yabluchanskiy A, Tucsek Z, Hertelendy P, Kiss T, Gautam T, Zhang XA, Sonntag WE, de Cabo R, Farkas E, Elliott MH, Kinter MT, Deak F, Ungvari Z, Csiszar A. Nrf2 deficiency exacerbates obesity-induced oxidative stress, neurovascular dysfunction, blood-brain barrier disruption, neuroinflammation, amyloidogenic gene expression, and cognitive decline in mice, mimicking the aging phenotype. J Gerontol A Biol Sci Med Sci. 2018;73:853–863. doi: 10.1093/gerona/glx177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatem KS, Quinn JL, Phadke A, Yu Q, Gordish-Dressman H, Nagaraju K. Behavioral and locomotor measurements using an open field activity monitoring system for skeletal muscle diseases. J Vis Exp. 2014:51785. 10.3791/51785. [DOI] [PMC free article] [PubMed]
- Tiehuis AM, et al. Cerebral perfusion in relation to cognitive function and type 2 diabetes. Diabetologia. 2008;51:1321–1326. doi: 10.1007/s00125-008-1041-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P, et al. Resveratrol treatment rescues neurovascular coupling in aged mice: role of improved cerebromicrovascular endothelial function and downregulation of NADPH oxidase. Am J Physiol Heart Circ Physiol. 2014;306:H299–H308. doi: 10.1152/ajpheart.00744.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P, et al. IGF-1 deficiency impairs neurovascular coupling in mice: implications for cerebromicrovascular aging. Aging Cell. 2015;14:1034–1044. doi: 10.1111/acel.12372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P, Tarantini S, Csiszar A, Ungvari Z. Functional vascular contributions to cognitive impairment and dementia: mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling in aging. Am J Physiol Heart Circ Physiol. 2017;312:H1–H20. doi: 10.1152/ajpheart.00581.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Tarantini S, Donato AJ, Galvan V, Csiszar A. Mechanisms of vascular aging. Circ Res. 2018;123:849–867. doi: 10.1161/CIRCRESAHA.118.311378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, et al. Endothelial dysfunction and angiogenesis impairment in the ageing vasculature. Nat Rev Cardiol. 2018;15:555–565. doi: 10.1038/s41569-018-0030-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verweij B. Current concepts of cerebral oxygen transport and energy metabolism after severe traumatic brain injury. Prog Brain Res. 2007;161C:111–124. doi: 10.1016/S0079-6123(06)61008-X. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Williams MT. Assessing spatial learning and memory in rodents. ILAR J. 2014;55:310–332. doi: 10.1093/ilar/ilu013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, et al. Role of autoregulation of cerebral blood flow in diabetes-related Alzheimer-like cognitive deficits. FASEB J. 2017;31:1013.1015. [Google Scholar]
- Wang S, Jiao F, Guo Y, Booz GW, Roman RJ, Fan F. Role of vascular smooth muscle cells in diabetes-related vascular cognitive impairment. Stroke. 2019;50:ATP556. [Google Scholar]
- Wang S, et al. Sex differences in the structure and function of rat middle cerebral arteries. Am J Physiol Heart Circ Physiol. 2020. 10.1152/ajpheart.00722.2019. [DOI] [PMC free article] [PubMed]
- Warrington JP, Fan F, Murphy SR, Roman RJ, Drummond HA, Granger JP, et al. Placental ischemia in pregnant rats impairs cerebral blood flow autoregulation and increases blood-brain barrier permeability. Physiol Rep. 2014;2. 10.14814/phy2.12134. [DOI] [PMC free article] [PubMed]
- Watson AN, Berthiaume AA, Faino AV, McDowell KP, Bhat NR, Hartmann DA, et al. Mild pericyte deficiency is associated with aberrant brain microvascular flow in aged PDGFRbeta(+/-) mice. J Cereb Blood Flow Metab. 2020:271678X19900543. 10.1177/0271678X19900543. [DOI] [PMC free article] [PubMed]
- Yarchoan M, Arnold SE. Repurposing diabetes drugs for brain insulin resistance in Alzheimer disease. Diabetes. 2014;63:2253–2261. doi: 10.2337/db14-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoi N, et al. A novel rat model of type 2 diabetes: the Zucker fatty diabetes mellitus ZFDM rat. J Diabetes Res. 2013;2013:103731. doi: 10.1155/2013/103731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, et al. Influence of dual-specificity protein phosphatase 5 on mechanical properties of rat cerebral and renal arterioles. Physiol Rep. 2020;8:e14345. doi: 10.14814/phy2.14345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann PA, Knot HJ, Stevenson AS, Nelson MT. Increased myogenic tone and diminished responsiveness to ATP-sensitive K+ channel openers in cerebral arteries from diabetic rats. Circ Res. 1997;81:996–1004. doi: 10.1161/01.RES.81.6.996. [DOI] [PubMed] [Google Scholar]
- Zudaire E, Gambardella L, Kurcz C, Vermeren S. A computational tool for quantitative analysis of vascular networks. PLoS One. 2011;6:e27385. doi: 10.1371/journal.pone.0027385. [DOI] [PMC free article] [PubMed] [Google Scholar]