Abstract
Background
A remarkably better prognosis is associated with oropharyngeal squamous cell carcinomas (OPSCC) driven by human papillomaviruses (HPV) compared with HPV-negative OPSCC. Consequently, de-escalation of standard treatment has been suggested. Due to modest specificity rates, debates are ongoing, whether p16INK4a, a surrogate marker for HPV-driven OPSCC, is sufficient to correctly identify those tumours and avoid substantial HPV misattribution and thus undertreatment of patients by de-escalation. Robust data estimating the proportion of potentially undertreated patients are missing.
Methods
We assessed a large-scale cohort of consecutively included OPSCC diagnosed between 2000 and 2017 for HPV–DNA, HPV genotypes, p16INK4a expression and multiple tumour- and patient-related risk factors, and investigated their impact on patients’ survival in comprehensive uni- and multivariate analyses.
Results
Aetiological relevance of HPV (p16INK4a- and high-risk HPV–DNA-positivity) was detected in 27.1% (n = 192) of OPSCC, with HPV16 being the most abundant HPV type (94.6%). In 5.5% patients (n = 39), p16INK4a overexpression but no HPV–DNA was detected. Principal component and survival analyses revealed that 60.6% of these p16INK4a-positive OPSCC lacking HPV–DNA did not resemble HPV16-driven but HPV-negative OPSCC regarding risk-factor profile and overall survival. Notably, this group represented 10.6% of all p16INK4a-overexpressing OPSCC.
Conclusions
p16INK4a as a single marker appears insufficient to indicate OPSCC patients suitable for treatment de-escalation.
Subject terms: Prognostic markers, Oral cancer, Tumour biomarkers, Tumour virus infections, Cancer therapy
Background
Persistent infections with high-risk (HR) human papillomavirus (HPV) types are causative for about 31% of oropharyngeal squamous cell carcinomas (OPSCC).1 Importantly, the incidence of HPV-driven OPSCC has been rising in several countries worldwide,1–3 albeit heterogeneity exists regarding anatomic subsite, geography and sex.4 Patients with HPV-driven OPSCC are reported to have a different risk profile (younger age, reduced tobacco/alcohol consumption) in comparison with HPV-negative OPSCC.5,6 Likewise, patients’ survival is remarkably better, although most patients present with smaller primary tumours, but more advanced cervical lymph node metastasis status compared with HPV-negative OPSCC patients.5 In view of the superior survival, several clinical phase I–III trials are currently investigating the benefits of de-escalating treatment for this patient group to spare them treatment-associated morbidity.7–11 However, de-escalation by replacement of cisplatin with cetuximab failed in the setting of cisplatin-based chemo–radiotherapy.12,13 This situation raises the central question which diagnostic test can reliably identify HPV-driven OPSCC patients suitable for treatment de-escalation.14
The detection of viral oncogene E6/E7 mRNA or protein can be considered as gold standard to define an aetiological relevance of HPV for carcinogenesis, since E6 and E7 expression is essential for initiation and maintenance of the transformed phenotype in HPV-induced tumours.15 However, the technically rather laborious detection procedure for E6/E7 products generally limits its application in routine laboratory testing. Among the various consequences of HPV E6/E7 oncogene signalling, the cellular cyclin-dependent kinase inhibitor 2A (p16INK4a) becomes overexpressed as a sequel to E7-induced epigenetic remodelling.16 Thus, p16INK4a has been suggested as a biomarker of HPV-transformed cells, and evaluation of p16INK4a expression can be easily performed by immunohistochemistry using formalin-fixed tissue. Detection of p16INK4a has been introduced as a surrogate marker in the AJCC-8/UICC-8 staging system for the classification of OPSCC.
Several studies, however, have demonstrated a lack of association between HPV–DNA or RNA detection and p16INK4a overexpression in about 5–20% of OPSCC, indicating that a considerable subset of p16INK4a-positive OPSCC is not causally driven by HPV.14,17–19 This fact has raised concerns whether a group of OPSCC patients could be undertreated if the decision for therapy de-escalation was solely based on p16INK4a positivity.14 However, there is currently a paucity of robust data estimating the proportion of potentially undertreated patients in this context.
The major goal of our study was to determine the proportion of potentially undertreated patients if p16INK4a represented the single-decision criterion for treatment de-escalation in OPSCC patients. We therefore assessed p16INK4a, HPV–DNA and HPV genotypes in relation to tumour- and patient-related risk factors and survival in a cohort of more than 800 consecutively included OPSCC patients at a large German University Hospital diagnosed between 2000 and 2017. Specifically, we aimed to determine risk groups among OPSCC with discordant test results for p16INK4a expression and HPV–DNA to assess whether the respective patient groups could be considered suitable for treatment de-escalation.
Methods
Patients and treatment
Between 2000 and 2017, 802 patients were diagnosed with OPSCC (according to ICD10) and treated at our hospital (Department of Otorhinolaryngology, Head and Neck Surgery of the University Hospital Giessen) by upfront surgery with or without neck dissection, or by definitive radio- or chemo–radiotherapy according to the local guidelines and upon patient’s decision (Table 1). Written informed consent was obtained from all patients, and tumour material was used in accordance with the local ethics committee. Those not treated with curative intent were not included in the analysis regarding survival.
Table 1.
Descriptive analysis of biometric data and treatment of OPSCC patients with concordant HPV–DNA and p16INK4a tests included in the analysis (N = 620).
| All | HPV-negative | HPV16-driven | P value | p16INK4a negative HPV16–DNA-positive | p16INK4a positive HPV–DNA-negative | |
|---|---|---|---|---|---|---|
| (N = 620) | (N = 436) | (N = 184) | (N = 29) | (N = 39) | ||
| Risk factors | N (%)* | N (%) | N (%) | N (%) | N (%) | |
| Gender | ||||||
| Male | 478 (77) | 340 (78) | 138 (75) | 0.420 | 23 (79) | 32 (82) |
| Female | 142 (23) | 96 (22) | 46 (25) | 6 (21) | 7 (18) | |
| Age (IQR) | ||||||
| Median | 60.8 | 60.0 | 62.6 | 0.002§ | 57.9 | 60.0 |
| Third quartile | 67.8 | 66.4 | 71.6 | 65.2 | 64.9 | |
| First quartile | 53.9 | 53.8 | 54.1 | 53.4 | 56.0 | |
| Comorbidity (ECOG) | ||||||
| 0 | 38 (6) | 21 (5) | 17 (9) | 0.008# | 4 (14) | 2 (5) |
| 1 | 398 (65) | 277 (64) | 121 (67) | 17 (61) | 26 (67) | |
| 2 | 152 (25) | 114 (26) | 38 (21) | 4 (14) | 9 (23) | |
| 3 | 22 (4) | 17 (4) | 5 (3) | 3 (11) | 2 (5) | |
| 4 | 5 (1) | 5 (1) | 0 (0) | 0 (0) | 0 (0) | |
| Unknown | 5 | 2 | 3 | 1 | 0 | |
| Healthy (0–1) | 436 (71) | 298 (69) | 138 (76) | 0.059 | 21 (75) | 28 (72) |
| Sick (2–4) | 179 (29) | 136 (31) | 43 (24) | 7 (25) | 11 (28) | |
| Alcohol | ||||||
| >2 standard drinks/day | 292 (53) | 269 (68) | 23 (15) | <0.001 | 20 (77) | 15 (44) |
| ≤2 standard drinks/ day | 260 (47) | 125 (32) | 135 (85) | 6 (23) | 19 (56) | |
| Unknown | 68 | 42 | 26 | 3 | 5 | |
| Smoking | ||||||
| Yes | 489 (81) | 392 (92) | 97 (56) | <0.001 | 26 (90) | 31 (82) |
| No | 112 (19) | 36 (8) | 76 (44) | 3 (10) | 7 (18) | |
| Unknown | 19 | 8 | 11 | 0 | 1 | |
| Pack years (IQR) | ||||||
| Median | 38.0 | 40.0 | 25.5 | <0.001§ | 40.0 | 33.0 |
| Third quartile | 50.0 | 52.5 | 40.0 | 61.5 | 43.5 | |
| First quartile | 25.0 | 29.8 | 15.0 | 30.0 | 25.5 | |
| Smokers (N), pack year unspecified | 76 | 60 | 16 | 5 | 4 | |
| Tumour characteristics | ||||||
| Localisation | ||||||
| Tonsil | 259 (42) | 150 (34) | 109 (59) | <0.001 | 12 (41) | 17 (44) |
| Other than tonsil | 361 (58) | 286 (66) | 75 (41) | 17 (59) | 22 (56) | |
| T stage | ||||||
| 1–2 | 319 (52) | 202 (47) | 117 (64) | <0.001 | 10 (34) | 23 (59) |
| 3–4 | 297 (48) | 230 (53) | 67 (36) | 19 (66) | 16 (41) | |
| 1 | 137 (22) | 93 (22) | 44 (24) | <0.001# | 8 (28) | 8 (21) |
| 2 | 182 (30) | 109 (25) | 73 (40) | 2 (7) | 15 (38) | |
| 3 | 128 (21) | 98 (23) | 30 (16) | 9 (31) | 7 (18) | |
| 4 | 36 (6) | 24 (6) | 12 (7) | 0 (0) | 1 (3) | |
| 4a | 56 (9) | 41 (9) | 15 (8) | 6 (21) | 3 (8) | |
| 4b | 77 (13) | 67 (16) | 10 (5) | 4 (14) | 5 (13) | |
| Unknown | 4 | 4 | 0 | 0 | 0 | |
| N stage | ||||||
| N0 | 157 (26) | 134 (31) | 23 (13) | <0.001 | 11 (38) | 7 (18) |
| N+ | 455 (74) | 295 (69) | 160 (87) | 18 (62) | 31 (82) | |
| N0 | 157 (26) | 134 (31) | 23 (13) | 0.630# | 11 (38) | 7 (18) |
| N1 | 106 (17) | 53 (12) | 53 (29) | 1 (3) | 6 (16) | |
| N2–N2a | 62 (10) | 34 (8) | 28 (15) | 2 (7) | 3 (8) | |
| N2b | 187 (31) | 129 (30) | 58 (32) | 8 (28) | 14 (37) | |
| N2c | 75 (12) | 59 (14) | 16 (9) | 4 (14) | 7 (18) | |
| N3 | 25 (4) | 20 (5) | 5 (3) | 3 (10) | 1 (3) | |
| Unknown | 8 | 7 | 1 | 0 | 1 | |
| M stage | ||||||
| M0 | 561 (93) | 396 (93) | 165 (93) | 0.985 | 25 (89) | 35 (92) |
| M+ | 44 (7) | 31 (7) | 13 (7) | 3 (11) | 3 (8) | |
| Unknown | 15 | 9 | 6 | 1 | 1 | |
| UICC7 stages | ||||||
| I–III | 218 (36) | 152 (35) | 66 (36) | 0.881 | 10 (34) | 13 (33) |
| >III | 394 (64) | 277 (65) | 117 (64) | 19 (66) | 26 (67) | |
| I | 52 (8) | 47 (11) | 5 (3) | 0.277# | 7 (24) | 1 (3) |
| II | 53 (9) | 43 (10) | 10 (5) | 1 (3) | 6 (15) | |
| III | 113 (18) | 62 (14) | 51 (28) | 2 (7) | 6 (15) | |
| IV–IVa | 268 (44) | 177 (41) | 91 (50) | 12 (41) | 18 (46) | |
| IVb | 83 (14) | 71 (17) | 12 (7) | 4 (14) | 5 (13) | |
| IVc | 43 (7) | 29 (7) | 14 (8) | 3 (10) | 3 (8) | |
| Unknown | 8 | 7 | 1 | 0 | 0 | |
| UICC-8 stages | ||||||
| I–III | 306 (54) | 149 (37) | 157 (91) | <0.001 | 12 (43) | 12 (32) |
| >III | 265 (46) | 249 (63) | 16 (9) | 16 (57) | 25 (68) | |
| I | 123 (22) | 45 (11) | 78 (45) | <0.001# | 8 (29) | 6 (16) |
| II | 90 (16) | 43 (11) | 47 (27) | 1 (4) | 4 (11) | |
| III | 93 (16) | 61 (15) | 32 (18) | 3 (11) | 2 (5) | |
| IV–IVa | 174 (30) | 158 (40) | 16 (9) | 11 (39) | 18 (49) | |
| IVb | 70 (12) | 70 (18) | 0 (0) | 3 (11) | 5 (14) | |
| IVc | 21 (4) | 21 (5) | 0 (0) | 2 (7) | 2 (5) | |
| Unknown | 49 | 38 | 11 | 1 | 2 | |
| Palliative treatment | 43 (7) | 35 (8) | 8 (5) | 0.091 | 2 (7) | 0 |
| Curative treatment type | ||||||
| Upfront surgery without neck dissection | 39 (7) | 31 (8) | 8 (5) | 0.022 | 5 (17) | 1 (3) |
| Upfront surgery with neck dissection | 325 (54) | 210 (54) | 115 (68) | 17 (59) | 20 (53) | |
| Definitive radiotherapy | 25 (4) | 20 (5) | 5 (3) | 0 (0) | 2 (5) | |
| Definitive chemo–radiotherapy | 167 (28) | 126 (33) | 41 (24) | 5 (17) | 15 (39) | |
| Unknown | 21 | 14 | 7 | 0 | 1 | |
| Resection status of surgery | ||||||
| R0 | 268 (79) | 177 (79) | 91 (81) | 0.690 | 17 (77) | 17 (81) |
| R+ | 70 (21) | 48 (21) | 22 (19) | 5 (23) | 4 (19) | |
| Unknown | 26 | 16 | 10 | 0 | 0 | |
OPSCC patients with discordant HPV–DNA and p16INK4a tests (N = 68) are indicated for comparison.
*Percentage based on total cases (N = 620) without missing values. P values for comparison of HPV-negative versus HPV16-driven OPSCC (asymptotic, two-sided) calculated by chi square, (§) t test or (#) Mantel–Haenszel test of trend; significant P values (P ≤ 0.05) in bold.
HPV status of OPSCC
We have analysed all primary tumours with sufficient formalin-fixed, paraffin-embedded tissue for the presence of HPV–DNA, HPV genotypes (n = 721) and expression of p16INK4a (n = 717) by immunostaining (Fig. 1) as previously described.2 Tumour tissue from 709 (88.4%) diagnostic biopsies (in the case of non-surgical treatment) and resected OPSCC was available to perform both tests.
Fig. 1. Expression of p16INK4a in oropharyngeal squamous cell carcinomas (OPSCC) detected by immunostaining.
a HPV–DNA-negative OPSCC without p16INK4a expression. b HPV16-DNA-positive OPSCC showing strong, diffuse overexpression of p16INK4a characteristic for HPV16-driven OPSCC. c HPV–DNA-negative OPSCC displaying p16INK4a overexpression similar to HPV16-driven OPSCC (b).
Statistics
We used principal component analysis (PCA)20,21 as an eigenvector-based multivariate method to reveal the internal structure of our data, especially regarding the four combinations of HPV–DNA testing and p16INK4a expression of OPSCC to define HPV status (HPV-negative and HPV-driven for concordant tests vs. discordant test results). Overall survival (OS, calculated from the date of histological diagnosis by routine biopsy to the date of death from any cause or date of last seen alive) was used to generate survival curves by the Kaplan–Meier method. SPSS Statistical Software (IBM SPSS 26.0) was used for statistical analysis, and significance was considered P ≤ .05 for all tests, unless otherwise indicated.
Results
HPV types in relation to p16INK4a expression
We detected HR–HPV–DNA in 234/721 (32.5%) OPSCC samples. DNA from non-HR–HPV types was detected in 5 (0.7%) OPSCC only, two of them corresponding to the low-risk (LR) types 6 and 11 (Table 2). HPV16 was the most frequent among all HPV types, identified as the exclusive type in 91.2% (and in 94.6% overall) of HPV–DNA-positive cases. HR–HPV types other than 16 were detected alone or in combination with one or two other types in 16 cases (Table 2).
Table 2.
Prevalence of HPV-driven OPSCC determined by HPV–DNA detection and p16INK4a expression and total frequency of HPV types 2000–2017.
| n | % | p16INK4a overexpression | ||||||
|---|---|---|---|---|---|---|---|---|
| Negative | Positive | Unknown | ||||||
| n | % | n | % | n | % | |||
| All OPSCC | 802 | 100.0* | 480 | 59.9* | 237 | 29.6* | 85 | 10.6* |
| HPV–DNA-negative | 482 | 66.9 | 436 | 61.5 | 39 | 5.5 | 7 | |
| HR–HPV–DNA-positive | 234 | 32.5 | 37 | 5.2 | 192 | 27.1 | 5 | |
| Non-HR–HPV–DNA-positive | 5 | 0.7 | 1 | 0.1 | 4 | 0.6 | 0 | |
| Unknown | 81 | 10.1* | 6 | 2 | 73 | |||
| High-risk (HR) HPV types | ||||||||
| Single HR type | ||||||||
| 16 | 218 | 91.2 | 29 | 13.3 | 184 | 84.4 | 5 | 2.3 |
| 18 | 2 | 0.8 | – | – | 2 | 100.0 | – | – |
| 33 | 1 | 0.4 | – | – | 1 | 100.0 | – | – |
| 51 | 1 | 0.4 | 1 | 100.0 | – | – | – | – |
| 58 | 2 | 0.8 | – | – | 2 | 100.0 | – | – |
| HR double | ||||||||
| 16, 18 | 6 | 2.5 | 5 | 83.3 | 1 | 16.7 | – | – |
| 16, 53 | 1 | 0.4 | 1 | 100.0 | – | – | – | – |
| 35, 26 | 2 | 0.8 | – | – | 2 | 100.0 | – | – |
| HR triple | ||||||||
| 16, 18, 53 | 1 | 0.4 | 1 | 100.0 | – | – | – | – |
| Non-HR–HPV types | ||||||||
| Putative HR type | ||||||||
| 26 | 1 | 0.4 | – | – | 1 | 100.0 | – | – |
| Unspecified risk type | ||||||||
| 30 | 2 | 0.8 | – | – | 2 | 100.0 | – | – |
| HPV low-risk type | ||||||||
| 6 | 1 | 0.4 | – | – | 1 | 100.0 | – | – |
| 11 | 1 | 0.4 | 1 | 100.0 | – | – | – | – |
*Percentage based on total cases (n = 802). Bold numbers: patient groups further analysed.
About 27.1% of 709 OPSCC patients were positive for both p16INK4a and HR–HPV–DNA, representing the average prevalence of truly HPV-driven OPSCC during the study period of 18 years. About 61.5% of the samples showed no expression of p16INK4a and did not contain any HPV–DNA, thus being considered truly HPV-negative. Discordant results were observed in 10.7% of all samples analysed for both markers: 5.2% of samples were HPV–DNA-positive but lacked overexpression of p16INK4a, and 5.5% of cases displayed p16INK4a overexpression but no HR–HPV–DNA (Table 2).
p16INK4a overexpression was accompanied by positivity for HR–HPV–DNA in 81.7% (192/235) and specifically for HPV16–DNA in 78.7% (185/235) of cases. Importantly, in 39 (16.6%) OPSCC overexpressing p16INK4a, no HPV–DNA was detected. p16INK4a expression pattern in these HPV– DNA-negative tumours did not differ from HPV-driven OPSCC (Fig. 1). Only OPSCC positive for HPV16 or negative for any HPV–DNA (Table 2: highlighted in bold) were included in the descriptive analysis (Table 1).
Descriptive analysis of OPSCC according to HPV16 status
We analysed tumour characteristics, lifestyle- and patient-related risk factors for patients with “truly” HPV-negative (HPV–DNA-negative and p16INK4a-negative) and HPV16-driven (HPV16–DNA-positive and p16INK4a-positive) OPSCC. Cases with discordant results were not considered in the statistical comparison of this sub-analysis (Table 1).
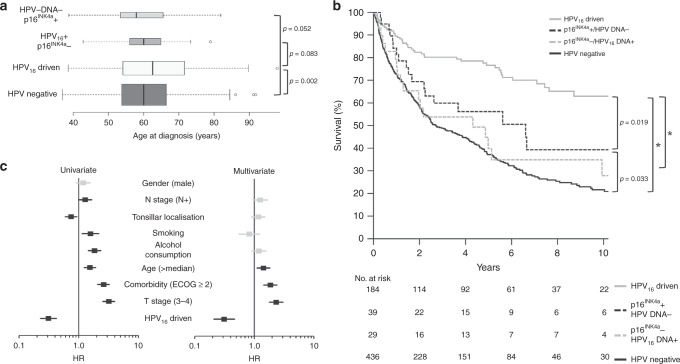
In contrast to most demographic characteristics and risk factors of OPSCC patients (Table 1), which are in line with the literature, patients with HPV16-driven OPSCC were older at diagnosis than patients with HPV-negative OPSCC. The difference in median age was 2.6 years and statistically significant (P = 0.002, Fig. 2a, Table 1).
Fig. 2. Age at diagnosis in comparison with HPV status and influence of HPV status and risk factors on overall survival (OS) of patients with OPSCC.
a Age of patients with HPV16-driven compared with patients with HPV-negative OPSCC and OPSCC with discordant markers for HPV (HPV16–DNA and p16INK4a). p: P value, t test, two-sided; widths of boxes are proportional to square roots of the sample size. b OS according to HPV status (including cases with discordant HPV tests). Significant differences in OS are indicated. p: P value, log-rank test; *: p < 0.001. c Univariate and multivariate analysis (Cox regression) of risk factors for OS of patients with concordant HPV tests (total: n = 620, HPV-negative: n = 436, HPV16-driven: n = 184). Boxes: hazard ratio (HR); horizontal lines: 95% confidence interval; black boxes/lines indicate statistical significance of risk factors.
The distribution of treatment modalities significantly differed between patients with HPV16-driven and HPV-negative OPSCC, with surgical tumour resection, including neck dissection performed more frequently in patients with HPV16-driven OPSCC (Table 1).
Uni- and multivariate OS analysis of OPSCC according to HPV status
Patients with HPV16-driven OPSCC had remarkably better overall survival (OS) than all other patient groups with HPV-negative OPSCC or discordant results for HPV–DNA and p16INK4a expression (P ≤ 0.019, Fig. 2b).
In univariate analysis of patients with concordant HPV–DNA and p16INK4a results (HPV16-driven and HPV-negative OPSCC), dichotomised variables for age, localisation, comorbidity, tobacco and alcohol consumption and T- and N-stage showed significant impact on patients’ overall survival (Fig. 2c, Supplementary Table S1). Hazard reduction by positive HPV status and low T stage was similar (3.2-fold, P < 0.001) and the highest in comparison with all other positive predictors (Supplementary Table S1).
We performed a multivariate Cox regression analysis of OS for patients with concordant markers for HPV status, including all dichotomised variables with significance in the univariate analysis. Age, HPV status, T stage and comorbidity remained independent factors for OS in this multivariate model (P < 0.005 each). Again, a 3.2-fold reduction of hazard in the case of HPV16-driven OPSCC had the strongest impact on survival (Fig. 2c, Supplementary Table S2).
Principal component analysis (PCA)
Patients with discordant HPV-test results differed from those with HPV-negative and HPV16-driven OPSCC regarding OS (Fig. 2b), tumour characteristics and lifestyle/patient-related risk factors (Supplementary Table S3). In this multivariate analysis, we included all patients (with complete datasets) and factors significant in univariate analysis, and reduced data dimensionality by principal component analysis (PCA) to two main components. The contribution of each included factor in both components is shown in Fig. 3a. Three clusters of factors with similar impact on the two components with respect to size and direction became evident: 1: p16INK4a expression and HPV16 –DNA detection, 2: alcohol consumption and smoking and 3: tumour size (T-) and lymph node (N-) stage together with patient’s performance (ECOG). Age and tonsillar localisation of the primary tumour did not cluster together with other factors.
Fig. 3. Principal component analysis (PCA) of tumour characteristics and lifestyle/patient-related risk factors and distribution of cases according to the resulting two main components (components 1 and 2).
a Loading plot of components 1 and 2 using the indicated variables (further details: Table 1). b Distribution of all cases (HPV16-driven, HPV-negative and discordant HPV tests) without missing data (n = 598) according to the resulting main component scores. Selected cases mentioned in detail in Supplementary Table S4 are labelled. c Component-1 score efficiently separates HPV16-driven and HPV-negative OPSCCs as indicated by the distribution plots next to the axis for both components. A separation border (dashed line) was defined at the central minimum. d Applying the separation border defined in (c) to cases with discordant HPV tests. The distribution plot of component 1 for HPV16-driven and HPV-negative OPSCCs is indicated for comparison.
The distribution of all OPSCCs plotted according to the two main components separated two main groups of patients (Fig. 3b). Risk profiles of selected cases with central or marginal position are shown in Supplementary Table S4.
HPV-negative and HPV16-driven OPSCC were clearly separated upon plotting of the individual cases, and a separation border regarding component 1 could be defined according to the distribution of cases. Eighteen of 385 (4.7%) HPV-negative and only one of 155 (0.6%) HPV16-driven OPSCC were located outside their respective clusters (Fig. 3c). Applying this separation to OPSCC with discordant HPV-test results showed that 21 of 25 (84%) OPSCC patients positive for HPV16–DNA, but lacking p16INK4a expression, clustered together with HPV-negative OPSCC on the left side of the plot (Fig. 3d). These 21 cases resembled patients with HPV-negative OPSCC with respect to risk factors and OS (Fig. 2b) and were not further investigated.
Notably, 20 of 33 (60.6%, group 1) OPSCC with p16INK4a expression but without detection of any HPV–DNA did not cluster together with HPV16-driven OPSCC (Fig. 3d). This resulted in two groups showing significant differences: patients of group 1 were characterised by alcohol consumption, smoking and non-tonsillar localisation of the primary tumour. This contrasts with patients of group 2, who were specified by the absence of tobacco and alcohol consumption and tonsillar localisation of the primary tumour (Supplementary Table S5), and clustered together with HPV16-driven OPSCC (Fig. 3).
Survival analysis revealed that group 1 patients had significantly worse survival compared with patients with HPV16-driven OPSCC (Fig. 4, P = 0.003), therefore representing “HPV-negative-like” OPSCC. In contrast, no difference in OS existed between group 2 patients in comparison with HPV16-driven (P = 0.845) but compared with HPV-negative OPSCC (P = 0.012, Fig. 4). Treatment of group 1 and 2 patients did not differ in our cohort (Supplementary Table S6). Notably, group 1 patients constituted 10.6% (20/188) of all p16INK4a-overexpressing OPSCC in this analysis.
Fig. 4. Overall survival (OS) of patients with OPSCC and positive test for p16INK4a expression but lacking detectable HPV–DNA (groups 1 and 2) in comparison with HPV-negative (n = 436, dashed black) and HPV16-driven (n = 184, dashed grey) OPSCC.
Patients were stratified applying the separation border defined by PCA (Fig. 3c, d), resulting in patient group 1 (black) with and group 2 (grey) without a more severe risk profile. p: P value, log-rank test.
Discussion
p16INK4a overexpression is currently applied as decision criterion for treatment de-escalation trials in OPSCC.14,22 This proceeding relies on the circumstance that p16INK4a overexpression is a surrogate marker of HPV-driven OPSCC, a patient group with a remarkably favourable prognosis. Hence, patients with HPV–driven OPSCC may benefit from de-escalated treatment by the reduction of therapy-related acute and long-term toxicity. This might become relevant for more patients in the future. However, considering the moderate specificity of p16INK4a for HPV-driven OPSCC, concerns have been raised that a subgroup of OPSCC patients could be undertreated in this scenario.14 Since robust data estimating the size of this group are presently not available, the major goal of our study was to determine the proportion of these potentially undertreated patients in a large cohort of consecutively characterised patients that allowed the distinction of patient risk-factor profiles in relation to HPV-related markers.
About 30% of OPSCC worldwide are caused by oncogenic HPV types.1 We observed an HPV prevalence of 27.1% by simultaneous detection of HR–HPV–DNA and p16INK4a overexpression in our cohort. This represents an average in a central region in Germany over the past 18 years, which is comparable to the global burden. However, we recently showed significant increase of incidence rates in Germany.2 In comparison with cervical cancers,23 a much higher contribution of HPV16 and HPV18 is reported for anal (87%) and head and neck (85%) cancers worldwide.1 In our study, HPV16 was almost the only relevant HPV type for OPSCC. Therefore, we excluded non-16 HPV types from our further investigation.
To our surprise, patients with HPV16-driven OPSCC were not younger, but significantly older than patients with HPV-negative OPSCC in our cohort. This is in contrast to the literature, although most published data rely on selected populations from clinical trials,5,6 comparatively small random cohorts or cancer registry-based data without inclusion of any experimental data regarding HPV.24 Interestingly, the unimodal age incidence distribution was peaking in the United States at ages 60–64 for all patients with HPV-positive OPSCC in a recent investigation,25 which is comparable to our data, and more importantly, not younger than HPV-negative OPSCC in our cohort.
However, other lifestyle-related risk factors were obviously reduced in these patients. Furthermore, the type of therapy differed in our cohort according to HPV status, which might be a direct consequence of patient (comorbidity) and tumour characteristics (primary size and N-/M-status) and, therefore, contributes to superior outcome of patients with HPV16-driven cancers. Age, HPV status, T stage and comorbidity remained independent risk factors in the Cox regression model. In contrast to other studies,6 tobacco and alcohol consumption did not have significant impact on this model. However, both are highly related with HPV status, and in a previous study we showed that tobacco and alcohol consumption were less important for survival prediction in our patients.26 In contrast to other studies,6 the frequency of smokers in our cohort was higher in general, and especially in HPV-negative OPSCC, which might cover its influence in our multivariate Cox model. This is supported by studies showing that HPV positivity in OPSCC is a significant prognostic factor, irrespective of smoking.27
In the PCA, variables for HPV16–DNA and p16INK4a had almost similar values of components 1 and 2, and were accompanied by smoking and alcohol consumption on the component-1 axis (Fig. 3a). In accordance with the literature, this demonstrates that p16INK4a overexpression is highly related to the presence of HPV16–DNA, and both are negatively related with smoking and alcohol consumption. By geometrical projecting in the PCA, data are transformed onto lower, uncorrelated dimensions with the goal that the first dimension (component 1) explains as much of the data as possible. The second dimension (component 2) explains as much as possible of the remaining, unexplained data (and so on), which reduces complexity by summarising the most contributing features of the dataset. In conclusion, HPV status, smoking and alcohol consumption explained most of the variance in our data by contributing (positively and negatively) to component 1, followed by patient’s performance (ECOG), T- and N-category as they have similar values contributing to component 2 (Fig. 3a). Age and tonsillar localisation were somewhere in-between the aforementioned clusters of factors (Fig. 3a), possibly explained by a closer relation to HPV status (supported by our descriptive data, Table 1 and Fig. 2a). Overall, clustering of the factors fits well to published risk models in OPSCC6,26,28 regarding the fact that HPV status is most important followed by ECOG, T- and N-stage. Smoking and alcohol consumption were not the highest in ranking because their association with HPV status most probably covers their influence.
Component-1 values efficiently separated cases with HPV16-driven and -negative OPSCC in the PCA (Fig. 3c). The distribution of cases with discordant HPV tests showed that those with HPV16–DNA-positive but p16INK4a-negative OPSCC resembled HPV-negative OPSCC (except 4/25, 16%), which is supported by similar OS of these patients. In contrast, only 39% (13/33) of OPSCC with p16INK4a overexpression in the absence of any HPV–DNA resembled HPV-driven OPSCC. The remaining patients were similar to patients with HPV-negative OPSCC (notably also with respect to OS) and constituted 10.6% (20/188) of all p16INK4a-overexpressing OPSCC included in this analysis. Consequently, if the selection of patients for therapy de-escalation solely relies on p16INK4a expression as an indicator of viral aetiology and improved survival, those patients would be incorrectly selected and thus be put at jeopardy for treatment failure. Our findings clearly show that patient selection for de-escalated treatments based on a single marker bears a substantial risk for OPSCC patients, particularly in populations with high tobacco consumption.
Summary and conclusion
We have analysed a consecutive cohort of patients and a relevant number of samples lacked either HPV–DNA or p16INK4a positivity. HPV16 is by far the most important HPV type in OPSCC in central Germany. Patients with HPV16-driven OPSCC consumed fewer amounts of cigarettes and alcohol, but were significantly older at diagnosis. OPSCC positive for p16INK4a but negative for any HPV– DNA, accounting for 10.6% of all p16INK4a-overexpressing OPSCC, resembled HPV-negative OPSCC in risk-factor profile and overall survival.
In conclusion, our data imply that p16INK4a expression as a single marker is insufficient to define HPV aetiology in OPSCC, and that stratification of patients for treatment de-escalation thus requires the integration of further risk factors and/or diagnostic tests, primarily confirmatory HPV testing.
Supplementary information
Acknowledgements
Not applicable.
Author contributions
Conceptualisation and methodology: S.W., E.S.P., Mv.K.D., S.G., J.P.K., C.W. and J.P.K.l.; data curation, investigation and validation: S.W., E.S.P., N.W., H.R., A.B., S.J.S., T.O., T.D., G.W. and J.P.K.; formal analysis, software and visualisation: S.W. and J.P.K.; funding acquisition, resources and supervision: S.W., E.S.P., Mv.K.D., S.G., C.W. and J.P.K.l.; project administration and writing—original draft: S.W., E.S.P., C.W. and J.P.K.l.; writing—review and editing: all authors.
Ethics approval and consent to participate
The study was performed in accordance with the Declaration of Helsinki. The study was approved by the local ethics committee (Ethik-Kommission am Fachbereich Medizin, University of Giessen, reference numbers 151/11 and 296/11). Written informed consent was obtained from all patients, and tumour material was used in accordance with the local ethics committee.
Consent to publish
Not applicable.
Data availability
Data described in this study are available, upon request, from the corresponding author for academic research within the constraints of the consent given by the patients.
Competing interests
Mv.K.D. was cofounder, shareholder and member of the supervisory board of mtm Laboratories, Heidelberg, Germany. J.P.K. is the principal investigator of a MISP-funded project and received funding from MSD Sharp & Dohme GmbH for this study. E.S.P. has received lecture fees from MSD Sharp & Dohme GmbH. All remaining authors have declared no conflict of interest.
Funding information
This work was supported by the Investigator Studies Program (MISP) from MSD Sharp & Dohme GmbH (grant number: 56606). MSD Sharp & Dohme GmbH was not involved in study design, in the collection, analysis and interpretation of data, in the writing of the report and in the decision to submit the data for publication. Open Access funding enabled and organized by Projekt DEAL
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Steffen Wagner, Elena-Sophie Prigge
These authors jointly supervised this work: Claus Wittekindt, Jens Peter Klussmann
Change history
6/10/2021
A Correction to this paper has been published: 10.1038/s41416-021-01457-z
Supplementary information
Supplementary information is available for this paper at 10.1038/s41416-020-0964-x.
References
- 1.de Martel C, Plummer M, Vignat J, Franceschi S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J. Cancer. 2017;141:664–670. doi: 10.1002/ijc.30716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wittekindt C, Wagner S, Bushnak A, Prigge ES, von Knebel Doeberitz M, Wurdemann N, et al. Increasing Incidence rates of oropharyngeal squamous cell carcinoma in germany and significance of disease burden attributed to human papillomavirus. Cancer Prev. Res (Philos.) 2019;12:375–382. doi: 10.1158/1940-6207.CAPR-19-0098. [DOI] [PubMed] [Google Scholar]
- 3.Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J. Clin. Oncol. 2011;29:4294–4301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castellsague X, Alemany L, Quer M, Halec G, Quiros B, Tous S, et al. HPV involvement in head and neck cancers: comprehensive assessment of biomarkers in 3680 patients. J. Natl. Cancer Inst. 2016;108:djv403. doi: 10.1093/jnci/djv403. [DOI] [PubMed] [Google Scholar]
- 5.O’Sullivan B, Huang SH, Siu LL, Waldron J, Zhao H, Perez-Ordonez B, et al. Deintensification candidate subgroups in human papillomavirus-related oropharyngeal cancer according to minimal risk of distant metastasis. J. Clin. Oncol. 2013;31:543–550. doi: 10.1200/JCO.2012.44.0164. [DOI] [PubMed] [Google Scholar]
- 6.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen AM, Felix C, Wang PC, Hsu S, Basehart V, Garst J, et al. Reduced-dose radiotherapy for human papillomavirus-associated squamous-cell carcinoma of the oropharynx: a single-arm, phase 2 study. Lancet Oncol. 2017;18:803–811. doi: 10.1016/S1470-2045(17)30246-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Owadally W, Hurt C, Timmins H, Parsons E, Townsend S, Patterson J, et al. PATHOS: a phase II/III trial of risk-stratified, reduced intensity adjuvant treatment in patients undergoing transoral surgery for Human papillomavirus (HPV) positive oropharyngeal cancer. BMC Cancer. 2015;15:602. doi: 10.1186/s12885-015-1598-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marur S, Li S, Cmelak AJ, Gillison ML, Zhao WJ, Ferris RL, et al. E1308: phase II trial of induction chemotherapy followed by reduced-dose radiation and weekly cetuximab in patients with HPV-associated resectable squamous cell carcinoma of the oropharynx- ECOG-ACRIN cancer research group. J. Clin. Oncol. 2017;35:490–497. doi: 10.1200/JCO.2016.68.3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nichols AC, Lang P, Prisman E, Berthelet E, Tran E, Hamilton S, et al. Treatment de-escalation for HPV-associated oropharyngeal squamous cell carcinoma with radiotherapy vs. trans-oral surgery (ORATOR2): study protocol for a randomized phase II trial. BMC Cancer. 2020;20:125. doi: 10.1186/s12885-020-6607-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel RR, Ludmir EB, Augustyn A, Zaorsky NG, Lehrer EJ, Ryali R, et al. De-intensification of therapy in human papillomavirus associated oropharyngeal cancer: A systematic review of prospective trials. Oral. Oncol. 2020;103:104608. doi: 10.1016/j.oraloncology.2020.104608. [DOI] [PubMed] [Google Scholar]
- 12.Gillison ML, Trotti AM, Harris J, Eisbruch A, Harari PM, Adelstein DJ, et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): a randomised, multicentre, non-inferiority trial. Lancet. 2019;393:40–50. doi: 10.1016/S0140-6736(18)32779-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehanna H, Robinson M, Hartley A, Kong A, Foran B, Fulton-Lieuw T, et al. Radiotherapy plus cisplatin or cetuximab in low-risk human papillomavirus-positive oropharyngeal cancer (De-ESCALaTE HPV): an open-label randomised controlled phase 3 trial. Lancet. 2019;393:51–60. doi: 10.1016/S0140-6736(18)32752-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mirghani H, Blanchard P. Treatment de-escalation for HPV-driven oropharyngeal cancer: Where do we stand? Clin. Transl. Radiat. Oncol. 2018;8:4–11. doi: 10.1016/j.ctro.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.von Knebel Doeberitz M. The causal role of human papillomavirus infections in non-anogenital cancers. It’s time to ask for the functional evidence. Int J. Cancer. 2016;139:9–11. doi: 10.1002/ijc.30059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McLaughlin-Drubin ME, Park D, Munger K. Tumor suppressor p16INK4A is necessary for survival of cervical carcinoma cell lines. Proc. Natl. Acad. Sci. USA. 2013;110:16175–16180. doi: 10.1073/pnas.1310432110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boscolo-Rizzo P, Pawlita M, Holzinger D. From HPV-positive towards HPV-driven oropharyngeal squamous cell carcinomas. Cancer Treat. Rev. 2016;42:24–29. doi: 10.1016/j.ctrv.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 18.Prigge ES, Arbyn M, von Knebel Doeberitz M, Reuschenbach M. Diagnostic accuracy of p16(INK4a) immunohistochemistry in oropharyngeal squamous cell carcinomas: A systematic review and meta-analysis. Int J. Cancer. 2017;140:1186–1198. doi: 10.1002/ijc.30516. [DOI] [PubMed] [Google Scholar]
- 19.Nauta IH, Rietbergen MM, van Bokhoven A, Bloemena E, Lissenberg-Witte BI, Heideman DAM, et al. Evaluation of the eighth TNM classification on p16-positive oropharyngeal squamous cell carcinomas in the Netherlands and the importance of additional HPV DNA testing. Ann. Oncol. 2018;29:1273–1279. doi: 10.1093/annonc/mdy060. [DOI] [PubMed] [Google Scholar]
- 20.Hotelling H. Relations Between Two Sets of Variates. Biometrika. 1936;28:321–377. doi: 10.1093/biomet/28.3-4.321. [DOI] [Google Scholar]
- 21.Pearson K. LIII. On lines and planes of closest fit to systems of points in space. Lond., Edinb., Dublin Philos. Mag. J. Sci. 1901;2:559–572. doi: 10.1080/14786440109462720. [DOI] [Google Scholar]
- 22.Mirghani H, Amen F, Blanchard P, Moreau F, Guigay J, Hartl DM, et al. Treatment de-escalation in HPV-positive oropharyngeal carcinoma: ongoing trials, critical issues and perspectives. Int J. Cancer. 2015;136:1494–1503. doi: 10.1002/ijc.28847. [DOI] [PubMed] [Google Scholar]
- 23.Clifford GM, Smith JS, Plummer M, Munoz N, Franceschi S. Human papillomavirus types in invasive cervical cancer worldwide: a meta-analysis. Br. J. Cancer. 2003;88:63–73. doi: 10.1038/sj.bjc.6600688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J. Clin. Oncol. 2008;26:612–619. doi: 10.1200/JCO.2007.14.1713. [DOI] [PubMed] [Google Scholar]
- 25.Mahal B. A., Catalano P. J., Haddad R. I., Hanna G. J., Kass J. I., Schoenfeld J. D. et al. Incidence and demographic burden of HPV-associated oropharyngeal head and neck cancers in the United States. Cancer Epidemiol Biomarkers Prev. 10.1158/1055-9965.EPI-19-0038 (2019). [DOI] [PubMed]
- 26.Wagner S, Wittekindt C, Sharma SJ, Wuerdemann N, Juttner T, Reuschenbach M, et al. Human papillomavirus association is the most important predictor for surgically treated patients with oropharyngeal cancer. Br. J. Cancer. 2017;116:1604–1611. doi: 10.1038/bjc.2017.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Broglie MA, Stoeckli SJ, Sauter R, Pasche P, Reinhard A, de Leval L, et al. Impact of human papillomavirus on outcome in patients with oropharyngeal cancer treated with primary surgery. Head. Neck. 2017;39:2004–2015. doi: 10.1002/hed.24865. [DOI] [PubMed] [Google Scholar]
- 28.Rietbergen MM, Brakenhoff RH, Bloemena E, Witte BI, Snijders PJ, Heideman DA, et al. Human papillomavirus detection and comorbidity: critical issues in selection of patients with oropharyngeal cancer for treatment De-escalation trials. Ann. Oncol. 2013;24:2740–2745. doi: 10.1093/annonc/mdt319. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in this study are available, upon request, from the corresponding author for academic research within the constraints of the consent given by the patients.