Abstract
Background & Aims
Our understanding of outcomes and disease time course of COVID-19 in patients with gastrointestinal (GI) symptoms remains limited. In this study we characterize the disease course and severity of COVID-19 among hospitalized patients with gastrointestinal manifestations in a large, diverse cohort from the Unites States.
Methods
This retrospective study evaluated hospitalized individuals with COVID-19 between March 11 and April 28, 2020 at two affiliated hospitals in New York City. We evaluated the association between GI symptoms and death, and also explored disease duration, from symptom onset to death or discharge.
Results
Of 2804 patients hospitalized with COVID-19, the 1,084 (38.7%) patients with GI symptoms were younger (aOR for age ≥75, 0.59; 95% CI, 0.45-0.77) and had more co-morbidities (aOR for modified Charlson comorbidity score ≥2, 1.22; 95% CI, 1.01-1.48) compared to those without GI symptoms. Individuals with GI symptoms had better outcomes, with a lower likelihood of intubation (aHR, 0.66; 95% CI, 0.55-0.79) and death (aHR, 0.71; 95% CI, 0.59-0.87), after adjusting for clinical factors. These patients had a longer median disease course from symptom onset to discharge (13.8 vs 10.8 days, log-rank p = .048; among 769 survivors with available symptom onset time), which was driven by longer time from symptom onset to hospitalization (7.4 vs 5.4 days, log-rank P < .01).
Conclusion
Hospitalized patients with GI manifestations of COVID-19 have a reduced risk of intubation and death, but may have a longer overall disease course driven by duration of symptoms prior to hospitalization.
Keywords: COVID-19, Mortality, Gastrointestinal, Disease Course
Abbreviations used in this paper: aOR, adjusted odds ratio; BMI, body mass index; COVID-19, coronavirus disease 2019; GI, gastrointestinal; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2
See related article on page 1426.
What You Need to Know.
Background
Although a significant proportion of patients with coronavirus disease 2019 (COVID-19) present with gastrointestinal symptoms, our understanding of disease time course and outcomes in this population is limited.
Findings
Patients with gastrointestinal symptoms had a significantly lower likelihood of intubation and death but longer times from symptom onset to hospitalization.
Implications for patient care
Hospitalized patients with gastrointestinal manifestations of COVID-19 have a less severe disease course, though they remain symptomatic for longer periods of time prior to hospitalization. Therefore, early recognition and testing in this population is indicated to limit further transmission of disease.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the viral pathogen responsible for causing coronavirus disease 2019 (COVID-19), a pandemic that has spread rapidly, infecting over 14 million people globally and causing over 600,000 deaths to date.1 , 2 While the hallmark of this infection is severe respiratory illness, involvement of other organ systems, including the gastrointestinal (GI) tract, has been documented. Reports have been mixed, showing anywhere from 11 to 63% of hospitalized patients have at least 1 GI symptom.3, 4, 5, 6, 7, 8, 9, 10, 11 Furthermore, SARS-CoV-2 nucleic acid has been identified in stool samples and on endoscopic biopsies, and absorptive enterocytes in the ileum and colon have been found to highly express angiotensin-converting enzyme 2 receptors, which are critical for viral cell entry.12, 13, 14, 15, 16, 17, 18 These findings indicate that the GI system may be a route for transmission and a moderator of both symptom manifestation and outcomes.
Nonetheless, our understanding of the prognostic implications of GI manifestations, particularly diarrhea, on outcomes has been inconsistent.19 Some studies suggest that GI symptoms may convey increased risk of poor outcomes,4 , 20 while others show no association or a potential protective association.6, 7, 8, 9 These studies vary in their inclusion criteria, ascertainment of GI symptoms, and definitions of severity of disease course.17 , 21 , 22
A recent case-control study from our center showed that patients hospitalized with GI manifestations had lower rates of death and were more likely to have a length of stay over 1 week; however, like other early reports of GI symptoms, it did not account for the potential impact of factors such as age and comorbidities on mortality, and did not assess the overall time course of disease from symptom onset to death or discharge.6, 7, 8, 9 Most reports on outcomes globally have assessed the impact of GI symptoms on incidence of severe disease, rather than on mortality.4 , 20
Furthermore, small studies from China have assessed how time course of disease is impacted by presence of GI symptoms, and some suggest that presence of diarrhea may be associated with prolonged symptoms.4 , 11 , 18 , 23 Here, we aimed to further characterize the duration and severity of COVID-19 among hospitalized patients with GI manifestations in a large, diverse cohort from New York City. We hypothesized that the presence of GI symptoms could portend a milder disease phenotype but longer disease time course, potentially due to differences in inflammatory response.
Materials and Methods
Study Design
This retrospective study at 2 affiliated academic hospitals (Columbia University Irving Medical Center and Allen Hospital) includes consecutive hospitalized patients who underwent testing for COVID-19 from March 11, 2020 (when institution-based testing began), to April 28, 2020, and includes a small percentage of patients described in our center’s prior report (<8%).6 A positive result was based upon real-time reverse-transcription polymerase chain reaction assay of nasopharyngeal swab specimens. The primary exposure of interest was presence of GI symptoms, including nausea, vomiting, diarrhea, and abdominal pain, documented within 3 days of COVID-19 testing. The primary outcome of interest was death. The secondary outcome of intubation was also assessed. In addition, we assessed how presence of GI symptoms impacted time course of disease, including duration of symptoms of COVID-19 prior to hospitalization, length of stay during hospitalization, and time from admission to death. Additional variables collected included age, sex, body mass index (BMI) (coded as a categorical value <18 kg/m2, 18–25 kg/m2, 25–30 kg/m2, >30 kg/m2, and unknown), race/ethnicity, comorbidities (including concomitant GI diseases such as diverticular disease, irritable bowel syndrome, and inflammatory bowel disease), laboratory values, presence of additional symptoms of COVID-19 (fever, fatigue, dizziness, headache, cough, sore throat, congestion, rhinorrhea, and shortness of breath), and exposure to COVID-19 treatments during hospitalization.
Data Collection
Demographic data, clinical symptoms, laboratory findings, comorbidities, treatment, and outcome data were collected from electronic medical records. Data on symptoms at the time of presentation were obtained using natural language processing algorithms applied to chart documentation, implemented in Python. Presence and absence of various iterations of specific symptoms including fever, fatigue, dizziness, headache, cough, sore throat, congestion, rhinorrhea, shortness of breath, nausea, vomiting, diarrhea, and abdominal pain were systematically identified in emergency room evaluation and admission notes, including in the history of present illness, chief complaint, review of systems, and assessment. The algorithm parsed relevant full text notes and was systematically refined based on chart review to include variations on spelling and phrasing of individual symptom keywords to ensure accurate capture of data. Algorithm extraction was then validated by investigators blinded to the outcomes of the study, who performed manual review of 1053 charts of the total 2804 COVID-19–positive patients. Data for symptom documentation between algorithm extraction and manual review was >90% concordant for individual GI symptoms. Exposure to COVID-19 treatments during the course of the hospitalization were also recorded, including steroids (prednisone, methylprednisolone, and dexamethasone), hydroxychloroquine, azithromycin, remdesivir, and tocilizumab. Patients were considered exposed if they received at least 1 dose of a given treatment.
Statistical Analysis
Continuous variables were expressed as medians and interquartile ranges, with Wilcoxon rank sum test used to assess differences in distributions between the groups. Categorical variables were summarized as counts and percentages, with chi-square or Fisher’s exact tests used for comparison. Univariable and multivariable regression analyses were done to compare characteristics of patients with and without GI symptoms in COVID-19. A modified Charlson comorbidity index was utilized in multivariable models, which incorporated weighted values of all components of the index (myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic obstructive pulmonary disease, connective tissue disease, peptic ulcer disease, diabetes mellitus, chronic kidney disease, hemiplegia, leukemia, lymphoma, solid tumor, liver disease, and acquired immunodeficiency syndrome) but excluded age because it was included separately in our model. Cox proportional hazards models were used to assess the association between GI symptoms and 30-day rate of intubation and death, adjusting for age, sex, BMI, comorbidities, and presence of respiratory and constitutional symptoms. In a subgroup of patients with available data on timing of symptom onset, Kaplan-Meier analysis was used to assess overall duration of illness, as well as time from symptom onset to admission and time from admission to discharge or death, stratified by the presence of GI symptoms. Patients with unknown symptom onset time as well as 1 outlier with a reported symptom duration over 3 months were excluded for this part of the analysis. Symptom onset prior to hospitalization was censored at 14 days to limit recall bias and ensure symptoms were likely associated with having COVID-19. For all analyses, an alpha of 0.05 was considered statistically significant, with calculations performed in STATA 16 (StataCorp, College Station, TX). This study was approved by the Institutional Review Board of Columbia University Medical Center.
Results
From March 11 to April 28, there were a total of 4670 individuals who were hospitalized and tested for SARS-CoV-2, including 2804 (60.0%) who tested positive.
Among these, 1084 (38.7%) reported any GI symptoms on presentation, including diarrhea (n = 657, 23.4%), nausea or vomiting (n = 648, 23.2%), and abdominal pain (n = 334, 11.9%) (Supplementary Table 1).
On univariable analysis (Table 1 ), patients with GI symptoms were more likely to be younger (P < .01) and have a higher BMI (P < .01), though there were no significant differences in sex or gender (P = .18), race or ethnicity (P = .63), or underlying comorbidities, other than a slightly higher proportion of patients with a history of stroke presenting without GI symptoms (9% vs 6%; P = .01; all other P values >.05). More patients with GI symptoms had diverticular disease compared with those without (2% vs 0.5%; P < .01), though there was no difference among individuals with inflammatory bowel disease or irritable bowel syndrome on univariable analysis. On multivariable analysis (Table 2 ), age ≥75 years (adjusted odds ratio [aOR], 0.59; P < .01) was associated with a lower odds of GI symptoms at presentation, whereas an increased number of comorbidities (aOR, 1.22; P = .04 for modified Charlson comorbidity score ≥2) was associated with a higher odds of having GI symptoms at presentation. Furthermore, presence of an underlying GI disease including diverticular disease, irritable bowel syndrome, and inflammatory bowel disease was associated with a significantly higher odds of presenting with GI symptoms (aOR, 3.04; P < .01). Male sex (P = .08) and BMI (all P values > .49) were not associated with GI symptoms at presentation on multivariable analysis.
Table 1.
Demographic Characteristics, Clinical Characteristics and Outcomes of COVID-19–Positive Patients Presenting With GI Symptoms Compared With No GI Symptoms
| All (N = 2804) | GI Symptoms (n = 1084) | No GI symptoms (n = 1720) | P value | |
|---|---|---|---|---|
| Age at diagnosis, y | ||||
| Mean ± SD | 63.4 ± 18.4 | 61.4 ± 17.9 | 64.6 ± 18.6 | <.01 |
| Median (interquartile range) | 65.4 (51.6–77.4) | 63.0 (50.5–73.7) | 67.1 (52.8–78.6) | <.01 |
| Distribution | ||||
| 18–39 y | 390 (14) | 163 (15) | 227 (13) | |
| 40–59 y | 636 (23) | 288 (27) | 348 (20) | <.01 |
| 60–74 y | 953 (34) | 378 (35) | 575 (34) | |
| 75+ y | 825 (29) | 255 (23) | 570 (33) | |
| Sex | ||||
| Female | 1239 (44) | 495 (46) | 743 (43) | .18 |
| Male | 1565 (56) | 588 (54) | 977 (57) | |
| BMI | ||||
| <18 kg/m2 | 46 (2) | 16 (2) | 30 (2) | |
| 18–25 kg/m2 | 514 (18) | 175 (16) | 339 (20) | |
| 25–30 kg/m2 | 748 (27) | 317 (29) | 431 (25) | <.01 |
| >30 kg/m2 | 814 (29) | 356 (33) | 458 (27) | |
| Unknown | 682 (24) | 220 (20) | 462 (27) | |
| Race/ethnicity | ||||
| Non-Hispanic White | 330 (12) | 130 (12) | 200 (12) | |
| Hispanic | 1203 (43) | 475 (44) | 728 (42) | |
| Black | 585 (21) | 225 (21) | 360 (21) | .63 |
| Asian | 36 (1) | 10 (1) | 26 (1) | |
| Unknown | 650 (23) | 244 (23) | 406 (24) | |
| Comorbidities | ||||
| Hypertension | 1552 (56) | 616 (57) | 936 (55) | .30 |
| Diabetes | 1033 (37) | 403 (37) | 630 (37) | .87 |
| Coronary disease | 311 (11) | 109 (10) | 202 (12) | .17 |
| Asthma/COPD/ILD | 461 (17) | 196 (18) | 265 (16) | .08 |
| Chronic kidney disease | 288 (10) | 110 (10) | 178 (10) | .82 |
| Cancer | 219 (8) | 93 (9) | 126 (7) | .25 |
| Congestive heart failure | 227 (8) | 83 (8) | 144 (8) | .47 |
| Cerebrovascular accident | 211 (8) | 65 (6) | 146 (9) | .01 |
| Peptic ulcer disease | 55 (2) | 26 (2) | 29 (2) | .20 |
| Iron deficiency anemia | 132 (5) | 50 (5) | 82 (5) | .82 |
| Liver disease | 47 (2) | 22 (2) | 25 (1) | .26 |
| History of GI bleed | 32 (1) | 12 (1) | 20 (1) | .88 |
| Diverticular disease | 32 (1) | 25 (2) | 7 (0.5) | <.01 |
| Irritable bowel syndrome | 11 (0.4) | 2 (0.2) | 9 (0.5) | .16 |
| Inflammatory bowel disease | 10 (0.4) | 6 (0.6) | 4 (0.2) | .17 |
| Laboratory findings on admissiona | ||||
| White blood cell count, ×109/L | 7.4 (5.5–10.2) | 7.1 (5.3–9.5) | 7.7 (5.6–10.6) | <.01 |
| Lymphocyte count, ×109/L | 1.1 (0.7–1.5) | 1.1 (0.8–1.5) | 1.0 (0.7–1.5) | .04 |
| Erythrocyte sedimentation rate, mm/h | 72 (49–97) | 68 (48–96) | 74 (50–98) | .06 |
| C-reactive protein, mg/L | 121.7 (59.9–207.9) | 114 (59–194) | 126 (61–218) | .05 |
| Lactate dehydrogenase, U/L | 420 (312–581) | 403 (301–545) | 434 (317–606) | <.01 |
| Creatine kinase, U/L | 180 (86–407) | 176 (81–390) | 181 (92–422) | .07 |
| D-dimer, μg/mL | 1.4 (0.8–3.3) | 1.2 (0.7–2.4) | 1.6 (0.9–4.2) | <.01 |
| Ferritin, μg/L | 729 (360–1300) | 736 (357–1342) | 724 (363–1283) | .83 |
| COVID-19 treatments during admission | ||||
| Steroids | 599 (21) | 233 (21) | 366 (21) | .89 |
| Hydroxychloroquine | 1383 (49) | 583 (54) | 800 (47) | <.01 |
| Azithromycin | 1006 (36) | 417 (38) | 589 (34) | .02 |
| Remdesivir | 46 (2) | 25 (2) | 21 (1) | .03 |
| Tocilizumab | 46 (2) | 17 (2) | 29 (2) | .81 |
| Disease time course and outcomes | ||||
| Required intubation | 565 (20) | 184 (17) | 381 (22) | <.01 |
| Time to intubation, d | 1.2 (0.2–4.4) | 2.0 (0.6–4.8) | 0.9 (0.1–3.9) | <.01 |
| Death | 542 (19) | 147 (14) | 395 (23) | <.01 |
| Time to death, d | 5.4 (2.4–10.2) | 7.5 (4.1–12.3) | 4.9 (2.1–9.2) | <.01 |
NOTE. Values are n (%) or median (interquartile range), unless otherwise indicated.
BMI, body mass index; COPD, Chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; GI, gastrointestinal; ILD, interstitial lung disease; SD, standard deviation.
For patients with specific laboratory values available: white blood cell count: n = 2579; lymphocyte count: n = 2078; erythrocyte sedimentation rate: n = 1862; C-reactive protein: n = 1996; lactate dehydrogenase : n = 1973; creatine kinase: n = 1717; D-dimer: n = 170; ferritin: n = 1956
Table 2.
Multivariable Model Assessing Predictors of GI Symptoms in COVID-19–Positive Patients (n = 2804)
| Odds ratio | 95% Confidence interval | P value | |
|---|---|---|---|
| Age | |||
| 18–39 y | (Reference) | — | |
| 40–59 y | 1.14 | 0.88–1.48 | .31 |
| 60–74 y | 0.87 | 0.68–1.12 | .29 |
| 75+ y | 0.59 | 0.45–0.77 | <.01 |
| Male | 0.87 | 0.74–1.01 | .08 |
| BMI | |||
| <18 kg/m2 | (Reference) | — | |
| 18–25 kg/m2 | 0.91 | 0.48–1.74 | .78 |
| 25–30 kg/m2 | 1.23 | 0.65–2.32 | .53 |
| >30 kg/m2 | 1.17 | 0.62–2.22 | .63 |
| Unknown | 0.80 | 0.42–1.51 | .49 |
|
Modified Charlson comorbidity score |
|||
| 0 | (Reference) | – | |
| 1 | 1.11 | 0.91–1.35 | .31 |
| 2+ | 1.22 | 1.01–1.48 | .04 |
| Concomitant GI diseasea | 3.04 | 1.71–5.43 | <.01 |
BMI, body mass index; COVID-19, coronavirus disease 2019; GI, gastrointestinal.
Concomitant GI disease includes diverticular disease, irritable bowel syndrome, and inflammatory bowel disease.
On presentation, COVID-19 patients with concomitant GI symptoms had a significantly lower white blood cell count (7.1 × 109/L vs 7.7 × 109/L; P < .01), C-reactive protein (114 mg/L vs 126 mg/L; P = .05), D-dimer (1.2 μg/mL vs 1.6 μg/mL; P < .01), and lactate dehydrogenase (403 U/L vs 434 U/L; P < .01) (Table 1). Although additional inflammatory markers were not significantly different between the groups, patients presenting with GI symptoms appeared to have lower median values of erythrocyte sedimentation rate (68 mm/h vs 74 mm/h; P = .06). Patients with GI symptoms were also less likely to be lymphopenic (1.1 × 109/L vs 1.0 × 109/L; P = .04). Of note, some of these differences such as extent of lymphopenia probably have limited clinical relevance despite being statistically significant.
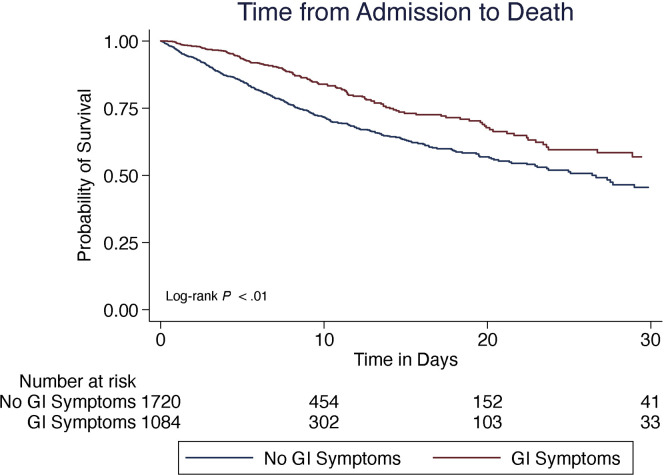
Patients presenting with GI symptoms had better outcomes than those without GI symptoms, including significantly lower rates of intubation (17% vs 22%; P < .01) and lower rates of death (15% vs 23%; P < .01) (Table 1). On Kaplan-Meier survival analysis, those presenting with GI symptoms had a significantly longer median time to death (7.5 days vs 4.9 days; P < .01) and lower rate of death than those without (log-rank P < .01) (Figure 1 ). This persisted on Cox proportional hazards analysis (adjusted hazard ratio, 0.71; P < .01) (Table 3 ) after adjusting for age, sex, BMI, comorbidities, exposure to COVID-19 therapies, and presence of respiratory or constitutional symptoms. Patients presenting with GI symptoms also had significantly longer median time to intubation (2.0 days vs 0.9 days; P < .01) and lower rates of intubation on Cox proportional hazards analysis after adjusting for the same variables (adjusted hazard ratio, 0.66; P < .01) (Supplementary Table 2).
Figure 1.
Survival analysis among 2804 COVID-19–positive patients, comparing 30-day mortality from the time of admission.
Table 3.
Cox Proportional Hazards Model Examining Time to Death in COVID-19–Positive Patients (n = 2804)
| Hazard ratio | 95% Confidence interval | P value | |
|---|---|---|---|
| Symptom presentation | |||
| Gastrointestinal | 0.71 | 0.59–0.87 | <.01 |
| Constitutional | 0.61 | 0.50–0.75 | <.01 |
| Respiratory | 1.68 | 1.27–2.22 | .01 |
| Age | |||
| 18–39 y | (Reference) | — | |
| 40–59 y | 2.02 | 0.90–4.53 | .09 |
| 60–74 y | 4.84 | 2.26–10.37 | <.01 |
| 75+ y | 12.14 | 5.68–25.92 | <.01 |
| Male | 1.12 | 0.94–1.34 | .19 |
| BMI | |||
| <18 kg/m2 | (Reference) | — | |
| 18–25 kg/m2 | 0.70 | 0.43–1.14 | .15 |
| 25–30 kg/m2 | 0.61 | 0.37–0.99 | .05 |
| >30 kg/m2 | 0.72 | 0.44–1.18 | .19 |
| Unknown | 1.94 | 1.20–3.13 | .01 |
| Modified Charlson comorbidity score | |||
| 0 | (Reference) | — | |
| 1 | 1.25 | 0.98–1.60 | .07 |
| 2+ | 1.35 | 1.08–1.70 | <.01 |
| COVID-19 treatments during admission | |||
| Steroids | 0.56 | 0.45–0.69 | <.01 |
| Other treatmentsa | 0.75 | 0.62-0.91 | <.01 |
BMI, body mass index; COVID-19, coronavirus disease 2019.
Other treatments include hydroxychloroquine, azithromycin, remdesivir, and tocilizumab.
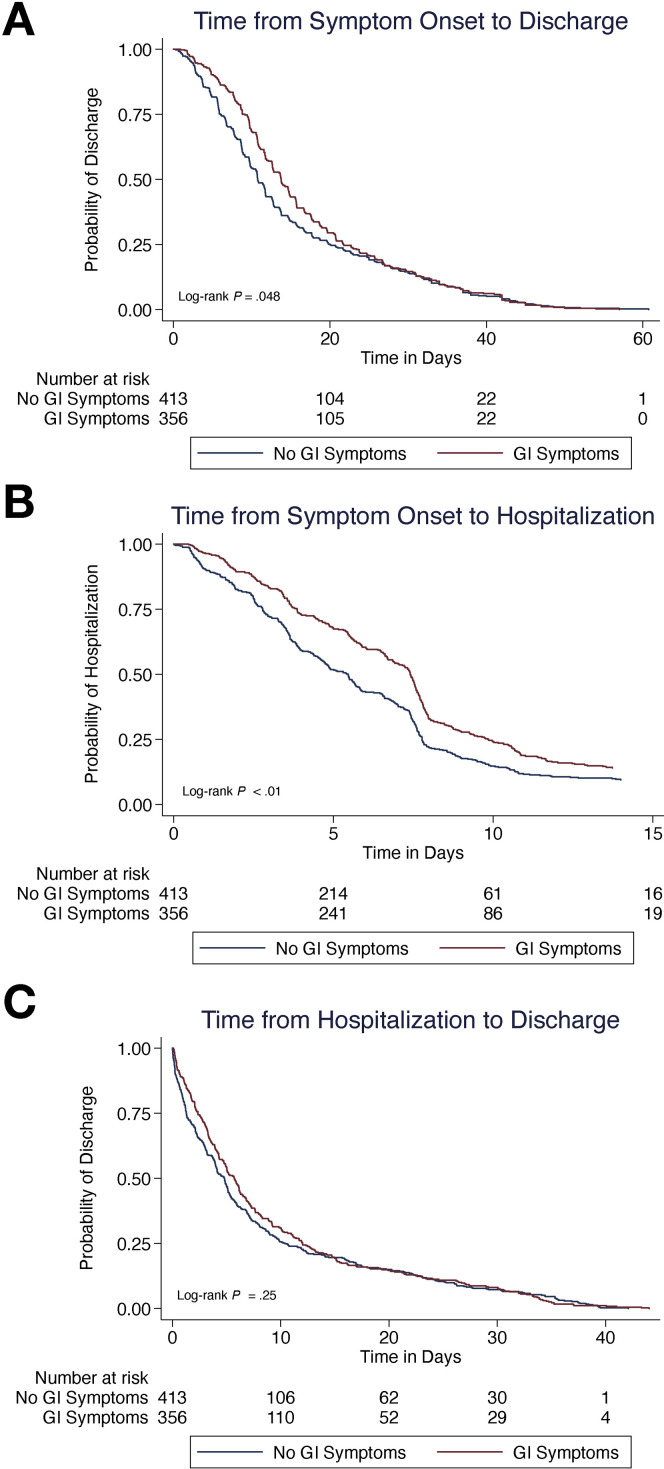
Next, we explored whether the presence of GI symptoms was associated with differences in disease time course among 945 COVID-19 patients with known symptom onset time. We conducted a time to event analysis looking at how the duration of time between symptom onset and discharge varied. Those who survived (n = 769) with GI symptoms had a significantly longer median disease course than did those who survived without GI symptoms (13.8 days vs 10.8 days; log-rank P = .048) (Figure 2 A). This was largely driven by longer median time from symptom onset to admission (7.4 days vs 5.4 days; log-rank P < .01) (Figure 2 B), rather than median length of stay (5.7 days vs 4.8 days; log-rank P = .25) (Figure 2 C). Although patients with GI symptoms who died also had a longer course of disease as compared with patients without GI symptoms who died, the result was not statistically significant (13.5 days vs 9.1 days; P = .08).
Figure 2.
(A) Time-to-event analysis from symptom onset to discharge among the 769 patients who survived to discharge and had known time of symptom onset. The breakdown of (B) time-to-event analysis from symptom onset to hospitalization and (C) time from hospitalization to discharge.
Discussion
In this study, we found that hospitalized individuals with COVID-19 who presented with GI symptoms of diarrhea, nausea, vomiting, or abdominal pain were younger but had more comorbidities than those presenting without GI symptoms. Individuals with GI symptoms had better outcomes than those without GI symptoms, including lower rates of intubation and death, even after adjusting for other relevant predictors such as age and comorbidities on multivariable analysis. Patients with GI symptoms also had a longer time-course of disease from symptom onset to discharge, which was largely driven by longer prehospital duration (from symptom onset to admission) rather than by length of stay.
One possible explanation for this longer but more indolent disease course in individuals with GI symptoms is that such patients may have less systemic inflammation secondary to COVID-19. We demonstrated that patients with GI symptoms had lower inflammatory markers on laboratory testing, including significantly lower C-reactive protein, D-Dimer, and lactate dehydrogenase, and trends toward lower erythrocyte sedimentation rates. These individuals were also less likely to have a leukocytosis. While further research is needed to elucidate the role of specific biomarkers in predicting severity of disease, we hypothesize that a lower degree of inflammation may modulate rates of adverse outcomes such as intubation and death, but the impaired immune response may result in a longer time to clear the virus, resulting in prolonged duration of illness. In contrast, some prior studies have suggested that GI symptoms may convey increased risk of poor outcomes.4 , 20 This discordance in findings may be due to differences in inclusion criteria and small size of prior studies, as well as differences in the symptoms that were assessed. For example, a study by Wan et al20 assessed the impact of diarrhea but not of other GI symptoms. Furthermore, patients were admitted with symptoms such as fever, cough, or dyspnea or CT scan abnormalities, but not necessarily a requirement for supplemental oxygen, which was included in admission criteria at our institution during the height of the pandemic. Data regarding inflammatory markers corresponding to GI symptoms have also been discordant. As such, individuals with diarrhea in the study by Wan et al20 (n = 84) had higher erythrocyte sedimentation rate but no difference in C-reactive protein or D-dimer, whereas no difference in inflammatory markers was seen in a small U.S. study by Redd et al7 (n = 318). It is possible that we were able to detect this difference given the larger size of our study.
Additionally, in this study we demonstrated that individuals with GI symptoms have a longer disease course, which is largely driven by the duration of symptoms prior to hospitalization. While this may raise concerns about a delayed diagnosis of disease in this population, the fact that they have better outcomes suggests that GI symptoms may instead be a marker of more indolent disease, or disease more localized to the GI tract. While these individuals may not need to present earlier for admission, it may be important to recognize and test them early in their disease course given concerns of potential outpatient fecal transmission and infectious spread.24 , 25 In addition, other studies have shown more familial clustering of disease among individuals with GI symptoms.4 This further emphasizes the importance of early recognition and testing in this population, which remains symptomatic at home longer, to limit spread.
To date, this is the largest study in the United States to assess outcomes in COVID-19–positive patients with GI symptoms. Our findings complement the findings of several other recent publications. A prior case-control study from our institution suggested that patients hospitalized with diarrhea, nausea, and vomiting had lower rates of death.6 Another study from a large center in New York City found that individuals with GI symptoms of diarrhea, abdominal pain, and nausea or vomiting had significantly lower rates of death on univariable analysis (but not on multivariable analysis, though this analysis was for the composite outcome of intensive care unit admission and death).8 Studies from China have also shown that patients with digestive symptoms have a longer time of symptom onset to admission, and have a longer duration between symptom onset and viral clearance, supporting our findings.23 Notably, other studies have reported conflicting implications of GI manifestations for disease severity. Inconsistencies in these reports are likely due to the heterogenous nature in which GI manifestations and disease severity have been defined and ascertained.7 , 9 , 22 For example, studies may have been confounded by use of antivirals with GI side effects, and many utilized different variations of composite outcomes without examining mortality specifically.4 , 20
Our study has many strengths. First, it is the largest study characterizing outcomes in individuals with GI manifestations of COVID-19. We utilized a large dataset from an academic institution at the epicenter of the COVID-19 pandemic in New York City, which represents a diverse population with robust data across many demographic and clinical variables. This is the first study to assess the impact of GI symptoms on mortality after adjusting for other relevant clinical factors such as age and comorbidities. Furthermore, this study was the first to document the timeline from symptom onset to hospitalization in a U.S. cohort. These data provide key insights into the clinical presentation of an indolent phenotype of disease, and further research to understand the mechanisms driving this phenotype will help identify those at highest risk for complications of this disease.
Our study does have limitations. First, symptom reporting is limited by recall bias of individual patients, as well as ascertainment bias among providers. To mitigate this, we utilized a natural language processing algorithm to flag symptom reporting across provider documentation 3 days before and after testing for COVID-19 to maximize accuracy, with validation on manual review. For patients presenting early in the epidemic before the widespread recognition of GI symptoms as a COVID-19 manifestation, it is possible that clinicians were not eliciting this history as commonly as later in the pandemic, or were not testing as many individuals with GI symptoms for SARS-CoV-2. Furthermore, when dealing with a large influx of patients in the setting of a pandemic, limitations to thorough history taking could have impacted results. Providers may be less likely to elicit a history of GI symptoms in patients with severe respiratory symptoms; such differential misclassification may produce an artifactual protective association between diarrhea and severe outcomes. In addition, details regarding attributes of specific symptoms, such as frequency and consistency of diarrhea, were not reliably recorded which is a limitation of the data. Another limitation is that this analysis only involved hospitalized patients. Further research of outpatients with and without GI manifestation may give additional insights into the broader population afflicted with COVID-19. Finally, our analysis did not account for impact of various investigational treatments on outcome.
In conclusion, our study demonstrates that individuals with GI symptoms including diarrhea, nausea, vomiting, and abdominal pain have lower rates of intubation and death than do those without GI symptoms. These individuals have a more indolent disease course, driven largely by longer time from symptom onset to admission. Given the lower inflammatory marker profiles in these patients, it is possible that GI manifestations convey a phenotype of disease with a dampened immune response—potentially improving outcomes but delaying viral clearance. Further research is needed to better understand the mechanisms underlying these differences, which may lead to an improved understanding of the natural history and time course of COVID-19.
Footnotes
Conflicts of Interest The authors disclose no conflicts.
Funding Adam S. Faye is supported by National Institutes of Health Grant No. T32DK083256. Benjamin Lebwohl is supported by the Louis and Gloria Flanzer Philanthropic Trust.
Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at https://doi.org/10.1016/j.cgh.2020.09.037.
Supplementary Material
Supplementary Table 1.
Symptoms of COVID-19–Positive Patients on Presentation
| Symptom | COVID-19–Positive patients (n = 2804) |
|---|---|
| Constitutional | |
| Fever | 2001 (71.4) |
| Fatigue | 964 (34.4) |
| Dizziness | 171 (6.1) |
| Headache | 278 (9.9) |
| Respiratory | |
| Cough | 1992 (71.0) |
| Sore throat | 272 (9.7) |
| Congestion | 316 (11.3) |
| Rhinorrhea | 122 (4.4) |
| Shortness of breath | 1987 (70.9) |
| Gastrointestinal | |
| Nausea/vomiting | 649 (23.2) |
| Diarrhea | 657 (23.4) |
| Abdominal pain | 334 (11.9) |
| Any GI symptoms (nausea, vomiting, diarrhea, abdominal pain) | 1084 (38.7) |
NOTE. Values are n (%).
COVID-19, coronavirus disease 2019; GI, gastrointestinal.
Supplementary Table 2.
Cox Proportional Hazards Model Examining Time to Intubation in COVID-19–Positive Patients (n = 2804)
| Hazard ratio | 95% Confidence interval | P value | |
|---|---|---|---|
| Symptom presentation | |||
| Gastrointestinal | 0.66 | 0.55–0.79 | <.01 |
| Constitutional | 0.61 | 0.49–0.76 | <.01 |
| Respiratory | 1.37 | 1.03–1.83 | .03 |
| Age | |||
| 18-39 y | (Reference) | — | |
| 40-59 y | 1.17 | 0.84–1.65 | .36 |
| 60-74 y | 1.21 | 0.88–1.68 | .25 |
| 75+ y | 0.93 | 0.66–1.32 | .68 |
| Male | 1.19 | 1.00–1.42 | .06 |
| BMI | |||
| <18 kg/m2 | (Reference) | — | |
| 18-25 kg/m2 | 1.16 | 0.56–2.39 | .69 |
| 25-30 kg/m2 | 1.25 | 0.61–2.55 | .54 |
| >30 kg/m2 | 1.46 | 0.72–2.98 | .30 |
| Unknown | 0.96 | 0.46–2.01 | .91 |
| Modified Charlson comorbidity score | |||
| 0 | (Reference) | — | |
| 1 | 1.27 | 1.03–1.57 | .03 |
| 2+ | 1.03 | 0.84–1.27 | .76 |
| COVID-19 treatments during admission | |||
| Steroids | 2.44 | 2.05–2.91 | <.01 |
| Other treatmentsa | 1.51 | 1.20–1.90 | <.01 |
BMI, body mass index; COVID-19, coronavirus disease 2019.
Other treatments include hydroxychloroquine, azithromycin, remdesivir, and tocilizumab.
References
- 1.Johns Hopkins University Center for Systems Science and Engineering. COVID-19 Dashboard. https://coronavirus.jhu.edu/map.html [DOI] [PMC free article] [PubMed]
- 2.World Health Organization Coronavirus Disease 2019 (COVID-19) Situation Report – 51. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200311-sitrep-51-covid-19.pdf?sfvrsn = 1ba62e57_10
- 3.Pan L., Mu M., Yang P. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study. Am J Gastroenterol. 2020;115:766–773. doi: 10.14309/ajg.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jin X., Lian J.-S., Hu J.-H. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;69:1002–1009. doi: 10.1136/gutjnl-2020-320926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luo S., Zhang X., Xu H. Don't overlook digestive symptoms in patients with 2019 novel coronavirus disease (COVID-19) Clin Gastroenterol Hepatol. 2020;18:1636–1637. doi: 10.1016/j.cgh.2020.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nobel Y.R., Phipps M., Zucker J. Gastrointestinal symptoms and COVID-19: case-control study from the United States. Gastroenterology. 2020;159:373–375.e2. doi: 10.1053/j.gastro.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Redd W.D., Zhou J.C., Hathorn K.E. Prevalence and characteristics of gastrointestinal symptoms in patients with SARS-CoV-2 infection in the United States: a multicenter cohort study. Gastroenterology. 2020;159:765–767.e2. doi: 10.1053/j.gastro.2020.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hajifathalian K., Krisko T., Mehta A. Gastrointestinal and hepatic manifestations of 2019 novel coronavirus disease in a large cohort of infected patients from New York: clinical implications. Gastroenterology. 2020;159:1137–1140.e2. doi: 10.1053/j.gastro.2020.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cholankeril G., Podboy A., Aivaliotis V.I. High prevalence of concurrent gastrointestinal manifestations in patients with SARS-CoV-2: early experience from California. Gastroenterology. 2020;159:775–777. doi: 10.1053/j.gastro.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parasa S., Desai M., Thoguluva Chandrasekar V. Prevalence of gastrointestinal symptoms and fecal viral shedding in patients with coronavirus disease 2019: a systematic review and meta-analysis. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.11335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mao R., Qiu Y., He J.S. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:667–678. doi: 10.1016/S2468-1253(20)30126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang H., Kang Z., Gong H. Digestive system is a potential route of COVID-19: an analysis of single-cell coexpression pattern of key proteins in viral entry process. Gut. 2020;69:1010–1018. [Google Scholar]
- 13.Ng S.C., Tilg H. COVID-19 and the gastrointestinal tract: more than meets the eye. Gut. 2020;69:973–974. doi: 10.1136/gutjnl-2020-321195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holshue M.L., DeBolt C., Lindquist S. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin L., Jiang X., Zhang Z. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut. 2020;69:997–1001. doi: 10.1136/gutjnl-2020-321013. [DOI] [PubMed] [Google Scholar]
- 16.Xiao F., Tang M., Zheng X., Liu Y., Li X., Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158:1831–1833.e3. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang W., Feng Z., Rao S. Diarrhoea may be underestimated: a missing link in 2019 novel coronavirus. Gut. 2020;69:1141–1143. doi: 10.1136/gutjnl-2020-320832. [DOI] [PubMed] [Google Scholar]
- 18.Wei X.-S., Wang X., Niu Y.-R. Diarrhea is associated with prolonged symptoms and viral carriage in coronavirus disease 2019. Clin Gastroenterol Hepatol. 2020;18:1753–1759.e2. doi: 10.1016/j.cgh.2020.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D'Amico F., Baumgart D.C., Danese S., Peyrin-Biroulet L. Diarrhea during COVID-19 infection: pathogenesis, epidemiology, prevention, and management. Clin Gastroenterol Hepatol. 2020;18:1663–1672. doi: 10.1016/j.cgh.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wan Y., Li J., Shen L. Enteric involvement in hospitalised patients with COVID-19 outside Wuhan. Lancet Gastroenterol Hepatol. 2020;5:534–535. doi: 10.1016/S2468-1253(20)30118-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ong J., Young B.E., Ong S. COVID-19 in gastroenterology: a clinical perspective. Gut. 2020;69:1144–1145. doi: 10.1136/gutjnl-2020-321051. [DOI] [PubMed] [Google Scholar]
- 22.Henry BM, de Oliveira MHS, Benoit J, Lippi G. Gastrointestinal symptoms associated with severity of coronavirus disease 2019 (COVID-19): a pooled analysis. Intern Emerg Med 2020 Apr 17 [E-pub ahead of print]. [DOI] [PMC free article] [PubMed]
- 23.Han C., Duan C., Zhang S. Digestive symptoms in COVID-19 patients with mild disease severity: clinical presentation, stool viral RNA testing, and outcomes. Am J Gastroenterol. 2020;115:916–923. doi: 10.14309/ajg.0000000000000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arslan M., Xu B., Gamal El-Din M. Transmission of SARS-CoV-2 via fecal-oral and aerosols-borne routes: Environmental dynamics and implications for wastewater management in underprivileged societies. Sci Total Environ. 2020;743 doi: 10.1016/j.scitotenv.2020.140709. 140709–140709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baraniuk C. Sewage monitoring is the UK’s next defence against covid-19. BMJ. 2020;370:m2599. doi: 10.1136/bmj.m2599. [DOI] [PubMed] [Google Scholar]