Abstract
Purpose
There has been no clear data on the effectiveness of pulmonary metastasectomy on several original cancers, including head and neck. We aim to collect data about the metastasectomies performed in our center for eligible patients and elaborate more on predictors and prognosis.
Methods
A retrospective analysis of 56 patients who underwent metastasectomy from head and neck cancers at our facility between January 2000 and January 2016 (16 years). Statistical analysis was performed based on gender, disease-free interval (DFI), location of the original tumor, and histological subtypes to assess their effect and relevance to the prognosis and disease recurrence.
Results
Twenty-nine males and 27 females had lung metastasis from head and neck. The primary lesions of the lung metastasis were more often found in the thyroid (34%), followed by nasopharynx (32%). As for histology, the most common one was papillary cancer (34%), followed by squamous cell carcinomas (29%). The DFI was more than 2 years in 32 patients (57%). The survival rates were 79.5% at 3 years and 71.7% at 5 years. In the univariate analysis, histology was the only independent prognostic factor (p = 0.05). On the other hand, age (p = 0.6), DFI (p = 0.24), and site of the primary tumor (p = .06) showed no effect on the prognosis of head and neck cancers metastasizing to the lungs.
Conclusion
Pulmonary metastasectomy for lesions originating from head and neck provides good long-term survival. Histological subtype was the only statistically significant prognostic factor.
Keywords: Head and neck cancer, Pulmonary metastasis, Metastasectomy, Outcomes
Introduction
Surgical resection of lung metastases in selected patients provides better control of the disease that would not be possible with chemotherapy alone in most cases [1, 2]. However, some patients have bad outcomes following the surgical intervention despite choosing the most appropriate management. Nevertheless, not enough studies were performed to assess the survival benefit from pulmonary metastasectomy for different histological subtypes and other prognostic factors. For the above-mentioned facts, the question remains as to whether or not the apparent improvement in metastasectomy associated survival is due to the resection, or simply due to the selection of patients with favorable demographics.
As part of choosing the patients that would benefit from the metastasectomy, a multidisciplinary approach that includes both the oncologist and the thoracic surgeon is necessary to proceed with pulmonary metastasectomy. Prolonging the survival and optimizing the timing of the surgical intervention are the key objectives in providing a surgical procedure to those patients, who are most likely to benefit from it.
Although, there are no published guidelines from experts in this field (with the exception of guidelines for the resectability of the colorectal cancer lung metastases from the National Comprehensive Cancer Network (NCCN) [3]. Most clinicians would accept the following criteria for a pulmonary metastasectomy [1, 2, 4, 5]:
The pulmonary metastases appear to be completely resectable based on the preoperative imaging
Adequate cardiopulmonary reserve to undergo resection
Technical feasibility of the operation
Controlled primary tumor site
In the absence of extra-pulmonary metastatic disease, the disease must be controllable with surgical intervention or another treatment modality
One of the most common cancers that metastasize into the lungs is head and neck cancer [1, 2, 4]. It is considered to be the third common indication for pulmonary mastectomies after bone and soft tissues sarcomas in our center [5]. Although the number of surgeries for the pulmonary metastasis from head and neck cancer is increasing, few reports have assessed their outcome with different results about the prognostic factors that affect the survival [5–7]. Thus, this study examines the surgical procedures, indications, and survival after lung metastases resection of head and neck cancer in our institution. This particular retrospective design is aiming to study how survival is affected by different parameters in our practice at our tertiary care center.
Methodology
Type of study
Our study is a retrospective cohort study.
Primary and secondary endpoints
Examined the survival prognosis in 56 patients with pulmonary metastasis from head and neck cancers.
Examined the predictors of survival in 56 patients with pulmonary metastasis from head and neck cancers.
We examined the prognosis and predictors of survival in 56 patients with pulmonary metastasis from head and neck cancers. The cases included in the study encompassed surgeries done at our facility running during a 16-year period from January 2000 till January 2016. We examined epithelial malignant tumors with primary lesions in the oral and nasal cavities, with pharyngeal and thyroid cancer. The metastasectomy margin was primarily removed with possible segmentectomy, lobectomy, and additional mediastinal lymph node dissection when indicated.
Inclusion/exclusion criteria
Inclusion criteria for patients were determined based on controlled primary disease and a curative approach to surgery, which goes in congruence with Thomford’s criteria. We excluded any patient who does not fit these criteria. Fifty-six patients were included in the study as per the inclusion criteria.
Aim of the study
We aim to identify the impact of many different factors on lung metastasectomy of head and neck tumors as well as the outcomes and prognosis of the surgery.
Statistical consideration
The statistical analyses of data were performed using SAS version 9.3 software (SAS Institute, Inc., Cary, NC, USA). Descriptive statistics of variables are reported as a mean standard deviation and categorical variables as frequencies and percentages. Disease-free interval was defined as the time between the treatment of the primary tumor and the diagnosis of the metastasis1. The Kaplan-Meyer method was used for survival tables and curves. Age, sex, DFI, histology, surgical approach, and number and size of nodules were compared by the log-rank test and univariate logistic regression test. The level of significance was set at p < 0.05.
Results
Out of the 56 patients, 29 males and 27 females were showing a fair distribution across both genders. The mean age at the time of surgery for the lungs was 53.1 years, ranging from 18 years to 81 years. The primary lesion was most often confirmed in the thyroid (19) followed by nasopharynx (18), oral cavity (8), salivary glands (7), and larynx (4). For the histological type, papillary cancer, squamous cell carcinoma, adenocystic carcinoma, undifferentiated, and myoepithelial cancer were 19, 16, 11, 8, and 2 in number respectively.
The mean number of pulmonary metastases was 2.8 nodules, ranging from a single nodule to 23 nodules. The mean tumor diameter was 2.5 cm, with a range between 0.4 and 7.5 cm. Thirty-eight patients had unilateral lesions in the lungs while 18 had bilateral lesions. With respect to the surgical method, thoracotomy, video-assisted thoracoscopic surgery (VATS), and sternotomy were performed in 43, 12, and 1 patient respectively. The metastases were completely removed from all subjects with negative margins. Thirty-four patients underwent chemoradiotherapy alongside the operation as part of the management plan for the primary cancers. Also, 32 subjects had DFI of more than 2 years, 18 subjects had DFI of less than a year, and the other 6 had DFI between 1 and 2 years (Table 1).
Table 1.
The background of the subjects
| Characteristics of primary cancer | Number (N = 56) | Characteristics of metastatic lesion | Number (N = 56) |
|---|---|---|---|
| Gender | Age median (range) | 53.1 (18.0–81.0) | |
| Male | 29 | Number of tumors median (range) | 2.8 (1.0–23.0) |
| Female | 27 | Largest tumor size median (range) | 2.5 (0.4–7.5) |
| Site of primary | Laterality | ||
| Larynx | 4 | Unilateral | 38 |
| Nasopharynx | 18 | Bilateral | 18 |
| Oral cavity | 8 | Operation | |
| Salivary gland | 7 | Sternotomy | 1 |
| Thyroid | 19 | Thoracotomy | 43 |
| Histology | VATS*** | 12 | |
| Papillary | 19 | Margins | |
| SCC* | 16 | Negative | 56 |
| ACC** | 11 | Positive | 0 |
| Undifferentialted | 8 | Chemoradiotherapy for the primary cancer | |
| Myoepithelial | 2 | Yes | 34 |
| Disease-free interval | No | 22 | |
| < 1 year | 18 | Recurrence of Mets | |
| 1–2 years | 6 | Yes | 32 |
| > 2 years | 32 | No | 24 |
*Squamous cell carcinoma
**Adenoid cystic carcinoma
***Video-assisted thoracoscopic surgery
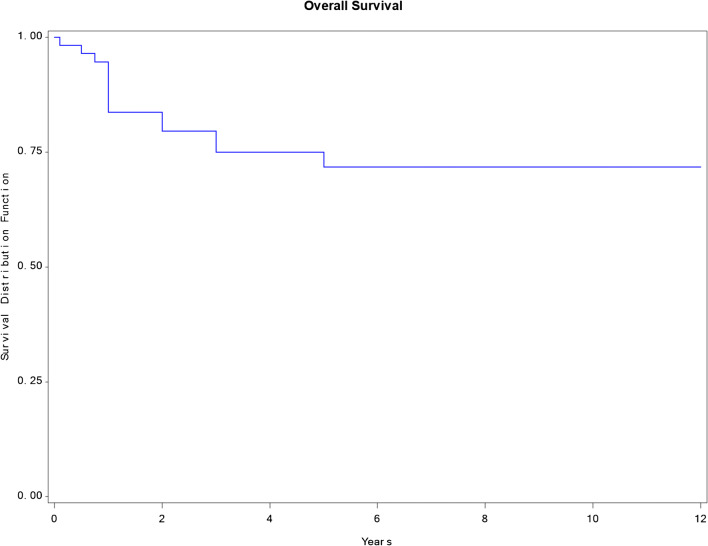
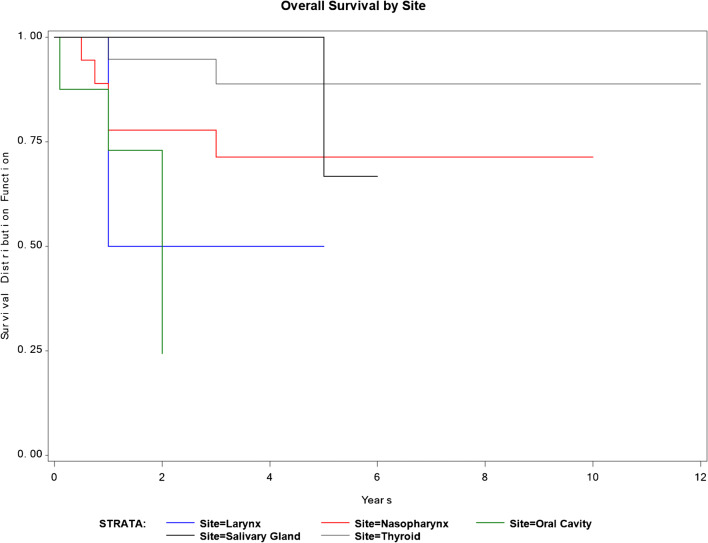
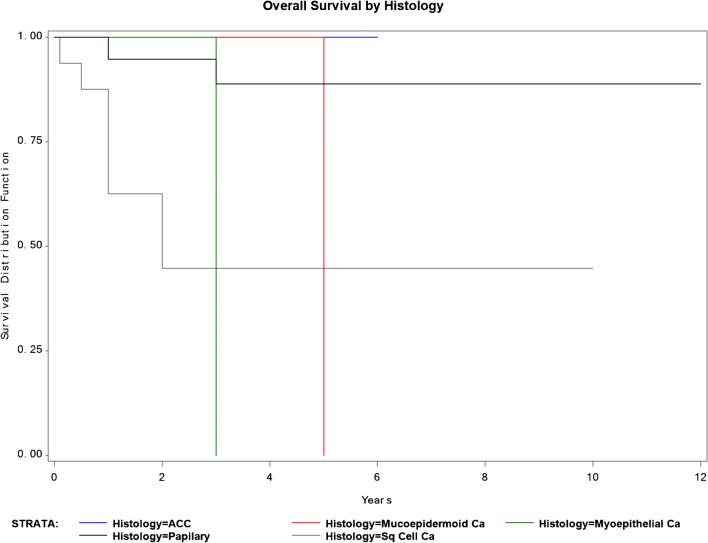
No postoperative complications of grade 2 and higher or surgery-related death occurred. Some postoperative complications included grade 1 complications, with pneumonia, atelectasis, and one case of wound infection. Survival with no recurrence, survival with recurrent cancer, deceased with no recurrence, and deceased with recurrence diseases occurred in 23, 19, 1, and 13 respectively. With regard to the recurrence site after metastasectomy surgery, 15 patients had chest recurrence, and 17 patients had other organ recurrences including the liver, bone, brain, and others (Table 2). In all of the subjects, the 3- and 5-year survival rates were 79.5 and 71.7%, respectively, and the median survival period was 4.2 years (Fig. 1). The survival rates according to the primary lesion site are presented in (Table 3) and Fig. 2, while the survival pertaining to the histology of the primary lesion is presented in (Table 4) and Fig. 3. Similarly, the survival rate based on DFI is presented in (Table 5) and Fig. 4. In a univariate analysis of the prognostic factors, the histological subtype of primary cancer showed a statistical significance with a p value of 0.05. However, age (p = 0.6), disease-free interval (p = 0.24), and site of primary cancer (p = 0.06) showed no significant prognostic accountability (Table 6).
Table 2.
Outcome and survival of 56 patients with pulmonary metastasis
| Outcome | |
| Alive with recurrence | 19 |
| Alive with no recurrence | 23 |
| Deceased with recurrence | 13 |
| Deceased with no recurrence | 1 |
| Recurrence site after metastasectomy | |
| Chest (lung/pleura/lymph nodes) | 15 |
| Other (liver/bone/brain) | 17 |
| Survival in years mean (range) | 4.2 (0.1–12) |
| 3-year survival | 79.5% |
| 5-year survival | 71.7% |
| Hospital stay in days mean (range) | 10.8 (3.0–49.0) |
| Operative/in-hospital mortality | 0 |
Fig. 1.
Overall survival graph. The survival rate was 79.5% at 3 years and 71.7% at 5 years
Table 3.
Survival of the patients after pulmonary metastasectomy according to the primary site using the Kaplan-Meier method and log-rank test
| Primary site | Number (n = 56) | 3-year survival (%) | 5-year survival (%) | p value |
|---|---|---|---|---|
| Larynx | 4 | 100 | 50 | 0.02 |
| Nasopharynx | 18 | 77.78 | 71.3 | |
| Oral cavity | 8 | 72.92 | 24.31 | |
| Salivary gland | 7 | 100 | 66.67 | |
| Thyroid | 19 | 94.74 | 88.82 |
Fig. 2.
Survival and site of original tumor
Table 4.
Survival of the patients after pulmonary metastasectomy according to histology of primary using the Kaplan-Meier method and log-rank test
| Primary site | Number (n = 56) | 3-year survival (%) | 5-year survival (%) | p value |
|---|---|---|---|---|
| ACC** | 11 | 100 | 100 | 0.002 |
| Myoepithelial | 2 | 100 | 100 | |
| Papillary | 19 | 94.74 | 88.82 | |
| SCC* | 16 | 62.5 | 44.64 | |
| Undifferentiated | 8 | 87.5 | 75 |
*Squamous cell carcinoma
**Adenoid cystic carcinoma
Fig. 3.
Survival and histology of original tumor
Table 5.
Survival of the patients after pulmonary metastasectomy according to disease-free interval (DFI) using the Kaplan-Meier method and log-rank test
| Primary site | Number (n = 56) | 3-year survival (%) | 5-year survival (%) | p value |
|---|---|---|---|---|
| < 1 year | 18 | 77.03 | 71.12 | 0.632 |
| 1–2 years | 6 | 83.33 | 66.66 | |
| > 2 years | 32 | 83.85 | 79.19 |
Fig. 4.
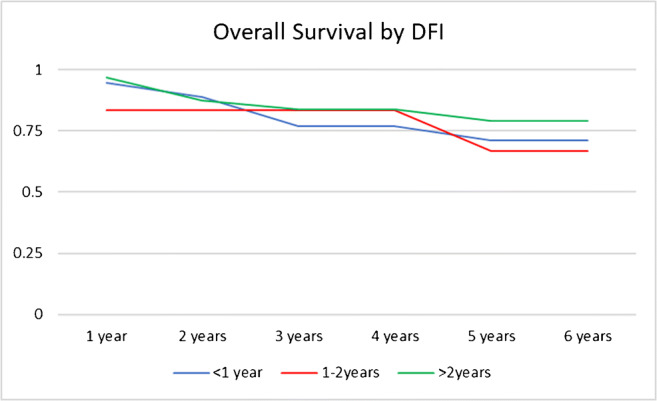
Survival and disease-free interval (DFI)
Table 6.
Univariate logistic regression test
| Characteristic | Number (n = 56) | Percentage | p value |
|---|---|---|---|
| Age | |||
| < 40 | 10 | 17.86 | 0.60 |
| 40–69 | 36 | 64.28 | |
| 70+ | 10 | 17.86 | |
| Histology | |||
| ACC** | 11 | 19.64 | 0.05 |
| Myoepithelial Ca | 2 | 3.57 | |
| Papillary | 19 | 33.92 | |
| SCC* | 16 | 28.57 | |
| Undifferentiated | 8 | 14.3 | |
| Site | |||
| Larynx | 4 | 7.14 | 0.06 |
| Nasopharynx | 18 | 32.14 | |
| Oral cavity | 8 | 14.29 | |
| Salivary gland | 7 | 12.50 | |
| Thyroid | 19 | 33.93 | |
| Disease-free interval | |||
| < 1 year | 18 | 32.14 | 0.24 |
| 1–2 years | 6 | 10.71 | |
| > 2 years | 32 | 57.14 | |
*Squamous cell carcinoma
**Adenoid cystic carcinoma
Discussion
Surgical resection and chemotherapy have been the mainstream treatments for pulmonary metastasis. The goal of curative resection of pulmonary metastases is complete identification and removal of all foci of malignancy with the maximal preservation of normal lung tissue. Surgical resection can be performed either by open thoracotomy or minimally invasive techniques. Many standard thoracic operations such as wedge resection, segmentectomy, lobectomy, and pneumonectomy are performed nowadays as part of the treatment plan for pulmonary metastasis. There have been studies suggesting survival benefits following surgical resection of lung metastasis from head and neck cancer [6–12]. A meta-analysis shows an absolute reduction of 29.1% in patients underwent pulmonary metastasectomy for head and neck tumors with metastasis to the lungs [7]. Furthermore, a 5-year survival rate between 20.9 and 59.9% has been reported from other studies in patients who underwent resection of pulmonary metastasis [2]. These studies strongly suggest a significantly favorable prognosis in the surgical resection group.
Our center, being oncology as well as a tertiary care referral center, has been receiving many patients with lung metastases for consideration for surgery. All patients in our center underwent a computed tomography (CT) scan for better evaluation of their nodules and since 2005, most patients had positron emission tomography (PET) scan as preoperative staging. Hence, we only operate on potential patients who are more likely to benefit from metastasectomy. It is known that multiple bilateral metastasis are less likely to be suitable for metastasectomy unless stable in number [1, 2, 4, 5], Therefore, we adopted to observe all such cases over 6–12 weeks and repeat the scan before surgery, and if there is an evidence of new metastasis appearing, we elect not to offer metastasectomy. Likewise, we adopted an aggressive approach to our patients with multiple or recurrent lung metastasis upon fulfilling the following criteria: (1) long disease-free interval, (2) absence of pleural based or lymph node involvement, (3) squamous carcinoma with good response to the treatment of the primary site, and (4) patients in good performance status. On the other hand, pre-operatively, we follow a strict policy to take a biopsy of the approachable nodule to confirm its pathology and correlate it with the primary diagnosis. The decision on the nature of the pathology and whether to go ahead with surgical intervention is taken jointly by different team members including pathologists, oncologist, radiologist, and surgeons during tumor board meetings and the examination is carried out again post-operatively. Additionally, for challenging special cases, we perform a CT-guided needle puncture/CT-guided microcoil insertions prior to VATS when lung nodules were not palpable. This being said and adding the fact that our analysis included patients with papillary thyroid carcinoma that has better prognosis may explain our slightly better outcome with 79.5% at 3 years and 71.7% at 5 years. Likewise, having a younger population with a mean age of 53.1 years, compared to the literature with older candidates, has added favorable results to our experience.
We ensure complete resection of lesions with negative margins but also are aware of minimizing resection of functional lung tissue as much as possible while leaving patients with adequate pulmonary function. Thus, wedge resection is the most common approach done by our team to ensure adequate outcomes. Moreover, segmentectomy is generally infrequently done by our section for pulmonary metastasectomy, but for lesions for which a wedge resection is technically not possible and lobectomy is not required, we do segmentectomy.
Also, the prognostic factors for patients with lung metastasis from head and neck cancer have also been studied in the literature. Some studies have shown gender, mediastinal lymph node metastasis, the number of pulmonary metastases, DFI, histological subtypes, and location of the original tumor [7, 8, 10–12].
Out of the significant factors being addressed in other studies, we have analyzed DFI, histological subtypes, and location of the original tumor in our study to compare our experience with that in the literature. Shiono et al. showed that worse prognosis is expected when DFI is less than 2 years compared to more than 2 years (p = 0.044) [11]. Regarding the site and histology of the primary tumors, most of the literature agrees that tumors that originate from the oral cavity and squamous cell carcinoma had the worst prognosis from all cancers of head and neck [6–8, 13].
Likewise, our study found that histological subtypes and the location of the original tumor had an effect on the prognosis of our patients; however, DFI was not statistically significant as a prognostic factor. In our subjects, the histological subtype is the most important prognostic factor on the 5-year survival rate for patients with metastatic head and neck tumors. Supporting the current literature, squamous cell carcinoma (SCC) has the worst 5-year survival rate of 44.64% compared to undifferentiated thyroid carcinoma (75%), papillary (88.82%), adenoid cystic carcinoma (100%), and myoepithelial carcinoma (100%) (Table 3). Thus, SCC needs aggressive chasing compared to other histological subtypes during the treatment process. For the location of the original tumor, oral cavity tumors had the worse prognosis. Oral and laryngeal cancers had a 5-year survival rate of 24.31% and 50% respectively compared to salivary glands tumors (66.67%), nasopharynx (71.3%), and thyroid cancer (88.82%). This could be related to the histological subtype associated with the original tumor location or the nature of the spreading process of these lesions to other organs. Thus, our study supports the current literature where squamous cell carcinoma and oral cancers have a very poor prognostic prediction when metastasized to the lung.
Limitations
In addition to the lack of a control group to standardize the results, we acknowledge limitations to our study. First, this was a retrospective study, so not all patients were treated similarly. This implies the potential of selection bias in some patients compared to others. However, by the nature of the study subjects and the consecutive recruitment of patients, we feel confident that such systematic bias may be excluded. Furthermore, the sample size was sufficient to attain statistically significant results.
Some patients underwent chemotherapy either prior to the primary surgery or after resection, which may influence the lesion size. However, the change in the size of all these patients was not significant in any of our analysis. Moreover, including papillary thyroid carcinomas with favorable prognosis may have affected our results; however, this could be explained by our center’s protocol in accepting patients with thyroid cancer under head and neck section. Lastly, we could not evaluate the histological involvement of lymph nodes and its effect on survival; nevertheless, it was not established in the literature as per our search. This study can be seen as a framework for further prospective analyses.
Conclusion
Pulmonary metastasectomy of tumor originating from head and neck has a survival rate of 79.5% survival at 3 years and 71.7% survival at 5 years, which is considered to be a good prognosis following surgery. Survival is significantly changed depending on two variables: histology and site of the original tumor. On univariate logistic regression test, histology is the only variable that is statistically significant as a prognostic factor. The site is also noteworthy to a certain measure, with no influence on hazard ratio. Our study supports the current literature and adds to its evidence about the prognostic factors for lung metastasectomy from head and neck tumors as a local/regional evidence. We also recommend further studies to help with the selection criteria for patients who undergo a pulmonary mastectomy to ensure the best outcomes.
Funding
None
Compliance with ethical standards
Ethical clearance
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Institutional Review Board (IRB) and the Office of Research Affairs (ORA) at our institute (RAC # 2151047) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animal rights
This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Abdullah AlShammari, Email: aalshammari@alfaisal.edu, Email: aalshammari@ymail.com.
Talal Almasri, Email: talmasri@alfaisal.edu.
Jumana Sarraj, Email: jsarraj@alfaisal.edu.
Omniyah AlAshgar, Email: oalashgar@kfshrc.edu.sa.
Mohamed Hussein Ahmed, Email: amohamedhussein@kfshrc.edu.sa.
Khaled AlKattan, Email: kkattan@alfaisal.edu.
Waleed Saleh, Email: waleedsaleh@kfshrc.edu.sa.
References
- 1.Kaifi JT, Gusani NJ, Deshaies I, et al. Indications and approach to surgical resection of lung metastases. J Surg Oncol. 2010;102:187–195. doi: 10.1002/jso.21596. [DOI] [PubMed] [Google Scholar]
- 2.Pfannschmidt J, Egerer G, Bischof M, Thomas M, Dienemann H. Surgical intervention for pulmonary metastases. Dtsch Arztebl Int. 2012;109:645–651. doi: 10.3238/arztebl.2012.0645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: adult cancer pain. Available from: External link http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#supportive.2012.
- 4.Kondo H, Okumura T, Ohde Y, Nakagawa K. Surgical treatment for metastatic malignancies. Pulmonary metastasis: indications and outcomes. Int J Clin Oncol. 2005;10:81–85. doi: 10.1007/s10147-004-0472-7. [DOI] [PubMed] [Google Scholar]
- 5.Saleh W, AlShammari A, Sarraj J, AlAshgar O, Ahmed MH, AlKattan K. Surgical treatment of pulmonary metastasis: report from a tertiary care center. Asian Cardiovasc Thorac Ann. 2018;26:296–301. doi: 10.1177/0218492318767795. [DOI] [PubMed] [Google Scholar]
- 6.Nakajima Y, Iijima Y, Kinoshita H, et al. Surgical treatment for pulmonary metastasis of head and neck cancer: study of 58 cases. Ann Thorac Cardiovasc Surg. 2007;23:169–174. doi: 10.5761/atcs.oa.16-00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Young ER, Diakos E, Khalid-Raja M, Mehanna H. Resection of subsequent pulmonary metastases from treated head and neck squamous cell carcinoma: systematic review and meta-analysis. Clin Otolaryngol. 2015;40:208–218. doi: 10.1111/coa.12348. [DOI] [PubMed] [Google Scholar]
- 8.Yotsukura M, Kinoshita T, Kohno M, et al. Survival predictors after resection of lung metastases of head or neck cancers. Thorac Cancer. 2015;6:579–583. doi: 10.1111/1759-7714.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schulz D, Wirth M, Piontek G, et al. Improved overall survival in head and neck cancer patients after specific therapy of distant metastases. Eur Arch Otorhinolaryngol. 2018;275:1239–1247. doi: 10.1007/s00405-018-4920-9. [DOI] [PubMed] [Google Scholar]
- 10.Oki T, Hishida T, Yoshida J, et al. Survival and prognostic factors after pulmonary metastasectomy of head and neck cancer: what are the clinically informative prognostic indicators? Eur J Cardiothorac Surg. 2019;55:942–7. [DOI] [PubMed]
- 11.Shiono S, Kawamura M, Sato T, et al. Pulmonary metastasectomy for pulmonary metastases of head and neck squamous cell carcinomas. Ann Thorac Surg. 2009;88:856–860. doi: 10.1016/j.athoracsur.2009.04.040. [DOI] [PubMed] [Google Scholar]
- 12.Winter H, Meimarakis G, Hoffmann G, et al. Does surgical resection of pulmonary metastases of head and neck cancer improve survival? Ann Surg Oncol. 2008;15:2915–2926. doi: 10.1245/s10434-008-0001-4. [DOI] [PubMed] [Google Scholar]
- 13.Mochizuki T, Okumura S, Ishii G, et al. Surgical resection for oral tongue cancer pulmonary metastases. Interact Cardiovasc Thorac Surg. 2010;11:56–59. doi: 10.1510/icvts.2009.226399. [DOI] [PubMed] [Google Scholar]