Abstract
Among gastrointestinal nematodes, haematophagous strongylids Haemonchus contortus and Ashworthius sidemi belong to the most pathogenic parasites of both domestic and wild ruminants. Correct identification of parasitic taxa is of crucial importance in many areas of parasite research, including monitoring of occurrence, epidemiological studies, or testing of effectiveness of therapy. In this study, we identified H. contortus and A. sidemi in a broad range of ruminant hosts that occur in the Czech Republic using morphological/morphometric and molecular approaches. As an advanced molecular method, we employed qPCR followed by High Resolution Melting analysis, specifically targeting the internal transcribed spacer 1 (ITS-1) sequence to distinguish the two nematode species. We demonstrate that High Resolution Melting curves allow for taxonomic affiliation, making it a convenient, rapid, and reliable identification tool.
Subject terms: Molecular biology, Zoology
Introduction
Infection of domestic and wild ruminants by helminth parasites, especially gastrointestinal nematodes (GINs), has a considerable social and economic impact throughout the world. These infections can lead to significant economic losses both in livestock industry and wildlife ranching due to decreased productivity or even animal death1–4.
Among the GINs, trichostrongylid Haemonchus contortus belongs to the most important parasites of a wide range of small ruminants in tropical and temperate regions around the globe5–7. It has a direct life cycle alternating between a parasitic and a free-living stage. Hosts become infected after accidental ingestion of third-stage infective larvae (L3) during grazing. Adult nematodes attach the abomasal mucosa where they feed on host’s blood, which poses a burden on animal’s health, significant especially in sheep husbandry8,9. H. contortus is currently emerging as a model organism of anthelmintic resistance in parasites, an issue that poses an increasing problem10–12.
Ashworthius sidemi is another haematophagous abomasal nematode, phylogenetically related to H. contortus. It is a typical parasite of Asiatic deer that was introduced into Europe probably via the sika deer (Cervus nippon)13–16. A highly successful invasive parasite has been dynamically spreading among various species of wild ruminants and into new regions17–20. In the European bison (Bison bonasus), a new susceptible host, the intensity of infection can reach thousands of nematodes per animal, which leads to massive histopathological changes20–22. Some studies highlight the danger of A. sidemi transmission from wildlife to livestock23–25. Based on available evidence, it is to be feared that A. sidemi has the potential of becoming one of the most widespread pathogenic gastrointestinal nematodes of autochthonous European ruminants.
As noted above, GINs of domestic and wild ruminants have a negative impact on animal health, which can translate into economic losses1–4. The impact of infection differs depending on ruminant species, age, environment, nutrition, management, the time of year, and obviously also parasitic species and its pathogenicity, therefore the proper identification of parasitic taxa is crucial both for veterinarians and producers25,26.
Correct differentiation between H. contortus and A. sidemi is complicated by morphological, biochemical, and biological similarities between the two species20,26,27. Taxonomic identification based on morphological/morphometric features is possible, especially in adult male specimens, but in adult females and immature nematodes, these methods are unreliable and in the larval stages and eggs they are not applicable at all. Moreover, because morphological examination is time-consuming and requires a helminthologist of considerable experience, we witness a growing use of molecular tools for species identification and demand for novel approaches28–30.
High Resolution Melting (HRM) analysis is a PCR-based technique available for routine diagnostic applications31–33. It can detect sequence alterations, such as small deletions, insertions, or even single nucleotide polymorphisms (SNPs) in dsDNA fragments amplified by qPCR, which are subsequently denatured by increasing temperature, i.e. by melting. Differences in melting profiles are visualised as the fluorescence of saturating dye that is gradually disassociated from the dsDNA amplicons. HRM is a fast, simple, and cost-effective approach for genotyping and mutation scanning, and it can easily be applied to the taxonomic identification of nematodes34–36.
The aim of this study was to develop a fast and usable qPCR-HRM method, which uses polymorphisms in the internal transcribed spacer 1 (ITS-1) region to distinguish between H. contortus and A. sidemi. This approach allowed us to evaluate the potential of HRM analysis for intravital diagnostics without any need for additional confirmation by e.g. electrophoretic separation or sequencing.
Results
Establishment and optimisation of HRM reference curves
Selection of specimens
We analysed adult males of H. contortus and A. sidemi nematodes (ten specimens from each species; 1M–20M) collected from the gastrointestinal tract of ruminants and constructed their HRM specific reference curves. Table 1 shows the range and area of origin of the host species present in the Czech Republic that were included in the study to assess possible sequential variability in the selected internal transcribed spacer (ITS-1) region. Prior to molecular analysis, we conducted a morphological identification of the male nematodes to confirm their species affiliation (see Supplementary Tables S1 and S2).
Table 1.
Geographical origin of hosts of individual specimens of H. contortus and A. sidemi adults.
| ID | Parasite | Host | Region | |
|---|---|---|---|---|
| Species | Sex | |||
| 1M | A. sidemi | Male | European bison (Bison bonasus) | Liberec |
| 2M | A. sidemi | Male | Roe deer (Capreolus capreolus) | Pilsen |
| 3M | A. sidemi | Male | Fallow deer (Dama dama) | Liberec |
| 4M | A. sidemi | Male | Red deer (Cervus elaphus) | Central Bohemia |
| 5M | A. sidemi | Male | Red deer (Cervus elaphus) | Pilsen |
| 6M | A. sidemi | Male | Red deer (Cervus elaphus) | Liberec |
| 7M | A. sidemi | Male | Roe deer (Capreolus capreolus) | Pilsen |
| 8M | A. sidemi | Male | Red deer (Cervus elaphus) | Liberec |
| 9M | A. sidemi | Male | Roe deer (Capreolus capreolus) | Pilsen |
| 10M | A. sidemi | Male | Fallow deer (Dama dama) | Liberec |
| 11M | H. contortus | Male | Mouflon (Ovis musimon) | Liberec |
| 12M | H. contortus | Male | White-tailed deer (Odocoileus virginianus) | Moravia–Silesia |
| 13M | H. contortus | Male | Roe deer (Capreolus capreolus) | Central Bohemia |
| 14M | H. contortus | Male | Mouflon (Ovis musimon) | Liberec |
| 15M | H. contortus | Male | Wild goat (Capra aegagrus) | Liberec |
| 16M | H. contortus | Male | Roe deer (Capreolus capreolus) | Central Bohemia |
| 17M | H. contortus | Male | Domestic sheep (Ovis aries) | South Bohemia |
| 18M | H. contortus | Male | Domestic sheep (Ovis aries) | South Bohemia |
| 19M | H. contortus | Male | Wild goat (Capra aegagrus) | Liberec |
| 20M | H. contortus | Male | Roe deer (Capreolus capreolus) | Central Bohemia |
| 1F | A. sidemi | Female | Red deer (Cervus elaphus) | Pilsen |
| 2F | A. sidemi | Female | Red deer (Cervus elaphus) | Pilsen |
| 3F | A. sidemi | Female | Red deer (Cervus elaphus) | Liberec |
| 4F | A. sidemi | Female | Red deer (Cervus elaphus) | Liberec |
| 5F | A. sidemi | Female | European bison (Bison bonasus) | Liberec |
| 6F | A. sidemi | Female | European bison (Bison bonasus) | Liberec |
| 7F | A. sidemi | Female | Moose (Alces alces) | Pilsen |
| 8F | A. sidemi | Female | Moose (Alces alces) | Pilsen |
| 9F | A. sidemi | Female | Roe deer (Capreolus capreolus) | Karlovy Vary |
| 10F | A. sidemi | Female | Roe deer (Capreolus capreolus) | Karlovy Vary |
| 11F | A. sidemi | Female | Red deer (Cervus elaphus) | Liberec |
| 12F | A. sidemi | Female | Red deer (Cervus elaphus) | Liberec |
| 13F | A. sidemi | Female | Fallow deer (Dama dama) | Liberec |
| 14F | A. sidemi | Female | Fallow deer (Dama dama) | Liberec |
| 15F | A. sidemi | Female | Fallow deer (Dama dama) | Liberec |
| 16F | H. contortus | Female | Mouflon (Ovis musimon) | Liberec |
| 17F | H. contortus | Female | White-tailed deer (Odocoileus virginianus) | Moravia-Silesia |
| 18F | H. contortus | Female | Roe deer (Capreolus capreolus) | Central Bohemia |
| 19F | H. contortus | Female | Mouflon (Ovis musimon) | Liberec |
| 20F | H. contortus | Female | Wild goat (Capra aegagrus) | Liberec |
| 21F | H. contortus | Female | Roe deer (Capreolus capreolus) | Central Bohemia |
| 22F | H. contortus | Female | Domestic goat (Capra hircus) | Central Bohemia |
| 23F | H. contortus | Female | Domestic sheep (Ovis aries) | South Bohemia |
| 24F | H. contortus | Female | Roe deer (Capreolus capreolus) | Central Bohemia |
| 25F | H. contortus | Female | Roe deer (Capreolus capreolus) | West Bohemia |
Melting temperatures
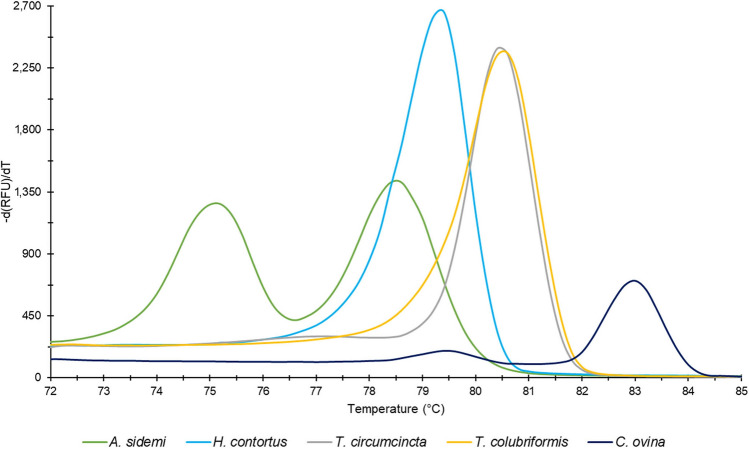
After qPCR-HRM analysis, values of melting temperatures Tm of amplified products with their corresponding peaks were generated by CFX Manager 3.0 software as one of the outputs. Two melting peaks at temperature mean 75.1 °C and 78.6 °C were identified as specific for the A. sidemi sequence, while one peak at 79.3 °C is characteristic of the H. contortus (Fig. 1).
Figure 1.
Melt peak chart. Median values of melting temperatures (Tm) of products amplified by qPCR-HRM primers corresponding to each species tested: A. sidemi (Tm: 75.1 °C; 78.6 °C), H. contortus (Tm: 79.3 °C), T. circumcincta (Tm: 80.5 °C), T. colubriformis (Tm: 80.6 °C), and C. ovina (Tm: 83.0 °C).
Primer specificity validation
Primer pair specificity to the target sequence was verified using qPCR-HRM analysis of genomic DNA isolated from several related gastrointestinal nematodes recovered from ruminants. In the case of Nematodirus battus, Cooperia curticei, and Oesophagostomum venulosum, no products of amplification were found. In Teladorsagia circumcincta, Trichostrongylus colubriformis, and Chabertia ovina, products with higher Tm values do appear (Fig. 1) but they can easily be distinguished from the Tm values of A. sidemi and H. contortus.
qPCR-HRM analysis: normalised data and difference plot
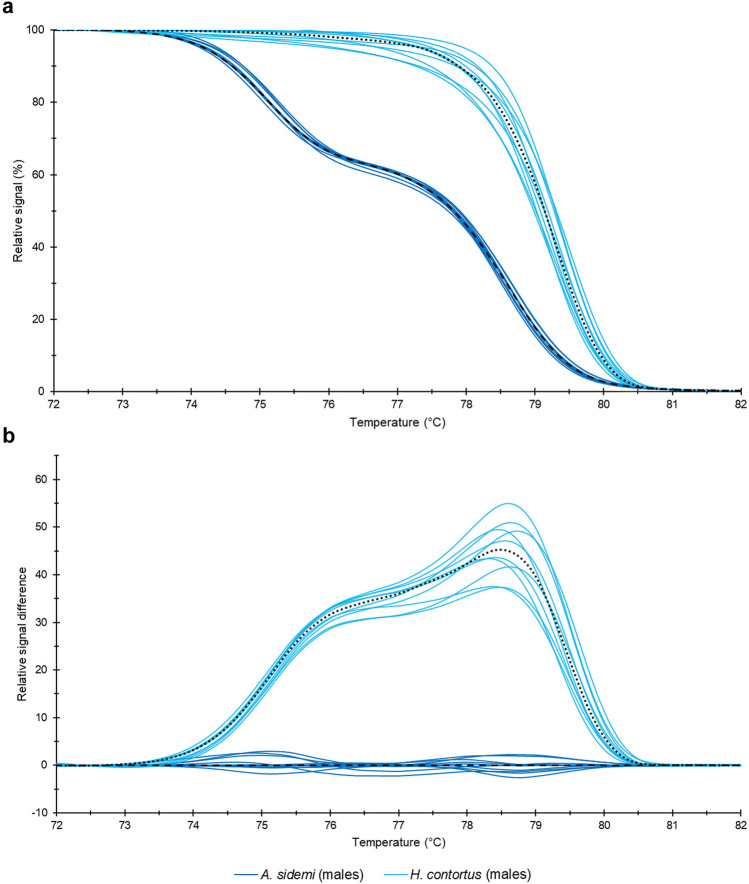
This analysis produced raw melting data, which were normalised and based on their shape and course of melting curves (relative fluorescence signal versus temperature) yielded two unambiguously differentiated groups corresponding to each species (Fig. 2a). To present these data as clearly as possible, we calculated from the HRM curves a difference plot (Fig. 2b).
Figure 2.
Normalised fluorescence versus temperature (a) and Difference plot (b). Data yielded by analysis of morphometrically identified H. contortus and A. sidemi adult male nematodes (samples 1M–20M). Median values are marked with a dashed line for A. sidemi and with a dotted line for H. contortus.
Sequencing
We amplified the ITS-1 fragments of the male nematodes (samples 1M–20M) which were used to construct the HRM reference curves with the same primer set as above. This resulted in products of 290 bp for A. sidemi and 281 bp for H. contortus (Fig. 3). Sequencing of these products yielded identical sequences for all samples of A. sidemi (1M–10M) used in this study. Comparison with the GenBank sequence EF467325.1 showed that the heterozygous genotype A/G in position 142 was in all these samples conserved. H. contortus samples (11M–20M) all yielded a fully identical sequence, namely one corresponding to GenBank sequence AB908961.1. Results obtained by sequencing confirmed genetic conformity within each species and the relevant differences between A. sidemi and H. contortus. This strongly supports our original assumption of specificity of HRM reference curves.
Figure 3.
Aligned consensus sequences of the amplified ITS-1 region. The sequences (290 bp for A. sidemi and 281 bp for H. contortus) correspond morphometrically identified H. contortus and A. sidemi adult male nematodes (samples 1M–20M). Identical nucleotides are represented by dots, gaps by hyphens. Variable sites are as indicated. Binding sites of the primers are shown in rectangles.
Application of qPCR-HRM analysis to other parasite forms
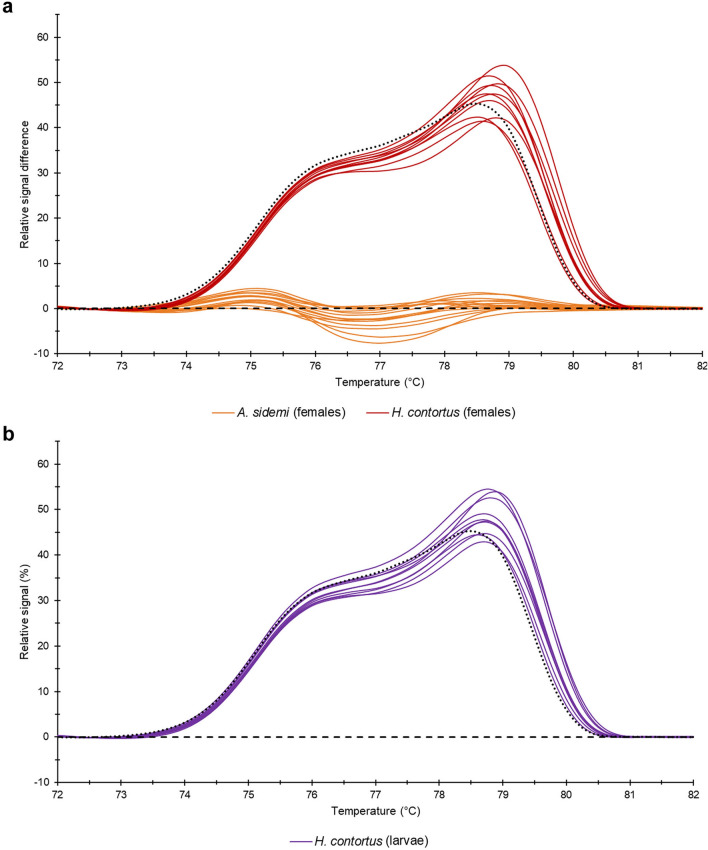
In this experiment, we identified female adult nematodes (1F–25F) using their morphological/morphometric characteristics (see Supplementary Tables S3 and S4). Then we subjected them to the same HRM analysis as the males and evaluated their data by comparing them with HRM reference curves based on male adult nematode data. All analysed samples clustered in their appropriate reference groups corresponding to the relevant species (Fig. 4a). Next, we applied HRM analysis to infective larvae of H. contortus (samples 1L–10L) and once again, a comparison with HRM curves based on adult male H. contortus showed a clear match (Fig. 4b). This suggests that the method yields correct results not only for adult male parasites but also for female adult specimens and for other developmental stages.
Figure 4.
Difference plots. (a) data yielded by analysis of H. contortus and A. sidemi adult female nematodes (samples 1F–25F); (b) data yielded by analysis of H. contortus L3 larvae (samples 1L–10L). Median values of normalised data of male samples are marked with a dashed line for A. sidemi and a dotted line for H. contortus.
Discussion
Different GINs, however, vary in their pathogenicity and if parasites are not properly taxonomically identified, nematode burdens cannot be correctly established37,38. Identification on at least the level of genus is crucial for mapping parasite spread, epidemiological studies, and efficacy of anthelmintic treatment.
Intravital diagnostics of GINs is still primarily based on coproscopy. Due to morphological similarities between the ‘trichostrongyle type’ eggs, the most prevalent species of nematodes in ruminants cannot be reliably differentiated, and further processing requiring faeces cultivation is needed39.
In the case of H. contortus and A. sidemi, the adult males can be distinguished due to differences in spicule length and presence or absence of a gubernaculum20,26,27. Also, adult females may be identified based on their specific morphological features, but it is often difficult to reliably differentiate between particular species.
The whole process of species differentiation using morphological characteristics is time-consuming and laborious, requires an experienced helminthologist, and is not always reliable. Therefore, molecular confirmation is appropriate if not downright necessary. For this reason, GIN diagnostics based on molecular methods is fast becoming a preferred method, because it allows for a rapid, safe, and sensitive species identification28–30.
Until now, multiplex PCR techniques for the detection of A. sidemi and H. contortus tended to target the nuclear ribosomal region and mitochondrial NADH dehydrogenase subunit 4 (ND4)40,41. These approaches required a subsequent visualisation by electrophoretic separation or sequencing. The protocol designed by Lehrter et al.40 called for a high input of DNA (optimally 100 ng), and our experiments showed that this method is not sufficiently robust and sensitive when it comes to individual larvae (data not shown).
In the present study, we used qPCR followed by HRM analysis as an advanced molecular method to differentiate H. contortus and A. sidemi species in a one-tube trial using a single pair of universal primers. Species identification was based on ITS-1 of the nuclear ribosomal region, a commonly used marker that sheds light on relationships between congeneric species and closely related genera of many nematodes42–44. For qPCR-HRM, we selected the ITS-1 fragment, making use of the fact that this region differs between the studied species (distinct nucleotide sequences composition, length, and GC contents) and exhibits a high within-species conservation. We found that melting temperatures Tm, and unequivocally separated HRM curves provide an easy, rapid, and unambiguous species differentiation that requires no subsequent confirmation by molecular analysis.
Furthermore, our results revealed that qPCR-HRM analysis is suitable for species identification using DNA isolated not only from adult parasites but also from other nematode developmental stages, such as infective larvae. For optimisation purposes, we used only H. contortus larvae (mono-infection of A. sidemi required for this analysis was not available during the experiment). Because individual larvae produce only a small volume of genomic DNA, we selected a suitable extraction protocol established in a previous study36. All in all, however, the qPCR-HRM shows promise of significant improvement of intravital GIN diagnostics that does not require post-mortem examination of host animals.
Our investigation confirmed that H. contortus is a generalist nematode that can infect a wide range of species of domestic and wild ruminants45. In the Czech Republic, the occurrence of non-native related nematode A. sidemi was first described in sika deer (Cervus nippon) in 197315 and since that time, it became a widespread parasite of wild ruminants in this country20,46. Results of the current study also demonstrate the presence of A. sidemi in European bison (Bison bonasus), red deer (Cervus elaphus), roe deer (Capreolus capreolus), fallow deer (Dama dama), and moose (Alces alces).
Given the wide range of actual and potential host species susceptible to A. sidemi, it is most advisable to monitor the infection in wild and domestic ruminants precisely, because this parasite poses a considerable potential threat to naive hosts. Introduction of this parasite through European bison relocated into the Czech Republic can serve as evidence of failure of intravital diagnostics20. This example clearly demonstrates that accurate parasite identification is essential if we want to prevent further spread of A. sidemi into new areas.
Conclusions
The results of our qPCR-HRM study based on ITS-1 fragment of the ribosomal region allow for a rapid and reliable differentiation of parasitic nematodes H. contortus and A. sidemi on a species level without the need for electrophoretic separation of PCR products or/and sequencing. Based on specific melting curves, we identified a total of 45 specimens of adult nematodes that came from a wide range of domestic and wild ruminant species living in various parts of the Czech Republic. We also confirmed that qPCR-HRM analysis is applicable to the infective larval stages of the nematodes, which promises a significant improvement in intravital diagnostics.
Methods
Ethical approval
All wild ruminants were culled as part of periodic control of game populations during the hunting seasons of 2017, 2018, and 2019; domestic sheep were slaughtered for human consumption. The handling of animals was carried out in accordance with relevant guidelines and regulations valid in the Czech Republic. The moose died due to collision with a vehicle. The digestive tracts dissected in this study were provided by responsible authorities, state-owned forestry company and farmers.
Collection of specimens
Adult nematodes were recovered from gastrointestinal tracts using post-mortem examination of ruminants; in total, we necropsied ten host species (Tab.1). The host animals came from various areas of the Czech Republic and sampling was conducted in 2017–2019. The abomasa were processed within a few hours of host culling/slaughtering using standard parasitological post-mortem examination techniques47,48. Organ contents were washed with tap water repeatedly and passed through mesh sieves with openings sized 200 μm and 150 μm to recover adult nematodes. Recovered worms were washed in a saline solution, preserved in 70% ethanol, and prior to morphological and molecular processing stored at 4 °C. To obtain H. contortus infective larvae (L3 stage), faecal samples from domestic sheep with artificial mono-infection were incubated for 7 days at 27 °C and subsequently processed using Baermann’s technique39. The larvae were preserved in tap water and prior to molecular processing stored at 4 °C.
Morphological identification
The recovered nematodes were identified based on their dominant distinguishing morphological/morphometric characters as described elsewhere20,26,27. The individual nematode specimens were evaluated using a light microscope Olympus BX51 and measurement of their dominant morphological characteristics carried out by QuickPHOTO MICRO 3.0 software (PROMICRA).
gDNA extraction
Molecular analysis was performed on a total of 45 adult nematode specimens morphologically identified as H. contortus and A. sidemi. In all cases, total genomic DNA was extracted separately from one half of individual adult nematodes using a slightly adjusted protocol adopted from previous studies36,49. The tissue was incubated in 50 μl of extraction buffer (100 mM Tris–HCl, 10 mM EDTA, 100 mM NaCl, 1% SDS, 1.5 mM dithiothreitol) containing 0.06 mg proteinase K. Lysis took place in a Thermomixer 5350 Mixer (Eppendorf) at 55 °C, with continuous mixing at 300 rpm overnight. 3 M sodium acetate (1/3 of the lysate volume), 5 μl oyster glycogen (20 mg/ml stock; SERVA Electrophoresis), and ice-cold isopropanol (2/3 of the lysate volume) were immediately added to the lysate. The sample was briefly vortexed and subsequently incubated at − 80 °C for 30 min to accelerate DNA precipitation. After centrifugation for 2 min at 14,000×g, the upper aqueous phase was carefully discarded. The pellet containing gDNA was washed with 200 μl of ice-cold 70% ethanol without disturbing the pellet in order to remove any salts that may be present. Another centrifugation was carried out for 2 min at 14,000×g and the supernatant removed. Finally, the DNA pellet was dried in a heater and dissolved in 25 μl of molecular-grade water. All samples were stored at − 20 °C until subsequent processing. gDNA was extracted from the individual larvae (n = 10) according to the same protocol, with reduction of the total extraction volume to 30 μl. In the case of larvae (6L–10L), a step of repeated thawing (at − 80 °C) and boiling (at 99 °C) was added prior to overnight lysis but no difference in DNA yields was observed. Concentration, yield, and purity of extracted gDNA were measured by NanoDrop 8000 Spectrophotometer (Thermo Fisher Scientific).
qPCR-HRM primers design and validation
A pair of universal primers, Fw: 5′-GCAATTCGTGGTAAATAACGCAG-3′ and Rev: 5′-GGGTGTATTGCTAWAATACTGCCTC-3′ targeting ITS-1 fragment of the ribosomal region, was used for species differentiation of H. contortus and A. sidemi nematodes that parasitised ruminants in the Czech Republic. These primers were designed according to available GenBank sequences for H. contortus (AB908961.1) and A. sidemi (EF467325.1). Basic Local Alignment Search Tool (BLAST) was used to predict primer targets in the massive GenBank database to assure both divergence among species and sequence conservation within each species. Target specificity of the primer set was verified experimentally using a qPCR-HRM analysis of related species of parasitic GINs of ruminants (Teladorsagia circumcincta, Trichostrongylus colubriformis, Nematodirus battus, Cooperia curticei, Chabertia ovina, and Oesophagostomum venulosum).
qPCR assay and HRM analysis
The isolated genomic DNA was used as a template for qPCR, which was performed immediately prior to the HRM analysis by C1000 Touch Thermal Cycler combined with CFX96 optical module (Bio-Rad Laboratories). The mixture and conditions of the reaction were adopted from our previous study36. In short, the qPCR was performed in a reaction mixture comprised of 1X Kapa HRM FAST Master Mix (Kapa Biosystems) containing Eva Green saturating dye, 2.5 mM MgCl2, 250 nM of each primer, and 50 ng of DNA, with molecular-grade water added to a final volume of 20 μl. The qPCR cycling conditions were as follows: initial denaturation at 95 °C for 3 min, followed by 45 cycles of denaturation at 95 °C for 5 s, annealing/extension at 57 °C for 40 s, and a final cooling step at 40 °C for 30 s. Following the qPCR, amplicon dissociation was initiated by a melting step in the same machine. Temperature range was set at 70–87 °C, with a data increment of 0.2 °C per 10 s. All samples were tested in duplicate.
Data analysis
Melting temperatures Tm of samples were evaluated by CFX Manager 3.0 software (Bio-Rad Laboratories). For subsequent evaluation, raw data representing the sample melting curves were extracted from this software. Data normalisation to uniform relative values from 100 to 0% for each sample was carried out by a mathematical conversion50 and average values calculated from duplicates. Pre- and post-melt fluorescent signals were set to 71.0–73.0 °C and 83.0–85.0 °C. To evaluate the melting process, we constructed a difference plot as an optimally transparent expression of the melting curves. First, we chose the median value of normalised data of male A. sidemi adult worms (samples 1M–10M) as a baseline. Then we subtracted from this baseline normalised data of all samples. Finally, we evaluated the data obtained from female adult worms (samples 1F–25F) and larvae (samples 1L–10L) according to HRM reference curves established on the basis of adult male worm data.
DNA sequence analysis
To verify that the HRM reference curves correspond to morphometrically identified specimens of male worms (samples 1M–20M), we sequenced the gene region used to establish the HRM matrix curves. PCR products were purified using a MinElute PCR Purification Kit (Qiagen) and sequenced. All sequences were compared with the NCBI GenBank nucleotide database using the BLAST.
Supplementary information
Acknowledgements
The authors would like to thank Doc. MVDr. Marian Varady, DrSc. (Institute of Parasitology, Slovak Academy of Sciences, Košice, 04001, Slovakia) for providing the infective larvae of H. contortus and Anna Pilatova, Ph.D. for proofreading the manuscript. The work was supported by a grant of the Ministry of Education, Youth and Sport (LTC19018).
Author contributions
L.S. designed the study, analysed data and sequences, created sequence alignments, and wrote the manuscript. N.R. contributed to the molecular work and consulted on experimental work. J.M. performed morphometric measurements. J.V. secured the biological material, contributed to morphometric data interpretation, and revised the manuscript. M.K. provided financial support, contributed to molecular data interpretation, and revised the manuscript. All authors contributed to the final version of the manuscript.
Data availability
All data generated or analysed during this study are included in this article and its Supplementary Information file.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-73037-9.
References
- 1.Charlier J, et al. Econohealth: Placing helminth infections of livestock in an economic and social context. Vet. Parasitol. 2015;212:62–67. doi: 10.1016/j.vetpar.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 2.Gunn A, Irvine RJ. Subclinical parasitism and ruminant foraging strategies—A review. Wildl. Soc. Bull. 2003;31:117–126. [Google Scholar]
- 3.Mavrot F, Hertzberg H, Torgerson P. Effect of gastro-intestinal nematode infection on sheep performance: A systematic review and meta-analysis. Parasites Vectors. 2015;8:557. doi: 10.1186/s13071-015-1164-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stien A, et al. The impact of gastrointestinal nematodes on wild reindeer: Experimental and cross-sectional studies. J. Anim. Ecol. 2002;71:937–945. [Google Scholar]
- 5.O'Connor LJ, Kahn LP, Walkden-Brown SW. Moisture requirements for the free-living development of Haemonchus contortus: Quantitative and temporal effects under conditions of low evaporation. Vet. Parasitol. 2007;150:128–138. doi: 10.1016/j.vetpar.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 6.Rinaldi L, et al. Haemonchus contortus: spatial risk distribution for infection in sheep in Europe. Geospat. Health. 2015;9:325–331. doi: 10.4081/gh.2015.355. [DOI] [PubMed] [Google Scholar]
- 7.Whitley NC, et al. Impact of integrated gastrointestinal nematode management training for US goat and sheep producers. Vet. Parasitol. 2014;200:271–275. doi: 10.1016/j.vetpar.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 8.Angulo-Cubillan FJ, Garcia-Coiradas L, Alunda JM, Cuquerella M, de la Fuente C. Biological characterization and pathogenicity of three Haemonchus contortus isolates in primary infections in lambs. Vet. Parasitol. 2010;171:99–105. doi: 10.1016/j.vetpar.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Rowe JB, Nolan JV, Dechaneet G, Teleni E, Holmes PH. The effect of haemonchosis and blood-loss into the abomasum on digestion in sheep. Br. J. Nutr. 1988;59:125–139. doi: 10.1079/bjn19880016. [DOI] [PubMed] [Google Scholar]
- 10.Doyle SR, et al. Population genomic and evolutionary modelling analyses reveal a single major QTL for ivermectin drug resistance in the pathogenic nematode, Haemonchus contortus. BMC Genomics. 2019;20:218. doi: 10.1186/s12864-019-5592-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mohammedsalih KM, et al. New codon 198 beta-tubulin polymorphisms in highly benzimidazole resistant Haemonchus contortus from goats in three different states in Sudan. Parasites Vectors. 2020;13:1–5. doi: 10.1186/s13071-020-3978-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riou M, et al. Effects of cholesterol content on activity of P-glycoproteins and membrane physical state, and consequences for anthelmintic resistance in the nematode Haemonchus contortus. Parasite. 2020;27:13. doi: 10.1051/parasite/2019079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drozdz J. Materials contributing to the knowledge of the helminth fauna of Cervus (Russa) unicolor Kerr and Muntjacus muntjak Zimm. of Vietnam, including two new nematode species: Oesophagostomum labiatum sp. n., and Trichocephalus muntjaci sp. n. Acta Parasitol. 1973;33:465–474. [Google Scholar]
- 14.Kotrla B, Kotrly A. The first finding of the nematode Aschworthius sidemi Schulz, 1933 in Sika nippon from Czechoslovakia. Folia Parasitol. 1973;20:377–378. [Google Scholar]
- 15.Kotrla B, Kotrly A. Helminths of wild ruminants introduced into Czechoslovakia. Folia Parasitol. 1977;24:35–40. [PubMed] [Google Scholar]
- 16.Schulz RE. Ashwoethius sidemi n. sp. (Nematoda, Trichostrongylidae) aus einem Hirsch (Pseudaxis hortulorum) des fernen Ostens. Zschr. Parasitenk. 1933;5:735–739. [Google Scholar]
- 17.Demiaszkiewicz AW, Merta D, Kobielski J, Filip KJ. A further increase in the prevalence and intensity of infection with Ashworthius sidemi nematodes in red deer in the Lower Silesian Wilderness. Ann. Parasitol. 2018;64:189–192. doi: 10.17420/ap6403.150. [DOI] [PubMed] [Google Scholar]
- 18.Kuzmina TA, Kharchenko VA, Malega AM. Helminth fauna of roe deer (Capreolus Capreolus) in Ukraine: Biodiversity and parasite community. Vestn. Zool. 2010;44:15–22. [Google Scholar]
- 19.Kuznetsov D, Romashova N, Romashov B. The first detection of Ashworthius sidemi (Nematoda, Trichostrongylidae) in roe deer (Capreolus capreolus) in Russia. Vet. Parasitol. Reg. Stud. Rep. 2018;14:200–203. doi: 10.1016/j.vprsr.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Vadlejch J, Kyrianova IA, Rylkova K, Zikmund M, Langrova I. Health risks associated with wild animal translocation: A case of the European bison and an alien parasite. Biol. Invasions. 2017;19:1121–1125. [Google Scholar]
- 21.Demiaszkiewicz AW, Lachowicz J, Osinska B. Ashworthius sidemi (Nematoda, Trichostrongylidae) in wild ruminants in Bialowieza Forest. Pol. J. Vet. Sci. 2009;12:385–388. [PubMed] [Google Scholar]
- 22.Osinska B, Demiaszkiewicz AW, Lachowicz J. Pathological lesions in European bison (Bison bonasus) with infestation by Ashworthius sidemi (Nematoda, Trichostrongylidae) Pol. J. Vet. Sci. 2010;13:63–67. [PubMed] [Google Scholar]
- 23.Drozdz J, Demiaszkiewicz AW, Lachowicz J. Expansion of the Asiatic parasite Ashworthius sidemi (Nematoda, Trichostrongylidae) in wild ruminants in Polish territory. Parasitol. Res. 2003;89:94–97. doi: 10.1007/s00436-002-0675-7. [DOI] [PubMed] [Google Scholar]
- 24.Kotrla B, Kotrly A, Kozdon O. Studies on the specifity of the nematode Ashworthius sidemi Schulz, 1933. Acta Vet. Brno. 1976;45:123–126. [Google Scholar]
- 25.Moskwa B, et al. The first identification of a blood-sucking abomasal nematode Ashworthius sidemi in cattle (Bos taurus) using simple polymerase chain reaction (PCR) Vet. Parasitol. 2015;211:106–109. doi: 10.1016/j.vetpar.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 26.Lichtenfels JR, Pilitt PA, Hoberg EP. New morphological characters for identifying individual specimens of Haemonchus spp. (Nematoda: Trichostrongyloidea) and a key to species in ruminants of North America. J. Parasitol. 1994;80:107–119. [PubMed] [Google Scholar]
- 27.Pike AW. A revision of the genus Ashworthius Le Roux, 1930 (Nematoda: Trichostrongylidae) J. Helminthol. 1969;43:135–144. doi: 10.1017/s0022149x00003977. [DOI] [PubMed] [Google Scholar]
- 28.Baltrisis P, Halvarsson P, Hoglund J. Molecular detection of two major gastrointestinal parasite genera in cattle using a novel droplet digital PCR approach. Parasitol. Res. 2019;118:2901–2907. doi: 10.1007/s00436-019-06414-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Said Y, Gharbi M, Mhadhbi M, Dhibi M, Lahmar S. Molecular identification of parasitic nematodes (Nematoda: Strongylida) in feces of wild ruminants from Tunisia. Parasitology. 2018;145:901–911. doi: 10.1017/S0031182017001895. [DOI] [PubMed] [Google Scholar]
- 30.Santos LL, et al. Molecular method for the semiquantitative identification of gastrointestinal nematodes in domestic ruminants. Parasitol. Res. 2020;119:529–543. doi: 10.1007/s00436-019-06569-3. [DOI] [PubMed] [Google Scholar]
- 31.Ababneh M, Ababneh O, Al-Zghoul MB. High-resolution melting curve analysis for infectious bronchitis virus strain differentiation. Vet. World. 2020;13:400–406. doi: 10.14202/vetworld.2020.400-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dehbashi S, Tahmasebi H, Sedighi P, Davarian F, Arabestani MR. Development of high-resolution melting curve analysis in rapid detection of vanA gene, Enterococcus faecalis, and Enterococcus faecium from clinical isolates. Trop. Med. Health. 2020;48:1–2. doi: 10.1186/s41182-020-00197-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang C, et al. Rapid screening of MMACHC gene mutations by high-resolution melting curve analysis. Mol. Genet. Genomic Med. 2020;6:e1221. doi: 10.1002/mgg3.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arbabi M, Hooshyar H, Lotfinia M, Bakhshi MA. Molecular detection of Trichostrongylus species through PCR followed by high resolution melt analysis of ITS-2 rDNA sequences. Mol. Biochem. Parasitol. 2020;236:111260. doi: 10.1016/j.molbiopara.2020.111260. [DOI] [PubMed] [Google Scholar]
- 35.Filipiak A, Hasiow-Jaroszewska B. The use of real-time polymerase chain reaction with high resolution melting (real-time PCR-HRM) analysis for the detection and discrimination of nematodes Bursaphelenchus xylophilus and Bursaphelenchus mucronatus. Mol. Cell. Probes. 2016;30:113–117. doi: 10.1016/j.mcp.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 36.Reslova N, Skorpikova L, Slany M, Pozio E, Kasny M. Fast and reliable differentiation of eight Trichinella species using a high resolution melting assay. Sci. Rep. 2017;7:16210. doi: 10.1038/s41598-017-16329-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Irvine RJ, Corbishley H, Pilkington JG, Albon SD. Low-level parasitic worm burdens may reduce body condition in free-ranging red deer (Cervus elaphus) Parasitology. 2006;133:465–475. doi: 10.1017/S0031182006000606. [DOI] [PubMed] [Google Scholar]
- 38.Kemper KE, et al. Reduction of faecal worm egg count, worm numbers and worm fecundity in sheep selected for worm resistance following artificial infection with Teladorsagia circumcincta and Trichostrongylus colubriformis. Vet. Parasitol. 2010;171:238–246. doi: 10.1016/j.vetpar.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 39.van Wyk, J. A. & Mayhew, E. Morphological identification of parasitic nematode infective larvae of small ruminants and cattle: A practical lab guide. Onderstepoort J. Vet. Res.80, 539 (2013). [DOI] [PubMed]
- 40.Lehrter V, Jouet D, Lienard E, Decors A, Patrelle C. Ashworthius sidemi Schulz, 1933 and Haemonchus contortus (Rudolphi, 1803) in cervids in France: Integrative approach for species identification. Infect. Genet. Evol. 2016;46:94–101. doi: 10.1016/j.meegid.2016.10.027. [DOI] [PubMed] [Google Scholar]
- 41.Moskwa B, Bien J, Gozdzik K, Cabaj W. The usefulness of DNA derived from third stage larvae in the detection of Ashworthius sidemiinfection in European bison, by a simple polymerase chain reaction. Parasites Vectors. 2014;7:1–5. doi: 10.1186/1756-3305-7-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nabavi R, et al. Comparison of internal transcribed spacers and intergenic spacer regions of five common Iranian sheep bursate nematodes. Iran. J. Parasitol. 2014;9:350–357. [PMC free article] [PubMed] [Google Scholar]
- 43.Otranto D, et al. Differentiation among three species of bovine Thelazia (Nematoda: Thelaziidae) by polymerase chain reaction-restriction fragment length polymorphism of the first internal transcribed spacer ITS-1 (rDNA) Int. J. Parasitol. 2001;31:1693–1698. doi: 10.1016/s0020-7519(01)00279-x. [DOI] [PubMed] [Google Scholar]
- 44.Zarlenga DS, Gasbarre LC, Boyd P, Leighton E, Lichtenfels JR. Identification and semi-quantitation of Ostertagia ostertagi eggs by enzymatic amplification of ITS-1 sequences. Vet. Parasitol. 1998;77:245–257. doi: 10.1016/s0304-4017(98)00114-9. [DOI] [PubMed] [Google Scholar]
- 45.Hoberg, E. P. & Zarlenga, D. S. Evolution and biogeography of Haemonchus contortus: linking faunal dynamics in space and time. In Haemonchus Contortus and Haemonchosis - Past, Present and Future Trends Vol. 93, 1–30 (2016) [DOI] [PubMed]
- 46.Magdalek, J., Kyrianova, I. A. & Vadlejch, J. Ashworthius sidemiin wild cervids in the Czech Republic. In 9th Workshop on biodiversity 66–71 (Česká zemědělská univerzita v Praze, Praha, 2017). https://www.researchgate.net/publication/328902164 Accessed 29 July 2020.
- 47.Hansen, J. & Perry, B. The epidemiology, diagnosis and control of helminth parasites of ruminants. In FAO Animal Health Manual, 67–82 (1994).
- 48.Wood IB, et al. World Association for the Advancement of Veterinary Parasitology (W.A.A.V.P.) second edition of guidelines for evaluating the efficacy of anthelmintics in ruminants (bovine, ovine, caprine) Vet. Parasitol. 1995;58:181–213. doi: 10.1016/0304-4017(95)00806-2. [DOI] [PubMed] [Google Scholar]
- 49.Zavodna M, Sandland GJ, Minchella DJ. Effects of intermediate host genetic background on parasite transmission dynamics: A case study using Schistosoma mansoni. Exp. Parasitol. 2008;120:57–61. doi: 10.1016/j.exppara.2008.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Palais R, Wittwer CT. Mathematical algorithms for high-resolution DNA melting analysis. Methods Enzymol. 2009;454:323–343. doi: 10.1016/S0076-6879(08)03813-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this article and its Supplementary Information file.