Abstract
Purpose
When a mass develops around the staple line after lung cancer surgery, differential diagnosis between lung cancer recurrence and benign granuloma can be clinically problematic. Therefore, we investigated the clinical characteristics of benign granuloma and cancer recurrence around the staple line to determine clinical factors that can distinguish staple line granuloma and cancer recurrence.
Methods
We retrospectively investigated the clinical records of 25 patients who developed a nodule around the staple line after pulmonary resection for lung cancer and conducted a comparative study of staple line granuloma and cancer recurrence.
Results
Among 25 patients, the nodule was diagnosed as benign granuloma in 9, recurrence of primary lung cancer in 8, and recurrence of metastatic lung cancer in 8. Among these three groups, there were no significant differences in age, maximum standardized uptake value of fluoro-deoxyglucose, laboratory data, or radiological findings. However, in comparison with the cancer recurrence cases, the proportion of patients who had undergone segmentectomy as initial surgery was significantly higher in the granuloma group. Moreover, in five patients in the granuloma group, mycobacterium was detected.
Conclusion
It seemed difficult to differentiate between cancer recurrence and granuloma on the basis of radiological examination and laboratory findings. However, if a mass shadow around the staple line appeared after segmentectomy, the mass is likely to be a granuloma. Mycobacterial infection may be an important factor for development of granuloma on the staple line.
Keywords: Staple line, Granuloma, Segmentectomy
Introduction
The staples of an automatic suturing device are made mainly of titanium. Although titanium has been considered to be inert within the body, granuloma arising around the staple line has been reported with increasing frequency [1–17], especially as the use of thoracoscopic surgery requiring automatic mechanical suturing equipment becomes more widespread.
When a mass is detected around the staple line after lung cancer surgery, differential diagnosis between cancer recurrence and benign granuloma can be clinically problematic. Although the usefulness of fluoro-deoxyglucose (FDG) positron emission tomography (PET) for differentiating between benign and malignant nodules has been reported, uptake of FDG may be observed even in granulomatous diseases such as mycobacterial infection and sarcoidosis. Because staple line granulomas are expected to increase in the future, clinical factors that allow differential diagnosis between staple line granuloma and cancer recurrence are required. We examined 25 cases of nodules around the staple line after pulmonary resection and investigated the clinical characteristics of benign granuloma and cancer recurrence.
Aim of the study
The aims of this study is to investigate various clinical factors on staple line granuloma and cancer recurrence and to determine clinical factors that can distinguish staple line granuloma and cancer recurrence.
Patients and methods
Type of study: retrospective case-control study.
Clinical findings in 25 patients who developed nodular shadows around the staple line between January 2012 and March 2017 were retrospectively investigated. Among these patients, benign granuloma was diagnosed in nine, recurrence of primary lung cancer in eight, and recurrence of metastatic lung cancer in eight.
During the same period, 2128 patients underwent lung resection for lung cancer; 1865 (87.6%) for primary lung cancer and 263 (12.4%) for metastatic lung cancer. As a surgical procedure, 65 patients (3.1%) underwent pneumonectomy, 1493 (70.2%) underwent lobectomy, 7 (0.3%) underwent lobectomy + segmentectomy, 238 (11.2%) underwent segmentectomy, and 325 (15.3%) underwent wedge resection. Among the nine patients with granuloma, seven were diagnosed by surgical resection and the remaining two because the nodule size was reduced during the pretreatment examination period. Although transbronchial biopsy and bronchial washing using a bronchofiberscope were performed in three granuloma cases, definitive diagnosis of granuloma could not be obtained and bacteria such as mycobacterium could not be detected. Among the 18 patients with cancer recurrence, 4 were diagnosed by transbronchial biopsy using bronchofiberscope, 1 by transcutaneous biopsy under computed tomography (CT) guidance, and 13 by surgical resection.
In order to investigate the clinical features of benign granuloma around the staple line, we examined the characteristics of these patients and compared several clinical factors between patients with benign granuloma and those with lung cancer recurrence. The patients were divided into three groups: granuloma, recurrence of primary lung cancer, and recurrence of metastatic lung cancer.
The Institutional Review Board approved this retrospective study. The need for subsequent individual consent from patients whose records were evaluated was waived because the individuals were not identified in this study.
Statistical analysis
Counts were compared using the Chi-square test. The Mann-Whitney U test was used to assess associations between quantitative variables. Differences at p < 0.05 were considered to be significant. Statistical analysis was performed using the StatView 5.0 software package (SAS Institute Inc., Cary, NC, USA).
Results
Patient characteristics
The characteristics of the patients in each group are shown in Table 1. Although there was no evident tendency in terms of age and laboratory findings, the granuloma group includes more female patients than lung cancer recurrence groups.
Table 1.
The characteristics of the patients in each group
| Granuloma (n = 9) | Recurrence of primary lung cancer (n = 8) | Recurrence of metastatic lung cancer (n = 8) | |
|---|---|---|---|
| Male/female | 5/4 | 7/1 | 7/1 |
| Age | 71.8 ± 6.2 (73.0) | 70.4 ± 6.7 (68.0) | 68.4 ± 4.9 (69.5) |
| WBC (/μl) | 5100 ± 1257 (5200) | 6062 ± 2098 (5550) | 6138 ± 1597 (5700) |
| CRP (mg/dl) | 0.09 ± 0.07 (0.06) | 0.21 ± 0.23 (0.11) | 0.81 ± 1.74 (0.17) |
| CEA (mg/ml) | 4.0 ± 2.0 (3.9) | 10.5 ± 13.9 (3.2) | 7.7 ± 7.1 (5.6) |
mean ± standard deviation (median)
WBC white blood cell count, CRP C-reactive protein, CEA carcinoembryonic antigen
Chest CT findings
Representative chest CT films of staple line granuloma and cancer recurrence are demonstrated in Fig. 1. In the granuloma cases, an irregular nodule appeared to grow from the staple line towards the inside of the lung. Chest CT of some granuloma cases demonstrated a low-density area in the nodule, and chest CT of other granuloma case demonstrated a contrasted mass. In both the primary lung cancer recurrence group and metastatic cancer recurrence group, the mass had grown from the staple line toward the inside of the lung parenchyma in one direction, as was seen in the granuloma cases. In some cases of cancer recurrence, lung abscess was suspected from the CT findings, which showed a rectilinear margin and a low concentration area within.
Fig. 1.
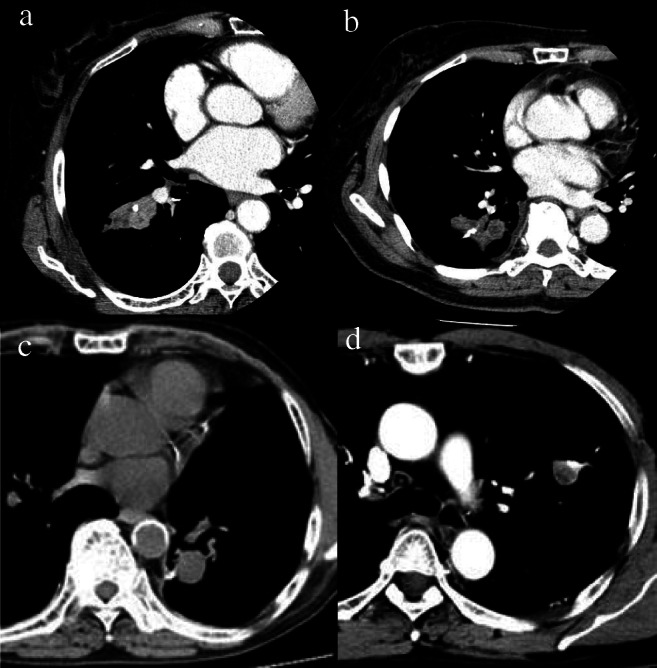
Representative chest CTimages for the patient of granuloma and cancer recurrence around the staple line. a, b Benign granuloma, c recurrence of primary lung cancer, d recurrence of metastatic lung cancer
FDG-PET findings
FDG-PET imaging was performed in eight of the nine cases in the granuloma group, five of the eight cases in the primary lung cancer recurrence group, and three of the eight cases in metastatic lung cancer recurrence group. In the granuloma group, uptake of FDG was observed in all cases except one. In one of granuloma cases, FDG-PET was performed at other hospitals and there was no data for the maximum standardized uptake value (SUVmax). Therefore, SUVmax values were compared in the remaining 15 cases (Fig. 2). The SUVmax value tended to decrease in the order: recurrence of metastatic lung cancer > recurrence of primary lung cancer > granuloma, but even in the granuloma cases, some had a high SUVmax value of 10 or more.
Fig. 2.
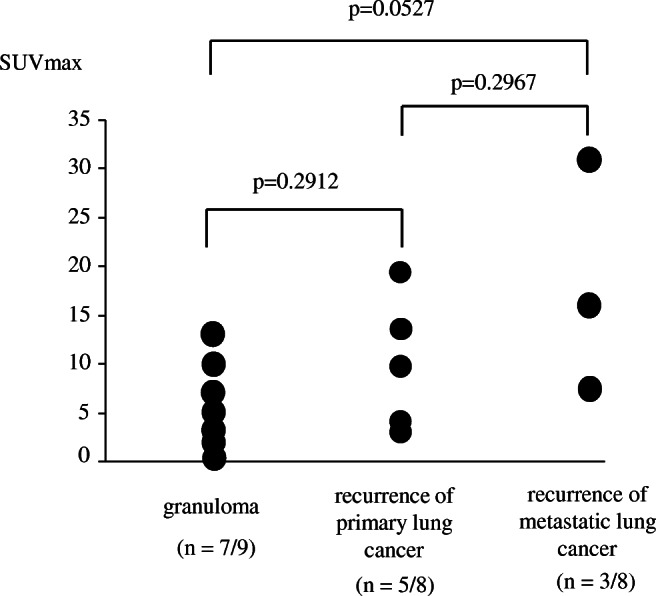
Comparison of SUVmax value among granuloma, recurrence of primary lung cancer, and recurrence of metastatic lung cancer
Period from initial surgery to discovery of shadow
The median period from initial surgery to the discovery of the current shadow in the various groups was as follows: granuloma group: 15.0 months (range: 3–84 months), primary lung cancer recurrence group 34.0 months (12–72 months), and metastatic lung cancer recurrence group 10.9 months (3–24 months) (Fig. 3). Compared with recurrence of primary lung cancer, recurrence of metastatic lung cancer appeared within a significant short period of time after initial surgery. However, the period from initial surgery until discovery of the current shadow varied widely for granuloma and recurrence of primary lung cancer, and no particular tendency was evident.
Fig. 3.

The period from initial surgery to discovery of the shadow around the staple line
Procedure used for initial surgery
At initial surgery, segmentectomy was performed in six of nine cases in granuloma group. On the other hand, in the cancer recurrence cases, the procedure for initial surgery was wedge resection in 14 patients, lobectomy in 1, and segmentectomy in 1. The proportion of patients undergoing segmentectomy at initial surgery was significantly higher for granuloma cases (p = 0.0025).
Postoperative findings
In five of seven granuloma cases treated by surgical resection, pathological examination revealed epithelioid granulomas with multinucleated giant cells, and these were diagnosed as non-tuberculous mycobacteriosis histologically. White pus was observed during surgery in these five cases, and all cases were positive for Mycobacterium avium by polymerase chain reaction.
Discussion
Granuloma cases and cancer recurrence cases showed differences in the procedure used for initial surgery. The proportion of patients undergoing segmentectomy at initial surgery was significantly higher for granuloma (6/9, 66.7%), while 14 of 16 cases (87.5%) of recurrent cancer occurred after wedge resection. Even though segmentectomy accounts for only about 10% of all pulmonary resections in Japan [18] and our institution, 66.7% of staple line granuloma occurred after segmentectomy in this series. There have been 22 previously reported cases of granuloma around the staple line [1–17] (Table 2), in which the use of segmentectomy for initial surgery was as frequent (50%, 11/22) as that in the present study. Disturbance of ventilation and blood flow near the resection line is reported to be the main cause of infection and granuloma formation around the staple line [14]. The pulmonary parenchyma to be dissected is usually longer and thicker in segmentectomy than in lobectomy and wedge resection. Therefore, ventilation and blood flow disturbance near the resection line are thought to be more significant after segmentectomy than after lobectomy and wedge resection, and granuloma may be more likely to occur after the former.
Table 2.
Previously reported cases of granulomas around staple line: 22 cases
| Sex | Male/female | 10/12 | |
| Previous surgery |
Lobectomy Lobectomy + segmentectomy Segmentectomy Wedge resection |
2 1 10 9 |
|
| Mycobacterium | Positive | 9/20 (45%) | |
| Range | Mean ± SD | Median | |
| Age (year-old) | 42–74 | 64.4 ± 9.5 | 67 |
| Interval (months) | 8–144 | 50.0 ± 34.8 | 48.0 |
| PET (FDG uptake) | Positive (12/12) | ||
| SUV max | 1.5–11.1 | 5.6 ± 3.3 | 4.8 |
SD standard deviation, PET positron emission tomography, SUVmax maximum standardized uptake value
In the present study, among seven patients with granuloma who underwent bacterial examination, mycobacterium was detected in five. In the 20 previously reported cases for which bacterial examination was performed, mycobacterium was detected in 9 (45%). Because mycobacterium was detected at a high frequency in the staple line granulomas we experienced and in previous studies, mycobacterium infection may be an important factor in the formation of staple line granuloma. However, in the present study, mycobacterium could not be detected by transbronchial biopsy and bronchial washing using a bronchofiberscope. If a definitive diagnosis of mycobacterial infection or benign granuloma can be obtained before surgery, medical treatment is an important option for compromised patients who may not be amenable to surgery. A previous report has demonstrated that percutaneous needle biopsy is useful for diagnosis of staple line granuloma and detection of mycobacterium [14]. If a staple line granuloma is suspected from the clinical findings, then percutaneous needle biopsy and bacterial examination may be considered for management of staple line nodules.
For distinguishing between lung cancer recurrence and granulomatous lesions around the staple line on chest CT, previous reports have considered the following to be useful: (1) whether a border is smooth or irregular, (2) whether calcification, or vascular and bronchial involvement is present, and (3) whether there is a difference in growth pattern [14]. It is reported that tumor recurrence tends to proliferate centered on staple, whereas granulomas tend to grow from the staple line towards the inside of the lung parenchyma in one direction [14]. However, in this study, many recurrent tumors grew from the staple line toward the inside of the lung parenchyma in one direction, as was the case for granuloma. In addition, some cases of cancer recurrence had a smooth border and a low-density area inside the mass, and a lung abscess was suspected from the CT findings. Therefore, on the basis of CT findings alone, differential diagnosis between cancer recurrence and granuloma on the staple line is difficult. Moreover, the SUVmax value also differed greatly among the granuloma cases. In previous reports, uptake of FDG was observed in all granuloma cases and SUVmax values varied widely, as was the case in this study. Although the SUVmax value in granuloma cases tended to be lower than that in cancer recurrence cases, it is difficult to differentiate granuloma from cancer recurrence on the basis of SUVmax value alone. Because mycobacterium was detected in staple line granuloma at high frequency, mycobacterium infection in nodules may account for the increase of SUVmax value.
The period from initial surgery until the appearance of the staple line nodule was shorter in cases of recurrent metastatic lung cancer. In cases of metastatic lung cancer, recurrent tumors around the staple line appeared within 2 years after initial surgery in all cases. However, in cases of recurrent primary lung cancer, the period from initial surgery until the appearance of staple line nodules varied and showed no constant tendency. The length of time from resection to cancer recurrence seems to be proportional to the degree of tumor malignancy. Because metastatic lung cancer is considered to be a high-grade malignancy, tumors will recur in a short period of time. However, because primary lung cancer has various degree of malignancy, the period until recurrence will vary. A recurrent tumor around the staple line 13 years after wedge resection of primary lung cancer [19] and three cases of delayed cut-end recurrence after limited resection for ground-glass opacity adenocarcinoma [20] were reported previously. In this study, the period until detection of primary lung cancer recurrence varied from 12 to 72 months. In granuloma cases, the period from initial surgery until the appearance of staple line nodules also varied and showed no constant tendency. In previous reports, the period until granuloma detection also varied from 8 to 144 months, similarly to the present results. If a nodule appears on the staple line 2 years or more after resection of metastatic lung cancer, then the possibility of granuloma would appear to be high. However, after primary lung cancer surgery, it seems difficult to distinguish between cancer recurrence and granuloma based purely on the period from initial surgery to the appearance of a nodule around the staple line.
Limitation
This study was limited in view of its retrospective design, small size, and the fact it was performed at a single institution. It is stated that the tumor has been removed with a sufficient margin in the operation record of initial surgery, but the exact distance from the tumor to the resected end at initial surgery is unknown. Detailed pathological examination of tumor at initial surgery was not possible. A patient selection bias may have existed and a further large-scale study will be necessary to investigate in detail the clinical characteristics of granuloma and cancer recurrence around the staple line. However, because staple line granulomas are expected to increase in the future, they seem to become a clinically important problem in the future.
Conclusion
When growing shadows are observed around the staple line, it seemed difficult to differentiate between cancer recurrence and granuloma on the basis of chest CT/PET and laboratory findings alone. Although definite diagnosis of staple line granuloma and cancer recurrence can only be obtained by histological examination after biopsy or surgical excision, if a mass shadow around the staple line appeared after segmentectomy, the mass is considered likely to be a granuloma. Segmentectomy as initial surgery and mycobacterial infection are thought to be important factors affecting the occurrence of staple line granuloma.
Compliance with ethical standards
The need for subsequent individual consent from patients whose records were evaluated was waived because the individuals were not identified in this study.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tanaka H, Iuchi K, Matsumura A, et al. Pulmonary tuberculosis post staple-segmentectomy for lung cancer. Jpn J Chest Surg. 2003;17:86–89. doi: 10.2995/jacsurg.17.794. [DOI] [Google Scholar]
- 2.Tomita M, Matsuzaki Y, Edagawa M, Shimizu T, Hara M, Onitsuka T. Pulmonary granuloma possibly caused by staples after video-assisted thoracoscopic surgery. Ann Thorac Cardiovasc Surg. 2003;9:123–125. [PubMed] [Google Scholar]
- 3.Kono Y, Endo S, Otani S, et al. Non-tuberculous mycobacterial infection along the staple-suture line after segmentectomy for small peripheral lung cancer; report of a case. Kyobu Geka. 2005;58:165–168. [PubMed] [Google Scholar]
- 4.Furukawa M, Ikeda H, Takeo M, Yamamoto M. Pulmonary granuloma associated with nontuberculous mycobacteriosis occurring in the staple used for operation of lung cancer. Jpn J Chest Surg. 2007;21:942–945. doi: 10.2995/jacsurg.21.942. [DOI] [Google Scholar]
- 5.Matsuoka T, Sugi K, Matsuda E, Okabe K, Hirazawa K, Azuma T. Mycobacterium avium complex (MAC) infection needed differential diagnosis of the recurrence after surgery for double lung cancer; report of a case. Kyobu Geka. 2007;60:1200–3. [PubMed]
- 6.Sawada T, Watanabe Y, Oura H, Handa M. Pulmonary staple granuloma mimicking lung cancer; report of a case. Kyobu Geka. 2008;61:591–594. [PubMed] [Google Scholar]
- 7.Ohtsuka H, Kanzaki M, Kikkawa T, Obara T, Ishizawa M. A case of pulmonary granuloma occurring along the staple. Jpn J Chest Dis. 2008;67:977–980. [Google Scholar]
- 8.Eguchi T, Kurai M, Kato K, et al. A case of non-tuberculous mycobacterial infection along the pulmonary suture line after partial resction for aspergilloma. Jpn J Chest Surg. 2008;22:35–38. doi: 10.2995/jacsurg.22.035. [DOI] [Google Scholar]
- 9.Murakami S, Saito H, Tsuboi M, Nakayama H, Kameda Y, Yamada K. Mycobacterial granuloma on the staple-line eight years after middle lobectomy for adenocarcinoma of the lung. Jpn J Lung Cancer. 2009;49:1038–42.
- 10.Tempaku H, Takao M, Suzuki H, Shimamoto A, Hideto S. Pulmonary foreign body granuloma on the staple line five years after wedge resection for metastatic lung cancer. Jpn J Chest Surg. 2012;26:52–5.
- 11.Motono N, Okada A. Togashi K. A foreign body granuloma on the staple-line after pulmonary resection for metastatic renal cell carcinoma. Jpn J Lung Cancer. 2012;52:23–36. doi: 10.2482/haigan.52.23. [DOI] [Google Scholar]
- 12.Yoshino M, Sekine Y, Koh E, Hata A, Katsura H, Hiroshima K.. Pulmonary granuloma associated with non-tuberculous mycobacteriosis occurring at the staple line after segmentectomy for lung cancer: report of a case. Jpn J Lung Cancer. 2014;54:790–4.
- 13.Yoshida S, Matsumoto I, Saito D, Takata M, Tamura M, Takemura H. A case of pulmonary stapler granuloma suspected to be lung cancer. Jpn J Chest Surg. 2015;29:452–455. doi: 10.2995/jacsurg.29.452. [DOI] [Google Scholar]
- 14.Kamata T, Watanabe S, Sakurai H, Nakagawa K. Two cases of pulmonary granuloma difficult to differentially diagnosis from resection stump recurrence of lung cancer. Jpn J Chest Surg. 2015;29:700–705. doi: 10.2995/jacsurg.29.700. [DOI] [Google Scholar]
- 15.Miyauchi S, Maki Y, Ueno T, Sugimoto R, Yamashita M, Takahata H.Two cases of inflammatory tumor and nontuberculous mycobacteriosis around the staple line after lung cancer surgery. Jpn J Chest Surg. 2017;31:598–603.
- 16.Hashimoto S, Yamasaki N, Doi R, et al. Granuloma by foreign body reaction to the stapler used for partial resection of the lung. Kyobu Geka. 2017;70:187–190. [PubMed] [Google Scholar]
- 17.Mizuno K, Ohde Y, Hayashi S, et al. Clinical differentiations in stump granuloma and stump recurrence after lung resection for malignancy using a stapler. Jpn J Lung Cancer. 2017;57:826–31.
- 18.Masuda M, Okumura M, Doki Y, et al. Thoracic and cardiovascular surgery in Japan during 2014 :Annual report by The Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg. 2016;64:665–97. [DOI] [PMC free article] [PubMed]
- 19.Hanaoka T, Kurai M, Okada M ,et al. Pulmonary adenocarcinoma possibly developed from the cut-end of small-sized adenocarcinoma in the lung periphery as recurrence 13 years after its wedge resection. Surg Case Rep. 2018;4:2. 10.1186/s40792-017-0413-0. [DOI] [PMC free article] [PubMed]
- 20.Yoshida J, Ishii G, Yokose T, et al. Possible delayed cut-end recurrence after limited resection for groundglass opacity adenocarcinoma, intraoperatively diagnosed as Noguchi type B, in three patients. J Thorac Oncol. 2010;5:546–50. [DOI] [PubMed]


