Abstract
Background
The present retrospective study was designed to review the clinicopathological features and outcome of surgical treatment of pulmonary hamartoma who underwent surgical operation between January 2008 and January 2018.
Methods
The information about the age and gender of patients, symptoms, history of tobacco consumption, the presence of malignancies, radiological and imaging findings, calcification in the hamartoma, location and size of the lesions, findings of preoperative diagnostic investigations, operative procedures, operative time, tube drainage duration, surgical complication, hospital stay after tumor resection, duration of follow-up, and outcome were recorded.
Results
The average size of the neoplasms was 2.72 cm. Five patients (20.8%) had malignancies, which occurred previously in two patients, and concomitantly in three patients. Twenty-four patients underwent surgical treatment which included enucleation in 14 (four cases had thoracoscopic surgery), wedge resection in 8 (six cases had thoracoscopic surgery), and lobectomy in 2 patients. A total of four postoperative complications were noted. The patients were followed up for 2–98 months.
Conclusion
Enucleation was the main choice in our series. The follow-up for a long period revealed no malignant transformation and recurrence. Due to lack of the malignance after operation in our series, we presumed that the enucleation for pulmonary hamartoma was safe enough.
Keywords: Hamartoma, Treatment, Outcome
Introduction
Pulmonary hamartomas, the most common benign tumor of the lung, are usually an incidental finding and range in size between 1 and 5 cm. The incidence of pulmonary hamartoma is 0.25% with a two- to fourfold male predominance. Most are found in the sixth decade. Clinical presentation is usually asymptomatic solitary nodule on the routine chest X-ray [1–3]. They are usually peripheral parenchymal lesions. Between January 2008 and January 2018, 24 patients with pulmonary hamartoma underwent surgical operation at our hospital. All patients were pathologically diagnosed. The present paper aimed to summarize the clinicopathological features and the long-term follow-up results of surgical treatment of pulmonary hamartoma.
Materials and methods
From January 2008 to January 2018, 24 consecutive patients with pulmonary hamartoma underwent surgical resection in the Department of Thoracic Surgery, Konya Training and Research Hospital. Medical records and the hospital electronic database were reviewed for each patient. The information about the age and gender of patients, symptoms, history of tobacco consumption, the presence of malignancies, radiological and imaging findings, calcification in the hamartoma, location and size of the lesions, findings of preoperative diagnostic investigations, operative procedures, operative time, tube drainage duration, surgical complication, hospital stay after tumor resection, duration of follow-up, and outcome were recorded. Intraoperative frozen section examination was performed in some of the patients, and the histological diagnosis in other patients was obtained postoperatively from the resected specimen. All patients had clinical follow-up by the same team of surgeons. Stable clinical outcome was also confirmed by telephoning the patients.
Results
In our series, 15 (69.2%) patients were male and the sex ratio (male/female) was 1.66. The mean age was 57 years (range, 24–74 years). Fourteen of the patients (58.3%) were between 50 and 70 years, and only one patient (4.1%) was under 30 (24 years old). With respect to smoking, 18 patients (75%) were smokers (mean, 20.5 pack-years), and six (25%) had never smoked.
One patient had accepted adrenalectomy before admission, one had the history of thoracic blunt injury, and one had multiple renal cysts. Five patients (20.8%) had malignancies, which occurred previously in two patients, and concomitantly in three patients. The patients with previous malignancies were smokers. Two patients were asymptomatic. Radiologically, two patients had solitary pulmonary nodules with no findings associated with malignancy. Fluorodeoxyglucose positron emission tomography-computed tomography (FDG PET-CT) was performed in two patients. In the patient with larynx carcinoma, the maximum standard uptake value (SUV max) was 7.4 for the malignant lesion and ametabolic for the pulmonary hamartoma. In the other patient with timoma, the SUV max was 5.8 for the malignant lesion and 2.4 for the pulmonary hamartoma.
Of the three patients with concomitant malignancies, two were males and one was female. Three patients were smokers, with a mean duration of 47.1 pack-years (range, 30–55 pack-years). Malignancies originated from the lung in three patients. Coexistence of a hamartoma and malignancy was not diagnosed preoperatively in three patients. Malignancies were diagnosed before surgery and hamartomas after surgery in three patients. Pulmonary hamartomas and malignancies were located in the ipsilateral lung in three patients and in the same lobe in two patients. FDG PET-CT was performed in three patients, all of whom had ametabolic SUV max for hamartomas. The SUV max for malignancies ranged from 3.4 to 25.5, with a mean value of 12.4.
Chest X-ray examination was performed preoperatively in all cases, the most common manifestation of chest roentgenogram was an irregular high-density margin with small lobulations located peripherally. Calcification formation was found in nine cases (37.5%), only three of them (12.5%) were the typical popcorn calcification, and the remaining six (24.5%) had patching calcification on chest roentgenogram (Table 1, Fig. 1). The mean tumor diameter was 2.72 cm, ranging between 1.5 and 5.4 cm. The edges of the 17 cases were rough and the rest were smooth. Preoperatively, eight patients were considered hamartoma, 16 patients were considered solitary pulmonary nodules. The diagnosis of pulmonary hamartomas was established via bronchoscopic biopsies in two patients, computed tomography-guided transthoracic fine-needle aspiration in six patients, and surgical biopsies in 16 patients (Figs. 2 and 3).
Table 1.
Clinical and surgical date
| n (%) | ||
|---|---|---|
| Symptoms | Symptom-free | 7 (29) |
| Cough | 7 (29) | |
| Chest pain | 6 (24) | |
| Dyspnea | 4 (16) | |
| Hemoptysis | 4 (16) | |
| Fever | 3 (12) | |
| Sputum production | 2 (8) | |
| Constitutional symptoms | 7 (29) | |
| Radiological appearance | Calcification formation | 9 (37) |
| Popcorn calcification | 3 (12) | |
| Patching calcification | 6 (24) | |
| Fat density | 7 (29) | |
| Localization | Right upper lobe | 5 (20) |
| Right middle lobe | 1 (4) | |
| Right lower lobe | 11 (45) | |
| Left upper lobe | 4 (16) | |
| Left lower lobe | 3 (12) | |
| Surgical treatment | Enucleation (thoracotomy) | 10 (41) |
| Enucleation (thoracoscopy) | 4 (16) | |
| Wedge resection (thoracotomy) | 2 (8) | |
| Wedge resection (thoracoscopy) | 4 (16) | |
| Lobectomy | 2 (8) |
Fig. 1.
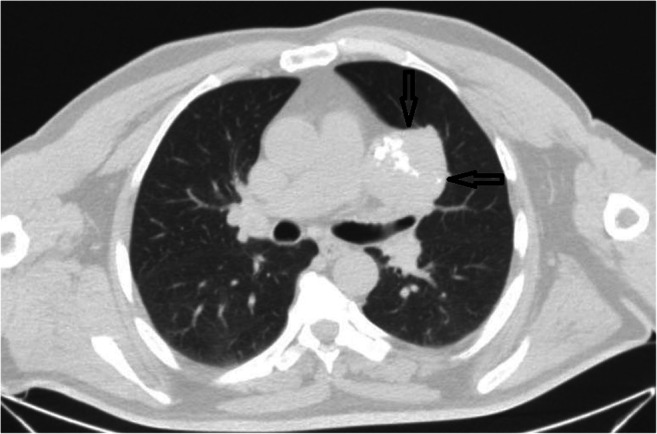
Thorax computed tomography revealed a large inhomogeneous density mass with varying degrees of calcification
Fig. 2.
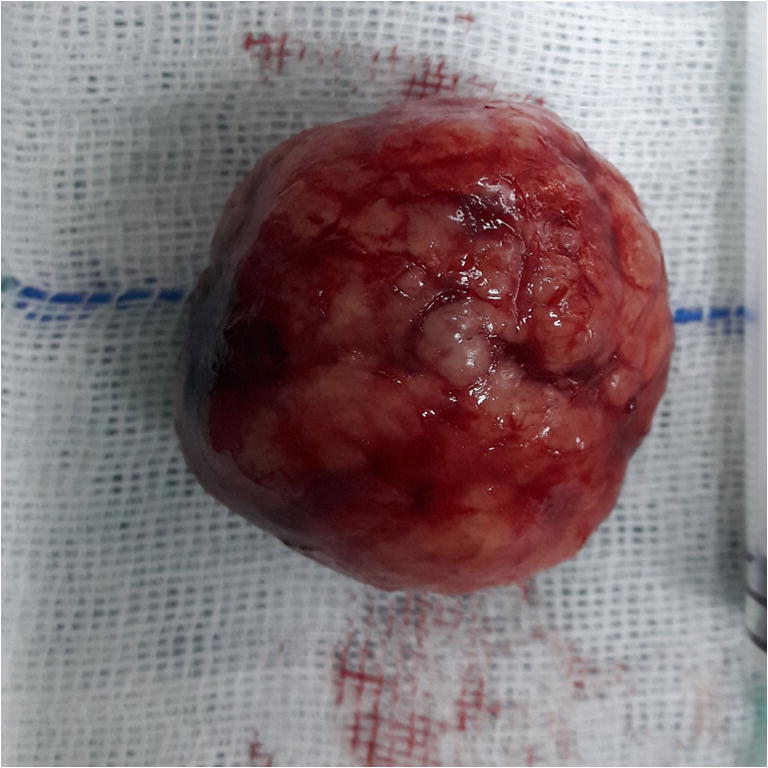
Gross appearance of the specimen showing lobulated solid mass
Fig. 3.
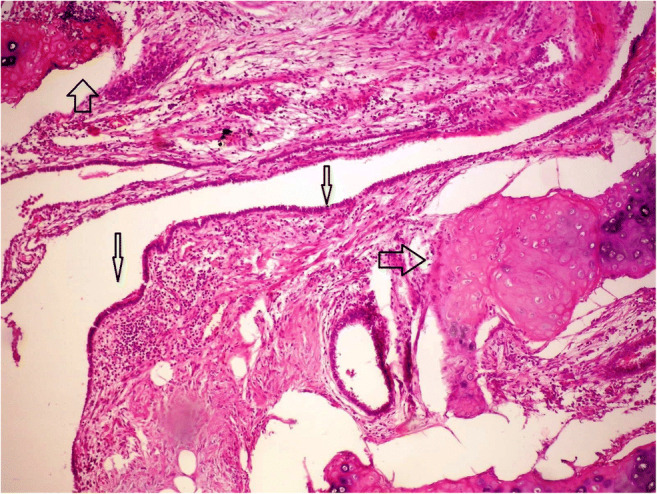
Hematoxylin and eosin staining of chondromatous hamartoma (magnification, × 100)
No mortality occurred during surgery. The operative duration ranged between 45 and 185 min (mean, 86.25 ± 22 min). No patient required a blood transfusion. All patients, with the exception of four patients, had an uneventful postoperative course (83.3%). Of the four complications, two were postoperative encapsulated pleural effusion and one was pulmonary infection. These three patients recovered well following percutaneous catheter drainage by CT-guided and anti-infection therapy. Postoperative pulmonary atelectasis occurred in one patient at the operation day, and the blood coagulum obstructing the left main bronchus was found by emergent bronchofibroscope examination. The drainage duration ranged between 2 and 7 days (mean, 3.5 ± 1.8 days) and the postoperative hospital duration ranged between 3 and 11 days (mean, 4.7 ± 2.3 days). The mean follow-up was 29.3 months (range, 2–98 months). During follow-up, 21 patients were alive without recurrence, one patient succumbed to larynx cancer after 12 months post-surgery, and two patients succumbed to lung cancer after 26 and 36 months post-surgery (Table 2).
Table 2.
Clinical date and follow up
| Patient no. | Age (years) | Size (mm) | Tumor | Diagnosis | Treatment | Follow-up (month) | Survival |
|---|---|---|---|---|---|---|---|
| 1 | 58 | 34 | No | Frozen | Enucleation | 24 | Alive |
| 2 | 63 | 22 | No | Fine-needle aspiration | Wedge resection | 15 | Alive |
| 3 | 66 | 20 | Larynx carcinoma | Frozen | Enucleation | 12 | Dead |
| 4 | 62 | 17 | No | Fine-needle aspiration | Wedge resection | 24 | Alive |
| 5 | 68 | 30 | No | Fine-needle aspiration | Wedge resection | 66 | Alive |
| 6 | 70 | 24 | No | Frozen | Enucleation | 45 | Alive |
| 7 | 56 | 28 | Lung squamous carcinoma | Frozen | Enucleation | 36 | Alive |
| 8 | 24 | 28 | No | Fine-needle aspiration | Wedge resection | 24 | Alive |
| 9 | 68 | 15 | Timoma | Frozen | Enucleation | 24 | Alive |
| 10 | 50 | 39 | No | Frozen | Enucleation | 35 | Alive |
| 11 | 68 | 23 | No | Frozen | Enucleation | 24 | Alive |
| 12 | 74 | 29 | No | Frozen | Enucleation | 42 | Alive |
| 13 | 63 | 23 | Lung squamous carcinoma | Frozen | Lobectomy | 18 | Alive |
| 14 | 24 | 25 | No | Bronchoscopy | Enucleation | 45 | Alive |
| 15 | 74 | 26 | No | Frozen | Enucleation | 98 | Alive |
| 16 | 65 | 19 | Lung adenocarcinoma | Frozen | Wedge resection | 26 | Dead |
| 17 | 30 | 24 | No | Fine-needle aspiration | Wedge resection | 18 | Alive |
| 18 | 64 | 54 | No | Frozen | Wedge resection | 24 | Alive |
| 19 | 59 | 28 | No | Frozen | Enucleation | 5 | Alive |
| 20 | 66 | 32 | No | Frozen | Enucleation | 2 | Alive |
| 21 | 57 | 22 | No | Frozen | Enucleation | 48 | Alive |
| 22 | 46 | 30 | No | Bronchoscopy | Lobectomy | 36 | Alive |
| 23 | 58 | 36 | No | Frozen | Enucleation | 12 | Alive |
| 24 | 65 | 25 | No | Fine-needle aspiration | Wedge resection | 2 | Alive |
Discussion
Pulmonary hamartomas are tumor-like malformations resulting from abnormal mixing or abnormal development of normal tissue components in the organ where they occur [4]. Pulmonary hamartomas constitute 5–8% of all solitary lung tumors, account for 75% of all benign lung tumors, and are primarily (90%) solitary [5]. Endobronchial hamartoma was reported in approximately 3 to 19.5% cases [3, 6–8] but has not been found in our series. Pulmonary hamartoma may occur in each pulmonary lobe, particularly the right lower lobe. Of the 24 patients, 11 exhibited pulmonary hamartoma located in the right lower lobe and seven were located in the left lung, also inconsistent with the previous literature. Pulmonary hamartomas usually range in size between 1 and 5 cm. In the present study, the average diameter was 2.72 cm (1.5–5.4 cm).
Hamartomas are usually solitary, well-demarcated nodules on roentgenogram. Multiple hamartomas are a rare entity and only 19 cases have been described in the world [9–11]. Pulmonary hamartomas are predominantly composed of cartilage; other components include fibromyxoid connective tissue, fat, bone, and smooth muscle [1]. Intranodular fat (attenuation, − 40 to − 120 HU) and popcorn-like chondroid calcifications are reliable indicators of hamartomas. However, some hamartomas contain neither fat nor calcification. Siegelman et al. reported that fat is only observed in approximately 34% of hamartomas and fat with calcification is present in approximately 21% of hamartomas [12]. In the present study, calcification formation was found in nine cases (37.5%), only three of them (12.5%) were the typical popcorn calcification, and the remaining six (24.5%) had patching calcification on chest roentgenogram. The image of fat density was found in seven cases (29.1%).
There have been several reports insisting on an increased risk to lung cancer in patients with a chondromatous hamartoma. Karasik et al. [13] reported that the risk to lung cancer in patients with a chondromatous hamartoma of the lung was estimated to be 6.3 times higher than the age-sex-ethnic adjusted rate expected for the general population. Ribet et al. [11] showed that the risk to bronchial cancer developing in patients with hamartoma was multiplied by 6.6 compared with that of the general population. Van den Bosch et al. [1] found bronchial carcinomas in 11 of 154 patients with pulmonary hamartomas, synchronousin six, and metachronous in five. Some reports suggested an etiologic association between hamartomas and malignancies [13]. Smoking and environmental carcinogenic factors have also been implicated as important risk factors for the development of lung carcinoma in patients with pulmonary hamartomas [2, 11]. We found concomitant lung malignancies in three patients (12.5%). Three patients were smokers, with a mean duration of 47.1 pack-years (range, 30–55 pack-years). It has been recommended that patients with hamartoma should be evaluated and closely followed up with respect to the risk for associated malignancies.
Because the symptoms of patients with pulmonary hamartoma were frequently indistinguishable from lung carcinoma or benign pulmonary diseases such as tuberculosis, differential diagnosis was necessary for diagnosis of pulmonary hamartoma. Patients with a previous history of tuberculosis were usually considered to have pulmonary tuberculoma [14]. Diagnosis can be achieved by a combination of radiological evidence and fine-needle biopsy. Radiologically, it is sometimes difficult to differentiate a pulmonary hamartoma from malignant nodules. Aspirates of pulmonary hamartoma were often scanty because of the dense nature of the lesion. The diagnostic accuracy has been shown to be less for pulmonary hamartoma than for malignant tumors, partly because of the difficulty in obtaining diagnostic material from cartilaginous lesions and partly because of difficulty in the interpretation of the findings [15–17]. In the present study, the diagnosis of pulmonary hamartomas was established via bronchoscopic biopsies in two patients, computed tomography-guided transthoracic fine-needle aspiration in six patients, and surgical biopsies in 16 patients. If we cannot rule out malignant causes and patients agree to undergo resection, we can remove the mass for diagnosis and intent to cure.
Today, even with the advancements in medical therapy, pulmonary resection remains the most important measure of treatment for patient with hamartoma of the lung [14]. Surgical treatment is the gold standard in intraparenchymal hamartomas including enucleation, wedge resection, segmentectomy, lobectomy or sleeve resection, and pneumonectomy. Lien et al. [18] conducted a retrospective study over a 31-year period on 61 patients who had resection of their pulmonary hamartoma. There were no operative-related deaths and a mean follow-up period of 8.9 years revealed no tumor recurrence. The authors concluded that resection of pulmonary hamartomas was safe with good prognosis. Guo et al. [14] reviewed their experience with resection of pulmonary hamartomas over a 20-year period. Thirty-nine cases were included. Three patients needed a lobectomy and one patient needed a pneumonectomy (4/39 = 10%). There was no operative mortality and two cases developed morbidities. Wedge resection and enucleation were the main choices in our series for patients with pulmonary hamartoma. Two patients needed a lobectomy in our series. All patients, with the exception of four patients, had an uneventful postoperative course. There was postoperative encapsulated pleural effusion in two patients, pulmonary infection and atelectasis occurred in two patients.
In conclusion, pulmonary hamartoma is a rare benign tumor of the lung. It grows slowly with hidden clinical symptoms, often identified by routine medical examination. Pulmonary hamartoma can be easily misdiagnosed as a tuberculosis tumor and peripheral lung cancer. If we cannot rule out malignant causes and patients agree to undergo resection, we can remove the mass for diagnosis and intent to cure. With the advantages of less trauma and faster recovery, thoracoscopy or minithoracotomy was used as the preferred treatment. Enucleation was the main choice in our series. The follow-up for a long period revealed no malignant transformation and recurrence. Due to lack of the malignance after operation in our series, we presumed that the enucleation was safe enough.
Compliance with ethical standards
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Van den Bosch J, Wagenaar S, Corrin B, Elbers JR, Knaepen PJ, Westermann CJ. Mesenchymoma of the lung (so-called hamartoma): a review of 154 parenchymal and endobronchial cases. Thorax. 1987;42:790–3. [DOI] [PMC free article] [PubMed]
- 2.Gjevre JA, Myers JL, Prakash UB. Pulmonary hamartomas. Mayo Cline Proc. 1996;71:14–20. [DOI] [PubMed]
- 3.Tomashefski JF., Jr Benign endobronchial mesenchymal tumors: their relationship to parenchymal pulmonary hamartomas. Am J Surg Pathol. 1982;6:531–540. doi: 10.1097/00000478-198209000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Itoga M, Kobayashi Y, Takeda M, et al. A case of pulmonary hamartoma showing rapid growth. Case Rep Med. 2013;2013:231652. 10.1155/2013/231652. [DOI] [PMC free article] [PubMed]
- 5.Lu Z, Qian F, Chen S, Yu G. Pulmonary hamartoma resembling multiple metastases: a case report. Oncol Lett. 2014;7:1885–1888. doi: 10.3892/ol.2014.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hansen CP, Holtveg H, Francis D, Rasch L, Bertelsen S. Pulmonary hamartoma. J Thorac Cardiovasc Surg. 1992;104:674–678. [PubMed] [Google Scholar]
- 7.Bateson EM. Relationship between intrapulmonary and endobronchial cartilage-containing tumors (so-called hamartoma) Thorax. 1965;20:447–461. doi: 10.1136/thx.20.5.447. [DOI] [Google Scholar]
- 8.Bergh NP, Hafstrom LO, Scherson T. Hamartoma of the lung: with special reference to the endobronchial localization. Scand J Respir Dis. 1967;48:201–207. [PubMed] [Google Scholar]
- 9.Kang MW, Han JH, Yu JH, et al. Multiple central endobronchial chondroid hamartoma. Ann Thorac Surg. 2007;83:691–693. doi: 10.1016/j.athoracsur.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 10.Minami Y, Iijima T, Yamamoto T, et al. Diffuse pulmonary hamartoma: a case report. Pathol Res Pract. 2005;200:813–816. doi: 10.1016/j.prp.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 11.Ribet M, Jaillard-Thery S, Nuttens MC. Pulmonary hamartoma and malignancy. J Thorac Cardiovasc Surg. 1994;107:611–614. [PubMed] [Google Scholar]
- 12.Siegelman SS, Khouri NF, Scott WW, Jr, et al. Pulmonary hamartoma: CT findings. Radiology. 1986;160:313–317. doi: 10.1148/radiology.160.2.3726106. [DOI] [PubMed] [Google Scholar]
- 13.Karasik A, Modan M, Jacob CO, Lieberman Y. Increased risk of lung cancer in patient with chondromatous hamartoma. J Thorac Cardiovasc Surg. 1980;80:217–220. [PubMed] [Google Scholar]
- 14.Guo W, Zhao YP, Jiang YG, Wang RW, Ma Z. Surgical treatment and outcome of pulmonary hamartoma: a retrospective study of 20-year experience. J Exp Clin Cancer Res. 2008;27:8. 10.1186/1756-9966-27-8. [DOI] [PMC free article] [PubMed]
- 15.Hamper UM, Khouri NF, Stitik FP, Siegelman SS. Pulmonary hamartoma: diagnosis by transthoracic needle-aspiration biopsy. Radiology. 1985;155:15–18. doi: 10.1148/radiology.155.1.3975394. [DOI] [PubMed] [Google Scholar]
- 16.Dunbar F, Leiman G. The aspiration cytology of pulmonary hamartomas. Diagn Cytopathol. 1989;5:174–180. doi: 10.1002/dc.2840050212. [DOI] [PubMed] [Google Scholar]
- 17.Hummel P, Cangiarella JF, Cohen JM, Yang G, Waisman J, Chhieng DC. Transthoracic fine-needle aspiration biopsy of pulmonary spindle cell and mesenchymal lesions: a study of 61 cases. Cancer. 2001;93:187–198. doi: 10.1002/cncr.9028. [DOI] [PubMed] [Google Scholar]
- 18.Lien YC, Hsu HS, Li WY, et al. Pulmonary hamartoma. J Chin Med Assoc. 2004;67:21–26. [PubMed] [Google Scholar]


