Abstract
The frozen elephant trunk might be considered one of the most appreciated innovations during the last decades to treat complex thoracic aortic lesions. Many useful tips have been progressively introduced to standardize the procedures and, nowadays, the improved post-operative outcomes contributed to spread the procedure. The following article will provide a comprehensive review analysis of the Frozen Elephant Trunk (FET) technique, throughout the current available devices, possible surgical indications and primary post-operative complications.
Keywords: Frozen elephant trunk, Frozen, Aortic arch
Introduction
Patients with complex and extensive lesions of the entire thoracic aorta are usually treated with different surgical and hybrid procedures, in single or double-step approach. Until the early 2000s, the combined pathologies of the arch and of the descending thoracic aorta were mainly treated by a two-stage approach, the Elephant Trunk technique (ET) described in 1983 by Borst [1]. Despite the excellent results obtained with this technique, its main limitation is represented by the fact that more than half of the patients did not arrive at the second surgical step, either because they died between the first and the second step or because some patients refused another surgical operation [2, 3]. With the advent of transfemoral stent-graft for the treatment of descending thoracic aorta aneurysms, it became possible to perform the second step of ET through a combined endovascular approach avoiding the second surgical step. In 1996, Suto and Kato, in order to treat combined lesions of the thoracic aorta during a single-stage procedure, introduced a new technique: they placed a stent-graft in the descending thoracic aorta in antegrade fashion directly from the arch. This new procedure was called “open stent-grafting technique” [4, 5]. In 2003, the technique introduced by the Japanese surgeons was modified by the group of Hannover using a custom-made hybrid prosthesis and the procedure was re-named FET. The hybrid prosthesis consisted in a distal endovascular stent graft and a proximal conventional surgical [6]. The FET technique can be used to treat a variety of acute and chronic pathologies of the thoracic aorta. Main indications are chronic aneurysms involving the distal part of the aortic arch and the upper part of the descending aorta, as well as acute type A aortic dissection whose primary entry tear is localized in the aortic arch or in the proximal part of the descending aorta, or type B aortic dissection when thoracic endovascular aortic repair (TEVAR) is not anatomically feasible and when there is a coexisting ascending and/or arch aneurysm, or in case of retrograde dissection.
The aim of this review is to examine the state of the art in the use of the FET procedure in terms of surgical indications, early results, and long-term follow-up.
Methods
Literature search criteria
Selection of literature articles was performed using PubMed databases from inception to October 2018, using “frozen elephant trunk” OR “stented elephant trunk” OR “antegrade stenting of the descending thoracic aorta” as either keywords or MeSH terms. Case reports, editorial, and expert opinion or recommendation types of publication were excluded as well as review articles because of potential doubling of results. Among series coming from the same group, only the most recent ones were considered.
Objectives
Primary endpoints included description of the devices and technique adopted to perform the FET, in-hospital death, and occurrence of paraplegia as early outcomes and FU analysis including survival and freedom from aortic reintervention (including the type of second stage operation).
Current available devices
In Europe, at the moment, there are two available grafts for the FET procedure that obtained the Conformite Europeene (CE) mark. The E-vita Open Plus (Jotec GmbH, Hechingen, Germany) was the first commercially available hybrid prosthesis and it is composed of a proximal part consisting of vascular prosthesis and a distal part of self-expandable nitinol stent graft. In 2012, a new kind of hybrid prosthesis was introduced by Vascutek, the Thoraflex hybrid device (Vascutek, Terumo, Inchinnan, Scotland, UK). The proximal part consists in a quadruple-branched vascular prosthesis and the distal part is a self-expandable nitinol stent graft with a different stent shape. The multi-branched portion allows to perform the individual arch vessel reimplantation and to restart the perfusion of the lower part of the body, through the fourth branch, once the distal anastomosis is completed. Both prosthesis have a sewing collar to facilitate distal anastomosis and are available in different diameter (28–40 mm for the Thoraflex and 20–40 mm for the Evita Open Plus) and length (100 or 150 mm for the Thoraflex and 130,150, or 160 mm for the Evita Open Plus).
Outside Europe instead, Sun from Beijing designed in 2003 a different hybrid device, the Cronus open stented graft (MircoPort, Shanghai, China), consisting of a regular Dacron vascular graft and interconnected Z-shaped stents made from a specific metal alloy. At the proximal and distal ends, there is an extra centimeter of Dacron sewing cuff. The Cronus always requires an extravascular graft, straight, or branched, to complete the reconstruction of the aortic arch [7]. More recently (July 2014), a new device was developed in Japan, the Frozenix J Graft Open Stent graft (Japan Lifeline Co. Ltd., Tokyo, Japan). It consists of a distal-stented part that is made of a polyester tube with oval shaped nitinol stent, and a proximal unstented graft. Before deployment, the stented portion is bent to conform to the curvature of the aorta in order to facilitate the insertion of the device. After deployment and distal anastomosis, the arch vessels are reconstructed with a separate branched graft in an end-to-end fashion [8].
Alternatives techniques with assembled stent graft have been also described. In 2009, Desai and Pochettino described the “Penn technique” for hybrid hemi-arch replacement with antegrade deployment of a standard 10 to 15-cm GORE-TAG thoracic aortic stent-graft (W.L. Gore and Associates, Flagstaff, AZ) [9]. Once the stent-graft is deployed and balloon-dilated into the descending thoracic aorta under direct vision, it is fixed to the native aorta. The hemi-arch anastomosis is then completed in a standard fashion with the TAG stent-graft incorporated into the arch suture line. In 2013, a slight modification of the “Penn technique” was proposed by Roselli et al. [10]. In this case, the stent GORE-TAG (W.L. Gore and Associates, Flagstaff, AZ) is released more proximally into the arch. Two of the flares on the proximal stent-graft are resected in order to create a “fenestration” around the supra-aortic vessels. The stent-graft at the base of the fenestration is sutured to the base of the branch artery. The distal aortic hemi-arch anastomosis is then performed in the usual fashion with a standard Dacron graft including the stent graft for 220° of the circumference.
Surgical technique: “tips and tricks”
The FET technique has gained more popularity due to a more standardized approach and to the introduction of useful modifications. One of the most appreciated innovations is represented by the possibility to make a more proximal distal anastomosis in the arch. In Bologna, if anatomically feasible, we prefer to perform the distal anastomosis in arch zone 2. In particular, arch ZONE 2 is prepared as follows: the LSA is distally clamped and proximally ligated. After being detached from the arch, the LSA origin is always secured with a pledgeted U-stitch, and then the distal anastomosis is prepared using an external Teflon felt fixed with four internal pledgeted U-stitches [11].
Other useful tips are represented by a facilitated LSA reimplantation with fenestration of the grafts or with sutureless endoluminal anastomosis using Hybrid prosthesis, and also the possibility to choose among different hybrid grafts.
Here are summarized ten useful and practical recommendations for FET implantation in case of acute type A aortic dissection, chronic type A aortic dissection, aneurysmal disease of distal arch/proximal DTA, and Type B dissection:
Surgical “tips and tricks” during the hybrid prosthesis implantation in acute type A aortic dissection
Evaluation of the preoperative angio-CT scans: we strongly suggest a careful evaluation of the preoperative angio-CT scan in order to better define the entry tear and the “more proximal” and “more distal” entry tears and the relationship between the true and false lumen and the origin of visceral arteries;
Perioperative CSF drainage (spinal pressure < 12 mmHg): unnecessary;
Antegrade selective bilateral cerebral perfusion as method of cerebral protection: Strongly suggested;
Angioscopy before and immediately after the stent-graft implantation: strongly suggested;
Intraoperative use of transesophageal echo-guidance during guide-wire positioning before the device implantation: suggested but not strictly mandatory;
Restart of the systemic perfusion and “rewarming” immediately after the distal aortic arch anastomosis: strongly suggested;
Prosthesis oversizing: not recommended;
Use of branched aortic arch grafts in case of epiaortic vessels involvement: strongly suggested;
Reduction of the risk of spinal cord injury using the long stent graft trunk (≥ 150 mm) only if strictly necessary: strongly suggested;
Avoidance of hypotension (mean systemic pressure > 80 mmHg) after device implantation: strongly suggested.
Surgical “tips and tricks” during the hybrid prosthesis implantation in chronic type A aortic dissection
Evaluation of the preoperative angio-CT scans: we strongly suggest a careful evaluation of the preoperative angio-CT scan in order to better define the “more proximal” and “more distal” entry tears and the relationship between the true and false lumen and the origin of visceral arteries;
Perioperative CSF drainage (spinal pressure < 12 mmHg): strongly suggested;
Antegrade selective bilateral cerebral perfusion as method of cerebral protection: strongly suggested;
Angioscopy before and immediately after the stent-graft implantation: strongly suggested;
Intraoperative use of transesophageal echo-guidance during guide-wire positioning before the device implantation: suggested but not strictly mandatory;
Restart of the systemic perfusion and “rewarming” immediately after the distal aortic arch anastomosis: strongly suggested;
Prosthesis oversizing: not recommended;
Use of branched aortic arch grafts in case of epiaortic vessels involvement: strongly suggested;
Reduction of the risk of spinal cord injury using the long stent graft trunk (≥ 150 mm) only if strictly necessary: strongly suggested;
Avoidance of hypotension (mean systemic pressure > 80 mmHg) after device implantation: strongly suggested.
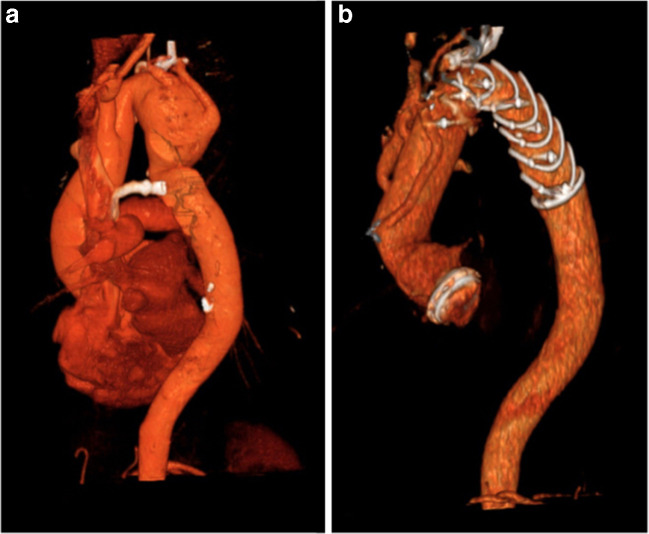
Surgical “tips and tricks” during the hybrid prosthesis implantation in aneurysmal disease of distal arch/proximal DTA (Fig. 1)
Fig. 1.
Angio-CT showing a degenerative distal aortic arch aneurysm: a before and b after FET surgery
Evaluation of the preoperative angio-CT scans: we strongly suggest a careful evaluation of the preoperative angio-CT scan in order to better define the extension of the aneurysm and the correct sizing of the hybrid prosthesis;
Perioperative CSF drainage (spinal pressure < 12 mmHg): strongly suggested;
Antegrade selective bilateral cerebral perfusion as method of cerebral protection: strongly suggested;
Angioscopy before and immediately after the stent-graft implantation: strongly suggested;
Intraoperative use of transesophageal echo-guidance during guide-wire positioning before the device implantation: unnecessary,
Restart of the systemic perfusion and “rewarming” immediately after the distal aortic arch anastomosis: strongly suggested;
Prosthesis oversizing: 10–20% oversizing is recommended;
Use of branched aortic arch grafts in case of epiaortic vessels involvement: strongly suggested;
Reduction of the risk of spinal cord injury using the long stent graft trunk (≥ 150 mm) only if strictly necessary: Strongly suggested;
Avoidance of hypotension (mean systemic pressure > 80 mmHg) after device implantation: strongly suggested.
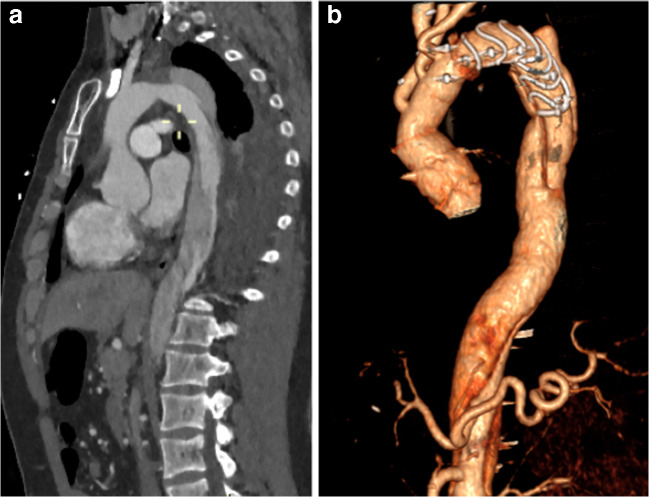
Surgical “tips and tricks” during the hybrid prosthesis implantation in type B dissection (Fig. 2)
Fig. 2.
Chronic type B aortic dissection: a before and b after FET surgery
Evaluation of the preoperative angio-CT scans: we strongly suggest a careful evaluation of the preoperative angio-CT scan in order to better define the extension of the dissection, the entry tear and the “reentries,” the relationship between the true and false lumen and the origin of the visceral arteries;
Perioperative CSF drainage (spinal pressure < 12 mmHg): unnecessary;
Antegrade selective bilateral cerebral perfusion as method of cerebral protection: strongly suggested;
Angioscopy before and immediately after the stent-graft implantation: strongly suggested;
Intraoperative use of transesophageal echo-guidance during guide-wire positioning before the device implantation: suggested but not strictly mandatory;
Restart of the systemic perfusion and “rewarming” immediately after the distal aortic arch anastomosis: strongly suggested;
Prosthesis oversizing: recommended;
Use of branched aortic arch grafts in case of epiaortic vessels involvement: strongly suggested;
Reduction of the risk of spinal cord injury using the long stent graft trunk (≥ 150 mm) only if strictly necessary: strongly suggested;
Avoidance of hypotension (mean systemic pressure > 80 mmHg) after device implantation: strongly suggested.
Results
In Table 1 are summarized retrospective studies analyzing early clinical outcomes of Frozen Elephant Trunk surgery. We selected 20 studies from 2009 to 2018 with a number of patients ranging from 14 to 251 from single centers as well as from international registries. The prosthesis used in the different reviewed series vary as we tried to represent the real world scenario. Most of the studies analyze the use of FET in type A acute aortic dissection, or at least in either aneurysms or dissections, with only one study from the Kobe group [29] focusing on aneurysms and two studies, from Beijing and from Freiburg [26, 28], on type B acute aortic dissections. The distal anastomosis was performed in arch zone 2 in almost the 65% of the series, even if there are studies in which we can observe the evolution of the surgical technique over the years with arch zone 3 anastomosis in the oldest cases and arch zone 2 anastomosis more recently. In-hospital mortality and vary from 0% in the studies on type B aortic dissections and on isolated aortic arch aneurysms to 15.3% observed by the Stuttgart group [27] in DeBakey type I aortic dissections and 17.2% reported by the Bologna group [31] in 250 cases including emergency operations and redo cases. The rate of spinal cord injury goes from 0% as assessed by different German groups [16, 27, 29] as well as by Japanese [14, 20] and Chinese [13] groups, to 9% in the study from the Pennsylvania group [12] in patients with DeBakey type I aortic dissection that is the oldest one we admitted in our review.
Table 1.
Results of the principal observational studies reporting early outcomes after FET surgery
| Author/year | Institution | Number patients | Prosthesis | Etiology | Arch zone | In-hospital mortality (%) | Paraplegia (%) |
|---|---|---|---|---|---|---|---|
| Pochettino et al. 2009 [12] | Hospital of the University of Pennsylvania (USA) | 36 | Dacron graft (Vascutec Ltd., Renfrewshire, Scotland) with Gore TAG stent (W.L. Gore and Associates, Flagstaff, AZ, USA) | DeBakey Type I dissection | 2 | 14.0 | 9 |
| Chen et al. 2010 [13] | Nanjing First Hospital (China) | 28 | Woven Dacron graft with Gianturco-type self-expandable metallic stent (Microport Medical Corp., Shanghai, China) | DeBakey Type I dissection 1 | 2 | 14.3 | 0 |
| Sun et al. 2011 [7] | Fuwai Hospital (China) | 148 | Four-branch prosthetic graft (Boston Scientific, Inc., Boston, MA) with stent-graft (Microport Medical Corp., Shanghai, China) | Type A Dissection | 2 | 4.7 | 1.4 |
| Uchida et al. 2011 [14] | Hiroshima-city General Hospital (Japan) | 80 | Polyester fabric prosthesis (UBE, Tokyo, Japan) with self-expanding Z-shaped stent | Type A Dissection | 2 | 5.0 | 0 |
| Shen et al. 2012 [15] | The Second Xiangya Hospital (China) | 22 | Four-branch graft with self-expanding stent (Yuhengjia Sci-Tech Co. Ltd., Beijing, China) | Type A Dissection | 2 | 9.1 | 4.5 |
| Hoffman et al. 2013 [16] | University Hospital RWTH (Germany) | 32 | E-vita open hybrid stent-graft (JOTEC, Hechingen, Germany) | Type A Dissection | 3 | 3.1 | 0 |
| Dohle et al. 2016 [17] | University Hospital Essen (Germany) | 70 | E-vita open hybrid stent-graft (JOTEC, Hechingen, Germany) | Acute and chronic Dissection | 3 | 10 | NA |
| Uchida et al. 2016 [18] | 10 Japanese centers | 60 | J Graft Open Stent Graft (Frozenix, Japan Life Line, Tokyo) | Aneurysm | NA | 5 | 6.7 |
| Acute e Chronic Dissection | |||||||
| Shrestha et al. 2016 [19] | Hannover Medical School, (Germany) | 100 | Thoraflex (Vascutek, Terumo, Inchinnan, Scotland, UK) | Aneurysm | NA | 7 | 7 |
| Acute e Chronic Dissection | |||||||
| Yamane et al. 2017 [20] | Hiroshima-city General Hospital (Japan) | 24 | J Graft open stent graft (Frozenix, Japan Life Line, Tokyo) | Type A Dissection | 2 | 8.3 | 0 |
| Shrestha 2017 [21] | Hannover Medical School, (Germany) | 251 | Thoraflex (Vascutek, Terumo, Inchinnan, Scotland, UK); E-vita open hybrid stent-graft (JOTEC, Hechingen, Germany); custom-made Chavan–Haverich | Aneurysm | 2 | 12 | 2 |
| Acute e Chronic Dissection Type A and B | |||||||
| Roselli et al. 2018 [22] | Cleveland Clinic (USA) | 72 | Dacron graft with GORE stent-grafts | DeBakey Type I Dissection | 2 | 4.2 | 4.2 |
| (Gore Medical, Flagstaff, AZ, USA) | |||||||
| Iafrancesco 2017 [23] | International E-vita Open Registry | 137 | E-vita open plus hybrid stent-graft (JOTEC, Hechingen, Germany | Acute and chronic Dissection | 2–3 | NA | 3.6 |
| Chu et al. 2019 [24] | 9 Canadian centers on behalf of the Canadian Thoracic Aortic Collaborative (CTAC) Investigators | 40 | Thoraflex Hybrid Prosthesis (Vascutek, Terumo, Inchinnan, Scotland, UK) | Aneurysm, Acute e Chronic Dissection | 2–3 | 5 | 5 |
| Qi et al. 2018 [25] | Beijing Institute of Heart, Lung and Blood Vessel Diseases (China) | 53 | Four-branch prosthetic graft (Boston Scientific, Inc., Boston, MA) with stent-graft (Microport Medical Corp., Shanghai, China) | Type B Acute Aortic Dissection | 2 | 0 | NA |
| Goebel et al. 2018 [26] | Robert-Bosch-Hospital, Stuttgart (Germany) | 72 | E-vita open plus hybrid stent-graft (JOTEC, Hechingen, Germany | DeBakey type I Aortic Dissection | 3 | 15.3 | 4.2 |
| Kreibich et al. 2018 [27] | Heart Centre Freiburg University (Germany) | 14 | Thoraflex Hybrid Prosthesis (Vascutek, Terumo, Inchinnan, Scotland, UK) | Type B Acute Aortic Dissection | 3 | 0 | 0 |
| Koizumi et al. 2018 [28] | Department of Cardiovascular Surgery, Kobe City Medical Center General Hospital (Japan) | 30 | J Graft open stent graft (Frozenix, Japan Life Line, Tokyo | Aortic Arch Aneurysms | 2 | 0 | 3.3 |
| Berger et al. 2018 [29] | University Heart Center Freiburg University (Germany) | 65 | Thoraflex Hybrid Prosthesis (Vascutek, Terumo, Inchinnan, Scotland, UK) | Acute and Chronic Aortic Dissection | 3 | 6 | 0 |
| Di Bartolomeo et al. 2019 [30] | S. Orsola-Malpighi Hospital, University of Bologna (Italy) | 250 | Thoraflex (Vascutek, Terumo, Inchinnan, Scotland, UK); E-vita open hybrid stent-graft (JOTEC, Hechingen, Germany); | Aneurysm | 2 | 17.2 | 4.8 |
| Acute e Chronic Dissection Type A and B |
NA not applicable
In Table 2, we grouped the data related to follow-up. Mean follow-up time goes from 10 months [28] to 5 years [7, 13, 22, 23], with a good survival rate that goes from a minimum of 75% [14, 23, 26] to a peak of 100% at 1 year [12, 27, 29]. Freedom from reoperation vary from 100% with none required second stage procedures on the descending aorta [15, 20] to 72% reoperations [22, 29] with 66.1% of them consisting in TEVAR [29]. The preferred type of second stage procedure varied extensively, ranging from the exclusive use of endovascular approaches and open surgical options to intermediate situations (both techniques were adopted). In general, there seems to be a prevalence of endovascular treatments and it can be easily understood since they are generally less invasive than the open surgical approach.
Table 2.
Results of the principal observational studies reporting follow-up outcomes after FET surgery
| Author/year | FU time | Survival (%) | Freedom from reoperation (%) | Second stage procedure on TAAA (%) | Type of procedure |
|---|---|---|---|---|---|
| Pochettino et al. 2009 [12] | 1 year | 100 | 74 | 26 | Tevar, 100 |
| Open, 0 | |||||
| Chen et al. 2010 [13] | 5 years | 87.5 | 91.7 | 3.5 | Tevar, 0 |
| Open, 100 | |||||
| Sun et al. 2011 [7] | 5 years | 97 | 99 | 0.68 | Tevar, 0 |
| Open, 100 | |||||
| Uchida et al. 2011 [14] | 10 years | 75 | 92.5 | 7.5 | Tevar, 16.7 |
| Open, 83.3 | |||||
| Shen et al. 2012 [15] | 1 year | 91 | 100 | 0 | Tevar, 0 |
| Open, 0 | |||||
| Hoffman et al. 2013 [16] | 2 year | 97 | 75 | 25 | Tevar, 100 |
| Open, 0 | |||||
| Dohle et al. 2016 [17] | 1 year | 90 | 88.5 | 11.5 | Tevar, 60.9 |
| Open, 39.1 | |||||
| Uchida et al. 2016 [18] | 3 years | 76.7 | 80 | 20 | Tevar, 83.4 |
| Open, 16.6 | |||||
| Shrestha et al. 2016 [19] | 3 years | 81 | 78 | 22 | Tevar, 59 |
| Open, 41 | |||||
| Yamane et al. 2017 [20] | 1 years | 91.6 | 100 | 0 | Tevar, 0 |
| Open, 0 | |||||
| Shrestha et al. 2017 [21] | 3 years | 75 | 80.5 | 19.5 | Tevar, 48.7 |
| Open, 51.3 | |||||
| Roselli et al. 2018 [22] | 5 years | 80 | 72 | 10 | Tevar, 100 |
| Open, 0 | |||||
| Iafrancesco 2017 [23] | 5 years | 93 | 83.2 | 16.8 | Tevar, 69.6 |
| Open, 30.4 | |||||
| Chu et al. 2019 [24] | 2 years | 90 | 85 | 2.5 | Tevar, 100 |
| Open, 0 | |||||
| Qi et al. 2018 [25] | 4 years | 98 | 96 | 2 | Tevar, 0 |
| Open, 100 | |||||
| Goebel et al. 2018 [26] | 4 years | 75 | 96.7 | 3.3 | Tevar, 0 |
| Open, 100 | |||||
| Kreibich et al. 2018 [27] | 1 years | 100 | 78.5 | 21.5 | Tevar, 100 |
| Open, 0 | |||||
| Koizumi et al. 2018 [28] | 10 months | 93 | 87 | 0 | Tevar, 0 |
| Open, 0 | |||||
| Berger et al. 2018 [29] | 1 year | 100 | 72 | 28 | Tevar, 66.1 |
| Open, 33.9 | |||||
| Di Bartolomeo et al. 2019 [30] | NA | NA | 81.6 | 18.4 | Tevar, 82.2 |
| Open, 17.8 |
TAAA thoracoabdomina laortic aneurysm false lumen, NA not applicable
The Hannover group [21] is the one with the biggest number of patients, having analyzed 251 patients who, from 2001 to 2016, underwent total aortic arch replacements with three different FET prostheses: the custom-made Chavan–Haverich (2001–2007), the Jotec E-vita (2007–2010) and the Vascutek Thoraflex (2010–2016) hybrid prostheses. Of the 251 patients, 82 had aortic aneurysms, 96 acute aortic dissection type A, 4 acute type B dissections, 52 chronic aortic dissection type A, and 17 chronic type B dissection. The incidence of rethoracotomy for bleeding, stroke, spinal cord injury, prolonged ventilatory support (> 96 h), and long-term dialysis were 18, 14, 2, 24, and 2%, respectively. The in-hospital mortality rate was 11% (in acute aortic dissection type A, 12%). There were 49-s-stage procedures in the downstream aorta: either open surgical [n = 25 (thoraco-abdominal, n = 15; descending, n = 6; infrarenal (n = 4)] or transfemoral endovascular (n = 23).
The Bologna experience [30] is equally important, with 250 patients from 2007 who underwent extensive thoracic aorta surgery using the FET approach with an E-vita open (n = 164) or the Thoraflex Hybrid (n = 86) prosthesis. The most frequent indications for surgery included residual type A chronic dissection (40%), extensive degenerative aneurysm of the thoracic aorta (27%), acute type A (12%) and type B (4%) aortic dissection, and chronic type A (5%) and type B aortic dissection (12%). They reported a rate of spinal cord deficits of 4.8% that was observed more frequently at the beginning of the experience with the use of the E-vita prosthesis. At the moment, with the use of Thoraflex-branched graft paraplegia was never observed. At follow-up, they observed that 46 patients (18.4%) necessitated a secondary intervention. Of them, 82.2% were endovascular procedures for distal aneurysms extension or to treat endoleaks; the remaining 17.8% were open thoracoabdominal procedures.
In the study from the International E-Vita Open Registry [23], they included only the patients who survived the initial repair with at least a 1-year follow-up CT scan to evaluate aortic remodeling after FET. Results showed as the FET technique provides an effective treatment for aortic dissections, promoting false lumen thrombosis, and remodeling of the descending thoracic aorta in a similar way in acute and chronic scenarios. However, increased FL thrombosis and positive remodeling rates were not maintained at the level of the abdominal aorta [23].
Discussion
“Even since the elephant trunk crashed into surgery, its trunk has pursued with amazing gyrations” this Hans Borst citation emphasize the evolution and diffusion of the elephant trunk procedure in the aortic community. Well known is how the FET took advance of the introduction of the endoprosthesis to further refine and facilitate the initial trunk techniques. Now, it has become an important tool in the treatment of complex thoracic aortic disease by providing one-stage repair or a useful proximal landing zone to further coverage the remaining downstream aorta.
In this literature, review analysis is relevant to observe how the widespread of the FET procedures turn into a progressively increase in the number of implants worldwide and better postoperative results [12–30]. More of 28,000 prostheses were implanted until 2015 and interestingly more than a half were used in China. In a recent review, Ma et al. reported an early mortality rate ranging from 6.4 to 15.8% [31]. Similar results have been reported by the recent position paper of the vascular domain of the European Association of Cardio-Thoracic Surgery [32]. Results coming from the most recent series reported even more positive results concerning hospital mortality and neurological injuries. In particular, Qi et al. [25], Kreibich [27], and Koizumi [28] reported a 0% of in-hospital mortality.
The reasons should be found in a more appropriate patient selection, a better management of the left subclavian artery and the introduction of newer hybrid grafts [11].
From a technical perspective, moving the arch anastomosis more proximally from zone 3 to zone 2 greatly facilitated distal anastomosis. Additionally, other advantages can be considered a reduced hypothermic circulatory arrest times, risk of the left laryngeal nerve palsy and paraplegia/paraparesis rate due to a shorter coverage of the descending thoracic aorta. In Bologna, anatomically feasible, we also prefer to perform the anastomosis in arch zone 2. Of course, the possibility to make a more proximal anastomosis in the arch sometimes can pose to a partially exclusion of a distal aortic aneurysm. Therefore, it might be necessary to adopt longer stent graft or a second endovascular treatment to achieve a complete coverage of the lesion.
On the most appreciated manufactured hybrid arch graft was the Thoraflex Hybrid graft (CryoLife, Kennesaw, GA) which offers in a single prosthesis the possibility to perform a separated epiaortic arch vessels reconstruction and a distal antegrade stenting of the descending thoracic aorta over a guidewire. Moreover, the ante-Flo side branch enabled a more rapid reperfusion reducing visceral ischemic times. The Hannover group have the longer experience with the use of this prosthesis with a median follow-up of approximately 3 years in which they found low rates of unplanned aortic reinterventions and significant positive aortic remodeling [19].
Another main target of the FET procedure is to reduce late aortic events in case of aortic dissections. It might be achieved by closing distal entry tears and promote FL shrinkage in the downstream thoraco-abdominal aorta in case of aortic dissection [23]. In our review analysis, only limited series reported a long follow-up analysis (> 5 years). Emphasizing how this technique is still under investigation regarding the incidence of aortic reinterventions.
In summary, according to this 10-year literature review analysis, the FET technique became a consolidated option to treat complex arch pathologies both in a single or multiple endovascular stage approach. Performing the distal anastomosis more proximally in aortic arch (from zone 0 to zone 2) represented the most appreciated modification as it facilitates arch reconstruction and reduce the risk of paraplegia. However, all the articles mentioned above presented limitations represented by the retrospective analysis of the studies, the short follow-up time, and the lack of a group of comparison. Further investigations with longer follow-up time and specifically designed protocols are still necessary to effectively consider the procedure a definitive treatment or to prompt recognize patients at major risk for secondary reintervention.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
Not required being a review article.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Borst HG, Walterbusch G, Schaps D. Extensive aortic replacement using “elephant trunk” prosthesis. Thorac Cardiovasc Surg. 1983;31:37–40. doi: 10.1055/s-2007-1020290. [DOI] [PubMed] [Google Scholar]
- 2.Shrestha M, Beckmann E, Krueger H, et al. The elephant trunk is freezing: The Hannover experience. J Thorac Cardiovasc Surg. 2015;149:1286–1293. doi: 10.1016/j.jtcvs.2015.01.044. [DOI] [PubMed] [Google Scholar]
- 3.Castrovinci S, Murana G, de Maat GE, et al. The classic elephant trunk technique for staged thoracic and thoracoabdominal aortic repair: long-term results. J Thorac Cardiovasc Surg. 2015;149:416–422. doi: 10.1016/j.jtcvs.2014.09.078. [DOI] [PubMed] [Google Scholar]
- 4.Suto Y, Yasuda K, Shiiya N, et al. Stented elephant trunk procedure for an extensive aneurysm involving distal aortic arch and descending aorta. J Thorac Cardiovasc Surg. 1996;112:1389–1390. doi: 10.1016/S0022-5223(96)70157-5. [DOI] [PubMed] [Google Scholar]
- 5.Kato M, Ohnishi K, Kaneko M, et al. New graft-implanting method for thoracic aortic aneurysm or dissection with a stented graft. Circulation. 1996;94:II188–II193. doi: 10.1161/01.CIR.94.6.1379. [DOI] [PubMed] [Google Scholar]
- 6.Karck M, Chavan A, Hagl C, Friedrich H, Galanski M, Haverich A. The frozen elephant trunk technique:a new treatment for thoracic aortic aneurysms. J Thorac Cardiovasc Surg. 2003;125:1550–1553. doi: 10.1016/S0022-5223(03)00045-X. [DOI] [PubMed] [Google Scholar]
- 7.Sun L, Qi R, Zhu J, Liu Y, Zheng J. Total arch replacement combined with stented elephant trunk implantation: a new “standard” therapy for type a dissection involving repair of the aortic arch? Circulation. 2011;123:971–978. doi: 10.1161/CIRCULATIONAHA.110.015081. [DOI] [PubMed] [Google Scholar]
- 8.Hata M, Akiyama K, Orime Y, Wakui S, Nakamura T, Shiono M. Less invasive quick open stenting using a J graft open stent for distal arch aneurysms. Thorac Cardiovasc Surg. 2016;64:330–332. doi: 10.1055/s-0035-1546299. [DOI] [PubMed] [Google Scholar]
- 9.Desai ND, Pochettino A. Distal aortic remodeling using endovascular repair in acute DeBakey I aortic dissection. Semin Thorac Cardiovasc Surg. 2009;21:387–392. doi: 10.1053/j.semtcvs.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Roselli EE, Rafael A, Soltesz EG, Canale L, Lytle BW. Simplified frozen elephant trunk repair for acute DeBakey type I dissection. J Thorac Cardiovasc Surg. 2013;145:S197–S201. doi: 10.1016/j.jtcvs.2012.11.068. [DOI] [PubMed] [Google Scholar]
- 11.Pacini D, Murana G, Di Marco L. Frozen elephant trunk technique: Ready to get back to the future? J Thorac Cardiovasc Surg. 2018;156:e79–e80. doi: 10.1016/j.jtcvs.2018.04.071. [DOI] [PubMed] [Google Scholar]
- 12.Pochettino A, Brinkman WT, Moeller P, et al. Antegrade thoracic stent grafting during repair of acute DeBakey I dissection prevents development of thoracoabdominal aortic aneurysms. Ann Thorac Surg. 2009;88:482–489. doi: 10.1016/j.athoracsur.2009.04.046. [DOI] [PubMed] [Google Scholar]
- 13.Chen X, Huang F, Xu M, et al. The stented elephant trunk procedure combined total arch replacement for DeBakey I aortic dissection: operative result and follow-up. Interact Cardiovasc Thorac Surg. 2010;11:594–598. doi: 10.1510/icvts.2010.238212. [DOI] [PubMed] [Google Scholar]
- 14.Uchida N, Katayama A, Tamura K, Sutoh M, Kuraoka M, Ishihara H. Frozen elephant trunk technique and partial remodeling for acute type A aortic dissection. Eur J Cardiothorac Surg. 2011;40:1066–1071. doi: 10.1016/j.ejcts.2011.02.074. [DOI] [PubMed] [Google Scholar]
- 15.Shen K, Tang H, Jing R, Liu F, Zhou X. Application of triple-branched stent graft for Stanford type A aortic dissection: potential risks. Eur J Cardiothorac Surg. 2012;41:e12–e17. doi: 10.1093/ejcts/ezr259. [DOI] [PubMed] [Google Scholar]
- 16.Hoffman A, Damberg AL, Schälte G, et al. Thoracic stent graft sizing for frozen elephant trunk repair in acute type A dissection. J Thorac Cardiovasc Surg. 2013;145:964–969. doi: 10.1016/j.jtcvs.2012.03.059. [DOI] [PubMed] [Google Scholar]
- 17.Dohle DS, Tsagakis K, Janosi RA, et al. Aortic remodelling in aortic dissection after frozen elephant trunk. Eur J Cardiothorac Surg. 2016;49:111–117. doi: 10.1093/ejcts/ezv045. [DOI] [PubMed] [Google Scholar]
- 18.Uchida N, Katayama A, Higashiue S, et al. A new device as an open stent graft for extended aortic repair: a multicentre early experience in Japan. Eur J Cardiothorac Surg. 2016;49:1270–1278. doi: 10.1093/ejcts/ezv310. [DOI] [PubMed] [Google Scholar]
- 19.Shrestha M, Kaufeld T, Beckmann E, et al. Total aortic arch replacement with a novel 4-branched frozen elephant trunk prosthesis: Single-center results of the first 100 patients. J Thorac Cardiovasc Surg. 2016;152:148–159. doi: 10.1016/j.jtcvs.2016.02.077. [DOI] [PubMed] [Google Scholar]
- 20.Yamane Y, Uchida N, Mochizuki S, Furukawa T, Yamada K. Early- and mid-term aortic remodelling after the frozen elephant trunk technique for retrograde type A acute aortic dissection using the new Japanese J Graft open stent graft. Interact Cardiovasc Thorac Surg. 2017;25:720–726. doi: 10.1093/icvts/ivx144. [DOI] [PubMed] [Google Scholar]
- 21.Shrestha M, Martens A, Kaufeld T, et al. Single-centre experience with the frozen elephant trunk technique in 251 patients over 15 years. Eur J Cardiothorac Surg. 2017;52:858–866. doi: 10.1093/ejcts/ezx218. [DOI] [PubMed] [Google Scholar]
- 22.Roselli EE, Idrees JJ, Bakaeen FG, et al. Evolution of Simplified Frozen Elephant Trunk Repair for Acute DeBakey Type I Dissection: Midterm Outcomes. Ann Thorac Surg. 2018;105:749–755. doi: 10.1016/j.athoracsur.2017.08.037. [DOI] [PubMed] [Google Scholar]
- 23.Iafrancesco M, Goebel N, Mascaro J, et al. Aortic diameter remodelling after the frozen elephant trunk technique in aortic dissection: results from an international multicentre registry. Eur J Cardiothorac Surg. 2017;52:310–318. doi: 10.1093/ejcts/ezx131. [DOI] [PubMed] [Google Scholar]
- 24.Chu MWA, Losenno KL, Dubois LA, et al. Early Clinical Outcomes of Hybrid Arch Frozen Elephant Trunk Repair With the Thoraflex Hybrid Graft. Ann Thorac Surg. 2019;107:47–53. doi: 10.1016/j.athoracsur.2018.07.091. [DOI] [PubMed] [Google Scholar]
- 25.Qi R, Zhu JM, Liu YM, et al. Frozen Elephant Trunk for Acute Type B Dissection Involving the Distal Arch in the Hybrid Repair Era. Ann Thorac Surg. 2018;106:1182–1188. doi: 10.1016/j.athoracsur.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 26.Goebel N, Nagib R, Salehi-Gilani S, et al. One-stage hybrid aortic repair using the frozen elephant trunk in acute DeBakey type I aortic dissection. J Thorac Dis. 2018;10:4195–4203. doi: 10.21037/jtd.2018.06.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kreibich M, Berger T, Morlock J, et al. The frozen elephant trunk technique for the treatment of acute complicated Type B aortic dissection. Eur J Cardiothorac Surg. 2018;53:525–530. doi: 10.1093/ejcts/ezx281. [DOI] [PubMed] [Google Scholar]
- 28.Koizumi S, Nagasawa A, Koyama T. Total aortic arch replacement using frozen elephant trunk technique with J Graft Open Stent Graft for distal aortic arch aneurysm. Gen Thorac Cardiovasc Surg. 2018;66:91–94. doi: 10.1007/s11748-017-0856-z. [DOI] [PubMed] [Google Scholar]
- 29.Berger T, Kreibich M, Morlock J, et al. True-lumen and false-lumen diameter changes in the downstream aorta after frozen elephant trunk implantation. Eur J Cardiothorac Surg. 2018;54:375–381. doi: 10.1093/ejcts/ezy031. [DOI] [PubMed] [Google Scholar]
- 30.Di Bartolomeo R, Murana M, Di Marco L, et al. Is the frozen elephant trunk frozen? Gen Thorac Cardiovasc Surg. 2019;67:111–117. doi: 10.1007/s11748-018-0911-4. [DOI] [PubMed] [Google Scholar]
- 31.Ma WG, Zheng J, Sun LZ, Elefteriades JA. Open stented grafts for frozen elephant trunk technique: technical aspects and current outcomes. Aorta. 2015;3:122–135. doi: 10.12945/j.aorta.2015.14-062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shrestha M, Bachet J, Bavaria J, et al. Current status and recommendations for use of the frozen elephant trunk technique. A position paper by the vascular domain of EACTS. Eur J Cardiothorac Surg. 2015;47:759–769. doi: 10.1093/ejcts/ezv085. [DOI] [PubMed] [Google Scholar]