Abstract
Thoracic aortic aneurysm is a complex disease. The consequences of such silent and indolent disease include acute aortic syndrome if not recognized early and treated appropriately. Aortic aneurysm size was a reliable clinical marker to aid clinical intervention; however, aneurysm growth is variable and is influenced by many factors such as age, presence of connective tissue disorders, genetic disorders, hypertension, inflammatory conditions of the aorta, autoimmune diseases, smoking, and history of previous cardiac surgery. Therefore, aortic size became a non-specific disease surrogate and prediction tool on outcome and intervention. In this review article, we examined the current literature for evidence about aneurysm size and its relation to type A aortic dissection.
Keywords: Aorta, Aortic dissection, Dissection size
Is aortic dissection predictable?
Acute type A aortic dissection (ATAAD) is a surgical emergency with high mortality and morbidity rates. The reported incidence is of 3–4/100,000 of the population [1, 2]. The majority of aortic dissections, especially in the elderly patients, are of degenerative origin and present as an emergency condition [3].
Historically, aortic aneurysm size was the key criteria for undergoing elective aortic repair. The current recommended aneurysmal size for intervention was based on a diameter of > 55 mm in non-syndromic patients [4]; however, for syndromic patients with a connective tissue disorder, the threshold for intervention is an ascending aorta size of ≥ 45 mm [4, 5]. Current guidelines can be challenged due to the lack of constructive prediction of aortic dissection while using aneurysm size as a key surrogate factor [6, 7]. According to the International Registry of Aortic Dissection (IRAD) database, more than 60% of patients who presented with ATAAD had an aortic aneurysm size below 55 mm, such findings have been reinforced by two separate studies. Neri et al. and Williams et al. [8, 9] both studied human aorta dissection and concluded that aortic size alone was not sufficient factor to predict ATAAD. They also commented that the mean diameter at which the dissection occurred was 41 mm which is much lower than the current guidelines. The results were also supported by a further study by Parish et al. [7]. However, their Marfan group of patients had aortic root diameter of ≥ 45 mm [7, 10]. Furthermore, in a recent study by De Beaufort et al. [11] utilizing IRAD database, they identified 258 Marfan patients who underwent aortic dissection repair. Among this cohort, 164 had ATAAD, with average ascending aorta diameter of 45 mm at time of presentation in comparison to non-Marfan patients who had a diameter of 46 mm at time of ATAAD presentation, which again goes against what has been broadly stated in the guidelines.
With advancement in understanding aortic aneurysm, many other factors have been found to be contributing factors in predicting acute aortic dissection other than having an isolated aortic aneurysm alone, and those factors have direct correlation and a strong impact on emergency presentation of such patients [3, 12–14]. These factors have been summarized in Table 1.
Table 1.
Summary of the major risk factors contributing to prediction of type A aortic dissection
| Aortic tissue related | Patient related |
|---|---|
| Bicuspid aortic valve | Connective tissue disorder |
| Inflammatory conditions of aorta | Male sex |
| Familiar thoracic aortic aneurysm | Smoking |
| Aortic trauma either blunt or iatrogenic | Age > 65 years |
| Previous cardiac surgery | |
| Acute hemodynamic stress (heavy weight lifting, cocaine use, pheochromocytoma) | |
| Pregnancy | |
| Hypertension |
The above-mentioned risk factors have been correlated with occurrence of acute aortic syndrome and in particular ATAAD; among all the mentioned factors, connective tissue disorders such as Marfan, Vascular Ehlers-Danlos, Loeys-Dietz syndrome, and patients with bicuspid aortic valve remain the highly suggestive risks for the occurrence of ATAAD under the age of 40 years old [15]. The presence of inflammatory conditions of the aorta such as Giant cell and Takayasu arteritis, Behcet’s disease, aortitis, systemic lupus erythematous, and syphilis have been reported to increase prediction of aortic dissection regardless of the size of the aortic aneurysm. Estrera et al. [16] published their results of 330 patients with ATAAD repair, and the study identified aortic aneurysm size as a predicting factor for presentation with ATAAD; however, the presence of other risk factors, such as previous cardiac surgery, renders the aneurysm size-based dissection prediction unreliable.
Overall, the above risk factors are useful when the aneurysm size is below 55 mm [12, 14]; however, the influence of such risk factors will be less likely when the aneurysm size is 60 mm or more, as the chance of dissection is more than 30% in such large aneurysms; Therefore, the aneurysm size at this stage remains the core guide for intervention [17]. These findings have been largely supported by a study from Elefteriades et al. [18, 19].
Is size alone a surrogate marker for when to operate?
Until less than a decade ago, the key indication for elective repair of ascending aortic aneurysm was size of the aneurysm itself. The European and American guidelines [4, 5] recommended open repair with interposition graft for isolated ascending aorta or root aneurysm of ≥ 55 mm in patients with no elastopathy (5, class IIa C recommendation). Such measures differ when there are pre-existing aortic pathologies such as connective tissue disorders or genetic predisposition and therefore the recommendation for surgery is at a lower sized aorta (≥ 45 and ≥ 50 mm, respectively). These size-based guidelines have been challenged by many international studies and therefore the exact size of the aneurysm where complications are anticipated noted to be variable, inconsistent, and multi-factorial. Summary of those studies are in Table 2.
Table 2.
Diameter of ascending aorta at timings of complications
| Variables | Mean size at complications (mm) | Source |
|---|---|---|
| Hypertensive | 60 | Cetin et al. [47] |
| Marfan syndrome | 51 | Roman et al. [48] |
| 56 | Lazarevic et al. [49] | |
| 50–59 | Jondeau et al. [50] | |
| Bicuspid aortic valve | 52 | Della Corte et al. [51] |
| Familial (non-syndromic) | No data | No data |
| Loeys-Dietz syndrome | 40–50 | Loeys et al. [52] |
| Ehlers-Danlos syndrome | No data | No data |
The size of aneurysm itself should serve a purpose in the presence of other co-factors especially the underline pathology [20], such factors include presence of bicuspid aortic valve, hypertension, male sex, positive family history, presence of connective tissue disorder, body surface area, previous cardiac surgery, annual growth of aneurysm of 5 mm or more, and presence of severe aortic regurgitation [5, 13, 21, 22].
In a recent large study by Ziganshin et al. [23], they analyzed several factors contributing to the presentation of ATAAD in patients with TAD; in addition to the size of aneurysm, presence of family history with genetic predisposition increases the rate of complications from the aortic aneurysm and size itself alone is not useful in predicting a condition as lethal as ATAAD. Therefore, a combination of presence of aneurysm, positive family history, high blood pressure, and previous cardiac surgery puts the individual at high risk for early aortic dissection.
In a separate study by Parish et al. [7] where they studied 177 non-Marfan patients over a period of 10 years following presentation with ATAAD, they concluded that the majority of their patients presented when the aortic diameter was < 55 mm and therefore did not fall within current guidelines for elective aortic root replacement. Such findings were also supported by an earlier separate study from Neri et al. [8] in which they analyzed 220 patients, concluding that although aortic root diameter is a strong predicting factor for ATAAD presentation. It rarely occurs within the suggested root size especially in the presence of a connective tissue disorder, as one third of the patients included in their study presented with ATAAD had normal or near-normal aortic size. In a larger cohort and a more recent study by Geisbusch et al. [24], they have studied 507 patients with small to moderate ascending aortic aneurysm, and the mean mid-ascending aortic aneurysm size was 44 ± 6 mm, such patients were also at risk of developing aortic dissection and rupture and therefore aggressive surveillance and early surgery is advised to prevent the catastrophe of ATAAD.
Can our genetic unraveling help?
Genetics involvement in thoracic aortic aneurysm (TAA) has been a focus of interested in the last few years, as almost 95% of TAA diseases are asymptomatic prior to presentation as acute aortic dissection or rupture, hence the silent killer name [25]. Genetics plays an important role in addition to size for prediction of acute aortic events. In two separate studies by Yale group [26, 27], they have identified several genes that can help in predicting TAA and therefore to be put under surveillance and planning early surgery to prevent acute aortic dissection or rupture. The use of ribonucleic acid (RNA) signature sequence has created a new platform to predict the disease itself and plan management of such a lethal condition in advance. The results of Chau et al. [28] who analyzed 33,000 RNA expressions noted a particular genetic presence, namely 41 single nucleotide polymorphism panel which was found to be distinctive among patients with TAA compared to those without evidence of TAA with an accuracy rate of over 80%. The same group have also isolated selected genetic mutations which can be found in patients with TAA; however, the sensitivity and selectivity of such tests are yet to be determined by a larger trial [29]. A further ground-breaking study has been performed by Milewicz and colleagues [29–32], supporting the genetic mutations identification in patients with TAA and using them as biomarkers. Among such genes are ACTA2, MYLK, FBN1, and KIF6, although these genetic mutations only account for 20% of the TAA diseases. With further studies, a greater role may be discovered.
What about biomarkers and prediction of aortic dissection?
The role of biomarkers is significant in assisting to predict ATAAD in patients with TAA diseases, among such biomarkers are D-Dimer study, C-reactive protein, interleukins, and extracelullar matrix remodeling elements such as matrix metalloproteinase, osteopontin, soluble elastin fragments, and tissue inhibitor of metalloproteases [33, 34]. The drawback of such markers is the fact that they are only released at the time of the event and hence it cannot be of use to predict the disease progress and preventative measures. Lack of randomized studies has put the use of such markers into question. In an extensive literature review by Balmforth et al., they concluded that no biomarkers exist in the diagnosis of the presence of TAA or the exact rate of expansion [35] in such a cohort of patients. In a recent study by Suzuki et al. [36], their focus was on the contribution of the biomarkers to the Aortic Dissection Detection Risk Score (ADD-RS) in the American and European guidelines [4, 5], such biomarkers can score up to 3 (high risk patients) if found and detected, and the finding of such biomarkers was proven to be effective based on the outcomes from the IRAD database of studying 2538 patients. Therefore, the ultimate conclusion from this study led towards using the biomarkers as a serious tool in aiding predicting such a lethal condition. Therefore size alone cannot be relied on for prediction of TAAD.
The immunology behind prediction of aortic dissection
Immunity and inflammation play an important role in patients with thoracic aortic aneurysm and progression to ATAAD. In a study by del Porto et al. [37], they concluded that innate immunity plays a crucial role in the genesis of ATAAD in patients with TAA. In their study, they analyzed 35 patients, who presented with ATAAD. For hemochrome and lymphocyte subpopulations using flow cytometry, they identified that there is a significant increase in the level of natural killer B cells and CD8 + CD28 cells, while a significant decrease in the level of T lymphocytes and T helper fractions. Furthermore, they have also noted an increase in the level of CRP, IL-6, IL-8, IL-10, TNF-alpha, and MCP-1 which correlates with an increased level of inflammatory response in such acute circumstances. Such findings have been supported later by a further study, Peng et al. [38], which have identified how the levels of such immune cells alter significantly in ATAAD and a trend can be predicted and used as a marker for the disease progression in patients with TAA in correlation with the size of the aneurysm.
The subtleties of aortic dissection and small aortic size
It is well known that the aortic dissection is higher in patients with a large aortic aneurysm; however, recently, with the presence of enhanced international studies, it has been published that patients with small aortic root have higher rate of ATAAD in the presence of TAA [23]. The database from IRAD has published data supporting such phenomenon, as a significant portion of the patients with small aortic root who presented with ATAAD had an aneurysm of less than 5.5 cm [39]; despite this fact from IRAD, the European and American guidelines remained the same at 5.5 cm and as did the cutoff dimension for operating on elective basis. This topic remained controversial and debatable at international platform [23, 39, 40]. In a separate study by Paruchuri et al. [40], they concluded that, although patients who have a small aortic root and suffer from TAA have a greater chance of ATAAD, the incidence of ATAAD remained 6000 times more common in patient with aortic size of > 4.5 cm than those with aortic root of 3.5 cm. Parish et al. [7] also predicted that small aortic size patients are more prone to dissection than average aortic size patients. Currently, the data supporting this claim is very limited and therefore there is a demand for a high-quality study to prove this concept and perhaps in future it may be used to develop future guidelines.
The arm of aortic engineering and imaging
Recently, Chau et al. [28] assessed the role of engineering calculations with six full independent parameters which could predict acute aortic dissection in patients with TAA, such parameters include aortic pressure, thickness, and diameter in systole and diastole periods which are based on epi-aortic measurements in the operating room. Their study has shown that as the aorta gets older and larger, the elasticity is lost and it transforms from a flexible vessel to rather a “rigid tube” and hence the chance of aortic dissection is high. In separate studies, the role of imaging, transesophageal echocardiography, computerized tomography (CT), and magnetic resonance imaging (MRI) has been found to assist in measuring the size of the aneurysm and rate of expansion in order to determine the severity of the disease. The use of positron emission tomography has been demonstrated to be useful in assessing the metabolic activities, of which an increase in the uptake of F18-fluorodexoyyglucose (FDG) correlates directly with the activities of the inflammatory process within the aorta and therefore perhaps predicting the severity of disease progress with risk of rupture or dissection [41, 42], such use of position emission tomography (PET) scan is currently limited to abdominal aorta; however, perhaps in future studies, it will allow us to develop a greater understanding of TAA disease.
Currently, studies are ongoing in several European centers [43] to identify if such parameters can be thoroughly assessed through transesophageal echocardiography on a regular basis as a surveillance tool; moreover in certain centers in Europe, magnetic resonance imaging is being used to calculate such parameters and it is used to correlate clinically and predict the regions of the aneurysm that are at high risk of dissection or rupture [43, 44].
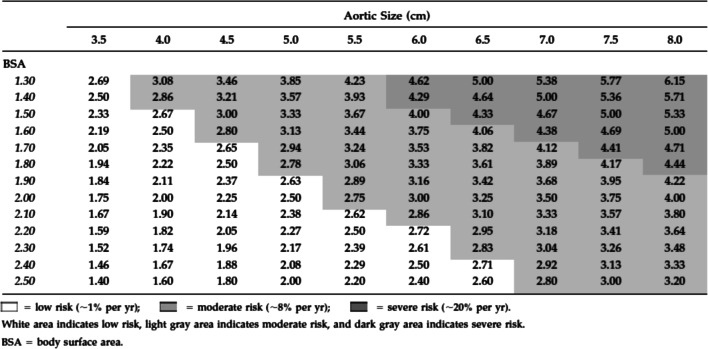
On the contrary to the above, several studies have been published by Yale group which demonstrated that size is the sole factor in predicting rupture and dissection and hence an increased rate of mortality and morbidity [16, 21, 28, 45] when the aneurysm is increasing in size. The group took the lead in publishing studies related to the size-dissection relationship using their extensive and large-scale database. They concluded that the larger the size of the aorta the higher risk of acute aortic syndrome and death, such data is summarized in Table 3; therefore, they have used aneurysm size as the basis of their practice in intervention for TAA disease, including all types of aneurysms affecting the ascending aorta. However, Coady et al. [45] identified in their study that the size-rupture relationship for TAA diseases still remains debatable and is subject to many other factors that can lead to earlier presentation of acute aortic syndrome than predicted in the presence of an aneurysm; in particular, they have noted that patients with low body surface area have a higher rate of such complications compared to patients with a similar size aortic aneurysm but with a higher body surface area (Fig. 1).
Table 3.
Annual risk of complications based on thoracic aortic aneurysm size. With permission from Chau et al. [28]
| Annual risk (%) | ||||
|---|---|---|---|---|
| Aortic size | Rupture | Dissection | Death | Rupture/dissection/death |
| > 3.5 cm | 0.0 | 2.2 | 5.9 | 7.2 |
| > 4.0 cm | 0.3 | 1.5 | 4.6 | 5.3 |
| > 5.0 cm | 1.7 | 2.5 | 4.8 | 6.5 |
| > 6.0 cm | 3.6 | 3.7 | 10.8 | 14.1 |
Fig. 1.
Risk of complications by aortic diameter and body surface area with aortic size index given within chart, with permission from Davies et al. [46]
Nevertheless, Yale group have noted that there is a selected point where occurrence of aortic dissection is inevitable; 31% of patients with ascending aortic size of 60 mm at time of screening had already developed dissection or rupture of the aneurysm, while this rate goes to 43% when the aneurysm size is 70 mm. Such data from Yale was also re-enforced by a large cohort study carried out by Davies et al. [46]; they looked at 721 patients analyzing the yearly rate of dissection and rupture based on the aortic size only, and they suggested that a careful radiological follow-up and an elective surgical intervention was strongly recommended for TAA diseases as relative size of the aorta is more important than absolute aortic size in predicting complications. A further study by Pape et al. [6] from the IRAD database has also demonstrated that size is not the only matter for predicting acute aortic dissection in patients with aortic aneurysm, as a matter of fact more than half of the patients with aortic dissections had an aortic diameter of less than 55 mm which is below the recommended size for surgical intervention. Therefore, size of the aortic aneurysm itself remains a loop hole which is surrounded by ongoing debate.
Moving beyond size
While both American and European guidelines use size as key base for elective repair of aortic root aneurysm to prevent the occurrence of ATAAD, several large international studies confronted those with evidences that size itself cannot be the only predictive risk factor of presentation as an acute aortic dissection. Although size-related dissection forms a strong pillar in managing patients with TAA, on its own it is not sufficient. Parallel assessment of other factors that can contribute to acute unpredicted presentation of TAA is evolving [28].
Conclusion
There is no perfect way of predicting acute aortic dissection or rupture in patients with pre-existing thoracic aortic aneurysm disease; however, size of the aortic aneurysm remains an important predictor of acute complications from TAA; however, it cannot be used as a sole base for planning necessary surgical intervention as acute complications can happen in aortic sizes that are below the current recommended American and European guidelines for surgical intervention, therefore a multi-disciplinary approach for patients with thoracic aortic aneurysm is important to prevent such catastrophe. Yet, we hope the future studies can give us more concrete answers about the role of biomarkers, metabolic activities, mechanics and genetics in predicting such lethal conditions.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest. Ethical approval, Statement of Human and Animal Rights, Informed consent–Being a Review article, these are not required.
References
- 1.Olsson C, Thelin S, Stahle E, Ekbom A, Granath F. Thoracic aortic aneurysm and dissection: increasing prevalence and improved outcomes reported in a nationwide population-based study of more than 14000 cases from 1987 to 2002. Circulation. 2006;114:2611–2618. doi: 10.1161/CIRCULATIONAHA.106.630400. [DOI] [PubMed] [Google Scholar]
- 2.Meszaros I, Morocz J, Szlavi J, et al. Epidemiology and clinic opathology of aortic dissection. Chest. 2000;117:1271–1278. doi: 10.1378/chest.117.5.1271. [DOI] [PubMed] [Google Scholar]
- 3.Goldfinger JZ, Halperin JL, Marin ML, Stewart AS, Eagle KA, Fuster V. Thoracic aortic aneurysm and dissection. J Am Coll Cardiol. 2014;64:1725–1739. doi: 10.1016/j.jacc.2014.08.025. [DOI] [PubMed] [Google Scholar]
- 4.Hiratzka LF, Bakris GL, Beckman JA, et al. ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/ SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: a Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation. 2010;121:e266–e369. doi: 10.1161/CIR.0b013e3181d4739e. [DOI] [PubMed] [Google Scholar]
- 5.Erbel R, Aboyans V, Boileau C, et al. ESC Committee for Practice Guidelines. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC) Eur Heart J. 2014;35:2873–2926. doi: 10.1093/eurheartj/ehu281. [DOI] [PubMed] [Google Scholar]
- 6.Pape LA, Tsai TT, Isselbacher EM, et al. International Registry of Acute Aortic Dissection (IRAD) Investigators. Aortic diameter >or = 5.5 cm is not a good predictor of type A aortic dissection: observations from the International Registry of Acute Aortic Dissection (IRAD) Circulation. 2007;116:1120–1127. doi: 10.1161/CIRCULATIONAHA.107.702720. [DOI] [PubMed] [Google Scholar]
- 7.Parish LM, Gorman JH, 3rd, Kahn S, et al. Aortic size in acute type A dissection: implications for preventive ascending aortic replacement. Eur J Cardiothorac Surg. 2009;35:941–945. doi: 10.1016/j.ejcts.2008.12.047. [DOI] [PubMed] [Google Scholar]
- 8.Neri E, Barabesi L, Buklas D, et al. Limited role of aortic size in the genesis of acute type A aortic dissection. Eur J Cardiothorac Surg. 2005;28:857–863. doi: 10.1016/j.ejcts.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 9.Williams DM, LePage MA, Lee DY. The dissected aorta. Part I. Early anatomic changes in an in vitro model. Radiology. 1997;203:23–31. doi: 10.1148/radiology.203.1.9122399. [DOI] [PubMed] [Google Scholar]
- 10.Gott VL, Greene PS, Alejo DE, et al. Replacement of the aortic root in patients with Marfan’s syndrome. N Engl J Med. 1999;340:1307–1313. doi: 10.1056/NEJM199904293401702. [DOI] [PubMed] [Google Scholar]
- 11.de Beaufort HWL, Trimarchi S, Korach A, et al. Aortic dissection in patients with Marfan syndrome based on the IRAD data. Ann Cardiothorac Surg. 2017;6:633–641. doi: 10.21037/acs.2017.10.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erbel R, Eggebrecht H. Aortic dimensions and the risk of dissection. Heart. 2006;92:137–142. doi: 10.1136/hrt.2004.055111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gawinecka J, Schönrath F, von Eckardstein A. Acute aortic dissection: pathogenesis, risk factors and diagnosis. Swiss Med Wkly. 2017;147:w14489. doi: 10.4414/smw.2017.14489. [DOI] [PubMed] [Google Scholar]
- 14.Braverman AC. Acute aortic dissection: clinician update. Circulation. 2010;122:184–188. doi: 10.1161/CIRCULATIONAHA.110.958975. [DOI] [PubMed] [Google Scholar]
- 15.Januzzi JL, Isselbacher EM, Fattori R, et al. International Registry of Aortic Dissection (IRAD). Characterizing the young patient with aortic dissection: results from the International Registry of Aortic Dissection (IRAD) J Am Coll Cardiol. 2004;43:665–669. doi: 10.1016/j.jacc.2003.08.054. [DOI] [PubMed] [Google Scholar]
- 16.Estrera AL, Miller CC, Kaneko T, et al. Outcomes of acute type a aortic dissection after previous cardiac surgery. Ann Thorac Surg. 2010;89:1467–1474. doi: 10.1016/j.athoracsur.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 17.Davies RR, Gallo A, Coady MA, et al. Novel measurement of relative aortic size predicts rupture of thoracic aortic aneurysms. Ann Thorac Surg. 2006;81:169–177. doi: 10.1016/j.athoracsur.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 18.Elefteriades JA, Farkas EA. Thoracic Aortic Aneurysm Clinically Pertinent Controversies and Uncertainties. J Am Coll Cadiol. 2010;55:841–857. doi: 10.1016/j.jacc.2009.08.084. [DOI] [PubMed] [Google Scholar]
- 19.Elefteriades JA. Natural history of thoracic aortic aneurysms: indications for surgery, and surgical versus nonsurgical risks. Ann Thorac Surg. 2002;74:S1877–S1880. doi: 10.1016/S0003-4975(02)04147-4. [DOI] [PubMed] [Google Scholar]
- 20.Saliba E, Sia Y. In collaboration with Dore Annie ,El Hamamsy Ismael . The ascending aortic aneurysm: When to intervene? IJC Heart & Vasculature. 2015;6:91–100. doi: 10.1016/j.ijcha.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elefteriades JA, Ziganshin BA, Rizzo JA, et al. Indications and imaging for aortic surgery: size and other matters. J Thorac Cardiovasc Surg. 2015;149:S10–S13. doi: 10.1016/j.jtcvs.2014.07.066. [DOI] [PubMed] [Google Scholar]
- 22.Mokashi SA, Svensson LG. Guidelines for the management of thoracic aortic disease in 2017. Gen Thorac Cardiovasc Surg. 2017. 10.1007/s11748-017-0831-8. [DOI] [PubMed]
- 23.Ziganshin BA, Elefteriades JA. Treatment of Thoracic Aortic Aneurysm: Role of Earlier Intervention. Semin Thorac Cardiovasc Surg. 2015;27:135–143. doi: 10.1053/j.semtcvs.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 24.Geisbüsch S, Stefanovic A, Schray D, et al. A prospective study of growth and rupture risk of small-to-moderate size ascending aortic aneurysms. J Thorac Cardiovasc Surg. 2014;147:68–74. doi: 10.1016/j.jtcvs.2013.06.030. [DOI] [PubMed] [Google Scholar]
- 25.Kuzmik GA, Sang AX, Elefteriades JA. Natural history of thoracic aortic aneurysms. J Vasc Surg. 2012;56:565–571. doi: 10.1016/j.jvs.2012.04.053. [DOI] [PubMed] [Google Scholar]
- 26.Elefteriades JA, Pomianowski P. Practical genetics of thoracic aortic aneurysm. Prog Cardiovasc Dis. 2013;56:57–67. doi: 10.1016/j.pcad.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 27.Pomianowski P, Elefteriades JA. The genetics and genomics of thoracic aortic disease. Ann Cardiothorac Surg. 2013;2:271–279. doi: 10.3978/j.issn.2225-319X.2013.05.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chau KH, Elefteriades JA. Natural history of thoracic aortic aneurysms: size matters. plus moving beyond size. Prog Cardiovasc Dis. 2013;56:74–80. doi: 10.1016/j.pcad.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 29.Milewicz DM, Michael K, Fisher N, Coselli JS, Markello T, Biddinger A. Fibrillin-1 (FBN1) mutations in patients with thoracic aortic aneurysms. Circulation. 1996;94:2708–2711. doi: 10.1161/01.CIR.94.11.2708. [DOI] [PubMed] [Google Scholar]
- 30.Putnam EA, Zhang H, Ramirez F, Milewicz DM. Fibrillin-2 (FBN2) mutations result in the Marfan-like disorder, congenital contractural arachnodactyly. Nat Genet. 1995;11:456–458. doi: 10.1038/ng1295-456. [DOI] [PubMed] [Google Scholar]
- 31.Hasham SN, Lewin MR, Tran VT, et al. Nonsyndromic genetic predisposition to aortic dissection: a newly recognized, diagnosable, and preventable occurrence in families. Ann Emerg Med. 2004;43:79–82. doi: 10.1016/S0196-0644(03)00818-7. [DOI] [PubMed] [Google Scholar]
- 32.Hasham SN, Willing MC, Guo DC, et al. Mapping a locus for familial thoracic aortic aneurysms and dissections (TAAD2) to 3p24-25. Circulation. 2003;107:3184–3190. doi: 10.1161/01.CIR.0000078634.33124.95. [DOI] [PubMed] [Google Scholar]
- 33.Ohlmann P, Faure A, Morel O, et al. Diagnostic and prognostic value of circulating D-dimers in patients with acute aortic dissection. Crit Care Med. 2006;34:1358–1364. doi: 10.1097/01.CCM.0000216686.72457.EC. [DOI] [PubMed] [Google Scholar]
- 34.Parolari A, Trimoli E, Songia P, et al. Biology features of thoracic aortic diseases. Where are we now, where are we heading to: established and emerging biomarkers and molecular pathways. Eur J Cardiothorac Surg. 2013;44:9–23. doi: 10.1093/ejcts/ezs647. [DOI] [PubMed] [Google Scholar]
- 35.Balmforth D, Harky A, Adams B, et al. Is there a role for biomarkers in thoracic aortic aneurysm disease? Gen Thorac Cardiovasc Surg. 2017. 10.1007/s11748-017-0855-0. [DOI] [PubMed]
- 36.Suzuki T, Eagle KA. Biomarker-assisted diagnosis of acute aortic dissection. Circulation. 2018;137:270–272. doi: 10.1161/CIRCULATIONAHA.117.032048. [DOI] [PubMed] [Google Scholar]
- 37.Del Porto F, Proietta M, Tritapepe L, et al. Inflammation and immune response in acute aortic dissection. Ann Med. 2010;42:622–629. doi: 10.3109/07853890.2010.518156. [DOI] [PubMed] [Google Scholar]
- 38.Peng W, Zhu QY, Zhou XH, Chai XP. A simple emergency prediction tool for acute aortic dissection. Iran J Public Health. 2013;42:1085–1091. [PMC free article] [PubMed] [Google Scholar]
- 39.Rampoldi V, Trimarchi S, Eagle KA, et al. International Registry of Acute Aortic Dissection(IRAD) Investigators. Simple risk models to predict surgical mortality in acute type A aortic dissection: the International Registry of Acute Aortic Dissection score. Ann Thorac Surg. 2007;83:55–61. doi: 10.1016/j.athoracsur.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 40.Paruchuri V, Salhab KF, Kuzmik G, et al. Aortic size distribution in the general population: Explaining the size paradox in aortic dissection. Cardiology. 2015;131:265–272. doi: 10.1159/000381281. [DOI] [PubMed] [Google Scholar]
- 41.Reeps C, Essler M, Pelisek J, Seidl S, Eckstein HH, Krause BJ. Increased 18Ffluorodeoxyglucose uptake in abdominal aortic aneurysms in positron emission/computed tomography is associated with inflammation, aortic wall instability, and acute symptoms. J Vasc Surg. 2008;48:417–423. doi: 10.1016/j.jvs.2008.03.059. [DOI] [PubMed] [Google Scholar]
- 42.Truijers M, Kurvers HA, Bredie SJ, et al. In vivo imaging ofabdominal aortic aneurysms: increased FDG uptake suggests inflammation in the aneurysm wall. J Endovasc Ther. 2008;15:462–467. doi: 10.1583/08-2447.1. [DOI] [PubMed] [Google Scholar]
- 43.Leung JH, Wright AR, Cheshire N, et al. Fluid structure interaction of patient specific abdominal aortic aneurysms: a comparison with solid stress models. Biomed Eng Online. 2006;5:33. doi: 10.1186/1475-925X-5-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Poullis MP, Warwick R, Oo A, Poole RJ. Ascending aortic curvature as an independent risk factor for type A dissection, and ascending aortic aneurysm formation: a mathematical model. Eur J Cardiothorac Surg. 2008;33:995–1001. doi: 10.1016/j.ejcts.2008.02.029. [DOI] [PubMed] [Google Scholar]
- 45.Coady MA, Rizzo JA, Hammond GL, et al. What is the appropriate size criterion for resection of thoracic aortic aneurysms? J Thorac Cardiovasc Surg. 1997;113:476–491. doi: 10.1016/S0022-5223(97)70360-X. [DOI] [PubMed] [Google Scholar]
- 46.Davies RR, Goldstein LJ, Coady MA, et al. Yearly rupture or dissection rates for thoracic aortic aneurysms: simple prediction based on size. Ann Thorac Surg. 2002;73:17–27. doi: 10.1016/S0003-4975(01)03236-2. [DOI] [PubMed] [Google Scholar]
- 47.Çetin M, Kocaman SA, Durakoğlugil ME, et al. Independent determinants of ascending aortic dilatation in hypertensive patients: smoking, endothelial dysfunction, and increased epicardial adipose tissue. Blood Press Monit. 2012;17:223–230. doi: 10.1097/MBP.0b013e328359c4a7. [DOI] [PubMed] [Google Scholar]
- 48.Roman MJ, Rosen SE, Kramer-Fox R, Devereux RB. Prognostic significance of the pattern of aortic root dilation in the Marfan syndrome. J Am Coll Cardiol. 1993;22:1470–1476. doi: 10.1016/0735-1097(93)90559-J. [DOI] [PubMed] [Google Scholar]
- 49.Lazarevic AM, Nakatani S, Okita Y, et al. Determinants of rapid progression of aortic root dilatation and complications in Marfan syndrome. Int J Cardiol. 2006;106:177–182. doi: 10.1016/j.ijcard.2005.01.040. [DOI] [PubMed] [Google Scholar]
- 50.Jondeau G, Detaint D, Tubach F, et al. Aortic event rate in the Marfan population: a cohort study. Circulation. 2012;125:226–232. doi: 10.1161/CIRCULATIONAHA.111.054676. [DOI] [PubMed] [Google Scholar]
- 51.Della Corte A, Bancone C, Quarto C, et al. Predictors of ascending aortic dilatation with bicuspid aortic valve: a wide spectrum of disease expression. Eur J Cardiothorac Surg. 2007;31:397–404. doi: 10.1016/j.ejcts.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 52.Loeys BL, Schwarze U, Holm T, et al. Aneurysm syndromes caused by mutations in the TGF-beta receptor. N Engl J Med. 2006;355:788–798. doi: 10.1056/NEJMoa055695. [DOI] [PubMed] [Google Scholar]