Abstract
The thalamocortical pathway is the main route of communication between the eye and the cerebral cortex. During embryonic development, thalamocortical afferents travel to cortical layer 4 and are sorted by receptive field position, eye of origin and contrast polarity (preference for light or dark stimuli). In primates and carnivores, this sorting involves a large number of afferents most of which sample a limited region of the binocular field. Devoting abundant thalamocortical resources to process a limited visual field has a clear advantage: it allows sampling a large number of stimulus combinations at each spatial location. Moreover, the sampling efficiency can be further enhanced by organizing the afferents in a cortical grid for eye input and contrast polarity. We argue that thalamocortical interactions within this eye/polarity grid can be used to represent multiple stimulus combinations found in nature and build an accurate cortical map for multi-dimensional stimulus space.
Keywords: thalamus, visual cortex, thalamocortical, cortical map, receptive field, visual development
INTRODUCTION
The primary visual cortex (area V1) receives its main sensory input from the dorsal Lateral Geniculate Nucleus (LGN) of the thalamus. During thalamocortical development, the geniculate afferents become densely packed within cortical layer 4, forming a rich functional network that can be used to build a precise map of stimulus space. It has been known for many decades that thalamic afferents are arranged in visual cortex based on retinotopy (retinal location of the stimulus) and eye input (Daniel & Whitteridge 1961, Hubel & Wiesel 1972). However, processing visual stimuli not only requires knowing the location of a stimulus within each retina but also knowing the light/dark polarity and orientation of the different stimulus parts. Both light/dark polarity (Kremkow et al 2016, Lee et al 2016, Smith et al 2015, Wang et al 2015) and stimulus orientation (Bonhoeffer & Grinvald 1991, Hubel & Wiesel 1962, Obermayer & Blasdel 1993) are systematically mapped in the primary visual cortex of carnivores as are other stimulus dimensions such as spatial frequency (Hubener et al 1997, Issa et al 2000, Yu et al 2005), retinal disparity (Kara & Boyd 2009), and direction of motion (Shmuel & Grinvald 1996, Weliky et al 1996). The cortical map for stimulus orientation has been studied in great detail (Bonhoeffer & Grinvald 1991, Hubener et al 1997, Kaschube et al 2010, Obermayer & Blasdel 1993), however, its relations with the maps for other stimulus dimensions remain unclear (Bosking et al 2002, Das & Gilbert 1997, Hubener et al 1997, Nauhaus et al 2016, Nauhaus et al 2012, Yu et al 2005). Recent work suggests that the maps for all stimulus dimensions have a common origin: the thalamocortical network (Erwin & Miller 1998, Jin et al 2011b, Jin et al 2008, Kremkow et al 2016, Nauhaus & Nielsen 2014, Paik & Ringach 2011, Wang et al 2015). This common origin should generate systematic relations across the maps for different stimulus dimensions, which can be experimentally tested. Here, we review our current understanding of the geniculocortical network within layer 4 of area V1 and its relations with the visual cortical topography for the different stimulus dimensions.
GENICULOCORTICAL CIRCUITS AND CORTICAL RECEPTIVE FIELDS
Each V1 neuron in layer 4 receives input from a limited number of thalamic afferents that shape the geometry of the cortical receptive field. This section reviews the organization of the main geniculocortical pathways converging on cortical layer 4 and the geniculocortical circuits involved in receptive field construction. The geniculocortical pathway also projects to other cortical layers (Callaway 1998, Casagrande & Xu 2004, Chatterjee & Callaway 2003, Sherman & Guillery 1996), however, these projections are usually weaker (Sherman & Guillery 1998) and are beyond the scope of this review.
Functional organization of geniculocortical pathways
The human eyes have an average axial length of 24 mm (Hitzenberger 1991) with an average separation of 63 mm (Dodgson 2004). The eye separation varies as a function of age, gender and race by as much as 36 mm (44 to 80 mm), although the range in most adults is from 50 and 75 mm (Dodgson 2004). This eye separation provides humans with a horizontal visual field of 220 degrees, which extends 110 degrees temporally (Traquair 1938) and overlaps nasally 120 degrees (Figure 1, top). The field of view in humans is considerably smaller than the nearly panoramic view of other animals such as rabbits, which can cover 350 degrees (Hughes 1971). Moreover, the visual field of primates is further restricted by a pronounced retinal bias towards central vision. For example, 30% of all retinal ganglion cells in squirrel monkeys are located within the central 8 degrees and 90% are located within the central 50 degrees (Adams & Horton 2003b).
Figure 1. Human visual pathway.
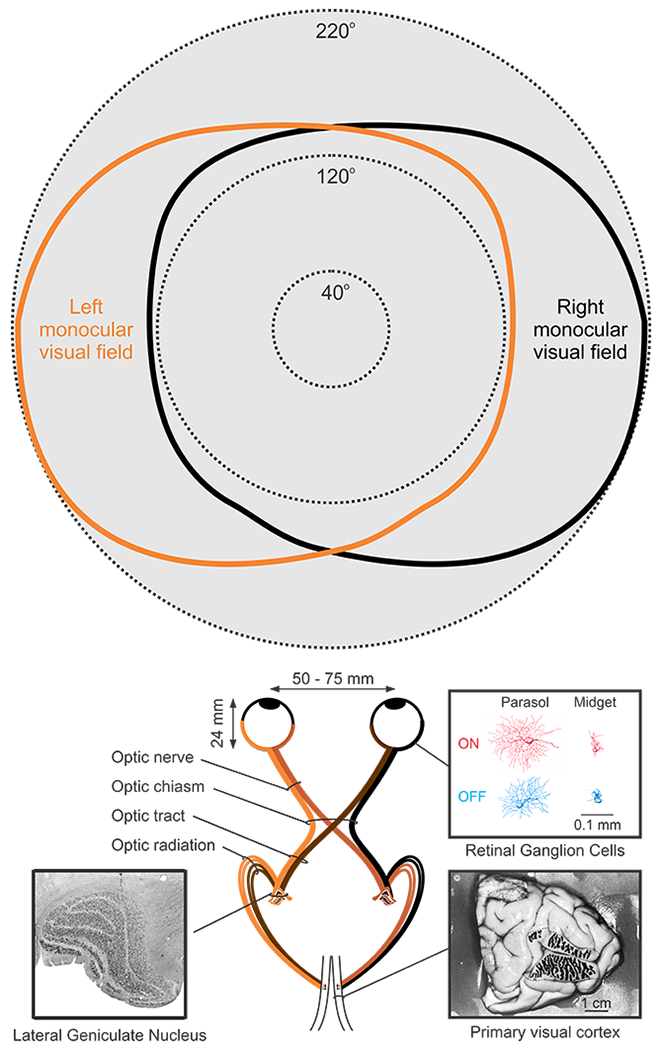
Top. Monocular and binocular visual field in humans (redrawn from (Traquair 1938)). Bottom. Visual pathway from the eye to primary visual cortex. Insets illustrate the human Lateral Geniculate Nucleus (left, reproduced from (Briggs & Usrey 2011)), retinal ganglion cells (right, top, reproduced from (Dacey & Petersen 1992)) and ocular dominance bands in primary visual cortex (right, bottom, reproduced from (Horton & Hedley-Whyte 1984)).
Such pronounced dedication of retinal resources to central vision is common in primates and carnivores. In these animals, the majority of retinal ganglion cells project to the LGN (Figure 1, bottom), leaving only a minority to target other subcortical structures (Bunt et al 1975, Illing & Wassle 1981, Perry & Cowey 1984). This strong retinal bias towards the LGN projection further reflects the pronounced dedication of primates and carnivores to central vision, which is important to process visual detail. For comparison, the majority of retinal ganglion cells in the mouse project to the superior colliculus (Ellis et al 2016) and other subcortical structures that are important for visual navigation but not high-resolution vision (Dhande et al 2015). As evolutionary pressure for binocular vision increases, the angle between the visual axes of the eyes decreases, the binocular field becomes larger and the percentage of retinal ganglion cells projecting to the LGN also increases (Figure 2a). The increase in the number of retinogeniculate connections is associated with an increase in the number of geniculate afferents and the V1 area available to accommodate them. Importantly, the size of area V1 does not increase linearly with the number of afferents but exponentially (Mazade & Alonso 2017, Stevens 2001), which leads to an overexpansion of the cortical space (Figure 2b–c) available to sort the different afferent types (Mazade & Alonso 2017).
Figure 2. Overexpansion of area V1 as the number of LGN afferents increases during evolution.
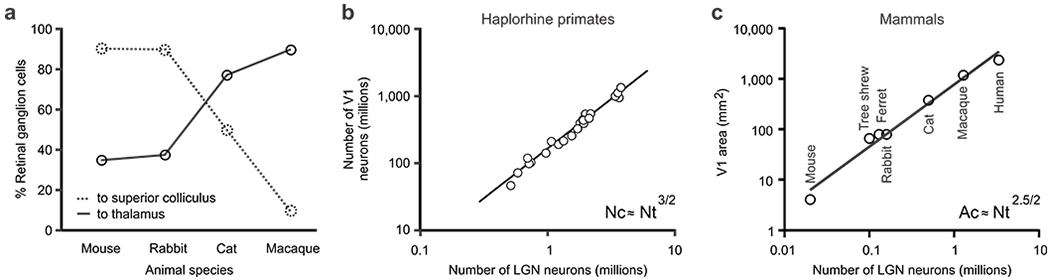
a. Percentage of retinal ganglion cells projecting to thalamus and superior colliculus in the mouse (Ellis et al 2016, Martin 1986, Seabrook et al 2017), rabbit (Vaney et al 1981), cat (Illing & Wassle 1981, Wassle & Illing 1980) and macaque (Bunt et al 1975, Perry & Cowey 1984). b. Relation between the number of LGN and V1 cells in haplorhine primates (reproduced from (Stevens 2001). c. Relation between the number of LGN cells and V1 area in mammals commonly used to study vision (reproduced from (Mazade & Alonso 2017)). Both relations in b and c follow a similar power law with an exponent close to 3/2. Nc: number of cortical neurons. Nt: number of thalamic geniculate neurons. Ac: area of cortex (V1) in mm2.
The afferents from the retina to LGN are organized in two main streams that signal different contrast polarities of the stimulus, light and dark. The ON pathway responds to light increments (light ON) and the OFF pathway to light decrements (light OFF). Each of these pathways split in three other parallel pathways that differ in spatial resolution, temporal resolution, contrast sensitivity and conduction velocity among other properties. These retinal pathways, called Parvocellular, Magnocellular and Koniocellular in primates, and X, Y and W in cats (Casagrande & Xu 2004, Chatterjee & Callaway 2003, Enroth-Cugell & Robson 1966, Hendry & Reid 2000), remain segregated in LGN and form separate geniculocortical pathways that also segregate through the depth of the cortex. The Parvocellular/Magnocellular/Koniocellular pathways in primates are far from being the functional equivalent to the X/Y/W pathways in cats (Kaplan & Shapley 1982), however, they have similar laminar projections in visual cortex. The Parvocellular/X pathway projects most densely to the bottom of layer 4, the Magnocellular/Y to the top of layer 4 and the Koniocellular/W to layer 3 and the top of layer 4 (Blasdel & Lund 1983, Chatterjee & Callaway 2003, Gilbert & Wiesel 1979, Humphrey et al 1985). Unlike the Parvocellular/X, Magnocellular/Y and Koniocellular/W pathways, the ON and OFF visual pathways are well preserved across all animals with form vision, from invertebrates to humans (Dacey & Petersen 1992, Joesch et al 2010). Moreover, just as for retinotopy and eye input, ON and OFF afferents segregate horizontally in the visual cortex of carnivores (Jin et al 2008, McConnell & LeVay 1984, Zahs & Stryker 1988) and probably also primates (Kremkow et al 2016).
The geniculocortical pathways of humans, macaques and cats share several important features. They all show a strong bias towards central vision, sample a limited horizontal visual field and an extensive binocular field, and ~90% of their V1 areas are used to process binocular vision. Due to the pronounced cortical magnification of central vision, each square millimeter of cortex can accommodate hundreds of geniculate afferents with the same retinotopy in these animals. Consequently, the afferents with the same retinotopy can be sorted by eye input and contrast polarity to sample a large number of eye/polarity stimulus combinations (Kremkow et al 2016). Because neurons with similar properties are more likely to be interconnected (Bosking et al 1997, Cossell et al 2015, Gilbert & Wiesel 1989, Ko et al 2011) and share inputs and targets (Alonso et al 1996, Morgenstern et al 2016), total axon length can be minimized by keeping neurons and afferents with similar properties close together in cortical space (Cajal 1995, Chklovskii et al 2002, Mitchison 1991). In cat visual cortex, the geniculate afferents are arranged in an eye/polarity grid that can be used to build cortical maps for multiple stimulus dimensions (Jin et al 2011b, Kremkow et al 2016, Wang et al 2015). We hypothesize that this eye/polarity grid is also present in the visual cortex of other animals with a strong bias towards central binocular vision. Consistent with this hypothesis, the sorting of geniculate afferents by eye input has been demonstrated in the visual cortex of tree shrews (Kretz et al 1986), ferrets (Law et al 1988), cats (Anderson et al 1988), macaques (Hubel & Wiesel 1972) and humans (Horton & Hedley-Whyte 1984), and the sorting by contrast polarity has been demonstrated in the visual cortex of tree shrews (Hubel 1975, Kretz et al 1986), minks (McConnell & LeVay 1984), ferrets (Zahs & Stryker 1988), cats (Jin et al 2008), and is probably also present in macaques (Kremkow et al 2016). In most of these animals, the thalamic afferents are sorted by eye input and contrast polarity through the horizontal dimension of visual cortex. In tree shrews, the sorting is done through the vertical dimension of layer 4 (e.g. ON afferents at the top of OFF afferents), for reasons that remain unclear. Interestingly, unlike rodents, cats and primates, the thalamic terminals in tree shrews have an extremely thin elliptical shape, with the ellipse major axis being almost an order of magnitude longer than the shortest axis. Because of this unusual shape and arrangement, we have previously suggested that the packing of thalamic afferents in tree shrew cortex may provide the best compromise between the need for binocular vision and the limited cortical space available to accommodate the afferents in a small brain (Mazade & Alonso 2017).
Development of geniculocortical pathways
The thalamic afferents to visual cortex are sorted during embryonic development through mechanisms that are still poorly understood. In humans, cortical neurons are born in the wall of the lateral ventricle around 6-18 weeks of gestation (Rakic 1995) at an outstanding rate of 225,000 neurons per minute (Uylings 2001). The newborn neurons start migrating through radial glial fibers towards the brain surface (marginal zone) forming an intermediate zone of migration and a primordial layer of polymorphic neurons (Marin-Padilla 1971). This primordial layer then splits into two tiers (Figure 3a): a cortical plate that will become the mature cortex and a transitory subplate that plays a major role in guiding the thalamocortical innervation (Allendoerfer & Shatz 1994, Hoerder-Suabedissen & Molnar 2015). The thalamic afferents accumulate in the subplate until the newborn cortical neurons reach cortical layer 4 (Figure 3b), a temporal period that lasts just three days in rats (Lund & Mustari 1977) but extends to several weeks in cats and primates (Rakic 1977, Shatz & Luskin 1986). The prolonged period that the thalamic afferents spend in the subplate seems ideal to build a primordial map of stimulus space through thalamic afferent sorting. Consistent with its important role in afferent sorting, sensory maps cannot properly develop when the subplate is ablated (Kanold & Shatz 2006).
Figure 3. Development of thalamocortical afferents.
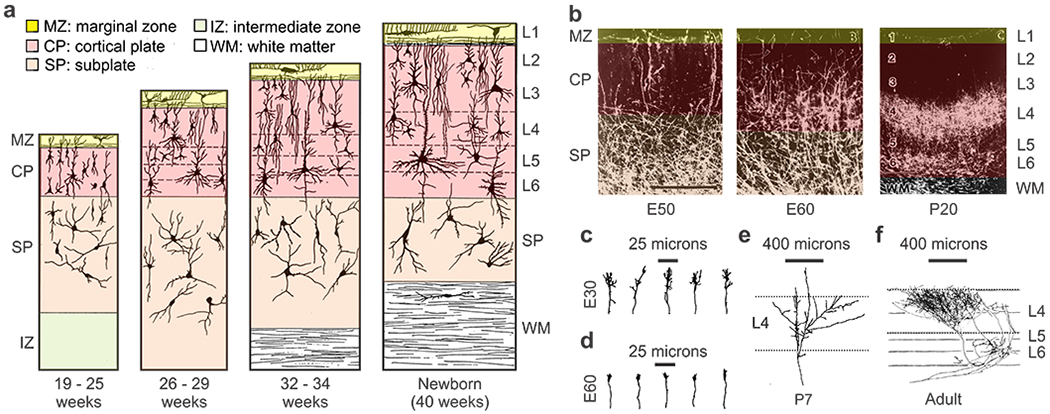
a. Prenatal development of human frontal cortex. b. Prenatal development of geniculate afferents in the cat at embryonic day 50 (left), 60 (middle) and 20 days after birth (right). Cats are born at embryonic day 65 (E65). c-f. Morphology of single geniculate afferents in the cat at embryonic days 30 (c), 60 (d), postnatal day 7 (e) and the adult (f). Reproduced from (Mrzljak et al 1988) for a, (Ghosh & Shatz 1992) for b-e and (Humphrey et al 1985) for f.
Axon interactions within the optic radiation also play a major role in afferent sorting (Reese & Cowey 1988). The growth cones of cat geniculate afferents are more elaborate as they travel through the optic radiations at embryonic day 30 (Figure 3c) than when they start growing towards their specific targets at embryonic day 60 (Figure 3d, (Ghosh & Shatz 1992)), as is also the case for retinal afferents traveling within the optic nerve (Bovolenta & Mason 1987). In their journey through the optic radiations, the geniculate afferents are continuously remodeling their paths and often send collaterals to nonvisual areas (e.g. auditory cortex) that are later removed (Ghosh & Shatz 1992). When they finally reach layer 4, the afferents start branching rapidly (Figure 3e) to form the dense and highly localized axonal arbor typical of the adult cortex (Figure 3f).
Afferent sorting by retinotopy and eye input
The sorting of retinal afferents by retinotopy and eye input in LGN is guided by a combination of molecular cues and spontaneous neuronal activity (Seabrook et al 2017). The retinal afferents from each eye originate in different half-retinas (nasal or temporal) and, therefore, a retinal gradient of ephrins can control the position and shape of the LGN layers (Huberman et al 2005b). In cat LGN, retinal afferents from the contralateral eye arrive three days earlier than afferents from the ipsilateral eye (embryonic days 32 and 35) and afferent convergence makes most geniculate cells binocular (Shatz 1983, Shatz & Kirkwood 1984). Around embryonic day 47, the afferents start segregating (Shatz 1983) and each geniculate neuron loses the inputs from one eye while strengthening the inputs from the other (Shatz & Kirkwood 1984). The segregation of retinal afferents by eye input occurs even earlier in primates and is complete around the second trimester of gestation in both macaques and humans (Hevner 2000, Huberman et al 2005a, Rakic 1976).
The sorting of geniculate afferents by retinotopy is also guided by a gradient of ephrins in mouse visual cortex (Cang et al 2005) and is followed by a segregation by eye input that is heavily dependent on neuronal activity (Crair et al 1998, Crowley & Katz 1999, LeVay et al 1978, Rakic 1976, Ruthazer et al 1999). While the precise temporal windows for eye-input segregation remain unknown, the afferents from contralateral and ipsilateral eyes are already segregated three weeks before birth in macaques (Rakic 1976) and two weeks after birth in cats (LeVay et al 1978). The late segregation of geniculate afferents by eye input provides an excellent opportunity for visual experience to fine tune the geniculocortical network underlying the cortical map of visual space (Adams et al 2007, Hubel et al 1977, Wiesel & Hubel 1963).
Afferent sorting by ON-OFF contrast polarity
Retinal afferents segregate by ON-OFF contrast polarity later than by eye input (Speer et al 2010), and the ON-OFF segregation is likely to occur simultaneously in retina, LGN and visual cortex. In the retina, the dendrites from ON and OFF retinal ganglion cells are mixed within the inner plexiform layer at early stages of development and segregate very slowly after. In cats, the segregation process starts 2 weeks before birth and can take several months to be completed (Bodnarenko et al 1995). If the ON pathway is inactivated during this period (e.g. one month after birth), the number of retinal ganglion cells with mixed ON and OFF responses increases (Bisti et al 1998).
ON and OFF retinal afferents segregate in different sublayers of LGN in ferrets (Stryker & Zahs 1983), tree shrews (Conway & Schiller 1983), minks (LeVay & McConnell 1982), cats (Berman & Payne 1989, Bowling & Wieniawa-Narkiewicz 1986) and macaques (Schiller & Malpeli 1978). In all these animals, OFF afferents are in layers/sublayers closer to the optic tract than ON afferents for reasons that remain unclear. In ferrets, the segregation of retinal afferents by ON-OFF polarity starts 10 days after the segregation by eye input (20 days after birth) and takes 5 days to be completed (Figure 4) (Speer et al 2010).
Figure 4. Development of ON-OFF segregation in ferret LGN.
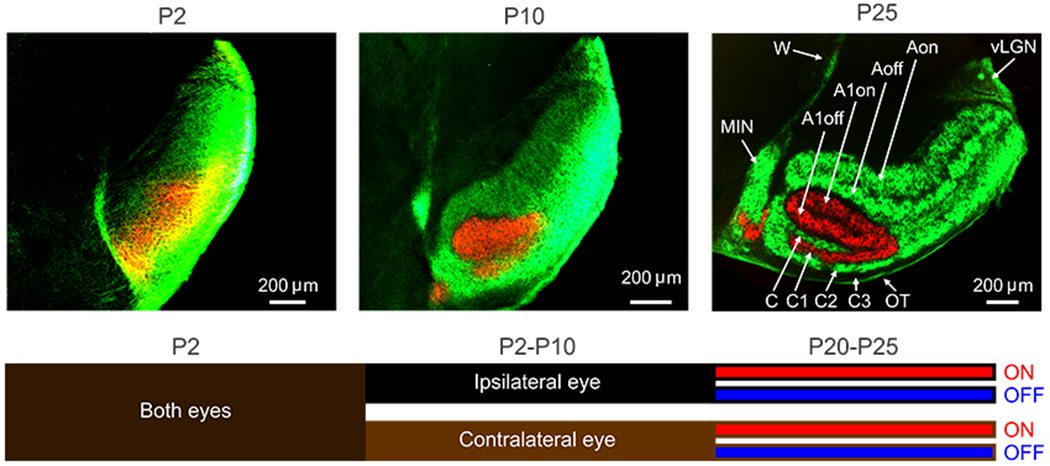
Top. Ferret LGN 2 days (P2), 10 days (P10) and 25 days (P25) after birth. Eye segregation is completed at P10 and ON-OFF segregation is completed at P25. A, C, C2: contralateral layers, A1, C1: ipsilateral layers. C3: layer not receiving retinal input, OT: optic tract, MIN: medial interlaminar nucleus, vLGN: ventral lateral geniculate nucleus, W: wing of the geniculate, on: on layers, off: off layers. Reproduced from (Speer et al 2010). Bottom. Cartoon illustrating the stages of retinal afferent segregation in LGN, first by eye and later by ON-OFF contrast polarity (based on (Speer et al 2010)).
While many questions remain about the process of ON-OFF segregation in visual cortex (Hubel 1975, Jin et al 2008, Kremkow et al 2016, Kretz et al 1986, Zahs & Stryker 1988), its temporal sequence is likely to follow the afferent segregation in LGN: retinotopy eye input ON-OFF polarity (Figure 4). OFF afferents may also arrive earlier to the visual cortex than ON afferents since most cortical responses are OFF dominated at early postnatal stages of development in cats (Albus & Wolf 1984). While the precise temporal windows for ON-OFF cortical segregation remain unknown, the process is likely to be slow since the ON-OFF segregation can last several months in the cat retina (Bodnarenko et al 1995) and the binocular matching of contrast polarity is also a slow developmental process in mouse visual cortex (Sarnaik et al 2014). The slow process for ON-OFF cortical segregation provides another excellent opportunity for visual experience to fine tune the geniculocortical network and the cortical map of stimulus space.
Geniculocortical circuits and cortical receptive fields
Each layer 4 neuron receives convergent input from a small number of geniculate afferents that has been estimated to range between 20 to 50 in cat visual cortex (Alonso et al 2001, Freund et al 1985, Jin et al 2011b, Peters & Payne 1993, Stanley et al 2012). The geniculocortical connections made by this subset of afferents are very specific for retinotopy, eye input and contrast polarity. ON geniculate inputs from each eye are retinotopically aligned with the ON subregions of each layer 4 cortical receptive field and OFF geniculate inputs with the OFF subregions (Alonso et al 1996, Alonso et al 2001, Reid & Alonso 1995, Sedigh-Sarvestani et al 2017, Tanaka 1985). In addition, geniculate afferents with the same retinotopy and contrast polarity share input from the same retinal afferent (Alonso et al 1996), providing the entire retino-geniculo-cortical network with exquisite connection specificity (Figure 5a). Such connection specificity is important to maximize the spatial resolution of the visual system and to build receptive fields with parallel ON and OFF subregions that make cortical cells orientation selective (Alonso et al 2001, Ferster 1987, Ferster et al 1996, Hirsch et al 1998, Hubel & Wiesel 1962, Lien & Scanziani 2013, Reid & Alonso 1995).
Figure 5. Geniculocortical convergence in visual cortex.
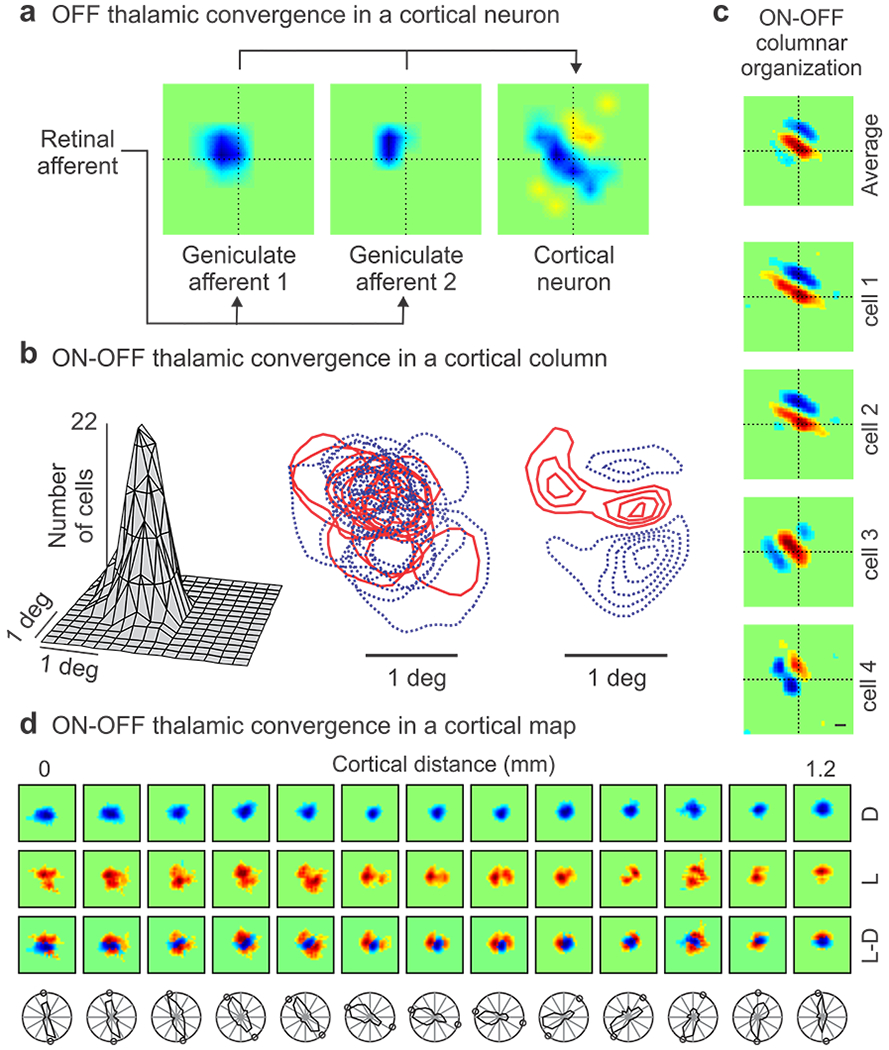
a. Receptive fields from two OFF geniculate neurons (left and middle) that share input from the same retinal afferent and make monosynaptic connection with the same OFF-dominated cortical cell (right). b. Receptive field scatter from geniculate afferents converging within a cortical column of ~ 200 microns in diameter. From left to right, receptive-field spread-function, superimposed ON (red) and OFF (blue) receptive fields, population receptive field obtained by ON-OFF subtraction. c. ON and OFF receptive field subregions in single neurons recorded within a vertical track in cortical layer 4 and their average receptive field. d. Top. Change in retinotopy through the horizontal dimension of visual cortex for responses to dark (D), light (L) and light-dark (L-D). Bottom. Same for orientation preference. Circles show predicted orientation from L-D receptive fields. Reproduced from (Alonso et al 1996) for a, (Jin et al 2011b) for b, (Wang et al 2015) for c and (Kremkow et al 2016) for d.
The geniculocortical specificity is lower for inhibitory than excitatory neurons, making cortical inhibition less selective but more sensitive to stimuli than cortical excitation (Alonso & Swadlow 2005, Sedigh-Sarvestani et al 2017). The same difference in specificity between excitatory and inhibitory neurons is found in intracortical connections. Intracortical excitatory connections link cells with similar response properties (Bosking et al 1997, Cossell et al 2015, Gilbert & Wiesel 1989, Ko et al 2011) and often share common geniculate inputs (Morgenstern et al 2016). In contrast, intracortical inhibitory connections are less selective (Karnani et al 2014) and can be linked by electrical gap junctions with poor connection specificity (Fukuda et al 2006). Thus, the entire geniculocortical network is organized in clusters of afferents and cortical targets that share similar response properties and reserve the highest connection specificity for cortical excitation.
The connection specificity of the geniculocortical network is facilitated in great part by the local sorting of geniculate afferents within the cortex. Geniculate afferents making monosynaptic connections within the same cortical column share the same retinotopy and eye input. However, their ON and OFF receptive fields overlap extensively while representing the same point in visual space (Figure 5b, left). This prioritization of retinotopy in afferent sorting helps maximize visual resolution and provides each cortical column with enough diversity of ON and OFF receptive field positions (Figure 5b, middle) to represent multiple combinations of light and dark stimulus arrangements. At the same time, the population receptive field of the geniculate afferents (ON-OFF average) is strongly biased towards a specific orientation and ON-OFF geometry (horizontal, ON-flanked-by-OFF in Figure 5b, right). This bias creates clusters of cortical neurons with receptive fields sharing dominant subregions of similar retinotopy and contrast polarity (e.g. ON-dominant subregion centered at the intersection of dotted lines in figure 5c). Moreover, because the ON and OFF geniculate afferents cluster in cortical space (Jin et al 2008), the ON-OFF dominance of the cortical receptive fields changes through the horizontal axes of the visual cortex. In regions dominated by OFF afferents, ON receptive fields tend to rotate around OFF receptive fields (as in the example from Figure 5d), and in regions dominated by ON afferents, OFF receptive fields tend to rotate around ON receptive fields. This receptive field rotation within the geniculocortical network is likely to play an important role in generating cortical maps for stimulus orientation.
GENICULOCORTICAL CIRCUITS AND CORTICAL MAPS
The horizontal organization of the geniculocortical network provides a rich substrate to map multiple stimulus dimensions within the cortex that include not only retinotopy and eye input but also light/dark polarity, stimulus orientation, direction, retinal disparity and spatial frequency. This section reviews the functional organization of the cortical maps for different stimulus dimensions and their relation with the horizontal organization of the geniculocortical network.
Cortical map for retinotopy
Probably the most important dimension to map in visual cortex is the retinal position of the stimulus. Humans, macaques and carnivores devote a large portion of their V1 areas to map the central retina, with a nearly equal split between upper and lower visual fields (Figure 6a–b, e–f). This retinotopic arrangement reflects the importance of high-resolution vision at the center of gaze in these animals. In contrast, the smaller V1 areas of rabbits and mice are used to map a much larger portion of the visual field with a heavy bias towards the upper half, as most predators approach these animals from above (Figure 6c–d, g–h). The differences in cortical retinotopy across animals is strongly determined by the density of retinal afferents sampling different parts of the visual field (Adams & Horton 2003b, Azzopardi & Cowey 1993), which is replicated by the density of geniculate afferents (Azzopardi & Cowey 1996). The highest density is at the center of gaze in humans (Curcio & Allen 1990), macaques (Adams & Horton 2003b) and cats (Hughes 1975), and at the retinal region aligned with the visual horizon (horizontal streak) in rabbits and mice (Drager & Olsen 1981, Hughes 1971). These retinotopic maps provide clear examples of how the geniculocortical network builds a cortical map of visual space by closely replicating and further amplifying sampling asymmetries that are already present at the retina (Adams & Horton 2003b, Azzopardi & Cowey 1993, Azzopardi & Cowey 1996).
Figure 6. Cortical map for retinotopy.
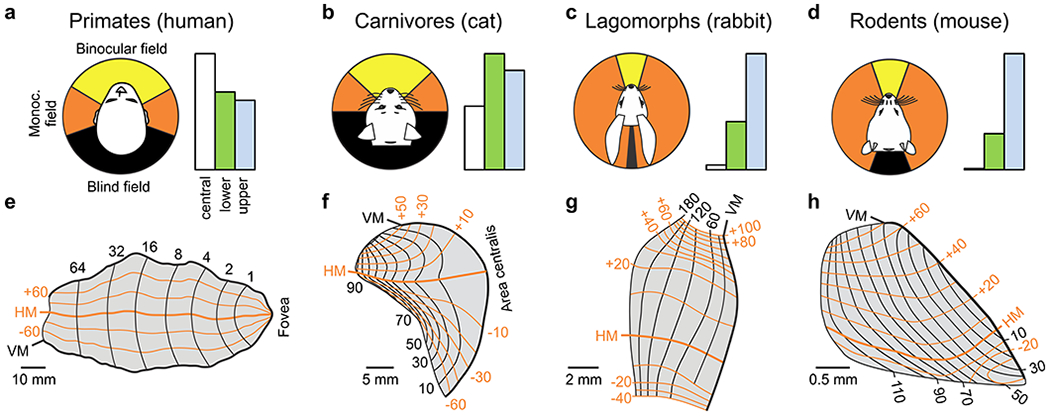
a-d. Visual fields and their cortical representation in humans (a), cats (b), rabbits (c) and mice (d). The left panel shows the binocular field (yellow), monocular field (orange) and the blind field (black). Values from (Mazade & Alonso 2017). The right panel shows the percentage of area V1 devoted to central vision (white, central 10 degrees), lower (green) and upper visual fields (blue). Percentages of blue bars are 26% (a), 36% (b), 69% (c), and 76% (d). Values obtained from retinotopic maps in e-h. e-h. Retinotopic maps of area V1 in the human, cat, rabbit and mouse. Redrawn from (Adams et al 2007) for a, (Tusa et al 1978) for b, (Hughes 1971) for c, and (Ji et al 2015) for d.
Cortical map for ocular dominance
A second important dimension to map in visual cortex is eye input. In animals with a limited binocular field, the afferents from the contralateral eye are more numerous, have better spatial resolution (Salinas et al 2017) and spread less within the cortex (Raczkowski & Fitzpatrick 1990) than the afferents from the ipsilateral eye. As the afferents from the ipsilateral eye increase in number and start covering a larger cortical territory, they also start segregating. The importance of afferent number for ocular-dominance segregation has been clearly demonstrated in the optic tectum of frogs and fishes. The optic tectum normally receives input from only one eye, however, when retinal afferents from two eyes are forced within the same tectum, they segregate in different eye domains (Boss & Schmidt 1984, Constantine-Paton & Law 1978). The importance of ipsilateral cortical spread for ocular-dominance segregation has been demonstrated in rodents. As the afferents from the ipsilateral eye start covering a larger cortical territory, a rudimentary segregation for ocular dominance emerges in both mutant mice (Merlin et al 2013) and certain types of rats (Laing et al 2015). Finally, in animals that devote most of their cortex to binocular vision, the ratio between contralateral/ipsilateral axonal projections approaches equality although still showing a bias towards the contralateral eye (Adams et al 2007, Anderson et al 1988, Shatz & Stryker 1978). The magnitude of ocular dominance segregation in binocular cortex can vary greatly across animals, even within the same species (Adams & Horton 2003a), probably reflecting variations in the number of afferents and amount of cortical area available to accommodate them.
The afferent segregation by eye input splits the map of cortical retinotopy into two intercalated copies, one for each eye. At the same time, asymmetries in cortical retinotopy distort the cortical map for ocular dominance (Blasdel & Campbell 2001, Hubel & Wiesel 1977, Tootell et al 1988, Van Essen et al 1984). Asymmetries in cortical retinotopy can explain why ocular dominance columns take different shapes in different animals and why ocular dominance columns tend to run perpendicular to the border between areas V1 and V2 in macaques and humans (Figure 7a,b, (Adams et al 2007, Blasdel & Campbell 2001)). Because retinotopy changes continuously across the cortex (Bosking et al 2002, Kremkow et al 2016), maximizing the retinotopy binocular match requires aligning the width ocular dominance bands along the cortical axis with the slowest retinotopy change. The cortical axis with the slowest retinotopic change is the axis with the largest magnification factor (i.e. the axis that devotes more cortical area to represent one degree of visual field, (Daniel & Whitteridge 1961)). Because the cortical magnification factor is 1.6 times greater along the length than the width of the V1/V2 border in macaques (Blasdel & Campbell 2001, Tootell et al 1988, Van Essen et al 1984), the ocular dominance bands become perpendicular to this border. Consistent with this interpretation, the main axis of the ocular dominance bands tends to be perpendicular to the cortical axis with the largest magnification factor in macaques, and the angle between both is tightly constrained within 60-90 degrees (Blasdel & Campbell 2001). Also consistent with this interpretation, ocular dominance bands are parallel in cortical regions with strong magnification asymmetries but break apart in regions with smaller asymmetries (Blasdel & Campbell 2001). Finally, primates and carnivores with limited asymmetries in cortical magnification do not show ocular dominance bands running perpendicular to the V1/V2 border or show a much weaker trend (Anderson et al 1988, Blasdel & Campbell 2001, Schmidt et al 2002, Takahata et al 2014).
Figure 7. Cortical map for ocular dominance.
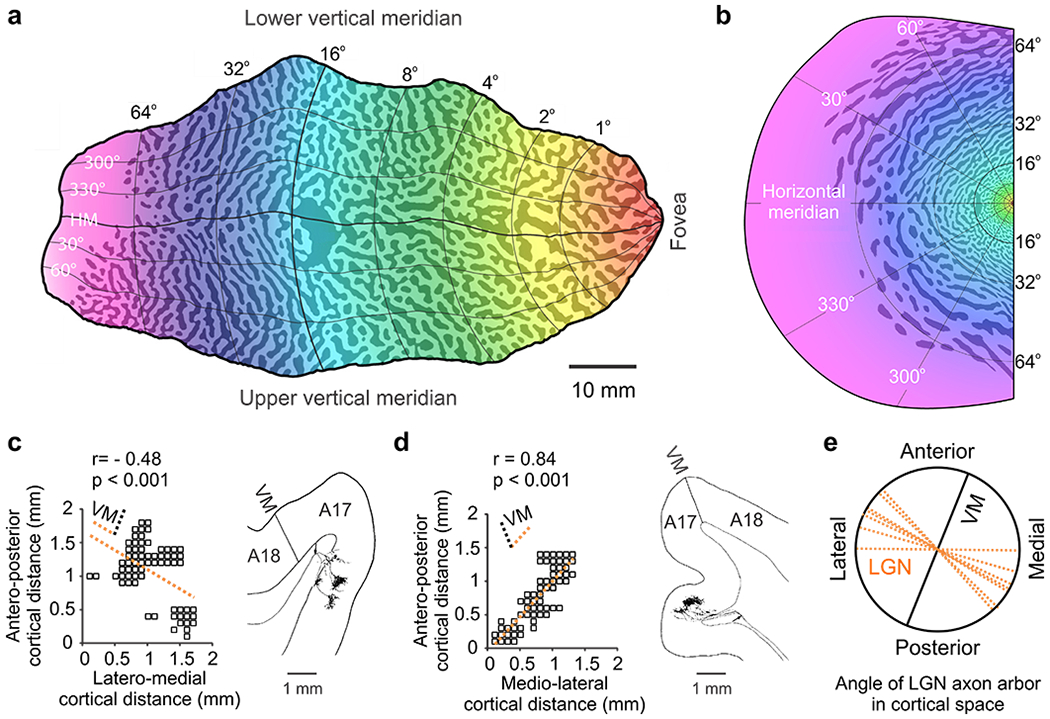
a. Ocular dominance map in human V1 (the color gradient represents changes in retinotopy). b. Ocular dominance map superimposed on the human visual field. Notice how the ocular dominance bands rotate around the center of vision and are perpendicular to the vertical meridian, which is represented in the V1/V2 border. c-d. Geniculate axonal arbors from cat visual cortex showing their spread in cortical space in horizontal (left) and coronal sections (right). Notice that the main axis of the arbor (dotted orange line) tends to be tilted to the left in the left hemisphere (c) and right in the right hemisphere (d), and perpendicular to the vertical meridian (VM). Notice also how the axon arbor splits in clusters along the longest axis, providing the anatomical basis for ocular dominance columns. e. The main axis of the axon arbors in cat area V1 shows a strong tendency to be perpendicular to the vertical meridian. Reproduced from (Adams et al 2007) for a-b and from (Humphrey et al 1985) for c-e.
Although not previously reported (Humphrey et al 1985), the main axes of geniculate axonal arbors also show a strong tendency to run perpendicular to the V1/V2 border in cat area V1 (Figure 7c–e). This tendency matches the cortical retinotopic asymmetries in this animal (e.g. greater magnification along the width than the length of the V1/V2 border, particularly within the lower visual field, Figure 6f). Importantly, the axon arbors showing the largest length/width ratios in cat area V1 (Figure 7c–d) tend to split in patches to accommodate ocular dominance columns, as in macaques (Blasdel & Lund 1983). Therefore, regardless of differences in cortical retinotopy across animals, the width of the ocular dominance columns tends to be aligned with the cortical axis of largest magnification factor, probably to maximize the binocular match in cortical retinotopy. Consequently, asymmetries in visual sampling within the retina shape not only the retinotopic map but also the ocular dominance map in visual cortex.
Cortical map for light/dark polarity
A third dimension that needs to be mapped in visual cortex is the light-dark polarity of the stimulus. This map is likely to be particularly important in animals that need to discriminate different stimulus patterns of lights and darks in addition to the stimulus position. Having an accurate map of light-dark polarity requires splitting the retinotopic map not only into two but four separate copies, two for each eye and two for each polarity. Because afferents with similar retinotopy share similar cortical space, the four afferent types can be organized in an eye/polarity grid within the cortex. In cat visual cortex (Kremkow et al 2016), eye input changes every ~0.5 mm along the eye axis of the grid and polarity changes also every ~0.5 mm along the polarity axis (Figure 8). Therefore, ~1 mm2 of cortex can represent most combinations of eye input and polarity for each position of visual space. Importantly, because retinotopy changes slowly within the cortex at a rate of 0.5 receptive field centers per mm, all neurons within a ~1 mm2 can represent the same point of visual space (Kremkow et al 2016). The same point is covered by cortical receptive field centers at the center of the ~1 mm2 surface and by half receptive field centers at the edge. The slow retinotopy progression through the eye/polarity grid avoids the need for abrupt retinotopy jumps at the ocular dominance border, as originally proposed by Hubel and Wiesel (Hubel & Wiesel 1977). Moreover, by aligning the eye/polarity axes with the slowest/fastest retinotopic gradients of the cortical map, the eye/polarity grid maximizes both the binocular retinotopic match and the ON-OFF retinotopy separation needed to generate selectivity to stimulus orientation.
Figure 8. Cortical map for light/dark polarity.
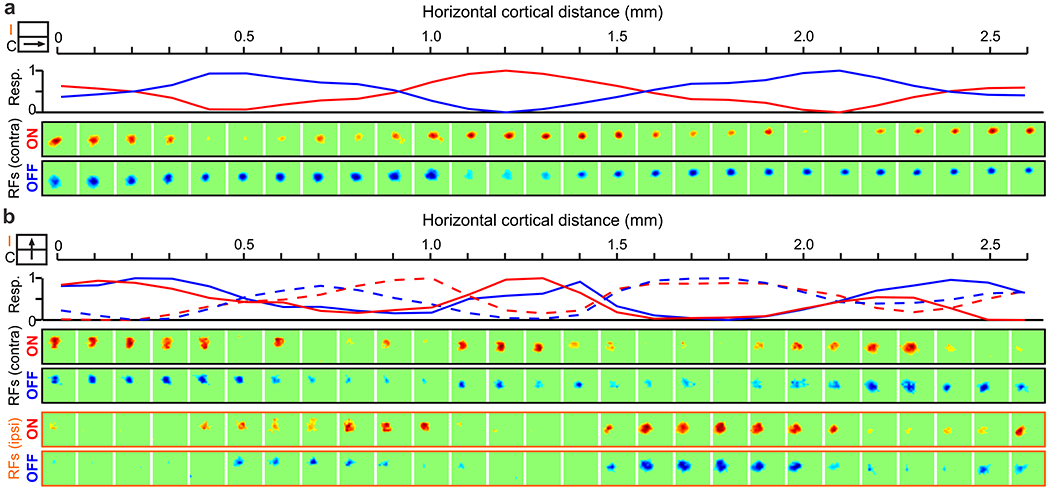
a. ON and OFF domains in a horizontal cortical track running along the length of an ocular dominance column (illustrated by cartoon in inset). From top to bottom, line plot showing normalized maximum ON (red) and OFF responses (blue), and receptive fields measured with light (ON) and dark stimuli (OFF). b. ON and OFF domains in a horizontal track running orthogonal to ocular dominance columns (inset). From top to bottom, normalized ON and OFF responses from contralateral (continuous lines) and ipsilateral eyes (dashed lines), and receptive fields mapped with dark and light stimuli from the contralateral eye (black frame) and ipsilateral eyes (orange frame). Reproduced from (Kremkow et al 2016).
The eye polarity grid does not represent equally the four types of afferents. Just as there is a cortical bias towards the contralateral eye, there is also a bias towards OFF geniculate afferents. OFF cortical responses are stronger, have better spatiotemporal resolution and show less spatial scatter in cortical retinotopy than ON cortical responses (Jin et al 2011a, Jin et al 2011b, Jin et al 2008, Komban et al 2014, Kremkow et al 2014, Kremkow et al 2016, Lee et al 2016, Wang et al 2015, Xing et al 2010, Yeh et al 2009, Zemon et al 1988). These ON-OFF asymmetries make the cortical map OFF dominated (OFF responses are stronger) and OFF centric (OFF retinotopy is more precise). ON-OFF cortical asymmetries in spatial resolution could be the result of the neuronal blur caused by the luminance/response saturation within the ON pathway (Kremkow et al 2014). Because this neuronal blur is likely to originate at the photoreceptor, it can also explain ON-OFF spatial asymmetries in the retina (Chichilnisky & Kalmar 2002, Dacey & Petersen 1992, Wassle et al 1981). Therefore, just as for retinotopy and ocular dominance, the cortical map for light-dark polarity replicates and amplifies asymmetries in visual sampling that are already present at the retina.
The eye/polarity grid and the multi-dimensional map of stimulus space
The eye/polarity grid (Figure 9a) formed by the geniculocortical network provides the seed to build cortical maps for multiple stimulus dimensions that include not only retinotopy, eye input and contrast polarity but also orientation, direction, retinal disparity and spatial frequency. Given their common origin, the gradients for the different stimulus dimensions should show systematic relations that can be experimentally tested. Some of these predicted relations are briefly discussed here and are currently being tested in our laboratories.
Figure 9. The eye/polarity grid and the multi-dimensional map of stimulus space.
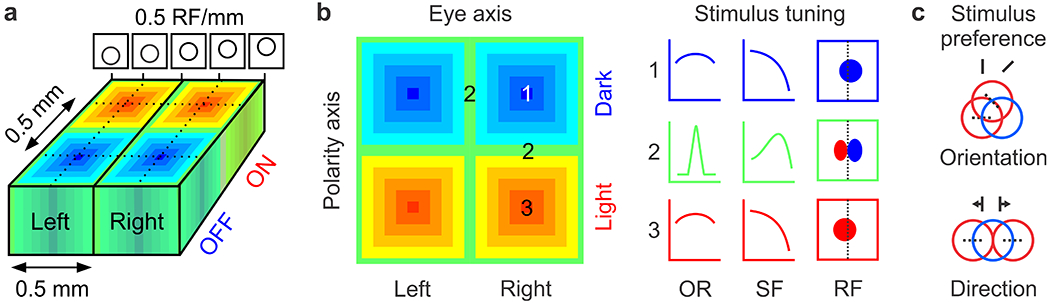
a. Cortical organization for retinotopy, eye input and ON-OFF contrast polarity. Top. Retinotopy illustrated as circular receptive fields (RF) changing position in the vertical axis at a slow rate of 0.5 RF/mm. Bottom. The orthogonal arrangement of geniculate afferents sorted by eye input and ON-OFF polarity forms ocular dominance columns and ON-OFF cortical domains. b. Left. Cortical responses within the eye/polarity grid can be OFF dominated (blue), ON dominated (red) or ON-OFF balanced (green). Right. Changes in ON-OFF balance should be associated with changes in orientation tuning (OR), spatial frequency tuning (SF) and spatial resolution (spatial frequency cutoff). The OR/SF tuning and spatial resolution should be lowest at the center of ON and OFF domains and increase in surrounding regions. c. Changes in ON-OFF retinotopy should be associated with changes in orientation (e.g. when ON receptive fields rotate around OFF receptive fields in the geniculocortical network) and direction (e.g. when ON receptive fields jump over OFF receptive fields). a,c Modified from (Kremkow et al 2016).
Multiple systematic relations should emerge from variations in ON-OFF response balance across the cortex. Because the centers of ON and OFF cortical domains are dominated by one afferent type, they should show the weakest ON-OFF response antagonism within the cortical map. In turn, the weak ON-OFF antagonism should make neurons responsive to a wide variety of stimulus orientations and spatial frequencies (Figure 9b, red and blue). By comparison, the cortical regions surrounding the centers of ON and OFF domains have more balanced ON and OFF afferent numbers, which should make the ON-OFF response antagonism stronger. In turn, the stronger ON-OFF antagonism should make cortical neurons more selective for orientation and spatial frequency (Figure 9b, green) while increasing their spatial resolution by narrowing the ON and OFF receptive field subregions (Figure 9b, right, green). These variations in ON-OFF response balance should generate strong correlations between orientation and spatial frequency tuning across the cortex. In addition, they should reserve the highest stimulus selectivity and spatial resolution for regions with the highest ON-OFF balance, which are at the borders of ocular dominance columns and ON-OFF domains (Figure 9b, right, green).
Changes in ON and OFF retinotopy should also be associated with changes in orientation and direction preference across the cortical map (Figure 9c, (Kremkow et al 2016)). Because the contrast polarity remains constant along the eye axis of the grid (Figure 9a), the monocular receptive fields of binocular neurons should have dominant subregions of the same polarity (Erwin & Miller 1998, Kremkow et al 2016, Sarnaik et al 2014, Wang et al 2015). Consequently, the retinal disparity of binocular neurons should emerge mostly from variations in the position of non-dominant receptive field subregions at the border of ocular dominance columns (Kremkow et al 2016). Moreover, because the cortical map is OFF dominated and OFF-centric, the most accurate mapping of spatial position and retinal disparity should be provided by OFF-dominated receptive fields, making spatial resolution and binocular vision higher for darks than lights to sample more efficiently the predominance of dark features in natural scenes (Cooper & Norcia 2015, Ratliff et al 2010). Finally, the functional map originating from variations in ON-OFF response balance should be further amplified and refined by the recurrent cortical network (Koch et al 2016). At regions strongly dominated by ON or OFF afferents, recurrent cortical excitation should reduce orientation selectivity, response suppression and increase luminance/response linearity, making these regions more effective at discriminating light-dark patterns with multiple orientations. At cortical regions with more balanced ON and OFF afferents, recurrent cortical excitation should increase orientation selectivity, cross-orientation suppression and contrast sensitivity, making these regions more effective at detecting moving borders (Koch et al 2016). In summary, sampling asymmetries already present at the retina are replicated and amplified by the geniculocortical network, and further refined by the intracortical network.
General implications of thalamocortical organization
The sorting of geniculate afferents by retinotopy, eye input and contrast polarity could be just one example of a common principle in thalamocortical organization. The thalamocortical network represents the most relevant stimulus dimensions for each sensory modality within the cortex (e.g. visual space, body surface, sound frequency). As the size of the thalamocortical network increases during evolution, the larger number of afferents representing different sensory modalities may force the cortical map to split in regions representing separate stimulus dimensions to form a multi-dimension grid of stimulus space. This grid can then help to combine different stimulus dimensions more efficiently and magnify the combinations that are most behaviorally relevant such as central vision, the contralateral eye and dark stimuli in carnivores and primates. A precise reconstruction of the thalamocortical network will be important in the future to understand the functional architecture of the visual cortex and the cortical substrate of visual deficits. Just as accurate geographical maps were essential for world exploration, a precise reconstruction of visual cortical topography will be essential to understand visual function and visual disease.
SUMMARY POINTS.
The increase in the number of geniculate afferents during evolution is associated with an overexpansion of the cortical area available to accommodate them. This overexpansion allows sorting afferents by multiple stimulus dimensions within the cortex.
Afferent sorting follows a temporal sequence that starts with retinotopy followed by eye input and light-dark polarity. This sequence has important functional implications as it should make the segregation for light-dark polarity the most strongly affected by visual experience.
Geniculate afferents with different eye input and contrast polarity are organized in an eye/polarity grid within the carnivore cortex, which allows representing multiple eye/polarity combinations for each point of visual space.
The eye/polarity grid provides a general framework to build a cortical map for multiple stimulus dimensions that represents some stimulus combinations better than others. Because the maps for all dimensions have a common origin, the different dimension gradients within the cortical map should show predictable relations that can be experimentally tested.
FUTURE ISSUES.
How are different stimulus patterns represented in visual cortex? Research over the past decades revealed maps for individual stimulus dimensions. However, we need a better understanding of how the different stimulus dimensions and stimulus patterns are combined within the cortical map.
We need to improve the accuracy of our visual cortical maps both at the level of the thalamocortical and intracortical networks. More accurate maps will lead to a better understanding of visual function and cortical deficits. The cortical substrate of some visual disorders may remain unknown due to the coarseness of our current cortical maps.
We need better tools to refine our measures of cortical topography. New multielectrode arrays and imaging methods hold great promise but still need to circumvent important technical limitations.
ACKNOWLEDGEMENTS
This work was supported by US National Institutes of Health grants EY005253 and EY027361 (JMA) and DFG (Emmy-Noether grant KR 4062/4-1, J.K.). We thank Mario Carta for his drawings to illustrate Figure 6 and Marty Usrey, Carla Shatz, Andrew Huberman, Colenso Speer, and Jonathan Horton for sending us original images from their work to illustrate Figures 1, 3, 4 and 7.
LITERATURE CITED
- Adams DL, Horton JC. 2003a. Capricious expression of cortical columns in the primate brain. Nature neuroscience 6: 113–4 [DOI] [PubMed] [Google Scholar]
- Adams DL, Horton JC. 2003b. A precise retinotopic map of primate striate cortex generated from the representation of angioscotomas. The Journal of neuroscience : the official journal of the Society for Neuroscience 23: 3771–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams DL, Sincich LC, Horton JC. 2007. Complete pattern of ocular dominance columns in human primary visual cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience 27: 10391–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albus K, Wolf W. 1984. Early post-natal development of neuronal function in the kitten’s visual cortex: a laminar analysis. The Journal of physiology 348: 153–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allendoerfer KL, Shatz CJ. 1994. The subplate, a transient neocortical structure: its role in the development of connections between thalamus and cortex. Annual review of neuroscience 17: 185–218 [DOI] [PubMed] [Google Scholar]
- Alonso JM, Swadlow HA. 2005. Thalamocortical specificity and the synthesis of sensory cortical receptive fields. Journal of neurophysiology 94: 26–32 [DOI] [PubMed] [Google Scholar]
- Alonso JM, Usrey WM, Reid RC. 1996. Precisely correlated firing in cells of the lateral geniculate nucleus. Nature 383: 815–9 [DOI] [PubMed] [Google Scholar]
- Alonso JM, Usrey WM, Reid RC. 2001. Rules of connectivity between geniculate cells and simple cells in cat primary visual cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience 21: 4002–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson PA, Olavarria J, Van Sluyters RC. 1988. The overall pattern of ocular dominance bands in cat visual cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience 8: 2183–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzopardi P, Cowey A. 1993. Preferential representation of the fovea in the primary visual cortex. Nature 361: 719–21 [DOI] [PubMed] [Google Scholar]
- Azzopardi P, Cowey A. 1996. The overrepresentation of the fovea and adjacent retina in the striate cortex and dorsal lateral geniculate nucleus of the macaque monkey. Neuroscience 72: 627–39 [DOI] [PubMed] [Google Scholar]
- Berman NE, Payne BR. 1989. Modular organization of ON and OFF responses in the cat lateral geniculate nucleus. Neuroscience 32: 721–37 [DOI] [PubMed] [Google Scholar]
- Bisti S, Gargini C, Chalupa LM. 1998. Blockade of glutamate-mediated activity in the developing retina perturbs the functional segregation of ON and OFF pathways. The Journal of neuroscience : the official journal of the Society for Neuroscience 18: 5019–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasdel G, Campbell D. 2001. Functional retinotopy of monkey visual cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience 21: 8286–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasdel GG, Lund JS. 1983. Termination of afferent axons in macaque striate cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience 3: 1389–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnarenko SR, Jeyarasasingam G, Chalupa LM. 1995. Development and regulation of dendritic stratification in retinal ganglion cells by glutamate-mediated afferent activity. The Journal of neuroscience : the official journal of the Society for Neuroscience 15: 7037–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonhoeffer T, Grinvald A. 1991. Iso-orientation domains in cat visual cortex are arranged in pinwheel-like patterns. Nature 353: 429–31 [DOI] [PubMed] [Google Scholar]
- Bosking WH, Crowley JC, Fitzpatrick D. 2002. Spatial coding of position and orientation in primary visual cortex. Nature neuroscience 5: 874–82 [DOI] [PubMed] [Google Scholar]
- Bosking WH, Zhang Y, Schofield B, Fitzpatrick D. 1997. Orientation selectivity and the arrangement of horizontal connections in tree shrew striate cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience 17: 2112–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boss VC, Schmidt JT. 1984. Activity and the formation of ocular dominance patches in dually innervated tectum of goldfish. The Journal of neuroscience : the official journal of the Society for Neuroscience 4: 2891–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovolenta P, Mason C. 1987. Growth cone morphology varies with position in the developing mouse visual pathway from retina to first targets. The Journal of neuroscience : the official journal of the Society for Neuroscience 7: 1447–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowling DB, Wieniawa-Narkiewicz E. 1986. The distribution of on- and off-centre X- and Y-like cells in the A layers of the cat’s lateral geniculate nucleus. The Journal of physiology 375: 561–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs F, Usrey WM. 2011. Corticogeniculate feedback and visual processing in the primate. The Journal of physiology 589: 33–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunt AH, Hendrickson AE, Lund JS, Lund RD, Fuchs AF. 1975. Monkey retinal ganglion cells: morphometric analysis and tracing of axonal projections, with a consideration of the peroxidase technique. The Journal of comparative neurology 164: 265–85 [DOI] [PubMed] [Google Scholar]
- Cajal SR. 1995. Histology of the nervous system of man and vertebrates. . Oxford University Press, USA. [Google Scholar]
- Callaway EM. 1998. Local circuits in primary visual cortex of the macaque monkey. Annu Rev Neurosci 21: 47–74 [DOI] [PubMed] [Google Scholar]
- Cang J, Kaneko M, Yamada J, Woods G, Stryker MP, Feldheim DA. 2005. Ephrin-as guide the formation of functional maps in the visual cortex. Neuron 48: 577–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casagrande VA, Xu X. 2004. Parallel visual pathways: a comparative perspective In The Visual Neurosciences, ed. Chalupa L, Werner JS, pp. 494–506: MIT Press [Google Scholar]
- Chatterjee S, Callaway EM. 2003. Parallel colour-opponent pathways to primary visual cortex. Nature 426: 668–71 [DOI] [PubMed] [Google Scholar]
- Chichilnisky EJ, Kalmar RS. 2002. Functional asymmetries in ON and OFF ganglion cells of primate retina. The Journal of neuroscience : the official journal of the Society for Neuroscience 22: 2737–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chklovskii DB, Schikorski T, Stevens CF. 2002. Wiring optimization in cortical circuits. Neuron 34: 341–7 [DOI] [PubMed] [Google Scholar]
- Constantine-Paton M, Law MI. 1978. Eye-specific termination bands in tecta of three-eyed frogs. Science 202: 639–41 [DOI] [PubMed] [Google Scholar]
- Conway JL, Schiller PH. 1983. Laminar organization of tree shrew dorsal lateral geniculate nucleus. Journal of neurophysiology 50: 1330–42 [DOI] [PubMed] [Google Scholar]
- Cooper EA, Norcia AM. 2015. Predicting cortical dark/bright asymmetries from natural image statistics and early visual transforms. PLoS computational biology 11: e1004268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossell L, Iacaruso MF, Muir DR, Houlton R, Sader EN, et al. 2015. Functional organization of excitatory synaptic strength in primary visual cortex. Nature 518: 399–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crair MC, Gillespie DC, Stryker MP. 1998. The role of visual experience in the development of columns in cat visual cortex. Science 279: 566–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley JC, Katz LC. 1999. Development of ocular dominance columns in the absence of retinal input. Nature neuroscience 2: 1125–30 [DOI] [PubMed] [Google Scholar]
- Curcio CA, Allen KA. 1990. Topography of ganglion cells in human retina. The Journal of comparative neurology 300: 5–25 [DOI] [PubMed] [Google Scholar]
- Dacey DM, Petersen MR. 1992. Dendritic field size and morphology of midget and parasol ganglion cells of the human retina. Proceedings of the National Academy of Sciences of the United States of America 89: 9666–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel PM, Whitteridge D. 1961. The representation of the visual field on the cerebral cortex in monkeys. The Journal of physiology 159: 203–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Gilbert CD. 1997. Distortions of visuotopic map match orientation singularities in primary visual cortex. Nature 387: 594–8 [DOI] [PubMed] [Google Scholar]
- Dhande OS, Stafford BK, Lim JA, Huberman AD. 2015. Contributions of Retinal Ganglion Cells to Subcortical Visual Processing and Behaviors. Annual review of vision science 1: 291–328 [DOI] [PubMed] [Google Scholar]
- Dodgson NA. 2004. Variation and extrema of human interpupillary distance In Stereoscopic Displays and Virtual Reality Systems XI, ed. Woods AJ, Merritt JO, Benton SA, Bolas MT, pp. 36–46. San Jose, California, USA: Proc. SPIE [Google Scholar]
- Drager UC, Olsen JF. 1981. Ganglion cell distribution in the retina of the mouse. Investigative ophthalmology & visual science 20: 285–93 [PubMed] [Google Scholar]
- Ellis EM, Gauvain G, Sivyer B, Murphy GJ. 2016. Shared and distinct retinal input to the mouse superior colliculus and dorsal lateral geniculate nucleus. Journal of neurophysiology 116: 602–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C, Robson JG. 1966. The contrast sensitivity of retinal ganglion cells of the cat. The Journal of physiology 187: 517–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erwin E, Miller KD. 1998. Correlation-based development of ocularly matched orientation and ocular dominance maps: determination of required input activities. The Journal of neuroscience : the official journal of the Society for Neuroscience 18: 9870–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferster D 1987. Origin of orientation-selective EPSPs in simple cells of cat visual cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience 7: 1780–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferster D, Chung S, Wheat H. 1996. Orientation selectivity of thalamic input to simple cells of cat visual cortex. Nature 380: 249–52 [DOI] [PubMed] [Google Scholar]
- Freund TF, Martin KA, Somogyi P, Whitteridge D. 1985. Innervation of cat visual areas 17 and 18 by physiologically identified X- and Y- type thalamic afferents. II. Identification of postsynaptic targets by GABA immunocytochemistry and Golgi impregnation. The Journal of comparative neurology 242: 275–91 [DOI] [PubMed] [Google Scholar]
- Fukuda T, Kosaka T, Singer W, Galuske RA. 2006. Gap junctions among dendrites of cortical GABAergic neurons establish a dense and widespread intercolumnar network. The Journal of neuroscience : the official journal of the Society for Neuroscience 26: 3434–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Shatz CJ. 1992. Pathfinding and target selection by developing geniculocortical axons. The Journal of neuroscience : the official journal of the Society for Neuroscience 12: 39–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert CD, Wiesel TN. 1979. Morphology and intracortical projections of functionally characterised neurones in the cat visual cortex. Nature 280: 120–5 [DOI] [PubMed] [Google Scholar]
- Gilbert CD, Wiesel TN. 1989. Columnar specificity of intrinsic horizontal and corticocortical connections in cat visual cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience 9: 2432–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry SH, Reid RC. 2000. The koniocellular pathway in primate vision. Annual review of neuroscience 23: 127–53 [DOI] [PubMed] [Google Scholar]
- Hevner RF. 2000. Development of connections in the human visual system during fetal mid-gestation: a DiI-tracing study. Journal of neuropathology and experimental neurology 59: 385–92 [DOI] [PubMed] [Google Scholar]
- Hirsch JA, Alonso JM, Reid RC, Martinez LM. 1998. Synaptic integration in striate cortical simple cells. The Journal of neuroscience : the official journal of the Society for Neuroscience 18: 9517–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitzenberger CK. 1991. Optical measurement of the axial eye length by laser Doppler interferometry. Investigative ophthalmology & visual science 32: 616–24 [PubMed] [Google Scholar]
- Hoerder-Suabedissen A, Molnar Z. 2015. Development, evolution and pathology of neocortical subplate neurons. Nature reviews. Neuroscience 16: 133–46 [DOI] [PubMed] [Google Scholar]
- Horton JC, Hedley-Whyte ET. 1984. Mapping of cytochrome oxidase patches and ocular dominance columns in human visual cortex. Philosophical transactions of the Royal Society of London. Series B, Biological sciences 304: 255–72 [DOI] [PubMed] [Google Scholar]
- Hubel DH. 1975. An autoradiographic study of the retino-cortical projections in the tree shrew (Tupaia glis). Brain Res 96: 41–50 [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. 1962. Receptive fields, binocular interaction and functional architecture in the cat’s visual cortex. The Journal of physiology 160: 106–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. 1972. Laminar and columnar distribution of geniculo-cortical fibers in the macaque monkey. The Journal of comparative neurology 146: 421–50 [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. 1977. Ferrier lecture. Functional architecture of macaque monkey visual cortex. Proceedings of the Royal Society of London. Series B, Biological sciences 198: 1–59 [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN, LeVay S. 1977. Plasticity of ocular dominance columns in monkey striate cortex. Philosophical transactions of the Royal Society of London. Series B, Biological sciences 278: 377–409 [DOI] [PubMed] [Google Scholar]
- Hubener M, Shoham D, Grinvald A, Bonhoeffer T. 1997. Spatial relationships among three columnar systems in cat area 17. The Journal of neuroscience : the official journal of the Society for Neuroscience 17: 9270–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman AD, Dehay C, Berland M, Chalupa LM, Kennedy H. 2005a. Early and rapid targeting of eye-specific axonal projections to the dorsal lateral geniculate nucleus in the fetal macaque. The Journal of neuroscience : the official journal of the Society for Neuroscience 25: 4014–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman AD, Murray KD, Warland DK, Feldheim DA, Chapman B. 2005b. Ephrin-As mediate targeting of eye-specific projections to the lateral geniculate nucleus. Nature neuroscience 8: 1013–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes A 1971. Topographical relationships between the anatomy and physiology of the rabbit visual system. Documenta Ophthalmologica 30:33–159. [DOI] [PubMed] [Google Scholar]
- Hughes A 1975. A quantitative analysis of the cat retinal ganglion cell topography. The Journal of comparative neurology 163: 107–28 [DOI] [PubMed] [Google Scholar]
- Humphrey AL, Sur M, Uhlrich DJ, Sherman SM. 1985. Projection patterns of individual X- and Y-cell axons from the lateral geniculate nucleus to cortical area 17 in the cat. The Journal of comparative neurology 233: 159–89 [DOI] [PubMed] [Google Scholar]
- Illing RB, Wassle H. 1981. The retinal projection to the thalamus in the cat: a quantitative investigation and a comparison with the retinotectal pathway. The Journal of comparative neurology 202: 265–85 [DOI] [PubMed] [Google Scholar]
- Issa NP, Trepel C, Stryker MP. 2000. Spatial frequency maps in cat visual cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience 20: 8504–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji W, Gamanut R, Bista P, D’Souza RD, Wang Q, Burkhalter A. 2015. Modularity in the Organization of Mouse Primary Visual Cortex. Neuron 87: 632–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Wang Y, Lashgari R, Swadlow HA, Alonso JM. 2011a. Faster thalamocortical processing for dark than light visual targets. The Journal of neuroscience : the official journal of the Society for Neuroscience 31: 17471–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Wang Y, Swadlow HA, Alonso JM. 2011b. Population receptive fields of ON and OFF thalamic inputs to an orientation column in visual cortex. Nature neuroscience 14: 232–8 [DOI] [PubMed] [Google Scholar]
- Jin JZ, Weng C, Yeh CI, Gordon JA, Ruthazer ES, et al. 2008. On and off domains of geniculate afferents in cat primary visual cortex. Nature neuroscience 11: 88–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joesch M, Schnell B, Raghu SV, Reiff DF, Borst A. 2010. ON and OFF pathways in Drosophila motion vision. Nature 468: 300–4 [DOI] [PubMed] [Google Scholar]
- Kanold PO, Shatz CJ. 2006. Subplate neurons regulate maturation of cortical inhibition and outcome of ocular dominance plasticity. Neuron 51: 627–38 [DOI] [PubMed] [Google Scholar]
- Kaplan E, Shapley RM. 1982. X and Y cells in the lateral geniculate nucleus of macaque monkeys. J Physiol 330: 125–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kara P, Boyd JD. 2009. A micro-architecture for binocular disparity and ocular dominance in visual cortex. Nature 458: 627–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnani MM, Agetsuma M, Yuste R. 2014. A blanket of inhibition: functional inferences from dense inhibitory connectivity. Current opinion in neurobiology 26: 96–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaschube M, Schnabel M, Lowel S, Coppola DM, White LE, Wolf F. 2010. Universality in the evolution of orientation columns in the visual cortex. Science 330: 1113–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko H, Hofer SB, Pichler B, Buchanan KA, Sjostrom PJ, Mrsic-Flogel TD. 2011. Functional specificity of local synaptic connections in neocortical networks. Nature 473: 87–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch E, Jin J, Alonso JM, Zaidi Q. 2016. Functional implications of orientation maps in primary visual cortex. Nature communications 7: 13529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komban SJ, Kremkow J, Jin J, Wang Y, Lashgari R, et al. 2014. Neuronal and perceptual differences in the temporal processing of darks and lights. Neuron 82: 224–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremkow J, Jin J, Komban SJ, Wang Y, Lashgari R, et al. 2014. Neuronal nonlinearity explains greater visual spatial resolution for darks than lights. Proceedings of the National Academy of Sciences of the United States of America 111: 3170–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremkow J, Jin J, Wang Y, Alonso JM. 2016. Principles underlying sensory map topography in primary visual cortex. Nature 533: 52–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretz R, Rager G, Norton TT. 1986. Laminar organization of ON and OFF regions and ocular dominance in the striate cortex of the tree shrew (Tupaia belangeri). The Journal of comparative neurology 251: 135–45 [DOI] [PubMed] [Google Scholar]
- Laing RJ, Turecek J, Takahata T, Olavarria JF. 2015. Identification of Eye-Specific Domains and Their Relation to Callosal Connections in Primary Visual Cortex of Long Evans Rats. Cerebral cortex 25: 3314–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law MI, Zahs KR, Stryker MP. 1988. Organization of primary visual cortex (area 17) in the ferret. The Journal of comparative neurology 278: 157–80 [DOI] [PubMed] [Google Scholar]
- Lee KS, Huang X, Fitzpatrick D. 2016. Topology of ON and OFF inputs in visual cortex enables an invariant columnar architecture. Nature 533: 90–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeVay S, McConnell SK. 1982. ON and OFF layers in the lateral geniculate nucleus of the mink. Nature 300: 350–1 [DOI] [PubMed] [Google Scholar]
- LeVay S, Stryker MP, Shatz CJ. 1978. Ocular dominance columns and their development in layer IV of the cat’s visual cortex: a quantitative study. The Journal of comparative neurology 179: 223–44 [DOI] [PubMed] [Google Scholar]
- Lien AD, Scanziani M. 2013. Tuned thalamic excitation is amplified by visual cortical circuits. Nature neuroscience 16: 1315–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund RD, Mustari MJ. 1977. Development of the geniculocortical pathway in rats. The Journal of comparative neurology 173: 289–306 [DOI] [PubMed] [Google Scholar]
- Marin-Padilla M 1971. Early prenatal ontogenesis of the cerebral cortex (neocortex) of the cat (Felis domestica). A Golgi study. I. The primordial neocortical organization. Zeitschrift fur Anatomie und Entwicklungsgeschichte 134: 117–45 [DOI] [PubMed] [Google Scholar]
- Martin PR. 1986. The projection of different retinal ganglion cell classes to the dorsal lateral geniculate nucleus in the hooded rat. Experimental brain research 62: 77–88 [DOI] [PubMed] [Google Scholar]
- Mazade R, Alonso JM. 2017. Thalamocortical processing in vision. Visual Neuroscience 34: e007 , 9 pages doi: 10.1017/S0952523817000049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell SK, LeVay S. 1984. Segregation of on- and off-center afferents in mink visual cortex. Proceedings of the National Academy of Sciences of the United States of America 81: 1590–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlin S, Horng S, Marotte LR, Sur M, Sawatari A, Leamey CA. 2013. Deletion of Ten-m3 induces the formation of eye dominance domains in mouse visual cortex. Cerebral cortex 23: 763–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison G 1991. Neuronal branching patterns and the economy of cortical wiring. Proceedings. Biological sciences 245: 151–8 [DOI] [PubMed] [Google Scholar]
- Morgenstern NA, Bourg J, Petreanu L. 2016. Multilaminar networks of cortical neurons integrate common inputs from sensory thalamus. Nature neuroscience 19: 1034–40 [DOI] [PubMed] [Google Scholar]
- Mrzljak L, Uylings HB, Kostovic I, Van Eden CG. 1988. Prenatal development of neurons in the human prefrontal cortex: I. A qualitative Golgi study. The Journal of comparative neurology 271: 355–86 [DOI] [PubMed] [Google Scholar]
- Nauhaus I, Nielsen KJ. 2014. Building maps from maps in primary visual cortex. Current opinion in neurobiology 24: 1–6 [DOI] [PubMed] [Google Scholar]
- Nauhaus I, Nielsen KJ, Callaway EM. 2016. Efficient Receptive Field Tiling in Primate V1. Neuron 91: 893–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauhaus I, Nielsen KJ, Disney AA, Callaway EM. 2012. Orthogonal micro-organization of orientation and spatial frequency in primate primary visual cortex. Nature neuroscience 15: 1683–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermayer K, Blasdel GG. 1993. Geometry of orientation and ocular dominance columns in monkey striate cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience 13: 4114–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik SB, Ringach DL. 2011. Retinal origin of orientation maps in visual cortex. Nature neuroscience 14: 919–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry VH, Cowey A. 1984. Retinal ganglion cells that project to the superior colliculus and pretectum in the macaque monkey. Neuroscience 12: 1125–37 [DOI] [PubMed] [Google Scholar]
- Peters A, Payne BR. 1993. Numerical relationships between geniculocortical afferents and pyramidal cell modules in cat primary visual cortex. Cerebral cortex 3: 69–78 [DOI] [PubMed] [Google Scholar]
- Raczkowski D, Fitzpatrick D. 1990. Terminal arbors of individual, physiologically identified geniculocortical axons in the tree shrew’s striate cortex. The Journal of comparative neurology 302: 500–14 [DOI] [PubMed] [Google Scholar]
- Rakic P 1976. Prenatal genesis of connections subserving ocular dominance in the rhesus monkey. Nature 261: 467–71 [DOI] [PubMed] [Google Scholar]
- Rakic P 1977. Prenatal development of the visual system in rhesus monkey. Philosophical transactions of the Royal Society of London. Series B, Biological sciences 278: 245–60 [DOI] [PubMed] [Google Scholar]
- Rakic P 1995. A small step for the cell, a giant leap for mankind: a hypothesis of neocortical expansion during evolution. Trends in neurosciences 18: 383–8 [DOI] [PubMed] [Google Scholar]
- Ratliff CP, Borghuis BG, Kao YH, Sterling P, Balasubramanian V. 2010. Retina is structured to process an excess of darkness in natural scenes. Proceedings of the National Academy of Sciences of the United States of America 107: 17368–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese BE, Cowey A. 1988. Segregation of functionally distinct axons in the monkey’s optic tract. Nature 331: 350–1 [DOI] [PubMed] [Google Scholar]
- Reid RC, Alonso JM. 1995. Specificity of monosynaptic connections from thalamus to visual cortex. Nature 378: 281–4 [DOI] [PubMed] [Google Scholar]
- Ruthazer ES, Baker GE, Stryker MP. 1999. Development and organization of ocular dominance bands in primary visual cortex of the sable ferret. The Journal of comparative neurology 407: 151–65 [PMC free article] [PubMed] [Google Scholar]
- Salinas KJ, Figueroa Velez DX, Zeitoun JH, Kim H, Gandhi SP. 2017. Contralateral bias of high spatial frequency tuning and cardinal direction selectivity in mouse visual cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnaik R, Wang BS, Cang J. 2014. Experience-dependent and independent binocular correspondence of receptive field subregions in mouse visual cortex. Cerebral cortex 24: 1658–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller PH, Malpeli JG. 1978. Functional specificity of lateral geniculate nucleus laminae of the rhesus monkey. Journal of neurophysiology 41: 788–97 [DOI] [PubMed] [Google Scholar]
- Schmidt KE, Stephan M, Singer W, Lowel S. 2002. Spatial analysis of ocular dominance patterns in monocularly deprived cats. Cerebral cortex 12: 783–96 [DOI] [PubMed] [Google Scholar]
- Seabrook TA, Burbridge TJ, Crair MC, Huberman AD. 2017. Architecture, Function, and Assembly of the Mouse Visual System. Annual review of neuroscience 40: 499–538 [DOI] [PubMed] [Google Scholar]
- Sedigh-Sarvestani M, Vigeland L, Fernandez-Lamo I, Taylor MM, Palmer LA, Contreras D. 2017. Intracellular, In Vivo, Dynamics of Thalamocortical Synapses in Visual Cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience 37: 5250–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatz CJ. 1983. The prenatal development of the cat’s retinogeniculate pathway. The Journal of neuroscience : the official journal of the Society for Neuroscience 3: 482–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatz CJ, Kirkwood PA. 1984. Prenatal development of functional connections in the cat’s retinogeniculate pathway. The Journal of neuroscience : the official journal of the Society for Neuroscience 4: 1378–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatz CJ, Luskin MB. 1986. The relationship between the geniculocortical afferents and their cortical target cells during development of the cat’s primary visual cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience 6: 3655–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatz CJ, Stryker MP. 1978. Ocular dominance in layer IV of the cat’s visual cortex and the effects of monocular deprivation. The Journal of physiology 281: 267–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. 1996. Functional organization of thalamocortical relays. J Neurophysiol 76: 1367–95 [DOI] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. 1998. On the actions that one nerve cell can have on another: distinguishing “drivers” from “modulators”. Proc Natl Acad Sci U S A 95: 7121–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmuel A, Grinvald A. 1996. Functional organization for direction of motion and its relationship to orientation maps in cat area 18. The Journal of neuroscience : the official journal of the Society for Neuroscience 16: 6945–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GB, Whitney DE, Fitzpatrick D. 2015. Modular Representation of Luminance Polarity in the Superficial Layers of Primary Visual Cortex. Neuron 88: 805–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speer CM, Mikula S, Huberman AD, Chapman B. 2010. The developmental remodeling of eye-specific afferents in the ferret dorsal lateral geniculate nucleus. Anatomical record 293: 1–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley GB, Jin J, Wang Y, Desbordes G, Wang Q, et al. 2012. Visual orientation and directional selectivity through thalamic synchrony. The Journal of neuroscience : the official journal of the Society for Neuroscience 32: 9073–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens CF. 2001. An evolutionary scaling law for the primate visual system and its basis in cortical function. Nature 411: 193–5 [DOI] [PubMed] [Google Scholar]
- Stryker MP, Zahs KR. 1983. On and off sublaminae in the lateral geniculate nucleus of the ferret. The Journal of neuroscience : the official journal of the Society for Neuroscience 3: 1943–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahata T, Miyashita M, Tanaka S, Kaas JH. 2014. Identification of ocular dominance domains in New World owl monkeys by immediate-early gene expression. Proceedings of the National Academy of Sciences of the United States of America 111: 4297–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K 1985. Organization of geniculate inputs to visual cortical cells in the cat. Vision research 25: 357–64 [DOI] [PubMed] [Google Scholar]
- Tootell RB, Switkes E, Silverman MS, Hamilton SL. 1988. Functional anatomy of macaque striate cortex. II. Retinotopic organization. The Journal of neuroscience : the official journal of the Society for Neuroscience 8: 1531–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traquair HM. 1938. An introduction to clinical perimetry, ed. Kimpton H, pp. 4–5. London [Google Scholar]
- Tusa RJ, Palmer LA, Rosenquist AC. 1978. The retinotopic organization of area 17 (striate cortex) in the cat. The Journal of comparative neurology 177: 213–35 [DOI] [PubMed] [Google Scholar]
- Uylings HBM, ed. 2001. The human cerebral cortex in development. Amsterdam:: Kluwer Academic; 63–80 pp. [Google Scholar]
- Van Essen DC, Newsome WT, Maunsell JH. 1984. The visual field representation in striate cortex of the macaque monkey: asymmetries, anisotropies, and individual variability. Vision research 24: 429–48 [DOI] [PubMed] [Google Scholar]
- Vaney DI, Peichl L, Wassle H, Illing RB. 1981. Almost all ganglion cells in the rabbit retina project to the superior colliculus. Brain research 212: 447–53 [DOI] [PubMed] [Google Scholar]
- Wang Y, Jin J, Kremkow J, Lashgari R, Komban SJ, Alonso JM. 2015. Columnar organization of spatial phase in visual cortex. Nature neuroscience 18: 97–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassle H, Boycott BB, Illing RB. 1981. Morphology and mosaic of on- and off-beta cells in the cat retina and some functional considerations. Proceedings of the Royal Society of London. Series B, Biological sciences 212: 177–95 [DOI] [PubMed] [Google Scholar]
- Wassle H, Illing RB. 1980. The retinal projection to the superior colliculus in the cat: a quantitative study with HRP. The Journal of comparative neurology 190: 333–56 [DOI] [PubMed] [Google Scholar]
- Weliky M, Bosking WH, Fitzpatrick D. 1996. A systematic map of direction preference in primary visual cortex. Nature 379: 725–8 [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. 1963. Effects of Visual Deprivation on Morphology and Physiology of Cells in the Cats Lateral Geniculate Body. Journal of neurophysiology 26: 978–93 [DOI] [PubMed] [Google Scholar]
- Xing D, Yeh CI, Shapley RM. 2010. Generation of black-dominant responses in V1 cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience 30: 13504–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh CI, Xing D, Shapley RM. 2009. “Black” responses dominate macaque primary visual cortex v1. The Journal of neuroscience : the official journal of the Society for Neuroscience 29: 11753–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Farley BJ, Jin DZ, Sur M. 2005. The coordinated mapping of visual space and response features in visual cortex. Neuron 47: 267–80 [DOI] [PubMed] [Google Scholar]
- Zahs KR, Stryker MP. 1988. Segregation of ON and OFF afferents to ferret visual cortex. Journal of neurophysiology 59: 1410–29 [DOI] [PubMed] [Google Scholar]
- Zemon V, Gordon J, Welch J. 1988. Asymmetries in ON and OFF visual pathways of humans revealed using contrast-evoked cortical potentials. Visual neuroscience 1: 145–50 [DOI] [PubMed] [Google Scholar]


