Abstract
Objective
To compare the outcomes of isolated coronary artery bypass grafting (CABG) versus surgical ventricular restoration (SVR) with or without CABG for patients with ischemic cardiomyopathy (ICM).
Methods
Retrospectively, 49 patients with ICM and severe LV dysfunction (LVEF < 35%) who underwent SVR with or without CABG from January 2009 to December 2016 at a single institution was compared with 49 patients who underwent isolated CABG. The two groups were matched for preoperative clinical and echocardiographic parameters including left ventricular end-diastolic diameter (LVIDd), left ventricular end-systolic diameter (LVIDs), left ventricular ejection fraction (LVEF), left ventricular end-diastolic volume (LVEDV), and left ventricular end-systolic volume (LVESV). Primary outcomes analyzed included early mortality, late mortality, and major adverse cardiac or cerebrovascular events (MACCE). Secondary outcomes analyzed included echocardiographic parameters of left ventricular volume and function-indexed left ventricular end-diastolic volume (LVEDVi), indexed left ventricular end-systolic volume (LVESVi), and LVEF. Cox and survival analysis was performed.
Results
Early and late mortality in SVR vs. CABG groups were 4 (8.1%) and 6 (12.2%) vs. 1 (2%) and 5 (10.2%) respectively. Mean improvement in LVEF was 3.39 ± 7.51 compared to 4.97 ± 5.45 between the two groups at 3-month follow-up. Mean improvement in LVEF was 5.1 ± 8.3 in the SVR group vs 5.9 ± 7.1 in the CABG group at the last follow-up. There was no statistically significant improvement between the two groups in terms of LVEF at 3 months or the last follow-up. There were statistically significant differences between LVEDVi and LVESVi between the two groups at 3 months and the last follow-up. The 5-year rates of survival were 85 ± 6 and 82 ± 9% for SVR and CABG groups respectively. The 5-year rates of freedom from MACCE were 75 ± 7 and 60 ± 11% for SVR and CABG groups respectively.
Conclusion
Compared with isolated CABG, SVR plus CABG results in equivalent late mortality and better left ventricular reverse remodeling (as evidenced by LV volume reduction) and better freedom from MACCE at 5-year follow-up.
Keywords: Stich trial, Dor’s ventriculoplasty, MACCE
Introduction
The optimal surgical therapy for the management of ischemic cardiomyopathy is a matter of debate following the publication of surgical treatment of heart failure (STICH) trial. Nearly 70% of patients with heart failure have coronary artery disease and nearly all have had a myocardial infarction [1]. With the advent of active revascularization therapy for acute myocardial infarction, the incidence of transmural infarction, and hence aneurysm formation, decreased substantially, such that the nonaneurysmal poorly contracting dilated left ventricle (LV) became the dominant cause of advanced ICM. Focus of ventricular reconstructive surgeries shifted from treatment of aneurysms to treatment of (nonaneurysmal) dilated ICM. The spectrum of post infarction left ventricular scar ranges from classic left ventricular LV aneurysm at one end to diffuse scattered and punctate scars on the other end. Between these two extremes, continuous spectrum of left ventricular scarring exists. Revascularization of significant coronary artery disease has been proven to be beneficial and forms the basis of coronary bypass grafting in this subset of patients with ICM [2]. Addressing concomitant mitral valve insufficiency underlines the concept of addressing ventricle, vessel, and valve simultaneously as proposed by Buckberg [3, 4].
Materials and methods
Study design
Retrospective analysis of 49 patients with (ICM) and severe LV dysfunction (LVEF < 35%) who underwent SVR with or without CABG from January 2009 to December 2016 at a single institution was compared with 49 patients who underwent isolated CABG. The two groups were matched for preoperative clinical and echocardiographic parameters including LVIDd, LVIDs, LVEF, LVEDV, and LVESV. Primary outcomes analyzed included early mortality, late mortality, and MACCE. Secondary outcomes analyzed included echocardiographic parameters of left ventricular volume and function-LVEDVi, LVESVi, LVEF. Cox and survival analysis was performed.
Preoperative, intraoperative, postoperative-clinical and echocardiographic data, and follow-up data were analyzed to identify the risk factors for mortality.
Echocardiographic evaluation
Echocardiographic parameters assessed for left ventricular size, function, and volume include LVIDd, LVIDs, LVEF, LVEDV, LVEDVi, LVESV, LV stroke volume (SV), and LVESVi. Mitral regurgitation, tricuspid regurgitation, pulmonary artery hypertension, and RV dysfunction were graded as mild, moderate, and severe based on established guidelines. The presence of LV clot was documented as well. Left ventricular diastolic dysfunction was grade as types 1, 2, and 3. All these parameters were assessed preoperatively, at 3 months and at the last follow-up.
Surgical procedure
All patients with significant stenosis of the coronaries were grafted as per established guidelines. Patients with LVEF ≤ 35% with dilated LV were identified. Patients with predominant anterior wall akinesia or dyskinesia underwent surgical ventricular restoration by Dor’s ventriculoplasty under cardiopulmonary bypass and cardioplegia to achieve LV volume reduction with exclusion of akinetic or dyskinetic septum while preserving the elliptical shape of LV. Fontan stitch was not used. LV clot was removed whenever found per operatively. Concomitant chronic ischemic mitral valve regurgitation was addressed by restrictive annuloplasty or mitral valve replacement. Patients with predominantly global hypokinesia, inferior wall myocardial infarction, and negligible anterior or septal scar not amenable to SVR underwent isolated CABG.
Follow-up
All patients were followed up in the cardiology and cardiac surgical outpatient clinic at 3 months and yearly till the last follow-up. The latest information was obtained by telephonic contact with the patients or the relatives of the patients.
Statistical analysis
Categorical variables are expressed as counts and percentages. Normally, distributed continuous variables are expressed as mean ± standard deviation. Comparison between two groups was performed using unpaired two-tailed t test for normally distributed variables and Pearson’s Chi square test for categorical variables. Pre- and postoperative continuous data of the same patients were compared by paired t test for normal distribution. Stepwise logistic regression was performed to evaluate the independent risk factors for in-hospital mortality, late mortality, and MACCE. The overall model fit and the Hosmer-Lemeshow test was used to evaluate model predictivity. Survival was analyzed by the Kaplan-Meier method. Results of Cox analysis were reported as the instantaneous relative risk (hazard ratio), 95% confidence intervals (CIs) and P value. All variables listed in (Tables 1, 2, 3, and 4) were entered into univariate analysis to predict risk factors for mortality. For all tests, a P value < 0.05 was considered statistically significant. The SPSS software (SPSS, Inc., Chicago, IL, USA) was used. The sample size was calculated based on the following formula considering the incidence of SVR in ICM:
Table 1.
Clinical and echocardiographic preoperative characteristics
| Variable | Isolated CABG group | SVR group | P value |
|---|---|---|---|
| Total number of patients (n) | 49 | 49 | |
| Gender | |||
| Male | 46 (93.9%) | 43 (87.8%) | 0.294 |
| Female | 3 (6.1%) | 6 (12.2%) | |
| Age | 62.63 ± 9.08 | 57.96 ± 9.74 | 0.016 |
| BSA | 1.74 ± 0.13 | 1.68 ± 0.16 | 0.047 |
| Co morbidities | |||
| Diabetes mellitus | 30 (61.2%) | 22 (44.9%) | 0.105 |
| Hypertension | 19 (38.8%) | 14 (28.5%) | 0.285 |
| COPD | 2 (4.1%) | 2 (4.1%) | 1 |
| CKD | 1 (2.0%) | 2 (4.1%) | 0.399 |
| Creatinine | 1.09 ± 0.38 | 0.98 ± 0.25 | 0.096 |
| NYHA class | 0.005 | ||
| Class 1 | 8 (16.3%) | 0 (0%) | |
| Class 2 | 31 (63.3%) | 43 (87.8%) | |
| Class 3 | 6 (12.2%) | 6 (12.2%) | |
| Class 4 | 3 (6.1%) | 0 (0%) | |
| Documented MI | |||
| IWMI | 18 (36.7%) | 3 (6.1%) | < 0.001 |
| AWMI | 29 (59.2%) | 37 (75.5%) | 0.085 |
| Emergency surgery | 0 (0%) | 3 (6.1%) | 0.079 |
| Echocardiographic parameters | |||
| Documented LV aneurysm | 0 (0%) | 21 (42.9%) | < 0.001 |
| Mitral regurgitation | 0.001 | ||
| No | 12 (24.5%) | 1 (2.0%) | |
| Mild | 22 (44.9%) | 26 (53.1%) | |
| Moderate | 15 (30.6%) | 15 (30.6%) | |
| Severe | 0 (0%) | 7 (14.3%) | |
| Tricuspid regurgitation | < 0.001 | ||
| No | 30 (61.2%) | 12 (24.5%) | |
| Mild | 17 (34.7%) | 23 (46.9%) | |
| Moderate | 2 (4.1%) | 10 (20.4%) | |
| Severe | 0 (0%) | 4 (8.2%) | |
| PHT | 0.032 | ||
| No | 31 (63.3%) | 30 (61.2%) | |
| Mild | 11 (22.4%) | 4(8.2%) | |
| Moderate | 4 (8.2%) | 3(6.1%) | |
| Severe | 3 (6.1%) | 12(24.5%) | |
| RV dysfunction | 0.264 | ||
| No | 45 (91.8%) | 45 (91.8%) | |
| Mild | 2 (4.1%) | 4 (8.2%) | |
| Moderate | 2 (4.1%) | 0 (0%) | |
| Severe | 0 (0%) | 0 (0%) | |
| LV size, volume, and function assessment | |||
| LV diastolic dysfunction | 0.126 | ||
| No | 15 (30.6%) | 23 (46.9%) | |
| Type 1 | 17 (34.7%) | 8 (16.3%) | |
| Type2 | 11 (22.4%) | 9 (18.4%) | |
| Type 3 | 6 (12.2%) | 9 (18.4%) | |
| LV ejection fraction | 31.78 ± 2.99 | 33.04 ± 6.93 | 0.245 |
| LVIDd | 57.29 ± 4.44 | 59.14 ± 8.03 | 0.161 |
| LVIDs | 48.33 ± 4.02 | 49.22 ± 8.63 | 0.511 |
| IVS | 8.84 ± 1.03 | 8.51 ± 1.31 | 0.173 |
| LVEDV | 163.45 ± 28.73 | 178.86 ± 54.69 | 0.085 |
| LVESV | 110.44 ± 21.33 | 119.12 ± 46.42 | 0.239 |
| SV | 52.88 ± 9.37 | 59.74 ± 12.79 | 0.003 |
| LVEDVi | 94.34 ± 16.70 | 107.59 ± 34.07 | 0.017 |
| LVESVi | 63.79 ± 12.64 | 71.67 ± 28.68 | 0.083 |
Statistically significant p values
Table 2.
Intraoperative characteristics
| Variable | Isolated CABG group | SVR group | P value |
|---|---|---|---|
| SVR | 0 (0%) | 49 (100%) | < 0.001 |
| LV clot removal | 0 (0%) | 9 (18.4%) | 0.002 |
| Number of bypass grafts | 3.18 ± 0.95 | 2.27 ± 1.42 | 0.003 |
| 0 | 0 | 6 | |
| 1 | 1 | 11 | |
| 2 | 11 | 9 | |
| 3 | 19 | 12 | |
| 4 | 14 | 9 | |
| 5 | 4 | 2 | |
| Mitral valve intervention | 0.001 | ||
| No | 49 (100%) | 36 (73.5%) | |
| MV replacement | 0 (0%) | 5 (10.2%) | |
| MV repair | 0 (0%) | 8 (16.3%) | |
| Aortic valve replacement | 0 (0%) | 1 (2.0%) | 0.315 |
| Tricuspid valve repair | 0 (0%) | 2 (4.1%) | 0.153 |
| Cardiopulmonary bypass time | 100.33 ± 18.38 | 157 ± 51.17 | < 0.001 |
| Aortic cross clamp time | 53 ± 11.46 | 101.86 ± 37.3 | < 0.001 |
| Intraaortic balloon pump | 1 (2.0%) | 9 (18.4%) | 0.002 |
Statistically significant p values
Table 3.
Postoperative characteristics
| Variable | Isolated CABG group | SVR group | P value |
|---|---|---|---|
| Early mortality | 1 (2.0%) | 4 (8.2%) | 0.168 |
| Stroke | 0 (0%) | 0 (0%) | |
| Dialysis | 0 (0%) | 2 (4.1%) | 0.157 |
| Reintubation | 2 (4.1%) | 6 (12.2%) | 0.013 |
| AICD | 0 (0%) | 2 (4.1%) | 0.153 |
| Cardiac resynchronization | 0 (0%) | 0 (0%) | |
| ICU stay (in days) | 3.25 ± 0.91 | 4.80 ± 2.65 | 0.000 |
| Length of hospital stay (in days) | 7.44 ± 1.15 | 13.94 ± 9.48 | 0.000 |
Statistically significant p values
Table 4.
Follow-up characteristics
| Variable | Isolated CABG group | SVR group | P value |
|---|---|---|---|
| Late mortality | 5 (10.4%) | 6 (13.3%) | |
| MACCE | 11 (22.9%) | 11 (24.4%) | |
| NYHA class in survivors (till the last follow-up) | 0.008 | ||
| Class 1 | 31 (73.8%) | 12 (35.3%) | |
| Class 2 | 8 (19.0%) | 18 (52.9%) | |
| Class 3 | 1 (2.4%) | 1 (2.9%) | |
| Class 4 | 2 (4.8%) | 3 (8.8%) | |
| Mitral regurgitation | 0.906 | ||
| No | 12 (26.6%) | 11 (32.4%) | |
| Mild | 17 (37.8%) | 13 (38.2%) | |
| Moderate | 15 (33.3%) | 9 (26.5%) | |
| Severe | 1 (2.2%) | 1 (2.9%) | |
| Tricuspid regurgitation | 0.006 | ||
| No | 23 (51.1%) | 8 (23.5%) | |
| Mild | 17 (37.8%) | 25 (73.5%) | |
| Moderate | 5 (11.1%) | 1 (2.9%) | |
| Severe | 0 (0%) | 0 (0%) | |
| PHT | 0.952 | ||
| No | 23 (51.1%) | 19 (55.9%) | |
| Mild | 14 (31.1%) | 10 (29.4) | |
| Moderate | 7 (15.5%) | 4 (11.8%) | |
| Severe | 1 (2.2%) | 1 (2.9%) | |
| RV dysfunction | 0.264 | ||
| No | 37 (82.2%) | 32 (94.1%) | |
| Mild | 7 (15.6%) | 2 (5.9%) | |
| Moderate | 1 (2.2%) | 0 (0%) | |
| Severe | 0 (0%) | 0 (0%) | |
| LV size, volume, and function assessment | |||
| LV diastolic dysfunction | 0.063 | ||
| No | 19 (42.2.6%) | 17 (50.0%) | |
| Type 1 | 14 (31.1%) | 11 (32.4%) | |
| Type 2 | 8 (17.8%) | 4 (11.8%) | |
| Type 3 | 4 (8.9%) | 2 (5.9%) | |
| LV ejection fraction | 37.64 ± 6.02 | 37.91 ± 6.26 | 0.848 |
| LVIDd | 57.00 ± 6.38 | 56.74 ± 7.03 | 0.862 |
| LVIDs | 46.29 ± 6.61 | 46.12 ± 7.60 | 0.915 |
| IVS | 9.02 ± 0.89 | 8.97 ± 0.72 | 0.783 |
| LVEDV | 162.68 ± 41.05 | 161.64 ± 45.39 | 0.915 |
| LVESV | 101.79 ± 32.98 | 101.80 ± 39.88 | 0.998 |
| SV | 60.90 ± 11.12 | 59.84 ± 13.15 | 0.699 |
| LVEDVi | 94.31 ± 23.49 | 96.92 ± 25.85 | 0.641 |
| LVESVi | 58.95 ± 18.71 | 60.84 ± 22.13 | 0.683 |
Statistically significant p values
The confidence level is estimated at 95%
with a z value of 1.96
the confidence interval or margin of error is estimated at ± 15
Assuming p% = 68.2 and q% = 31.8
n = 37.03 (the minimum size needed for conclusion)
Results
Clinical and echocardiographic preoperative characteristics
Isolated CABG group
All 49 patients in the CABG group had an elective procedure. Table 1 shows the clinical and echocardiographic preoperative characteristics between the two groups.
SVR group
SVR was performed in 49 patients, as an elective procedure in 46 (93.9%) and on an emergency basis in 3 (6.1%).
Surgical data
The intra op characteristics are described in Table 2.
Isolated CABG group
This group of patients underwent isolated CABG procedure. Diseased coronary arteries with significant stenosis were revascularised whenever feasible according to established guidelines. The mean number of grafts in this group was 3.2 ± 0.9.
SVR group
SVR was targeted mainly towards anterior wall, septum, and lateral wall all by Dor’s ventriculoplasty technique. No inferior/posterior SVR was done. LV clot removal was done in 9 (18.4%) patients. Mitral valve surgery was performed in 13 patients (26.5%), including repair in 8 (16.3%) and replacement in 5 (10.2%). Tricuspid valve repair was done in 2 (4.1%) of patients. Diseased coronary arteries with significant stenosis were revascularised whenever feasible according to established guidelines. The SVR group had 2.3 ± 1.4 mean number of bypass grafts.
Early survival and postoperative outcome
The post op characteristics are given in Table 3.
Isolated CABG group
One patient (2%) had early in-hospital mortality secondary to low cardiac output syndrome. All patients required inotropic support and IABP was required for one (2%) patient. Two (4.1%) patients required reintubation.
SVR group
Four patients (8.2%) had early in-hospital mortality secondary to cardiac causes. All patients required inotropic support and IABP was required for nine (18.4%) patients. Six (12.2%) patients required reintubation.
Late survival and follow-up outcomes
The mean follow-up for isolated CABG group was 45.2 ± 16.6 months as compared to a mean follow-up of 59 ± 43.9 months.
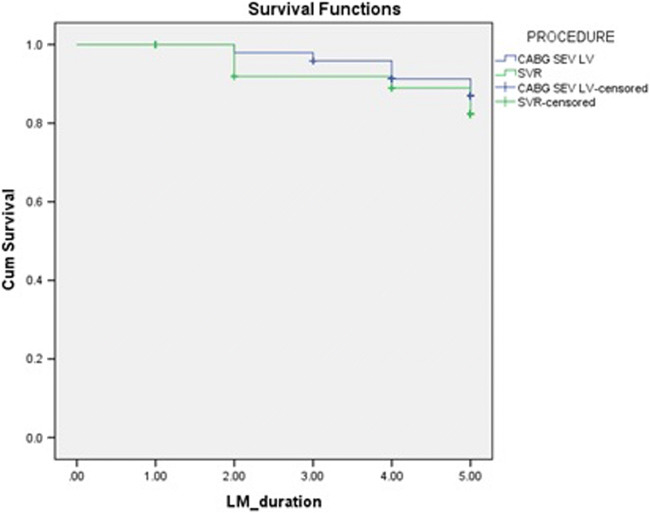
Freedom from late mortality in the isolated CABG group was 82 ± 9% compared to the SVR group which was 76 ± 9% as shown in Fig. 1. Late mortality hazard ratio of SVR compared to CABG is 1.395 (CI 0.4220–4.611), p = 0.585.
Fig. 1.
Freedom from late mortality
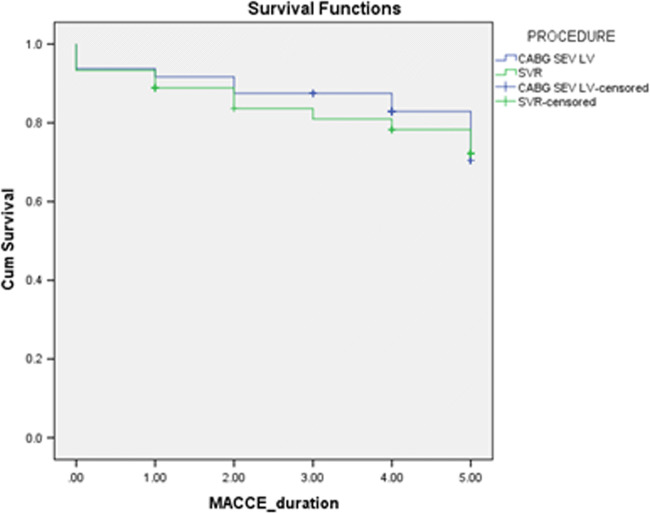
Freedom from MACCE in the isolated CABG group was 60 ± 11% compared to the SVR group which was 67 ± 9% as shown in Fig. 2. MACCE hazard ratio of SVR compared to CABG is 1.063 (CI 0.459–2.46), p = 0.887.
Fig. 2.
Freedom from MACCE
Three (7.2%) patients in the isolated CABG group had NYHA class III/IV when compared to four (11.7%) patients in the SVR group. Sixteen (35.5%) patients in the isolated CABG group had ≥ moderate mitral regurgitation as compared to ten (29.4%) patients in the SVR group. Five (11.1%) patients in the isolated CABG group had ≥ moderate tricuspid regurgitation whereas only one (2.9%) patient had ≥ moderate tricuspid regurgitation in the SVR group as shown in Table 4. (The data for the follow-up NYHA class was missing for three patients which is not included in the table. The follow-up is not 100% complete as due to the loss of data of two patients).
The comparison of the pre op and follow-up characteristics within the isolated CABG group is shown in Table 5. (The follow-up is not 100% complete as due to the loss of data for five patients).
Table 5.
Isolated CABG group—changes from preop to the last follow-up
| Preop | Last follow-up | |
|---|---|---|
| LV ejection fraction | 31.78 ± 2.99 | 37.64 ± 6.02 |
| LVIDd | 57.29 ± 4.44 | 57.00 ± 6.38 |
| LVIDs | 48.33 ± 4.02 | 46.29 ± 6.61 |
| IVS | 8.84 ± 1.03 | 9.02 ± 0.89 |
| LVEDV | 163.45 ± 28.73 | 162.68 ± 41.05 |
| LVESV | 110.44 ± 21.33 | 101.79 ± 32.98 |
| SV | 52.88 ± 9.37 | 60.90 ± 11.12 |
| LVEDVi | 94.34 ± 16.70 | 94.31 ± 23.49 |
| LVESVi | 63.79 ± 12.64 | 58.95 ± 18.71 |
| NYHA class in survivors (till the last follow-up) | ||
| Class 1 | ||
| Class 2 | 8 (16.3%) | 31 (73.8%) |
| Class 3 | 31 (63.3%) | 8 (19.0%) |
| Class 4 | 6 (12.2%) | 1 (2.4%) |
| 3 (6.1%) | 2 (4.8%) | |
| Mitral regurgitation | ||
| No | 12 (24.5%) | 12 (26.6%) |
| Mild | 22 (44.9%) | 17 (37.8%) |
| Moderate | 15 (30.6%) | 15 (33.3%) |
| Severe | 0 (0%) | 1 (2.2%) |
| Tricuspid regurgitation | ||
| No | 30 (61.2%) | 23 (51.1%) |
| Mild | 17 (34.7%) | 17 (37.8%) |
| Moderate | 2 (4.1%) | 5 (11.1%) |
| Severe | 0 (0%) | 0 (0%) |
| PHT | ||
| No | 31 (63.3%) | 23 (51.1%) |
| Mild | 11 (22.4%) | 14 (31.1%) |
| Moderate | 4 (8.2%) | 7 (15.5%) |
| Severe | 3 (6.1%) | 1 (2.2%) |
| RV dysfunction | ||
| No | 45 (91.8%) | 37 (82.2%) |
| Mild | 2 (4.1%) | 7 (15.6%) |
| Moderate | 2 (4.1%) | 1 (2.2%) |
| Severe | 0 (0%) | 0 (0%) |
| LV diastolic dysfunction | ||
| No | 15 (30.6%) | 19 (42.2.6%) |
| Type 1 | 17 (34.7%) | 14 (31.1%) |
| Type2 | 11 (22.4%) | 8 (17.8%) |
| Type 3 | 6 (12.2%) | 4 (8.9%) |
Univariate analysis for mortality in the isolated CABG group was performed as shown in Table 6.
Table 6.
Risk factors associated with increased mortality in the isolated CABG group
| Variable | P value |
|---|---|
| Diabetes mellitus | 0.017 |
| Chronic kidney disease | 0.016 |
| Single coronary bypass graft | 0.040 |
| Severe tricuspid regurgitation | 0.022 |
| Severe pulmonary artery hypertension | 0.000 |
| RV dysfunction | 0.003 |
The comparison of the preop and follow-up characteristics within the SVR group is shown in Table 7.
Table 7.
SVR group changes from preop to the last follow-up
| Preop | Last follow-up | |
|---|---|---|
| LV ejection fraction | 33.04 ± 6.93 | 37.91 ± 6.26 |
| LVIDd | 59.14 ± 8.03 | 56.74 ± 7.03 |
| LVIDs | 49.22 ± 8.63 | 46.12 ± 7.60 |
| IVS | 8.51 ± 1.31 | 8.97 ± 0.72 |
| LVEDV | 178.86 ± 54.69 | 161.64 ± 45.39 |
| LVESV | 119.12 ± 46.42 | 101.80 ± 39.88 |
| SV | 59.74 ± 12.79 | 59.84 ± 13.15 |
| LVEDVi | 107.59 ± 34.07 | 96.92 ± 25.85 |
| LVESVi | 71.67 ± 28.68 | 60.84 ± 22.13 |
| NYHA class in survivors (till the last follow-up) | ||
| Class 1 | 0 (0%) | 12 (35.3%) |
| Class 2 | 43 (87.8%) | 18 (52.9%) |
| Class 3 | 6 (12.2%) | 1 (2.9%) |
| Class 4 | 0 (0%) | 3 (8.8%) |
| Mitral regurgitation | ||
| No | 1 (2.0%) | 11 (32.4%) |
| Mild | 26 (53.1%) | 13 (38.2%) |
| Moderate | 15 (30.6%) | 9 (26.5%) |
| Severe | 7 (14.3%) | 1 (2.9%) |
| Tricuspid regurgitation | ||
| No | 12 (24.5%) | 8 (23.5%) |
| Mild | 23 (46.9%) | 25 (73.5%) |
| Moderate | 10 (20.4%) | 1 (2.9%) |
| Severe | 4 (8.2%) | 0 (0%) |
| PHT | ||
| No | 30 (61.2%) | 19 (55.9%) |
| Mild | 4 (8.2%) | 10 (29.4) |
| Moderate | 3 (6.1%) | 4 (11.8%) |
| Severe | 12 (24.5%) | 1 (2.9%) |
| RV dysfunction | ||
| No | 45 (91.8%) | 32 (94.1%) |
| Mild | 4 (8.2%) | 2 (5.9%) |
| Moderate | 0 (0%) | 0 (0%) |
| Severe | 0 (0%) | 0 (0%) |
| LV diastolic dysfunction | ||
| No | 23 (46.9%) | 17 (50.0%) |
| Type 1 | 8 (16.3%) | 11 (32.4%) |
| Type 2 | 9 (18.4%) | 4 (11.8%) |
| Type 3 | 9 (18.4%) | 2 (5.9%) |
Univariate analysis for risk factors for mortality was performed in the SVR group as shown in Table 8.
Table 8.
Risk factors associated with increased mortality in the SVR group
| Variable | P value |
|---|---|
| Aortic valve replacement | 0.046 |
| Dialysis | 0.004 |
| Severe mitral regurgitation | 0.011 |
| ≥ moderate tricuspid regurgitation | 0.004 |
| Increased length of hospital stay | 0.032 |
| Reintubation | 0.020 |
| Increased LVDi | 0.003 |
LV ejection fraction increased by 18.8% in the isolated CABG group as compared to 15.3% in the SVR group between preop and the last follow-up. LVEDVi decreased by 0% in the isolated CABG group as compared to 14.6% in the SVR group. LVESVi decreased by 8% in the isolated CABG group as compared to 20.6% in the SVR group. Stroke volume increased by 15.4% in the isolated CABG group as compared to 2% in the SVR group. In the SVR group, reduction in LVEDVi and LVESVi and the absence of significant increase in stroke volume was statistically significant.
Discussion
Surgical ventricular restoration for patients with ICM has evolved considerably since the first successful LV aneurysmectomy using linear closure technique under extracorporeal circulation by Cooley et al. in 1958 [5]. In 1985, two highly innovative and entirely new approaches to the surgical treatment of left ventricular aneurysms were introduced almost simultaneously and completely independently by Jatene in Sao Paulo, Brazil, and Dor et al. then at the University of Nice, France, and now at the Centre Cardio-Thoracique de Monaco [6, 7]. The concept was to exclude all diseased akinetic and dyskinetic segments from the cavity by endoventricular patch and is still considered a fundamental improvement [7]. Appropriate candidates for LV reconstruction have suffered an MI, have a dilated left ventricle, and have an asynergic area (dyskinetic or akinetic) of 35% or more with symptoms of heart failure, angina, or intractable ventricular arrhythmias. The guiding principle in the current era is to restore LV shape, volume, and geometry to address the biomechanical model for heart failure as described by Mann and Bristow [8]. Consistent with the Laplace law, which states that wall tension is directly proportional to LV pressure and chamber radius and inversely proportional to wall thickness the GUSTO (global utilization of streptokinase and t-PA for occluded arteries) trial (LVESVi ≥ 40 ml/m2), SAVE (survival and ventricular enlargement) trial (≥ 60 ml/m2), and work by Yamaguchi et al. (LVESVi ≥ 100 ml/m2) have shown that increase in end-diastolic and end-systolic volumes are associated with adverse outcomes [9–11]. Menicanti et al. proposed the term exhausted myocardium for dysfunctional nonischemic myocardium in dilated cardiomyopathy which implies recovery of function if the hemodynamic burden that imposes a high wall tension is relieved by SVR [12].
In contrary to the work of all these pioneers in the field of cardiology and cardiac surgery, the STICH trial demonstrated that there was no survival or functional benefit after SVR at a median follow-up of 48 months. In STICH trial, SVR reduced LVESVi by 19% as compared with a reduction of 6% with CABG alone. In comparison in our study, SVR reduced LVESVi by 20.6% as compared with a reduction of 8% with CABG alone. The eligibility criteria for SVR in STICH trial was dominant LV anterior akinesia or dyskinesia. These patients were randomized for CABG alone versus SVR in STICH trial. Our indication for SVR was the same as well. However, with our study being retrospective in nature, we selected equal number of isolated CABG patients with severe LV dysfunction (LVEF ≤ 35%) and dilated LV, operated during the same period as a comparative group. The major differences in our groups were:
The presence of 30.6% ≥ moderate MR (with no severe MR) in isolated CABG group compared to 44.9% ≥ moderate MR (14.5% severe MR) in the SVR group.
The presence of 4.1% ≥ moderate TR (with no severe TR) in the isolated CABG group compared to 28.6% ≥ moderate TR (with 8.1% severe TR) in the SVR group.
The presence of 14.3% ≥ moderate PAH (with no 6.1% severe PAH) in the isolated CABG group compared to 30.6% ≥ moderate PAH (with 24.5% severe PAH) in the SVR group.
All elective versus 6.1% emergency surgery in the isolated CABG and SVR group respectively.
Use of 2% versus 18.4% IABP in the isolated CABG and SVR group respectively.
All of these suggest that the SVR group was a higher risk subset compared to the isolated CABG group. Thus, a consistent reduction in LV volumes in the SVR group in our study did not translate into an improved survival/freedom from MACCE and functional (NYHA class) compared to the isolated CABG group like the STICH trial. Thus, we believe the conclusion derived from the STICH trial is flawed and requires further in-depth analysis than the published data.
As a counterpoint to the STICH trial, Calafiore et al. published their SVR results in 91 patients with a median follow-up of 31 months [13]. In their study, ≥ moderate MR was identified in 72.6% of patients versus 18% in the experience of Menicanti et al. [12], 17% in the STICH trial [2], 41.9% in the report by Dor et al. [14], and 44.9% in our study. The EF increased by 20% postoperatively, but SVi remained unchanged and they concluded that EF increase does not reflect increased pump function as long as SV remains equal. In comparison, EF increased by 15.3% with a minimal change of 2% in SV in our SVR study group. Extrapolating their results, we concur with them on the fact that an increase in EF does not reflect increased pump function as long as SV remains equal. Furthermore, in our isolated CABG group, EF increased by 18.8% and SV increased by 15.4% which could be due to revascularization of hibernating myocardium in the large spectrum of LV scarring as we discussed earlier. This also implies that LV scarring requires being quantified and myocardial viability predicted by late Gadolinium-enhanced magnetic resonance imaging (MRI) to identify the subset which will benefit most from each procedure [15]. Even though cardiovascular MRI radionuclide tagging remains the gold standard for calculation of strain and strain rate and regional wall motion abnormalities [16], tissue Doppler imaging and speckle tracking analysis by echocardiography is a practical option and we believe will help in identifying patients who would benefit from either intervention [17, 18]. Thus, identifying the extent of asynergy (akinesia or dyskinesia) may determine the outcome of SVR. STICH trial and similarly our study failed to identify the patients with ≥ 35% asynergy for the proper application of SVR. Calafiore et al. also report a 38% reduction in LVESVi and a 40% reduction in LVEDVi by echocardiography compared to 20.6% and 14.6% reduction in our group. This could be due to the increased percentage of CIMR in their group as well. Freedom from late mortality at 5 years is similar between their SVR group and our SVR group (73 ± 5% vs. 76 ± 9%). Freedom from MACCE at 5 years is also similar between their SVR group and our SVR group (58 ± 5% vs. 67 ± 9%). Thus, we speculate that a greater than 30% reduction in volume after SVR as advocated by Buckberg et al. [19] is dependent also on reducing concomitant CIMR in the presence of a very dilated LV with significant asynergy which can be excluded and may be a non-independent variable for good long-term results.
The keynote finding by Dor [20] of similarly absent function in aneurysm without reperfusion (in which thinned scar collapses during venting) and akinesia after reperfusion (in which the thick myocardium with inner shell scar is covered by normal anterior myocardium that does not collapse during venting) introduced a new SVR target which requires to be identified by cardiac MRI and initiates a learning curve for procedure application to see the benefits as propagated by the RESTORE (reconstructive endoventricular surgery) group registry and stalwarts of SVR surgery [21, 22].
Limitations of study
Retrospective design of the study
It is difficult to obtain a control group for SVR which matches all preoperative characteristics and shares the same risk profile.
The asynergy was not quantified by cardiac MRI. Greater than 35% asynergy was not mandatory for undergoing SVR.
The status of remote areas and myocardial viability was not checked routinely in all patients to identify patients in whom SVR would be harmful or beneficial.
Cardiac volume assessment by echocardiography is inferior to cardiac MRI which is the gold standard and all patients who underwent SVR did not have LVESVi ≥ 60 ml/m2.
Conclusion
The STICH trial results created a confusion in the mind of many a surgeons regarding the success of SVR and the doubts surrounding it. This study gives evidence doing SVR and the efforts around it. As long-term results of SVR are based on the LV reverse remodeling and quality of life depends on the better contractile property of the left ventricle, it is always better to have a good geometrically aligned ventricle. SVR helps to achieve the same. Compared with isolated CABG, SVR plus CABG results in equivalent late mortality and better left ventricular reverse remodeling (as evidenced by LV volume reduction) and better freedom from MACCE at 5-year follow-up. Hence, whenever feasible and if the patient meets the indications, SVR should be done with no hesitation.
Acknowledgments
We acknowledge the statistical analysis and support provided by Dr. Balaji, Assistant Professor, Department of Community Medicine, The Madras Medical College.
Presentation at meetings
Nil
Compliance with ethical standards
Institutional review board approval has been obtained for the publishing of patient profile and this article.
Conflict of interest
The authors declare that they have no conflict of interest.
Statement of human rights
For this type of study formal consent is not required.
Footnotes
Discussant
Editor in Chief, IJTC
Question 1: How was the degree of akinesia/dyskinesia calculated pre-operatively? Were the echocardiographers blinded to the study? Was it a single person doing all echocardiographies or two independent observations were taken?
Answer: The degree of akinesia/ dyskinesia was calculated pre-operatively by transthoracic echocardiography. The lack of objective quantification of the akinesia/ dyskinesia by MRI has been mentioned as a limitation of the study. Since it is a retrospective study, the echo cardiographers were not blinded to the study. Two independent observations were not taken but all echocardiographies were done at the same core lab according to our institute protocol.
Question 2: Were there any crossovers? Was the analysis “Intent to treat’ or “As Treated”?
Answer: There were no cross overs in the study and the analysis was “As Treated.”
Question 3: How was the size and the shape of the ventricle decided? Was it just eyeballing or some form of mannequin for objectivity was used for the same?
Answer: The size and shape of the ventricle was assessed by echocardiography. The intraoperative judgement was by eyeballing. No mannequin for objective reduction was used.
Question 4: Was any viability study done pre-operatively and if yes, its correlation with results and hard end-points.
Answer: All patients did not undergo viability testing preoperatively. Hence a correlation with results and hard end points could not be obtained.
Question 5: What was the experience of the surgeon/s doing the procedure and did the surgeon’s experience and the volumes reflect in results?
Answer: All the cases were performed by surgeons who had been performing SVR much before the commencement of the study. There was no discrepancy between the surgeons who performed SVR with respect to prior SVR experience. Hence, surgeon’s experience and results cannot be correlated.
Question 6: One of the lacunae with STICH Trial, and probably a major reason for its inability to demonstrate major benefit with SVR, was its failure to achieve a volume reduction of 30% as stipulated and planned. In your study too, volume reduction post SVR is to the tune of 20.6%, which is sub-optimum. How do you therefore justify your conclusions as valid?
Answer: There is a subset of patients within the SVR group who do not have a significantly dilated left ventricle but still have a significantly large area of akinesia or dyskinesia. When SVR is performed in such patients, the volume reduction will be less despite achieving adequate reconstruction of left ventricle. Thus volumetric analysis of SVR alone cannot be used to arrive at conclusions and requires to be correlated with the reduction in area of dyskinesia/ akinesia achieved. This quantification requires LV strain and MRI assessment for achieving further conclusions. This is mentioned as a limitation in our original paper.
Question 7: Was any separate analysis done for the patients who achieved more than 30% volume reduction post SVR?
Answer: No separate analysis was done for the patients who achieved more than 30% volume reduction post SVR.
Question 8: How do you claim that the 2 groups were matched with multiple confounding variables like Arterial Vs Venous CABG, Mitral Valve Interventions, varying levels of PAH, IABP use etc.?
Answer: As mentioned in the limitations in the study, it was difficult to obtain perfect matching between the two groups with multiple confounding variables.
Question 9: Was any form of functional testing like six minute walk test performed postoperatively?
Answer: No objective evidence of functional testing like six minute walk test was performed postoperatively.
Question 10: Did you find any threshold value of post-operative LV end-systolic volume index at which benefits of SVR were lost and what do you think is the best time for performing SVR?
Answer: We did not analyze any threshold value of postoperative end-systolic volume index at which benefits of SVR were lost. Best time for performing SVR is after scar formation since it helps in effective reconstruction of dilated LV. It also makes the operation technically easier to perform without increasing intraoperative bleeding.
Question 11: STICH2 study by Choi et al. reported reduced survival with rising Sphericity Index. Did you look at the Sphericity index in your study and its correlation to end results and functional status?
Answer: Sphericity index was not analyzed and hence its correlation to end results and functional status cannot be commented based on our study.
References
- 1.Gheorghiade M, Bonow RO. Chronic heart failure in the United States: A manifestation of coronary artery disease. Circulation. 1998;97:282–289. doi: 10.1161/01.CIR.97.3.282. [DOI] [PubMed] [Google Scholar]
- 2.Jones RH, Velazquez EJ, Michler RE, et al. Coronary bypass surgery with or without surgical ventricular reconstruction. N Engl J Med. 2009;360:1705–1717. doi: 10.1056/NEJMoa0900559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buckberg GD, Coghlan HC, Torrent-Guasp F. The structure and function of the helical heart and its buttress wrapping.V. Anatomic and physiologic considerations in the healthy and failing heart. Semin Thorac Cardiovasc Surg. 2001;13:358–385. doi: 10.1053/stcs.2001.29957. [DOI] [PubMed] [Google Scholar]
- 4.Buckberg G. Left ventricular reconstruction for dilated ischaemic cardiomyopathy: biology, registry, randomisation and credibility. Eur J Thorac Cardiovasc Surg. 2006;30:753–761. doi: 10.1016/j.ejcts.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 5.Cooley DA, Collins HA, Morris GC, Chapman DW. Ventricular aneurysm after myocardial infarction. Surgical excision with use of temporary cardiopulmonary bypass. JAMA. 1958;167:557. doi: 10.1001/jama.1958.02990220027008. [DOI] [PubMed] [Google Scholar]
- 6.Jatene AD. Left ventricular aneurysmectomy. Resection or reconstruction. J Thorac Cardiovasc Surg. 1985;89:321–331. [PubMed] [Google Scholar]
- 7.Dor V, Kreitmann P, Jourdan J, et al. Interest of physiological closure (circumferential plasty on contractile areas) of left ventricle after resection and endocardiectomy for aneurysm or akinetic zone. Comparison with classical technique about a series of 209 left ventricular resections. J Cardiovasc Surg. 1985;26:73. [Google Scholar]
- 8.Mann DL, Bristow MR. Mechanisms and models in heart failure. The biomechanical model and beyond. Circulation. 2005;111:2837–2849. doi: 10.1161/CIRCULATIONAHA.104.500546. [DOI] [PubMed] [Google Scholar]
- 9.Migrino RQ, Young JB, Ellis SG, et al. End-systolic volume index at 90 to 180 minutes into reperfusion therapy for acute myocardial infarction is a strong predictor of early and late mortality. Circulation. 1997;96:116–121. doi: 10.1161/01.CIR.96.1.116. [DOI] [PubMed] [Google Scholar]
- 10.Sutton MSJ, Pfeffer MA, Moye L, et al. Cardiovascular death and left ventricular remodeling two years after myocardial infarction baseline predictors and impact of long-term use of captopril: Information from the Survival and Ventricular Enlargement (SAVE) trial. Circulation. 1997;96:3294–3299. doi: 10.1161/01.CIR.96.10.3294. [DOI] [PubMed] [Google Scholar]
- 11.Yamaguchi A, Ino T, Adachi H, et al. Left ventricular volume predicts postoperative course in patients with ischemic cardiomyopathy. Ann Thorac Surg. 1998;65:434–438. doi: 10.1016/S0003-4975(97)01155-7. [DOI] [PubMed] [Google Scholar]
- 12.Menicanti L, Castelvecchio S, Ranucci M, et al. Surgical therapy for ischemic heart failure: single center experience with surgical anterior ventricular restoration. J Thorac Cardiovasc Surg. 2007;134:433–441. doi: 10.1016/j.jtcvs.2006.12.027. [DOI] [PubMed] [Google Scholar]
- 13.Calafiore AM, Iaco AL, Kheirallah H, et al. Outcome of left ventricular surgical remodelling after the STICH trial. Eur J Cardiothorac Surg. 2016;50:694–702. doi: 10.1093/ejcts/ezw103. [DOI] [PubMed] [Google Scholar]
- 14.Dor V, Civaia F, Alexandrescu C, Sabatier M, Montiglio F. Favorable effects of left ventricular reconstruction in patients excluded from the Surgical Treatments for Ischemic Heart Failure (STICH) trial. J Thorac Cardiovasc Surg. 2011;141:905–916. doi: 10.1016/j.jtcvs.2010.10.026. [DOI] [PubMed] [Google Scholar]
- 15.Kim RJ, Wu E, Rafael A, et al. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343:1445–1453. doi: 10.1056/NEJM200011163432003. [DOI] [PubMed] [Google Scholar]
- 16.Götte MJ, Germans T, Rüssel IK, et al. Myocardial strain and torsion quantified by cardiovascular magnetic resonance tissue tagging. studies in normal and impaired left ventricular function. J Am Coll Cardiol. 2006;48:2002–2011. doi: 10.1016/j.jacc.2006.07.048. [DOI] [PubMed] [Google Scholar]
- 17.Reisner SA, Lysyansky P, Agmon Y, Mutlak D, Lessick J, Friedman Z. Global Longitudinal Strain: A Novel Index of Left Ventricular Systolic Function. J Am Soc Echocardiogr. 2004;17:630–633. doi: 10.1016/j.echo.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 18.Artis NJ, Oxborough DL, Williams G, Pepper CB, Tan LB. Two-dimensional strain imaging: A new echocardiographic advance with research and clinical applications. Int J Cardiol. 2008;123:240–248. doi: 10.1016/j.ijcard.2007.02.046. [DOI] [PubMed] [Google Scholar]
- 19.Buckberg GD, Athanasuleas CL. The STICH trial: misguided conclusions. J Thorac Cardiovasc Surg. 2009;138:1060–1064. doi: 10.1016/j.jtcvs.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 20.Dor V. Left ventricular reconstruction: the aim and the reality after twenty years. J Thorac Cardiovasc Surg. 2004;128:17–20. doi: 10.1016/j.jtcvs.2004.02.026. [DOI] [PubMed] [Google Scholar]
- 21.Athanasuleas CL, Buckberg GD, Stanley AW, Siler W, Dor V, Di Donato M, et al. Surgical ventricular restoration in the treatment of congestive heart failure due to post-infarction ventricular dilation. J Am Coll Cardiol. 2004;44:1439–1445. doi: 10.1016/j.jacc.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 22.Shah PJ, Hare DL, Raman JS, et al. Survival after myocardial revascularization for ischemic cardiomyopathy: a prospective ten-year follow-up study. J Thorac Cardiovasc Surg. 2003;126:1320–1327. doi: 10.1016/S0022-5223(03)00809-2. [DOI] [PubMed] [Google Scholar]