Abstract
In this review, we discuss various patch materials used for reconstruction of the right ventricular outflow tract. Their relative merits and demerits are discussed. Traditional patches and their results are detailed along with a brief description of newer developments in the field.
Keywords: Patch materials, Right ventricular outflow tract, Tetralogy of Fallot
Introduction
During intracardiac repair of tetralogy of Fallot (TOF), the insertion of an outflow patch across the infundibulum, pulmonary valve ring, and pulmonary artery provides satisfactory relief of the right ventricular outflow tract (RVOT) obstruction. However, it also leads to significant pulmonary valve incompetence (PI). The long-term significance of PI is still controversial, although it is increasingly being realized that PI produces chronic volume load of the right ventricle (RV) that is known to adversely impact its function, leading to arrhythmias in the long term. Traditionally, autologous pericardium (either fresh and untreated or fresh but fixed with glutaraldehyde) was used for RVOT reconstruction. However, individual practices vary [1] and any discussion on the use of patch material for RVOT reconstruction is expected to elicit multiple, very strongly held opinions, each of them based on individual practices rather than on clinical data [1]. Each choice has advocates who promote their viewpoint with great fervor. Similarly, there is no dearth of individuals who hold the counterpoint with equal intensity. Unfortunately, the body of evidence that supports one strategy or viewpoint over another is often limited and inconclusive.
An ideal patch material should have the minimum dyskinetic properties without propensity for progressive dilatation or aneurysm formation. It should not produce progressive stenosis of the RVOT because of patch contraction [2]. Besides autologous pericardium, the patch materials used clinically are limited to prosthetic materials, allogenic or glutaraldehyde fixed xenograft pericardium, homografts (antibiotic or cryopreserved), bovine jugular vein patches, and various types of extracellular matrices.
In order to understand the role of various patch materials for RVOT reconstruction, we must understand that everything we do is associated with potential clinical benefits, limitations, and potential harm, as well as increased cost. The primary goal of the review was to evaluate the strength of evidence for effectiveness of each type of material and its effect on PI, RV function, and other factors that impact outcomes after intracardiac repair of TOF. Secondary goals were the following: (1) to determine if the level of evidence for any strategy was sufficient to make recommendations concerning guidelines for clinical practice and (2) to provide results which could be helpful in planning future clinical trials or quality improvement initiatives.
Methods
We performed a systematic review of the literature describing RVOT reconstruction with different patch materials and to evaluate all clinical studies describing techniques or outcomes of RVOT reconstruction with various patch materials. The search engines employed were Pubmed, Cochrane data base, Ovid, Google scholar, and Embase. The search included literature in all languages. This strategy yielded 27 publications that provided the best answer to the primary and secondary goals. A detailed analysis of these papers is presented below.
Autologous pericardium
Pericardium (Fig. 1) has been used for decades for repair of TOF. Autologous pericardium is harvested during various cardiovascular procedures and has been widely used for surgical reconstruction of many areas like vena cava, atrial septum, ventricular septum, and right side of the heart. The use of autologous pericardium carries multiple advantages: ready availability, conformability, non-porosity, and lack of bleeding through needle holes. The ability to resist infections, less likelihood of thrombosis, and hemolysis are the other favorable factors that have made autologous pericardium the most widely used patch material for TOF repair. It is a low-cost biomaterial that is free of donor derived pathogens; thus, it does not provoke an immune response. Nevertheless, untreated autologous pericardium is difficult to handle and is susceptible to aneurysmal dilatation. The incidence of aneurysms of the RVOT after repair with pericardial patches is reported to range between 6 and 25% [3]. Etiology of aneurysmal dilatation is multifactorial: elevated right ventricular pressure, distal stenosis in the pulmonary artery, or a residual surgical lesion in the form of residual ventricular septal defect or residual right ventricular outflow tract gradient. The size of the pericardial patch is also considered to be one of the etiological factors leading to its aneurysmal dilatation [2]. Hawe et al. [3] reported a high incidence (18%) of RVOT aneurysm when a large pericardial patch was used. They also observed that this incidence reduced to less than 2%, when smaller, tear drop-shaped pericardial patches were placed. In addition, the pericardial patch may undergo retraction or fibrosis. Glutaraldehyde fixation of the autologous pericardium has been shown to improve the handling characteristics of the patch apart from improving the tensile strength and tissue resilience [4]. It results in cross-linking of collagen molecules and strengthens the pericardium as well as fixing its shape and by reducing its elasticity. Glutaraldehyde-fixed pericardium exhibits no tendency towards shrinkage or curling, making its tailoring and suturing easier [5]. Glutaraldehyde fixation is inexpensive, easy to perform, and does not prolong the duration of surgery. However, an optimal method of glutaraldehyde fixation of autologous pericardium is unknown. Lee et al. [6] conducted a study to evaluate the effects of glutaraldehyde concentration and fixation time on material characteristics and calcification of bovine pericardium. Fixation of bovine pericardium with 0.5 and 0.6% glutaraldehyde for 20 min produced superior results with regard to material characteristics (mechanical properties, degree of fixation, and resistance to enzymatic degradation) and post-implantation calcification. They concluded that these results may have implications for optimal fixation of autologous pericardium used for cardiovascular surgery.
Fig. 1.
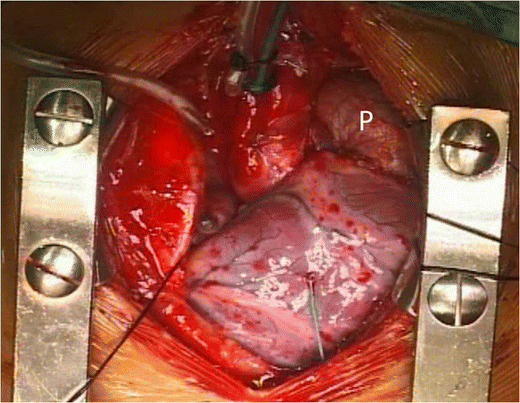
The autologous glutaraldehyde treated pericardium (P) used as a transannular patch
In their experience, Messina et al. [4] found that glutaraldehyde-treated autologous pericardium is both safe and efficacious for TOF repair. The limitation of this study was the short follow-up. Minor disadvantages which have been noted with glutaraldehyde fixation include predisposition to varying degrees of calcification depending upon the strength and duration of glutaraldehyde treatment. In 1992, Chiu et al. [7] investigated the fate of autologous pericardial valved conduits in 20 piglets. The autologous pericardium of 10 piglets was fixed using 0.6% glutaraldehyde and was used to form a conduit connected to the pulmonary trunk distally and RVOT proximally. In the remaining 10 piglets, the pericardium was not fixed. They noted an increased incidence of patch aneurysms in the untreated group at a mean interval of 104 days and an increased incidence of calcification in the glutaraldehyde treated group at a mean interval of 66 days.
Cryopreserved allograft pericardium
Cryopreserved allograft pericardium is an important patch material for repair of TOF. Benefits of allograft patches include excellent hemodynamics, resistance to infection, and decreased thromboembolic events without the need for anticoagulation [8]. However, their use is limited by their availability and commercially available allograft pericardium is expensive. In institutions such as ours where homograft valve banks exist, it is an extremely inexpensive patch material, especially in re-operations where the patient’s own native pericardium is not available. Nevertheless, cryopreserved allografts have sufficient immunogenicity to induce a marked antibody response, involving both class I and class II anti-HLA antibodies and may trigger rejection and result in early allograft failure. Besides this, there is a risk of transmission of viruses (HIV, hepatitis B and C), it is difficult to handle, and both unfixed and glutaraldehyde-fixed allograft pericardia are also liable to calcification. When the method of antibiotic preservation is used in institutions where there are no facilities for cryopreservation, the shelf-life is short.
Xenograft pericardium
Commercially available xenograft pericardium is either bovine, equine, or porcine and treated with glutaraldehyde. A xenograft pericardial patch is particularly useful when the patient has had a previous palliative surgery and when a sufficient amount of autologous pericardium may not be available for a subsequent repair [9]. Its primary disadvantage is that the combination of an immune response to the xenograft as well as the aldehyde residue may result in severe degree of calcification within a short duration. Crawford et al. [9] reported the use of bovine pericardium for repair of various congenital heart defects. These included atrial septal defects (n = 39), ventricular septal defects (n = 12), atrioventricular canal defects (n = 17), TOF (n = 10), and others. There were 12 early and 6 late deaths unrelated to the patch material. They observed that bovine pericardium was flexible, easy to suture, could easily be cut into the desired configuration, and did not show a tendency to roll. Gabbay et al. [10] demonstrated significant calcification 12 months following surgery in procedures where these patches were used to replace a segment of the atrial wall in dogs. They suggested that the tendency of bovine pericardium to calcify is influenced by the site of implantation and that those in contact with blood are more likely to calcify. The major disadvantage is availability, in addition to its higher costs (15 × 15 cm patch of bovine pericardium typically costs Rs, 25,000) compared to the synthetic patches. Another limitation is the need for thorough rinsing of the xenograft pericardium for 3–6 min prior to its use to remove the residual glutaraldehyde residue, failing which, it calcifies rapidly.
Cryopreserved allograft/ homograft arterial wall
Allograft arterial wall is a good material for patch enlargement of stenotic vessels, being quite hemostatic and conforming well to irregular contours. However, it suffers from similar drawbacks that allograft pericardium does. It can transmit viral diseases and requires time for thawing and rinsing. Youn et al. [8] reported short and mid-term results using cryopreserved homografts for RVOT reconstruction. Nineteen patients received pulmonary homografts and one patient received an aortic homograft. There were no early or late deaths at a mean follow-up period of 4.1 ± 1.9 years. Graft dysfunction was found in three patients (all less than 10 years old), one each due to homograft stenosis, homograft valve regurgitation, and mixed lesion. However, the unpredictability of allograft arterial wall, regarding the size it will assume or dilate to, once under pressure, is another concern. Aortic allografts are particularly more susceptible to calcification and obstruction as compared to pulmonary allografts [8]. This is particularly true, more with aortic allograft tube graft conduits than with aortic allograft patches.
Dacron
Dacron (polyethylene terephthalate), developed by DuPont company in 1950, is a synthetic polymer which was found to be more stable and resistant to deterioration when exposed to biological fluids. Simon et al. [11] compared the results of TOF repair in infants with a limited transannular patch versus Dacron annular sparing RVOT patch. Of the 94 infants, a limited transannular patch was placed in 48 patients and in the remaining 46 patients, an annular sparing Dacron patch was placed. Mean follow-up was 7.9 ± 3.4 years. The freedom from re-operation at 10 years was not significantly significant between the two groups. The authors used Dacron due to its non-distensibility, which limits the degree of annular dilation. They hypothesized that pericardial patches led to annular dilatation by exacerbating the chronic volume and pressure overload frequently encountered in TOF patients and increase the risk of patch aneurysm formation. On the other hand, Dacron being non-distensible remains static over time. Therefore, any increase in the pulmonary annular dimension is entirely related to native tissue growth.
However, Dacron stimulates an aggressive inflammatory process with fibrosis at follow-up. This may prove useful for residual peripatch ventricular septal defects but is a point of concern for Dacron conduits and RVOT patches. This is particularly true when Dacron is used close to a semi lunar valve, where this fibrosis may result in either stenosis or regurgitation [12]. Moreover, Dacron is much less elastic and its conformability is also less compared to pericardial patches. Nevertheless, it does not pose a concern when it is used as a flat patch to close septal defects but it may be worrisome when it is used to reconstruct baffles or transannular patches in TOF repair. Furthermore, the fibrosis induced by the inflammation may also result in stenosis of the baffle.
Expanded Polytetrafluoroethylene
Teflon is also a synthetic polymer. Expanded polytetrafluoroethylene (ePTFE) is microporous and is a form of Teflon in which the polymer is arranged as a lattice of nodes interconnected by filaments (Fig. 2). The presence of pores has the advantage of allowing ingrowth and anchoring of a fibrous pseudo intima. Also, PTFE stimulates less fibrosis when compared to Dacron.
Fig. 2.
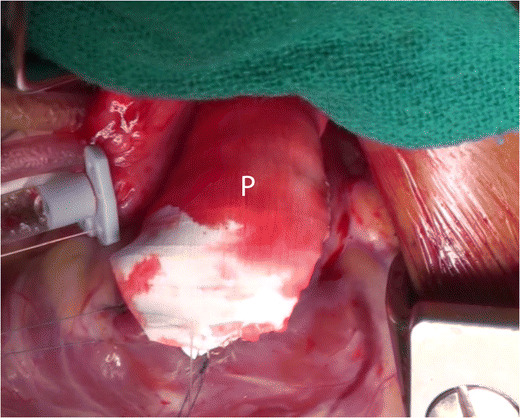
The polytetrafluoroethylene (PTFE) patch (P) fashioned out of a PTFE graft in use as a trans-annular patch
In an earlier study, we [13] randomized 53 consecutive patients of TOF into two groups, group I (pericardial patch) and group II (PTFE patch), and their postoperative outcomes in terms of postoperative rhythm, duration of mechanical ventilation, mediastinal and pleural drainage, intensive care unit (ICU), and hospital stay were assessed. There was one death; there were no differences between the two groups in the postoperative duration of mechanical ventilation, ICU, and hospital stay. The requirement of inotropes was less in the PTFE patch group compared to the pericardial patch group (12.80 ± 8.04 vs. 17.30 ± 7.21; P = 0.025). The re-exploration rate in the PTFE group was higher than the pericardial patch group (6 vs. 1). There was no difference in the RV systolic function between the two groups as assessed by tricuspid annular systolic plane excursion on echocardiograms before discharge. We concluded that a non-immunologic, non-degenerating, and relatively durable material such as PTFE can safely be used for transannular patch repair. Vascular grafts made of PTFE have been used for systemic-pulmonary artery shunts in neonates with complex cyanotic heart defects [14], for aortic arch reconstruction, for extra-anatomic aortic bypass grafts [15], and as an inferior vena cava to pulmonary artery conduit in total cavopulmonary connection [16]. The PTFE patches are suitable for closure of septal defects, plastic reconstruction of the right and the left ventricular outflow tracts, correction of coarctation of the aorta, and plastic repair of stenosis on the main pulmonary artery and its branches [13]. The major disadvantage which we observed was the higher re-exploration rate due to bleeding through the needle holes in the PTFE patch. However, our results were only in early follow-up and long-term results of our approach are unclear.
Miyazaki et al. [17] conducted a multicenter study with each patient followed up at respective institutes. In a 10-year period, a total of 794 patients underwent RVOT reconstruction using fan-shaped ePTFE valves and ePTFE valved conduits and patches with bulging sinuses in 52 Japanese institutes. Autologous pericardium was used to reconstruct the posterior wall of the RVOT in neonates and infants who had discontinuity between the right ventricle and the pulmonary artery. The anterior wall was covered with the ePTFE valved patches. Mean follow-up was 3.6 ± 2.2 years. Freedom from re-operation at 10 years was 92.3%. There was no or mild PI in 79.6% patients. The pressure gradient between the right ventricle and the pulmonary artery was 11.6 ± 11.6 mm [17]. They concluded that ePTFE valved conduits and patches with bulging sinuses prevent PI, with a high freedom from re-operation and area promising material for RVOT reconstruction.
ePTFE can be made to any size, has good biocompatibility, and is relatively inexpensive but is a little stiff for suturing than homografts and pericardial patches. In addition, bleeding from the needle holes can be cumbersome. This can be reduced to a great extent by using PTFE sutures. Rarely, heavy calcification can occur making these patches difficult to excise at a re-operation (Fig. 3).
Fig. 3.
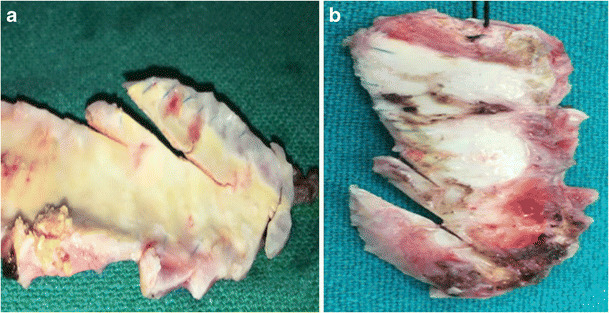
a, b The calcified polytetrafluoroethylene patch. The patch was found to be densely calcified, bony hard on palpation, and obliterated the original anatomy
Newer materials
Decellularized bovine pericardium (Syncroscaff®)
Decellularized bovine pericardium (Syncroscaff®) (Fig. 4) is a new biomaterial [18] which has recently been licensed for use as a cardiovascular patch material by the Central License Approving Authority working under the Drug Controller General of India. It is manufactured in India by SynkroMax Biotech Ltd., Chennai. The tissue is completely decellularized and cross-linked using a non-glutaraldehyde chemical process and has passed through stringent hemocompatibility and biocompatibility studies [18]. It is claimed to allow the patient’s own cells to grow into itself and integrates well with the host tissues. It is expected that this decellularized bovine pericardium may have growth potential due to repopulation by the host cells. In a study by Agarwal et al. [19], 18 patients underwent RVOT reconstruction using hand-sewn decellularized bovine pericardial conduits. Follow-up ranged from 1 to 24 months. There was no thrombosis or endocarditis even in patients with high pulmonary artery pressures or small-sized branch pulmonary arteries. Four out of 18 (22%) patients had mild conduit valve regurgitation on follow-up echocardiograms. There was no incidence of conduit stenosis at the site of distal conduit anastomosis during the follow-up duration. There was no mortality in the follow-up period.
Fig. 4.
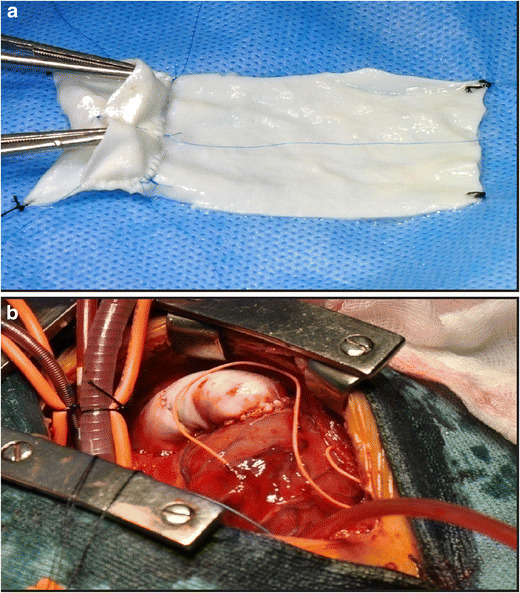
a The decellularized bovine pericardium [P] (Syncroscaff®) is being used to prepare a conduit. b After implantation. (Reproduced with kind permission from Agarwal R, Varghese R, Swaminathan V, Mubeena S. Hand-sewn valved bovine pericardial conduits for right ventricular outflow tract reconstruction—a single center experience. Presented at: 7th World Congress of Pediatric Cardiology & Cardiac Surgery, July 16–21, 2017, Barcelona, Spain)
Biodegradable polyester urethane urea
Fujimoto et al. [20] used an elastomeric, biodegradable polyester urethane urea (PEUU) which was processed into circular scaffolds and used to replace a surgical defect in the RVOT of adult rats. The objective of the study was to assess the performance of the PEUU scaffold in the reconstruction of a transmural defect created in the RVOT of the rat. After implantation periods of 4, 8, and 12 weeks, the RVOT was histologically examined to assess material degradation, inflammation, host cell migration, and endocardial endothelialization (Fig. 5). For control purposes, an ePTFE patch of the same dimensions was implanted in a separate group of animals and assessed at the same time points. There was no patch dehiscence or aneurysm formation at the site of the implanted patch in the RVOT at each time point. Both PEUU and expanded polytetrafluoroethylene were encapsulated with fibrous tissue and had complete endothelialization on the endocardial surface with no aneurysm formation or thrombus. With PEUU patching, fibroblast ingrowth occurred at 4 weeks, increasing with time. In contrast, expanded polytetrafluoroethylene patches exhibited no ingrowth and elicited local inflammation that moderated with time.
Fig. 5.
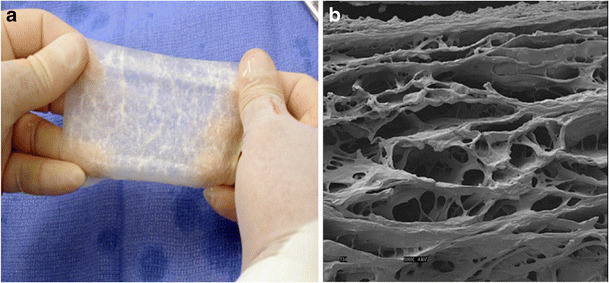
a CorMatrix ECM1. b Scanning electron micrograph of CorMatrix ECM1; magnification × 500 at 5 kV. (Reproduced with kind permission from Holubec T, Caliskan E, Sundermann SH, Starck CT, Plass A, Bettex D, et al. The Use of Extracellular Matrix Patches in Cardiac Surgery. J Card Surg 2014)
Extracellular matrix
Quarti et al. [21] described their experience with an extracellular matrix for cardiac and vascular tissue repair. Between August 2009 and April 2011, 26 patients underwent cardiac surgery using the CorMatrix patch (Fig. 6) for vascular repair (10 pulmonary artery, four ascending aortas, three aortic arch and one right ventricular outflow tract) or for valve reconstruction (five aortic, two tricuspid, one mitral and one pulmonary valve). There were no hospital deaths. The mean follow-up period was 13.2 months. Recently, extracellular matrix obtained from porcine small intestinal submucosa has been introduced into cardiac surgery. It is an acellular biomaterial that surrounds cells in most of the tissues. Composed of structural proteins (elastin and collagen), adhesion glycoproteins, glycosaminoglycan, proteoglycans, and matricellular proteins (thrombospondins, osteopontin and tenascins), the CorMatrix is a small intestinal submucosal extracellular matrix [21]. The material is hypothesized to provide an interim bio scaffold that allows patient’s own cells to repopulate and repair damage tissues (Figs. 5 and 6). None of the patients have had patch related complications.
Fig. 6.
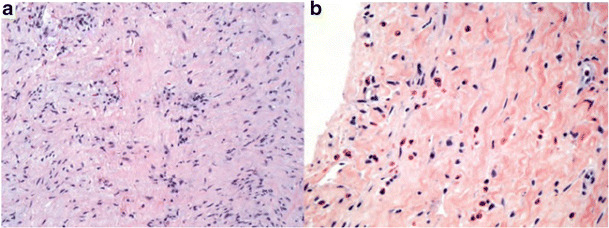
a Explanted SIS-ECM RVOT patch (day 555); H&E staining demonstrates similar chronic inflammation and fibrous collagenous tissue (magnification × 20). b Biopsy of SIS-ECM ASD patch taken 336 days after implantation (magnification × 40). H&E staining demonstrates chronic inflammation including eosinophils and diffuse myxoid degeneration. (Reproduced with kind permission from Witt RG, Raff G, Gundy JV, Rodgers-Ohlau M, Si MS. Short-term experience of porcine small intestinal submucosa patches in paediatric cardiovascular surgery. Eur J Cardiothorac Surg 2013; 44 72–76)
Scholl et al. [22] used small intestinal submucosa-extracellular matrix (SIS-ECM) patches (Fig. 7) in 43 operations on 40 patients aged 2 days to 13 years. In 16 patients, the SIS-ECM was used for pericardial closure. The SIS-ECM was used for cardiac or great vessel repair in 37 patients: atrial septal defect repair in 11, pulmonary arterioplasty in 10, RVOT patch in six, pulmonary monocusp valve creation in five, superior vena cava patch in two, aortoplasty in two, valve leaflet augmentation in two, and repair of unroofed coronary sinus in one patient. There were five deaths, all unrelated to the SIS-ECM. Mean follow-up was 7.85 months. No pericardial effusions or intracardiac or intravascular thromboses occurred related to the SIS-ECM. The patches did not shrink or calcify. Explanted tissue showed resorption of the SIS-ECM, replacement with organized collagen, and re-endothelialization [22].
Fig. 7.
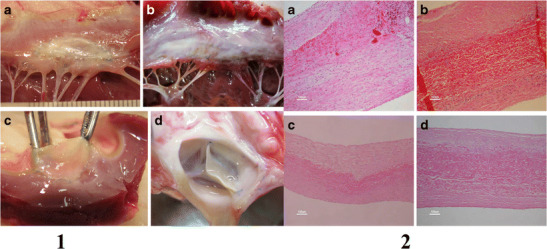
Label 1: Macroscopy. a and b—both the autologous and the CardioCel patches have the same macroscopic aspect. c and d—both the autologous patch and the CardioCel are flexible. The CardioCel patch 8 months after implantation is remarkably comparable to a fresh patch. Label 2: Hematoxylin and eosin. a, b, c, and d—all slides show a central layer made of the original patch and two outer layers of new collagen. An endothelial layer is continuous on the outer surface of the new collagen layers. Some mononucleated cells and lymphocytes can be identified in the autologous group mostly in the mitral slide (a). Reproduced with permission from Brizard et al. [27]
3D cardiac extracellular matrix
Wainwright et al. [23] hypothesized that a 3D cardiac extracellular matrix (C-ECM) biologic scaffold material would logically have structural and functional advantages over materials such as Dacron. A porcine C-ECM patch/scaffold (UBM) or a Dacron patch was used to reconstruct a full-thickness right ventricular outflow tract defect in a rat model with end points of structural remodeling at 16 weeks. The objective of this study was to assess the ability of C-ECM as a patch material to replace and remodel a full-thickness RVOT defect in a Lewis rat model. The Dacron patch was encapsulated by dense fibrous tissue and showed little cellular infiltration. Echocardiographic analysis showed that the right ventricle of hearts patched with Dacron was dilated at 16 weeks compared to pre-surgery baseline values. The C-ECM patch remodeled into dense, cellular connective tissue with scattered small islands of cardiomyocytes. The hearts patched with C-ECM showed no difference in the size or function of the ventricles as compared to baseline values at both 4 and 16 weeks. In 2013, Remlinger et al. [24] compared the effectiveness of an organ-specific C-ECM patch with a commonly used ECM scaffold for myocardial tissue repair of the RVOT and to examine the role of bone marrow derived cells in the remodeling response. A chimeric rat model in which all bone marrow cells express green fluorescent protein (GFP) was generated and used to show the ability of ECM scaffolds derived from the heart and bladder to support cardiac function and cellular growth in the right ventricular outflow tract [24]. Both scaffolds were able to preserve cardiac performance throughout the study, and both patches were able to support cell infiltration for up to 16 weeks after reconstruction. Neither scaffold showed signs of fibrotic encapsulation, and both scaffolds expressed a continuous endothelial lining along the endocardial wall of the RV at the site of repair. However, UBM patches were rapidly degraded and remodeled, with the formation of new host tissue in the reconstructed area by 16 weeks. The results of this study showed that UBM scaffolds may provide a more viable option for myocardial reconstruction than a scaffold derived from a cardiac location. The ability for the UBM patches to completely and rapidly degrade, while being replaced with newly formed site-appropriate tissue, was a significant finding.
Naik et al. [25] compared subjects undergoing non-transannular bovine pericardial patch repair of TOF with the extracellular matrix. In contrast to the above studies, which found favorable results for extracellular matrix, Naik et al. reported no significant difference in right ventricular function between groups having extracellular matrix versus bovine pericardium patches followed up for more than 1 year. Lower right ventricular longitudinal strain was noted in both the groups compared to healthy children.
Tissue engineered patches: a new concept
In 2013, Neethling et al. [26] reported the use of tissue engineered bovine pericardial patch—ADAPT-treated CardioCel patch (ABPP), in repair of congenital heart defects in 30 pediatric patients. In the 30-day postoperative period, no graft-related morbidity was observed. There were five deaths due to comorbid non-graft-related events. Echocardiography assessment at 6 and 12 months revealed intact anatomical and hemodynamically stable repairs without any visible calcification of the patch. Magnetic resonance imaging assessment in 10 patients at 12 months revealed no signs of calcification. In 19 patients, echocardiographic data were available at 18–36 months with no evidence of patch calcification, infection, thromboembolic events, or patch failure. They concluded that tissue engineered CardioCel patch (Figs. 6 and 7) is a safe and efficacious substitute for surgical repair of both simple and more complex congenital cardiac defects. In a study on growing lamb model by Brizzard et al. [27], the mechanical properties of CardioCel were preserved after 7 months with more controlled healing than treated autologous pericardium with a higher resistance to calcification (Fig. 7).
Another animal study was performed by Stock et al. [28] in which they tested a fast absorbing biopolymer, poly-4-hydroxybutyric acid, with autologous cell seeding for patch augmentation of the pulmonary artery in a juvenile sheep model (Fig. 8). Follow-up was performed with echocardiography after 1 week before explantation and after 1, 7, and 24 weeks (two animals each) for the seeded control patches and after 20 weeks for the non-seeded control patch. All animals survived the procedure. Postoperative echocardiography of the seeded patches demonstrated a smooth surface without dilatation or stenosis. Macroscopic appearance showed a smooth internal surface with increasing tissue formation. Histology at 169 days demonstrated a near-complete resorption of the polymer and formation of organized and functional tissue. Biochemical assays revealed increasing cellular and extracellular matrix contents. They suggested that this material is a feasible patch material particularly in the pulmonary circulation.
Fig. 8.
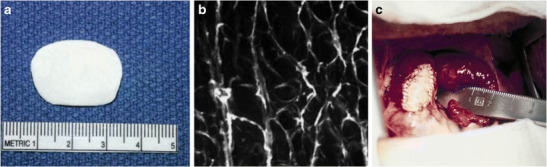
a Polymer scaffold (P-4HB) 10 × 25 mm with a thickness of 1.6 mm. b Surface electron microscopy of highly porous (porosity > 95%) P-4HB (PHA4400) (original magnification × 500). c Implanted patch in the main pulmonary artery. (Reproduced with permission from Stock UA, Sakamoto T, Hatsuoka S, Martin DP, Nagashima M, Moran AM, et al. Patch augmentation of the pulmonary artery with bioabsorbable polymers and autologous cell seeding. J Thorac Cardiovasc Surg. 2000 Dec;120(6):1158–1167)
Conclusion
Despite a wide array of patches available for RVOT reconstruction in the current era, there is no evidence to prove that one patch is superior over the other. Current practices are therefore based more on individual practices rather than hard scientific data. The bioengineered patches may be the future.
Compliance with ethical standards
Statement of human rights/ethical approval
All procedures performed in this study were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Dunning J, Prendergast B, Mackway-Jones K. Towards evidence-based medicine in cardiothoracic surgery: best BETS. Interact Cardiovasc Thorac Surg. 2003;2:405–409. doi: 10.1016/S1569-9293(03)00191-9. [DOI] [PubMed] [Google Scholar]
- 2.Rosenthal A, Fross RE, Pasternac A. Aneurysms of right ventricular outflow patches. J Thorac Cardiovasc Surg. 1972;63:735. [PubMed] [Google Scholar]
- 3.Hawe A, Rastelli GC, Ritter DG, et al. Management of the right ventricular outflow tract in severe tetralogy of Fallot. J Thorac Cardiovasc Surg. 1970;60:131. [PubMed] [Google Scholar]
- 4.Messina JJ, O’Loughlin J, Isom OW, Klein AA, Engle MA, Gold JP. Glutaraldehyde treated autologous pericardium in complete repair of tetralogy of Fallot. J Card Surg. 1994;9:298–303. doi: 10.1111/j.1540-8191.1994.tb00848.x. [DOI] [PubMed] [Google Scholar]
- 5.D’Andrilli A, Ibrahim M, Venuta F, De Giacomo T, Coloni GF, Rendina EA. Glutaraldehyde preserved autologous pericardium for patch reconstruction of the pulmonary artery and superior vena 507 cava. Ann Thorac Surg. 2005;80:357–358. doi: 10.1016/j.athoracsur.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 6.Lee C, Lim HG, Lee CH, Kim YJ. Effects of glutaraldehyde concen-510 tration and fixation time on material characteristics and calcification 511 of bovine pericardium: implications for the optimal method of fix-512 ation of autologous pericardium used for cardiovascular surgery. Interact Cardiovasc Thorac Surg. 2017;24:402–406. doi: 10.1093/icvts/ivx005. [DOI] [PubMed] [Google Scholar]
- 7.Chiu IS, How SW, Hou SH, Wang E. Fate of the autologous tri-cusp-valved pericardial conduit in the right ventricular outflow tract of growing pigs. Proc Natl Sci Counc Repub China B. 1992;16:23–30. [PubMed] [Google Scholar]
- 8.Youn YN, Park HK, Kim D, Park SY, Yi G, Park YH. Mid-Term results of reconstruction of the right ventricular outflow tract using cryopreserved homografts. Yonsei Med J. 2007;48:639–644. doi: 10.3349/ymj.2007.48.4.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crawford FA, Jr, Sade RM, Spinale F. Bovine pericardium for correction of congenital heart defects. Ann Thorac Surg. 1986;41:602–605. doi: 10.1016/S0003-4975(10)63068-8. [DOI] [PubMed] [Google Scholar]
- 10.Gabbay S, Bortolotti U, Factor S, Shore DF, Frater RW. Calcification of implanted xenograft pericardium. Influence of site and function. J Thorac Cardiovasc Surg. 1984;87:782–787. [PubMed] [Google Scholar]
- 11.Simon BV, Swartz MF, Egan M, Cholette JM, Gensini F, Alfieris GM. Use of a dacron annular sparing versus limited transannular patch with nominal pulmonary annular expansion in infants with tetralogy of fallot. Ann Thorac Surg. 2017;103:186–192. doi: 10.1016/j.athoracsur.2016.05.056. [DOI] [PubMed] [Google Scholar]
- 12.Jonas RA. Choosing the right biomaterial. In: Jonas RA, editor. Comprehensive surgical management of congenital heart disease. Boca Raton: CRC Press; 2014. pp. 247–266. [Google Scholar]
- 13.Talwar S, Selvam M, Rajasekhar P, Ramakrishnan S, Choudhary S, Airan B. Polytetrafluoroethylene patch versus autologous pericardial patch for right ventricular outflow tract reconstruction. J Practice Cardiovasc Sci. 2016;2:175–180. doi: 10.4103/2395-5414.201372. [DOI] [Google Scholar]
- 14.de Leval MR, McKay R, Jones M, Stark J, Macartney FJ. Modified Blalock-Taussig shunt. Use of subclavian artery orifice as flow regulator in prosthetic systemicpulmonary artery shunts. J Thorac Cardiovasc Surg. 1981;81:112–119. [PubMed] [Google Scholar]
- 15.Kumar MV, Choudhary SK, Talwar S, et al. Extraanatomic bypass to supraceliac abdominal aorta for complex thoracic aortic obstruction. Ann Thorac Surg. 2016;101:1552–1557. doi: 10.1016/j.athoracsur.2015.10.080. [DOI] [PubMed] [Google Scholar]
- 16.Marcelletti C, Corno A, Giannico S, Marino B. Inferior vena cava-pulmonary artery extracardiac conduit. A new form of right heart bypass. J Thorac Cardiovasc Surg. 1990;100:228–232. [PubMed] [Google Scholar]
- 17.Miyazaki T, Yamagishi M, Maeda Y, et al. Expanded polytetrafluoroethylene conduits and patches with bulging sinuses and fan-shaped valves in right ventricular outflow tract reconstruction: multicenter study in Japan. J Thorac Cardiovasc Surg. 2011;142:1122–1129. doi: 10.1016/j.jtcvs.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 18.Guhathakurta S, Balasubramanian V, Ananthakrishnan B, et al. Thrombogenicity studies of three different variants of processed bovine pericardium. IRBM. 2008;29:223–230. doi: 10.1016/j.rbmret.2007.07.003. [DOI] [Google Scholar]
- 19.Agarwal R, Varghese R, Swaminathan V, Mubeena S. Hand-sewn valved bovine pericardial conduits for right ventricular outflow tract reconstruction—a single center experience.WCPCCS2017. http://wcpccs2017.org/files/downloads/WCPCCS%202017%20Full%20Program-.pdf.
- 20.Fujimoto KL, Guan J, Oshima H, Sakai T, Wagner WR. In vivo evaluation of a porous, elastic, biodegradable patch for reconstructive cardiac procedures. Ann Thorac Surg. 2007;83:648–654. doi: 10.1016/j.athoracsur.2006.06.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quarti A, Nardone S, Colaneri M, Santoro G, Pozzi M. Preliminary experience in the use of an extracellular matrix to repair congenital heart diseases. Interact Cardiovasc Thorac Surg. 2011;13:569–572. doi: 10.1510/icvts.2011.280016. [DOI] [PubMed] [Google Scholar]
- 22.Scholl FG, Boucek MM, Chan KC, Valdes-Cruz L, Perryman R. Preliminary experience with cardiac reconstruction using decellularized porcine extracellular matrix scaffold: human applications in congenital heart disease. World J Pediatr Congenit Heart Surg. 2010;1:132–136. doi: 10.1177/2150135110362092. [DOI] [PubMed] [Google Scholar]
- 23.Wainwright JM, Hashizume R, Fujimoto KL, et al. Right ventricular outflow tract repair with a cardiac biologic scaffold. Cells Tissues Organs. 2012;195:159–170. doi: 10.1159/000331400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Remlinger NT, Gilbert TW, Yoshida M, et al. Urinary bladder matrix promotes site appropriate tissue formation following right ventricle outflow tract repair. Organogenesis. 2013;9:149–160. doi: 10.4161/org.25394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naik R, Johnson J, Kumar TKS, Philip R, Boston U, Knott-Craig CJ. Right ventricular function after repair of tetralogy of Fallot: a comparison between bovine pericardium and porcine small intestinal extracellular matrix. Cardiol Young. 2017;1–7. [DOI] [PubMed]
- 26.Neethling WM, Strange G, Firth L, Smit FE. Evaluation of a tissue-engineered bovine pericardial patch in paediatric patients with congenital cardiac anomalies: initial experience with the ADAPT-treated CardioCel(R) patch. Interact Cardiovasc Thorac Surg. 2013;17:698–702. doi: 10.1093/icvts/ivt268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brizard CP, Brink J, Horton SB, Edwards GA, Galati JC, Neethling WM. New engineering treatment of bovine pericardium confers outstanding resistance to calcification in mitral and pulmonary implantations in a juvenile sheep model. J Thorac Cardiovasc Surg. 2014;148:3194–3201. doi: 10.1016/j.jtcvs.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 28.Stock UA, Sakamoto T, Hatsuoka S, et al. Patch augmentation of the pulmonary artery with bioabsorbable polymers and autologous cell seeding. J Thorac Cardiovasc Surg. 2000;120:1158–1167. doi: 10.1067/mtc.2000.109539. [DOI] [PubMed] [Google Scholar]


