Abstract
Background
Surgical aortic valve replacement (SAVR) has shown safe, robust results in elderly populations, and up until recently, was the gold standard for management of severe aortic stenosis. The approach to severe aortic stenosis in high-risk populations, such as octogenarians, has been challenged with the development of transcatheter-based strategies. We sought to systematically analyse outcomes between surgical and transcatheter aortic valve replacement (TAVI) in octogenarians.
Method
Electronic databases were searched from their inception until November 2018 for studies comparing SAVR to TAVI in octogenarians, according to a predefined search criterion. The primary end point was mortality, and secondary end points included post-procedural complications.
Results
The review yielded four observational studies. The total number of patients included was 1221 including 395 who underwent TAVI and 826 SAVR. On average, patients from both subgroups carried a high number of cardiac risk factors, and STS-PROM scoring yielded mean values equating to high-risk population groups, with significantly higher values for TAVI patients across the board. The presence of post-procedural moderate aortic regurgitation was noted only in the TAVI population (OR = 8.88; 95% CI (1.47–53.64), χ2 = 1.22; p = 0.02; I2 = 0%). Otherwise, there were no significant differences when accounting for mortality (OR = 0.68; 95% CI (0.44–1.05), χ2 = 1.88; p = 0.60; I2 = 0%), permanent pacemaker implantation groups (OR = 0.45; 95% CI (0.44–1.49), χ2 = 0.11; p = 0.19; I2 = 0%), and neurological events (OR = 0.72; 95% CI (0.42–1.23), χ2 = 2.57; p = 0.23; I2 = 22%).
Discussion
The analysed data on TAVI versus SAVR in the octogenarian population show that TAVI shows similar outcomes with relation to mortality and inpatient admission times, in a population with significantly higher risk profiles than their SAVR counterparts. TAVI has higher occurrences of post-procedural AR. TAVI still does not have robust long-term data to ensure its efficacy and rate of complications, but is showing promising results nonetheless.
Keywords: TAVI, transcatheter aortic valve implantation; SAVR; Surgical aortic valve replacement; Octogenarians
Introduction
Aortic stenosis (AS) is the most common cardiac valve pathology encountered in the elderly population [1]. The Australian population aged over 65 years has been steadily increasing [2]. The older population has a higher prevalence of AS [3]. Aortic stenosis also carries a significant burden of morbidity and mortality, with 1 year mortality rates close to 32% [4], however, variable depending on symptomatology associated with AS.
Up until recently, SAVR was undoubtedly the gold standard option for management of severe AS; however, new guidelines from the most recent European Society of Cardiology [5] suggest involvement of a heart team, and discussions to determine the most appropriate form of intervention for an individual prior to offering surgical or percutaneous aortic valve replacement. Survival at 3 years differed from 87% for patients who underwent SAVR compared with 21% for medically management patients [6]. The development of TAVI has shed new options for management of high-risk patients who were initially deemed to be non-operative candidates [7], and has recently been noted to be non-inferior to SAVR even for intermediate-risk patients, when evaluating death and stroke risk [8].
Extensive long-term data, existing for the surgical population, however, is still lacking in the TAVI group. Nevertheless, the advances in minimally invasive techniques challenge the current state of conventional SAVR. The aim of this review is to evaluate outcomes of SAVR and TAVI in the octogenarian population, and assess post-operative complications, together with mortality, so as to analyse the major differences in outcomes in this growing age group.
Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Guidelines were followed in this analysis [9].
Search strategy and study selection
A systematic search was performed utilising several databases, such as PubMed, MEDLINE, Embase, Google Scholar, Science Direct, and Web of Science from conception to November 2018. The following search terms, including MeSH terms where appropriate, were utilised: “Octogenarians” OR “Aged over 80” AND “TAVI” OR “TAVR” OR “transcatheter aortic valve” OR “transfemoral aortic valve” OR “transcutaneous aortic valve” OR “percutaneous aortic valve” OR “transapical aortic valve” AND “SAVR” OR “Surgical Aortic Valve Replacement” AND “Aortic stenosis”. Studies were limited to human studies, written in the English language. Conference abstracts, letters to the editor, and editorials were also excluded. The references of records screened were also reviewed in an attempt to identify potential additional articles.
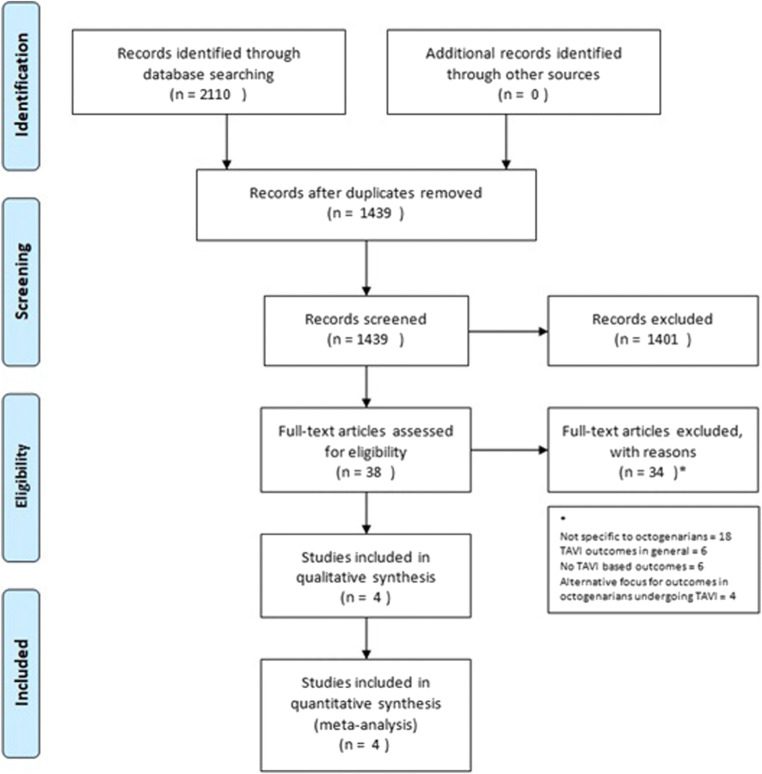
All studies which compared TAVI versus SAVR in the octogenarian population were included. The full text articles of relevant abstracts were retrieved and re-reviewed for inclusion as demonstrated in (Fig. 1). The screening process removed 1401 articles as they did not meet the criteria of comparing SAVR and TAVI based on initial assessment from titles and abstracts.
Fig. 1.
PRISMA guidelines flow chart for systematic analysis [9]
Data collection process and quality assessment
All data was extracted by published text, tables, and graphs, including online supplemental material by a single reviewed (SM), and reviewed by a co-author (MD). Any inconsistencies were resolved by discussion in conjunction with all authors and agreement by all parties.
Data analysis
Data items included patient demographics, pre-operative cardiovascular comorbidities, renal function, and risk stratification scores.
Primary end point for the review was all cause mortality. Secondary end points assess post-procedural complications: (1) Residual aortic regurgitation (AR) as assessed by echocardiography; (2) Requirement for permanent pacemaker (PPM) implantation; (3) Neurological events; and (4) Acute kidney injury.
Statistical analyses were performed utilising Review Manager (RevMan 5.3, Cochrane [10]). In order to obtain odds ratio (OR), the Mantel-Haenszel statistical method was utilised with a fixed effect analysis model, maintaining a confidence interval (CI) of 95%. Heterogeneity was calculated utilising the χ2 test, and assumed to be significant if I2 was greater than 0.5.
Quality of studies was assessed by utilising the Newcastle-Ottawa quality assessment form for cohort studies [11]. The domains utilised for assessing quality included selection, comparability, and outcomes. Selection was assessed by analysing if populations included were truly representative of the exposed cohort, drawn from the same community, with data obtained from reliable sources, and no suggestion of bias from the study outset. Similarly, comparability was assessed by the quality of comparability of cohorts, based on design and analysis. The final assessment of outcome analysed if outcomes were obtained in an independent or reliably secure manner, follow-up time, and degree of loss to follow-up.
Results
This study identified four major studies which compared TAVI and SAVR in the octogenarian population [12–15]. The papers were comprised of three prospective, and one retrospective observational study which enrolled patients from as far back as 2002, and up to 2015.
In total, there were 1070 octogenarians who underwent SAVR, whilst 656 underwent TAVI. There was also the inclusion of 42 octogenarians who underwent non-procedural intervention. Table 1 demonstrates summary of the articles, including patient baseline characteristics. All papers explore common cardiac risk factors, such as previous myocardial infarctions and presence of coronary artery disease (CAD), hypertension, diabetes, New York Heart Association (NYHA) Class III and IV symptoms, previous cerebrovascular accidents, and renal function on admission. Each author provided median risk stratification scores in the form of Society of Thoracic Surgeons predicted risk of mortality (STS-PROM) which ranged from 5.2 to 7 for the SAVR groups, and 6.8–14 for the TAVI groups [12–15]. EuroSCORE and logEuroSCORE were also included in two papers [13, 14], and are difficult to compare directly to STS-PROM; however, they depict a high-risk population for both groups, with significantly higher risks identified in the TAVI population.
Table 1.
Comparison of patient demographics and pre-procedural comorbidities
| Author | Hirji et al. [12] | Im et al. [13] | Strauch et al. [14] | Martinez-Selles et al. [15] | ||||||||||
| Year | 2017 | 2013 | 2012 | 2013 | ||||||||||
| Country | USA | South Korea | Germany | Spain | ||||||||||
| Type of study | Pros, Obs, SC | Pros, Obs, SC | Retro, Obs, SC | Pros, Obs, MC | ||||||||||
| Subgroups analysed | SAVR | TAVI | p | SAVR | TAVI | OMT | p | SAVR | TAVI | p | SAVR | TAVI | Conserv | p |
| Number of Patients | 722 | 306 | – | 14 | 10 | 42 | – | 90 | 79 | – | 244 | 261 | 423 | |
| Age (years ± SD) | 84.1 ± 3.2 | 86.2 ± 3.9 | Y | 83 | 82.5 | 84 | N | 83.2 ± 0.2 | 83.8 ± 0.6 | N | 82.2 ± 2 | 84.7 ± 3.3 | 85.1 ± 3.8 | |
| Male (%) | 47.8 | 47.4 | N | 4 (28.6) | 4 (40) | 19 (45.2) | N | 35 (39) | 23 (29) | N | 113 (46.3) | 108 (41.4) | 161 (38.1) | |
| Previous AMI (n (%)) | 65 (9) | 74 (24.2) | Y | 1 (7.1) | 3 (30) | 2 (4.8) | N | – | – | – | 19 (7.8) | 33 (12.6) | 67 (15.8) | |
| AMI in the last 6 months (n (%)) | – | – | – | – | – | – | – | 6 (7) | 8 (10) | N | – | – | – | – |
| Hypertension (n (%)) | 574 (79.5) | 245 (80.1) | N | 10 (71.4) | 8 (80) | 32 (76.2) | N | 21 (23) | 25 (32) | N | 190 (77.9) | 186 (71.3) | 335 (79.2) | |
| Hypercholesterolemia (n (%)) | – | – | – | – | – | – | – | 72 (80) | 56 (71) | N | 113 (46.3) | 114 (43.7) | 164 (38.8) | |
| NYHA III/IV | 369 (51.1) | 242 (79.1) | Y | 12 (85.7) | 8 (80) | 15 (35.7) | Y | 76 (84) | 77 (97) | Y | – | – | – | – |
| Previous cardiac surgery (n (%)) | 134 (18.6) | 97 (31.7) | N | 1 (7.1) | 1 (10) | 2 (4.8) | N | 14 (16) | 36 (45) | Y | – | – | – | – |
| Previous SAVR (n (%)) | 21 (2.9) | 37 (12.1) | Y | – | – | – | – | – | – | – | – | – | – | – |
| Previous stroke (n (%)) | 41 (5.7) | 39 (12.7) | Y | 2 (14.3) | 1 (10) | 9 (21.4) | N | 2 (2) | 7 (9) | N | 18 (7.4) | 24 (9.2) | 46 (10.9) | |
| Diabetes (n (%)) | 139 (19.3) | 107 (35) | Y | 4 (28.6) | 1 (10) | 9 (21.4) | N | 17 (19) | 39 (49) | Y | 61 (25) | 60 (23) | 126 (29.8) | |
| Mean Creat (mg/dL ± SD) | 1.12 ± 0.4 | 1.36 ± 1.2 | Y | – | – | – | – | – | – | – | – | – | – | – |
| Mean eGFR (mL/min/m2) | – | – | – | 54.5 | 47.5 | 51 | N | – | – | – | – | – | – | – |
| Serum Creat > 1.1 mg/dL (n (%)) | – | – | – | – | – | – | – | 28 (31) | 45 (56) | Y | – | – | – | – |
| Mean CrCl (mL/min) | – | – | – | – | – | – | – | – | – | – | 50.1 ± 16.6 | 40.2 ± 15.1 | 40.4 ± 17.5 | |
| STS-PROM (Mean% ± SD) | 5.5 ± 3.4 | 6.81 ± 4.5 | Y | 5.2 | 6.9 | 7.4 | N | 7 ± 0.5 | 14 ± 0.5 | Y | – | – | – | – |
| LogEuroSCORE (Mean% ± SD) | – | – | – | 11.7 | 24.7 | 23.4 | N | – | – | – | – | – | – | – |
| EuroSCORE (Mean ± SD) | – | – | – | – | – | – | – | 11 ± 1.2 | 38 ± 1.3 | Y | 20.9 ± 13.1 | 31.4 ± 17.9 | 33.3 ± 17.4 | – |
Pros, prospective; Retro, retrospective; Obs, observational; SC, single centre; MC, multi-centre; CAD, coronary artery disease; AMI, acute myocardial infarction; CCF, congestive cardiac failure; NYHA, New York Heart Association; Prev, previous; SAVR, surgical aortic valve replacement; Creat, creatinine; eGFR, estimated glomerular filtration rate; CrCl, creatinine clearance; STS-PROM, Society of Thoracic Surgeons Predicted Risk of Mortality; p, statistically significant p value reported; TAVI, transcatheter aortic valve implantation; OMT, optimal medical therapy; Y, yes; N, no; − indicates no data available
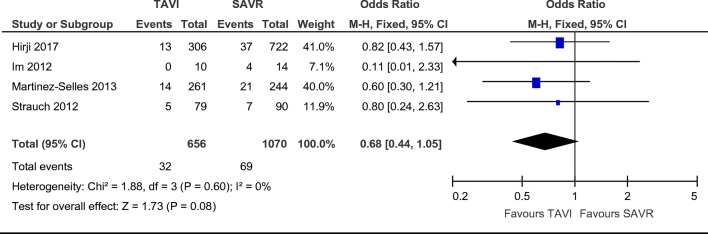
Combined 30-day all-cause mortality was reported in all four papers, and occurred in 70 SAVR patients and 34 TAVI patients, with no statistically significant difference between the groups for each paper [12–15]. Of note, SAVR mortality rates in the Im et al. paper was higher than that which is reported in the literature on valvular mortality [13]. Mortality rates were not significantly different between groups (Fig. 2. OR = 0.68; 95% CI (0.44–1.05), χ2 = 1.88; p = 0.60; I2 = 0%), with a non-statistically significant favour towards TAVI. Length of follow-up varied between studies and long-term mortality is shown in further detail in Table 2. Median follow-up lengths were 3 months [13], 6 months [14], 15 months [15], and 35 months [12] in the studies included. Long-term follow-up was not analysed in the meta-analysis due to significant differences in reporting of mortality between studies.
Fig. 2.
Odds ratio and forest plot of all cause peri-procedural mortality
Table 2.
Comparison of post-interventional outcomes
| Author | Hirji et al. [12] | Im et al. [13] | Strauch et al. [14] | Martinez-Selles et al. [15] | ||||||||||
| Subgroups analysed | SAVR | TAVI | p | SAVR | TAVI | OMT | p | SAVR | TAVI | p | SAVR | TAVI | Conserv | p |
| Residual AR (n (%)) | ||||||||||||||
| None | 722 (100) | 248 (81) | – | – | – | – | – | – | – | – | – | – | – | – |
| Trace | 0 (0) | 32 (10.5) | – | – | – | – | – | – | – | – | – | – | – | – |
| Mild | 0 (0) | 21 (6.9) | – | – | – | – | – | – | – | – | – | – | – | – |
| Moderate | 0 (0) | 6 (2) | – | 0 (0) | 1 (10) | – | N | 0 (0) | 1 (1.3) | N | – | – | – | – |
| Severe | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| PPM (n (%)) | – | – | – | 2 (14.3) | 1 (10%) | 0 (0) | Y | 8 (8.9) | 3 (3.8) | N | – | – | – | – |
| Acute myocardial infarction | – | – | – | 2 (14.3) | 0 (0) | 1 (2.4) | N | 0 (0) | 1 (1.3) | N | – | – | – | – |
| Neurological Event (n (%)) | 46 (6.1) | 17 (5.6) | N | 4 (28.6) | 0 (0) | 2 (4.8) | N | 4 (4.4) | 1 (1.3) | N | – | – | – | – |
| Renal injury (n (%)) | 27 (3.7) | 0 (0) | – | 4 (28.6) | 0 (0) | – | N | 9 (10) | 25 (31.5) | – | – | – | – | – |
| ICU LOS (median) | 51 h | 6 h | Y | 7 days (3–50) | 4 days (3–5) | – | N | 3.6 days ± 0.5 | 3.8 days ± 0.2 | N | – | – | – | – |
| Total LOS (days, median) | 8 | 3 | Y | – | – | – | – | 14.3 ± 0.6 | 12.8 ± 0.6 | N | – | – | – | – |
| Operative mortality (n (%)) | 37 (5.1) | 13 (4.2) | N | 4 (28.6) | 0 (0) | 9 (21.4) | N | 5 (5.6) | 7 (8.8) | N | 21 (8.6) | 14 (5.3) | – | Y |
| 6 months mortality (n (%)) | – | – | – | – | – | – | – | 76 (89) | 67 (85) | N | – | – | – | – |
| 12 months mortality (n (%)) | 101 (14%) | 52 (17) | Y | – | – | – | – | 66 (78) | – | – | 74 (30.3) | 91 (34.6) | 191 (45.3) | Y |
AR, aortic regurgitation; PPM, permanent pacemaker; ICU, intensive care unit; LOS, length of stay; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation; p, statistically significant p value reported; OMT, optimal medical therapy; Conserv, conservative medical management; Y, yes; N, no; − indicates no data available
Post-interventional outcomes are depicted in Table 2. The paper by Martinez-Selles et al., reporting mortality, however, did not report any other post-procedural outcomes, and hence was not included in the meta-analysis for any of the other outcomes analysed.
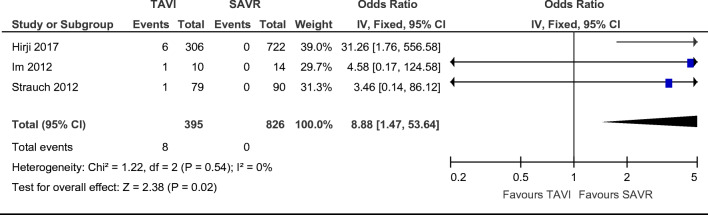
Three papers commented on the presence of moderate post-procedural aortic regurgitation. It is important to note that there were no events showing aortic regurgitation in the surgical groups. The TAVI studies reported a total of 8 patients with moderate post-insertion AR [12–14]. There were statistically significant differences in moderate post-procedural aortic regurgitation, with an increase towards TAVI and favouring SAVR (Fig. 3. OR = 8.88; 95% CI (1.47–53.64), χ2 = 1.22; p = 0.02; I2 = 0%), with a non-statistically significant favour towards SAVR.
Fig. 3.
Odds ratio and forest plot of moderate aortic regurgitation post-procedure
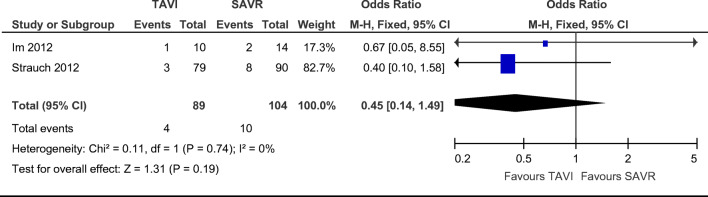
Requirement for permanent pacemaker implantation (PPM) were reported in a combined total of 10 SAVR and 4 TAVI patients without any significant difference found on statistical analysis of both papers reporting this finding [13, 14]. There were no statistically significant differences in the need for permanent pacemaker implantation post-procedure between groups (Fig. 4. OR = 0.45; 95% CI (0.44–1.49), χ2 = 0.11; p = 0.19; I2 = 0%), with a non-statistically significant favour towards TAVI.
Fig. 4.
Odds ratio and forest plot of requirement for permanent pacemaker insertion post-procedure
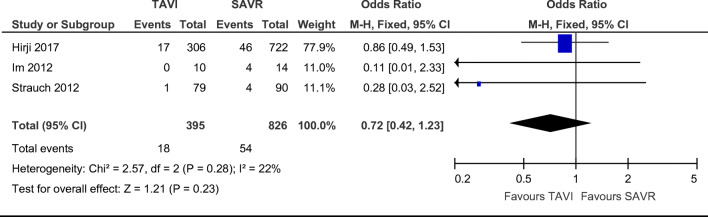
The presence of a neurological event, which encompasses both strokes and transient ischaemic attacks (TIAs), was reported in a total of 54 SAVR patients and 18 TAVI patients. Of note, however, the authors Im et al. stated that, despite reporting 4 neurological events, there was only one patient who experienced a major stroke in their study [13]. There was no statistically significant difference between groups regarding post-procedural neurological events (Fig. 5. OR = 0.72; 95% CI (0.42–1.23), χ2 = 2.57; p = 0.23; I2 = 22%), with a non-statistically significant favour towards TAVI.
Fig. 5.
Odds ratio and forest plot of a neurological event post-procedure
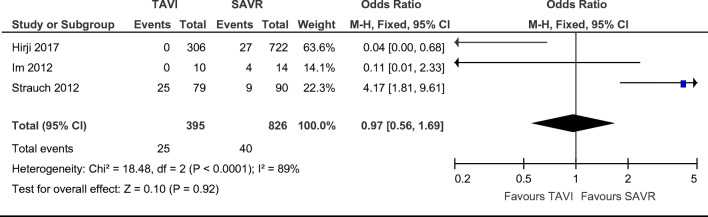
Acute kidney injury was reported in a combined total of 40 SAVR and 25 TAVI patients without any significant difference found on statistical analysis of all three papers reporting this finding [12–14]. There were no statistically significant differences in renal injury post-procedure between groups (Fig. 6. OR = 0.97; 95% CI (0.56–1.69), χ2 = 18.48; p = 0.92; I2 = 89%), with a non-statistically significant favour towards TAVI.
Fig. 6.
Odds ratio and forest plot of renal injury post-procedure
The risk of bias assessment table (Table 3) based on the Newcastle-Ottawa scale [11] has deemed two studies to be of good quality, whilst one classified as fair, due to lack of points in outcome/exposure, and one study classed as poor quality due to lack of points in the comparability domain and outcome/exposure sections.
Table 3.
Newcastle-Ottawa quality assessment form for cohort studies [11]
| Questions | Hirji et al. [12] | Im et al. [13] | Martinez-Selles et al. [15] | Strauch et al. [14] |
|---|---|---|---|---|
| Representativeness of the exposed cohort | Yes | Yes | Yes | Yes |
| Selection of the non-exposed cohort | Drawn from same community | Drawn from same community | Drawn from same community | Drawn from same community |
| Ascertainment of exposure | Secure record | Secure record | Secure record | Secure record |
| Demonstration that outcome of interest as not present at the start of the study | No | No | No | No |
| Comparability of cohorts on the basis of design or analysis controlled for confounders | Yes | Yes | Yes | Yes |
| Assessment of outcome | Several secure resources | No description | Performed by telephone | No description |
| Follow-up of 12 months | Yes | No | Yes | No |
| Adequacy of follow-up | 99% complete | Not described | 99.7% complete | Not described |
| Overall quality result | Good | Poor | Good | Fair |
Discussion
The technological advancements and ongoing development of percutaneous modalities for aortic valve replacement have sparked the interest in TAVI as a suitable alternative for aortic valve replacement. The use of less invasive techniques seems even more attractive when dealing with the octogenarian population, whom often are considered higher risk due to their age, and are more likely to be burdened with several co-morbidities, when compared with their younger counterparts. Only recently, we have seen the ongoing support of TAVI in large-scale publications, such as the PARTNER trial. These studies were not included in this study as they did not solely include octogenarians; however, they provide significant support towards TAVI in the management of aortic valve pathology.
This review has analysed four published articles comparing the outcomes of the octogenarian population undergoing TAVI and SAVR. There was no consistent significant difference in age between the studies. Even though there are slight differences between each paper, the overall picture portrayed is that octogenarians carry high burden of comorbidities which may be of some significance towards their overall outcomes following procedural intervention. This “higher-risk” population group findings are to be expected, and are the reason why alternatives to conventional SAVR were sought in the first place.
There are no differences in mortality between the TAVI and SAVR populations at 30 days, 1 year, and 2 years [7, 8, 16, 17]. Regarding secondary end points, SAVR yielded higher rates of AKI and bleeding [16, 17]. TAVI yielded higher rates of PPM implantation, vascular complications, and post-procedural aortic regurgitation [16, 17]. There were no differences in stroke rates [16, 17]. One anomaly however appeared to be the 4 patients (28%) who died in the 30-day follow-up reported by Im et al., one patient passed away from a peri-procedural AMI, and the other three were related to bleeding and renal impairment [13].
Post-procedural end points were analysed in three papers, and their reporting system was reasonably consistent. The implementation of standardised definitions for clinical end points was sought to be resolved by the use of the Valve Academic Research Consortium 2 (VARC-2) initiative [18]. The use of standardised end points will allow better comparison, and focus on what is considered an important finding to provide evidence on effectiveness of TAVI.
The PARTNER trial noted higher levels of stroke following TAVI [7, 8]. Out of the 3 papers which reported neurological events, there were no significant differences in outcomes between SAVR and TAVI. There was also no difference in PPM, AMI, or renal injury when comparing both SAVR and TAVI cohorts.
The advantage of less invasive techniques is decreased hospital length of stay. Significant differences in total hospital and intensive care unit (ICU) length of stays were noted by Hirji et al. The other studies suggested a non-statistically significant trend towards shortened ICU and total hospital length of stay for their TAVI groups. A unique disadvantage of TAVI is post-procedural aortic regurgitation which has been associated with an increased mortality in the long run, and has been reported in 11% of patients in the PARTNER B trial [7, 8]. Three studies reported on at least moderate post-procedural AR [12–14]. The total number of at least moderate AR in the TAVI population remains relatively low (n = 8) over the three studies which analysed post-procedural AR [12–14]; this finding is still quite different compared with the absence of at least moderate AR in the SAVR population reported in the studies [12–14].
A unique late complication of TAVI is that of delayed coronary artery occlusion (DCAO). The incidence of DCAO is not quite established; however, it has been reported as a potential complication, present most commonly in urgent cases, and within the first week following procedure [19]. There is still higher onset of post-operative atrial fibrillation (AF) in SAVR compared with TAVI (31–64% and 4–32%, respectively) [20]. Post-operative AF is an independent risk factor for increased mortality, stroke, and hospital admission time [20].
At present, there are two different styles of valves available for implantation: self-expanding and balloon-expandable valves. The most recent trial comparing both valves, the second-generation self-expandable versus balloon-expandable valves and general versus local anaesthesia in TAVI patients (SOLVE-TAVI) trial, showed no difference in mortality, stroke, at least moderate aortic regurgitation, and pacemaker implantation at 30 days [21]. There was some suggestion of a trend towards higher stroke rates in balloon-expandable valves [21].
This review included both transfemoral and transapical approaches to TAVI implantation. A recent study, which performed post hoc analysis of 7973 cases of TAVI, showed strong favour towards a transfemoral approach with respect to in-hospital mortality and AKI [22].
Limitations
The limitations of this review include large variability in number of patients included, the small amount of trials looking at SAVR and TAVI which solely include octogenarians, as well as the fact that these trials were all observational, and in one case retrospective. There is limitation from publication bias, and also some variability in reported outcomes despite similar cohorts being compared. No study strictly adhered to the VARC/VARC2 recommendations for reporting in TAVI studies. It is also important to note that studies did not select for a single type of valve being implanted, and this could potentially yield difference in outcomes. Different centres would mean there is an element of operator bias that cannot be accounted for, as well as variability in experience between proceduralists throughout the centres included. A large component of performing a meta-analysis in such small numbers includes the widespread heterogeneity which is likely to be encountered. In this instance, the statistical analysis was performed with a fixed effect analysis model with a confidence interval of 95%. If this were to have changed to a random effect analysis model, and lower confidence interval, the issue of heterogeneity would have been addressed to some degree; however, this would decrease the significance of results reported.
In general, TAVI is showing promising results, and we eagerly await results of randomised controlled trials to ascertain the pros and cons of this new modality in the general and octogenarian population. This review has not looked at concomitant percutaneous coronary intervention, which carries additional risks compared with TAVI alone, and a surgical approach is still strongly preferred when dealing with coronary and other valvular intervention. The long-term data for TAVI valves is still being questioned, and will not be available for years to come. Long-term follow-up and continuing maturation of clinical experience will be essential to provide gold standard patient care.
In conclusion, TAVI is quickly becoming the main option for intervention in octogenarians who require intervention for significant aortic valve stenosis.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Informed consent
Informed consent was not sought, as this study utilised previously published material, and all data utilised was already de-identified.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lung B, Baron G, Butchart EG, et al. A prospective survey of patients with valvular heart disease in Europe: the Euro Heart Survey on valvular heart disease. Euro Heart J. 2003;24:1231–1243. doi: 10.1016/S0195-668X(03)96147-1. [DOI] [PubMed] [Google Scholar]
- 2.ABS 2017. Australian demographic statistics, Jun 2016. ABS cat. no. 3101.0. Canberra: ABS.
- 3.Eveborn GW, Schirmer H, Heggelund G, Lunde P, Rasmussen K. The evolving epidemiology of valvular aortic stenosis. The Tromsø Study Heart. 2013;99:396–400. doi: 10.1136/heartjnl-2012-302265. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Dor I, Pichard AD, Gonzalez MA, et.al. Correlates and causes of death in patients with severe symptomatic aortic stenosis who are not eligible to participate in a clinical trial of transcatheter aortic valve implantation. Circulation. 2010;122:S37–S42. [DOI] [PubMed]
- 5.Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Euro Heart J. 2017;38:2739–2791. doi: 10.1093/eurheartj/ehx391. [DOI] [PubMed] [Google Scholar]
- 6.Schwarz F, Baumann P, Manthey J, Hoffmann M, Schuler G, Mehmel HC, Schmitz W, Kübler W. The effect of aortic valve replacement on survival. Circulation. 1982;66:1105–1110. doi: 10.1161/01.CIR.66.5.1105. [DOI] [PubMed] [Google Scholar]
- 7.Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Williams M, Dewey T, Kapadia S, Babaliaros V, Thourani VH, Corso P, Pichard AD, Bavaria JE, Herrmann HC, Akin JJ, Anderson WN, Wang D, Pocock SJ, PARTNER Trial Investigators Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187–2198. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 8.Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, Thourani VH, Tuzcu EM, Miller DC, Herrmann HC, Doshi D, Cohen DJ, Pichard AD, Kapadia S, Dewey T, Babaliaros V, Szeto WY, Williams MR, Kereiakes D, Zajarias A, Greason KL, Whisenant BK, Hodson RW, Moses JW, Trento A, Brown DL, Fearon WF, Pibarot P, Hahn RT, Jaber WA, Anderson WN, Alu MC, Webb JG, PARTNER 2 Investigators Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016;374(17):1609–1620. doi: 10.1056/NEJMoa1514616. [DOI] [PubMed] [Google Scholar]
- 9.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
- 11.Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa Hospital Research Institute; 2011.
- 12.Hirji SA, Ramirez-Del Val F, Kolkailah AA, et al. Outcomes of surgical and transcatheter aortic valve replacement in the octogenarians—surgery still the gold standard? Ann Cardiothorac Surg. 2017;6:453–462. doi: 10.21037/acs.2017.08.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Im E, Hong MK, Ko YG, Shin DH, Kim JS, Kim BK, Choi D, Shim CY, Chang HJ, Shim JK, Kwak YL, Lee S, Chang BC, Jang Y. Comparison of early clinical outcomes following transcatheter aortic valve implantation versus surgical aortic valve replacement versus optimal medical therapy in patients older than 80 years with symptomatic severe aortic stenosis. Yonsei Med J. 2013;54:596–602. doi: 10.3349/ymj.2013.54.3.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strauch JT, Scherner M, Haldenwang PL, Madershahian N, Pfister R, Kuhn EW, Liakopoulos OJ, Wippermann J, Wahlers T. Transapical minimally invasive aortic valve implantation and conventional aortic valve replacement in octogenarians. Thorac Cardiovasc Surg. 2012;60:335–342. doi: 10.1055/s-0032-1304538. [DOI] [PubMed] [Google Scholar]
- 15.Martínez-Sellés M, Gómez Doblas J, Carro Hevia A, García de la Villa B, Ferreira-González I, Alonso Tello A, Andión Ogando R, Ripoll Vera T, Arribas Jiménez A, Carrillo P, Rodríguez Pascual C, Casares i Romeva M, Borras X, Cornide L, López-Palop R, PEGASO Registry Group Prospective registry of symptomatic severe aortic stenosis in octogenarians: a need for intervention. J Intern Med. 2014;275:608–620. doi: 10.1111/joim.12174. [DOI] [PubMed] [Google Scholar]
- 16.Cao C, Ang S, Indraratna P, Manganas C, Bannon P, Black D, Tian D, Yan TD. Systematic review and meta-analysis of transcatheter aortic valve implantation versus surgical aortic valve replacement for severe aortic stenosis. Ann Cardiothorac Surg. 2013;2:10–23. doi: 10.3978/j.issn.2225-319X.2012.11.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Indraratna P, Tian DH, Yan TD, Doyle MP, Cao C. Transcatheter aortic valve implantation versus surgical aortic valve replacement: a meta-analysis of randomized controlled trials. Int J Cardiol. 2016;224:382–387. doi: 10.1016/j.ijcard.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 18.Kappetein AP, Head SJ, Genereux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. Euro Heart J. 2012;33:2403–2418. doi: 10.1093/eurheartj/ehs255. [DOI] [PubMed] [Google Scholar]
- 19.Jabbour R, Tanaka A, Colombo A, Latib A. Delayed coronary occlusion after transcatheter aortic valve implantation: implications for new transcatheter heart valve design and patient management. Interv Cardiol. 2018;13:137–139. doi: 10.15420/icr.2018.24.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jorgensen TH, Thygesen JB, Thyregod HG, Svendsen JH, Sondergaard L. New-onset atrial fibrillation after surgical aortic valve replacement and transcatheter aortic valve implantation: a concise review. J Invasive Cardiol 2015;27:41–7. [PubMed]
- 21.Thiele H. SOLVE-TAVI: self-expanding vs. balloon-expandable valves and local vs. general anesthesia F. Transcatheter Cardiovascular Therapeutics Conference. September 2018, San Diego.
- 22.Kumar N, Khera R, Fonarow GC, Bhatt DL. Comparison of outcomes of transfermoral versus transapical approach for transcatheter aortic valve implantation. Am J Cardiol. 2018;122:1520–1526. doi: 10.1016/j.amjcard.2018.07.025. [DOI] [PubMed] [Google Scholar]