Main text
Metformin is the most widely used oral antihyperglycemic agent. Because it is eliminated unmodified in urine, patients with renal insufficiency can accumulate metformin and may develop lactic acidosis [1]. Recent guidelines only restrict the use of metformin in patients with severe chronic kidney disease (CKD) because the benefit is considered larger than the risk for lactic acidosis [2]. Lactate measurement has a central role in identifying and monitoring critical illness [3]. A better understanding of the impact of metformin on lactate levels could improve clinical assessment of the critically ill.
Data were collected by combining data from Danish nationwide medical databases with laboratory data [4]. This multicenter cohort included all adults (≥ 18 years) hospitalized and surviving 24 h of intensive care unit (ICU) treatment in northern Denmark between January 2010 and August 2017. We required ≥ 3 lactate measurements between 6 h before until 24 h after ICU admission, with ≥ 12 h between first and last measurement. Patients receiving dialysis before ICU admission were excluded.
Metformin use was defined as a filled prescription for metformin within 90 days before ICU admission [4]. CKD stage was assessed by the mean estimated glomerular filtration rate (eGFR) 365 days until 7 days before ICU admission [5]. Acute kidney injury (AKI) within 24 h after ICU admission was defined and staged according to the KDIGO creatinine criteria. Lactate trajectories over time for metformin users and nonusers were fitted by a mixed-effects model assuming unstructured covariance and including individual-level random intercept and slope. Time was modeled as a natural cubic spline with knot locations at − 1 h, + 4 h, and + 12 h relative to ICU admission. Time-by-group interaction was entered as a covariate, and analyses were subsequently stratified by eGFR level or AKI stage. Differences in maximum lactate level with 95% confidence intervals between metformin users and nonusers were model-based.
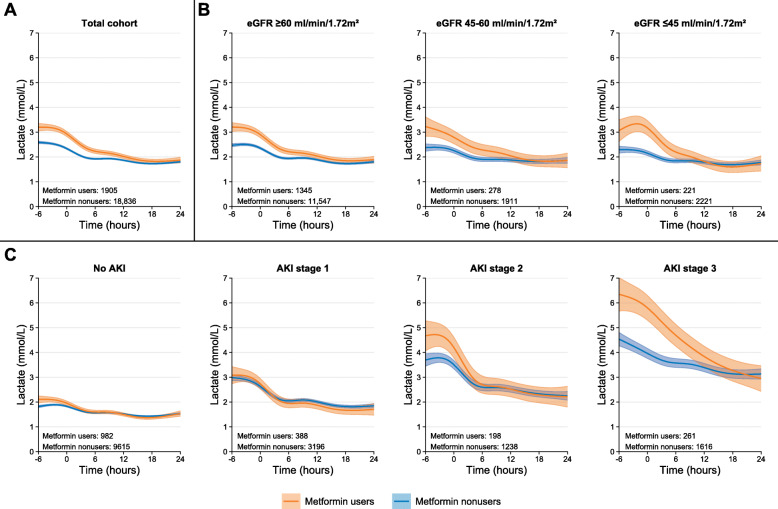
We studied 20,741 patients with a total of 209,394 lactate measurements, of whom 1905 (9%) patients used metformin (Table 1). Compared with nonusers, metformin users had a similar preadmission eGFR but had more often AKI stage 2 or 3. Metformin users had 0.61 (0.45–0.77) mmol/L higher maximum lactate levels than nonusers (Fig. 1a). This difference was highest for patients with eGFR ≤ 45 ml/min/1.73 m2 (1.06 [0.72–1.39] mmol/L; Fig. 1b). Differences in maximum lactate levels between metformin users and nonusers were more pronounced in patients with AKI stage 2 or 3 (Fig. 1c), with a difference of 0.30 (0.15–0.45) mmol/L for patients without AKI, and 0.12 (− 0.24 to 0.48), 1.00 (0.35–1.65), and 1.75 (1.03–2.47) mmol/L among patients with AKI stage 1, 2, or 3, respectively. The difference between metformin users and nonusers disappeared within 24 h of ICU admission. However, the time until this difference disappeared was longer for patients with moderate to severe CKD or AKI (Fig. 1).
Table 1.
Characteristics of metformin users and nonusers
| Characteristic | Total (N = 20,741) | Metformin users (N = 1905) | Metformin nonusers (N = 18,836) | SMD* |
|---|---|---|---|---|
| Age, median [IQR], years | 69 [58–77] | 70 [63–76] | 69 [58–77] | 0.24 |
| Male sex | 11,697 (56) | 1193 (63) | 10,504 (56) | 0.14 |
| Charlson Comorbidity Index | 0.52 | |||
| 0 | 6894 (33) | 305 (16) | 6589 (35) | |
| 1 or 2 | 8147 (39) | 737 (39) | 7410 (39) | |
| 3 or higher | 5700 (27) | 863 (45) | 4837 (26) | |
| Diabetes mellitus | 4594 (22) | 1903 (100) | 2691 (14) | 3.45 |
| Sulfonylureas | 476 (2) | 250 (13) | 226 (1) | 0.48 |
| Insulin | 1473 (7) | 443 (23) | 1030 (5) | 0.52 |
| Other antihyperglycemic agents | 445 (2) | 259 (14) | 186 (1) | 0.50 |
| Preadmission eGFR, median [IQR], ml/min/1.73 m2 | 80 [58–95] | 77 [58–92] | 80 [58–95] | 0.07 |
| ≥ 60 ml/min/1.73 m2 | 12,892 (62) | 1345 (71) | 11,547 (61) | 0.48 |
| 45–60 ml/min/1.73 m2 | 2189 (11) | 278 (15) | 1911 (10) | |
| ≤ 45 ml/min/1.73 m2 | 2442 (12) | 221 (12) | 2221 (12) | |
| Missing | 3218 (16) | 61 (3) | 3157 (17) | |
| ICU admission type | 0.20 | |||
| Medical | 9942 (48) | 1019 (53) | 8923 (47) | |
| Emergency surgical | 6344 (31) | 456 (24) | 5888 (31) | |
| Elective surgical | 3149 (15) | 345 (18) | 2804 (15) | |
| Missing | 1306 (6) | 85 (4) | 1221 (6) | |
| Time from hospital admission to ICU admission, median [IQR], h† | 5.1 [0.0–30.5] | 5.3 [0.0–29.4] | 5.1 [0.0–30.7] | 0.06 |
| SAPS-II score, median [IQR] | 40 [30–52] | 42 [31–53] | 40 [30–52] | 0.09 |
| Missing | 11,456 (55) | 1024 (54) | 10,432 (55) | |
| Mechanical ventilation | 9305 (45) | 815 (43) | 8490 (45) | 0.05 |
| Inotropes or vasopressors | 8943 (43) | 854 (45) | 8089 (43) | 0.04 |
| Renal replacement therapy | 1257 (6) | 152 (8) | 1105 (6) | 0.09 |
| AKI stage within 24 h | 0.47 | |||
| No AKI | 10,597 (51) | 982 (52) | 9615 (51) | |
| 1 | 3584 (17) | 388 (20) | 3196 (17) | |
| 2 | 1436 (7) | 198 (10) | 1238 (7) | |
| 3 | 1877 (9) | 261 (14) | 1616 (9) | |
| Missing | 3247 (16) | 76 (4) | 3171 (17) | |
| 30-day mortality | 4367 (21) | 346 (18) | 4021 (21) | 0.08 |
Data are expressed as no. (%) or median [IQR]
*As general guidance, it is suggested that effect sizes are likely to be “small” when an SMD approximates 0.2, likely to be “medium” when an SMD is 0.5, and “large” when an SMD is higher than 0.8
†In total, data are missing for 19 (0.1%) patients
SMD standardized mean difference, eGFR estimated glomerular filtration rate, ICU intensive care unit, SAPS-II Simple Acute Physiology Score II, AKI acute kidney injury
Fig. 1.
Lactate levels for metformin users and nonusers according to estimated glomerular filtration rate and acute kidney injury stage. Mean lactate trajectories over time with 95% confidence interval for metformin users and nonusers were fitted by a mixed-effect model with individual-level random intercept and slope. Time after ICU admission was modeled as natural cubic spline with knot location at − 1 h, + 4 h, and + 12 h surrounding intensive care unit admission. a Total population. Subsequently, analyses were stratified according to b chronic kidney disease stage based on mean estimated glomerular filtration rate (eGFR) 1 year before ICU admission or c acute kidney injury (AKI) stage within 24 h of ICU admission
In this large cohort of critically ill patients, metformin users had higher lactate levels than nonusers in the early phase of critical illness, which disappeared within 24 h of ICU admission. Importantly, the difference in lactate levels between metformin users and nonusers was higher in patients with more severe AKI, while the difference was almost similar across preadmission eGFR subgroups. This may be explained by reduced clearance of metformin or lactate, or both. A limitation is that blood metformin concentrations were unavailable to confirm this because such correlation was found in patients receiving renal replacement therapy for metformin-associated lactic acidosis [6].
The monitoring of lactate trajectories is recommended during critical illness [3]. Awareness of factors affecting this biomarker will improve its interpretation. We report that the association of metformin use with increased lactate levels is more pronounced in patients who develop AKI stage 2 or 3 than in patients without AKI or who develop AKI stage 1.
Acknowledgements
We want to thank Trine Frøslev, MSc (Department of Clinical Epidemiology, Aarhus University Hospital, Aarhus, Denmark), for the acquisition of data and Daan J. Touw, PharmD, PhD (Department of Clinical Pharmacy and Pharmacology, University Medical Center Groningen, Groningen, The Netherlands), for interpretation of data and critically revising the manuscript for important intellectual content. Both received no additional compensation for the work provided.
Abbreviations
- CKD
Chronic kidney disease
- ICU
Intensive care unit
- eGFR
Estimated glomerular filtration rate
- AKI
Acute kidney injury
- KDIGO
Kidney Disease Improving Global Outcomes
- SMD
Standardized mean difference
Authors’ contributions
All authors contributed to the study conception and design, and analysis and/or interpretation of the data. RAP, AH, and CFC collected data and performed the analyses. RAP wrote the first draft of the manuscript. All authors critically reviewed and edited the manuscript, and all authors read and approved the final version. RAP and CFC had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Funding
RAP was supported by a grant from the Aarhus University Research Fund and the Van Leersum Grant of the Royal Netherlands Academy of Arts and Sciences. AH received support provided by the Steno Diabetes Center Aarhus, which is partially funded by an unrestricted donation from the Novo Nordisk Foundation. The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Availability of data and materials
Parts of the data that support the findings of this study are available from the Danish Health Data Authority (Sundhedsdatastyrelsen), but restrictions apply to the availability of these data, which were used under license for the present study and are thus not publicly available.
Ethics approval and consent to participate
The Danish Data Protection Agency approved the study (record number 2015-57-0002, Aarhus University record number 2016-051-000001/432). According to Danish law, no ethical approval or informed consent was required for this registry-based study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Rene A. Posma, Email: r.a.posma@umcg.nl
Adam Hulman, Email: adahul@rm.dk.
Reimar W. Thomsen, Email: rwt@clin.au.dk
Bente Jespersen, Email: bente.jespersen@clin.au.dk.
Maarten W. Nijsten, Email: m.w.n.nijsten@umcg.nl
Christian F. Christiansen, Email: cfc@clin.au.dk
References
- 1.Inzucchi SE, Lipska KJ, Mayo H, Bailey CJ, McGuire DK. Metformin in patients with type 2 diabetes and kidney disease: a systematic review. JAMA. 2014;312:2668–2675. doi: 10.1001/jama.2014.15298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davies MJ, D'Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2018;41:2669–2701. doi: 10.2337/dci18-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levy MM, Evans LE, Rhodes A. The Surviving Sepsis Campaign bundle: 2018 update. Crit Care Med. 2018;46(6):997–1000. doi: 10.1097/CCM.0000000000003119. [DOI] [PubMed] [Google Scholar]
- 4.Posma RA, Frøslev T, Jespersen B, et al. Prognostic impact of elevated lactate levels on mortality in critically ill patients with and without preadmission metformin treatment: a Danish registry-based cohort study. Ann Intensive Care. 2020;10:36. doi: 10.1186/s13613-020-00652-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siew ED, Ikizler TA, Matheny ME, et al. Estimating baseline kidney function in hospitalized patients with impaired kidney function. Clin J Am Soc Nephrol. 2012;7:712–719. doi: 10.2215/CJN.10821011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yeh HC, Ting IW, Tsai CW, Wu JY, Kuo CC. Serum lactate level and mortality in metformin-associated lactic acidosis requiring renal replacement therapy: a systematic review of case reports and case series. BMC Nephrol. 2017;18:229. doi: 10.1186/s12882-017-0640-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Parts of the data that support the findings of this study are available from the Danish Health Data Authority (Sundhedsdatastyrelsen), but restrictions apply to the availability of these data, which were used under license for the present study and are thus not publicly available.