Abstract
Objective.
Tumor-associated macrophages are known to be associated with decreased survival of patients with endometrial cancer. Given the physiological link of circulating monocytes as a progenitor of tumor-associated macrophages, monocyte counts were examined for tumor characteristics and survival in endometrial cancer.
Methods.
A retrospective study was conducted to examine consecutive patients with endometrial cancer with all histologic types who underwent hysterectomy-based surgical staging between 2003 and 2013 (n = 541). Preoperative monocyte counts were correlated to patient demographics, pathological findings, complete blood count results, and survival outcomes.
Results.
Median monocyte counts were 0.5 × 109/L. Monocyte counts significantly correlated with all other complete blood count components, with neutrophil counts having the most significant association (r = 0.52, p < 0.001). Elevated monocyte counts (defined as >0.7 × 109/L) when compared to lower counts were significantly associated with an increased risk of >50% myometrial tumor invasion (29.2% versus 22.0%, odds ratio [OR] 1.59, 95% confidence interval [CI] 1.01–2.45, p = 0.045), pelvic lymph node metastasis (39.0% versus 18.8%, OR 2.76, 95%CI 1.35–5.62, p = 0.007), and advanced-stage (stage I through IV, 18.5%, 24.6%, 32.5%, and 41.5%, p = 0.001). In survival analysis, elevated monocyte counts were associated with decreased disease-free survival (5-year rates, 71.0% versus 84.5%, p = 0.001) and overall survival (77.2% versus 89.3%, p < 0.001). In multivariate analysis, elevated monocyte counts remained an independent prognostic factor for decreased disease-free (hazard ratio [HR] 1.74, 95% CI 1.02–2.96, p = 0.041) and overall (HR 2.63, 95% CI 1.37–5.05, p = 0.004) survival.
Conclusions.
Elevated monocyte counts were associated with aggressive tumor features and poor survival outcomes of patients with endometrial cancer.
Keywords: Endometrial cancer, Monocyte, Survival outcome
1. Introduction
In 2015, endometrial cancer continues to be the most common gynecologic malignancy in the United States, with more than 54,000 new cases estimated to be diagnosed this year [1]. The majority of endometrial cancers are low-grade tumors with early-stage disease, and the mainstay of treatment approach for endometrial cancer is surgery, which includes hysterectomy, adnexectomy, and possible lymphadenectomy. In a fraction of endometrial cancer patients, additional systemic chemotherapy and/or radiotherapy are indicated [2]. Due to the favorable tumor characteristics in endometrial cancer, long remission and cure of disease is possible in the majority of patients. However, despite a multidisciplinary approach with surgery, chemotherapy, and radiotherapy, certain endometrial cancer patients develop disease recurrence where cure can be difficult and challenging. Therefore, novel approaches for identifying these tumors that are likely to recur may allow for optimization of treatment in these patients and improved survival.
The interaction between host immune cells and cancer cells has been identified as a key to tumor suppression or progression in various types of malignancies including endometrial cancer [3,4]. Tumor-associated macrophages (TAMs) appear to be a key mediator of this interaction, and multiple studies have shown that increased accumulation of TAMs in the tumor site of the uterus is associated with aggressive tumor behaviors (higher stage and grade, lymphovascular space invasion [LVSI], and deep myometrial tumor invasion) and decreased survival outcome of endometrial cancer patients [5-10]. TAMs are the differentiated form of monocytes outside of the vasculature (monocyte–macrophage lineage) [11,12], and are one of the inflammatory cell types found in the tumor microenvironment known to have a pivotal role in the immune response to cancer [13,14]. That is, TAMs in the tumor microenvironment are recruited from tumors and differentiated into polarized M2 macrophages that are a source and target of cytokines, chemokines, and growth factors, which results in cancer progression and suppression of anti-tumor immune system [12,15]. While TAMs adversely impact the prognosis of endometrial cancer patients, the association of monocyte counts and endometrial cancer progression has not been well studied. Given that circulating monocytes in blood vessels function as a precursor and potential origin of TAMs, monocyte counts were examined and correlated to tumor characteristics and survival outcomes of endometrial cancer patients in this study.
2. Patients and Methods
2.1. Eligibility
After Institutional Review Board (IRB) approval was obtained at the University of Southern California, the institutional database for endometrial cancer was utilized to identify the patients. This study included consecutive endometrial cancer patients with all histologic subtypes who were treated with hysterectomy-based surgical staging at Los Angeles County Medical Center between April 2003 and March 2013. Excluded cases were those without laboratory results at the time of cancer diagnosis, or if the final diagnosis was uterine sarcoma or endometrial hyperplasia. Among eligible cases, the following information were abstracted from medical records: (i) patient demographics, (ii) pathology results for hysterectomy-based surgical staging, (iii) laboratory results for complete blood counts obtained at the time of endometrial cancer diagnosis prior to surgical staging, and (iv) survival outcome. The STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) guidelines were consulted for reporting in a retrospective cohort study [16]. Some of the patients in this study were within the context of our previous studies [17-20].
2.2. Clinical information
Archived medical records were reviewed to abstract the clinical information for eligible cases. (i) Patient demographics included age at surgery, ethnicity, and body mass index (BMI, expressed in kg/m2). (ii) Pathology results for hysterectomy-based surgical staging included histology subtype, grade, stage, depth of myometrial tumor invasion (%), presence of LVSI, and pelvic/aortic lymph node metastasis. For lymph node results, total number of sampled nodes and number of positive lymph nodes were also recorded. (iii) Laboratory results for complete blood counts included absolute neutrophil, lymphocyte, monocyte, eosinophil, and basophil counts (expressed in × 109/L), hemoglobin (expressed in g/dL), and platelet counts (expressed in × 109/L) were abstracted. (iv) For survival outcomes, disease-free survival (DFS) and disease-specific overall survival (OS) were abstracted.
2.3. Definition
The cutoff values for complete blood counts were based on the previous studies for endometrial cancer: Neutrophil-to-lymphocyte ratio (N/L ratio, defined as the proportional ratio of absolute neutrophil counts over lymphocyte counts) of 3.0 [21], hemoglobin of 12.0 g/dL [22], and platelet of 400 × 109/L [20]. Because no prior study examined the significance of monocyte counts on endometrial cancer survival, monocyte counts were categorized into three groups (1%–33%ile ≤0.4, 34%–66%ile 0.5–0.6, and 67%–100%ile, ≥0.7 × 109/L). Various cutoff values for monocyte counts were examined and a count of 0.7 × 109/L was confirmed as the cutoff value to maximize the survival outcomes for DFS and OS (Table S1A). Neutrophil counts of 5.5 × 109/L were used for the cutoff value to maximize the survival outcome for DFS and OS in our study (Table S1B). Lymphocyte, eosinophil, and basophil counts were not associated with survival outcome and therefore median values were used for the cutoff.
Lymph node ratio (LNR) was defined as the percent ratio of positive metastatic lymph node number per total sampled lymph nodes per case [23]. Endometrial cancer grade was based on the International Federation of Gynecology and Obstetrics (FIGO), and cancer stage was reclassified based on the 2009 FIGO staging system [24]. DFS was defined as the time interval between hysterectomy-based surgical staging and the date of first recurrence or the date of last follow-up date if there was no recurrence. OS was defined as the time interval between the date of hysterectomy-based surgical staging and the date of death due to endometrial cancer of the last follow-up date if the patient was alive.
2.4. Statistical analysis
The primary objective of the analysis was to correlate monocyte counts to tumor factors from the hysterectomy specimen. The secondary objective of the analysis was to determine the survival significance of monocyte counts in endometrial cancer patients. Continuous variables were examined for normality by Kolmogorov-Smirnov test expressed with mean (±standard deviation) or median (range) as appropriate, and Spearman’s correlation coefficient was performed for statistical evaluation among the continuous variables. Median values of monocyte counts among multiple groups were examined by Mann–Whitney U test or Kruskal–Wallis test as appropriate. Using a cutoff value of elevated monocyte counts (≥0.7 × 109/L), the statistical accuracy for pelvic and aortic lymph node metastasis was determined (sensitivity, specificity, positive predictive value [PPV], negative predictive value [NPV], and accuracy). Categorical or ordinal variables were expressed as a percentage (%), and Fisher’s exact test or chi-square test was used to determine the statistical significance expressed with odds ratio (OR) and 95% confidence interval (CI).
For survival analysis, log-rank test was used for univariate analysis. A Cox proportional hazard regression model was used for multivariate analysis with conditional backward method given the degree of freedom in this sample size. That is, all covariates with p < 0.10 in univariate analysis were initially entered into the model. Then, the least significant covariate was removed from the model until all the covariates in the final model remained p < 0.10. These covariates entered in the initial model included age (<60 versus ≥60), ethnicity (non-Hispanic versus Hispanic), BMI (<30 versus ≥30 kg/m2), histologic subtype (endometrioid versus non-endometrioid), grade (1–2 versus 3), stage (I versus II-IV), depth of myometrial tumor invasion (≤50% versus >50%), LVSI (absence versus presence), neutrophil counts (<5.5 versus ≥5.5 × 109/L), N/L ratio (<3.0 versus ≥3.0), monocyte counts (<0.7 versus ≥0.7 × 109/L), hemoglobin (≥12.0 versus <12.0 g/dL), and platelet counts (<400 versus ≥400 × 109/L). Statistical significance of survival analysis was expressed with hazard ratio (HR) and 95% CI. Kaplan–Meier method was used to construct survival curves. All statistical analyses were two-tailed, and p < 0.05 was considered statistically significant. Statistical Package of Social Science (SPSS, Inc., version 12.0, Chicago, IL) was used for statistical analysis.
3. Results
The schema of patient selection is shown in Figure S1. There were 694 cases of endometrial cancer identified during the study period. Of those, 69 patients did not undergo surgical staging (chemotherapy alone n = 34, hormonal therapy for fertility preservation n = 18, or lost to follow-up after endometrial biopsy for cancer diagnosis n = 17). The remaining 625 patients underwent hysterectomy-based surgical staging for endometrial cancer. Of those, 29 had no preoperative complete blood counts and another 55 cases did not have cell differential counts. The study population was comprised of the remaining 541 patients with endometrial cancer who underwent hysterectomy-based surgical staging and had preoperative complete blood counts with cell differential counts.
Patient demographics are shown in Table 1. The mean age of the study population was 52.1. The majority of patients were Hispanic (68.9%) and obese (BMI ≥30 kg/m2, 70.8%). The majority of endometrial cancers were of endometrioid histology (81.9%), low grade (grade 1–2, 77.6%), and stage I disease (67.1%). Deep myometrial tumor invasion (>50%) was seen in approximately one-quarter of tumors (24.1%). Pelvic and para-aortic lymphadenectomy was performed in 48.8% and 21.3% of cases, respectively. Among lymph node metastasis cases, LNR was 17.6% in pelvic metastasis and 63.5% in aortic metastasis, respectively. Median follow-up time of the study was 35.0 months. There were 84 (15.5%) cases of recurrence and 56 (10.4%) deaths observed in the study cohort.
Table 1.
Patient demographics of endometrial cancer.
| N = 541 (100%) | |
|---|---|
| Age (years) | 52.1 (±10.5) |
| <60 | 409 (75.6%) |
| ≥60 | 132 (24.4%) |
| Ethnicity | |
| Caucasian | 56 (10.4%) |
| African | 26 (4.8%) |
| Hispanic | 373 (68.9%) |
| Asian | 86 (15.9%) |
| BMI (kg/m2) | 36.1 (±9.9) |
| <30 | 157 (29.2%) |
| ≥30 | 381 (70.8%) |
| Histology | |
| Endometrioid | 443 (81.9%) |
| Serous | 37 (6.8%) |
| Clear cell | 27 (5.0%) |
| Othersa | 34 (6.3%) |
| Grade | |
| 1 | 291 (53.8%) |
| 2 | 129 (23.8%) |
| 3 | 121 (22.4%) |
| Stage | |
| I | 363 (67.1%) |
| II | 57 (10.5%) |
| III | 80 (14.8%) |
| IV | 41 (7.6%) |
| Myometrial invasion | 20 (0–100) |
| 0% | 88 (16.7%) |
| ≤50% | 312 (59.2%) |
| >50% | 127 (24.1%) |
| LVSI | |
| Absence | 438 (81.9%) |
| Presence | 97 (18.1%) |
| Pelvic lymph node | n = 264 |
| No metastasis | 223 (84.4%) |
| Metastasis | 41 (15.5%) |
| Lymph node ratio (%)b | 17.9 (3–100) |
| Aortic lymph node | n = 115 |
| No metastasis | 88 (76.5%) |
| Metastasis | 27 (23.5%) |
| Lymph node ratio (%)b | 63.5 (4–100) |
Number (%), mean (±SD), or median (range) are shown. Abbreviations: BMI, body mass index; and LVSI, lymphovascular space invasion.
3 missing for BMI, 14 missing for myometrial invasion, and 6 missing for LVSI.
Among positive lymph node cases.
The median monocyte count was 0.5 × 109/L (range, 0.0–1.8). The median total white blood cell count was 8.1 × 109/L (range, 3.3–37.2), and the median monocyte proportion was 6.2% (range, 0%–15.6%). Monocyte counts were then correlated to various clinicopathological factors (Tables 2 and 3). Monocyte counts were positively correlated to all of the white blood cell components, with neutrophil count holding the strongest correlation (r=0.52, p < 0.001), followed by platelet (r=0.26, p < 0.001), lymphocyte (r = 0.23, p < 0.001), basophil (r = 0.21, p < 0.001), N/L ratio (r = 0.20, p < 0.001), and eosinophil (r = 0.12, p = 0.004) counts. Monocyte counts were negatively correlated to hemoglobin levels (r=−0.17, p < 0.001). Monocyte counts were also significantly correlated to total white blood cell counts (r = 0.62, p < 0.001). Results of other correlations are shown in Table S2.
Table 2.
Correlations between monocyte counts and clinicopathological factors (continuous variables).
| Mean (±SD) | Median (range) | r value | p value | |
|---|---|---|---|---|
| Age (year) | 52.1 (±10.5) | 53 (24–87) | 0.03 | 0.46 |
| BMI (kg/m2) | 36.1 (±9.9) | 34.6 (15.6–84.3) | 0.03 | 0.44 |
| Neutrophil (×109/L) | 5.8 (±3.0) | 5.3 (1.3–29.6) | 0.52 | <0.001 |
| Lymphocyte (×109/L) | 2.2 (±1.5) | 2.1 (0.5–30.0) | 0.23 | <0.001 |
| N/L ratio | 3.2 (±3.0) | 2.5 (0.2–37.0) | 0.20 | <0.001 |
| Eosinophil (×109/L) | 0.2 (±0.2) | 0.1 (0–1.6) | 0.12 | 0.004 |
| Basophil (×109/L) | 0.1 (±0.1) | 0 (0–0.5) | 0.21 | <0.001 |
| Hemoglobin (g/dL) | 12.1 (±2.1) | 12.6 (4.8–15.8) | −0.17 | <0.001 |
| Platelet (×109/L) | 306 (±97) | 292 (101–985) | 0.26 | <0.001 |
Spearman’s correlation coefficient to monocyte counts for p values. Significant p values with r value ≥0.20 or≤−0.20 are in bold. Mean (±SD) and median (range) of monocyte counts were 0.5 (±0.2) and 0.5 (0–1.8), respectively. Abbreviations: SD, standard deviation; BMI, body mass index; and N/L ratio, neutrophil-to-lymphocyte ratio. Metadata are shown in Table S2.
Table 3.
Correlations between monocyte counts and clinicopathological factors (categorical and ordinal variables).
| |
|
Monocyte counts |
≤0.4 × 109/L |
0.5–0.6 × 109/L |
≥0.7 × 109/L |
p value |
|---|---|---|---|---|---|---|
| No. | median (range) | n = 190 | n = 227 | n = 124 | ||
| Ethnicity | <0.001 | |||||
| Caucasian | 56 | 0.55 (0.3–0.9) | 10 (17.9%) | 30 (53.6%) | 16 (28.6%) | |
| African | 26 | 0.4 (0.1–1.8) | 17 (65.4%) | 3 (11.5%) | 6 (23.1%) | |
| Hispanic | 373 | 0.5 (0–1.6) | 122 (47.7%) | 160 (39.5%) | 91 (12.8%) | |
| Asian | 86 | 0.5 (0.1–1.0) | 41 (35.1%) | 34 (42.0%) | 11 (12.8%) | |
| Histology | 0.25 | |||||
| Endometrioid | 443 | 0.5 (0.1–1.6) | 157 (35.4%) | 187 (42.2%) | 99 (22.3%) | |
| Serous | 37 | 0.5 (0–1.8) | 15 (40.5%) | 15 (40.5%) | 7 (18.9%) | |
| Clear cell | 27 | 0.6 (0.3–1.2) | 8 (29.6%) | 8 (29.6%) | 11 (40.7%) | |
| Others | 34 | 0.5 (0.2–1.2) | 10 (29.4%) | 17 (50.0%) | 7 (20.6%) | |
| Grade | 0.51 | |||||
| 1 | 291 | 0.5 (0–1.3) | 103 (35.4%) | 125 (43.0%) | 63 (21.6%) | |
| 2 | 129 | 0.5 (0.1–1.6) | 48 (37.2%) | 53 (41.1%) | 28 (21.7%) | |
| 3 | 121 | 0.5 (0.1–1.8) | 39 (32.2%) | 49 (40.5%) | 33 (27.3%) | |
| Stage | 0.0003 | |||||
| I | 363 | 0.5 (0–1.8) | 143 (39.4%) | 153 (42.1%) | 67 (18.5%) | |
| II | 57 | 0.5 (0.3–1.4) | 18 (31.6%) | 25 (43.9%) | 14 (24.6%) | |
| III | 80 | 0.5 (0.2–1.5) | 17 (21.3%) | 37 (29.3%) | 26 (32.5%) | |
| IV | 41 | 0.6 (0.1–1.3) | 12 (29.3%) | 12 (29.3%) | 17 (41.5%) | |
| LVSI | 0.19 | |||||
| Absence | 438 | 0.5 (0–1.8) | 158 (36.1%) | 186 (42.5%) | 94 (21.5%) | |
| Presence | 97 | 0.5 (0.1–1.1) | 30 (30.9%) | 40 (41.2%) | 27 (27.8%) | |
| Myometrial invasion | 0.005 | |||||
| ≤50% | 403 | 0.5 (0–1.8) | 154 (38.2%) | 166 (41.2%) | 83 (20.6%) | |
| >50% | 127 | 0.5 (0.1–1.4) | 31 (24.4%) | 59 (46.5%) | 37 (29.1%) | |
| Pelvic lymph nodes | 0.039 | |||||
| No metastasis | 223 | 0.5 (0.1–1.3) | 86 (38.6%) | 95 (42.6%) | 42 (18.8%) | |
| Metastasis | 41 | 0.6 (0.3–1.2) | 11 (26.8%) | 14 (34.1%) | 16 (39.0%) |
Mann-Whitney U test or Kruskal-Wallis test for p values. Significant p values are in bold. Abbreviation: LVSI, lymphovascular space invasion.
Of the tumor factors, monocyte counts were positively correlated to the depth of myometrial tumor invasion (r = 0.11, p = 0.014) and to pelvic LNR (r = 0.15, p = 0.021). Elevated monocyte counts ≥0.7 × 109/L were significantly associated with tumor invasion into the outer half of the myometrial layer (monocyte counts ≥0.7 versus <0.7 × 109/L, 29.1% versus 20.6%, OR 1.59, 95% CI 1.01–2.49, p = 0.045). Elevated monocyte counts ≥0.7 × 109/L predicted pelvic lymph node metastasis (monocyte counts ≥0.7 versus <0.7 × 109/L, 39.0% versus 18.8%, OR 2.76, 95% CI 1.35–5.62, p=0.007). Sensitivity, specificity, PPV, and NPV, accuracy of elevated monocyte counts ≥0.7 × 109/L for pelvic lymph node metastasis were 39.0%, 81.2%, 27.6%, 87.9%, and 74.6%, respectively. When the depths of myometrial tumor invasion and monocyte counts were combined, it significantly stratified the risk of pelvic lymph node metastasis (≤50% invasion + monocyte counts <0.7 × 109/L 0.3%, ≤50% invasion + monocyte counts ≥0.7 × 109/L 18.8%, >50% invasion + monocyte counts <0.7 × 109/L 31.3%, and >50% invasion + monocyte counts ≥0.7 × 109/L40.0%, p < 0.001). Elevated monocyte counts ≥0.7 × 109/L were also correlated with advanced-stage disease (proportion of monocyte counts ≥0.7 × 109/L, stage I 18.5%, stage II 24.6%, stage III 32.5%, and stage IV 41.5%, respectively, p=0.001, chi-square test). Monocyte counts were not associated with age, BMI, grade, or histology (all p > 0.05).
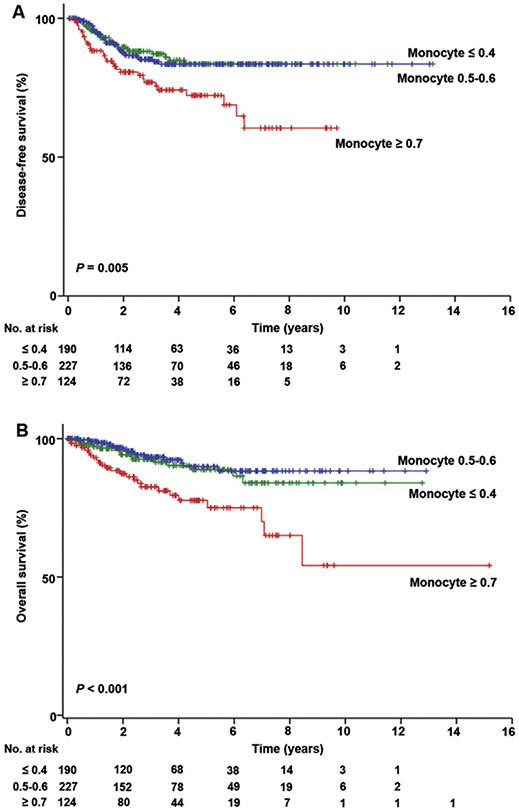
Next, survival analyses were performed (Tables 4 and 5). In univariate analysis, elevated monocyte counts were significantly associated with decreased DFS (5-year rates, ≥0.7 versus <0.7 × 109/L, 71.0% versus 84.5%, HR 2.22, 95% CI 1.36–3.64, p = 0.001). When monocyte counts were grouped into 1%–33%ile (<0.4 × 109/L), 34%–66%ile (0.5–0.6 × 109/L), and 67%–100%ile (≥0.7 × 109/L), 5-year DFS rates showed a threshold pattern with monocyte counts ≥0.7 × 109/L, demonstrating the poorest DFS rate compared to other groups (83.6%, 83.3%, and 59.3%, respectively, p = 0.005; Fig. 1A). Other clinicopathological factors significantly associated with decreased PFS included age ≥60, non-endometrioid histology, grade 3 tumor, stage II–IV disease, myometrial tumor invasion >50%, LVSI, neutrophil counts ≥5.5 × 109/L, N/L ratio ≥3.0, hemoglobin <12.0 g/dL, and platelet counts ≥400 × 109/L (all p < 0.05). Being Hispanic and obesity (BMI ≥30 kg/m2) were associated with improved DFS (both p < 0.05). After controlling for these significant covariates found in univariate analysis, multivariate analysis showed that monocyte counts ≥0.7 × 109/L remained an independent prognostic factor associated with decreased DFS (adjusted-HR 1.74, p = 0.041). Other independent prognostic factors for decreased DFS included non-endometrioid histology (adjusted-HR 1.98, p = 0.007), myometrial tumor invasion >50% (adjusted-HR 2.35, p < 0.001), grade 3 tumors (adjusted-HR 2.59, p = 0.001), and stage II-IV disease (adjusted-HR 4.91, p < 0.001).
Table 4.
Disease-free survival of endometrial cancer.
| No. | 5 years (%) | Univariate |
Multivariate |
|||
|---|---|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |||
| Age (years) | <0.001 | 0.086 | ||||
| <60 | 409 | 83.8% | 1 | 1 | ||
| ≥60 | 132 | 67.2% | 2.17 (1.39–3.40) | 1.57 (0.94–2.63) | ||
| Ethnicity | 0.013 | |||||
| Non-Hispanic | 168 | 70.9% | 1 | |||
| Hispanic | 373 | 83.9% | 0.58 (0.37–0.89) | |||
| BMI (kg/m2) | 0.003 | |||||
| <30 | 157 | 71.6% | 1 | |||
| ≥30 | 381 | 84.3% | 0.53 (0.34–0.82) | |||
| Histology | <0.001 | 0.007 | ||||
| Endometrioid | 443 | 87.1% | 1 | 1 | ||
| Non-endometrioid | 98 | 50.8% | 5.21 (3.39–8.00) | 1.98 (1.20–3.25) | ||
| Grade | <0.001 | 0.001 | ||||
| 1–2 | 420 | 89.7% | 1 | 1 | ||
| 3 | 121 | 49.1% | 8.27 (5.29–12.9) | 2.59 (1.48–4.52) | ||
| Stage | <0.001 | <0.001 | ||||
| I | 363 | 94.2% | 1 | 1 | ||
| II-IV | 178 | 55.9% | 11.2 (6.31–19.9) | 4.91 (2.60–9.27) | ||
| Myometrial invasion | <0.001 | <0.001 | ||||
| ≤50% | 403 | 89.8% | 1 | 1 | ||
| >50% | 127 | 56.9% | 5.36 (3.40–8.43) | 2.35 (1.45–3.79) | ||
| LVSI | <0.001 | |||||
| Absence | 438 | 87.7% | 1 | |||
| Presence | 97 | 52.6% | 4.57 (2.96–7.05) | |||
| Neutrophil (×109/L) | 0.014 | |||||
| <5.5 | 284 | 90.6% | 1 | |||
| ≥5.5 | 257 | 82.8% | 1.83 (1.12–2.99) | |||
| N/L ratio | 0.038 | |||||
| <3.0 | 339 | 83.3% | 1 | |||
| ≥3.0 | 202 | 75.7% | 1.65 (1.02–2.65) | |||
| Monocyte (×109/L) | 0.001 | 0.041 | ||||
| <0.7 | 417 | 83.4% | 1 | 1 | ||
| ≥0.7 | 124 | 71.0% | 2.22 (1.36–3.64) | 1.74 (1.02–2.96) | ||
| Hemoglobin (g/dL) | 0.047 | |||||
| ≥12.0 | 326 | 83.6% | 1 | |||
| <12.0 | 215 | 76.0% | 1.54 (1.00–2.36) | |||
| Platelet (×109/L) | <0.001 | |||||
| <400 | 465 | 83.3% | 1 | |||
| ≥400 | 73 | 64.5% | 2.71 (1.69–4.36) | |||
Log-rank test for univariate analysis (listed only significant variables among all the covariates tested). Cox proportional hazard regression test for multivariate analysis (conditional backward). Significant p values are in bold. Abbreviations: HR, hazard ratio; CI, confidence interval; BMI, body mass index; LVSI, lymphovascular space invasion; and N/L ratio, neutrophil-to-lymphocyte ratio.
Table 5.
Overall survival of endometrial cancer.
| No. | 5 years (%) | Univariate |
Multivariate |
|||
|---|---|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |||
| Age (years) | 0.001 | |||||
| <60 | 409 | 89.2% | 1 | |||
| ≥60 | 132 | 75.6% | 2.39 (1.38–4.15) | |||
| Ethnicity | 0.007 | 0.086 | ||||
| Non-Hispanic | 168 | 78.5% | 1 | 1 | ||
| Hispanic | 373 | 89.5% | 0.49 (0.29–0.83) | 0.59 (0.32–1.08) | ||
| BMI (kg/m2) | 0.004 | |||||
| <30 | 157 | 79.1% | 1 | |||
| ≥30 | 381 | 89.9% | 0.47 (0.28–0.80) | |||
| Histology | <0.001 | 0.003 | ||||
| Endometrioid | 443 | 92.1% | 1 | 1 | ||
| Non-endometrioid | 98 | 61.8% | 6.24 (3.68–10.6) | 2.63 (1.39–4.95) | ||
| Grade | <0.001 | 0.002 | ||||
| 1–2 | 420 | 94.0% | 1 | 1 | ||
| 3 | 121 | 61.4% | 8.51 (4.86–14.9) | 2.85 (1.45–5.61) | ||
| Stage | <0.001 | 0.003 | ||||
| I | 363 | 96.4% | 1 | 1 | ||
| II-IV | 178 | 70.2% | 11.2 (5.28–23.6) | 3.47 (1.55–7.79) | ||
| Myometrial invasion | <0.001 | <0.001 | ||||
| ≤50% | 403 | 93.3% | 1 | 1 | ||
| >50% | 127 | 72.2% | 5.34 (3.01–9.45) | 2.91 (1.60–5.32) | ||
| LVSI | <0.001 | |||||
| Absence | 438 | 92.1% | 1 | |||
| Presence | 97 | 66.9% | 4.75 (2.78–8.11) | |||
| Neutrophil (×109/L) | 0.034 | |||||
| <5.5 | 284 | 90.6% | 1 | |||
| ≥5.5 | 257 | 82.8% | 1.92 (1.04–3.52) | |||
| N/L ratio | 0.008 | |||||
| <3.0 | 339 | 91.0% | 1 | |||
| ≥3.0 | 202 | 80.2% | 2.18 (1.21–3.93) | |||
| Monocyte (×109/L) | <0.001 | 0.004 | ||||
| <0.7 | 417 | 89.3% | 1 | 1 | ||
| ≥0.7 | 124 | 77.2% | 3.13 (1.74–5.62) | 2.63 (1.37–5.05) | ||
| Hemoglobin (g/dL) | 0.047 | |||||
| ≥12.0 | 326 | 89.8% | 1 | |||
| <12.0 | 215 | 81.8% | 1.70 (1.00–2.87) | |||
| Platelet (×109/L) | <0.001 | |||||
| <400 | 465 | 88.6% | 1 | |||
| ≥400 | 73 | 73.7% | 2.59 (1.47–4.58) | |||
Log-rank test for univariate analysis (listed only significant variables among all the covariates tested). Cox proportional hazard regression test for multivariate analysis (conditional backward). Significant p values are in bold. Abbreviations: HR, hazard ratio; CI, confidence interval; BMI, body mass index; LVSI, lymphovascular space invasion; and N/L ratio, neutrophil-to-lymphocyte ratio.
Fig. 1.
Monocyte counts and survival outcome of endometrial cancer. Kaplan-Meier methods for survival curves per monocyte counts (×109/L). (A) Disease-free survival and (B) overall survival are shown based on monocyte count at the time of endometrial cancer diagnosis.
For OS analysis (Table 5), an elevated monocyte count ≥0.7 × 109/L was significantly associated with decreased OS (5-year rates, ≥0.7 versus <0.7 × 109/L, 77.2% versus 89.3%, HR 3.13, 95% CI 1.74–5.62, p < 0.001) in univariate analysis. Monocyte counts ≥0.7 × 109/L had the poorest 5-year OS rate compared to other groups (≤0.4, 0.5–0.6, and ≥0.7 × 109/L; 88.9%, 89.8%, and 77.2%, p < 0.001, Fig. 1B). In multivariate analysis, an elevated monocyte count ≥0.7 × 109/L remained an independent predictor for decreased OS (adjusted-HR, 2.63, p = 0.004). In addition, non-endometrioid histology (adjusted HR 2.63, p = 0.003), grade 3 tumors (adjusted HR 2.85, p = 0.002), stage II–IV disease (adjusted HR 3.47, p = 0.003), and myometrial tumor invasion >50% (adjusted HR 2.91, p < 0.001) remained independent prognostic factors for decreased OS.
4. Discussion
The key findings of our study were that elevated monocyte counts were significantly associated with an increased risk of deep myometrial tumor invasion, pelvic lymph node metastasis, and advanced stage disease, which resulted in decreased survival in endometrial cancer patients. These results support previous findings demonstrating the association between increased accumulation of TAMs and decreased survival in endometrial cancer. While our study did not address the direct correlation between circulating monocyte counts and TAMs in the uterine specimen, our results indirectly point toward a potential biological plausibility of monocyte–macrophage lineage in endometrial cancer biology.
There have been few previous studies that examined the significance of monocytes in endometrial cancer [25,26]. Patients with endometrial cancer have significantly higher monocyte counts in pretreatment blood samples compared to healthy non-cancer controls [26]. In other types of gynecologic malignancy, an elevated circulating monocyte count was associated with decreased survival in both ovarian and cervical cancers [27,28]. However, to date, no study has reported the impact of monocyte counts on endometrial cancer survival. Our study therefore validates the prognostic significance of monocyte counts in endometrial cancer. Altered functionality of circulating monocytes has been demonstrated in patients with endometrial cancer including increased vascular endothelial growth factor (VEGF) receptor 1 expression on the cell surface [25]. VEGF produced by the tumor is one of the major driving forces for recruitment of circulating monocytes into the tumor microenvironment. It is speculated then, that tumors not only have paracrine effects on macrophages to shape them into TAMs in the local tumor microenvironment, but they also may have distant effects that modulate global monocyte functions in endometrial cancer. TAMs also produce various signals including tumor growth factors, angiogenic factors, and matrix metalloproteases; and these ultimately promote cancer progression and invasion [12]. Whether the elevated monocyte counts in endometrial cancer is secondary to a paraneoplastic syndrome via the tumor and bone marrow circuit remains unknown and warrants further study.
Leukocytosis is known to be a prognostic factor for decreased survival in endometrial cancer patients [21,22,29]. The hypothesis behind this association includes tumor-related leukocytosis (TRL) driven by granulocyte colony-stimulating factor (GCSF) produced from the tumor [21]. However, these studies examined the survival impacts of total white blood cell counts and neutrophil counts on endometrial cancer, but not monocyte counts. Therefore, it is not known if TRL is related to GCSF produced from the tumor (direct effect) or to the TAM-related pro-inflammatory cytokine response (indirect effect). Our results showed that both elevated neutrophil counts and monocyte counts were associated with decreased DFS and OS in univariate analysis. In addition, monocyte counts and neutrophil counts were positively and strongly correlated with each other (r = 0.52). However, when neutrophil and monocyte counts were entered into the multivariate model for DFS and OS, only monocyte count remained an independent prognostic factor for decreased DFS and OS (Tables 4 and 5). As the generally accepted model of the immune-mediated response places macrophages as the upstream cell player to neutrophils, it is speculated that TAMs can also be an alternative or complementary cause of TRL to the tumor-related GCSF pathway.
A prior study showed that neutrophil counts ≥7.2 × 109/L were seen in 8.3% of patients with endometrial cancer and were associated with decreased survival outcomes [21]. In our study, neutrophil counts ≥7.2 × 109/L were seen in 26.1% of patients and this was a significantly higher proportion compared to the prior study (OR 2.90, 95% CI 1.98–4.23, p < 0.001). In addition, neutrophil counts ≥7.2 × 109/L were not associated with survival outcomes in our study (Table S1B). Our study and the prior study were similar with regard to study sample size (541 versus 501 patients) and treatment failure rate (15.5% versus 12.2%, p = 0.13). The main difference between the two studies may be that our study is characterized by a markedly obese population compared to the prior study (mean BMI, 36.1 versus 23.0 kg/m2). As shown in our study (Table S2), BMI is significantly positively correlated to neutrophil counts (r = 0.14, p = 0.001). Increased neutrophil counts in obese patients may occur due to the chronic inflammation related to excess adipose tissue [31]. Therefore, increased neutrophil counts in endometrial cancer may be partly related to non-tumor factors such as obesity. Importantly, our study showed that monocyte counts are not related to BMI (r = 0.03, p = 0.44; Table S2). This implies that monocyte counts are more reliable markers for prognosis of endometrial cancer than neutrophil counts.
Possible clinical utility of our results may include an application to immunotherapy in endometrial cancer patients with elevated monocyte counts. That is, TAMs are known to inhibit T cell activation via the interaction between programmed cell death 1 (PD-1) and its ligand (PD-L1), leading to suppression in anti-tumor activity [30]. Because increased monocyte counts may reflect increased TAM accumulation with PD-1 mediated anti-tumor immunosuppression, blocking this PD-1/PD-L1 interaction may be an attractive pathway for anti-tumor immunotherapy [32]. Evaluation of monocyte counts is relatively simple compared to TAMs in the uterine specimen obtained by hysterectomy, and therefore, assessing monocyte counts can be a possible screening strategy to triage a group of endometrial cancer patients as candidates for PD-1/PD-L1 immunotherapy. Another clinical opportunity for targeting TAMs may include bisphosphonates. For example, zoledronic acid inhibits the production of matrix metalloproteases from TAMs and reverses TAM phenotype from immunosupressive M2 macrophages to tumoricidal M1 macrophages [33]. Indeed, bisphosphonates are recognized for potential roles in cancer prevention [34]. Further study is warranted for therapeutic implications of bisphosphonates in patients with endometrial cancer.
In summary, our study highlighted the importance of monocyte counts in the management of endometrial cancer patients. Adopting monocyte count evaluation into practice may potentially have a wide variety of implications for surgical management, treatment strategy, and survival assessment. Lastly, our results propose the definition of tumor-related monocytosis as monocyte counts of ≥0.7 × 109/L based on survival impact with this cutoff value. Further validation in other populations is needed.
Supplementary Material
HIGHLIGHTS.
Monocyte counts obtained prior to hysterectomy-based surgical staging were correlated to tumor characteristics and survival of patients with endometrial cancer.
Elevated monocyte counts were associated with aggressive tumor behavior including deep myometrial tumor invasion, lymph node metastasis, and advanced stage.
Elevated monocyte counts were an independent prognostic factor for decreased survival outcome of endometrial cancer patients.
Acknowledgments
Support: Ensign Endowment for Ovarian Cancer Research (KM and LDR).
Footnotes
Disclosure
The authors declare no conflict of interest.
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ygyno.2015.05.019.
References
- [1].Siegel RL, Miller KD, Jemal A, Cancer statistics, 2015, CA Cancer J. Clin 65 (1) (Jan-Feb 2015)5–29. [DOI] [PubMed] [Google Scholar]
- [2].Wright JD, Barrena Medel NI, Sehouli J, Fujiwara K, Herzog TJ, Contemporary management of endometrial cancer, Lancet 379 (9823) (April 7 2012) 1352–1360. [DOI] [PubMed] [Google Scholar]
- [3].Finn OJ, Cancer immunology, N. Engl. J. Med 358 (25) (June 19 2008) 2704–2715. [DOI] [PubMed] [Google Scholar]
- [4].Zsiros E, Odunsi K, Tumor-associated macrophages: co-conspirators and orchestrators of immune suppression in endometrial adenocarcinoma, Gynecol. Oncol 135 (2) (November 2014) 173–175. [DOI] [PubMed] [Google Scholar]
- [5].Ohno S, Ohno Y, Suzuki N, Kamei T, Koike K, Inagawa H, et al. , Correlation of histological localization of tumor-associated macrophages with clinicopathological features in endometrial cancer, Anticancer Res. 24 (5C) (Sep-Oct 2004) 3335–3342. [PubMed] [Google Scholar]
- [6].Soeda S, Nakamura N, Ozeki T, Nishiyama H, Hojo H, Yamada H, et al. , Tumor-associated macrophages correlate with vascular space invasion and myometrial invasion in endometrial carcinoma, Gynecol. Oncol 109 (1) (April 2008) 122–128. [DOI] [PubMed] [Google Scholar]
- [7].de Jong RA, Leffers N, Boezen HM, ten Hoor KA, van der Zee AG, Hollema H, et al. , Presence of tumor-infiltrating lymphocytes is an independent prognostic factor in type I and II endometrial cancer, Gynecol. Oncol 114 (1) (July 2009) 105–110. [DOI] [PubMed] [Google Scholar]
- [8].Espinosa I, Jose Carnicer M, Catasus L, Canet B, D'Angelo E, Zannoni GF, et al. , Myometrial invasion and lymph node metastasis in endometrioid carcinomas: tumor-associated macrophages, microvessel density, and HIF1A have a crucial role, Am. J. Surg. Pathol 34 (11) (November 2010) 1708–1714. [DOI] [PubMed] [Google Scholar]
- [9].Jiang XF, Tang QL, Li HG, Shen XM, Luo X, Wang XY, et al. , Tumor-associated macrophages correlate with progesterone receptor loss in endometrial endometrioid adenocarcinoma, J. Obstet. Gynaecol. Res 39 (4) (April 2012) 855–863. [DOI] [PubMed] [Google Scholar]
- [10].Kubler K, Ayub TH, Weber SK, Zivanovic O, Abramian A, Keyver-Paik MD, et al. , Prognostic significance of tumor-associated macrophages in endometrial adenocarcinoma, Gynecol. Oncol 135 (2) (November 2014) 176–183. [DOI] [PubMed] [Google Scholar]
- [11].Sica A, Mantovani A, Macrophage plasticity and polarization: in vivo veritas, J. Clin. Invest 122 (3) (March 2012) 787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Mantovani A, Sozzani S, Locati M, Allavena P, Sica A, Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes, Trends Immunol. 23 (11) (November 2002) 549–555. [DOI] [PubMed] [Google Scholar]
- [13].Mantovani A, Allavena P, The interaction of anticancer therapies with tumor-associated macrophages, J. Exp. Med 212 (4) (April 6 2015) 435–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Noy R, Pollard JW, Tumor-associated macrophages: from mechanisms to therapy, Immunity 41 (1) (July 17 2014) 49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Condeelis J, Pollard JW, Macrophages: obligate partners for tumor cell migration, invasion, and metastasis, Cell 124 (2) (January 27 2006) 263–266. [DOI] [PubMed] [Google Scholar]
- [16].STROBE guideline at http://www.strobe-statement.org/.
- [17].Matsuo K, Cahoon SS, Gualtieri M, Scannell CA, Jung CE, Takano T, et al. , Significance of adenomyosis on tumor progression and survival outcome of endometrial cancer, Ann. Surg. Oncol 21 (13) (December 2014) 4246–4255. [DOI] [PubMed] [Google Scholar]
- [18].Matsuo K, Gray MJ, Yang DY, Srivastava SA, Tripathi PB, Sonoda LA, et al. , The endoplasmic reticulum stress marker, glucose-regulated protein-78 (GRP78) in visceral adipocytes predicts endometrial cancer progression and patient survival, Gynecol. Oncol 128 (3) (March 2013) 552–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Matsuo K, R Opper N, Ciccone MA, Garcia J, Tierney KE, Baba T, et al. , Time interval between endometrial biopsy and surgical staging for type I endometrial cancer: association between tumor characteristics and survival outcome, Obstet. Gynecol 125 (February 2015)424–433. [DOI] [PubMed] [Google Scholar]
- [20].Matsuo K, Yessaian AA, Lin YG, Pham HQ, Muderspach LI, Liebman HA, et al. , Predictive model of venous thromboembolism in endometrial cancer, Gynecol. Oncol 128 (3) (March 2013) 544–551. [DOI] [PubMed] [Google Scholar]
- [21].Takahashi R, Mabuchi S, Kawano M, Sasano T, Matsumoto Y, Kuroda H, et al. , Prognostic significance of systemic neutrophil and leukocyte alterations in surgically treated endometrial cancer patients: A monoinstitutional study, Gynecol. Oncol 137 (April 2015) 112–118. [DOI] [PubMed] [Google Scholar]
- [22].Njolstad TS, Engerud H, Werner HM, Salvesen HB, Trovik J, Preoperative anemia, leukocytosis and thrombocytosis identify aggressive endometrial carcinomas, Gynecol. Oncol 131 (2) (November 2013) 410–415. [DOI] [PubMed] [Google Scholar]
- [23].Chan JK, Kapp DS, Cheung MK, Osann K, Shin JY, Cohn D, et al. , The impact of the absolute number and ratio of positive lymph nodes on survival of endometrioid uterine cancer patients, Br. J. Cancer 97 (5) (September 3 2007) 605–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Pecorelli S, Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium, Int. J. Gynaecol. Obstet 105 (2) (May 2009) 103–104. [DOI] [PubMed] [Google Scholar]
- [25].Brooks N, Stojanovska L, Grant P, Apostolopoulos V, McDonald CF, Pouniotis DS, Characterization of blood monocyte phenotype in patients with endometrial cancer, Int. J. Gynecol. Cancer 22 (9) (November 2012) 1500–1508. [DOI] [PubMed] [Google Scholar]
- [26].Kim BW, Jeon YE, Cho H, Nam EJ, Kim SW, Kim S, et al. , Pre-treatment diagnosis of endometrial cancer through a combination of CA125 and multiplication of neutrophil and monocyte, J. Obstet. Gynaecol. Res 38 (1) (January 2012) 48–56. [DOI] [PubMed] [Google Scholar]
- [27].Armaiz-Pena GN, Gonzalez-Villasana V, Nagaraja AS, Rodriguez-Aguayo C, Sadaoui NC, Stone RL, et al. , Adrenergic regulation of monocyte chemotactic protein 1 leads to enhanced macrophage recruitment and ovarian carcinoma growth, Oncotarget 6 (6) (February 28 2015) 4266–4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lee YY, Choi CH, Sung CO, Do IG, Huh S, Song T, et al. , Prognostic value of pretreatment circulating monocyte count in patients with cervical cancer: comparison with SCC-Ag level, Gynecol. Oncol 124 (1) (January 2012) 92–97. [DOI] [PubMed] [Google Scholar]
- [29].Worley MJ Jr., Nitschmann CC, Shoni M, Vitonis AF, Rauh-Hain JA, Feltmate CM, The significance of preoperative leukocytosis in endometrial carcinoma, Gynecol. Oncol 125 (3) (June 2012) 561–565. [DOI] [PubMed] [Google Scholar]
- [30].Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, et al. , PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation, J. Exp. Med 211 (5) (May 5 2014) 781–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Neill AS, Nagle CM, Protani MM, Obermair A, Spurdle AB, Webb PM, Aspirin, nonsteroidal anti-inflammatory drugs, paracetamol and risk of endometrial cancer: A case-control study, systematic review and meta-analysis, Int. J. Cancer 132 (2013) 1146–1155. [DOI] [PubMed] [Google Scholar]
- [32].Ribas A, Tumor immunotherapy directed at PD-1, N. Engl. J. Med 366 (2012) 2517–2519. [DOI] [PubMed] [Google Scholar]
- [33].Rogers TL, Holen I, Tumour macrophages as potential targets of bisphosphonates, J. Transl. Med 9 (2011) 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Gronich N, Rennert G, Beyond aspirin–cancer prevention with statins, metformin and bisphosphonates, Nat. Rev. Clin. Oncol 10 (11) (November 2013) 625–642. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.