Abstract
This study seeks to identify risk factors associated with ovarian metastasis and to characterize a population with minimum risk of ovarian metastasis in young women with stage IB–IIB cervical cancer. This was a nation-wide multicenter retrospective study in Japan examining consecutive cases of surgically-treated women with clinical stage IB–IIB cervical cancer who had oophorectomy at radical hysterectomy (n=5,697). Multivariable analysis was performed to identify independent risk factors for ovarian metastasis. Ovarian metastasis was seen in 70 (1.2%, 95% confidence interval 0.9–1.5) cases. In the entire cohort, adenocarcinoma, lympho-vascular space invasion, uterine corpus tumor invasion, and pelvic/para-aortic nodal metastases remained independent risk factors for ovarian metastasis (all, adjusted-p<0.05). In a sensitivity analysis of 3,165 women aged <50 years (ovarian metastasis, 1.0%), adenocarcinoma, parametrial tumor involvement, uterine corpus tumor involvement, and pelvic/para-aortic nodal metastases remained independent risk factors for ovarian metastasis (all, adjusted-P<0.05). In the absence of these five risk factors (representing 46.1% of women aged <50 years), the incidence of ovarian metastasis was 0.14%. With the presence of adenocarcinoma alone (representing 18.9% of women aged <50 years), the incidence of ovarian metastasis was 0.17% and was not associated with increased risk of ovarian metastasis compared to the subgroup without any risk factors (p=0.87). In conclusion, nearly two thirds of women aged <50 years with clinical stage IB–IIB cervical cancer had no risk factor for ovarian metastasis or had adenocarcinoma alone: these subgroups had ovarian metastasis rates of around 0.1% and may be a candidate population for ovarian conservation at surgical treatment.
Keywords: cervical cancer, ovarian metastasis, early stage, radical hysterectomy, risk factor
Cervical cancer is the most common gynecologic malignancy in the world, and >500,000 women were estimated to be diagnosed with this disease as per the 2012 global statistics.1,2 Typically, cervical cancer is a disease of young women related to persistent oncogenic human papillomavirous infection.3,4 Indeed, cervical cancer is the leading cause of gynecologic malignancy death in women of reproductive age in the United States.5 In Japan, the incidence of cervical cancer among women younger than 40 years of age has been gradually increasing, and >40% of women with cervical cancer were younger than 50 years of age and had stage I–II disease, a situation in Japan where surgical treatment plays a pivotal role in disease management.6–8
The recent evidence-based guidelines per the Japan Society of Gynecologic Oncology indicate that either a surgical treatment approach with radical hysterectomy and a non-surgical approach with concurrent chemoradiotherapy are acceptable treatment options for women with stage IB–IIB cervical cancer.8 Both treatment approaches have unique risks and benefits related to its treatment modalities. Surgical treatment with radical hysterectomy has the benefit in that it allows the patient to have an opportunity for ovarian conservation at the time of surgery that is particularly applicable to premenopausal young women. The benefits of ovarian conservation for young women include both short-term and long-term aspects including prevention of osteoporosis and cardiovascular disease.9–13 Nevertheless, current available guidelines have not clearly specified the recommendation criteria for oophorectomy versus ovarian conservation at the time of surgical treatment for stage IB–IIB cervical cancer.8,14
A concern for ovarian conservation at surgical treatment for women with cervical cancer is a possibility of leaving tumor already metastasized to the ovary. While the risk of ovarian metastasis seems low as demonstrated in multiple prior studies,15–21 there is currently little reliable risk stratification available for the prediction of ovarian metastasis, especially in stages IB–IIB disease. Because ovarian conservation at the time of surgical treatment can potentially impact woman’s health and there are likely a substantial number of women who are candidates for ovarian conservation at surgery, establishing a risk stratification model of ovarian metastasis is of utmost importance in the management of young women with cervical cancer when surgical treatment is scheduled.
The objective of the study was (i) to identify independent predictors for ovarian metastasis and (ii) to characterize a population with minimum risk of ovarian metastasis in young women with clinical stage IB–IIB cervical cancer.
Patients and Methods
Eligibility
A nation-wide large-scale retrospective observational study was conducted in 116 Japanese Gynecologic Oncology Group designated institutions. Institutional Review Board approval was obtained at Tottori University which served as the host institution, and the Japanese Gynecologic Oncology Group-participating institutions reviewed the study protocol and obtained their own Institutional Review Board approvals at each site as indicated. Eligible cases were women with clinical stage IB–IIB cervical cancer who underwent type III radical hysterectomy, bilateral salpingo-oophorectomy, and pelvic lymphadenectomy between January 1, 2004 and December 31, 2008. The data acquisition period at the participating sites was set between October 1, 2012 and February 28, 2013. Women who had ovarian conservation or were of unknown oophorectomy status were excluded from the study. The STROBE guidelines were used to outline this observational study.22
Clinical information
A de-identified universal data entry form was used to collect clinical, tumor, and survival information from archived medical records at each study site. Upon the completion of data collection, the anonymous data sheet was sent to the host institution. The data was then compiled into a master excel data sheet by the research personnel. Clinical demographics included calendar year of surgery, patient age at diagnosis of cervical cancer (<40, 40–49 and ≥50 years), and clinical stage per the International Federation of Gynecology and Obstetrics grouped into stage IB1, IB2, IIA and IIB.
Surgical-pathological factors examined for this study included histologic subtype (squamous cell carcinoma, adenocarcinoma, adenosquamous, and other), tumor size (>4 vs. ≤4 cm), parametrial tumor involvement (yes vs. no), deep stromal invasion (outer half vs. inner half), presence of LVSI (yes vs. no), uterine corpus tumor invasion (yes vs. no), use of peritoneal cytology (yes vs. no), and ovarian metastasis (yes vs. no). Among the cases with ovarian metastasis, laterality (unilateral vs. bilateral) was further specified.
Survival outcomes examined for this study were disease-free survival and cause-specific survival. Disease-free survival was defined as the time interval between the date of radical hysterectomy and the date of the first recurrence. Cases were censored if there is no recurrence at the last follow-up. Cause-specific survival was defined as the time interval between the date of radical hysterectomy and the date of death due to cervical cancer. The patients were censored if patients were alive at the last follow-up or died of another cause.
Statistical analysis
The primary objective of analysis was to identify the independent risk factors for ovarian metastasis at the time of surgical treatment for women with clinical stage IB–IIB cervical cancer. The secondary objective of analysis was to characterize a population with minimum risk of ovarian metastasis in women aged <50 years in a sensitivity analysis. In addition, an association of ovarian metastasis and survival outcome was examined. On univariable analysis, continuous variables were assessed with the Student’s t test and categorical variables were assessed with the χ2 test or a Fisher exact test as appropriate.
A binary logistic regression model was used to determine the independent risk factors for ovarian metastasis (yes vs. no). Covariates entered in the initial full model were age, histology, tumor size, parametrial involvement, deep stromal invasion, LVSI, uterine corpus tumor invasion, pelvic and para-aortic nodal metastasis, and peritoneal cytology use. Because we estimated that the risk of ovarian metastasis in clinical stage IB–IIB cervical cancer is approximately 1% based on our previous study,15 this full model with ten covariates may result in overfitting in the model. Therefore, to avoid overfitting, we performed a conditional backward method to remove the least significant covariate from the model until all the retained covariates remained significant.23 Magnitude of statistical significance was expressed with OR and 95%CI. In addition, the Hosmer-Lemeshow goodness-of-fit test was used to assess the final model fitting of the binary logistic regression analysis, and a p values of >0.05 of the test was interpreted as a good-fit.24
Sensitivity analyses for the binary logistic regression model were further performed in various clinical settings. These included squamous histology cases alone and adenocarcinoma/adenosquamous histology cases alone given that the tumor biology and clinical behaviors differ between the two histology subtypes.25 Also, a group of women aged younger than 50 years were examined because this age group of women are the best candidates for ovarian conservation to maximize the cardioprotective effects of ovarian hormones.26 This arbitrary age cutoff was based on the average age of menopause in developed countries (51 years).27 Lastly, cases with clinical stage IB disease were examined because this sub-stage disease is the most common indication for radical hysterectomy in Japan.7
For survival analysis, the Kaplan–Meier method was used to construct survival curves for disease-free and cause-specific survival stratified by ovarian metastasis status,28 and statistical significance between the curves was assessed with a log-rank test. A Cox proportional hazard regression model was used for multivariable analysis.29 In this model, patient age and all collected tumor factors were entered in the final model due to an adequate survival event number. Magnitude of statistical significance was expressed with HR and 95%CI. Over-adjustment was examined by the ratio of event-of-interest per variables in the final model, and the ratio of <10 was considered absence of over-adjustment. All statistical analyses were two-sided, and a p values of <0.05 was considered statistical significant. Statistical Package for Social Sciences (version 24.0, Armonk, NY) was used for all the analyses.
Results
There were 6,003 cases identified in the database. Among those, 235 (3.9%) cases of unknown oophorectomy status, 32 (0.5%) cases of ovarian conservation, and 39 (0.6%) cases outside of our study period were excluded. The remaining 5,697 cases of clinical stage IB–IIB cervical cancer that underwent radical hysterectomy with oophorectomy composed the study population. Ovarian metastasis was seen in 70 (1.2%, 95%CI 0.9–1.5) cases including 43 cases (0.8%) cases of unilateral metastasis and 27 (0.5%) cases of bilateral metastasis.
Demographics are shown in Table 1. There was a trend of increasing incidence of ovarian metastasis in older age women although it did not reach statistical significance (<40, 40–49 and ≥50 years, 0.8, 1.1 and 1.5%; p=0.11). Women with clinical stage IB disease had a significantly lower incidence of ovarian metastasis relative to stage II disease (IB1 0.7%, IB2 0.9%, IIA 1.2% and IIB 2.7%; p<0.001). Adenocarcinoma histology (2.5%) had the highest incidence of ovarian metastasis followed by adenosquamous type (1.3%) with squamous type holding the lowest incidence of ovarian metastasis (0.7%, p<0.001). The two surgical-pathological factors associated with the highest risk for ovarian metastasis were para-aortic lymph node metastasis (13.8%) followed by uterine corpus tumor invasion (5.7%). All the collected surgical-pathological tumor factors were significantly associated with ovarian metastasis (all, p<0.001).
Table 1.
Demographics of the study cohort (N = 5,697)
| Ovarian metastasis |
|||
|---|---|---|---|
| Characteristic | Yes | No | p-value |
| Number | n = 70 (1.2%) | n = 5,627 (98.8%) | |
| Year | 0.30 | ||
| 2004 | 11 (1.1%) | 995 (98.9%) | |
| 2005 | 20 (1.8%) | 1,107 (98.2%) | |
| 2006 | 16 (1.4%) | 1,131 (98.6%) | |
| 2007 | 13 (1.0%) | 1,236 (99.0%) | |
| 2008 | 10 (0.9%) | 1,158 (99.1%) | |
| Age (years) | 50.4 (±11.8) | 48.1 (±12.0) | 0.11 |
| <40 | 13 (0.8%) | 1,566 (99.2%) | |
| 40–49 | 18 (1.1%) | 1,568 (98.9%) | |
| ≥50 | 39 (1.5%) | 2,491 (98.5%) | |
| Clinical stage | <0.001 | ||
| IB1 | 21 (0.7%) | 3,001 (99.3%) | |
| IB2 | 8 (0.9%) | 842 (99.1%) | |
| IIA | 7 (1.2%) | 576 (98.8%) | |
| IIB | 34 (2.7%) | 1,208 (97.3%) | |
| Histology | <0.001 | ||
| SCC | 27 (0.7%) | 3,683 (99.3%) | |
| Adeno | 36 (2.5%) | 1,409 (97.5%) | |
| AS | 6 (1.3%) | 464 (98.7%) | |
| Others | 1 (1.4%) | 71 (98.6%) | |
| Tumor size | <0.001 | ||
| ≤4 cm | 27 (0.7%) | 3,659 (99.3%) | |
| >4 cm | 35 (2.1%) | 1,595 (97.9%) | |
| Parametria | <0.001 | ||
| Not involved | 29 (0.6%) | 4,548 (99.4%) | |
| Involved | 41 (3.7%) | 1,077 (96.3%) | |
| Deep stromal invasion | <0.001 | ||
| Not involved | 8 (0.3%) | 2,532 (99.7%) | |
| Involved | 56 (1.9%) | 2,847 (98.1%) | |
| LVSI | <0.001 | ||
| No | 7 (0.3%) | 2,450 (99.7%) | |
| Yes | 62 (2.0%) | 3,004 (98.0%) | |
| Uterine corpus | <0.001 | ||
| Not involved | 25 (0.5%) | 4,861 (99.5%) | |
| Involved | 45 (5.7%) | 741 (94.3%) | |
| Pelvic lymph node | <0.001 | ||
| Not involved | 18 (0.4%) | 4,164 (99.6%) | |
| Involved | 52 (3.5%) | 1,426 (96.5%) | |
| Para-aortic lymph node | <0.001 | ||
| Not involved | 10 (1.2%) | 807 (98.8%) | |
| Involved | 16 (13.8%) | 100 (86.2%) | |
| Clinically not involved | 44 (0.9%) | 4,720 (99.1%) | |
| Peritoneal cytology | <0.001 | ||
| No | 36 (0.8%) | 4,280 (99.2%) | |
| Yes | 34 (2.5%) | 1,338 (97.5%) | |
Mean (±SD) or number (%) per row is shown. Student’s t test or Fisher exact test, or χ2 test for p-values. Significant p-values are emboldened.
Abbreviations: SCC: squamous cell carcinoma; Adeno: adenocarcinoma; AS: adenosquamous; LVSI: lympho-vascular space invasion.
On multivariable analysis (Table 2), adenocarcinoma histology (adjusted-OR 3.92, 95%CI 2.29–6.72, p<0.001), LVSI (adjusted-OR 2.60, 95%CI 1.09–6.19, p=0.031), uterine corpus tumor invasion (adjusted-OR 6.05, 95% CI 3.56–10.3, p<0.001), pelvic lymph node metastasis (adjusted-OR 3.74, 2.02–6.95, p<0.001) and para-aortic lymph node metastasis (adjusted-OR 4.96, 95%CI 2.02–12.9, p<0.001) remained independent predictors for an increased risk of ovarian metastasis. The tumor factors with the highest magnitude of statistical significance for ovarian metastasis were uterine corpus tumor invasion followed by para-aortic lymph node metastasis and adenocarcinoma histology.
Table 2.
Independent risk factors for ovarian metastasis
| Full model |
Conditional backward |
|||
|---|---|---|---|---|
| Characteristic | OR (95%CI) | p-value | OR (95%CI) | p-value |
| Age (years) | 0.93 | |||
| <40 | 1 | |||
| 40–49 | 1.16 (0.52–2.60) | 0.71 | ||
| ≥50 | 1.12 (0.55–2.30) | 0.76 | ||
| Histology type | <0.001 | <0.001 | ||
| SCC | 1 | 1 | ||
| Adeno | 4.68 (2.55–8.61) | <0.001 | 3.92 (2.29–6.72) | <0.001 |
| AS | 1.98 (0.75–5.21) | 0.17 | 1.54 (0.61–3.89) | 0.36 |
| Others | 1.42 (0.16–12.8) | 0.76 | 0.91 (0.11–7.50) | 0.93 |
| Tumor size | ||||
| ≤4 cm | 1 | |||
| > cm | 1.28 (0.71–2.31) | 0.42 | ||
| Parametria | ||||
| Not involved | 1 | |||
| Involved | 1.23 (0.66–2.30) | 0.52 | ||
| Deep stromal invasion | ||||
| No | 1 | |||
| Yes | 1.80 (0.62–5.21) | 0.28 | ||
| LVSI | ||||
| No | 1 | 1 | ||
| Yes | 2.34 (0.83–6.65) | 0.11 | 2.60 (1.09–6.19) | 0.031 |
| Uterine corpus | ||||
| Not involved | 1 | 1 | ||
| Involved | 6.21 (3.31–11.6) | <0.001 | 6.05 (3.56–10.3) | <0.001 |
| Pelvic lymph node | ||||
| Not involved | 1 | 1 | ||
| Involved | 3.19 (1.61–6.33) | 0.001 | 3.74 (2.02–6.95) | <0.001 |
| Para-aortic lymph node | <0.001 | <0.001 | ||
| Not involved | 1 | 1 | ||
| Involved | 4.75 (1.76–12.9) | 0.002 | 4.96 (2.02–12.2) | <0.001 |
| Clinically not involved | 0.94 (0.43–2.05) | 0.87 | 0.96 (0.47–1.96) | 0.90 |
| Peritoneal cytology | ||||
| No | 1 | |||
| Yes | 1.05 (0.58–1.89) | 0.88 | ||
| Hosmer and Lemeshow test | p = 0.44 | p = 0.80 | ||
Binary logistic regression models for multivariable analysis. In a full model, all the listed covariates were entered, and only statistically significant covariates in the final model were listed for a conditional backward model. Adjusted-odds ratio with 95% confidence interval are shown. Significant P-values (cutoff, p < 0.05) are emboldened.
Abbreviations: OR: odds ratio; CI: confidence interval; SCC: squamous cell carcinoma; Adeno: adenocarcinoma; AS: adenosquamous; LVSI: lymphovascular space invasion.
Sensitivity analyses were performed to explore the association of tumor factors and ovarian metastasis (Table 3). Notably, across the four analyses, both uterine corpus tumor invasion and pelvic lymph node metastasis were the common risk factors for ovarian metastasis. In the squamous and adenocarcinoma histology types, both uterine corpus tumor invasion and pelvic lymph node metastasis were the common tumor factors for increased risk of ovarian metastasis. However, para-aortic lymph node metastasis was an independent risk factor only in the squamous group but not in the adenocarcinoma group. Parametrial tumor involvement was an independent risk factor only in the adenocarcinoma group but not in the squamous group.
Table 3.
Sensitivity analysis for risk factors related to ovarian metastasis
| SCC |
Adeno/AS |
Age < 50 |
Clinical stage IB |
|||||
|---|---|---|---|---|---|---|---|---|
| (%)† | OR (95%CI) | (%)† | OR (95%CI) | (%)† | OR (95%CI) | (%)† | OR (95%CI) | |
| Histology | ||||||||
| SCC | 0.6% | 1 | 0.7% | 1 | ||||
| Adeno | 2.0% | 5.54 (2.46–12.5)* | 2.5% | 2.70 (1.25–5.84)** | ||||
| AS | 0.6% | 0.84 (0.17–4.02) | 1.3% | 0.65 (0.08–5.06) | ||||
| Others | 0.0% | na | 1.4% | na | ||||
| Parametrea | ||||||||
| Not involved | 0.9% | 1 | 0.5% | 1 | ||||
| Involved | 8.7% | 3.40 (1.67–6.93)* | 3.5% | 2.33 (1.01–5.36)** | ||||
| Uterine corpus | ||||||||
| Not involved | 0.4% | 1 | 0.8% | 1 | 0.5% | 1 | 0.5% | 1 |
| Involved | 3.3% | 5.55 (2.50–12.3)* | 9.4% | 6.50 (3.19–13.2)* | 5.0% | 4.58 (2.10–9.96)* | 5.7% | 8.49 (3.91–18.4)* |
| Pelvic lymph node | ||||||||
| Not involved | 0.2% | 1 | 0.9% | 1 | 0.3% | 1 | 0.4% | 1 |
| Involved | 2.2% | 6.09 (2.17–17.3)* | 6.7% | 3.91 (1.93–7.93)* | 3.0% | 6.10 (2.38–15.6)* | 3.5% | 6.64 (2.91–15.1)* |
| Para-aortic lymph node | ||||||||
| Not involved | 0.6% | 1 | 0.7% | 1 | ||||
| Involved | 11.0% | 6.78 (1.67–27.6)* | 11.7% | 7.42 (1.68–32.9) | ||||
| Clinically not involved | 0.5% | 0.85 (0.25–2.98) | 0.8% | 1.44 (0.41–5.04) | ||||
| Hosmer and Lemeshow test | p = 0.41 | p = 0.36 | p = 0.78 | p = 0.22 | ||||
Binary logistic regression models (conditional backward) for multivariable analysis (*p < 0.01, **p < 0.05). All the collected covariates were initially entered in the model, and only the statistically significant covariates (cutoff, p < 0.05) were listed in the table. Adjusted-odds ratio with 95% confidence interval are shown.
Ovarian metastasis based on risk factor.
Abbreviations: SCC: squamous cell carcinoma; Adeno: adenocarcinoma; AS: adenosquamous.
Independent risk factors for ovarian metastasis in women aged <50 years were similar to those in the entire cohort except that parametrial tumor involvement but not LVSI remained independent risk factors (Table 3). This difference between the entire cohort and the younger age group is likely due to the difference in baseline tumor characteristics (Supporting Information Table S1). That is, younger women were less likely to have tumors with squamous histology, parametrial involvement, deep stromal invasion, LVSI, uterine corpus invasion, and peritoneal cytology use compared to those who were older. Among the cases with clinical stage IB disease, adenocarcinoma, uterine corpus tumor invasion, and pelvic lymph node metastasis remained independent risk factors for ovarian metastasis, and tumor size was not associated with ovarian metastasis.
The incidence of ovarian metastasis was examined based on combination patterns of risk factors for ovarian metastasis (Table 4). Among the entire cohort, the incidence of ovarian metastasis was 0.14% without the presence of any risk factors (n=1,423). The presence of adenocarcinoma alone did not increase the risk of ovarian metastasis compared to the no risk factor group (0.29% vs. 0.14%, p=0.46). When tumors exhibit multiple risk factors, the incidence of ovarian metastasis increased significantly: two risk factors 0–2.33%, three risk factors 2.03–7.89%, and four or more risk factors 17.7–36.4% (p<0.001).
Table 4.
Incidence of ovarian metastasis based on risk factor patterns
| Whole cohort (n = 5,481) | ||||||
| Adeno | LVSI | Uterine corpus | Pelvic node | Aortic node | No. (%) | Ov mets (%) |
| 1,423 (26.0%) | 0.14% | |||||
| + | 683 (12.5%) | 0.29% | ||||
| + | 1,248 (22.8%) | 0.16% | ||||
| + | 83 (1.5%) | 0% | ||||
| + | 130 (2.4%) | 0% | ||||
| + | + | 287 (5.2%) | 1.39% | |||
| + | + | 54 (1.0%) | 0% | |||
| + | + | 43 (0.8%) | 2.33% | |||
| + | + | 201 (3.7%) | 0.99% | |||
| + | + | 696 (1.27%) | 1.0% | |||
| + | + | 15 (0.3%) | 0% | |||
| + | + | + | 76 (1.4%) | 7.89% | ||
| + | + | + | 148 (2.7%) | 2.03% | ||
| + | + | + | 207 (3.8%) | 5.80% | ||
| + | + | + | + | 79 (1.4%) | 17.7% | |
| + | + | + | + | 23 (0.4%) | 26.1% | |
| + | + | + | + | + | 11 (0.2%) | 36.4% |
| Age <50 cohort (n = 3,137) | ||||||
| Adeno | Parametrea | Uterine corpus | Pelvic node | Aortic node | No. (%) | Ov mets (%) |
| 1,446 (46.1%) | 0.14% | |||||
| + | 594 (18.9%) | 0.17% | ||||
| + | 113 (3.6%) | 0% | ||||
| + | 74 (2.4%) | 0% | ||||
| + | 337 (10.7%) | 0.89% | ||||
| + | + | 25 (0.8%) | 4.0% | |||
| + | + | 38 (1.2%) | 5.26% | |||
| + | + | 86 (2.7%) | 1.16% | |||
| + | + | 35 (1.1%) | 0% | |||
| + | + | 145 (4.6%) | 0% | |||
| + | + | 37 (1.2%) | 5.41% | |||
| + | + | + | 16 (0.5%) | 6.25% | ||
| + | + | + | 31 (1.0%) | 9.68% | ||
| + | + | + | 20 (0.6%) | 15.0% | ||
| + | + | + | 68 (2.2%) | 4.41% | ||
| + | + | + | + | 12 (0.4%) | 16.7% | |
| + | + | + | + | 12 (0.4%) | 16.7% | |
Only cases with available results for all the risk factors were analyzed for incidence of ovarian metastasis. Only combination patterns with more than five cases are shown. Abbreviations: Adeno, adenocarcinoma; LVSI, lymphovascular space invasion; and Ov mets, ovarian metastasis.
Among the subgroup with age younger than 50 years (Table 4), similar results were observed as for the entire cohort. With absence of these five risk factors (representing 46.1% of women aged <50 years), the incidence of ovarian metastasis was 0.14%. Adenocarcinoma alone (representing 18.9% of women aged <50 years) was not associated with an increased risk of ovarian metastasis (0.17% vs. 0.14%, p=0.87) while pelvic lymph node metastasis alone was (0.89% vs. 0.14%, p=0.041) compared to those without any risk factors. Presence of multiple risk factors was associated with increased incidence of ovarian metastasis: two risk factors 0–5.41%, three risk factors 4.41–15.0%, and four risk factors, 16.7% (p<0.001).
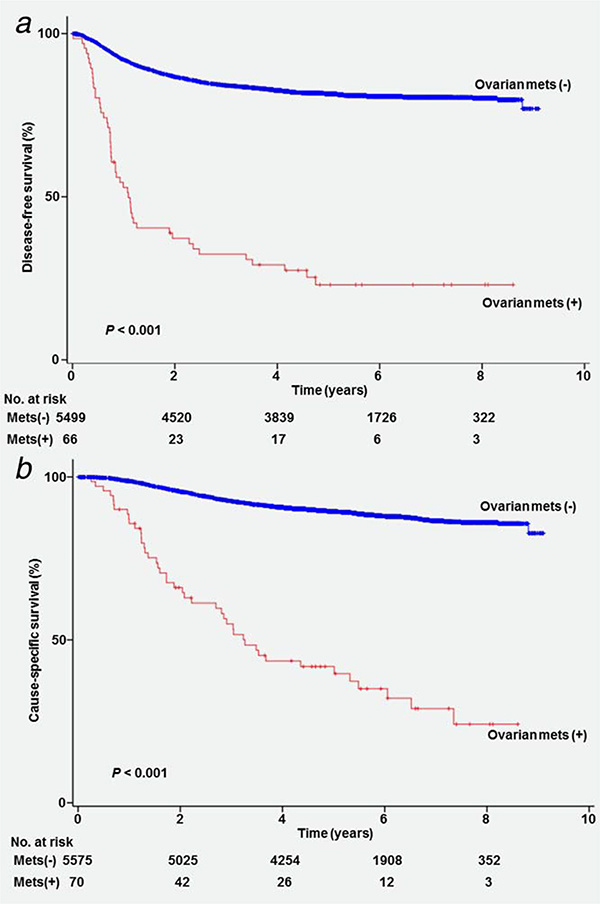
Survival analysis was performed. Median follow-up time for women without any survival events was 64.8 months. There were 1,153 recurrences and 661 deaths from cervical cancer recorded in the study cohort. On univariable analysis, women who had cervical cancer metastasized to the ovary had a significantly lower disease-free survival (5-year rates, 22.9% vs. 81.5%, p<0.001; Fig. 1a) and cause-specific survival (41.5% vs. 89.3%, p<0.001; Fig. 1b) compared to those who did not have ovarian metastasis. There was no difference in disease-free survival (5-year rates, 24.2% vs. 20.7%, p=0.26) and cause-specific survival (46.9% vs. 32.3%, p=0.16) between unilateral and bilateral ovarian metastasis.
Figure 1.
Survival curves based on ovarian metastasis. Log-rank test for p-values. Survival curves are shown for (a) disease-free survival and (b) cause-specific survival based on ovarian metastasis. Abbreviation: mets: metastasis.
On multivariable analysis controlling for a priori survival factors (Table 5), ovarian metastasis remained an independent prognostic factor for decreased disease-free survival (adjusted-HR 2.19, 95%CI 1.56–3.09, p<0.001) and cause-specific survival (adjusted-HR 2.32, 95%CI 1.60–3.36, P<0.001). Ovarian metastasis had the largest magnitude of statistical significance for disease-free survival among the 10 examined tumor factors, similar to the impact of pelvic lymph node metastasis (adjusted-HRs, 2.19 vs. 2.16). Similarly, ovarian metastasis had the largest impact on cause-specific survival among the tumor factors examined in the analysis.
Table 5.
Multivariable analysis for survival outcome (N = 5,697)
| Disease-free survival |
Cause-specific survival |
|||||
|---|---|---|---|---|---|---|
| Characteristic | 5-yr (%) | HR (95%CI) | P-value | 5-yr (%) | HR (95%CI) | p-value |
| Age (years) | 0.23 | 0.20 | ||||
| <40 | 83.4% | 1 | 88.8% | 1 | ||
| 40–49 | 81.9% | 0.98 (0.81–1.17) | 0.81 | 89.6% | 0.84 (0.67–1.05) | 0.13 |
| ≥50 | 78.5% | 1.11 (0.94–1.30) | 0.22 | 88.1% | 0.85 (0.69–1.03) | 0.10 |
| Histology | <0.001 | <0.001 | ||||
| SCC | 83.2% | 1 | 90.2% | 1 | ||
| Adeno | 76.6% | 1.73 (1.49–2.00) | <0.001 | 87.5% | 1.62 (1.33–1.97) | <0.001 |
| AS | 77.8% | 1.56 (1.25–1.96) | <0.001 | 83.5% | 1.91 (1.47–2.47) | <0.001 |
| Others | 63.1% | 2.13 (1.31–3.46) | 0.002 | 70.9% | 2.04 (1.14–3.65) | 0.016 |
| Tumor size | ||||||
| ≤4 cm | 84.5% | 1 | 92.0% | 1 | ||
| >4 cm | 71.4% | 1.27 (1.10–1.46) | 0.001 | 81.0% | 1.43 (1.20–1.71) | <0.001 |
| Parametrea | ||||||
| Not involved | 85.9% | 1 | 92.8% | 1 | ||
| Involved | 59.4% | 1.80 (1.55–2.11) | <0.001 | 72.0% | 1.71 (1.41–2.07) | <0.001 |
| Deep stromal invasion | ||||||
| Not involved | 89.8% | 1 | 96.2% | 1 | ||
| Involved | 72.9% | 1.34 (1.12–1.60) | 0.001 | 82.5% | 1.80 (1.39–2.33) | <0.001 |
| LVSI | ||||||
| No | 90.2% | 1 | 96.1% | 1 | ||
| Yes | 73.1% | 1.56 (1.31–1.86) | <0.001 | 83.0% | 1.97 (1.52–2.55) | <0.001 |
| Uterine corpus | ||||||
| Not involved | 83.5% | 1 | 91.1% | 1 | ||
| Involved | 64.1% | 1.10 (0.93–1.30) | 0.28 | 74.0% | 1.33 (1.09–1.62) | 0.005 |
| Pelvic lymph node | ||||||
| Not involved | 87.5% | 1 | 94.1% | 1 | ||
| Involved | 61.1% | 2.16 (1.86–2.50) | <0.001 | 73.4% | 2.31 (1.91–2.79) | <0.001 |
| Para–aortic lymph node | 0.09 | 0.11 | ||||
| Not involved | 81.3% | 1 | 87.7% | 1 | ||
| Involved | 35.1% | 1.47 (1.04–2.06) | 0.028 | 50.1% | 1.47 (0.99–2.17) | 0.05 |
| Clinically not involved | 81.7% | 1.11 (0.93–1.33) | 0.26 | 89.8% | 1.03 (0.82–1.29) | 0.82 |
| Peritoneal cytology | ||||||
| No | 83.5% | 1 | 90.8% | 1 | ||
| Yes | 72.2% | 1.10 (0.95–1.27) | 0.22 | 81.9% | 1.05 (0.88–1.27) | 0.59 |
| Ovarian metastasis | ||||||
| No | 81.5% | 1 | 89.3% | 1 | ||
| Yes | 22.9% | 2.19 (1.56–3.09) | <0.001 | 41.5% | 2.32 (1.61–3.36) | <0.001 |
Cox proportional hazard regression model for multivariable analysis. All the listed covariates were entered in the final model. Significant p-values (cutoff, p < 0.05) are emboldened.
Abbreviations: HR: hazard ratio; CI: confidence interval; SCC: squamous cell carcinoma; Adeno: adenocarcinoma; AS: adenosquamous; LVSI: lympho-vascular space invasion.
Discussion
Key findings of this study are that ovarian metastasis represents one of the strongest indicators of poor prognosis of women with stage IB–IIB cervical cancer and that the pathway leading to ovarian spread seem to be one of either anatomical proximity or through lymphatic spread to the ovary. Moreover, based on our risk stratification model, a large fraction of women with clinical stage IB–IIB cervical cancer have a minimum risk of ovarian metastasis and may be candidates for ovarian conservation.
Adenocarcinoma commonly arises in the endocervical glands while squamous carcinoma arises in the extocervix. This difference in the anatomical location of cancer origin is likely the causality of the difference in the risk of ovarian metastasis between the two histology subtypes. That is, the adenocarcinoma type is anatomically closer to the ovary compared to the squamous type with increased risk of uterine corpus tumor invasion resulting in a higher incidence of ovarian metastasis compared to the squamous type. This finding validates our previous study and others including a systematic literature review.15–21 Because our study examined a significantly larger sample size, our study is more reassuring to endorse this association.
A notable finding in our study is that presence of adenocarcinoma alone was not associated with an increased risk of ovarian metastasis particularly in young women (Table 4). We found that tumors with adenocarcinoma histology commonly exhibited other risk factors for ovarian metastasis. Thus, with the presence of additional risk factors other than adenocarcinoma, the risk of ovarian metastasis was significantly increased. This is likely the biological plausibility of adenocarcinoma being a risk factor for ovarian metastasis. Because adenocarcinoma is often seen in younger age women where ovarian conservation is an important consideration in the management of cervical cancer,30 our result of a non-increased risk of ovarian metastasis in women with adenocarcinoma alone is reassuring.
Moreover, this study showed that risk factors for ovarian metastasis differ slightly between adenocarcinoma and squamous histology subtypes (Table 3). While uterine corpus tumor invasion and pelvic nodal metastasis were risk factors for ovarian metastasis in both histology types, local factor (parametrial tumor involvement) was significant only in adenocarcinomas while lymphatic factor (para-aortic nodal metastasis) was significant only in squamous carcinomas. This finding may partly support the different patterns of tumor spread between the two histology types.25
This study found that uterine corpus tumor invasion was one of the strong predictors for ovarian metastasis. Multiple studies have shown that uterine corpus tumor invasion is associated with an increased risk of ovarian metastasis.18,31–35 Uterine corpus tumor invasion is also known to be an independent prognostic factor for decreased survival of women with early-stage cervical cancer shown in a recent population-based study.36 It is likely that anatomical proximity to the ovary from the uterine body explains this association. Therefore, based on what we and others have observed, preoperative or intraoperative assessment of uterine corpus tumor invasion is strongly recommended if ovarian conservation is scheduled in a young woman. A previous study suggested utility of magnetic resonance imaging for the evaluation of uterine corpus tumor invasion.37 If there is tumor involvement of either the uterine corpus or lower uterine segment, oophorectomy is warranted.
Lymphatic factors such as LVSI and lymph node metastasis play a significant role in ovarian metastasis shown in our study as well as by others.17,18,33–35 This implies that lymphatic spread as opposed to hematologic spread may be a mode of ovarian metastasis in cervical cancer. Based on our results, thorough evaluation of the lymph nodes is necessary when ovarian conservation is considered including the para-aortic lymph node chain. Of note, the presence of para-aortic nodal metastasis is commonly associated with presence of other risk factors for ovarian metastasis, and para-aortic nodal metastasis alone without other risk factors was fairly uncommon (Table 4).
Risk of ovarian cancer after ovarian conservation merits in-depth discussion. Given the excess risk of secondary malignancy in women with cervical cancer due to various factors such as cigarette and radiotherapy uses,38,39 long-term safety assessment after ovarian conservation is particularly necessary; however, this was not applicable in our retrospective study with relatively short follow-up (median follow-up time, ~5 years). A recent population-based study has shown that 10- and 20-year cumulative incidences of metachronous ovarian cancer after ovarian conservation for young women with stage I cervical cancer are 0.2 and 0.5%, respectively (median follow-up time, >10 years).40 Because their study did not have a proper control arm from general population to estimate relative risk of ovarian cancer after ovarian conservation for young women with stage I cervical cancer, further study is warranted to assess this long-term safety issue after ovarian conservation.
Although there are no clear criteria to choose surgical approach over concurrent chemoradiotherapy for stage IB–IIB cervical cancer in Japan, current practice patterns in this country demonstrated that the vast majority of stage I disease and nearly half of stage II disease undergo surgical treatment with radical hysterectomy.7 Thus, this unique treatment approach undertaken in Japan allows an evaluation of ovarian metastasis in stage IB–IIB cervical cancer with the benefit of a robust sample size thus making the results more reliable and relevant.
Strengths of the study are that this study analyzed one of the largest sample sizes reported in the literature and the event numbers were sufficient to perform multivariable analyses. Moreover, follow-up time is adequate to interpret the results of survival analysis and overfitting was not observed in multivariable analysis for this study cohort. Limitations of the study include that this is a retrospective study and there may be a missing confounder in the analysis. For example, body habitus, cigarette use, HIV status, and immunosuppressive use were not captioned in the study but these factors may influence the tumor spread patterns to the ovary. Also, while the sample size was large, event number for ovarian metastasis is relatively small and the exact association of each tumor factor and ovarian metastasis was not completely assessed in a full model (Table 2). Lastly, this study was conducted in Japan, and reproducibility and generalization to different ethnic groups cannot be assumed.
A weakness of the study is that we do not have information for the details of ovarian metastasis other than laterality. Therefore, the incidence of occult microscopic metastasis as opposed to macroscopic ovarian metastasis was not able to be assessed in this study. Because macroscopically abnormal ovaries are likely to be resected during surgical treatment, the more clinically meaningful information is to identify the risk factors for occult ovarian metastasis among women with early-stage cervical cancer. We recognize this as the major limitation of this study. In addition, central pathology review was not performed to confirm ovarian metastasis versus synchronous ovarian cancer; however, such misclassification is unlikely because synchronous ovarian cancer is generally rare in cervical cancer.
The clinical implication of our results is in the candidate selection for ovarian conservation in young women with early-stage cervical cancer. A recent population-based study in the United States demonstrated that ovarian conservation was not associated with worse cervical cancer-specific survival as compared to oophorectomy in stage IB disease.26 Importantly, women who had ovarian conservation had higher overall survival compared to those who had oophorectomy although it did not reach statistical significance. Thus, it is paramount to proactively identify the patients who do not have an increased risk of ovarian metastasis.18,20 This is particularly applicable to the Japanese population where ovarian conservation is substantially low (0.5% for stage IB–IIB in Japan versus ~40% for stage IB in the United States).26,41
In this study, women without risk factors for ovarian metastasis represented nearly half of the study population of age <50 years with stage IB–IIB cervical cancer (ovarian metastasis risk, 0.14%). In addition, women aged <50 years with adenocarcinoma alone represented nearly one fifth of the study population (ovarian metastasis risk, 0.17%). If these two groups were added, nearly two thirds of women aged <50 years with stage IB–IIB cervical cancer had ovarian metastasis risks of around 0.1% in our study population. These statistics for ovarian metastasis may be acceptable to safely offer ovarian conservation, and further study is warranted including a cost-effectiveness assessment based on our risk stratification model.
Supplementary Material
What’s new?
Women with cervical cancer confined to the cervix and uterus may opt for radical hysterectomy, sometimes with oophorectomy. Ovary removal, however, has long-term health consequences in young women. Here, five risk factors for ovarian metastasis were identified in women with stage IB–IIB cervical cancer. Women under age 50 who lacked these factors had approximately 1 in 1000 risk of ovarian metastasis. Risk also was not increased for women with adenocarcinoma alone, one of the five risk factors. Young women with adenocarcinoma alone accounted for nearly one-fifth of cervical cancer cases in the study. Many young women with stage IB–IIB cervical cancer may benefit from ovarian conservation.
Acknowledgement
The authors thank all the JGOG institutions participated in this study and the JGOG Cervical Cancer Committee members for administrative work for the study. We also thank Dr. Lynda D. Roman, MD, for her scientific input to the study.
Grant support: none
Footnotes
Disclosure of Potential Conflict of Interest: All the authors have no conflict of interest in this study.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Islami F, Siegel RL, et al. Global Cancer in Women: burden and trends. Cancer Epidemiol Biomarkers Prev 2017;26:444–57. [DOI] [PubMed] [Google Scholar]
- 3.Surveillance, Epidemiology, End Result Program, Cancer Stat Facts: Cervix Uteri Cancer. Available at: https://seer.cancer.gov/statfacts/html/cervix.html (Accessed on March 16, 2017).
- 4.Schiffman M, Castle PE, Jeronimo J, et al. Human papillomavirus and cervical cancer. Lancet 2007;370:890–907. [DOI] [PubMed] [Google Scholar]
- 5.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- 6.Hamashima C, Aoki D, Miyagi E, et al. The Japanese guideline for cervical cancer screening. Jpn J Clin Oncol 2010;40:485–502. [DOI] [PubMed] [Google Scholar]
- 7.Saito T, Katabuchi H. Annual Report of the Committee on Gynecologic Oncology, Japan Society of Obstetrics and Gynecology: Patient Annual Report for 2013 and Treatment Annual Report for 2008. J Obstet Gynaecol Res 2016;42:1069–79. [DOI] [PubMed] [Google Scholar]
- 8.Ebina Y, Yaegashi N, Katabuchi H, et al. Japan Society of Gynecologic Oncology guidelines 2011 for the treatment of uterine cervical cancer. Int J Clin Oncol 2015;20:240–8. [DOI] [PubMed] [Google Scholar]
- 9.Shoupe D, Parker WH, Broder MS, et al. Elective oophorectomy for benign gynecological disorders. Menopause 2007;14:580–5. [DOI] [PubMed] [Google Scholar]
- 10.Rocca WA, Grossardt BR, de Andrade M, et al. Survival patterns after oophorectomy in premenopausal women: a population-based cohort study. Lancet Oncol 2006;7:821–8. [DOI] [PubMed] [Google Scholar]
- 11.Parker WH, Feskanich D, Broder MS, et al. Long-term mortality associated with oophorectomy compared with ovarian conservation in the nurses’ health study. Obstet Gynecol 2013;121:709–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mytton J, Evison F, Chilton PJ, Lilford RJ. Removal of all ovarian tissue versus conserving ovarian tissue at time of hysterectomy in premenopausal patients with benign disease: study using routine data and data linkage. Bmj 2017;356:j372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsuo K, Machida H, Shoupe D, et al. Ovarian conservation and overall survival in young women with early-stage low-grade endometrial cancer. Obstet Gynecol 2016;128:761–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Comprehensive Cancer Network Clinical Practice Guideline in Oncology. Cervical Cancer. Available at: https://www.nccn.org/professionals/physician_gls/pdf/cervical.pdf (Accessed on March 16, 2017).
- 15.Shimada M, Kigawa J, Nishimura R, et al. Ovarian metastasis in carcinoma of the uterine cervix. Gynecol Oncol 2006;101:234–7. [DOI] [PubMed] [Google Scholar]
- 16.Sutton GP, Bundy BN, Delgado G, et al. Ovarian metastases in stage IB carcinoma of the cervix: a Gynecologic Oncology Group study. Am J Obstet Gynecol 1992;166:50–3. [DOI] [PubMed] [Google Scholar]
- 17.Landoni F, Zanagnolo V, Lovato-Diaz L, et al. Ovarian metastases in early-stage cervical cancer (IA2-IIA): a multicenter retrospective study of 1965 patients (a Cooperative Task Force study). Int J Gynecol Cancer 2007;17:623–8. [DOI] [PubMed] [Google Scholar]
- 18.Hu T, Wu L, Xing H, et al. Development of criteria for ovarian preservation in cervical cancer patients treated with radical surgery with or without neoadjuvant chemotherapy: a multicenter retrospective study and meta-analysis. Ann Surg Oncol 2013;20:881–90. [DOI] [PubMed] [Google Scholar]
- 19.Lyu J, Sun T, Tan X. Ovarian preservation in young patients with stage I cervical adenocarcinoma: a surveillance, epidemiology, and end results study. Int J Gynecol Cancer 2014;24:1513–20. [DOI] [PubMed] [Google Scholar]
- 20.Touhami O, Plante M. Should ovaries be removed or not in (early-stage) adenocarcinoma of the uterine cervix: a review. Gynecol Oncol 2014;136:384–8. [DOI] [PubMed] [Google Scholar]
- 21.Jiao XB, Hu J, Zhu LR. The safety of ovarian preservation in early-stage adenocarcinoma compared with squamous cell carcinoma of uterine cervix: a systematic review and meta-analysis of observational studies. Int J Gynecol Cancer 2016; 26:1510–4. [DOI] [PubMed] [Google Scholar]
- 22.von Elm E, Altman DG, Egger M, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Bmj 2007;335: 806–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lawless JF, Singhal K. Efficient screening of non-normal regression models. Biometrics 1978;34: 318–27. [Google Scholar]
- 24.Hosmer DW, Hosmer T, Le Cessie S, et al. A comparison of goodness-of-fit tests for the logistic regression model. Stat Med 1997;16:965–80. [DOI] [PubMed] [Google Scholar]
- 25.Gien LT, Beauchemin MC, Thomas G. Adenocarcinoma: a unique cervical cancer. Gynecol Oncol 2009;116:140–6. [DOI] [PubMed] [Google Scholar]
- 26.Matsuo K, Machida H, Shoupe D, et al. Ovarian conservation and overall survival in young women with early-stage cervical cancer. Obstet Gynecol 2017;129:139–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sapre S, Thakur R. Lifestyle and dietary factors determine age at natural menopause. J Midlife Health 2014;5:3–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaplan EL, Meier P. Nonparametric Estimation from Incomplete Observations. J Am Stat Assoc 1958;53:457–81. [Google Scholar]
- 29.Cox DR. Regression Models and Life-Tables. J R Stat Soc Series B Stat Methodol 1972;34:187–220. [Google Scholar]
- 30.Galic V, Herzog TJ, Lewin SN, et al. Prognostic significance of adenocarcinoma histology in women with cervical cancer. Gynecol Oncol 2012; 125:287–91. [DOI] [PubMed] [Google Scholar]
- 31.Kim MJ, Chung HH, Kim JW, et al. Uterine corpus involvement as well as histologic type is an independent predictor of ovarian metastasis in uterine cervical cancer. J Gynecol Oncol 2008;19: 181–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tabata M, Ichinoe K, Sakuragi N, et al. Incidence of ovarian metastasis in patients with cancer of the uterine cervix. Gynecol Oncol 1987;28:255–61. [DOI] [PubMed] [Google Scholar]
- 33.Sakuragi N, Takeda N, Hareyama H, et al. A multivariate analysis of blood vessel and lymph vessel invasion as predictors of ovarian and lymph node metastases in patients with cervical carcinoma. Cancer 2000;88:2578–83. [PubMed] [Google Scholar]
- 34.Yamamoto R, Okamoto K, Yukiharu T, et al. A study of risk factors for ovarian metastases in stage Ib-IIIb cervical carcinoma and analysis of ovarian function after a transposition. Gynecol Oncol 2001;82:312–6. [DOI] [PubMed] [Google Scholar]
- 35.Nakanishi T, Wakai K, Ishikawa H, et al. A comparison of ovarian metastasis between squamous cell carcinoma and adenocarcinoma of the uterine cervix. Gynecol Oncol 2001;82:504–9. [DOI] [PubMed] [Google Scholar]
- 36.Matsuo K, Machida H, Blake EA, et al. Significance of uterine corpus tumor invasion in early-stage cervical cancer. Eur J Surg Oncol 2017;43: 725–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Boer P, Adam JA, Buist MR, et al. Role of MRI in detecting involvement of the uterine internal os in uterine cervical cancer: systematic review of diagnostic test accuracy. Eur J Radiol 2013;82:e422–8. [DOI] [PubMed] [Google Scholar]
- 38.Chaturvedi AK, Kleinerman RA, Hildesheim A, et al. Second cancers after squamous cell carcinoma and adenocarcinoma of the cervix. J Clin Oncol 2009;27:967–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kleinerman RA, Boice JD, Jr, Storm HH, et al. Second primary cancer after treatment for cervical cancer. An international cancer registries study. Cancer 1995;76:442–52. [DOI] [PubMed] [Google Scholar]
- 40.Matsuo K, Machida H, Horowitz MP, et al. Risk of metachronous ovarian cancer after ovarian conservation in young women with stage I cervical cancer. Am J Obstet Gynecol, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matsuo K, Shimada M, Mikami M. Ovarian conservation for young women with clinical stage IB-IIB cervical cancer in Japan . J Gynecol Oncol 2017;28:e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.