Abstract
Objective.
To examine trends and associated characteristics and outcomes of minimally invasive surgery (MIS) for women with early-stage ovarian cancer.
Methods.
The National Inpatient Sample was queried to examine early-stage ovarian cancer treated with MIS from 2001 to 2011. Annualized hospital surgical volume was defined in the unweighted model as the average number of procedures performed per year in which at least one case was performed. Trends, characteristics, and outcomes related to MIS use were assessed in the weighted model.
Results.
Among 73,707 oophorectomy cases, there were 4822 (6.5%) MIS cases. Utilization of MIS increased from 3.9% to 13.5% from 2001 to 2011 (3.5-fold increase, P < 0.001), and the number of MIS-offering centers also increased from 10.6% to 36.2% (3.4-fold increase, P < 0.001). MIS was associated with a decreased complication rate (20.3% versus 35.4%) and shorter hospital stay (median, 2 versus 4 days) compared to laparotomy (both, P < 0.001). Of the 472 hospitals at which MIS was performed, the majority were minimum-volume with one MIS oophorectomy per year (340 [72.0%], n = 1929 [40.0%]), followed by mid-volume (85 [18.0%], n = 1272 [26.4%]) and topdecile-volume (47 [10.0%] hospitals, n = 1621 [33.6%]). The topdecile-volume group had the highest rate of lymphadenectomy compared to other groups (62.2% versus 39.2–55.1%, P < 0.05). On multivariable analysis, a one increment increase in annualized hospital surgical volume was associated with an 11% decrease in multiple complications (adjusted-odds ratio 0.89, 95% confidence interval 0.82–0.97, P = 0.006).
Conclusion.
Utilization of MIS for early-stage ovarian cancer has significantly increased in the United States in 2000s. In 2011, one in eight surgeries performed for early ovarian cancer were performed via MIS. MIS procedures performed at hospitals with a higher surgical volume may be associated with improved short-term perioperative outcomes.
Keywords: Ovarian cancer, Minimally invasive surgery, Surgical volume, Morbidity, Mortality, Outcome
1. Introduction
In 2020, ovarian cancer will be the fifth most deadly female malignancy in the United States with approximately 13,940 women expected to succumb to this disease [1]. Treatment of ovarian cancer is based upon the stage [2], and women with a suspected ovarian cancer that appears to be confined to the ovary will generally undergo oophorectomy-based surgical treatment (bilateral salpingo-oophorectomy, total hysterectomy, and comprehensive staging with peritoneal biopsy, omentectomy and pelvic/para-aortic lymphadenectomy) [2,3]. This surgery has been historically performed via laparotomy [2], while minimally invasive surgery (MIS) for ovarian cancer is relatively new. The rationale of this approach is that laparotomy is believed to be superior at identifying occult metastatic lesions through increased exposure and palpation that could otherwise be missed via the MIS approach.
To examine the feasibility of and outcomes related to MIS for early-stage ovarian cancer, multiple researchers have compared the MIS approach to the historical standard, a laparotomy approach [4-19]. A 2012 systematic literature review concluded that the MIS is comparable to laparotomy with regards to accuracy and adequacy of surgical staging as well as oncologic outcome [20]. However, population trends and utilization of MIS for early-stage ovarian cancer have not been addressed in the United States. The National Comprehensive Cancer Network (NCCN) guidelines for ovarian cancer recommend that the use of MIS be limited to selected patients by experienced surgeons in the primary surgical treatment of early-stage ovarian cancer [2].
One metric to measure surgical experience is hospital surgical volume. The concept of volume-outcome relationship was originally proposed in 1979, and the fundamental idea is that higher surgical volume is associated with decreased surgical morbidity and mortality [21]. To date, the volume-outcome relationship has not been studied in MIS for early-stage ovarian cancer [22]. The objective of the present study was to examine trends and characteristics associated with and outcomes of MIS for women with early-stage ovarian cancer.
2. Materials and methods
2.1. Data resource
The National (Nationwide) Inpatient Sample (NIS) was queried for this study. NIS is a publicly available, de-identified population-based database, and is distributed as part of the Healthcare Cost and Utilization Project by the Agency for Healthcare Research and Quality [23]. The NIS includes hospital discharge data for >36 million hospitalizations annually, covering >90% of the U.S. population when weighted. The program provides patient characteristics and resource-use information, such as diagnoses and intervention types, length of stay and hospital charges, as well as hospital-specific data, including location, bed capacity, and teaching status. This study was deemed exempt by the University of Southern California Institutional Review Board due to the use of publicly available, de-identified data.
2.2. Study criteria
Women with early-stage ovarian cancer who underwent MIS oophorectomy between 2001 and 2011 were eligible for analysis. The International Classification of Disease 9th revision (ICD-9) codes for diagnoses and procedures remained the same during the study period (Table S1). Since the NIS program does not have specific information for cancer stage, ovarian malignancies with the absence of ICD-9 codes for metastatic disease and for prior chemotherapy treatment were used as a surrogate for early-stage ovarian cancer. The presence of the ICD-9 codes for laparoscopic surgery and oophorectomy was used to determine the MIS oophorectomy cases (Table S1). The study end period of 2011 was chosen as the NIS program randomly captured approximately 20% of the U.S. hospitals in each year and all the consecutive inpatient admissions within the chosen hospitals were recorded until 2011 [23].
2.3. Patient demographics
Variables abstracted from the NIS database included: (i) patient baseline characteristics, (ii) hospital information, (iii) surgical procedures, and (iv) perioperative outcomes. (i) Patient characteristics included age, calendar year, race/ethnicity (white, black, Hispanic, Asian, and others), obesity per the CDC classification (no, class I-II, and class III obesity), medical comorbidities classified using the Charlson Comorbidity Index (0, 1, 2, and ≥3), primary expected payer (Medicare, Medicaid, private insurance, and others), and median household income (<$39,000, $39,000-$47,999, $48,000-$62,999, and ≥$63,000). The Charlson Comorbidity Index was calculated for each patient based on the codes for the specified medical conditions in each category and weighted appropriately to determine a final score (Table S1).
(ii) Hospital information included hospital bed capacity (small, medium, and large) determined by the program rule [23], teaching status (rural, urban non-teaching, and urban teaching), and hospital region (Northeast, Midwest, South, and West). (iii) Surgical procedure information included laterality of oophorectomy (unilateral versus bilateral), use of lymphadenectomy (yes versus no), and use of hysterectomy (yes versus no).
(iv) For perioperative outcome information, hospital length of the index admission, total charge, and complications recorded during the index admission were assessed. The NIS program captures perioperative complications for both intraoperative and postoperative complications before hospital discharge. The following complications were assessed for outcome measures [24-26]: hemorrhage, shock, wound complications, thromboembolism, cerebrovascular disease or stroke, cardiac failure, myocardial infarction, pneumonia, respiratory failure, systemic inflammatory response syndrome (SIRS) or sepsis, ileus or small bowel obstruction, vascular injury, acute kidney injury, pyelonephritis, abscess, fistula, intestinal perforation, position-dependent complications, and death during the index admission (Table S1). Total charge was corrected for medical inflation as described previously [25].
2.4. Analytic approaches
The annualized hospital surgical volume for MIS oophorectomy was defined as the average number of procedures a hospital performed per year in which at least one case was performed [24,25]. Due to the narrow range, annualized hospital surgical volume was analyzed as a continuous variable as in prior studies [24,25]. A scatter plot diagram was plotted to assess the association of annualized hospital surgical volume and the number of perioperative complications. A hypothesis of inverse linear association was tested for analysis as before [25].
Curve estimation was also tested between annualized hospital MIS oophorectomy volume and annualized hospital oophorectomy surgical volume with any mode, and the number of overall oophorectomy surgical volume at which one MIS oophorectomy surgical procedure was performed was interpreted as the threshold to start MIS oophorectomy in the center as described previously [25].
The association of annualized hospital surgical volume and perioperative surgical complications was assessed in multivariable analysis, adjusting for a priori factors for surgical morbidity (age and Charlson Comorbidity Index). A linear regression model was fitted to assess the extent of perioperative complications (entered as continuous) per surgical volume, and a binary logistic regression model was fitted to assess the independent association for multiple perioperative outcomes (≥2 versus <2). The effect size was expressed with regression coefficient or odds ratio (OR) and 95% confidence interval (CI).
The National Cancer Institute's Joinpoint Regression Program (version 4.4.0.0) was utilized to evaluate temporal trends for MIS oophorectomy [27]. Time point data was examined annually to identify temporal changes. Temporal trends were examined with a linear segmented regression test, and log-transformation was performed to determine the annual percent change and 95%CI.
Various sensitivity analyses were performed to assess the robustness of the study findings. First, centers were divided into tertiles using clinically relevant cutoffs for annualized hospital surgical volume. The minimum-volume group was defined as performing an annualized hospital MIS oophorectomy volume of 1. The top decile-volume group was defined as performing an annualized hospital surgical volume of >90% ile, which was exploratory and adopted from recent studies demonstrating improved outcome at very high volume centers for complex surgeries [28,29]. The remaining surgical centers were grouped as the mid-volume. Next, complication types were assessed by surgical volume. Third, characteristics and outcome comparisons were performed between the MIS and laparotomy groups. Last, cases were stratified to those who had oophorectomy alone, oophorectomy and hysterectomy, and oophorectomy, hysterectomy and lymphadenectomy.
The annualized hospital surgical volume was determined in an unweighted model, and the remaining analyses were performed in weighted models. Multicollinearity was assessed with variance inflation factor, and a value of ≥2.5 was interpreted as multicollinearity in this study. All analyses were based on a two-tailed hypothesis, and a P < 0.05 was interpreted statistically significant. Statistical Package for Social Sciences (version 25.0, Armonk, NY, USA) was used for statistical analyses. The study adhered to the STROBE guidelines for the reporting of observational cohort study [30].
3. Results
There were 73,707 oophorectomies identified for analysis. Of those, 4822 (6.5%) oophorectomies were via the MIS approach, and the remaining 68,885 (93.5%) were via laparotomy. During the study period, the utilization of MIS had significantly increased (3.5-fold, Fig. 1), and in 2011 nearly one in eight women who had oophorectomy-based surgical treatment for early-stage ovarian cancer had surgery via the MIS approach (3.9% to 13.5%, P < 0.001). A total of 472 centers performed MIS oophorectomy, and the number of MIS-offering centers increased from 10.6% (40 centers) to 36.2% (98 centers) between 2001 and 2011 (3.4-fold, P < 0.001; Fig. 2).
Fig. 1.
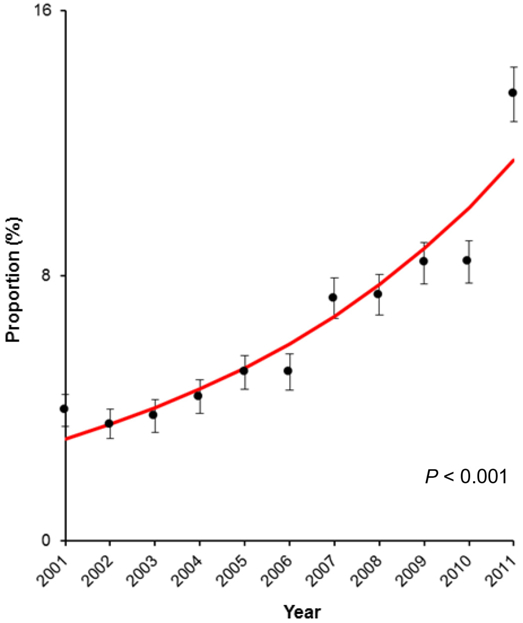
Trend of MIS cases for early-stage ovarian cancer between 2001 and 2011. The number of women with early-stage ovarian cancer who underwent oophorectomy with MIS approach increased from 3.9% to 13.5% between 2001 and 2011 (3.5-fold increase, P < 0.001). Annual percent change was 14.0 (95% confidence interval 10.6–17.6). Red line represents modeled value. Dots represent observed values, and bars represent 95% confidence interval. The Y-axis is truncated to 0–16% scale range to maximize the visibility.
Fig. 2.
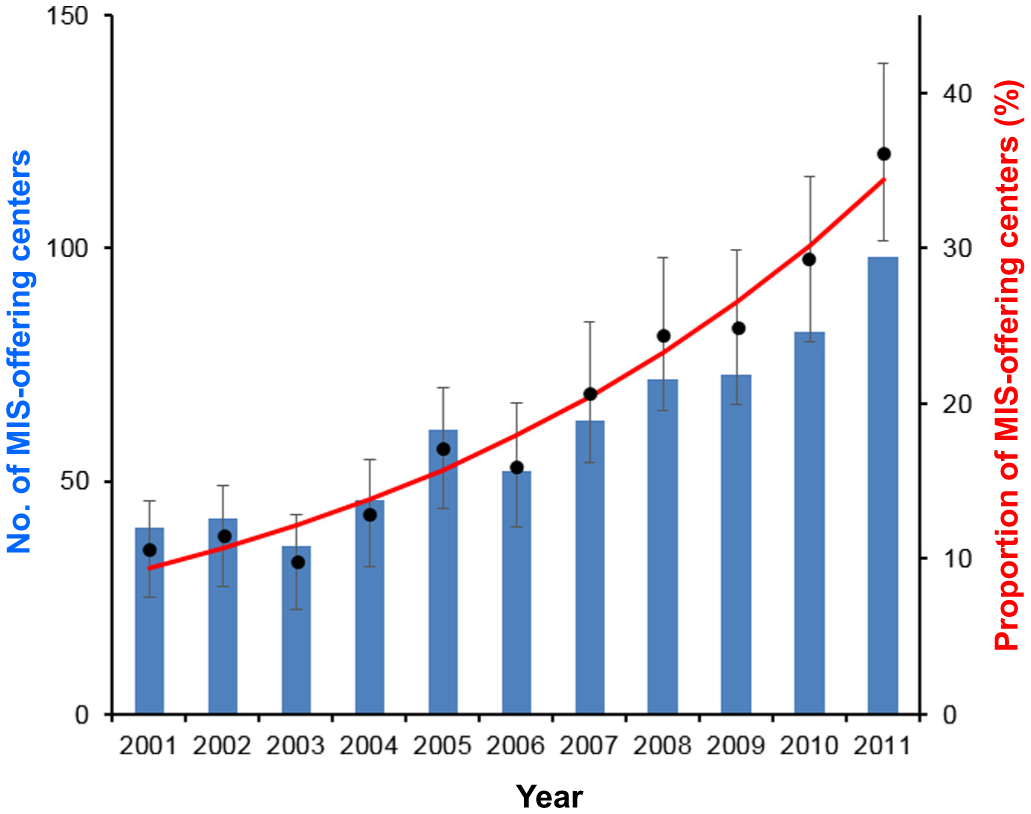
Trend of MIS-offering centers between 2001 and 2011. The number of MIS-offering center increased from 40 to 98 between 2001 and 2011 (2.5-fold increase) (blue bars). The proportion of MIS-offering center among oophorectomy-offering centers (any modes) has increased from 10.6% to 36.2% (3.4-fold increase; P < 0.001) (red line for modeled value and dots with 95% confidence interval for observed values). Abbreviation: MIS, minimally invasive oophorectomy for early-stage ovarian cancer.
Compared to women who had oophorectomy via laparotomy approach, those who underwent MIS oophorectomy were more likely to be young, white, more recently diagnosed, reside in a higher household income zip code, private insurance, and have had the oophorectomy performed at urban or teaching centers in the Northeast region of the United States (all, P < 0.05; Table 1). They were less likely to have a medical comorbidity when compared to those who underwent laparotomy (P < 0.05). Body habitus was not associated with MIS oophorectomy use (P= 0.998). There was a significant correlation between overall oophorectomy surgical volume (any approach) and MIS oophorectomy surgical volume (P< 0.001), with the centers performing MIS oophorectomy performing at least 3.3 oophorectomies via any approach (data not shown).
Table 1.
Patient demographics (open versus MIS cohorts, n = 73,707).
| Characteristic |
Open |
MIS |
P-value | aOR (95%CI)† | P-value† |
|---|---|---|---|---|---|
| Number | n = 68,885 | n = 4822 | |||
| Age (yr) | 55.5 (±16.0) | 52.4 (±17.0) | <0.001 | 0.98 (0.98–0.98) | <0.001 |
| Year | <0.001 | <0.001* | |||
| 2001–2004 | 23,912 (34.7%) | 971 (20.1%) | 1 | ||
| 2005–2008 | 25,834 (37.5%) | 1727 (35.8%) | 1.81 (1.67–1.96) | <0.001 | |
| 2009–2011 | 19,139 (27.8%) | 2124 (44.0%) | 3.09 (2.85–3.35) | <0.001 | |
| Race/ethnicity | <0.001 | <0.001* | |||
| White | 40,246 (58.4%) | 3166 (65.7%) | 1 | ||
| Black | 3809 (5.5%) | 214 (4.4%) | 0.77 (0.66–0.89) | 0.001 | |
| Hispanic | 5010 (7.3%) | 363 (7.5%) | 0.83 (0.74–0.94) | 0.003 | |
| Asian | 2086 (3.0%) | 204 (4.2%) | 0.94(0.81–1.10) | 0.465 | |
| Others | 1741 (2.5%) | 147 (3.0%) | 0.99 (0.83–1.18) | 0.899 | |
| Missing | 15,993 (23.2%) | 728 (15.1%) | 0.77 (0.70–0.84) | <0.001 | |
| Obesity | 0.998 | ||||
| No | 63,659 (92.4%) | 4455 (92.4%) | |||
| Class I-II | 3231 (4.7%) | 227 (4.7%) | |||
| Class III | 1995 (2.9%) | 140 (2.9%) | |||
| Charlson Index | 0.003 | <0.001* | |||
| 0 | 48,233 (70.0%) | 3710 (76.9%) | 1 | ||
| 1 | 14,217 (20.6%) | 833 (17.3%) | 0.80 (0.74–0.87) | <0.001 | |
| 2 | 4088 (5.9%) | 182 (3.8%) | 0.62 (0.53–0.72) | <0.001 | |
| ≥ 3 | 2347 (3.4%) | 97 (2.0%) | 0.55 (0.44–0.68) | <0.001 | |
| Median household income | <0.001 | <0.001* | |||
| < $39,000 | 12,196 (17.7%) | 648 (13.4%) | 1 | ||
| $39,000–$47,999 | 15,307 (22.2%) | 804 (16.7%) | 1.01 (0.91–1.13) | 0.856 | |
| $48,000–$62,999 | 17,387 (25.2%) | 1250 (25.9%) | 1.31 (1.19–1.45) | <0.001 | |
| ≥$63,000 | 22,549 (32.7%) | 2011 (41.7%) | 1.60 (1.45–1.76) | <0.001 | |
| Missing | 1446 (2.1%) | 109 (2.3%) | 1.29 (1.04–1.60) | 0.020 | |
| Primary expected payer | <0.001 | <0.001* | |||
| Medicare | 19,819 (28.8%) | 1186 (24.6%) | 1 | ||
| Medicaid | 5221 (7.6%) | 369 (7.7%) | 0.72 (0.63–0.84) | <0.001 | |
| Private including HMO | 37,744 (54.8%) | 2938 (60.9%) | 0.80 (0.73–0.88) | <0.001 | |
| Others | 5968 (8.7%) | 324 (6.7%) | 0.62 (0.54–0.71) | <0.001 | |
| Missing | 133 (0.2%) | a | 0.44 (0.18–1.07) | 0.070 | |
| Hospital bed size | 0.040 | ||||
| Small | 5105 (7.4%) | 389 (8.1%) | |||
| Medium | 14,105 (20.5%) | 968 (20.1%) | |||
| Large | 49,306 (71.6%) | 3427 (71.1%) | |||
| Missing | 369 (0.5%) | 38 (0.8%) | |||
| Hospital teaching status | <0.001 | <0.001* | |||
| Rural | 3933 (5.7%) | 152 (3.2%) | 1 | ||
| Urban non-teaching | 19,035 (27.6%) | 1451 (30.1%) | 1.46 (1.23–1.74) | <0.001 | |
| Urban teaching | 45,548 (66.1%) | 3181 (66.0%) | 1.29 (1.09–1.53) | 0.004 | |
| Missing | 369 (0.5%) | 38 (0.8%) | 1.54 (1.06–2.24) | 0.025 | |
| Hospital region | <0.001 | <0.001* | |||
| Northeast | 12,507 (18.2%) | 1294 (26.8%) | 1 | ||
| Midwest | 15,953 (23.2%) | 803 (16.7%) | 0.56 (0.50–0.61) | <0.001 | |
| South | 25,241 (36.6%) | 1421 (29.5%) | 0.60 (0.56–0.65) | <0.001 | |
| West | 15,184 (22.0%) | 1304 (27.0%) | 0.81 (0.75–0.89) | <0.001 | |
| Lymphadenectomy | 0.045 | ||||
| No | 31,093 (45.1%) | 2356 (48.9%) | |||
| Yes | 37,792 (54.9%) | 2465 (51.1%) | |||
| Hysterectomy | <0.001 | ||||
| No | 43,789 (63.6%) | 2268 (47.0%) | |||
| Yes | 25,096 (36.4%) | 2553 (53.0%) | |||
| Adnexectomy | <0.001 | ||||
| Unilateral | 10,220 (14.8%) | 1385 (28.7%) | |||
| Bilateral | 58,665 (85.2%) | 3437 (71.3%) | |||
| Complications (any) | <0.001 | ||||
| No | 44,477 (64.6%) | 3843 (79.7%) | |||
| Yes | 24,408 (35.4%) | 979 (20.3%) | |||
| Length of stay | 4 (IQR 3–6) | 2 (IQR 1–4) | <0.001 | ||
| Total charge ($) | 36,740 (IQR 24431–56,918) | 37,546 (IQR 24364–54,670) | 0.209 |
Mean (±SD) or number (percentage per column) is shown.
Suppressed per the HCUP requirement (1–10). Total number may not be 73,707 due to weighted values.
Multivariable analysis with a logistic regression model for the use of MIS approach. All the preoperative covariates with a P < 0.05 level were entered, and conditional backward method was used to retain only the covariates with P < 0.05 in the final model.
P-value for interaction.
There were 340 (72.0%) centers that had an average of one MIS-oophorectomy per year (minimum-volume group, n = 1929 [40.0%]; Table 2). Forty-seven (9.9%) centers had the top 10%ile surgical volume (average > 2 cases a year), and this top decile-volume group performed nearly one third of the total MIS-oophorectomies (n = 1626 [33.6%]). The mid-volume group, which averaged >1 but ≤2 oophorectomies a year, consisted of 85 (18.0%) centers including 1272 (26.4%) patients.
Table 2.
Annualized hospital surgical volume for MIS adnexectomy for early-stage ovarian cancer between 2001 and 2011.
| Annualized SV | Centers | (%) |
|---|---|---|
| 1 | 340 | 72.0% |
| 1.1–2.0 | 85 | 18.0% |
| 2.1–3.0 | 25 | 5.3% |
| 3.1–4.0 | 12 | 2.5% |
| 4.1–5.0 | 5 | 1.1% |
| >5.0 | 5 | 1.1% |
| Total | 472 | 100% |
Abbreviation: SV, surgical volume; and MIS, minimally invasive surgery.
Women in the top decile-volume group were more likely to be white, obese, have had any extent comorbidity, higher household income, and private insurance compared to the lower volume groups (all, P < 0.05; Table S2). The number of MIS oophorectomies performed in the larger volume centers increased during the study period (P < 0.001, Table S2). MIS oophorectomy performed at the top decile centers were more likely to have additional procedures for lymphadenectomy (62.2% versus 39.2–55.1%) and hysterectomy (61.8% versus 45.3–53.2%) compared to the lower volume centers (both,P < 0.001; Table S2). Overall, additional procedures of hysterectomy and lymphadenectomy were reported in 52.9% and 51.1%, respectively. The top decile centers were more likely to be urban teaching hospitals, have a large bed capacity, and located in the northeast region in the United State (all, P < 0.001).
There were 979 (20.3%) women who had any type of perioperative complication during the index admission for MIS oophorectomy. Of those who had a surgical complication, the majority had a single complication (n=686, 70.0%), while multiple complications were recorded in 293 (30.0%)women. There were 15 (0.3%)women who died during the index hospitalization.
There was a significant inverse linear association between annualized MIS oophorectomy surgical volume and the number of perioperative complication, and a large surgical volume was associated with decreased perioperative complications (estimated equation, y = −0.016x+0.338, P = 0.014; Fig. 3). After controlling for age and medical comorbidity, the inverse association between annualized hospital surgical volume and the extent of perioperative complications remained significant (regression coefficient per each annualized hospital surgical volume increase, −0.023, 95%CI −0.034 to −0.011, P < 0.001; Table 3).
Fig. 3.
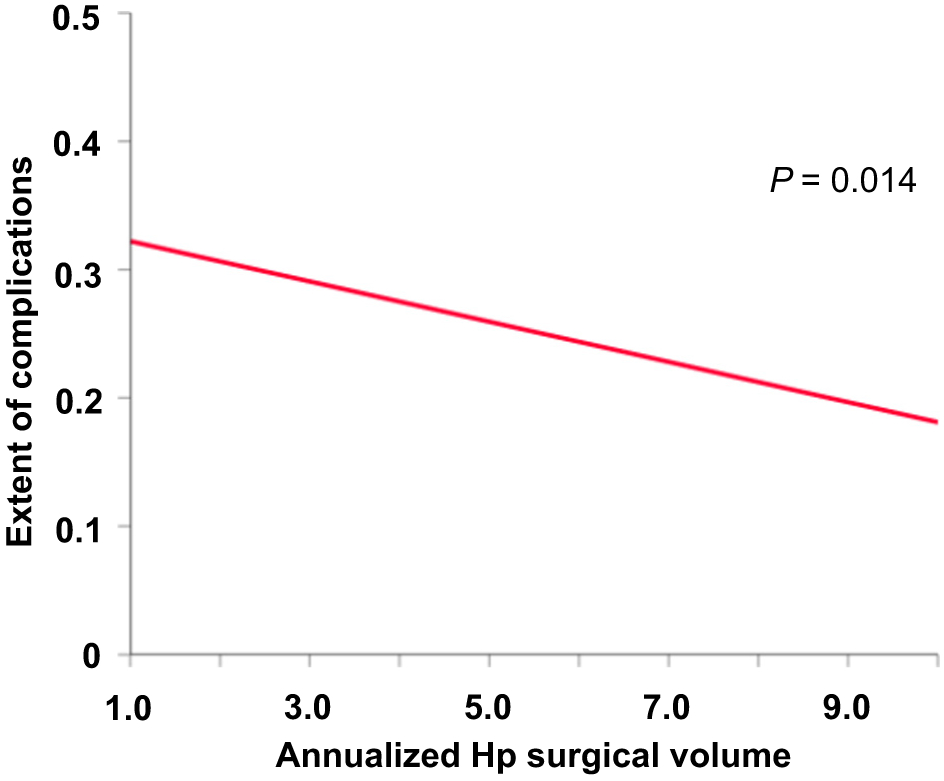
Association between surgical volume and perioperative complications in MIS for early-stage ovarian cancer. There is a significant inverse linear association between annualized hospital surgical volume and perioperative complications in women with early-stage ovarian cancer who underwent MIS surgery. Abbreviations: MIS, minimally invasive surgery; and Hp, hospital.
Table 3.
Association between annualized hospital surgical volume and perioperative complications (n=4822).
| Characteristic | Any complicationsa |
Multiple complicationsb |
||
|---|---|---|---|---|
| regression coefficient (95%CI) | P-value | Odds ratio (95%CI) | P-value | |
| Unadjusted | −0.016 (−0.028 to −0.003) | 0.014 | 0.91 (0.84 to 0.98) | 0.019 |
| Adjusted for age | −0.017 (−0.029 to −0.004) | 0.007 | 0.90 (0.83 to 0.98) | 0.017 |
| Adjusted for CCI | −0.023 (−0.035 to −0.011) | <0.001 | 0.87 (0.80 to 0.95) | 0.001 |
| Adjusted for age and CCI | −0.023 (−0.034 to −0.011) | <0.001 | 0.89 (0.82 to 0.97) | 0.006 |
Abbreviations: CI, confidence interval; and CCI, Charlson Comorbidity Index.
Linear regression models: the extent of perioperative complication was analyzed as a continuous variable. Intercept with regression coefficient value for perioperative complications per each increment increase of annualized hospital surgical volume for MIS is estimated.
Binary logistic regression models: outcome measures were dichotomized as ≥2 complications versus <2 complications. Odds ratio for multiple complications per each increment increase of annualized hospital surgical volume for MIS is estimated. Adjustment factors were a priori known factors for perioperative complications. Both age and CCI were examined as continuous variables.
Similarly, after controlling for age and medical comorbidity, annualized hospital MIS oophorectomy surgical volume was significantly associated with a decreased risk of multiple perioperative complications (Table 3): a one increment increase in annualized hospital surgical volume is associated with an 11% decrease in multiple complications (adjusted-odds ratio 0.89, 95%CI 0.82–0.97, P = 0.006).
When complication type was examined (Table S3), the minimum-volume group had higher incidences of abscess, acute kidney injury, bladder injury, ileus or small bowel obstruction, pneumonia, prolonged intubation, respiratory failure, and sepsis/SIRS compared to the higher volume groups (all, P < 0.05). The minimum-volume group also had the highest incidence of perioperative death (0.8% versus 0%) and prolonged hospitalization (≥28 days, 1.0% versus 0%) compared to the higher volume-groups (both, P < 0.05). In contrast, the top decile-volume center had higher incidence of atelectasis and venous thromboembolism compared to the lower volume centers (both, P < 0.05).
When cases were restricted to those who had oophorectomy, hysterectomy, and lymphadenectomy, higher annualized surgical volume was associated with lower number of perioperative complications (estimated equation,y = −0.062x+0.426, P = 0.004). After controlling for age and comorbidity, this association remained independent, and a one increment increase in annualized hospital surgical volume was associated with an 18% decrease in any perioperative complications (adjusted-odds ratio 0.82, 95% confidence interval 0.71–0.95, P = 0.008).
4. Discussion
Key findings of the current study are that U.S. surgeons began adopting minimally invasive surgery for early-stage ovarian cancer in the 2000s with the MIS-offering centers nearly tripling, but the overall hospital surgical volume remained modest with the majority of centers having only few cases a year. Moreover, there was a volume-outcome relationship for MIS oophorectomy, and a higher hospital surgical volume was associated with improved short-term surgical outcome.
MIS oophorectomy for early ovarian cancer is a relatively new surgical approach and has not been studied adequately. Prior studies that examined the feasibility and outcomes of MIS oophorectomy for early-stage ovarian cancer were predominantly retrospective and at single institutions with limited sample sizes. The majority included <100 MIS cases [7-19].None of prior studies examined volume-outcome relationships in MIS oophorectomy [4-19]. In addition, there is to date no level I evidence examining the safety and oncologic outcome for MIS in early-stage ovarian cancer [4-19]. Therefore, the results of current study add new information in the literature with regard to the surgical management of early-stage ovarian cancer.
Our study may partly support the NCCN guidelines for MIS oophorectomy in the treatment of early-stage ovarian cancer. They recommend that MIS should not be used in a universal fashion for ovarian cancer surgery and should be reserved for select women and be performed by experienced minimally invasive surgeons [2]. The observed volume-outcome relationship in our study suggests that it would be more appropriate for only experienced surgeons at higher volume centers to offer this surgical approach to improve perioperative outcomes. The reason for improved short-term perioperative outcomes in large hospital surgical volume is likely multifactorial [22]. Surgeon experience and operative skill as well as hospital infrastructure support and level of care likely influenced this association, but none of these were assessable in this study.
There was a nationwide expansion in the utilization of MIS oophorectomy in the United States in the 2000s. The number of MIS oophorectomy-offering centers has increased significantly in conjunction with the number of MIS oophorectomies. In 2011, there were ~100 centers offering this procedure in this study. As the NIS program captured ~20% of US hospitals during the study period, it is estimated that ~500 centers would have performed MIS oophorectomy in that year. Moreover, centers that had only 3 surgeries for early-stage ovarian cancer began adopting the MIS approach. This threshold seems low compared to other more complex and rare gynecologic surgeries [24,25]. Collectively, these statistics imply that surgical services of MIS oophorectomy for early-stage ovarian cancer are unlikely to be regionalized in the United States.
Despite the widespread utilization of MIS oophorectomy, the overall hospital surgical volume was modest in the 2000s in the United States. The majority of MIS-offering centers had one surgery per year during the study period. Performance of >2 cases of MIS oophorectomy met the criteria to be included as a top decile surgical volume center. This suggests that surgeon's experience and performance might also have varied significantly during the study period. In fact, there was a wide range of surgical performance at MIS oophorectomy related to hospital surgical volume. For instance, higher hospital surgical volume centers were more likely to have performed additional procedures such as lymphadenectomy and hysterectomy at the time of MIS oophorectomy. As the absence of surgical staging with lymphadenectomy is associated with increased cancer mortality in early-stage ovarian cancer [31], further study is warranted to examine if surgical volume for MIS oophorectomy is also associated with oncologic outcome in early-stage ovarian cancer.
Another possibility of limited hospital surgical volume is that this study examined an older time period. This is most likely the reason why the hospital surgical volume was so limited across the nation. It is possible that this study captured the learning curve period for MIS adnexectomy in this country. Therefore, the clinical utility of our study to the current practice may be limited. During the learning curve period, surgeons may have been reluctant to offer MIS approach for women with comorbidities to avoid long surgical hours as was observed in our study. There was a geographic disparity for hospital surgical volume for MIS oophorectomy for early-stage ovarian cancer in this study, most likely reflecting the heterogeneity in surgeon's adoption of MIS in the early-2000s.
Strengths of the current study include the use of population-based dataset and that this is likely the first study examining the volume-outcome association for MIS oophorectomy. There are several limitations in this study. First, unmeasured bias may be inherent to this type of retrospective study. For example, the exact cancer stage was not available in the database, and it is unknown if our study population truly represents early-stage disease. Similarly, histology types were not available in the database. Thus, it is unknown what proportion of the study population had borderline ovarian tumors or non-epithelial ovarian cancer. These two tumor types have distinct management approaches compared to epithelial ovarian cancer [3], so the absence of this information would be critical to long-term and oncologic outcomes. Relatively young age at surgery and low rate of hysterectomy and lymphadenectomy may indeed reflect that a considerable number of patients may have had one of these two tumor types in this study.
Second, tumor size and prior surgical history are not available in the database but would both likely impact the surgeon's choice for the route of surgery. Third, this study examined hospital surgical volume only, and it is unknown if an individual surgeon's surgical volume and experience also impacted outcomes. Fourth, the database captures short-term perioperative complications only for the index hospitalization, outpatient complications, longer term complications and oncologic outcomes after discharge were not assessable. MIS approach may be associated with increased risk of intraoperative capsule rupture compared to laparotomy that may negatively impact the oncologic outcome [20,32].
Fifth, both exposure and outcome measures relied on the ICD-9 codes alone without actual medical record review, and the accuracy of the data is unknown. This disadvantage is particularly applicable for surgical staging procedures. For example, omentectomy is one of key procedures for ovarian cancer staging, but there was no specific code for this procedure. Anatomic site of lymphadenectomy to distinguish pelvic versus para-aortic lymphadenectomy was also not available. Sixth, the cutoffs for hospital surgical volume used in this analysis were arbitrary and require further validation. Finally, the generalizability in different populations is unknown as this study examined the U.S. population alone.
In conclusion, the past several years have witnessed a populational-level increase in the utilization of MIS oophorectomy for early-stage ovarian cancer in the United States. In 2011, one in eight women undergoing this surgery was via the MIS approach. The number of MIS-offering centers has increased significantly during the same time, however, most of these centers perform modest number of these surgeries in a year. The observed volume-outcome relationship for MIS oophorectomy is reassuring, but further study examining oncologic outcome related to surgical volume for MIS oophorectomy is definitely warranted. Together with the NCCN guidelines recommending MIS oophorectomy for early ovarian cancer to be limited to experienced surgeons [2], this study offers an opportunity to consider the benefit of regionalizing care for MIS for ovarian cancer treatment.
Supplementary Material
HIGHLIGHTS.
Volume-outcome relationship for MIS was examined for early ovarian cancer from 2001 to 2011.
Utilization of MIS increased significantly for more than three-fold.
Nearly three quarters of MIS-performing centers had a minimum surgical volume of one case per year.
Higher volume centers were more likely to perform staging surgery compared to lower volume centers.
Higher annualized hospital surgical volume was associated with lower perioperative complication.
Acknowledgments
Funding support
Ensign Endowment for Gynecologic Cancer Research (K.M.).
Footnotes
Declaration of competing interest
Consultant, Clovis Oncology, Tesaro, research funding, Merck (J.D.W.); consultant, Quantgene (L.D.R.); advisory board, Tesaro, GSK (M.K.); research funding,MSD(S.M.); honorarium, Chugai, textbook editorial expense, Springer, and investigator meeting attendance expense, VBL therapeutics (K.M.); scientific consulting, Kiyatec, Merck, shareholder, Biopath, research funding, M-Trap (A.K.S.); none for others.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ygyno.2020.04.045.
References
- [1].Siegel RL, Miller KD, Jemal A, Cancer statistics, 2020, CA Cancer J. Clin 70 (2020) 7–30. [DOI] [PubMed] [Google Scholar]
- [2].Ovarian cancer including fallopean tube cancer and primary peritoneal cancer, National Comprehensive Cancer Network (US) NCCN Clinical Practice Guideline in Oncology. Version 1, 2020 http://nccn.org , accessed 3/23/2020.
- [3].Matsuo K, Sood AK, Gershenson DM, Management of early-stage ovarian cancer, in: Bristow RE, Karlan BY (Eds.), Surgery for Ovarian Cancer: Principles and Practice. Chapter 3, 3rd ed., 3, Informa Healthcare, New York: 2015, pp. 67–104. [Google Scholar]
- [4].Melamed A, Keating NL, Clemmer JT, Bregar AJ, Wright JD, Boruta DM, Schorge JO, Del Carmen MG, Rauh-Hain JA, Laparoscopic staging for apparent stage I epithelial ovarian cancer, Am J Obstet Gynecol 216 (2017) 50.e1–50.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Song T, Kim MK, Jung YW, Yun BS, Seong SJ, Choi CH, Kim TJ,Lee JW, Bae DS, Kim BG, Minimally invasive compared with open surgery in patients with borderline ovarian tumors, Gynecol. Oncol 145 (2017) 508–512. [DOI] [PubMed] [Google Scholar]
- [6].Bogani G, Borghi C, Ditto A, Signorelli M, Martinelli F, Chiappa V, Scaffa C, Perotto S, Leone Roberti Maggiore U, Montanelli L, Di Donato V, Infantino C, Lorusso D, Raspagliesi F, Impact of surgical route in influencing the risk of lymphatic complications after ovarian cancer staging, J. Minim. Invasive Gynecol 24 (2017) 739–746. [DOI] [PubMed] [Google Scholar]
- [7].Bogani G, Cromi A, Serati M, Di Naro E, Casarin J, Pinelli C, Ghezzi F, Laparoscopic and open abdominal staging for early-stage ovarian cancer: our experience, systematic review, and meta-analysis of comparative studies, Int. J. Gynecol. Cancer 24 (2014) 1241–1249. [DOI] [PubMed] [Google Scholar]
- [8].Liu M, Li L, He Y, Peng D, Wang X, Chen W, Fu X, Ma Y, Comparison of laparoscopy and laparotomy in the surgical management of early-stage ovarian cancer, Int. J. Gynecol. Cancer 24 (2014) 352–357. [DOI] [PubMed] [Google Scholar]
- [9].Park JY, Kim DY, Suh DS, Kim JH, Kim YM, Kim YT, Nam JH, Comparison of laparoscopy and laparotomy in surgical staging of early-stage ovarian and fallopian tubal cancer, Ann. Surg. Oncol 15 (2008) 2012–2019. [DOI] [PubMed] [Google Scholar]
- [10].Ditto A, Bogani G, Martinelli F, Signorelli M, Chiappa V, Scaffa C, Indini A, Leone Roberti Maggiore U, Lorusso D, Raspagliesi F, Minimally invasive surgical staging for ovarian carcinoma: a propensity-matched comparison with traditional open surgery, J. Minim. Invasive Gynecol 24 (2017) 98–102. [DOI] [PubMed] [Google Scholar]
- [11].Lu Q, Qu H, Liu C, Wang S, Zhang Z, Zhang Z, Comparison of laparoscopy and laparotomy in surgical staging of apparent early ovarian cancer: 13-year experience, Medicine (Baltimore) 95 (2016), e3655. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Minig L, Saadi J, Patrono MG, Giavedoni ME, Cardenas-Rebollo JM, Perrotta M, Laparoscopic surgical staging in women with early stage epithelial ovarian cancer performed by recently certified gynecologic oncologists, Eur. J. Obstet. Gynecol. Reprod. Biol 201 (2016) 94–100. [DOI] [PubMed] [Google Scholar]
- [13].Gallotta V, Petrillo M, Conte C, Vizzielli G, Fagotti A, Ferrandina G, Fanfani F, Costantini B, Carbone V, Scambia G, Laparoscopic versus laparotomic surgical staging for early-stage ovarian cancer: a case-control study, J. Minim. Invasive Gynecol 23 (2016) 769–774. [DOI] [PubMed] [Google Scholar]
- [14].Koo YJ, Kim JE, Kim YH, Hahn HS, Lee IH, Kim TJ, Lee KH, Shim JU, Lim KT, Comparison of laparoscopy and laparotomy for the management of early-stage ovarian cancer: surgical and oncological outcomes, J. Gynecol. Oncol 25 (2014) 111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Park JY, Bae J, Lim MC, Lim SY, Seo SS, Kang S, Park SY, Laparoscopic and laparotomic staging in stage I epithelial ovarian cancer: a comparison of feasibility and safety, Int. J. Gynecol. Cancer 18 (2008) 1202–1209. [DOI] [PubMed] [Google Scholar]
- [16].Bergamini A, Ferrandina G, Candiani M, Cormio G, Giorda G, Lauria R, Perrone AM, Scarfone G, Breda E, Savarese A, Frigerio L, Gadducci A, Mascilini F, Maneschi F, Cassani C, Marchetti C, Cecere SC, Biglia N, De Giorgi U, Raspagliesi F, Lorusso D, Mangili G, Laparoscopic surgery in the treatment of stage I adult granulosa cells tumors of the ovary: results from the MITO-9 study, Eur. J. Surg. Oncol 44 (2018) 766–770. [DOI] [PubMed] [Google Scholar]
- [17].Lee M, Kim SW, Paek J, Lee SH, Yim GW, Kim JH, Kim JW, Kim YT, Nam EJ, Comparisons of surgical outcomes, complications, and costs between laparotomy and laparoscopy in early-stage ovarian cancer, Int. J. Gynecol. Cancer 21 (2011) 251–256. [DOI] [PubMed] [Google Scholar]
- [18].Ghezzi F, Cromi A, Uccella S, Bergamini V, Tomera S, Franchi M, Bolis P, Laparoscopy versus laparotomy for the surgical management of apparent early stage ovarian cancer, Gynecol. Oncol 105 (2007) 409–413. [DOI] [PubMed] [Google Scholar]
- [19].Chi DS, Abu-Rustum NR, Sonoda Y, Ivy J, Rhee E, Moore K, Levine DA, Barakat RR, The safety and efficacy of laparoscopic surgical staging of apparent stage I ovarian and fallopian tube cancers, Am. J. Obstet Gynecol 192 (2005) 1614–1619. [DOI] [PubMed] [Google Scholar]
- [20].Covens AL, Dodge JE, Lacchetti C, Elit LM, Le T, Devries-Aboud M, Fung-Kee-Fung M, Gynecology Cancer Disease Site G, Surgical management of a suspicious adnexal mass: a systematic review, Gynecol. Oncol 126 (2012) 149–156. [DOI] [PubMed] [Google Scholar]
- [21].Luft HS, Bunker JP, Enthoven AC, Should operations be regionalized? The empirical relation between surgical volume and mortality, N. Engl. J. Med 301 (1979) 1364–1369. [DOI] [PubMed] [Google Scholar]
- [22].Wright JD, The volume-outcome paradigm for gynecologic surgery: clinical and policy implications, Clin Obstet Gynecol (2020) in-press. [DOI] [PubMed] [Google Scholar]
- [23].HCUP National Inpatient Sample (NIS), Healthcare Cost and Utilization Project (HCUP), Agency for Healthcare Research and Quality, Rockville, MD, 2001–2015. https://www.hcup-us.ahrq.gov/ accessed 1/2/2020. [Google Scholar]
- [24].Matsuo K, Matsuzaki S, Mandelbaum RS, Matsushima K, Klar M, Grubbs BH, Roman LD, Wright JD, Hospital surgical volume and perioperative mortality of pelvic exenteration for gynecologic malignancies, J. Surg. Oncol 121 (2020) 402–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Matsuo K, Matsuzaki S, Mandelbaum RS, Matsushima K, Klar M, Grubbs BH, Roman LD, Wright JD, Association between hospital surgical volume and perioperative outcomes of fertility-sparing trachelectomy for cervical cancer: a national study in the United States, Gynecol Oncol 157 (2020) 173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Mandelbaum RS, Chen L, Shoupe D, Paulson RJ, Roman LD, Wright JD, Matsuo K, Patterns of utilization and outcome of ovarian conservation for young women with minimal-risk endometrial cancer, Gynecol. Oncol 154 (2019) 45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Joinpoint trend analysis software. National Cancer Institute, https://surveillance.cancer.gov/joinpoint/ accessed 1/21/2020. [Google Scholar]
- [28].Matsuo K, Shimada M, Yamaguchi S, Matoda M, Nakanishi T, Kikkawa F, Ohmichi M, Okamoto A, Sugiyama T, Mikami M, Association of radical hysterectomy surgical volume and survival for early-stage cervical cancer, Obstet. Gynecol 133 (2019) 1086–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Trinh VT, Davies JM, Berger MS, Surgery for primary supratentorial brain tumors in the United States, 2000–2009: effect of provider and hospital caseload on complication rates, J. Neurosurg 122 (2015) 280–296. [DOI] [PubMed] [Google Scholar]
- [30].von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, S. Initiative, Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies, BMJ 335 (2007) 806–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Matsuo K, Machida H, Mariani A, Mandelbaum RS, Glaser GE, Gostout BS, Roman LD, Wright JD, Adequate pelvic lymphadenectomy and survival of women with early-stage epithelial ovarian cancer, J. Gynecol. Oncol 29 (2018), e69. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Matsuo K, Machida H, Yamagami W, Ebina Y, Kobayashi Y, Tabata T, Kaneuchi M, Nagase S, Enomoto T, Mikami M, Intraoperative capsule rupture, postoperative chemotherapy, and survival of women with stage I epithelial ovarian cancer, Obstet. Gynecol 134 (2019) 1017–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


