Abstract
Background and Objectives
To determine the significance of depth and extent of lymphovascular space invasion (LVSI) on lymph node metastasis and recurrence in endometrial cancer.
Methods
A case-control study was conducted to examine LVSI-positive (n=70) and LVSI-negative (n=641) stage I–III endometrial cancer cases that underwent hysterectomy-based surgical staging. The risk of lymph node metastasis and distant recurrence was estimated based on LVSI patterns.
Results
In multivariate analysis, deep (>50% invasion), and extensive (≥7 foci/slide) LVSI patterns had a significantly increased risk of lymph node metastasis (incidence 57.6% and 72.7%, odds ratio 33.8 and 49.9, respectively, P<0.001) as compared to other traditional uterine factors (>50% myometrial tumor invasion, cervical stromal invasion, and adnexal involvement: incidence range 30.4–37.9%, odds ratio range 3.80–7.03). Deep and extensive of LVSI patterns were both significantly correlated to distant recurrence (P<0.001). Among women who received postoperative chemotherapy, deep and extensive LVSI patterns did not have increased risks for distant recurrence compared to no LVSI (P=0.47 and 0.32, respectively). Among women who received postoperative radiotherapy, the depth of LVSI was significantly associated with recurrence outside the radiated field (P=0.02).
Conclusions
Depth and extent of LVSI are important predictors for lymph node metastasis and distant recurrence in endometrial cancer.
Keywords: endometrial cancer, lymphovascular space invasion, lymph node metastasis, recurrence, chemotherapy, radiotherapy
INTRODUCTION
In 2015, endometrial cancer continues to be the most common gynecologic malignancy in the United States with over 54,000 newly diagnosed cases projected to occur this year [1]. Surgery plays an important role in the management of endometrial cancer with regard to determination of extent of disease and to determine the need for adjuvant treatment [2]. The standard surgical procedure for endometrial cancer includes total hysterectomy and bilateral salpingo-oophorectomy with additional lymphadenectomy in selected cases [2]. Surgical specimens obtained from the hysterectomy-based surgical staging are valuable to identify histological factors that determine the patient’s prognosis such as lymphovascular space invasion (LVSI).
LVSI is known to be associated with an increased risk of lymph node metastasis and decreased survival outcome of women with various types of gynecological cancers including cervical [3–9], endometrial [10–14], and ovarian cancers [15–18]. In endometrial cancer, presence of LVSI in the uterine myometrial layer is a key finding that has implications for therapy and women with LVSI-positive endometrial cancers in a particular setting such as older age, higher tumor grade, and deeper myometrial tumor invasion benefit from radiotherapy after surgical staging to reduce the risk of disease recurrence [19]. However, interpretation of LVSI is generally done in a qualitative fashion as dichotomized into presence or absence, and the significance of quantitative measurement for extent of LVSI or anatomical location of LVSI within the myometrial layer of the uterus has not been completely elucidated. The aim of the study was to examine the impact of (i) the extent of LVSI foci and (ii) depth of LVSI within the myometrium on lymph node metastasis and recurrence patterns of women with endometrial cancer.
MATERIALS AND METHODS
Eligibility
After Institutional Review Board approval was obtained at University of Southern California, the institutional database for endometrial cancer was utilized to identify eligible cases. The database consists of consecutive patients with all histology type endometrial cancer who underwent hysterectomy-based surgical staging at Los Angeles County/University of Southern California Medical Center (January 2000 and December 2013) and the University of Southern California Keck Medical Center (December 2008 and December 2013). The case group consists of endometrial cancer patients with histopathologically confirmed LVSI in the hysterectomy specimen (LVSI-positive group). The control group consists of endometrial cancer with no LVSI in the hysterectomy specimen (LVSI-negative group). Cases receiving neoadjuvant chemotherapy or radiation, those with sarcoma, metastatic tumors to the endometrium, and endometrial hyperplasia were not included. Stage IV disease were also excluded. Among eligible patients for the case and control groups, the following information was abstracted from the medical records: (i) patient demographics, (ii) pathology results for hysterectomy-based surgical staging, (iii) treatment pattern for adjuvant therapy, and (iv) survival outcome. The STROBE guidelines were consulted for reporting in a case-control study [4]. Some of the patients in this study were within the context of our previous studies [20–24].
Clinical Information
Archived medical records were utilized to obtain the following information: (i) patient demographics included age at surgery, ethnicity, and body mass index (BMI, kg/m2); (ii) histopathology results included histologic subtypes, grade, stage, depth of myometrial tumor invasion (%), LVSI, cervical stromal invasion, adnexal tumor involvement, and pelvic/para-aortic nodal metastasis (number of nodal metastasis, and total lymph node number sampled); (iii) treatment pattern included use of neoadjuvant therapy (chemotherapy or radiotherapy), details of surgical staging (hysterectomy, salpingo-oophorectomy, and lymphadenectomy), postoperative radiotherapy (whole pelvic radiotherapy [WPRT], intracavitary brachytherapy [ICBT], and WPRT with extended field to para-aortic lymph node chain [WPRT+ext]), and postoperative chemotherapy (type and cycle); and (iv) survival outcomes including time to recurrence and its anatomical location that was grouped into vaginal cuff-recurrence, pelvic-recurrence (other than vaginal cuff), and distant-recurrence (abdomen including liver, spleen, para-aortic lymph nodes, and carcinomatosis; chest including lung, pleura, and mediastinum lymph nodes; osseous structure; and brain).
Evaluation of LVSI
Archived hematoxylin and eosin stained slides for the hysterectomy specimen were retrieved and examined by a gynecologic pathologist who was completely blind for clinical information. LVSI was defined as the presence of tumor cells within lymphatic or vascular spaces within the myometrial layer of the uterus but not the endometrial stromal layer [25]. The foci had to be considered unequivocal and potential artifacts or tumor cell contamination were not counted as LVSI. The distance between the serosa of the myometrium and the deepest focus of LVSI within the myometrial layer of the uterus was measured, and the depth of LVSI was expressed as percent distribution in the full thickness of the myometrial layer in the same histological section. For the extent of LVSI, all histopathology slides containing myometrium were examined, and the crude number of LVSI foci was manually counted in each case. In each case, the number of sections with LVSI, tumor and myometrial tumoral invasion was recorded and the number of LVSI foci was then adjusted for the number of slides representing myometrium and tumor.
Study Definition
Endometrial cancer grading was based on the International Federation of Gynecology and Obstetrics (FIGO) system, and cancer stage was re-classified based on the 2009 system [26]. In our study, deep LVSI was defined as the presence of LVSI in the outer half of the myometrial layer (>50%) as opposed to superficial LVSI (located in the inner half, ≤50%). Various cutoffs for LVSI foci adjusted for slide number were tested by correlating to endometrial cancer recurrence (Table S1). Based on the results, LVSI foci of ≥7 per slide was found to be the optimal cutoff to predict the risk of recurrence and was defined as extensive LVSI. LVSI foci<7 per slide was then defined as focal LVSI. Lymph node ratio (LNR) was defined as the percent proportion of positive lymph node count among total sampled lymph node counts [27]. Among patients who received postoperative radiotherapy, the recurrence sites were further categorized into the following two: recurrence within the radiation field (infield-recurrence) versus recurrence outside the radiation field (outfield-recurrence).
Statistical Analysis
The primary interest of analysis was to determine the significance of depth and extent of LVSI on lymph node metastasis risk. The secondary interest of analysis was to examine the pattern of recurrence based on LVSI patterns. Continuous variables were assessed for the normality by Kormogrov-Sminorov test and expressed as mean (± SD) or median (range) as appropriate. Statistical significance of continuous variables was assessed with Student t-test or Mann–Whitney U-test as appropriate. Categorical and ordinal variables were described as number (%), and statistical significance was assessed with chi-square test or Fisher’s exact test as appropriate, expressed with odds ratio (OR) and 95% confidence interval (CI). Spearman’s correlation coefficient was used to examine the correlation between depth of LVSI, number of LVSI foci per slide, depth of myometrial tumor invasion, and LNR.
In order to determine the independent risk factor for lymph node metastasis (no versus yes), a binary logistic regression model was used for the multivariate analysis. In this study, 3 models were examined to evaluate the significance of LVSI: (i) depth of LVSI (none vs. superficial versus deep); (ii) extent of LVSI (none vs. focal versus extensive); and (iii) combination of depth and extent (none, superficial/focal, superficial/extensive, deep/focal, and deep/extensive). Covariates entered in to the final model include age (≥60 vs. <60), ethnicity (Hispanic vs. non-Hispanic), BMI (≥30 vs. <30 kg/m2), histology (endometrioid vs. non-endometrioid), grade (1–2 vs. 3), depth of myometrial tumor invasion (≤50 vs. >50%), cervical stromal invasion (no vs. yes), adnexal involvement (no vs. yes), and LVSI patterns. Statistical significance was expressed with OR and 95%CI. Cumulative risks for time to develop recurrence were analyzed by Log-rank test. Kaplan–Meier method was used to construct survival and cumulative risk curves. All statistical analyses were two-tailed and P<0.05 was considered significant. Statistical Package for the Social Science (SPSS Inc, version 12.0, Chicago, IL) was used for the analysis.
RESULTS
There were 70 cases in the LVSI-positive group and 641 cases in the LVSI-negative group examined for statistical analysis (Table S2). Demographics of the two groups are shown in Table I. Patients in the LVSI-positive group were significantly older, less likely to be Hispanic, and had lower BMIs compared to patients in the LVSI-negative group (all, P<0.01). Tumor characteristics for the LVSI-positive group were significantly associated with non-endometrioid histology, grade 3 tumor, stage III disease, deep myometrial tumor invasion, cervical stromal invasion, adnexal tumor involvement, and lymph node metastasis (all, P<0.01). Patients in the LVSI-positive group were significantly more likely to receive postoperative radiotherapy and/or chemotherapy (both, P<0.001).
TABLE I.
Demographics of Endometrial Cancer
| LVSI-positive |
LVSI-negative |
||
|---|---|---|---|
| No. | n=70 | n=641 | P-value |
| Age | 57.8 (± 9.4) | 52.8 (± 11.0) | <0.001 |
| <60 | 38 (54.3%) | 475 (74.1%) | |
| ≥60 | 32 (45.7%) | 166 (25.9%) | |
| Ethnicity | 0.005 | ||
| Caucasian | 14 (20.0%) | 118 (18.4%) | |
| African American | 0 | 26 (4.1%) | |
| Hispanic | 38 (54.3%) | 419 (65.4%) | |
| Asian | 18 (25.7%) | 78 (12.2%) | |
| BMI (kg/m2) | 31.7 (± 7.9) | 36.3 (± 10.1) | <0.001 |
| <30 | 33 (47.1%) | 179 (28.2%) | |
| ≥30 | 37 (52.9%) | 456 (71.8%) | |
| Histology | 0.001 | ||
| Endometrioid | 53 (75.7%) | 582 (909.8%) | |
| Serous | 8 (11.4%) | 33 (5.1%) | |
| Clear | 5 (7.1%) | 13 (2.0%) | |
| Others | 4 (5.7%) | 13 (2.0%) | |
| Grade | <0.001 | ||
| 1 | 11 (15.7%) | 428 (66.8%) | |
| 2 | 22 (31.4%) | 138 (21.5%) | |
| 3 | 37 (42.9%) | 75 (11.7%) | |
| Stage | <0.001 | ||
| I | 35 (50.0%) | 551 (86.0%) | |
| II | 4 (5.7%) | 47 (7.3%) | |
| III | 31 (44.3%) | 43 (6.7%) | |
| Myometrial invasion (%) | 70 (1–100) | 13 (0–100) | <0.001 |
| ≤50% | 25 (35.7%) | 554 (87.5%) | |
| >50% | 45 (64.3%) | 79 (12.5%) | |
| Cervical stromal invasion | <0.001 | ||
| No | 54 (77.1%) | 598 (93.4%) | |
| Yes | 16 (22.9%) | 42 (6.6%) | |
| Adnexal involvement | <0.001 | ||
| No | 58 (82.9%) | 615 (93.6%) | |
| Yes | 12 (17.1%) | 42 (6.4%) | |
| Pelvic node metastasis | <0.001 | ||
| No | 33 (60.0%) | 236 (97.1%) | |
| Yes | 22 (40.0%) | 7 (2.9%) | |
| Para-aortic node metastasis | 0.007 | ||
| No | 18 (69.2%) | 62 (92.5%) | |
| Yes | 8 (30.8%) | 5 (7.5%) | |
| Radiotherapy type | <0.001 | ||
| None | 12 (17.1%) | 486 (76.2%) | |
| ICBT alone | 24 (34.3%) | 77 (12.1%) | |
| WPRT alone | 21 (30.0%) | 41 (6.4%) | |
| WPRT + ext | 4 (5.7%) | 1 (0.2%) | |
| WPRT + ICBT | 9 (12.9%) | 31 (4.9%) | |
| WPRT + ext + ICBT | 0 | 2 (0.3%) | |
| Chemotherapy type* | <0.001 | ||
| None | 28 (40.0%) | 554 (86.6%) | |
| Carboplatin + paclitaxel | 37 (52.9%) | 77 (12.0%) | |
| Other | 5 (7.1%) | 9 (1.4%) |
Number (%), mean (± SD), or median (range) are shown. Student t test, Mann–Whitney U test, chi-square test, or Fisher exact test for P-values. Significant P-values are in bold.
median 6 cycles (range 3–10) for chemotherapy recipients. 6 missing for BMI, 8 missing for myometrial tumor invasion, 2 missing for cervical stromal invasion, 1 missing for adnexal involvement. 3 missing for radiotherapy, 3 missing for chemotherapy. Pelvic and para-aortic lymphadenectomy were performed in 308 and 99 cases, respectively.
BMI, body mass index; LVSI, lymphovascular space invasion; ICBT, intracavitary brachytherapy; WPRT, whole pelvis radiotherapy; and ext, extended filed to cover aortic nodal chain.
Detailed patterns of LVSI are shown in Table S3. Median number of examined slides for myometrium with endometrial tumor was 6 (range, 2–22). All cases with LVSI had myometrial tumor invasion, and there were no cases with LVSI without myometrial invasion. Median of depth of LVSI within the myometrium was 66.7% (range, 3.9–99.8), and deep LVSI was seen in 60.0% of LVSI-positive cases. Median number of LVSI foci per slide was 2.4 (range, 0.1–148.8). Extensive LVSI was seen in 24.3% of LVSI-positive cases. When depth and extent of LVSI were combined, deep and extensive LVSI was seen in 25.3% of LVSI-positive cases. There was no case exhibiting superficial and extensive LVSI. Among 70 LVSI positive cases, depth of LVSI was significantly correlated with extent of LVSI (r=0.65, P<0.001), depth of myometrial tumor invasion (r=0.55, P<0.001), and LNR (pelvic and/or para-aortic, r=0.37, P=0.006). Similarly, extent of LVSI was significantly correlated with depth of myometrial tumor invasion (r=0.30, P=0.012).
To determine the independent risk factors for deep LVSI and extensive LVSI, multivariate analysis was performed (Table II). After controlling for patient demographics and tumor factors, grade 3 tumors (OR 10.3), myometrial tumor invasion >50% (OR 9.35), and stage III disease (OR 5.78) remained independent risk factors associated with increased risk of deep LVSI while obesity (OR 0.40) was independently associated with decreased risk of deep LVSI (all, P<0.05). In the same multivariate model, grade 3 tumors (OR 4.51), stage III disease (OR 6.42), and myometrial tumor invasion >50% (OR 17.6) remained independent risk factors for increased risk of extensive LVSI (all, P<0.05).
TABLE II.
Independent Risk Factors for Deep and Extensive Lymphovascular Space Invasion
| Deep LVSI |
Extensive LVSI |
|||
|---|---|---|---|---|
| OR (95%CI) |
P-value | OR (95%CI) |
P-value | |
| Age | 0.23 | 0.29 | ||
| <60 | 1 | 1 | ||
| ≥60 | 1.67 (0.72–3.86) |
0.50 (0.14–1.79) |
||
| Ethnicity | 0.73 | 0.023 | ||
| Non-hispanic | 1 | 1 | ||
| Hispanic | 0.86 (0.37–1.99) |
0.22 (0.06–0.81) |
||
| BMI (kg/m2) | 0.031 | 0.28 | ||
| <30 | 1 | 1 | ||
| ≥30 | 0.40 (0.18–0.92) |
0.49 (0.14–1.75) |
||
| Histology | 0.29 | 0.52 | ||
| Endometrioid | 1 | 1 | ||
| Non-endometrioid | 0.57 (0.20–1.64) |
1.59 (0.39–6.46) |
||
| Grade | <0.001 | 0.038 | ||
| 1–2 | 1 | 1 | ||
| 3 | 10.3 (4.03–26.6) |
4.51 (1.09–18.7) |
||
| Stage | 0.001 | 0.014 | ||
| I–II | 1 | 1 | ||
| III | 5.78 (1.97–16.9) |
6.42 (1.46–28.3) |
||
| Myometrial invasion (%) | <0.001 | <0.001 | ||
| ≤50% | 1 | 1 | ||
| >50% | 9.35 (3.93–22.2) |
17.6 (3.53–87.7) |
||
| Cervical stromal invasion | 0.92 | 0.75 | ||
| No | 1 | 1 | ||
| Yes | 1.06 (0.37–2.06) |
0.79 (0.18–3.40) |
||
| Adnexal involvement | 0.52 | 0.51 | ||
| No | 1 | 1 | ||
| Yes | 0.63 (0.16–2.55) |
0.55 (0.09–3.36) |
||
Multivariate analysis with binary logistic regression test for P-values. Covariates listed in table are entered in the final model. Significant P-values are in bold. BMI, body mass index; and LVSI, lymphovascular space invasion.
The association between LVSI patterns and lymph node metastasis was examined. Median numbers of pelvic and para-aortic lymph nodes sampled were 17.5 (n=298) and 7 (n=93), respectively. Depth of LVSI was significantly associated with an increased risk of multiple pelvic (deep, superficial, and no LVSI: 39.4%, 13.6%, 2.1%, P<0.001) and para-aortic nodal metastasis (37.5%,10%, and 4.5%, P=0.003). The risk of multiple lymph node metastases was similar between extensive and focal LVSI for both pelvic (27.3%, 29.5%, and 2.1%, P<0.001) and para-aortic (16.7%, 30%, and 4.5%, P=0.005) lymph nodes.
On multivariate analysis, controlling for patient demographics and tumor factors, all of the three tested models of LVSI pattern (depth of LVSI, extent of LVSI, and combination of depth and extent of LVSI) were independently associated with an increased risk of pelvic and/or para-aortic lymph node metastasis (Table III). In each model, there was a sequential increase of lymph node metastasis for (i) depth of LVSI (prevalence of lymph node metastasis, no LVSI 4.1%, superficial LVSI 22.7% [OR 6.48], and deep LVSI 57.6% [OR 33.8]), (ii) extent of LVSI (no LVSI 4.1%, focal LVSI 36.4% [OR 11.4], and extensive LVSI 72.7% [OR 49.9]), and (iii) combination of depth and extent of LVSI (no LVSI 4.1%, superficial/focal 22.7% [OR 6.43], deep/focal 50.0% [OR 26.3], and deep/extensive 72.7% [OR 63.9]). Among tested covariates, myometrial tumor invasion >50%, cervical stromal invasion, adnexal involvement, and LVSI were all significant for increased risk of lymph node metastasis with deep and extensive LVSI holding substantially higher risks compared to the other factors (nodal metastasis 30.4–37.9% versus 57.6–72.7%; and OR 3.80–7.03 versus 33.8–49.9). The combination of deep and extensive LVSI had the highest OR for risk of lymph node metastasis (OR 63.9).
TABLE III.
Independent Risk Factor for Pelvic and/or Para-Aortic Lymph Node Metastasis
| Depth of LVSI |
Extent of LVSI |
Depth/extent of LVSI |
||||||
|---|---|---|---|---|---|---|---|---|
| No. | Mets (%) | OR (95%CI) | P-value | OR (95%CI) | P-value | OR (95%CI) | P-value | |
| Age | 0.16 | 0.28 | 0.21 | |||||
| <60 | 212 | 11.3% | 1 | 1 | 1 | |||
| ≥60 | 88 | 11.4% | 0.42 (0.13–1.41) | 0.52 (0.16–1.71) | 0.45 (0.13–1.56) | |||
| Race | 0.19 | 0.12 | 0.15 | |||||
| Non-hispanic | 120 | 9.2% | 1 | 1 | 1 | |||
| Hispanic | 180 | 12.8% | 2.09 (0.69–6.34) | 2.58 (0.79–8.39) | 2.41 (0.74–7.91) | |||
| BMI (kg/m2) | 0.03 | 0.017 | 0.032 | |||||
| <30 | 110 | 18.2% | 1 | 1 | 1 | |||
| ≥30 | 188 | 7.4% | 0.30 (0.10–0.89) | 0.26 (0.09–0.79) | 0.30 (0.10–0.90) | |||
| Histology | 0.98 | 0.73 | 0.8 | |||||
| Endometrioid | 248 | 10.5% | 1 | 1 | 1 | |||
| Non-endometrioid | 52 | 15.4% | 0.98 (0.24–3.93) | 0.77 (0.18–3.37) | 0.83 (0.19–3.64) | |||
| Grade | 0.11 | 0.27 | 0.13 | |||||
| 1–2 | 216 | 7.9% | 1 | 1 | 1 | |||
| 3 | 84 | 20.2% | 0.31 (0.07–1.32) | 0.46 (0.12–1.81) | 0.32 (0.07–1.42) | |||
| Myometrial invasion (%) | 0.001 | 0.002 | 0.002 | |||||
| ≤50% | 207 | 2.9% | 1 | 1 | 1 | |||
| >50% | 92 | 30.4% | 7.03 (2.21–22.4) | 6.26 (1.92–20.4) | 6.33 (1.93–20.8) | |||
| Cervical stromal invasion | 0.019 | 0.017 | 0.017 | |||||
| No | 257 | 7.0% | 1 | 1 | 1 | |||
| Yes | 43 | 37.2% | 3.80 (1.24–11.6) | 3.95 (1.28–12.1) | 3.97 (1.28–12.3) | |||
| Adnexal involvement | 0.012 | 0.015 | 0.012 | |||||
| No | 271 | 8.5% | 1 | 1 | 1 | |||
| Yes | 29 | 37.9% | 5.92 (1.48–23.7) | 5.48 (1.39–21.6) | 6.00 (1.49–24.1) | |||
| Depth of LVSI | ||||||||
| None | 245 | 4.1% | 1 | |||||
| Superficial | 22 | 22.7% | 6.48 (1.52–27.6) | 0.011 | ||||
| Deep | 33 | 57.6% | 33.8 (7.36–155) | <0.001 | ||||
| Extent of LVSI | ||||||||
| None | 245 | 4.1% | 1 | |||||
| Focal | 44 | 36.4% | 11.4 (3.32–38.9) | <0.001 | ||||
| Extensive | 11 | 72.7% | 49.9 (5.81–428) | <0.001 | ||||
| Combination LVSI | ||||||||
| None | 245 | 4.1% | 1 | |||||
| Superficial/focal | 22 | 22.7% | 6.43 (1.51–27.4) | <0.001 | ||||
| Deep/focal | 22 | 50.0% | 26.3 (5.17–134) | <0.001 | ||||
| Deep/extensive | 11 | 72.7% | 63.9 (7.14–571) | <0.001 | ||||
Multivariate analysis with binary logistic regression test for P-values. Three hundered cases of pelvic and/or para-aortic lymphadenectomy were examined. Three different patterns of LVSI are tested in the analysis. Covariates listed in table are entered in the final model. Significant P-values are in bold.
BMI, body mass index; and LVSI, lymphovascular space invasion.
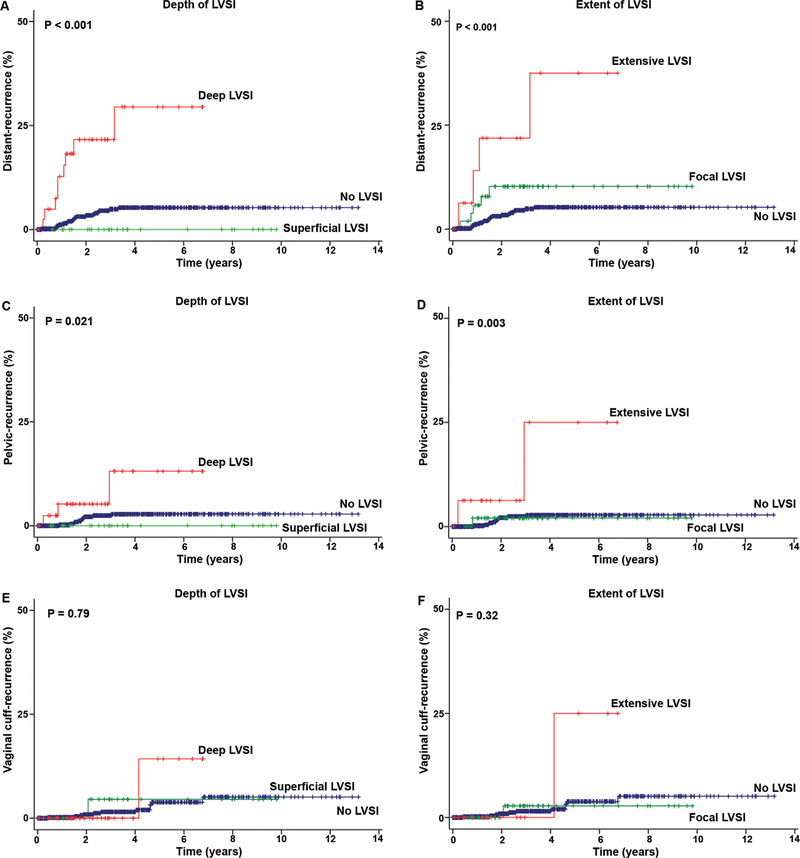
Median follow-up time of the study population was 31.2 months. There were 50 (7.0%) cases of recurrence reported (any distant-recurrence 31, pelvic-recurrence 14, and vaginal cuff-recurrence 13). Patterns for distant-recurrence were shown in Table S4. The depth of LVSI was significantly associated with distant-recurrence (5-year cumulative risks for deep, superficial, and no LVSI: 30.8%, 0%, and 5.2%, P<0.001, Fig. 1A), and deep LVSI had a significantly increased risk of distant-recurrence compared to no LVSI (hazard ratio [HR] 6.97, 95%CI 3.20–15.2, P<0.001). The extent of LVSI was also significantly associated with distant-recurrence (5-year cumulative risks for extensive, focal, and no LVSI: 39.7%, 10.1%, and 5.2%, P<0.001, Fig. 1B), and, extensive LVSI had a significantly increased risk of distant-recurrence compared to no LVSI (HR 8.55, 95%CI 2.95–24.8, P<0.001).
Fig. 1.
Depth and extent of LVSI and recurrence patterns in endometrial cancer. Kaplan–Meier methods for survival curves for (A) depth and (B) extent of LVSI for distant-recurrence, (C) depth, and (D) extent of LVSI for pelvic-recurrence, and (E) depth and (F) extent of LVSI for vaginal cuff-recurrence. Log-rank test for P-values.
The risks of pelvic-recurrence were examined. The depth of LVSI was significantly associated with pelvic-recurrence (5-year cumulative risks for deep, superficial, and no LVSI: 10.9%, 0%, and 2.7%, P=0.021, Fig. 1C). Deep LVSI had a significantly increased risk of pelvic-recurrence compared to no LVSI (HR 4.65, 95%CI 1.29–16.7, P=0.019). Extent of LVSI was significantly associated with pelvic-recurrence (5-year cumulative risks for extensive, focal, and no LVSI: 22.2%, 2.0%, and 3.6%, P=0.003, Fig. 1D). Extensive LVSI had a significantly increased risk of pelvic-recurrence compared to no LVSI (HR 8.72, 95%CI 1.93–39.4, P=0.005). Depth and extent of LVSI were not associated with vaginal cuff-recurrence (P=0.79 and 0.32, respectively, Fig. 1E–F).
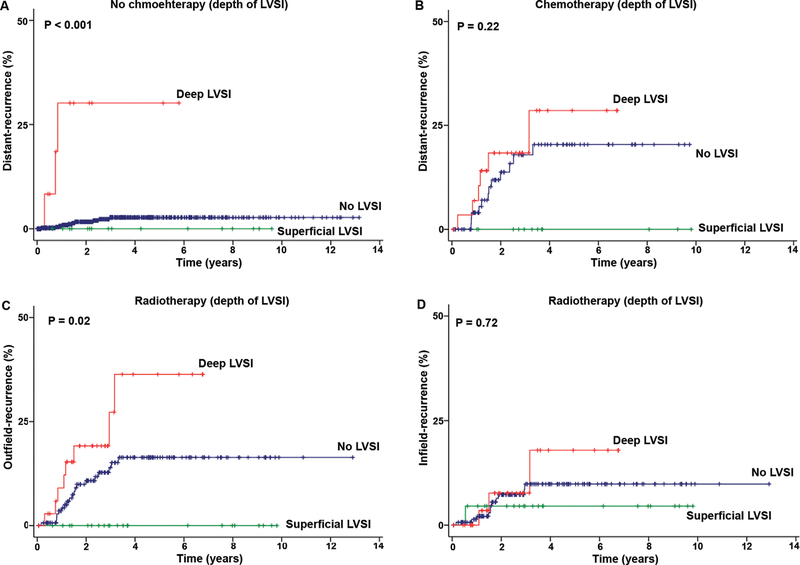
The effects of chemotherapy on distant-recurrences were examined (Table IV). While the depth of LVSI was significantly associated with distant-recurrence in patients who did not receive chemotherapy (P<0.001, Fig. 2A) with deep LVSI holding the highest risk compared to no LVSI (HR 21.2, P<0.001), the rate of distant-recurrence was similar across the LVSI groups in patients receiving chemotherapy (P=0.22, Fig. 2B), and deep LVSI had a non-significant risk of distant-recurrence compared to no LVSI (HR 1.43, P=0.47). The extent of LVSI was significantly associated with distant-recurrence in patients who did not receive chemotherapy (P<0.001) with extensive LVSI holding the highest risk compared to no LVSI (HR 22.1, P=0.004); however, the rates of distant-recurrence were not significantly different across the LVSI groups in patients who received chemotherapy (P=0.38), and extensive LVSI had a non-significant risk of distant-recurrence compared to no LVSI (HR 1.89, P=0.32). Effects of radiotherapy on outfield-recurrence were examined among patients who received radiotherapy. The depth of LVSI was significantly associated with outfield-recurrence (5-year cumulative risks for deep, superficial, and no LVSI: 36.4%, 0%, and 16.3%, P=0.02, Fig. 2C) but not associated with infield-recurrence (P=0.72, Fig. 2D). In-post hoc analysis, cases of endometrioid histology were analyzed (Table S5–8). The similar results were re-demonstrated and deep and extensive LVSI patterns were associated with the strongest risk factors for lymph node metastasis and with increased risk of distant-recurrence.
TABLE IV.
Effects of Chemotherapy on Distant-Recurrence in Endometrial Cancer
| No. | 5-yr (%) | HR (95%CI) | P-value | |
|---|---|---|---|---|
| Depth of LVSI | ||||
| Chemotherapy (−) | ||||
| None | 554 | 2.7% | 1 | |
| Superficial | 16 | 0% | n.a. | 0.98 |
| Deep | 12 | 28.6% | 21.2 (5.73–78.3) | <0.001 |
| Chemotherapy (+) | ||||
| None | 86 | 20.5% | 1 | |
| Superficial | 12 | 0% | n.a. | 0.98 |
| Deep | 30 | 30.8% | 1.43 (0.54–3.84) | 0.47 |
| Extent of LVSI | ||||
| Chemotherapy (−) | ||||
| None | 554 | 2.7% | 1 | |
| Focal | 23 | 8.9% | 4.71 (1.03–21.5) | 0.046 |
| Extensive | 5 | 28.6% | 22.1 (2.770176) | 0.004 |
| Chemotherapy (+) | ||||
| None | 86 | 20.5% | 1 | |
| Focal | 30 | 11.1% | 0.63 (0.18–2.24) | 0.48 |
| Extensive | 12 | 42.5% | 1.89 (0.53–6.71) | 0.32 |
Five-year cumulative distant-recurrence rates are shown. Cox proportional hazard regression test for P-values. Significant P-values are emboldened. 5-yr, 5 year: LVSI, lymphovascular space invasion; n.a., not available; HR, hazard ratio; and CI, confidence interval.
Fig. 2.
Postoperative therapy and distant-recurrence per LVSI patterns in endometrial cancer. Kaplan–Meier methods for survival curves for risk of distant-recurrence based on depth of LVSI for (A) no chemotherapy and (B) chemotherapy. Risks of recurrence in (C) outside the radiation field and (D) inside the radiation field are shown based on depth of LVSI. Log-rank test for P-values.
DISCUSSION
Our study showed that depth and extent of LVSI are important considerations in the management of endometrial cancer. The presence of both deep and extensive LVSI patterns was significantly associated with an increased risk of lymph node metastasis. This is particularly significant in that the magnitudes of significance of the deep and extensive LVSI patterns for lymph node metastasis were substantially larger than the other traditional prognostic tumor factors for endometrial cancer. This implies that determining the location and quantification of LVSI can be a useful tool to identify the patient at risk of lymph node metastasis during surgical staging for endometrial cancer in the era of selective lymph node dissection to avoid complication from lymphadenectomy in low risk patients [28].
Currently, widely accepted tumor factors that indicate additional lymphadenectomy is not required include grade 1–2 tumor, <50% myometrial tumor invasion, and <2 cm tumor size. LVSI is not considered as one of the criteria [29]. This is likely due to the difficulty and challenge in the evaluation of LVSI on frozen section. In fact, previous reports in the literature described that the accuracy for LVSI on frozen section varies across studies (68.3–92.4%) [30,31]. However, some institutions successfully integrated frozen section to evaluate LVSI as the standard algorithm for selective lymphadenectomy during surgical staging [32]. Because our study showed that deep and extensive LVSI patterns are the strongest predictors associated with lymph node metastasis, developing a technique and approach to improve the accuracy of LVSI in frozen section will be of utmost benefit to guide clinicians to identify women for lymphadenectomy during surgical staging. Possible clinical implication based on our results may be that additional lymphadenectomy is indicated if the evaluation of frozen section shows deep or extensive LVSI pattern even absence of other classic tumor factors for lymphadenectomy.
Therapeutic implication for depth and extent of LVSI merits further discussion. Our results showed that both deep and extensive LVSI patterns were associated with markedly high risk of distant-recurrence. Notably, the increased risks of distant-recurrence in such particular LVSI patterns were diminished in a group of patients who received postoperative chemotherapy. In addition, among patients who received radiotherapy, deep LVSI remained the highest risk factor for outfield-recurrence compared to focal and no LVSI. This implies that deep and extensive LVSI patterns may be associated with increased risk of metastasis to distant organs that can benefit from systemic chemotherapy to eliminate these metastatic implants but not from radiotherapy that cannot sterilize such microscopic metastasis outside the radiation field [9]. Indeed, LVSI is known to be associated with increased risk of distant-recurrence in other types of gynecologic malignancy [17], and systemic chemotherapy is suggested for LVSI-positive cases to reduce the risk of recurrence [17,18]. In support of this rationale, another study reported that additional chemotherapy reduces the risk of para-aortic lymph node recurrence among patients with pelvic lymph node metastasis, a surrogate marker for deep and extensive LVSI patterns found in our study [33].
Radiotherapy remains the mainstay of postoperative therapy in endometrial cancer and the role of adjuvant chemotherapy in early stage disease has not been completely elucidated [34]. LVSI is one of 3 components for high-intermediate risk stratification in early stage endometrial cancer that benefits from radiotherapy to reduce the risk of local recurrence [19]. Whether chemotherapy plays a role in controlling disease recurrence is currently being examined in multiple phase III clinical trials including PORTEC3 and GOG249 [35]. Both trials have a treatment arm for additional chemotherapy with carboplatin and paclitaxel after radiotherapy. In a preliminary result from GOG249, a randomized controlled trial for high-risk early-stage endometrial cancer comparing WPRT (n=287) versus ICBT followed by chemotherapy (carboplatin + paclitaxel 3 cycles, n=291), distant-recurrence rates were similar between the two arms (11.1% vs. 8.2%). It may be a future interest to analyze the data by stratifying depth and extent of LVSI based on our results to see if the study validates an impact of LVSI patterns on distant-recurrence and if chemotherapy reduces the risk of distant-recurrence in deep and extensive LVSI cases.
A strength of the study is that this study provides quantitative measurement of LVSI correlating to outcome in endometrial cancer. Potential weakness of the study is that this is a retrospective study that may have missed potential confounding factors. For instance, not all cases underwent systematic lymphadenectomy during surgical staging. In addition, sample size for the case group is relatively small. A limitation of the study is that additional immunohistochemistry study was not able to perform to distinguish lymphatic and vascular tumor involvements for LVSI.
CONCLUSION
Characterizing LVSI patterns by depth and quantification is a useful approach to identify a subgroup of endometrial cancer patients at high risk of distant-recurrence, suggesting that routine evaluation of LVSI patterns may be an important consideration in practice. Adaptation of LVSI patterns into surgical strategy for lymphadenectomy or indication for postoperative chemotherapy may potentially change patient outcome of endometrial cancer and thus further development will be warranted.
Supplementary Material
Acknowledgments
Grant sponsor: Ensign Endowment for Ovarian Cancer Research (KM and LDR).
Footnotes
Conflict of interest: None.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of this article at the publisher’s web-site.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A: Cancer statistics, 2015. CA Cancer J Clin 2015;65:5–29. [DOI] [PubMed] [Google Scholar]
- 2.Wright JD, Barrena Medel NI, et al. : Contemporary management of endometrial cancer. Lancet 2012;379:1352–1360. [DOI] [PubMed] [Google Scholar]
- 3.Chernofsky MR, Felix JC, Muderspach LI, et al. : Influence of quantity of lymph vascular space invasion on time to recurrence in women with early-stage squamous cancer of the cervix. Gynecol Oncol 2006;100:288–293. [DOI] [PubMed] [Google Scholar]
- 4.Lim CS, Alexander-Sefre F, Allam M, et al. : Clinical value of immunohistochemically detected lymphovascular space invasion in early stage cervical carcinoma. Ann Surg Oncol 2008;15: 2581–2588. [DOI] [PubMed] [Google Scholar]
- 5.Memarzadeh S, Natarajan S, Dandade DP, et al. : Lymphovascular and perineural invasion in the parametria: A prognostic factor for early-stage cervical cancer. Obstet Gynecol 2003;102:612–619. [DOI] [PubMed] [Google Scholar]
- 6.Morice P, Piovesan P, Rey A, et al. : Prognostic value of lymphovascular space invasion determined with hematoxylineosin staining in early stage cervical carcinoma: Results of a multivariate analysis. Ann Oncol 2003;14:1511–1517. [DOI] [PubMed] [Google Scholar]
- 7.Roman LD, Felix JC, Muderspach LI, et al. : Influence of quantity of lymph-vascular space invasion on the risk of nodal metastases in women with early-stage squamous cancer of the cervix. Gynecol Oncol 1998;68:220–225. [DOI] [PubMed] [Google Scholar]
- 8.Sakuragi N, Takeda N, Hareyama H, et al. : A multivariate analysis of blood vessel and lymph vessel invasion as predictors of ovarian and lymph node metastases in patients with cervical carcinoma. Cancer 2000;88:2578–2583. [PubMed] [Google Scholar]
- 9.Matsuo K, Mabuchi S, Okazawa M, et al. : Utility of risk-weighted surgical-pathological factors in early-stage cervical cancer. Br J Cancer 2013;108:1348–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hachisuga T, Kaku T, Fukuda K, et al. : The grading of lymphovascular space invasion in endometrial carcinoma. Cancer 1999;86:2090–2097. [PubMed] [Google Scholar]
- 11.O’Brien DJ, Flannelly G, Mooney EE, et al. : Lymphovascular space involvement in early stage well-differentiated endometrial cancer is associated with increased mortality. BJOG 2009;116: 991–994. [DOI] [PubMed] [Google Scholar]
- 12.Tsuruchi N, Kaku T, Kamura T, et al. : The prognostic significance of lymphovascular space invasion in endometrial cancer when conventional hemotoxylin and eosin staining is compared to immunohistochemical staining. Gynecol Oncol 1995;57:307–312. [DOI] [PubMed] [Google Scholar]
- 13.Guntupalli SR, Zighelboim I, Kizer NT, et al. : Lymphovascular space invasion is an independent risk factor for nodal disease and poor outcomes in endometrioid endometrial cancer. Gynecol Oncol 2011;124:31–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watanabe Y, Satou T, Nakai H, et al. : Evaluation of parametrial spread in endometrial carcinoma. Obstet Gynecol 2010;116: 1027–1034. [DOI] [PubMed] [Google Scholar]
- 15.Matsuo K, Sheridan TB, Yoshino K, et al. : Significance of lymphovascular space invasion in epithelial ovarian cancer. Cancer Med 2013;1:156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsuo K, Sheridan TB, Mabuchi S, et al. : Estrogen receptor expression and increased risk of lymphovascular space invasion in high-grade serous ovarian carcinoma. Gynecol Oncol 2014;133: 473–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsuo K, Yoshino K, Hiramatsu K, et al. : Effect of lymphovascular space invasion on survival of stage I epithelial ovarian cancer. Obstet Gynecol 2014;123:957–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsuo K, Yoshino K, Hasegawa K, et al. : Survival outcome of stage I ovarian clear cell carcinoma with lympho-vascular space invasion. Gynecol Oncol 2015;136:198–204. [DOI] [PubMed] [Google Scholar]
- 19.Keys HM, Roberts JA, Brunetto VL, et al. : A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: A Gynecologic Oncology Group study. Gynecol Oncol 2004;92:744–751. [DOI] [PubMed] [Google Scholar]
- 20.Matsuo K, Cahoon SS, Gualtieri M, et al. : Significance of adenomyosis on tumor progression and survival outcome of endometrial cancer. Ann Surg Oncol 2014;21:4246–4255. [DOI] [PubMed] [Google Scholar]
- 21.Matsuo K, Gray MJ, Yang DY, et al. : The endoplasmic reticulum stress marker, glucose-regulated protein-78 (GRP78) in visceral adipocytes predicts endometrial cancer progression and patient survival. Gynecol Oncol 2013;128:552–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsuo K, Opper NR, Ciccone MA, et al. : Time interval between endometrial biopsy and surgical staging for type I endometrial cancer: Association between tumor characteristics and survival outcome. Obstet Gynecol 2015;125:424–433. [DOI] [PubMed] [Google Scholar]
- 23.Matsuo K, Yessaian AA, Lin YG, et al. : Predictive model of venous thromboembolism in endometrial cancer. Gynecol Oncol 2013;128:544–551. [DOI] [PubMed] [Google Scholar]
- 24.Matsuo K, Hom MS, Moeini A, et al. : Significance of monocyte counts on tumor characteristics and survival outcome of women with endometrial cancer. Gynecol Oncol 2015;138:332–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsuo K, Sheridan TB, Yoshino K, et al. : Significance of lymphovascular space invasion in epithelial ovarian cancer. Cancer Med 2012;1:156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pecorelli S: Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet 2009;105: 103–104. [DOI] [PubMed] [Google Scholar]
- 27.Chan JK, Kapp DS, Cheung MK, et al. : The impact of the absolute number and ratio of positive lymph nodes on survival of endometrioid uterine cancer patients. Br J Cancer 2007;97:605–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yost KJ, Cheville AL, Al-Hilli MM, et al. : Lymphedema after surgery for endometrial cancer: Prevalence, risk factors, and quality of life. Obstet Gynecol 2014;124:307–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koh WJ, Greer BE, Abu-Rustum NR, et al. : Uterine neoplasms, version 1.2014. J Natl Compr Canc Netw 2014;12:248–280. [DOI] [PubMed] [Google Scholar]
- 30.Kumar S, Bandyopadhyay S, Semaan A, et al. : The role of frozen section in surgical staging of low risk endometrial cancer. PLoS ONE 2011;6:e21912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karabagli P, Ugras S, Yilmaz BS, et al. : The evaluation of reliability and contribution of frozen section pathology to staging endometrioid adenocarcinomas. Arch Gynecol Obstet 2015;292: 391–397. [DOI] [PubMed] [Google Scholar]
- 32.Kumar S, Medeiros F, Dowdy SC, et al. : A prospective assessment of the reliability of frozen section to direct intraoperative decision making in endometrial cancer. Gynecol Oncol 2012;127:525–531. [DOI] [PubMed] [Google Scholar]
- 33.Bogani G, Cromi A, Serati M, et al. : Chemotherapy reduces para-aortic node recurrences in endometrial cancer with positive pelvic and unknown para-aortic nodes. Int J Gynecol Cancer 2015;25: 263–268. [DOI] [PubMed] [Google Scholar]
- 34.Klopp A, Smith BD, Alektiar K, et al. : The role of postoperative radiation therapy for endometrial cancer: Executive summary of an American Society for Radiation Oncology evidence-based guideline. Pract Radiat Oncol 2014;4:137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deleon MC, Ammakkanavar NR, Matei D: Adjuvant therapy for endometrial cancer. J Gynecol Oncol 2014;25:136–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.