Abstract
Ovarian clear cell carcinoma (OCCC) is distinctive from other histological types of epithelial ovarian cancer, with genetic/epigenetic alterations, a specific immune-related molecular profile, and epidemiologic associations with ethnicity and endometriosis. These findings allow for the exploration of unique and specific treatments for OCCC. Two major mutated genes in OCCC are PIK3CA and ARID1A, which are frequently coexistent with each other. Other genes' alterations also contribute to activation of the PI3K (e.g. PIK3R1 and PTEN) and dysregulation of the chromatin remodeling complex (e.g. ARID1B, and SMARKA4). Although the number of focal copy number variations is small in OCCC, amplification is recurrently detected at chromosome 20q13.2 (including ZNF217), 8q, and 17q. Both expression and methylation profiling highlight the significance of adjustments to oxidative stress and inflammation. In particular, up-regulation of HNF-1β resulting from hypomethylation contributes to the switch from anaerobic to aerobic glucose metabolism. Additionally, up-regulation of HNF-1β activates STAT3 and NF-κB signaling, and leads to immune suppression via production of IL-6 and IL-8. Immune suppression may also be induced by the increased expression of PD-1, Tim-3 and LAG3. Mismatch repair deficient (microsatellite instable) tumors as found in Lynch syndrome also induce immune suppression in some OCCC. In a recent phase II clinical trial in heavily-treated platinum-resistant ovarian cancer, two out of twenty cases with a complete response to the anti-PD-1 antibody, nivolumab, were OCCC subtypes. Thus, the immune-suppressive state resulting from both genetic alterations and the unique tumor microenvironment may be associated with sensitivity to immune checkpoint inhibitors in OCCC. In this review, we highlight recent update and progress in OCCC from both the genomic and immunologic points of view, addressing the future candidate therapeutic options.
Keywords: Ovarian clear cell carcinoma, Genomics, Neoantigen, Immune checkpoint, Immunotherapy, Oncogenesis
1. Background
Ovarian clear cell carcinoma (OCCC) is a relatively rare histological type of epithelial ovarian carcinoma (EOC) with a unique distribution pattern across ethnicity [1]. While OCCC accounts for <10% of EOC in North America and Europe, in Japan, it may be up to 25% [2,3]. The increased incidence of endometriosis in Japan may be associated with an increased incidence of OCCC, as the histologic subtypes of ovarian cancers associated with endometriosis are predominantly OCCC and endometrioid ovarian carcinoma (EMOC) [4].
Recently, it has been proposed that EOC be classified as either type I or type II [5]. OCCC, EMOC, mucinous ovarian carcinoma, and low-grade serous ovarian carcinoma are categorized as type I, while type II tumors are represented by high-grade serous ovarian carcinoma (HGSOC). The type II tumors are considered to arise from the distal fallopian tube, and show distinct genetic profiling as compared to OCCC [5,6]. For example, TP53 mutations are found in <10% of OCCC, while they occur in over 96% of HGSOC [7,8]. In addition, BRCA mutations (both germline and somatic) are mainly observed in type II tumors [9,10].
Even among type I tumors, OCCC showed a significantly distinct molecular profiling pattern from other histologic types. Therefore, highlighting genetic, genomic and immunologic profiling of OCCC will assist in the development of precise therapeutics for these tumors. In this review, we focus on molecular subtypes of OCCC highlighting recent findings from both genomic and immunologic profiling.
2. Oncogenesis of OCCC
Although various histology-specific characterizations of OCCC have been unveiled, its oncogenic process is not fully understood. Although both OCCC and EMOC are well-known to be endometriosis-associated, it is still unclear how these tumors differentiate into this distinct morphology (and biology) [5,11]. The cellular origin of OCCC is also controversial. Proposed sources include (i) endometrium, (ii) endometrial cysts (endometriosis-derived epithelial cells), (iii) ovarian surface epithelia, and (iv) fallopian tube-derived cells [3,5,11-14]. Of note, most of these same characteristics in OCCC have been observed in endometriotic cysts without malignancy.
Oxidative stress has been implicated in the pathophysiology of endometriosis, which causes a particular inflammatory microenvironment (Fig. 1). Dysregulation of immune cells have also been reported in endometriotic lesions [15]. Epigenetic modifications induced by oxidative stress have also been suggested to exist in endometriosis [16]. Moreover, common mutations in OCCC, including ARID1A, PIK3CA, KRAS, and PPP2R1A, have been frequently identified in endometriosis without cancer (Fig. 1) [17].
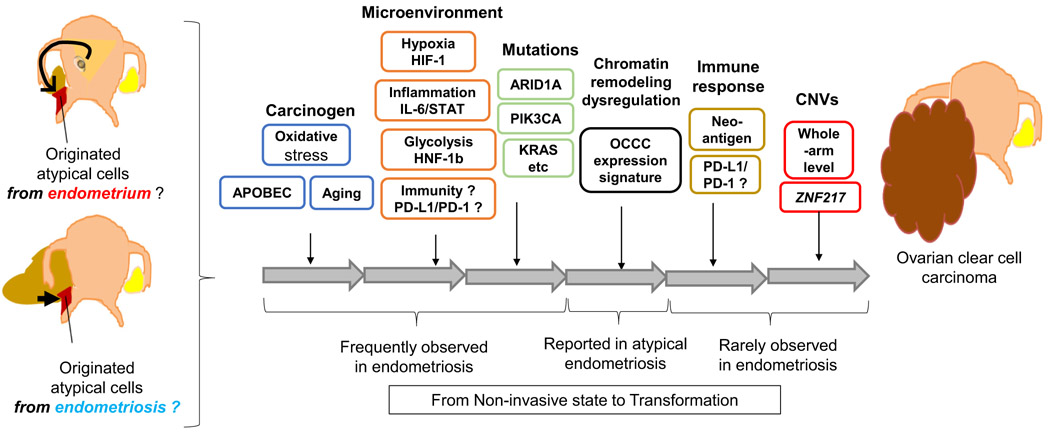
Fig. 1.
Proposed schema for oncogenesis of OCCC related to endometriosis. Various types of CCCC-specific microenvironment and genetic/epigenetic alterations may contribute to oncogenesis from the originated atypical cells (endometrium and/or possible endometriotic cells) to OCCC Exposure to the specific OCCC-related microenvironment such as oxidative stress, inflammation, glycolysis, and immune-suppressive state, possibly occurs before the malignant transformation. Genetic mutations may appear even in endometriotic cysts. The resultant gene disorders (induced by oncogenic activation of the PI3K, chromatin remodeling dysregulation, neoantigens, and etc.) and copy number variations (CNVs) may accelerate the cells to proceed to the malignant (invasive) state.
Loss of BAF250a (ARID1A gene) is also frequent in atypical endometriosis, suggesting its early contribution to the carcinogenesis [18]. Therefore, the majority of genomic/immunologic alterations may already exist before the transformation to OCCC. Overexpression of PD-L1 has not been reported yet in endometriosis, and copy number variations (CNVs) were rarely observed in endometriotic lesions [17], suggesting that acquisition of these biological characteristics may contribute to the transformation from non-invasive precursor lesion to OCCC (Fig. 1).
3. Genomic profiling of OCCC
3.1. Mutation profile of OCCC
Key molecules, pathways and molecular-targeted drugs are schematically summarized in Fig. 2. Two major mutated genes in OCCC are PIK3CA and ARID1A [19-21]. Oncogenic PIK3CA mutations activate the phosphatidylinositol 3-kinase (PI3K), whereas loss of function mutations in ARID1A, a component of the switch/sucrose non-fermentable (SWI/SNF) complex, results in dysregulation of chromatin remodeling [22,23]. The frequency of these mutations in OCCC is 40–62% for ARID1A and 33–51% for PIK3CA [24-26]. OCCC and EMOC showed a high frequency of mutations in PI3K, including PIK3CA and PTEN. PTEN mutations are less frequently observed in OCCC (~5%) than in EMOC (20%), whereas PIK3CA mutations are more commonly observed in OCCC than in EMOC (20%) [19,25,27]. Taken together with the high mutation frequency of ARID1A in EMOC (30%) [21], alterations in the PI3K pathway and the SWI/SNF complex are commonly shared in endometriosis-associated ovarian carcinomas.
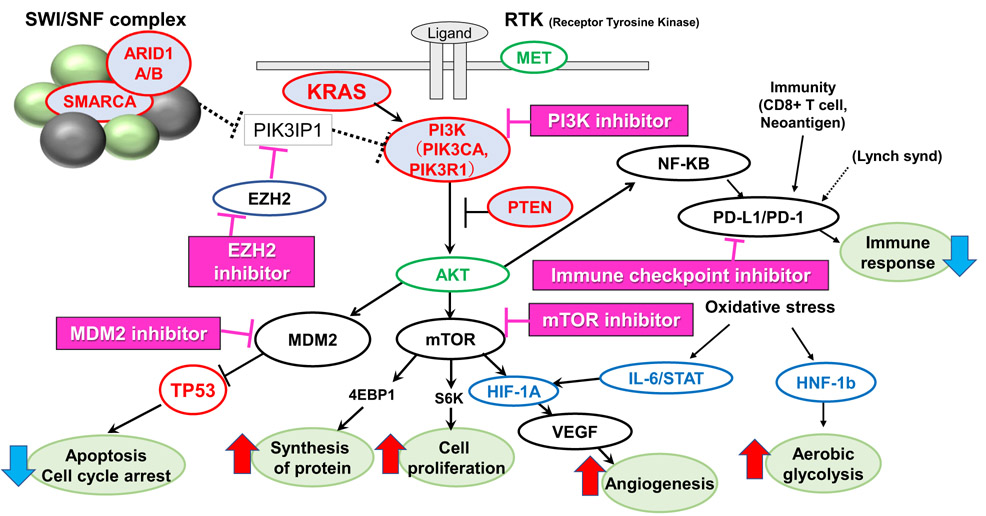
Fig. 2.
Candidate molecular targets and key pathways on basis of genomic characterization in OCCC. Frequently mutated genes (marked in red), frequently amplified genes (marked in green), and frequently up-regulated genes (marked in blue) cooperate to promote a unique cell survival advantage in OCCC. Genomic and/or immunologic-based candidate molecular targeted drugs are listed, which have been already approved or under clinical trials for other cancer types.
Mutational analysis by whole-exome sequencing in OCCC revealed other genetic mutations in the PI3K pathway and the SWI/SNF complex, such as ARID1B (10%), PIK3R1 (7–8%), and SMARKA4 (encoding ATP-dependent chromatic modeler BRG1) (5%) [24,25]. The other genes mutated in OCCC, which were also confirmed by whole-exome sequencing or targeted multiple gene panel testing, included PPP2R1A (encoding serine/threonine protein phosphatase 2 scaffold subunit alpha) (10–20%), KRAS (9–17%), TP53 (5–15%), and CTNNB1 (encoding betacatenin) (5–10%) [24-26,28,29].
3.2. Copy number variations of OCCC
Profiles of chromosomal CNVs in OCCC are also distinct from other histological subtypes [30,31]. Copy number analysis by single nucleotide polymorphism arrays revealed that the frequency of CNVs was significantly fewer in OCCC compared with that in HGSOC [32]. In contrast, the ratio of whole-arm CNVs among all CNVs (47%) in OCCC was significantly higher than that in HGSOC (21.6%). Thus, focal CNVs at the loci of specific genes were less frequent in OCCC than in HGSOC [32]. As whole-arm CNVs are associated with mitotic instability, each CNV might be less associated with the aberrant expression of cancer related genes in OCCC.
However, recurrent CNVs were identified at various loci [6,30,31]. At chromosome 20q13.2, including the ZNF217 (Zinc finger protein 217) locus, they were frequently amplified in OCCC (~36%). Amplification of chromosome 8 (8p11.21-q11.23 and 8q22.1-q24.13) was detected in 52% of OCCC [32]. Increased copy numbers of MET (chr7q31) (31%) and AKT2 (chr19q13.2) (24%) were also reported in OCCC (Fig. 2). Copy number loss (loss of heterozygosity or homozygous deletion) was detected at the loci of CDKN2A/2B (Cyclin-Dependent Kinase Inhibitor 2A/2B) (9p21.3) (17%) [33,34]. CNVs, evaluated by whole-exome sequencing, identified amplification at chr17q (46%) and deletion at chr13q (28%), 9q (21%) and 18q (21%) [25]. Although amplification of MET and AKT2 are potential candidate molecular targets, fewer CNVs at specific loci suggest that CNV-based targeted therapies may be limited in OCCC.
3.3. Expression signatures of OCCC
The gene expression profile of OCCC is also distinct from other histologic subtypes, especially as compared to HGSOC [32,35]. Expression arrays of OCCC and non-OCCC cell lines revealed that hepatocyte nuclear factor-1beta (HNF-1β) was the most abundantly up-regulated transcription factor in OCCC (Fig. 2) [35]. Overexpression of HNF-1β was also observed in 40% of endometriotic cysts without a malignancy [36]. Additional significantly up-regulated genes were identified in multiple microarray datasets, included versican (VCAN) and other genes related to oxidative stress [35]. Both oxidative stress-related and coagulation-related gene sets were up-regulated in OCCC, which is consistent with the increased frequency of endometriosis and venous thromboembolism in OCCC patients [37,38].
A set of 66 up-regulated genes was identified as a pathway network in OCCC. These genes included HNF-1β, HIF-1α, IL-6, p21, and Signal transducer and activator of transcription 3 (STAT3), highlighting the significance of the IL-6-STAT3-HIF pathway (Fig. 2) [35]. Glycogenrelated pathways were also enriched in this pathway network [35]. In endometriotic cysts, exposure to high concentrations of free iron induces persistent oxidative stress and may promote carcinogenesis [39]. The response to this persistent oxidative stress and inflammation may be reflected in the altered gene expression profile of OCCC.
Clear cell carcinoma is also a major histologic subtype in renal cell carcinoma (RCC). Hierarchical clustering by microarray data sets with various cancer types (merged data for the OCCC cell lines and NCI60 cell lines) discriminate a specific cluster enriched within both OCCC and RCC related to activation of HNF-1β and its downstream target genes [40]. Indeed, up-regulation of HIF-1α (by VHL mutations in RCC), amplification of VCAN, and a hypoxia-like mRNA expression signature are commonly observed in both OCCC and RCC [41-43]. Therefore, certain molecular targeted therapies against RCC that have not been tested in ovarian cancers or that have not demonstrated therapeutic benefit in unselected ovarian cancers may have efficacy if tested specifically for OCCC.
3.4. Epigenetic profiling of OCCC
Epigenetic alterations of OCCC are specific to histologic type. Hypo-methylation of HNF-1β is significantly detected in OCCC, suggesting that epigenetic silencing is one of the mechanisms of its overexpression [35,44]. Several other genes (14-3-3 sigma, TMS1/ASC, WT1, RASSF1A, CDH13, CACNA1A, HIN-1, and sFRP5) have also been reported as aberrantly methylated in OCCC [45-47]. Consensus clustering of DNA methylation profiles in ovarian cancer cell lines identified an OCCC-specific cluster, distinct from other histologic types [48]. In clinical samples, HGSOC was classified as a distinct cluster from non-HGSOC (type I). Sub-clustering of type I identified a specific methylation profile of OCCC, distinct from that of mucinous ovarian carcinoma and EMOC [48]. In OCCC, HNF-1 pathway genes (HNF-1A, HNF-1B, PAX8, and SGK2) were significantly hypomethylated, and the ER-α network genes were hypermethylated [48].
Unmethylated genes in OCCC (n = 22) were enriched for stress response-related gene ontology terms, while hyper-methylated genes in OCCC (n = 276) included “response to oxidative stress” gene ontology terms [48]. These data suggest that the expression profile of OCCC is closely associated with epigenetic regulation, possibly due to persistent oxidative stress. This unique epigenetic signature may lead to novel therapeutic strategies in OCCC.
3.5. Metabolic characteristics of OCCC
The Warburg effect is a phenomenon whereby tumor tissues tend to metabolize glucose to lactate, to a much greater degree than is seen in non-tumor cells (metabolization of glucose by glycolysis rather than oxidative phosphorylation) [49,50]. This is seen even under aerobic conditions. Although the mechanism of the Warburg effect in various cancer cells has not been fully clarified, there is growing evidence that high expression of HNF-1β plays an essential role in glucose metabolism [51]. Knocking down HNF-1β in OCCC cells significantly reduces the production of lactic acid (produced by anaerobic glycolysis) and increases that of citric acid (the first metabolite of the TCA cycle) indicating a switch from anaerobic to aerobic glucose metabolism [52].
Under hypoxic conditions, parental OCCC cells (with high HNF-1β) showed significant survival advantage, compared with HNF-1β-knockdown OCCC cells [52]. Overexpression of HNF-1β is also shown to reduce reactive oxygen species and contribute to protection of the cancer cells from the internal oxidative stress caused by the drastic changes in their cellular metabolism [50]. Thus, HNF-1β may be pivotal for cancer cell survival due to anti-stress effects, rather than increased proliferative potential. It is proposed that sustained high expression of HNF-1β cells may be associated with chemo-resistance in OCCC (Fig. 2) [50]. This can potentially be overcome, if aerobic glucose metabolism can be induced, or if HNF-1β-mediated anti-oxidative stress can be counteracted in these cancer cells.
3.6. Candidate molecular targets in OCCC
Based on the genetic and epigenetic alterations in OCCC, candidate molecular targets and possible molecular-targeted drugs are listed in Table 1. The PI3K pathway inhibitors may be considered for PIK3CA mutated (or PTEN mutated or PIK3R1 mutated) tumors, although clinical application of PI3K inhibitors has thus far been limited [53]. Copanlisib is a highly selective, pan-class I PI3K inhibitor, which preferentially inhibits p110α and p110δ isoforms, rather than the p110β and p110γ isoforms [54]. In 2017, copanlisib was approved for relapsed follicular lymphoma, in patients who have received at least two prior systemic therapies as a second FDA-approved PI3K Inhibitor [55]. As p110α is encoded by PIK3CA itself, this inhibitor may be a candidate drug for PIK3CA mutant OCCCs. Indeed, one endometrial cancer patient with coexistent mutations of PIK3CA and PTEN showed complete response to copanlisib [56].
Table 1.
Types of gene alterations and possible molecular targets in ovarian clear cell carcinoma.
| Genes | Type of alterations | Freq. (%) |
Targeted pathway/molecule(s) |
Drug availability | FDA approval (or clinical trials) | Reference |
|---|---|---|---|---|---|---|
| PIK3CA | Mutation | 51% | PI3K/mTOR | PI3K inhibitor (e.g., copanlisib) | Follicular lymphoma | [55] |
| PTEN | Mutation | 5% | ||||
| PIK3R1 | Mutation | 8% | mTORinhibitor (e.g., everolimus) | RCC and others | [99] | |
| ARID1A | Mutation Copy number loss | 60% | EZH2 | EZH2 inhibitor (e.g., Tazemetostat) | Phase I/II (solid tumors/B-cell lymphomas) | NCT01897571 |
| ARID1B | Mutation | 10% | ||||
| HNF-lbeta | Hypo-methylation Overexpression | >80% | Glucose metabolism? | Anti-diabetic? | N/A | N/A |
| HIF-lalpha | High expression | - | VEGF? | Bevacizumab | Ovarian cancer | [100] |
| MDM2 | High expression | - | MDM2-TP53 interaction | MDM2 inhibitor (RG-7112, DS-7423 etc.) | Phase I/II (solid tumors) | NCT03362723, NCT01877382 |
| NF-kB | - | N/A | N/A | |||
| PD-1/PD-L1 | Mutation (Lynch synd.) | 2–3% | MSI-high, MMR-deficient | Checkpoint inhibitor (e.g., pembrolizumab) | MSI-high/MMR-deficient solid tumor | [101] |
| PD-1/PD-L1 | High expression | - | PD-1/PD-L1 | Checkpoint inhibitor (e.g., nivolumab) | RCC, melanoma, lung cancer, gastric cancer | NCT03405454 NCT03355976 |
Frequently altered genes, their relevant molecules and pathways, candidate molecular-targeted drugs and their availability are listed. Multiple types of alterations may exist in a single gene with their correlation (i.e. mutation and copy number loss, hypo-methylation and overexpression).
We previously reported that a PI3K/mTOR dual inhibitor, DS-7423, showed anti-tumor activity in OCCC cell lines [57]. Therefore, the PI3K pathway remains a promising therapeutic target in OCCC. Inhibiting enhancer of zeste homolog 2 (EZH2) methyltransferase activity was reported to induce epigenetically synthetic lethality in ARID1A-mutated cancers by targeting of EZH2 methyltransferase activity in ARID1A-mutated cancers [58]. PIK3IP1 was found to be up-regulated by EZH2 inhibition as a direct target of ARID1A and EZH2, and it contributed to lethality through inhibition of PI3K signaling [58]. As several clinical trials with EZH2 inhibitors are ongoing for B-cell Lymphomas, as well as solid tumors [59], OCCC may be a good candidate of EZH2 inhibitors (Fig. 2).
MDM2 is a ubiquitin ligase, which degrades wild-type TP53 via proteasome-mediated ubiquitination [57]. We previously reported that the expression level of MDM2 in OCCC is significantly higher than that in HGSOC by expression arrays [60]. In addition, a MDM2 inhibitor, RG7112, showed anti-tumor effect and induced apoptosis of OCCC cells via TP53 activation [60]. MDM2 is known to be phosphorylated and activated by AKT (PI3K) signaling, and we showed that the PI3K pathway inhibition by DS-7423 dephosphorylated MDM2 and induced TP53-mediated apoptosis in OCCC cells [57]. Taken together, TP53 activation via suppression of MDM2 may be a possible therapeutic strategy against OCCC (Fig. 2), as clinical trials of MDM2 inhibitors are currently ongoing for solid tumors [61]. Immune checkpoint inhibitors are also promising in OCCC and are discussed in the following section.
4. Immunological aspects of OCCC
4.1. Immunobiology of OCCC
Several reports demonstrate that activation of oncogenes (MYC, RAS, PI3K) or inactivation of tumor suppressor genes (p53, PTEN, STAT3) induces an immune-suppressive state in the tumor microenvironment [62-66]. HNF-1β is preferentially activated in OCCC and has been reported to contribute to various malignant features including metastases, altered glucose metabolism and immune suppression via production of IL-6 and IL-8 through activated STAT-3 signaling as well as via NF-κB dependent pathways (Fig. 3) 67,68]. High levels of IL-6, IL-8, and overexpression of NF-κB related signaling in both serum and cancer ascites have been associated with a poor clinical outcome in ovarian cancer [67]. In all ovarian cancer including OCCC [69], NF-κB signal can also induce programmed death ligand 1 (PD-L1 and B7-H1), an inhibitory costimulating B7 family molecule (see Section 4.5 for the detail) (Fig. 3). These findings show that OCCC has a unique immune microenvironment, and thus, immunotherapy may be an attractive strategy for its treatment.
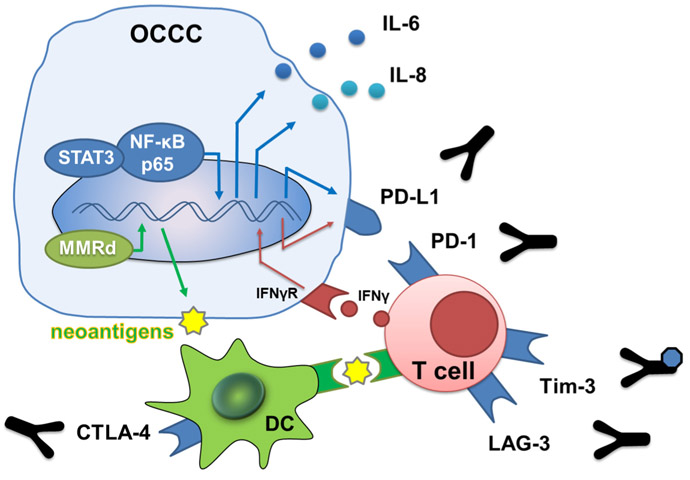
Fig. 3.
Immunobiology and target in OCCC. NF-κB and STAT3 induce immunosuppressive cytokine IL-6 and IL-8. NF-κB also induces PD-L1 on tumor cells. DNA mismatch repair deficient state increases the number of tumor mutation burden and neoantigen, and then dendritic cells and T cells recognize neoantigen and attack cancer cells. Activated immune cells produce IFN-γ and express PD-1, and then tumor cells express PD-L1 via IFN-γ/STAT1 signal. *DC, dendritic cell; MMR, DNA mismatch repair deficient; IFN-γ, interferon gamma; and IFN-γR, interferon gamma receptor. Y-shape figure indicates antibody.
4.2. Immune-related expression gene profile of OCCC
The gene expression profile signature of OCCC in a previous report identified up-regulation of IL-6, STAT3 related genes, as well as other inflammatory cytokines and immune-related genes which is suggestive of an immune-suppressive microenvironment [35]. Another report of the OCCC gene expression profile demonstrated that effector memory CD8+ T cell phenotype were overexpressed in tumors in stage I-II OCCC, as were cytotoxic T lymphocyte-associated antigen-4 (CTLA-4), PD-1, T cell immunoglobulin and mucin-domain containing-3 (Tim-3), and lymphocyte-activation gene-3 (LAG3) genes [70]. At the same time, expression of human leukocyte antigens (HLA) -A, -B, and -C were decreased. These changes result in an immune-suppressive microenvironment which may serve as a promising therapeutic target in OCCC.
4.3. Cancer antigen and application of cancer vaccine in OCCC
Several antigens have been identified in ovarian cancers which might allow for the development of a cancer vaccine therapy; however OCCC has yet been to be included in this spectrum. Glypican-3 (GPC3), a member of the glypican family of heparan sulfate proteoglycansone, is a potentially useful carcinoembryonic antigen for cancer vaccine immunotherapy and is overexpressed in both hepatocellular carcinoma and OCCC [71]. HLA-A24-restricted GPC3298-306 (EYILSLEEL) and HLAA2-restricted GPC3144–152 (FVGEFFTDV) peptides, are able to induce GPC3-reactive cytotoxic T cells without inducing autoimmunity [72]. In a small study, OCCC patients were treated with a GPC3-derived peptide vaccine, and the overall response rate was reported as 9.4% (2 partial response [PR] and 1 stable disease [SD]) with the disease control rate 17.9% in 32 patients [73].
4.4. Neoantigens in OCCC
With the success of immune checkpoint blockade therapy, increased attention has been paid to neoantigens. Neoantigens are derived from tumor-specific mutations, and because they are foreign to the host immune system, they can be potential targets for anti-tumor immunotherapy. Recent data has shown that these neoantigens make up a large part of the functional targets of immune checkpoint blockade therapy [74]. Very few reports are currently available regarding immune-targeting of neoantigens in OCCC. Matsushita and his colleagues assessed the neoantigen load, the depletion of expected antigenic mutations and immune signatures in 74 cases of OCCC using data from exome sequencing and expression arrays [70]. They found that the number of predicted neoantigens assessed in the tumor did not correlate with clinical outcomes, but the number of neoantigens per missense mutation did correlate with clinical outcomes.
Those with a lower number of neoantigens per missense mutation showed better clinical outcomes and demonstrated a phenotype consistent with T cell-mediated inflammation. This suggests that the cellular immune response functioned to eliminate neoantigen expressing subclones in tumors with a lower number of neoantigens per missense mutation. In contrast, decreased HLA class I expression as well as increased ratios of PD-1, Tim-3 and LAG3 were observed in tumors with higher number of neoantigens per missense mutation and worse clinical outcomes [70].
4.5. Immune checkpoint signals and DNA mismatch repair deficiency in OCCC
Although several immunological mechanisms contribute to immune suppression in the tumor microenvironment, the immune checkpoint signal PD-1/PD-L1 plays a central role in many cancer types [75,76]. The success of PD-1/PD-L1 inhibitors in various types of malignancies (such as RCC) has led to the expectation that they will be useful immunotherapy targets for gynecologic tumors including OCCC [77-80]. Some reports have identified the expression of PD-1 or/and PD-L1 on tumor cells and tumor-infiltrating lymphocytes (TILs) in OCCC and a correlation between these and clinical outcome [81,82].
Microsatellite instability (MSI) high tumors are associated with an enriched tumor mutation burden and a highly immunogenic phenotype. These tumors, including colorectal and endometrial cancer, which are also characterized as MSI high or mismatch repair deficient, are highly responsive to anti-tumor checkpoint inhibitors [83]. Women with Lynch syndrome, and a germline mutation of the mismatch repair genes (i.e. MLH1, MSH2, MSH6, and PMS2), have an increased life-time risk of colorectal, endometrial and ovarian cancer [84]. Lynch syndrome-associated ovarian cancer includes OCCC as well as EMOC [85]. Therefore, Lynch syndrome should also be taken into consideration with OCCC patients, especially with a Lynch syndromerelated family history. In a previous clinical sample analysis, PD-L1 expression was found in 43% of OCCC tumors, including 67% of OCCC with mismatch repair defects (Fig. 2) [86]. PD-L1 expression is common in mismatch repair proficient tumors and there is no correlation between PD-L1 expression and mismatch repair status in OCCC. Nevertheless, PD-L1 expression is prevalent in mismatch repair-intact OCCC [86].
Finally, OCCC with MSI exhibited a high number of CD8+ TILs and higher PD-1 expressing TILs compared with microsatellite stable (MSS) OCCC. PD-L1 expression in tumor cells or immune cells was also noted in all cases of OCCCs with MSI. MSI in specific subsets of OCCC was associated with endometriosis and ARID1A/BAF250A loss [82]. These observations may indicate an alternative therapeutic option for a subpopulation of the patients with OCCC.
4.6. Clinical response of immune checkpoint blockade for OCCC
In a recent phase II trial of pembrolizumab for recurrent ovarian cancer (KEYNOTE-100), anti–PD-1 antibody pembrolizumab for recurrent ovarian cancer (>300 patients) demonstrated that while the overall response rate of all cohorts was low (~8%), the response rate of clear cell histology was 15.8% [87]. Another phase II clinical trial of the anti-PD-1 antibody nivolumab for heavily-treated platinum-resistant recurrent ovarian cancer, included 10% with a complete response (CR) demonstrating a durable antitumor effect (2 out of 20) [88,89]. One of these CR patients had OCCC histologic subtype and another was diagnosed as having an OCCC-like phenotype following additional gene expression profile analysis of the tumor. Further experiments revealed that these two CR patients exhibited MSS by immunohistochemistry and wild-type ARID1A by exome sequencing (unpublished data).
In an anti-PD-1 antibody trial with pembrolizumab, an exceptional complete responder with chemo-/radiation-resistant OCCC was reported, and genomic analysis revealed a PD-L1-genetic rearrangement in the tumor sample [90]. In a phase I study of the anti-PD-L1 antibody avelumab in recurrent ovarian cancer, both patients with OCCC exhibited a PR [91]. In a phase I study of the anti-PD-L1 antibody durvalumab in combination with the poly (ADP-ribose) polymerase inhibitor olaparib for recurrent ovarian cancer, only one patient with OCCC was enrolled and exhibited a PR [92]. While the sample size of OCCC in these clinical trials was small and further verification is needed, anti-PD-1 or -PD-L1 antibodies appear to be a powerful new therapeutic agent for patients with OCCC [93].
A phase II/III trial of nivolumab for recurrent ovarian cancer (NINJA trial, JapicCTI-153004; target accrual, n = 300) is ongoing, and this study may directly validate the antitumor effect of anti-PD-1 therapy specific for OCCC in the near future [89].
5. Clinical trials in OCCC
5.1. Non-immunotherapeutic approaches
Salient trials related to OCCC are outlined in Table 2. Not surprisingly, OCCC-focused clinical trials have thus far been very limited. A randomized phase III study, which compared the efficacy and safety of irinotecan plus cisplatin to standard paclitaxel plus carboplatin in patients with stage I-IV OCCC, was conducted by JGOG as an international inter-group trial (JGOG-3017), and the combination chemotherapy regimen with irinotecan and cisplatin did not show superiority to standard regimen with paclitaxel and carboplatin in those patients [94].
Table 2.
Clinical trials in OCCC.
| Drug/combination | Molecular targets |
Study name | RCT | Phase | Status | Summary | Reference |
|---|---|---|---|---|---|---|---|
| Combination chemotherapy | |||||||
| Irinotecan + cisplatin | - | JGOG3017 | Yes | III | Completed | Non-taxane combination chemotherapy was not superior to standard paclitaxel + carboplatin | [94] |
| Targeted therapy | |||||||
| Temsirolimus + paclitaxel + carboplatin | mTOR | GOG268 | No | II | Completed | Temsirolimus + paclitaxel + carboplatin followed by temsirolimus consolidation was not superior to historical controls | [95] |
| Sunitinib | VEGFR, PDGFR | GOG254 | No | II | Completed | Minimal clinical activity | [96] |
| Nintedanib | VEGFR, PDGFR, FGFR | NiCCC (ENGOT-GYN1) | Yes | II | Ongoing | Nintedanib vs physician’s choice chemotherapy | NCT02866370 |
| Cabozantinib | MET, RET, VEGFR2 | NRG-GY001 | No | II | Completed | Minimal clinical activity | [97] |
| ENMD-2076 | Aurora A, VEGFR, FGFR | - | No | II | Completed | Median PFS 3.7 months. Loss of ARID1A was correlated with better PFS | [98] |
| CDX-014 | TIM-1 | - | No | I | Ongoing | A dose-escalation safety and activity study | NCT02837991 |
| Immunotherapy | |||||||
| Durvalumab | PD-L1 | MOCCA | Yes | II | Ongoing | Durvalumab vs physician’s choice chemotherapy | NCT03405454 |
| Nivolumab ± ipilimumab | PD-1, CTLA4 | BrUOG 354 | Yes | II | Ongoing | Nivolumab vs nivolumab + ipilimumab | NCT03355976 |
The mTOR-AKT pathway in OCCC is a candidate for therapeutic targeting, and Farley et al., reported the results of phase II study (GOG-268) in the 2016 ASCO meeting [95]. This study evaluated temsirolimus in combination with carboplatin and paclitaxel followed by temsirolimus consolidation as the first-line therapy in the treatment of stage III-IV OCCC. Nearly half (54%) of those with optimal debulking had a progression-free survival (PFS) longer than 12 months, however, this was not statistically significantly increased as compared to historical controls [95].
Multiple targeted tyrosine kinase inhibitors (TKI) have also been tested in OCCC. GOG-254, was a phase II trial of SU11248 (sunitinib), an oral multi-targeted tyrosine kinase inhibitor. This agent was studied for its efficacy and tolerability in persistent or recurrent OCCC (NCT00979992). The results were published recently and showed that the median PFS and overall survival (OS) was 2.7 and 12.8 months, respectively [96]. Sunitinib demonstrated minimal activity in those patients. Another TKI, BIBF1120 (nintedanib) is currently being tested in patients with relapsed OCCC (NCT02866370).
Cabozantinib, a MET, VRGFR2 and RET TKI, was also evaluated recently in patients with recurrent OCCC (NRG-GY001). No objective responses were observed. The median PFS and OS were 3.6 and 8.1 months, respectively. Cabozantinib showed minimal clinical activity in those patients [97]. ENMD-2076, an oral active kinase inhibitor, targeting the mitotic kinase Aurora A, VEGFRs, and FGFRs, was investigated in recurrent OCCC (NCT01914510). Somatic mutations in ARID1A, a key component of the SWI/SNF chromatin remodeling complex, may result in up-regulation and overexpression of Aurora A. Lheureux et al. presented the efficacy of ENMD-2076 at the 2017 ASCO meeting which showed that the median PFS was 3.7 months, and 6-months PFS rate was 20% for the evaluable patients (31% in ARID1A loss and 12% in ARID1A positive patients) [98].
T cell immunoglobulin mucin-1 (TIM-1) expression is up-regulated in several human cancers, most notably in RCC and OCCC but has minimum expression in normal tissues. A dose escalation trial of an antibody-drug conjugate, CDX-014 that targets TIM-1 and is linked to a potent cytotoxic, monomethyl auristatin E (NCT02837991), is currently under investigation in patients with advanced or metastatic RCC and OCCC.
5.2. Immunotherapeutic approaches
In a recent list of clinical trials for ovarian cancers including OCCC, over 50 are related to immunotherapies including immune checkpoint inhibitors, immune-modulating drugs, and T cell-engineered therapies either as a single agent or in combination with a traditional chemotherapeutic or a different immunotherapy. OCCC-specific clinical trials with immunotherapies are also listed in Table 2, including durvalumab (NCT03405454), a combination treatment of nivolumab and the anti-CTLA4 antibody ipilimumab (NCT03355976). Further studies characterizing the immunological, molecular, and genetic make-up of OCCC holds promise to open the door for more personalized treatment using specifically targeted immunotherapies.
6. Conclusion
OCCC is a distinct type of tumor from other histologic types of EOC. The compiled information regarding genetic/epigenetic disorders, expression profiling, glycolytic modification, and identified alterations in the immune-related response may shed light on the novel therapeutic options in OCCC. It may be categorized as one of rare tumors with an ethnicity-related distribution, however, candidate molecular targets generally overlap with various types of tumors from other organs (especially with RCC). This should allow for the testing of diverse molecular-targeted drugs in basket clinical trials. Additionally, clinical sequencing in OCCC may identify various types of actionable mutations and prove helpful in the development of precision medicine against ovarian cancer.
HIGHLIGHTS.
OCCC is distinct from other ovarian cancers in its genetic, epigenetic, metabolomic and immunologic profile.
Epigenetic/metabolomic modifications contribute to cell survival against oxidative stress.
A unique immune microenvironment causes immune-suppressive state in OCCC.
Genetic, epigenetic, metabolomic and immunologic differences of OCCC can be used to design treatments specific to OCCC
Acknowledgement
We thank Brendan H. Grubbs, MD, for his scientific input for this manuscript.
Funding support
Grants-in-Aid for Scientific Research (C) 18K09249 (K.O.), and 16K11152 (K.H.). Ensign Endowment for Gynecologic Cancer Research (K.M.).
Footnotes
Disclosure statement
K.O. received research grant from Daiichi-Sankyo Co., Ltd., lecture fee from Chugai Pharmaceutical Co., Ltd.; J.H. received research grant from MSD, Daiichi-Sankyo Co., Ltd., Ono Pharmaceutical Co., Ltd. and Dainihon-Sumitomo; K.H. received research grant from Pfizer Inc., Yakult Honsha Co., Ltd., OncoThreapy Science Inc., honoraria from Chugai Pharmaceutical Co., Ltd., Daiichi-Sankyo Co., Ltd., Ono Pharmaceutical Co., Ltd., Eisai Co., Ltd., Kyowa Hakko Kirin Co., Ltd., and Bayer Yakuhin Ltd., advisory role for Merck Sharp and Dohme K. K. None for others.
References
- [1].del-Carmen MG, Birrer M,Schorge JO, Clear cell carcinoma of the ovary: a review of the literature, Gynecol. Oncol 126 (2012) 481–490. [DOI] [PubMed] [Google Scholar]
- [2].Okamoto A, Glasspool RM, Mabuchi S, Matsumura N, Nomura H, Itamochi H, et al. , Gynecologic Cancer InterGroup (GCIG) consensus review for clear cell carcinoma of the ovary, Int. J. Gynecol. Cancer 24 (2014) S20–S25. [DOI] [PubMed] [Google Scholar]
- [3].Saito T, Takahashi F, Katabuchi H, Annual report of the Committee on Gynecologic Oncology, Japan Society of Obstetrics and Gynecology: patient annual report for 2014 and treatment annual report for 2009, J. Obstet. Gynaecol. Res 43 (2017) 1667–1677. [DOI] [PubMed] [Google Scholar]
- [4].Munksgaard PS,Blaakaer J, The association between endometriosis and ovarian cancer: a review of histological, genetic and molecular alterations, Gynecol. Oncol 124 (2012) 164–169. [DOI] [PubMed] [Google Scholar]
- [5].Kurman RJ, IeM Shih, The dualistic model of ovarian carcinogenesis: revisited, revised, and expanded, Am. J. Pathol 186 (2016) 733–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Mabuchi S, Sugiyama T, Kimura T, Clear cell carcinoma of the ovary: molecular insights and future therapeutic perspectives, J. Gynecol. Oncol 27 (2016) e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Genome Atlas Research Network Cancer, Integrated genomic analyses of ovarian carcinoma, Nature 474 (2011) 609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Oda K, Ikeda Y, Kashiyama T, Miyasaka A, Inaba K, Fukuda T, et al. , Characterization of TP53 and PI3K signaling pathways as molecular targets in gynecologic malignancies, J. Obstet. Gynaecol. Res 42 (2016) 757–762. [DOI] [PubMed] [Google Scholar]
- [9].Arts-de Jong M, de Bock GH, van Asperen CJ, Mourits MJ, JA de Hullu CM Kets, Germline BRCA1/2 mutation testing is indicated in every patient with epithelial ovarian cancer: a systematic review, Eur. J. Cancer 61 (2016) 137–145. [DOI] [PubMed] [Google Scholar]
- [10].Norquist BM, Harrell MI, Brady MF, Walsh T, Lee MK, Gulsuner S, et al. , Inherited mutations in women with ovarian carcinoma, JAMA Oncol. 2 (2016) 482–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Matias-Guiu X, Stewart CJR, Endometriosis-associated ovarian neoplasia, Pathology 50 (2018) 190–204. [DOI] [PubMed] [Google Scholar]
- [12].Kurman RJ, Shih IeM, Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer—shifting the paradigm, Hum. Pathol 42 (2011) 918–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Maeda D, Shih IeM, Pathogenesis and the role of ARID1A mutation in endometriosis-related ovarian neoplasms, Adv. Anat. Pathol 20 (2013) 45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wang Y, Mang M, Wang Y, Wang L, Klein R, Kong B, et al. , Tubal origin of ovarian endometriosis and clear cell and endometrioid carcinoma, Am. J. Cancer Res 5 (2015) 869–879. [PMC free article] [PubMed] [Google Scholar]
- [15].Cho YJ, Lee SH,Park JW, Han M, Park MJ, Han SJ, Dysfunctional signaling underlying endometriosis: current state of knowledge, J. Mol. Endocrinol 60 (2018) R97–R113. [DOI] [PubMed] [Google Scholar]
- [16].Ito F, Yamada Y, Shigemitsu A, Akinishi M, Kaniwa H, Miyake R, et al. , Role of oxidative stress in epigenetic modification in endometriosis, Reprod. Sci 24 (2017) 1493–1502. [DOI] [PubMed] [Google Scholar]
- [17].Anglesio MS, Papadopoulos N, Ayhan A, Nazeran TM, Noe M, Horlings HM, et al. , Cancer-associated mutations in endometriosis without cancer, N. Engl. J. Med 376 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Stamp JP, Gilks CB, Wesseling M, Eshragh S, Ceballos K, Anglesio MS, et al. , BAF250a expression in atypical endometriosis and endometriosis-associated ovarian cancer, Int. J. Gynecol. Cancer 26 (2016) 825–832. [DOI] [PubMed] [Google Scholar]
- [19].Kuo KT, L Mao T, Jones S, Veras E, Ayhan A, Wang TL, et al. , Frequent activating mutations of PIK3CA in ovarian clear cell carcinoma, Am. J. Pathol 174 (2009) 1597–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jones S, Wang TL, Shih IeM, Mao TL, Nakayama K, Roden R, et al. , Frequent mutations of chromatin remodeling gene ARID1A in ovarian clear cell carcinoma, Science 330 (2010) 228–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wiegand KC, Shah SP, Al-Agha OM, Zhao Y, Tse K, Zeng T, et al. , ARID1A mutations in endometriosis-associated ovarian carcinomas, N. Engl. J. Med 363 (2010) 1532–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Samuels Y, Ericson K, Oncogenic PI3K and its role in cancer, Curr. Opin. Oncol 18 (2006) 77–82. [DOI] [PubMed] [Google Scholar]
- [23].Wu JN, Roberts CW, ARID1A mutations in cancer: another epigenetic tumor suppressor? Cancer Discov. 3 (2013) 35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Itamochi H, Oishi T, Oumi N, Takeuchi S, Yoshihara K, Mikami M, et al. , Wholegenome sequencing revealed novel prognostic biomarkers and promising targets for therapy of ovarian clear cell carcinoma, Br. J. Cancer 117 (2017) 717–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Murakami R, Matsumura N, Brown JB, Higasa K, Tsutsumi T, Kamada M, et al. , Exome sequencing landscape analysis in ovarian clear cell carcinoma shed light on key chromosomal regions and mutation gene networks, Am. J. Pathol 187 (2017) 2246–2258. [DOI] [PubMed] [Google Scholar]
- [26].Kim SI,Lee JW, Lee M, Kim HS, Chung HH,Kim JW, et al. , Genomic landscape of ovarian clear cell carcinoma via whole exome sequencing, Gynecol. Oncol 148 (2018) 375–382. [DOI] [PubMed] [Google Scholar]
- [27].Campbell IG, Russell SE, Choong DY, Montgomery KG, L Ciavarella M, Hooi CS, et al. , Mutation of the PIK3CA gene in ovarian and breast cancer, Cancer Res. 64 (2004) 7678–7681. [DOI] [PubMed] [Google Scholar]
- [28].Maru Y, Tanaka N, Ohira M, Itami M, Hippo Y, Nagase H, Identification of novel mutations in Japanese ovarian clear cell carcinoma patients using optimized targeted NGS for clinical diagnosis, Gynecol. Oncol 144 (2017) 377–383. [DOI] [PubMed] [Google Scholar]
- [29].Shibuya Y, Tokunaga H, Saito S, Shimokawa K, Katsuoka F, Bin L, et al. , Identification of somatic genetic alterations in ovarian clear cell carcinoma with next generation sequencing, Genes Chromosom. Cancer 57 (2018) 51–60. [DOI] [PubMed] [Google Scholar]
- [30].Sung CO, Choi CH, Ko YH, Ju H, Choi YL, Kim N, et al. , Integrative analysis of copy number alteration and gene expression profiling in ovarian clear cell adenocarcinoma, Cancer Genet. 206 (2013) 145–153. [DOI] [PubMed] [Google Scholar]
- [31].Okamoto A, Sehouli J, Yanaihara N, Hirata Y, Braicu I, Kim BG, et al. , Somatic copy number alterations associated with Japanese or endometriosis in ovarian clear cell adenocarcinoma, PLoS One 10 (2015), e0116977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Uehara Y, Oda K, Ikeda Y, Koso T, Tsuji S, Yamamoto S, et al. , Integrated copy number and expression analysis identifies profiles of whole-arm chromosomal alterations and subgroups with favorable outcome in ovarian clear cell carcinomas, PLoS One 10 (2015), e0128066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Yamashita Y, Akatsuka S, Shinjo K, Yatabe Y, Kobayashi H, Seko H, et al. , Met is the most frequently amplified gene in endometriosis-associated ovarian clear cell adenocarcinoma and correlates with worsened prognosis, PLoS One 8 (2013), e57724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kuo KT, Mao TL, Chen X, Feng Y, Nakayama K, Wang Y, et al. , DNA copy numbers profiles in affinity-purified ovarian clear cell carcinoma, Clin. Cancer Res 16 (2010) 1997–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Yamaguchi K, Mandai M, Oura T, Matsumura N, Hamanishi J, Baba T, et al. , Identification of an ovarian clear cell carcinoma gene signature that reflects inherent disease biology and the carcinogenic processes, Oncogene 29 (2010) 1741–1752. [DOI] [PubMed] [Google Scholar]
- [36].Kato N, Sasou S, Motoyama T, Expression of hepatocyte nuclear factor-1beta (HNF-1beta) in clear cell tumors and endometriosis of the ovary, Mod. Pathol 19 (2006) 83–89. [DOI] [PubMed] [Google Scholar]
- [37].Diaz ES, Walts AE, Karlan BY, Walsh CS, Venous thromboembolism during primary treatment of ovarian clear cell carcinoma is associated with decreased survival, Gynecol. Oncol 131 (2013) 541–545. [DOI] [PubMed] [Google Scholar]
- [38].Matsuo K, Hasegawa K, Yoshino K, Murakami R, Hisamatsu T, Stone RL, et al. , Venous thromboembolism, interleukin-6 and survival outcomes in patients with advanced ovarian clear cell carcinoma, Eur. J. Cancer 51 (2015) 1978–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Yamaguchi K, Mandai M, Toyokuni S,Hamanishi J, Higuchi T, Takakura K, et al. , Contents of endometriotic cysts, especially the high concentration of free iron, are a possible cause of carcinogenesis in the cysts through the iron-induced persistent oxidative stress, Clin. Cancer Res 14 (2008) 32–40. [DOI] [PubMed] [Google Scholar]
- [40].Matsumura N, Mandai M, Okamoto T, Yamaguchi K, Yamamura S, Oura T, et al. , Sorafenib efficacy in ovarian clear cell carcinoma revealed by transcriptome profiling, Cancer Sci. 101 (2010) 2658–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Dondeti VR, Wubbenhorst B, Lal P, Gordan JD, D’Andrea K, Attiyeh EF, et al. , Integrative genomic analyses of sporadic clear cell renal cell carcinoma define disease subtypes and potential new therapeutic targets, Cancer Res. 72 (2012) 112–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Iida Y, Aoki K, Asakura T, Ueda K, Yanaihara N, Takakura S, et al. , Hypoxia promotes glycogen synthesis and accumulation in human ovarian clear cell carcinoma, Int. J. Oncol 40 (2012) 2122–2130. [DOI] [PubMed] [Google Scholar]
- [43].Ji JX, Wang YK, Cochrane DR, Huntsman DG, Clear cell carcinomas of the ovary and kidney: clarity through genomics, J. Pathol 244 (2018). [DOI] [PubMed] [Google Scholar]
- [44].Shen H, Fridley BL, Song H, Lawrenson K, Cunningham JM, Ramus SJ, et al. , Epigenetic analysis leads to identification of HNF1B as a subtype-specific susceptibility gene for ovarian cancer, Nat. Commun 4 (2013) 1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Terasawa K, Sagae S, Toyota M, Tsukada K, Ogi K, Satoh A, et al. , Epigenetic inactivation of TMS1/ASC in ovarian cancer, Clin. Cancer Res 10 (2004) 2000–2006. [DOI] [PubMed] [Google Scholar]
- [46].Asadollahi R, CA Hyde XY Zhong, Epigenetics of ovarian cancer: from the lab to the clinic, Gynecol. Oncol 118 (2010) 81–87. [DOI] [PubMed] [Google Scholar]
- [47].Ho CM, Huang CJ, Huang CY, Wu YY, Chang SF, Cheng WF, Promoter methylation status of HIN-1 associated with outcomes of ovarian clear cell adenocarcinoma, Mol. Cancer 11 (2012) 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Yamaguchi K, Huang Z, Matsumura N, Mandai M, Okamoto T, Baba T, et al. , Epigenetic determinants of ovarian clear cell carcinoma biology, Int. J. Cancer 135 (2014) 585–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Koppenol WH, Bounds PL, Dang CV, Otto Warburg's contributions to current concepts of cancer metabolism, Nat. Rev. Cancer 11 (2011) 325–337. [DOI] [PubMed] [Google Scholar]
- [50].Mandai M, Amano Y, Yamaguchi K, Matsumura N, Baba T, Konishi I, Ovarian clear cell carcinoma meets metabolism; HNF-1beta confers survival benefits through the Warburg effect and ROS reduction, Oncotarget 6 (2015) 30704–30714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Okamoto T, Mandai M, Matsumura N, Yamaguchi K, Kondoh H, Amano Y, et al. , Hepatocyte nuclear factor-1beta (HNF-1beta) promotes glucose uptake and glycolytic activity in ovarian clear cell carcinoma, Mol. Carcinog 54 (2015) 35–49. [DOI] [PubMed] [Google Scholar]
- [52].Amano Y, Mandai M, Yamaguchi K, Matsumura N, Kharma B, Baba T, et al. , Metabolic alterations caused by HNF1beta expression in ovarian clear cell carcinoma contribute to cell survival, Oncotarget 6 (2015) 26002–26017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Okkenhaug K, Graupera M, Vanhaesebroeck B, Targeting PI3K in cancer: impact on tumor cells, their protective stroma, angiogenesis, and immunotherapy, Cancer Discov. 6 (2016) 1090–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Liu N, Rowley BR, Bull CO, Schneider C, Haegebarth A, Schatz CA, et al. , BAY 80-6946 is a highly selective intravenous PI3K inhibitor with potent p110alpha and p110delta activities in tumor cell lines and xenograft models, Mol. Cancer Ther 12 (2013) 2319–2330. [DOI] [PubMed] [Google Scholar]
- [55].Janku F, Yap TA, Meric-Bernstam F, Targeting the PI3K pathway in cancer: are we making headway? Nat. Rev. Clin. Oncol 15 (2018) 273–291. [DOI] [PubMed] [Google Scholar]
- [56].Patnaik A, Appleman LJ, Tolcher AW, Papadopoulos KP, Beeram M, Rasco DW, et al. , First-in-human phase I study of copanlisib (BAY 80-6946), an intravenous pan-class I phosphatidylinositol 3-kinase inhibitor, in patients with advanced solid tumors and non-Hodgkin's lymphomas, Ann. Oncol 27 (2016) 1928–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Kashiyama T, Oda K, Ikeda Y, Shiose Y, Hirota Y, Inaba K, et al. , Antitumor activity and induction of TP53-dependent apoptosis toward ovarian clear cell adenocarcinoma by the dual PI3K/mTOR inhibitor DS-7423, PLoS One 9 (2014), e87220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Bitler BG, Aird KM, Garipov A, Li H, Amatangelo M, Kossenkov AV, et al. , Synthetic lethality by targeting EZH2 methyltransferase activity in ARID1A-mutated cancers, Nat. Med 21 (2015) 231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Italiano A, Soria JC, Toulmonde M, Michot JM, Lucchesi C, Varga A, et al. , Tazemetostat, an EZH2 inhibitor, in relapsed or refractory B-cell non-Hodgkin lymphoma and advanced solid tumours: a first-in-human, open-label, phase 1 study, Lancet Oncol. 19 (2018) 649–659. [DOI] [PubMed] [Google Scholar]
- [60].Makii C, Oda K, Ikeda Y, Sone K, Hasegawa K, Uehara Y, et al. , MDM2 is a potential therapeutic target and prognostic factor for ovarian clear cell carcinomas with wild type TP53, Oncotarget 7 (2016) 75328–75338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Wagner AJ, Banerji U, Mahipal A, Somaiah N, Hirsch H, Fancourt C, et al. , Phase I trial of the human double minute 2 inhibitor MK-8242 in patients with advanced solid tumors, J. Clin. Oncol 35 (2017) 1304–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Dong Y, Richards JA, Gupta R, Aung PP, Emley A, Kluger Y, et al. , PTEN functions as a melanoma tumor suppressor by promoting host immune response, Oncogene 33 (2014) 4632–4642. [DOI] [PubMed] [Google Scholar]
- [63].Lastwika KJ, Wilson W 3rd, Li QK, Norris J, Xu H, Ghazarian SR, et al. , Control of PD-L1 expression by oncogenic activation of the AKT-mTOR pathway in non-small cell lung cancer, Cancer Res. 76 (2016) 227–238. [DOI] [PubMed] [Google Scholar]
- [64].Guo G, Yu M, Xiao W, Celis E, Cui Y, Local activation of p53 in the tumor microenvironment overcomes immune suppression and enhances antitumor immunity, Cancer Res. 77 (2017) 2292–2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Coelho MA, de Carne Trecesson S, Rana S, Zecchin D, Moore C, Molina-Arcas M, et al. , Oncogenic RAS signaling promotes tumor immunoresistance by stabilizing PD-L1 mRNA, Immunity 47 (2017) 1083–1099.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Seton-Rogers S, Oncogenes: driving immune evasion, Nat. Rev. Cancer 18 (2018) 67. [DOI] [PubMed] [Google Scholar]
- [67].Nishio H, Yaguchi T, Sugiyama J, Sumimoto H, Umezawa K, Iwata T, et al. , Immunosuppression through constitutively activated NF-kappaB signalling in human ovarian cancer and its reversal by an NF-kappaB inhibitor, Br. J. Cancer 110 (2014) 2965–2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Yaguchi T, Kawakami Y, Cancer-induced heterogeneous immunosuppressive tumor microenvironments and their personalized modulation, Int. Immunol 28 (2016) 393–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Peng J, Hamanishi J, Matsumura N, Abiko K, Murat K, Baba T, et al. , Chemotherapy induces programmed cell death-ligand 1 overexpression via the nuclear factor-kappaB to foster an immunosuppressive tumor microenvironment in ovarian cancer, Cancer Res. 75 (2015) 5034–5045. [DOI] [PubMed] [Google Scholar]
- [70].Matsushita H, Hasegawa K, Oda K, Yamamoto S, Nishijima A, Imai Y, et al. , The frequency of neoantigens per somatic mutation rather than overall mutational load or number of predicted neoantigens per se is a prognostic factor in ovarian clear cell carcinoma, Oncoimmunology 6 (2017), e1338996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Komori H, Nakatsura T, Senju S, Yoshitake Y, Motomura Y, Ikuta Y, et al. , Identification of HLA-A2- or HLA-A24-restricted CTL epitopes possibly useful for glypican-3-specific immunotherapy of hepatocellular carcinoma, Clin. Cancer Res 12 (2006) 2689–2697. [DOI] [PubMed] [Google Scholar]
- [72].Motomura Y, Ikuta Y, Kuronuma T, Komori H, Ito M, Tsuchihara M, et al. , HLA-A2 and -A24-restricted glypican-3-derived peptide vaccine induces specific CTLs: preclinical study using mice, Int. J. Oncol 32 (2008) 985–990. [PubMed] [Google Scholar]
- [73].Suzuki S, Sakata J, Utsumi F, Sekiya R, Kajiyama H, Shibata K, et al. , Efficacy of glypican-3-derived peptide vaccine therapy on the survival of patients with refractory ovarian clear cell carcinoma, Oncoimmunology 5 (2016), e1238542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Riaz N, Morris L, Havel JJ, Makarov V, Desrichard A, Chan TA, The role of neoantigens in response to immune checkpoint blockade, Int. Immunol 28 (2016)411–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Keir ME, Butte MJ, Freeman GJ, Sharpe AH, PD-1 and its ligands in tolerance and immunity, Annu. Rev. Immunol 26 (2008) 677–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Iwai Y, Hamanishi J, Chamoto K, Honjo T, Cancer immunotherapies targeting the PD-1 signaling pathway, J. Biomed. Sci 24 (2017) 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. , Safety, activity, and immune correlates of anti-PD-1 antibody in cancer, N. Engl. J. Med 366 (2012) 2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. , Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma, N. Engl. J. Med 369 (2013) 134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Ribas A, Wolchok JD, Cancer immunotherapy using checkpoint blockade, Science 359 (2018) 1350–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Ochoa CE, Joseph RW, Nivolumab in renal cell carcinoma: current trends and future perspectives, J. Kidney Cancer VHL 5 (2018) 15–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Friedlander ML, Russell K, Millis S, Gatalica Z, Bender R, Voss A, Molecular profiling of clear cell ovarian cancers: identifying potential treatment targets for clinical trials, Int. J. Gynecol. Cancer 26 (2016) 648–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Howitt BE, Strickland KC, Sholl LM, Rodig S, Ritterhouse LL, Chowdhury D, et al. , Clear cell ovarian cancers with microsatellite instability: a unique subset of ovarian cancers with increased tumor-infiltrating lymphocytes and PD-1/PD-L1 expression, Oncoimmunology 6 (2017), e1277308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. , Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade, Science 357 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Lu KH, Daniels M, Endometrial and ovarian cancer in women with Lynch syndrome: update in screening and prevention, Familial Cancer 12 (2013) 273–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Helder-Woolderink JM, Blok EA, Vasen HF, Hollema H, Mourits MJ, De Bock GH, Ovarian cancer in Lynch syndrome; a systematic review, Eur. J. Cancer 55 (2016) 65–73. [DOI] [PubMed] [Google Scholar]
- [86].Willis BC, Sloan EA, Atkins KA, Stoler MH, Mills AM, Mismatch repair status and PD-L1 expression in clear cell carcinomas of the ovary and endometrium, Mod. Pathol 30 (2017) 1622–1632. [DOI] [PubMed] [Google Scholar]
- [87].Matulonis UA, Shapira-Frommer R, Santin A, Lisyanskaya AS, Pignata S, Vergote I, et al. , Antitumor activity and safety of pembrolizumab in patients with advanced recurrent ovarian cancer: interim results from the phase 2 KEYNOTE-100 study, J. Clin. Oncol 36 (2018) 5511. [DOI] [PubMed] [Google Scholar]
- [88].Hamanishi J, Mandai M, Ikeda T, Minami M, Kawaguchi A, Murayama T, et al. , Safety and antitumor activity of anti-PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer, J. Clin. Oncol 33 (2015) 4015–4022. [DOI] [PubMed] [Google Scholar]
- [89].Hamanishi J, Mandai M, Konishi I, Immune checkpoint inhibition in ovarian cancer, Int Immunol. 28 (2016) 339–348. https://rctportal.niph.go.jp/en/detai?trial_id=JapicCTI-153004. [DOI] [PubMed] [Google Scholar]
- [90].Bellone S, Buza N, Choi J, Zammataro L, Gay L, Elvin JA, et al. , Exceptional response to pembrolizumab in a metastatic, chemotherapy/radiation resistant ovarian cancer patient harboring a CD274/PD-L1-genetic rearrangement, Clin. Cancer Res (2018) 3282–3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Disis ML, Patel MR, Pant S, Hamilton EP, Lockhart AC, Kelly K, et al. , Avelumab (MSB0010718C; anti-PD-L1) in patients with recurrent/refractory ovarian cancer from the JAVELIN Solid Tumor phase Ib trial: safety and clinical activity, J. Clin. Oncol 34 (2016) 5533. [Google Scholar]
- [92].Lee JM, Cimino-Mathews A, Peer CJ, Zimmer A, Lipkowitz S, Annunziata CM, et al. , Safety and clinical activity of the programmed death-ligand 1 inhibitor durvalumab in combination with poly (ADP-ribose) polymerase inhibitor olaparib or vascular endothelial growth factor receptor 1-3 inhibitor cediranib in women's cancers: a dose-escalation, phase i study, J. Clin. Oncol 35 (2017) 2193–2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Hamanishi J, Murakami R, Mandai M, Matsumura N, Abiko K, Yamaguchi K, et al. , Specific gene signatures and oligo clonal expansion of b cell repertoire with responders of anti-PD-1 antibody, nivolumab for ovarian cancer: novel predictive biomarkers, J. Clin. Oncol 34 (2016) 5513. [Google Scholar]
- [94].Sugiyama T, Okamoto A, Enomoto T, Hamano T, Aotani E, Terao Y, et al. , Randomized phase III trial of irinotecan plus cisplatin compared with paclitaxel plus carboplatin as first-line chemotherapy for ovarian clear cell carcinoma: JGOG3017/GCIG trial, J. Clin. Oncol 34 (2016) 2881–2887. [DOI] [PubMed] [Google Scholar]
- [95].Farley JH, Brady WE, Fujiwara K, Nomura H, Yunokawa M, Tokunaga H, et al. , A phase II evaluation oftemsirolimus in combination with carboplatin and paclitaxel followed by temsirolimus consolidation as first-line therapy in the treatment of stage III-IV clear cell carcinoma of the ovary, J. Clin. Oncol 34 (2016) 5531. [Google Scholar]
- [96].Chan JK, Brady W, Monk BJ, Brown J, Shahin MS, Rose PG, et al. , A phase II evaluation of sunitinib in the treatment of persistent or recurrent clear cell ovarian carcinoma: an NRG Oncology/Gynecologic Oncology Group Study (GOG-254), Gynecol. Oncol 150 (2018) 247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Konstantinopoulos PA, Brady WE, Farley J, Armstrong A, Uyar DS, Gershenson DM, Phase II study of single-agent cabozantinib in patients with recurrent clear cell ovarian, primary peritoneal or fallopian tube cancer (NRG-GY001), Gynecol. Oncol 150 (2018) 9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Lheureux S, Burnier JV, Tan Q, Kanjanapan Y, Clarke B, Tinker A, et al. , Phase II clinical and molecular trial of oral ENMD-2076 in clear cell ovarian cancer (CCOC): a study of the Princess Margaret phase II consortium, J. Clin. Oncol 35 (2017) 5522. [Google Scholar]
- [99].Huang Z, Wu Y, Zhou X, Qian J, Zhu W, Shu Y, et al. , Clinical efficacy of mTOR inhibitors in solid tumors: a systematic review, Future Oncol. 11 (2015) 1687–1699. [DOI] [PubMed] [Google Scholar]
- [100].A Burger R, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H, et al. , Incorporation of bevacizumab in the primary treatment of ovarian cancer, N. Engl. J. Med 365 (2011) 2473–2483. [DOI] [PubMed] [Google Scholar]
- [101].Lemery S, Keegan P, Pazdur R, First FDA approval agnostic of cancer site - when a biomarker defines the indication, N. Engl. J. Med 377 (2017) 1409–1412. [DOI] [PubMed] [Google Scholar]