Abstract
Objective:
Recent studies have demonstrated that surgical menopause results in a significantly increased risk of nonalcoholic fatty liver disease (NAFLD) in women with endometrial cancer. In addition, venous thromboembolism (VTE) is known to be one of the major prognostic factors for decreased survival in endometrial cancer. Given the fact that coagulation factors are produced in the liver, the correlation between NAFLD and VTE was examined in endometrial cancer.
Methods:
A retrospective study was conducted to examine patients with endometrial cancer who underwent surgical staging including oophorectomy between 2000 and 2013 (n = 714). Cumulative risk of VTE was examined based on the NAFLD status. A Cox proportional hazard regression model was used to determine the independent risk predictors of VTE.
Results:
Venous thromboembolism and NAFLD were seen in 57 (8.0%) and 181 (25.4%) cases, respectively. Two-year cumulative risks of VTE and NAFLD were 7.9% and 19.3%, respectively. In univariate analysis, VTE was significantly associated with decreased disease-free survival (2-year rate, 43.6% vs 91.4%, P < .001) and overall survival (65.8% vs 96.8%, P < .001), whereas NAFLD was associated with decreased risk of VTE (1.7% vs 10.4%, P < .001). In multivariate analysis controlling for clinicopathological factors, NAFLD remained an independent predictor of decreased risk of VTE (hazard ratio [HR]: 0.24, 95% confidence interval [CI]: 0.07–0.79, P = .02). Thrombocytosis (HR: 2.30, 95% CI: 1.22-4.35, P = .01), cancer antigen 125 ≥ 35 (HR: 3.81,95% CI: 1.78-8.17, P < .001), and recurrent disease (HR: 4.57, 95% CI: 1.97-10.6, P < .001) remained as independent predictors of increased risk of VTE.
Conclusion:
Our results suggest that NAFLD may be associated with decreased VTE risk in women with endometrial cancer.
Keywords: endometrial cancer, venous thromboembolism, nonalcoholic fatty liver disease, survival
Introduction
Endometrial cancer is the most common of the gynecologic cancers.1 Surgery with hysterectomy and salpingo-oophorectomy remains the standard treatment of endometrial cancer. As a consequence of oophorectomy, young premenopausal women will experience estrogen withdrawal that impact both short- and long-term effects in health.2
Estrogen loss leads to several metabolic derangements including development of visceral adiposity, insulin resistance, and impaired glucose metabolism.3 Estrogen plays an important role in maintaining lipid metabolism, and its deficiency has been related to lipid accumulation in the hepatocyte (hepatic steatosis), development of nonalcoholic fatty liver disease (NAFLD), and insulin resistance. According to a recent study, NAFLD is one of the most prevalent conditions diagnosed after surgical menopause in young women with endometrial cancer.4 Nonalcoholic fatty liver disease entails a spectrum of liver damage, which ranges from simple steatosis to nonalcoholic steatohepatitis, advanced fibrosis, and cirrhosis and can lead to mild to moderate liver dysfunction.5 Maintenance of hemostasis is one of the many vital functions governed by the liver. The liver plays a central role in synthesizing several factors belonging to the pathways of coagulation, anticoagulation, fibrinolysis, and platelet production. Therefore, patients with chronic liver disease may experience coagulation and hemostatic disorders.
Venous thromboembolism (VTE) is another common complication in women with endometrial cancer, and it has been associated with decreased survival outcome.6 The development of a persistent hypercoagulable state mediated by tumor activity is considered to be the key feature in the pathogenesis of VTE.7 Paraneoplastic thrombocytosis is an important component of the serum milieu responsible for hypercoagulability, and the liver plays a crucial role in paraneoplastic thrombocytosis through interleukin 6 (IL-6) cascade.8 Given the high prevalence of NAFLD after surgical menopause and the major role of the liver in the coagulation system, especially the IL-6 cascade, we examined the association between NAFLD and the risk of VTE in patients with endometrial cancer.
Materials and Methods
Study Design and Eligibility
After institutional review board approval from the University of Southern California, patients with primary endometrial cancer who had undergone surgical staging including oophorectomy between 2000 and 2013 were retrospectively identified from the institutional database for endometrial cancer. All the patients had their follow-up at the University of Southern California and Los Angeles County Medical Center. Metastatic cancer to the endometrium, carcinosarcoma, uterine sarcoma, and endometrial hyperplasia were excluded from the study. Patients included in this study had been followed to assess VTE and/or NAFLD between the initial endometrial cancer diagnosis and subsequent follow-up time during treatment. Eligible cases were divided into 2 groups: patients with VTE and patients without VTE. There was no case with history of VTE in our study population. This study population of endometrial cancer was chosen because endometrial cancer is strongly associated with obesity, which is a well-known risk factor for developing NAFLD.3,4 The STrengthening the Reporting of OBservational studies in Epidemiology guidelines were consulted for reporting in a retrospective cohort study.
Clinical Information
Among cases eligible for analysis, medical records were examined to abstract the following information: (1) patient’s demographics, (2) histopathological findings at the time of initial endometrial cancer diagnosis, (3) laboratory tests and imaging findings at the time of NAFLD diagnosis, and (4) survival outcomes. Demographic characteristics analyzed included age at the time of surgical operation, ethnicity, body mass index, and medical comorbidities such as diabetes mellitus, hypertension, hypercholesterolemia, and liver disease. History of β-blocker, statin, and metformin use was collected. For histological findings, detailed information was obtained from pathology reports at the time of surgical staging: histology subtype, tumor grade, and stage. Laboratory tests abstracted from records included the cancer antigen 125 (CA-125) and platelet count. Radiology reports for imaging modalities used to diagnose NAFLD were reviewed. Information regarding cirrhosis and nonalcoholic steatohepatitis (NASH) was also abstracted from records. Disease-free survival (DFS) and overall survival (OS) were used to determine survival outcome.
Patients received care per clinical standards in our institution. Postoperative VTE prophylaxis includes early ambulation, pneumatic compression devices, and pharmacologic anticoagulation (enoxaparin or heparin, starting from post-operative day 1 until the day of discharge). Patients diagnosed with VTE were treated using therapeutic dosing of low-molecular-weight heparin for a minimum of 6 months or until resolution of active disease in patients with serum or radiologic evidence of malignancy. Serum testing including a liver function panel is performed at each clinic visit during postoperative follow-up (every 3 months for first 2 years, every 6 months until 5 years after surgery). Liver ultrasonography was performed as indicated by abnormal serum screening results or patient symptoms suspecting NAFLD. Systemic imaging with computed tomography (CT) was performed whenever recurrence was suspected.
Definition
Cancer grade and stage were reclassified based on the recent International Federation of Gynecology and Obstetrics guidelines.9 Advanced stage was defined as stage III and IV. Histologic subtypes were grouped as endometrioid, serous, clear cell, or other adenocarcinoma. Written medical records and radiology reports including Doppler study, CT scan, pulmonary angiogram, and ventilation perfusion lung scan were reviewed for the diagnosis of NAFLD and VTE. Nonalcoholic fatty liver disease was defined as abnormal liver function testing in addition to radiographic evidence of increased hepatic echogenicity on ultrasonography or attenuation of the liver on CT. Disease-free survival was defined as the time interval between the date of endometrial cancer diagnosis and the date of first recurrence, progression of disease, or last follow-up if there was no recurrence or progression. Overall survival was defined as the time interval between endometrial cancer diagnosis and the date of death or the last follow-up if the patient was still alive or died of other disease.
Statistical Analysis
The primary outcome was the association of NAFLD on the cumulative risk of VTE and survival outcome. The secondary outcome was to determine the independent risk factors for VTE in endometrial cancer. Continuous variables were assessed for normality (Kolmogorov-Smirnov test) and expressed as appropriate (mean [standard deviation, SD] or median [range]). Statistical significance of continuous variables was assessed with Student t test or Mann-Whitney U test as appropriate. Categorical or original variables were assessed with χ2 test or Fisher exact test as appropriate. Because VTE is a time-dependent event, log-rank test (univariate analysis) and a Cox proportional hazard regression model (multivariate analysis) were used for assessing the cumulative risk of VTE after hysterectomy-based surgical staging. Magnitude of statistical significance was expressed with hazard ratio (HR) and 95% confidence interval (CI). Similar survival analyses were performed for DFS and OS. All statistical tests were 2 tailed, and P < .05 was considered statistically significant. Statistical Package for the Social Sciences (version 12.0; SPSS Inc, Chicago, Illinois) was used for all analyses.
Results
A total of 714 patients with endometrial cancer were identified. Baseline characteristics of patients are shown in Table 1. The mean age of the entire cohort was 53.1 years. Most women in our study were Hispanic (67.2%), obese (68.6%), and had stage I (68.8%), grade 1 (52.4%), and endometrioid histology (82.8%). At the time of endometrial cancer diagnosis, hypertension, diabetes mellitus, and hypercholesterolemia were observed in 55.2%, 32.2%, and 24.7% of patients, respectively. Median follow-up time was 28.8 months, and there were 99 (13.9%) cases of recurrence and 67 (9.4%) cases of death due to endometrial cancer. There was no case of death due to cardiovascular disease. Abnormal laboratory results included elevated CA-125 level in 24.8% of patients and thrombocytosis in 11.1%.
Table 1.
Patient Demographics.a
| Characteristic | All, N = 714 (100%) | VTE, n = 57 (8.0%) | Non-VTE, n = 657 (92.0%) | P Value |
|---|---|---|---|---|
| Age | 53.1 (± 10.0) | 56.3 (± 9.2) | 52.8 (± 10.0) | .011 |
| <60 | 537 (75.2%) | 38 (7.1%) | 499 (92.9%) | |
| ≥60 | 177 (24.8%) | 19 (10.7%) | 158 (89.3%) | |
| Ethnicity | .84 | |||
| Caucasian | 85 (11.9%) | 6 (7.1%) | 79 (92.9%) | |
| African | 35 (4.9%) | 4 (11.4%) | 31 (88.6%) | |
| Hispanic | 480 (67.2%) | 39 (8.1%) | 441 (91.9%) | |
| Asian | 114 (16.0%) | 8 (7.0%) | 106 (93.0%) | |
| BMI (kg/m2) | 35.8 (± 9.9) | 33.9 (± 9.8) | 35.9 (± 9.9) | .13 |
| < 30 | 218 (30.5%) | 23 (10.6%) | 195 (89.4%) | |
| ≥30 | 490 (68.6%) | 34 (6.9%) | 456 (93.1%) | |
| Not available | 6 (0.8%) | |||
| Hypertension | .17 | |||
| No | 320 (44.8%) | 31 (9.7%) | 289 (90.3%) | |
| Yes | 394 (55.2%) | 26 (6.6%) | 368 (93.4%) | |
| β-Blocker use | .15 | |||
| No | 585 (81.9%) | 51 (8.7%) | 534 (91.3%) | |
| Yes | 129 (18.1%) | 6 (4.7%) | 123 (95.3%) | |
| Diabetes mellitus | .002 | |||
| No | 484 (67.8%) | 49 (10.1%) | 435 (89.9%) | |
| Yes | 230 (32.2%) | 8 (3.5%) | 222 (96.5%) | |
| Metformin use | .001 | |||
| No | 546 (76.5%) | 53 (9.7%) | 493 (90.3%) | |
| Yes | 168 (23.5%) | 4 (2.4%) | 164 (97.6%) | |
| Hypercholesterolemia | .42 | |||
| No | 537 (75.3%) | 46 (8.6%) | 491 (91.4%) | |
| Yes | 176 (24.7%) | 11 (6.3%) | 165 (93.8%) | |
| Not available | 1 (0.1%) | |||
| Statin use | .003 | |||
| No | 576 (80.8%) | 54 (9.4%) | 522 (90.6%) | |
| Yes | 138 (19.3%) | 3 (2.2%) | 135 (97.8%) | |
| NAFLD | <.001 | |||
| No | 533 (74.6%) | 54 (10.1%) | 479 (89.9%) | |
| Yes | 181 (25.4%) | 3 (1.7%) | 178 (98.3%) | |
| CA-125 (IU/L) | 18 (2-7192) | 142 (4-7192) | 17 (2-5421) | <.001 |
| <35 | 457 (64.0%) | 13 (2.8%) | 444 (97.2%) | |
| ≥35 | 177 (24.8%) | 38 (21.5%) | 139 (78.5%) | |
| Not available | 80 (11.2%) | |||
| Platelet count (× 109/L) | 301.7 (±92.9) | 361.9 (±117.3) | 296.7 (±88.9) | <.001 |
| <400 | 590 (82.6%) | 31 (5.3%) | 559 (94.7%) | |
| ≥400 | 79 (11.1%) | 20 (25.3%) | 59 (74.7%) | |
| Not available | 45 (6.3%) | |||
| Histology subtype | <.001 | |||
| Endometrioid | 591 (82.8%) | 33 (5.6%) | 558 (94.4%) | |
| Serous | 47 (6.6%) | 10 (21.3%) | 37 (78.7%) | |
| Clear cell | 36 (5.0%) | 9 (25.0%) | 27 (75.0%) | |
| Other | 40 (5.6%) | 5 (12.5%) | 35 (87.5%) | |
| Grade | <.001 | |||
| 1 | 374 (52.4%) | 13 (3.5%) | 361 (96.5%) | |
| 2 | 176 (24.6%) | 13 (7.4%) | 163 (92.6%) | |
| 3 | 164 (23.0%) | 31 (18.9%) | 133 (81.1%) | |
| Stage | <.001 | |||
| I | 491 (68.8%) | 15 (3.1%) | 476 (96.9%) | |
| II | 63 (8.8%) | 4 (6.3%) | 59 (93.7%) | |
| III | 109 (15.3%) | 21 (19.3%) | 88 (80.7%) | |
| IV | 51 (7.1%) | 17 (33.3%) | 34 (66.7%) |
Abbreviations: BMI, body mass index; CA, cancer antigen; NAFLD, nonalcoholic fatty liver disease; VTE, venous thromboembolism.
Number (%), mean (± standard deviation, SD), or median (range) is shown. Student t test, Fisher exact test, or χ2 test for P values. Significant P values are emboldened.
Nonalcoholic fatty liver disease was diagnosed in 181 (25.4%) cases during the follow-up after surgical management of endometrial cancer. There was 1 (0.1%) case of cirrhosis related to NAFLD, and no NASH case was reported in this study cohort. Women with NAFLD were less likely to develop VTE (P < .001). There were 57 (8.0%) patients with VTE reported in the cohort (DVT alone 57.9%, PE alone 17.5%, and DVT/PE 24.6%). Women who developed VTE were older (P = .011), had higher CA-125 levels (P < .001), higher platelet count (P < .001), serous and clear cell histology (P < .001), high-grade tumor (P < .001), and advanced stage (P < .001). Women with VTE were less likely to have diabetes mellitus (P = .002) and NAFLD (P < .001). Results of the time-dependent analysis for the risk of VTE after surgical staging for endometrial cancer are shown (Figure 1A). Two-year cumulative risks of VTE differed significantly in the presence of NAFLD (7.9% vs 19.3%).
Figure 1.
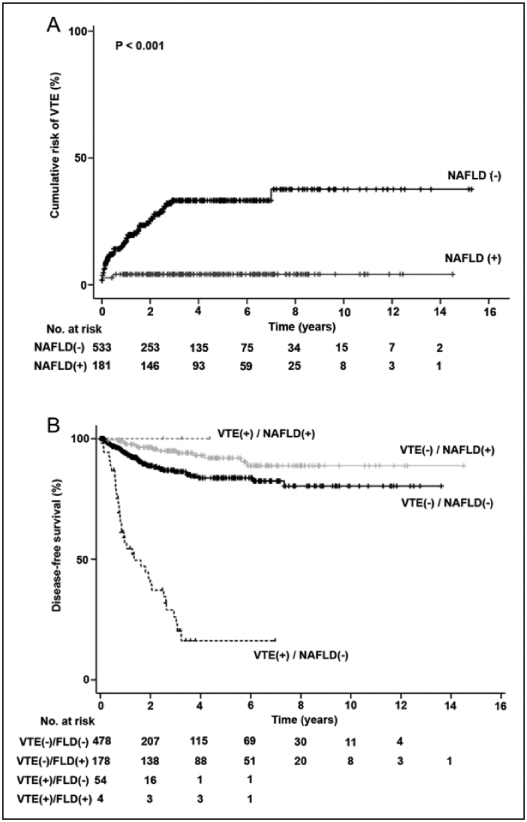
Cumulative risk of venous thromboembolism and disease-free interval of endometrial cancer. A, Cumulative risk of venous thromboembolism after surgical staging of endometrial cancer. B, Kaplan-Meier method for survival curves for disease-free survival of patient with endometrial cancer. Log-rank test for P value. P < 0.001 between VTE(−)/NAFLD(−) and VTE(+)/NAFLD(−). P = 0.012 between VTE(−)/NAFLD(−) and VTE(−)/NAFLD(+). P < 0.001 between VTE(+)/NAFLD(−) and VTE(−)/NAFLD(+). P = 0.035 between VTE(+)/NAFLD(−) and VTE(+)/NAFLD(+). NAFLD indicates nonalcoholic fatty liver disease; VTE, venous thromboembolism.
To identify independent risk factors for VTE, a multivariate analysis was performed (Table 2). On multivariate analysis controlling for clinicopathological factors, NAFLD remained an independent predictor of the decreased risk of VTE (adjusted HR: 0.24, 95% CI: 0.07-0.79, P = .02). Thrombocytosis was significantly associated with an increased risk of VTE (adjusted HR: 2.30, 95% CI: 1.22-4.35, P = .01). In addition, high CA-125 level (adjusted HR: 3.81, 95% CI: 1.78-8.17, P < .001) and recurrent disease (adjusted HR: 4.57, 95% CI: 1.97-10.6, P < .001) remained independent risk factors for increased risk of VTE.
Table 2.
Risk Factors for Venous Thromboembolism in Endometrial Cancer.a
| Characteristic | No. | 2-year (%) | Univariate |
Multivariate |
||
|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |||
| Age | .06 | .69 | ||||
| <60 | 537 | 6.8% | 1 | 1 | ||
| ≥60 | 177 | 11.4% | 1.70 (0.98-2.94) | 1.15 (0.57-2.32) | ||
| Ethnicity | .87 | .48 | ||||
| Non-Hispanic | 234 | 9.0% | 1 | 1 | ||
| Hispanic | 480 | 7.4% | 0.95 (0.55-1.67) | 1.26 (0.67-2.37) | ||
| BMI (kg/m2) | .10 | .41 | ||||
| <30 | 218 | 11.1% | 1 | 1 | ||
| ≥30 | 490 | 6.5% | 0.65 (0.38-1.10) | 1.31 (0.69-2.48) | ||
| Hypertension | .10 | .88 | ||||
| No | 320 | 9.5% | 1 | 1 | ||
| Yes | 394 | 6.6% | 0.65 (0.39-1.09) | 0.95 (0.49-1.84) | ||
| β-Blocker use | .11 | .40 | ||||
| No | 585 | 8.6% | 1 | 1 | ||
| Yes | 129 | 4.6% | 0.51 (0.22-1.19) | 0.64 (0.23-1.80) | ||
| Diabetes mellitus | .002 | .10 | ||||
| No | 484 | 10.1% | 1 | 1 | ||
| Yes | 230 | 3.4% | 0.32 (0.15-0.68) | 0.39 (0.12-1.22) | ||
| Metformin use | .001 | .54 | ||||
| No | 546 | 9.8% | 1 | 1 | ||
| Yes | 168 | 1.9% | 0.22 (0.08-0.61) | 0.65 (0.16-2.60) | ||
| Hypercholesterolemia | .25 | .25 | ||||
| No | 537 | 8.8% | 1 | 1 | ||
| Yes | 176 | 5.2% | 0.68 (0.35-1.31) | 1.59 (0.73-3.49) | ||
| Statin use | .003 | .14 | ||||
| No | 576 | 9.5% | 1 | 1 | ||
| Yes | 138 | 1.6% | 0.21 (0.06-0.66) | 0.38 (0.10-1.40) | ||
| NAFLD | <.001 | .02 | ||||
| No | 533 | 10.4% | 1 | 1 | ||
| Yes | 181 | 1.7% | 0.13 (0.04-0.41) | 0.24 (0.07-0.79) | ||
| CA-125 (IU/L) | <.001 | <.001 | ||||
| <35 | 457 | 2.8% | 1 | 1 | ||
| ≥35 | 177 | 22.3% | 8.51 (4.53-16.0) | 3.81 (1.78-8.17) | ||
| Platelet count (× 109/L) | <.001 | .01 | ||||
| <400 | 590 | 5.5% | 1 | 1 | ||
| ≥400 | 79 | 24.8% | 5.16 (2.94-9.06) | 2.30 (1.22-4.35) | ||
| Histology subtype | <.001 | .20 | ||||
| Endometrioid | 591 | 5.8% | 1 | 1 | ||
| Nonendometrioid | 123 | 17.4% | 3.57 (2.11-6.04) | 0.61 (0.29-1.30) | ||
| Grade | <.001 | .44 | ||||
| 1-2 | 550 | 4.9% | 1 | 1 | ||
| 3 | 164 | 17.8% | 4.30 (2.56-7.25) | 1.35 (0.64-2.85) | ||
| Stage | <.001 | .86 | ||||
| I-II | 554 | 3.6% | 1 | 1 | ||
| III-IV | 160 | 21.6% | 7.10 (4.10-12.3) | 1.08 (0.45-2.63) | ||
| Recurrence | <.001 | <.001 | ||||
| No | 615 | 3.7% | 1 | 1 | ||
| Yes | 99 | 30.9% | 12.1 (7.01-20.8) | 4.57 (1.97-10.6) | ||
Abbreviations: BMI, body mass index; CA, cancer antigen; CI, confidence interval; HR, hazard ratio; NAFLD, nonalcoholic fatty liver disease; VTE, venous thromboembolism; 2-yr cumulative rate (%), 2-year VTE proportion.
Multivariate analysis with Cox proportional hazards regression model for P values. Significant P values are emboldened.
To determine the independent risk factors for DFS in endometrial cancer, multivariate analysis was performed (Table 3). On univariate analysis, age >60 (69.2% vs 83.7%), CA-125 ≥35 IU/L (54.9% vs 91.5%), thrombocytosis (61.6% vs 85.4%), nonendometrioid histology (49.3% vs 87.6%), and VTE occurrence (19.5% vs 86.6%) were associated with decreased DFS (P < .001). On multivariate analysis, advanced stage (adjusted HR: 4.93, 95% CI: 2.62-9.28, P < .001), VTE (adjusted HR: 3.57, 95% CI: 2.01-6.36, P < .001), and CA-125 (adjusted HR: 2.79, 95% CI: 1.62-4.81, P < .001) remained independent prognostic factors for decreased DFS. Contrarily, NAFLD (adjusted HR: 0.46, 95% CI: 0.23-0.94, P = .034) was independently associated with improved DFS.
Table 3.
Disease-Free Survival of Patients With Endometrial Cancer.a
| Characteristic | No. | 5-year (%) | Univariate |
Multivariate |
||
|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |||
| Age | <.001 | .074 | ||||
| <60 | 537 | 83.7% | 1 | 1 | ||
| ≥60 | 177 | 69.2% | 2.29 (1.52-3.46) | 1.65 (0.95-2.84) | ||
| Ethnicity | .026 | .81 | ||||
| Non-Hispanic | 234 | 72.6% | 1 | 1 | ||
| Hispanic | 480 | 83.5% | 0.63 (0.42-0.95) | 0.94 (0.57-1.54) | ||
| BMI (kg/m2) | .044 | .27 | ||||
| <30 | 218 | 76.0% | 1 | 1 | ||
| ≥30 | 490 | 82.6% | 0.66 (0.44-0.99) | 1.34 (0.80-2.26) | ||
| Hypertension | .77 | .52 | ||||
| No | 320 | 78.8% | 1 | 1 | ||
| Yes | 394 | 81.8% | 0.94 (0.63-1.40) | 1.21 (0.67-2.18) | ||
| β-Blocker use | .62 | .77 | ||||
| No | 585 | 79.6% | 1 | 1 | ||
| Yes | 129 | 83.9% | 0.88 (0.52-1.48) | 1.10 (0.59-2.03) | ||
| Diabetes mellitus | .33 | .22 | ||||
| No | 484 | 78.9% | 1 | 1 | ||
| Yes | 230 | 83.9% | 0.81 (0.52-1.25) | 1.59 (0.76-3.36) | ||
| Metformin use | .19 | .47 | ||||
| No | 546 | 78.9% | 1 | 1 | ||
| Yes | 168 | 85.1% | 0.72 (0.44-1.18) | 0.74 (0.33-1.65) | ||
| Hypercholesterolemia | .83 | .94 | ||||
| No | 537 | 79.0% | 1 | 1 | ||
| Yes | 176 | 83.8% | 0.95 (0.61-1.50) | 0.97 (0.53-1.81) | ||
| Statin use | .24 | .62 | ||||
| No | 576 | 78.8% | 1 | 1 | ||
| Yes | 138 | 86.1% | 0.73 (0.43-1.24) | 1.22 (0.57-2.61) | ||
| NAFLD | <.001 | .034 | ||||
| No | 533 | 75.3% | 1 | 1 | ||
| Yes | 181 | 91.8% | 0.29 (0.16-0.51) | 0.46 (0.23-0.94) | ||
| CA-125 (IU/L) | <.001 | <.001 | ||||
| <35 | 457 | 91.5% | 1 | 1 | ||
| ≥35 | 177 | 54.9% | 6.09 (3.86-9.59) | 2.79 (1.62-4.81) | ||
| Platelet count (× 109/L) | <.001 | .56 | ||||
| <400 | 590 | 85.4% | 1 | 1 | ||
| ≥400 | 79 | 61.6% | 3.36 (2.12-5.35) | 0.86 (0.50-1.45) | ||
| Histology subtype | <.001 | .011 | ||||
| Endometrioid | 591 | 87.6% | 1 | 1 | ||
| Nonendometrioid | 123 | 49.3% | 5.69 (3.83-8.44) | 1.98 (1.17-3.37) | ||
| Grade | <0.001 | 0.013 | ||||
| 1-2 | 550 | 88.7% | 1 | 1 | ||
| 3 | 164 | 54.4% | 6.10 (4.08-9.13) | 2.12 (1.18-3.83) | ||
| Stage | <.001 | <.001 | ||||
| I-II | 554 | 92.3% | 1 | 1 | ||
| III-IV | 160 | 46.9% | 10.1 (6.48-15.7) | 4.93 (2.62-9.28) | ||
| VTE | <.001 | <.001 | ||||
| No | 657 | 86.6% | 1 | 1 | ||
| Yes | 57 | 19.5% | 10.2 (6.69-15.4) | 3.57 (2.01-6.36) | ||
Abbreviations: BMI, body mass index; CA, cancer antigen; CI, confidence interval; HR, hazard ratio; NAFLD, nonalcoholic fatty liver disease; VTE, venous thromboembolism; 5-yr (%), 5-year survival proportion.
Multivariate analysis with Cox proportional hazards regression test for P values. Significant P values are emboldened.
Survival analysis for OS was performed (Table 4). On univariate analysis, age >60 (74.8% vs 89.8%), CA-125 ≥35 IU/L (69.0% vs 93.9%), thrombocytosis (67.2% vs 90.8%), nonendometrioid histology (62.5% vs 92.4%), high-grade tumor (64.8% vs 93.6%), advanced stage (65.5% vs 94.5%), and VTE occurrence (34.8% vs 92.1%) were associated with decreased 5-year OS rates (all, P < .001). On multivariate analysis, advanced stage (adjusted HR: 5.42, 95% CI: 2.38-12.3, P < .001) and VTE (HR: 4.45, 95% CI: 2.16-9.17, P < .001) remained independent prognostic factors for decreased OS. Conversely, NAFLD (adjusted HR: 0.35, 95% CI: 0.13-0.94, P = .038) remained an independent factor for improved OS.
Table 4.
Overall Survival of Patients With Endometrial Cancer.a
| Characteristic | No. | 5-year (%) | Univariate |
Multivariate |
||
|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |||
| Age | <.001 | .61 | ||||
| <60 | 537 | 89.8% | 1 | 1 | ||
| ≥60 | 177 | 74.8% | 2.88 (1.75-4.74) | 1.20 (0.60-2.41) | ||
| Ethnicity | .015 | .26 | ||||
| Non-Hispanic | 234 | 80.1% | 1 | 1 | ||
| Hispanic | 480 | 89.4% | 0.55 (0.34-0.90) | 0.70 (0.38-1.30) | ||
| BMI (kg/m2) | .16 | .75 | ||||
| <30 | 218 | 84.0% | 1 | 1 | ||
| ≥30 | 490 | 88.1% | 0.70 (0.43-1.15) | 1.11 (0.59-2.07) | ||
| Hypertension | .84 | .15 | ||||
| No | 320 | 86.1% | 1 | 1 | ||
| Yes | 394 | 87.7% | 0.95 (0.59-1.54) | 1.69 (0.82-3.46) | ||
| β-Blocker use | .34 | .40 | ||||
| No | 585 | 86.0% | 1 | 1 | ||
| Yes | 129 | 89.3% | 0.72 (0.37-1.41) | 0.72 (0.33-1.57) | ||
| Diabetes Mellitus | .29 | .25 | ||||
| No | 484 | 86.0% | 1 | 1 | ||
| Yes | 230 | 87.1% | 0.75 (0.44-1.28) | 1.73 (0.68-4.38) | ||
| Metformin use | .12 | .86 | ||||
| No | 546 | 86.2% | 1 | 1 | ||
| Yes | 168 | 88.1% | 0.61 (0.33-1.14) | 0.91 (0.34-2.46) | ||
| Hypercholesterolemia | .44 | .99 | ||||
| No | 537 | 86.4% | 1 | 1 | ||
| Yes | 176 | 87.1% | 0.80 (0.45-1.42) | 1.01 (0.45-2.25) | ||
| Statin use | .03 | .88 | ||||
| No | 576 | 84.9% | 1 | 1 | ||
| Yes | 138 | 92.8% | 0.45 (0.22-0.94) | 1.08 (0.40-2.93) | ||
| NAFLD | <.001 | .038 | ||||
| No | 533 | 82.2% | 1 | 1 | ||
| Yes | 181 | 95.8% | 0.25 (0.12-0.51) | 0.35 (0.13-0.94) | ||
| CA-125 (IU/L) | <.001 | .027 | ||||
| <35 | 457 | 93.9% | 1 | 1 | ||
| ≥35 | 177 | 69.0% | 6.40 (3.68-11.1) | 2.12 (1.09-4.13) | ||
| Platelet count (× 109/L) | <.001 | .72 | ||||
| <400 | 590 | 90.8% | 1 | 1 | ||
| ≥400 | 79 | 67.2% | 3.47 (2.03-5.96) | 0.89 (0.48-1.65) | ||
| Histology subtype | <.001 | .038 | ||||
| Endometrioid | 591 | 92.4% | 1 | 1 | ||
| Nonendometrioid | 123 | 62.5% | 6.52 (4.02-10.6) | 1.95 (1.04-3.68) | ||
| Grade | <.001 | .064 | ||||
| 1-2 | 550 | 93.6% | 1 | 1 | ||
| 3 | 164 | 64.8% | 6.43 (3.92-10.6) | 1.92 (0.96-3.83) | ||
| Stage | <.001 | <.001 | ||||
| I-II | 554 | 94.5% | 1 | 1 | ||
| III-IV | 160 | 65.5% | 10.9 (6.15-19.4) | 5.42 (2.38-12.3) | ||
| VTE | <.001 | <.001 | ||||
| No | 657 | 92.1% | 1 | 1 | ||
| Yes | 57 | 34.8% | 13.3 (8.05-22.1) | 4.45 (2.16-9.17) | ||
Abbreviations: BMI, body mass index; CA, cancer antigen; CI, confidence interval; HR, hazard ratio; NAFLD, nonalcoholic fatty liver disease; VTE, venous thromboembolism; 5-yr (%), 5-year survival proportion.
Multivariate analysis with Cox proportional hazards regression test for P values. Significant P values are emboldened.
When patterns of NAFLD and VTE were combined (Figure 1B), it demonstrated a distinct survival outcome for DFS. That is, among non-NAFLD cases, the presence of VTE was associated with decreased DFS: VTE(−)/NAFLD(−) versus VTE(+)/NAFLD(−), P < .001. Among VTE cases, the presence of NAFLD was associated with improved survival: VTE (+)/NAFLD(−) versus VTE (+)/NAFLD(+), P = .035.
Discussion
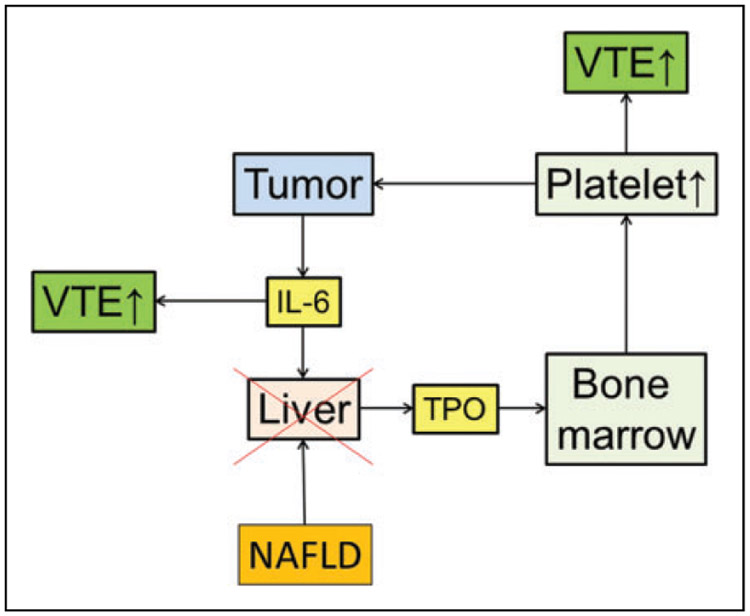
In this study, NAFLD was associated with the decreased risk of VTE in patients with endometrial cancer. Patients who developed NAFLD also had better survival outcome. In contrast, VTE was associated with poor survival outcome, and thrombocytosis was an independent risk factor for VTE, which highlights the important role of paraneoplastic thrombocytosis in VTE formation and reduced cancer survival. Based on our results, we speculated that NAFLD may disrupt the feed-forward loop of paraneoplastic thrombocytosis in cancer progression and therefore decreases the risk of VTE formation (Figure 2).
Figure 2.
Schema of paraneoplastic thrombocytosis disruption and venous thromboembolism. In this feed-forward loop theory, IL-6 derived from the tumor stimulates the hepatocyte to produce TPO. Thrombopoietin stimulates bone marrow to produce platelets. Interleukin 6 and thrombocytosis reflect the development of VTE. Disruption of the loop by NAFLD can therefore decrease the VTE risk. IL-6 indicates interleukin 6; NAFLD, nonalcoholic fatty liver disease; TPO, thrombopoietin; VTE, venous thromboembolism.
The association between cancer and VTE has been well established. Cancer-associated thrombosis (Trousseau syndrome) is the second leading cause of death in patients with cancer.10 The pathogenesis and risk factors for cancer-associated thrombosis are multifactorial and may be classified into 3 different categories: patient-related factors (older age, immobility, history of thrombosis, obesity, elevated leukocyte, and platelet counts), treatment-related factors (surgery, platinum-based chemotherapy, hormonal agents, and antiangiogenic factors), and malignancy-related factors (primary site such as adenocarcinoma of ovary, uterus, pancreas stomach, and lung, advanced and metastatic disease, and invasion to large vessels).5,6 A recent study identified 4 independent risk factors for VTE in endometrial cancer including elevated CA-125 levels, extrauterine disease, thrombocytosis, and high-risk histology.7 Our findings corroborated those of the previous study; patients with older age, advanced stage, high-grade tumor, and nonendometrioid histology were more likely to develop VTE.
Previous studies have demonstrated that platelet count and inflammation are dominant contributors to hypercoagulability.8 Alterations in serum components in patients with cancer include increased coagulation activation and activation of the inflammatory cascade, reduced red blood cell deformability, elevated plasma fibrinogen concentration, and possibly hemoconcentration due to third spacing, all of which can increase blood viscosity and reduce blood flow.9 It has been suggested that thrombocytosis, leukocytosis, and anemia are all associated with VTE.10,11 In our study, thrombocytosis was significantly associated with an increased risk of VTE and VTE was correlated with decreased survival outcome.
Thrombocytosis not only leads to a hypercoagulable status but also plays a major role in tumor growth, tissue invasion, and metastasis. Platelets act as protective “cloaks” helping tumor cells evade immune surveillance in the blood and promote tumor cell extravasation and metastasis.12,13 In fact, paraneoplastic thrombocytosis appears to involve a “positive feedback loop,” in which tumor cells secrete cytokines such as IL-6 that stimulate thrombocytosis.
The IL-6 cascade plays a particularly crucial role in the mechanism of paraneoplastic thrombocytosis. Interleukin 6 induces thrombopoietin (TPO) synthesis in the hepatocytes, which consequently increases platelet production by the bone marrow.8 Thrombopoietin is the major physiological regulator of platelet production. Thrombopoietin is produced in the liver at a constant rate and cleared by TPO receptors on platelets.14 In addition, IL-1, IL-3, IL-6, IL-11, and oncostatin M have been suggested as possible factors contributing to increased TPO production.15 The IL-6 effect is mediated through the induction of TPO messenger RNA (mRNA) expression and protein production in the liver, and TPO-neutralizing antibodies can abrogate the process of paraneoplastic thrombocytosis.16
Given the important role of the liver in the production of coagulation factors, it is not unexpected that liver disease may be associated with changes in the serum milieu due to defects in synthesis or clearance of proteins involved in coagulation. Estrogen deprivation is a recognized risk factor for NAFLD development. Additionally, laboratory abnormalities including aspartate aminotransferase/alanine aminotransferase ratio of >1, thrombocytopenia, hypoalbuminemia, and elevated prothrombin time may be found in patients with NAFLD.17 There are a limited number of published studies on the abnormalities associated with NAFLD including alteration in the coagulation cascade and thrombocytopenia in NAFLD, and those that exist have inconsistent findings. Some literature endorses TPO as a primary mechanism for these serum abnormalities, whereas others report contradictory results such as a normal or an increased level of TPO.5,18-23 In a normal physiologic state, TPO is produced in the liver, released into the circulation without any stored form, and its production is thought to be dependent on the hepatocyte function. After liver resection or liver disease, TPO levels and subsequent platelet counts may decrease.21-23 Serum TPO levels correlate inversely with the severity of liver disease as reflected by the degree of fibrosis, Child-Pugh class, and other synthetic measures of the liver function.24 The decrease in TPO production due to the reduction in functional hepatocytes can disrupt the main loop of paraneoplastic thrombocytosis through the IL-6 cascade. Therefore, it can be hypothesized that alterations in the hepatic production of TPO may be partially responsible for the association found between NAFLD, decreased platelet count, and reduced risk of VTE.
Notably, emerging evidence has linked the presence of NAFLD with low-level activation of the coagulation system. Hypercoagulability and thrombophilia in NAFLD can be described through different mechanisms. The most often reported factor is an increase in plasminogen activator inhibitor 1 (PAI-1) level, which results in the reduction of fibrinolysis. The level of PAI-1 is closely related to visceral adiposity in NAFLD.25,26 The second factor is an increase in the fibrinogen and factor VII level, as well as an increase in the activities of coagulation factors VIII, IX, XI, and XII.25-27 Additionally, clotting kinetics in NAFLD cases showed significantly stronger clot development and reduced clot lysis; this effect has not been related to platelet counts.28
Although there are multiple studies that have investigated NAFLD hypercoagulable status in general population, there is currently no prior study examining the NAFLD effects on coagulation status in cancer population.29-31 It is well known that cancer is a state of hypercoagulopathy.10,11,15 That is, tumor-derived IL-6 stimulates the hepatocyte to produce TPO to induce thrombocytosis via bone marrow stimulation as well as increase coagulation factors of which these two pathways will increase the risk of VTE.32,33 In endometrial cancer, high serum levels of IL-6 are reported in serous histology that is associated with an increased risk of VTE.7,27 In the absence of chronic liver disease, tumor-derived IL-6 increases hepatic TPO steady-state synthesis, leading to paraneoplastic thrombocytosis. However, in patients with chronic liver disease, secreted TPO levels are decreased due to impaired production.25 Decreased hepatic TPO mRNA in the hepatocytes diminishes the total amount of TPO available in the tissue to bind both megakaryocytes and platelets.22
There are diversities in the association between serum TPO levels and various liver conditions.28 Serum TPO levels seem not to always correlated with its hepatic productivity, platelet counts, or severity of liver disease because the serum TPO level is posttranscriptionally regulated by platelets and megakaryocytes and by platelet–megakaryocyte TPO receptor-mediated uptake and destruction.22,24 On the other hand, there is an inverse relationship between hepatic TPO production and the severity of underlying liver disease.26 Therefore, IL-6-mediated hepatic TPO production in paraneoplastic thrombocytosis in patients with cancer may be more vulnerable to the liver dysfunction than patients without cancer, and it is speculated that the disruption of coagulation pathway due to impairment of liver function after developing NAFLD may cause relative reduction in coagulation status resulting in reduced risk of VTE in patients with cancer. Because this study did not have results for biomarkers related to coagulation factors, further study to validate our findings will be warranted.
It has been reported that NAFLD is characterized by a procoagulant imbalance progressing from the less severe (steatosis) to the most severe form of the disease (metabolic cirrhosis).34,35 This imbalance appears to result from increased factor VIII and reduced protein C and might play a role in the risk of cardiovascular events and liver fibrosis commonly observed in NAFLD.29,35 Therefore, NAFLD may be associated with higher cardiovascular disease morbidity and mortality.36,37 In this study cohort, there was no death related to cardiovascular disease, but this is likely due to the short follow-up time in the study cohort.
As with any retrospective study, there is inherent bias and many potential confounders. This study did not have data regarding TPO levels to support the proposed mechanism of the relationship between NAFLD and VTE. A lack of laboratory or biopsy results for evaluating the severity of liver damage also prevents this study from evaluating the effect of liver disease severity on platelet count. Despite these limitations, this study has a rare focused look at several risk factors for VTE in patients with endometrial cancer. We identified several useful clinical and laboratory characteristics that may help clinicians working with patients with postoperative endometrial cancer.
Conclusion
Nonalcoholic fatty liver disease is significantly associated with decreased development of VTE in women with endometrial cancer. In addition, women with NAFLD have improved survival outcome. This association suggests a possible role of the liver in the feed-forward loop in paraneoplastic thrombocytosis in thrombosis formation and cancer progression. Further preclinical and preclinical studies will be warranted to examine the role of liver disease in disruption of this cancer-related thrombosis.
Acknowledgments
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Ensign Endowment for Gynecologic Cancer Research (K.M.).
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2.Riant E, Waget A, Cogo H, Arnal JF, Burcelin R, Gourdy P. Estrogens protect against high-fat diet-induced insulin resistance and glucose intolerance in mice. Endocrinol. 2009;150(5):2109–2117. [DOI] [PubMed] [Google Scholar]
- 3.Wise MR, Jordan V, Lagas A, et al. Obesity and endometrial hyperplasia and cancer in premenopausal women: a systematic review. Am J Obstet Gynecol. 2016;214(6):689.e681–689.e617. [DOI] [PubMed] [Google Scholar]
- 4.Li L, Liu DW, Yan HY, Wang ZY, Zhao SH, Wang B. Obesity is an independent risk factor for nonalcoholic fatty liver disease: evidence from a meta-analysis of 21 cohort studies. Obes Rev. 2016;17(6):510–519. [DOI] [PubMed] [Google Scholar]
- 5.Ikushima S, Ono R, Fukuda K, Sakayori M, Awano N, Kondo K. Trousseau’s syndrome: cancer-associated thrombosis. Jpn J Clin Oncol. 2016;46(3):204–208. [DOI] [PubMed] [Google Scholar]
- 6.Wun T, White RH. Epidemiology of cancer-related venous thromboembolism. Best Pract Res Clin Haematol. 2009;22(1):9–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsuo K, Yessaian AA, Lin YG, et al. Predictive model of venous thromboembolism in endometrial cancer. Gynecol Oncol. 2013;128(3):544–551. [DOI] [PubMed] [Google Scholar]
- 8.Albertin CL, Uppal S, Al-Niaimi AN, Seo S, Hinshaw JL, Hartenbach EM. Thrombocytosis is predictive of postoperative pulmonary embolism in patients with gynecologic cancer. Int J Gynecol Cancer. 2015;25(6):1096–1101. [DOI] [PubMed] [Google Scholar]
- 9.Tateo S, Mereu L, Salamano S, et al. Ovarian cancer and venous thromboembolic risk. Gynecol Oncol. 2005;99(1):119–125. [DOI] [PubMed] [Google Scholar]
- 10.Lyman GH, Khorana AA, Falanga A. Thrombosis and cancer: emerging data for the practicing oncologist. Am Soc Clin Oncol Educ Book. 2013. [DOI] [PubMed] [Google Scholar]
- 11.Kyrle PA. Predicting recurrent venous thromboembolism in cancer: is it possible? Thromb Res. 2014;133 suppl 2:S17–S22. [DOI] [PubMed] [Google Scholar]
- 12.Palumbo JS, Talmage KE, Massari JV, et al. Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood. 2005;105(1):178–185. [DOI] [PubMed] [Google Scholar]
- 13.Kim YJ, Borsig L, Varki NM, Varki A. P-selectin deficiency attenuates tumor growth and metastasis. Proc Natl Acad Sci U S A. 1998;95(16):9325–9330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuter DJ. The biology of thrombopoietin and thrombopoietin receptor agonists. Int J Hematol. 2013;98(1):10–23. [DOI] [PubMed] [Google Scholar]
- 15.Buergy D, Wenz F, Groden C, Brockmann MA. Tumor-platelet interaction in solid tumors. Int J Cancer. 2012;130(12):2747–2760. [DOI] [PubMed] [Google Scholar]
- 16.Kaser A, Brandacher G, Steurer W, et al. Interleukin-6 stimulates thrombopoiesis through thrombopoietin: role in inflammatory thrombocytosis. Blood. 2001;98(9):2720–2725. [DOI] [PubMed] [Google Scholar]
- 17.Targher G, Byrne CD. Diagnosis and management of nonalcoholic fatty liver disease and its hemostatic/thrombotic and vascular complications. Semin Thromb Hemost. 2013;39(2):214–228. [DOI] [PubMed] [Google Scholar]
- 18.Balcik OS, Akdeniz D, Cipil H, et al. Serum thrombopoietin levels in patients with nonalcoholic fatty liver disease. Saudi Med J. 2012;33(1):30–33. [PubMed] [Google Scholar]
- 19.Verrijken A, Francque S, Mertens I, et al. Prothrombotic factors in histologically proven nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology. 2014;59(1):121–129. [DOI] [PubMed] [Google Scholar]
- 20.Dasanu CA, Lamana S, Trikudanathan G. Thrombocytopenia in NAFLD: is thrombopoietin involved? South Med J. 2010;103(12):1278–1279. [DOI] [PubMed] [Google Scholar]
- 21.Kawasaki T, Takeshita A, Souda K, et al. Serum thrombopoietin levels in patients with chronic hepatitis and liver cirrhosis. Am J Gastroenterol. 1999;94(7):1918–1922. [DOI] [PubMed] [Google Scholar]
- 22.Ishikawa T, Ichida T, Matsuda Y, et al. Reduced expression of thrombopoietin is involved in thrombocytopenia in human and rat liver cirrhosis. J Gastroenterol Hepatol. 1998;13(9):907–913. [DOI] [PubMed] [Google Scholar]
- 23.Peck-Radosavljevic M, Wichlas M, Zacherl J, et al. Thrombopoietin induces rapid resolution of thrombocytopenia after orthotopic liver transplantation through increased platelet production. Blood. 2000;95(3):795–801. [PubMed] [Google Scholar]
- 24.Gangireddy VG, Kanneganti PC, Sridhar S, Talla S, Coleman T. Management of thrombocytopenia in advanced liver disease. Can J Gastroenterol Hepatol. 2014;28(10):558–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitchell O, Feldman DM, Diakow M, Sigal SH. The pathophysiology of thrombocytopenia in chronic liver disease. Hepat Med. 2016;8:39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giannini E, Botta F, Borro P, et al. Relationship between thrombopoietin serum levels and liver function in patients with chronic liver disease related to hepatitis C virus infection. Am J Gastroenterol. 2003;98(11):2516–2520. [DOI] [PubMed] [Google Scholar]
- 27.Bellone S, Watts K, Cane S, et al. High serum levels of interleukin-6 in endometrial carcinoma are associated with uterine serous papillary histology, a highly aggressive and chemotherapy-resistant variant of endometrial cancer. Gynecol Oncol. 2005;98(1):92–98. [DOI] [PubMed] [Google Scholar]
- 28.Ruiz-Arguelles GJ, Velazquez-Sanchez-De-Cima S, Zamora-Ortiz G, Hernandez-Reyes J, Ruiz-Delgado GJ. Nonalcoholic fatty liver disease may cause thrombocytopenia. Acta Haematol. 2014;132(2):159–162. [DOI] [PubMed] [Google Scholar]
- 29.Potze W, Siddiqui MS, Sanyal AJ. Vascular disease in patients with nonalcoholic fatty liver disease. Semin Thromb Hemost. 2015;41(5):488–493. [DOI] [PubMed] [Google Scholar]
- 30.Hickman IJ, Sullivan CM, Flight S, et al. Altered clot kinetics in patients with nonalcoholic fatty liver disease. Ann Hepatol. 2009;8(4):331–338. [PubMed] [Google Scholar]
- 31.Northup PG, Argo CK, Shah N, Caldwell SH. Hypercoagulation and thrombophilia in nonalcoholic fatty liver disease: mechanisms, human evidence, therapeutic implications, and preventive implications. Semin Liver Dis. 2012;32(1):39–48. [DOI] [PubMed] [Google Scholar]
- 32.Stone RL, Nick AM, McNeish IA, et al. Paraneoplastic thrombocytosis in ovarian cancer. N Engl J Med. 2012;366(7):610–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grignani G, Maiolo A. Cytokines and hemostasis. Haematologica. 2000;85(9):967–972. [PubMed] [Google Scholar]
- 34.Targher G, Bertolini L, Rodella S, et al. NASH predicts plasma inflammatory biomarkers independently of visceral fat in men. Obesity (Silver Spring). 2008;16(6):1394–1399. [DOI] [PubMed] [Google Scholar]
- 35.Tripodi A, Fracanzani AL, Primignani M, et al. Procoagulant imbalance in patients with nonalcoholic fatty liver disease. J Hepatol. 2014;61(1):148–154. [DOI] [PubMed] [Google Scholar]
- 36.Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010;363(14):1341–1350. [DOI] [PubMed] [Google Scholar]
- 37.Ekstedt M, Hagstrom H, Nasr P, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61(5):1547–1554. [DOI] [PubMed] [Google Scholar]