Abstract
Objective.
To propose an ideal patient candidate with early-stage cervical cancer for undergoing fertility-sparing trachelectomy.
Methods.
This nationwide, multicenter, retrospective study was conducted by the Japan Society of Obstetrics and Gynecology involving women aged <45 years with clinical stage I-II cervical cancer who had planned fertility-sparing trachelectomy and pelvic lymphadenectomy between 2009 and 2013 (n = 393). Ideal candidates were defined to have a tumor size of ≤2 cm, no lymph node metastasis, no deep stromal invasion, and no high-risk histology (n = 284, 69.6%). Less-ideal candidates were defined to have any one of these four characteristics (n = 109, 30.4%). Propensity score inverse probability of treatment weighting was used to assess survival outcomes.
Results.
Less-ideal candidates were more likely to undergo hysterectomy conversion (22.9% versus 3.2%), receive postoperative radiotherapy (11.9% versus 0.4%), or chemotherapy (32.1% versus 3.2%) compared with ideal candidates (all, P < 0.05). The weighted model revealed that among those who underwent trachelectomy (ideal candidates, n = 275 and less-ideal candidates, n = 84), less-ideal candidates had significantly decreased disease-free survival (5-year rates: 85.5% versus 95.5%; HR 3.93, 95% CI 1.99–7.74; P < 0.001) and cause-specific survival (92.5% versus 98.6%; HR 5.47, 95% CI 1.68–17.8, P = 0.001) compared with ideal candidates. Similarly, less-ideal candidates were significantly associated with decreased disease-free survival compared with ideal candidates among those who were young age, had small tumors or squamous histology, and underwent surgery alone (all, P < 0.05).
Conclusion.
Less-ideal candidates had approximately four-fold higher recurrence risk and cancer mortality compared with ideal candidates. Ideal candidates for fertility-sparing trachelectomy for early-stage cervical cancer proposed in our study may be useful as the future framework for developing guidelines for fertility-sparing trachelectomy in Japan.
Keywords: Cervical cancer, Trachelectomy, Tumor size, Deep stromal invasion, Lymph node metastasis, Survival
1. Introduction
Cervical cancer remains the most common gynecologic malignancy in young women, the incidence of which has been steadily increasing in Japan [1]. The National Cancer Center in Japan estimated that approximately 11,200 women have been newly diagnosed with cervical cancer in 2018 [1]. Women with early-stage cervical cancer generally have a favorable prognosis with either hysterectomy-based surgical and/or radiological treatment. When tumors exhibit nodal metastasis, large tumor size, or specific histological subtypes, risk of cancer recurrence increases [2,3].
The standard surgical management of stage IB1-IIA1 cervical cancer includes radical hysterectomy [4,5]. While this management provides curative treatment, it negatively impacts future fertility in reproductive aged women. The National Comprehensive Cancer Network (NCCN) and the Japan Society of Gynecologic Oncology (JSGO) have suggested that trachelectomy, involving the removal of the uterine cervix and adjacent tissues, is an optional treatment for young women with early-stage cervical cancer with a tumor size of ≤2 cm who wish to preserve fertility [5,6]. Utilization of fertility-sparing trachelectomy is increasing in young women with early-stage cervical cancer in recently [7,8].
The NCCN guideline modified the criteria for fertility-sparing trachelectomy in the 2019 version. The previously specified exclusion criteria of high-risk pathological features with the Sedlis criteria and/or with nodal metastasis have been removed from the current criteria [5]. Additionally, women with selected stage IB2 disease (tumor size 2–3.9 cm) can be considered for trachelectomy after weighing its risks and benefits [5,9]. These fluctuating changes imply that proper patient selection for fertility-sparing trachelectomy is yet to be set and fixed.
Japan is currently witnessing an increase in the number of single women and their age at first marriage [10]. As a result, more young women with cervical cancer are the potential candidates for this procedure. The lack of evidence for the safety of this procedure performed for large tumors and high-risk pathologic features, as well as efficacy of the procedure for early-stage cervical cancer, are obstacles in its implementation. Therefore, we aimed to identify an ideal candidate with early-stage cervical cancer for performing fertility-sparing trachelectomy.
2. Materials and methods
2.1. Study design
A nationwide multicenter retrospective observational study was conducted at 439 member institutions of the Japanese Gynecologic Oncology Group (JGOG). After receiving approval from the Institutional Review Board from the JSOG Ethics Committee (2018-68), the study concept and participation were called for from all 41 JGOG-designated centers where fertility-sparing trachelectomy was in practice during the study period. Of those, 27 centers voluntarily participated in the study where in the study approval was obtained by their own discretion as appropriate.
2.2. Eligibility and clinical information
The eligible criteria included women aged <45 years with clinical stage I-II cervical cancer who underwent trachelectomy from 2009 to 2013. Women were excluded if cancer histology or tumor size was unknown or if they did not undergo nodal evaluation. For eligible patients, information on demographics, tumor information, treatment types, and survival outcomes were extracted by clinicians in each site. The institutional cervical cancer database was analyzed to identify the patients with cervical cancer at each participating site.
Patient demographics included age, year at diagnosis, marital status (single, married, and others), and parity (nulliparous versus multiparous). Preoperative tumor characteristics included cancer stage (IA, IB, and II), histologic subtype (squamous, adenocarcinoma, adenosquamous, and others), tumor size (≤2 versus >2 cm), lymphvascular invasion (LVSI), lymph node status (metastasis versus no metastasis), deep stromal invasion (outer half), and surgical margins (positive versus negative). Treatment types included use of neoadjuvant chemotherapy, preoperative diagnostic conization, surgical intervention (abdominal, laparoscopic, and vaginal), trachelectomy type (simple, modified radical, and radical), and postoperative treatment (none, concurrent chemoradiotherapy, or systemic chemotherapy). Survival information included follow-up time after surgery, recurrence, and vital status. The cause of death was identified, if deceased.
2.3. Proposed criteria for trachelectomy
The study-specific proposal for the ideal candidates for undergoing fertility sparing trachelectomy is shown in Table 1. To examine the suitable model for ideal candidates, cox proportional hazard regression analysis and IPTW analysis were performed (Supplemental Figs. S1 and S2). The model for ideal candidate criteria was determined the Model 4 for the largest magnitude of the hazard ratio in disease-free survival at IPTW analysis (Mode1: HR 2.54, Model 2: HR 2.28, Model 3: HR 3.80, Model 4: HR 3.93, all P < 0.05). The ideal candidates were those whose tumor factors met the following four criteria (Model 4): (i) size ≤2 cm, (ii) no nodal metastasis, (iii) histologic types with squamous, adenocarcinoma, and adenosquamous, and (iv) without deep stromal invasion. The less-ideal candidates were defined if women did not meet any of these four criteria.
Table 1.
A proposal for an ideal candidate for fertility-sparing trachelectomy for early-stage cervical cancer.
| Model 1 | ||
|---|---|---|
| Characteristics | Ideal candidate | Less-ideal candidate |
| Histology | SCC, AC, AS | Other histology |
| Tumor size | ≤2.0 cm | >2 cm |
| LVSI | No | Yes |
| Model 2 | ||
| Characteristics | Ideal candidate | Less-ideal candidate |
| Histology | SCC, AC, AS | Other histology |
| Tumor size | ≤2.0 cm | >2 cm |
| LVSI | No | Yes |
| Deep stromal invasion | No | Yes |
| Model 3 | ||
| Characteristics | Ideal candidate | Less-ideal candidate |
| Histology | SCC, AC, AS | Other histology |
| Tumor size | ≤2.0 cm | >2 cm |
| Nodal metastasis | No | Yes |
| Model 4 | ||
| Characteristics | Ideal candidate | Less-ideal candidate |
| Histology | SCC, AC, AS | Other histology |
| Tumor size | ≤2.0 cm | >2 cm |
| Nodal metastasis | No | Yes |
| Deep stromal invasion | No | Yes |
Abbreviations: SCC, squamous cell cancer; AC, adenocarcinoma; AS, adenosquamous carcinoma; and LVSI, lymph-vascular space invasion.
2.4. Study definitions
In the analysis, women were grouped according to their age (<40 versus ≥40 years) [11]. Recorded cancer stage was classified based on the 2018 FIGO classification [12]. If women who had a preplanned trachelectomy ultimately resulted in hysterectomy due to intraoperative or postoperative decision, this was allocated to the hysterectomy conversion group. Tumor size was preoperatively measured in a largest diameter by conization specimens or magnetic resonance imaging of the pelvis. Surgery alone was defined as the absence of neoadjuvant chemotherapy or postoperative adjuvant treatment. Disease-free survival (DFS) was defined as the time interval between trachelectomy and the first disease recurrence/progression. Cause-specific survival (CSS) was defined as the time interval between trachelectomy and death due to cervical cancer. Women without survival event were censored at the last follow-up.
2.5. Statistical analysis
In the first-level analysis of the intention-to-treatment comparison (ITT), patient characteristics and trends of ideal trachelectomy candidates were assessed. In the second level-analysis of outcome comparison, survival discriminatory ability of our proposed criteria for fertility-sparing trachelectomy for early-stage cervical cancer was assessed. Specifically, DFS and CSS were compared between the ideal and less-ideal candidates who underwent trachelectomy.
Continuous variables were expressed as the mean (±standard deviation) or as the median (interquartile range, IQR) based on normality, and the statistical difference was assessed using the Student t-test or Mann-Whitney U test, as appropriate. Ordinal and categorical variables were examined using the chi-square test or Fisher exact test, as appropriate. A binary logistic regression model was fitted to identify the independent characteristics associated with the ideal candidates over the less-ideal candidates.
Survival outcomes of women who underwent trachelectomy were assessed by comparing the ideal and less-ideal candidates. The Kaplan-Meier method was used to construct survival curves, and differences between the curves were assessed using the log-rank test. Survival estimate was examined by fitting Cox proportional hazard regression models, expressed as hazard ratio (HR) and 95% confidence interval (CI).
Propensity score-based inverse probability of treatment weighting (PS-IPTW) was used to corroborate the background differences between the ideal and less-ideal groups [13]. PS was determined using a multivariable logistic regression model [14]. The PS model included age, marital status, parity, calendar year at disease diagnosis, neoadjuvant chemotherapy, surgical mode, lymphadenectomy, trachelectomy type, and postoperative treatment. Cancer stage, histology type, tumor size, nodal metastasis, LVSI, and deep stromal invasion were not included in this model owing to multicollinearity.
The IPTW approach assigned ideal candidates with a weight of 1/PS and less-ideal candidates with a weight of 1/(1-PS). To standardize the variability of IPTW and reduce the influence of extreme weights, a stabilized model was used followed by a trimming technique with thresholds of 1% and 99% [13]. After PS-IPTW, the balance of measured confounders was assessed via a weighted logistic regression model, in which each covariate was regressed on the treatment variable. DFS and CSS were assessed in the PS-IPTW models between the ideal and less-ideal candidates.
A series of sensitivity analyses was performed to ensure the robustness of the study findings. Among six subgroups (aged <40 years, tumor size ≤2 cm, squamous tumors, abdominal trachelectomy cases, lymphadenectomy, and surgery alone without pre/postoperative treatment), similar PS-IPTW models were fitted to assess the survival outcome. Moreover, the less-ideal candidates were further stratified based on tumor factor patterns as follows: large tumor size (>2 cm), deep stromal invasion, multiple risk factors without nodal status or lymph node metastasis regardless of tumor size. This was based on the rationale that the most recent NCCN guideline committee advocates that women with a large tumor size may be a possible candidate for trachelectomy [5].
The variance inflation factor was determined using covariates in the multivariable analysis, and a value of ≥2.0 was interpreted to have a multicollinearity. A P < 0.05 was considered to indicate statistical significance (two-tailed hypothesis). Statistical Package for Social Sciences (IBM SPSS, version 25.0, Armonk, NY) and Rversion3.6.0 (R Foundation for Statistical Computing, Vienna, Austria) were used for the statistical analyses. The STROBE guidelines for retrospective observational studies were followed [15].
3. Results
3.1. Cohort selection
The patient selection criteria are shown in Fig. 1. Of 401 women aged <45 years with FIGO stage I-II cervical cancer who underwent preplanned trachelectomy, eight without data on preoperative tumor size, histological subtypes, and nodal status were excluded. The remaining 393 women were included in the ITT cohort analysis and were further categorized into ideal [n = 284 (69.6%)] and less-ideal [n = 109 (30.4%)] candidates.
Fig. 1.
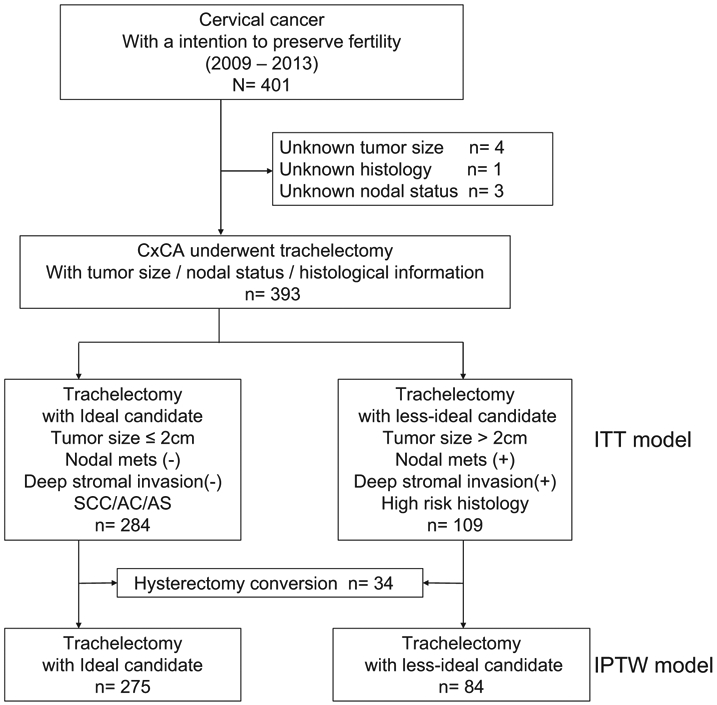
Schema for patient selection.
3.2. Patient characteristics
Patient characteristics in the ITT cohort were shown in Table 2. The majority of women in the ITT cohort were single marital status (52.5%), nullipara (80.5%), had a tumor with squamous cell carcinoma (73.8%), stage IB1 disease (73.5%), underwent abdominal radical trachelectomy (65.1%), and pelvic nodal dissection (74.1%). Only eight women (2.8%) underwent simple trachelectomy. The median age was similar between the two groups. Women in the less-ideal group were more likely to be multiparous, to have FIGO stage II disease, and to have a tumor with size of >2 cm, LVSI, and deep stromal invasion compared to those in ideal group (all, P < 0.05). Additionally, they were more likely to receive neoadjuvant chemotherapy, postoperative treatment, and undergo radical trachelectomy; however, a lesser number of women underwent diagnostic conization than those in the ideal candidates (all, P < 0.05).
Table 2.
Patient demographics for the ITT cohort in model 4 (n = 393).
| Characteristic | Ideal candidate | Less-ideal candidate | P value |
|---|---|---|---|
| Number (%) | 284 (69.6%) | 109 (30.4%) | |
| Age | 33 (±4.4) | 32 (±4.5) | 0.81 |
| ≥40 | 16 (5.6%) | 7 (6.4%) | |
| <40 | 268 (94.4%) | 102 (93.6%) | |
| Marital status | 0.58 | ||
| Single | 143 (50.4%) | 57 (52.3%) | |
| Married | 105 (37.0%) | 35 (32.1%) | |
| Others | 36 (12.7%) | 17 (15.6%) | |
| Parity | 0.002 | ||
| Nullipara | 238 (83.8%) | 76 (69.7%) | |
| Multipara | 46 (16.2%) | 33 (30.3%) | |
| Year at diagnosis | 0.13 | ||
| 2009 | 45 (15.8%) | 26 (23.9%) | |
| 2010 | 56 (19.7%) | 25 (22.9%) | |
| 2011 | 67 (23.6%) | 16 (14.7%) | |
| 2012 | 56 (20.8%) | 18 (16.5%) | |
| 2013 | 57 (20.1%) | 24 (22.0%) | |
| Histology | <0.001 | ||
| Squamous | 210 (73.9%) | 81 (74.3%) | |
| Adenocarcinoma | 60 (21.1%) | 14 (12.8%) | |
| AS | 14 (4.9%) | 6 (5.5%) | |
| Others | 0 | 8 (7.3%) | |
| Neoadjuvant chemotherapy | <0.001a | ||
| Not performed | 284 (100%) | 98 (89.9%) | |
| Performed | 0 | 11 (10.1%) | |
| Diagnostic conization | <0.001 | ||
| Not performed | 84 (29.6%) | 63 (57.8%) | |
| Performed | 200 (70.4%) | 46 (42.2%) | |
| FIGO 2018 | <0.001 | ||
| IA | 39 (13.7%) | 0 | |
| IB | 245 (86.3%) | 99 (90.8%) | |
| II | 0 | 10 (9.2%) | |
| Tumor size | <0.001 | ||
| ≤2 cm | 284 (100%) | 45 (41.3%) | |
| >2 cm | 0 | 64 (58.7%) | |
| Lymph-vascular invasion | <0.001 | ||
| No | 191 (67.3%) | 48 (44.0%) | |
| Yes | 85 (29.9%) | 57 (52.3%) | |
| Unknown | 8 (2.8%) | 4 (3.7%) | |
| Nodal metastasis | <0.001 | ||
| No | 284 (100%) | 77 (70.6%) | |
| Yes | 0 | 32 (29.4%) | |
| Deep stromal invasion | <0.001 | ||
| No | 273 (96.1%) | 57 (52.3%) | |
| Yes | 0 | 49 (45.0%) | |
| Unknown | 11 (3.9%) | 3 (2.8%) | |
| Surgical margins | 0.053a | ||
| Negative | 282 (99.3%) | 105 (96.3%) | |
| Positive | 2 (0.7%) | 4 (3.7%) | |
| Hysterectomy conversion | <0.001 | ||
| Not performed | 275 (96.8%) | 84 (77.1%) | |
| Performed | 9 (3.2%) | 25 (22.9%) | |
| Surgical type | <0.001 | ||
| Abdominal | 264 (93.0%) | 94 (86.2%) | |
| Vaginal | 3 (1.1%) | 11 (10.1%) | |
| Laparoscopica | 17 (6.0%) | 4 (3.7%) | |
| Surgery type | <0.001 | ||
| Simple | 8 (2.8%) | 0 | |
| Modified radical | 65 (22.9%) | 8 (7.3%) | |
| Radical | 211 (74.3%) | 101 (92.7%) | |
| Lymphadenectomy | 0.96 | ||
| Sentinel/biopsy | 71 (25.0%) | 27 (24.8%) | |
| Systematic | 213 (75.0%) | 82 (75.2%) | |
| Adjuvant treatment | <0.001 | ||
| Not performed | 274 (96.5%) | 61 (56.0%) | |
| CCRT | 1 (0.4%) | 13 (11.9%) | |
| Chemotherapy only | 9 (3.2%) | 35 (32.1%) |
Number (percentage per column) or mean (standard deviation) is shown. Univariate analysis with Student's t-test, chi-square test, or Fisher’s exact test for P values. Significant P values are in bold.
Laparoscopic trachelectomy including laparoscopic-assisted surgery.
Among six women with positive surgical margins, two women converted hysterectomy, two women underwent CCRT, and other women rejected to underwent adjuvant therapy. The rate of hysterectomy conversion in less-ideal candidates was significantly higher than that in ideal candidates (22.9% versus 3.2%, P< 0.001).
After excluding 34 women who converted to hysterectomy, 359 women underwent fertility-sparing trachelectomy, and 121 (33.7%) women underwent fertility treatments after this procedure. The rate of postoperative pregnancy with ideal candidates who underwent fertility-sparing trachelectomy was higher than that with less ideal candidates, but the difference was not significant (23.3% versus 14.1%, P = 0.09). Postoperative treatment type was not associated with pregnancy outcome (chemotherapy versus no adjuvant treatment, 15.1% versus 22.4%, P = 0.09). (data not shown).
3.3. Survival outcome
359 women who underwent fertility-sparing trachelectomy were included in the PS-IPTW analysis for assessing survival outcomes (Fig. 1): the ideal candidates n = 275 versus the less-ideal candidates n = 84. The PS-IPTW analysis demonstrated well-balanced baseline clinicopathological characteristics between the two groups (all, P > 0.05; Table 3).
Table 3.
Patient demographics after IPTW for survival analysis in model 4.
| Characteristic | Ideal candidate | Less-ideal candidate | P value |
|---|---|---|---|
| Number (%) | 304 (66.2%) | 155 (33.8%) | |
| Age (years) | 33.2 ± 4.5 | 32.1 ± 4.5 | 0.85 |
| ≥40 | 20 (6.6%) | 11 (7.1%) | |
| <40 | 284 (93.4%) | 144 (92.9%) | |
| Marital status | 0.63 | ||
| Single | 146 (47.9%) | 76 (48.7%) | |
| Married | 111 (36.4%) | 51 (32.7%) | |
| Others | 47 (15.7%) | 28 (18.6%) | |
| Parity | 0.91 | ||
| Nullipara | 241 (79.3%) | 122 (78.2%) | |
| Multipara | 63 (20.7%) | 33 (21.8%) | |
| Year at diagnosis | 0.74 | ||
| 2009 | 53 (17.4%) | 34 (21.8%) | |
| 2010 | 65 (21.4%) | 36 (23.1%) | |
| 2011 | 67 (22.0%) | 29 (18.6%) | |
| 2012 | 57 (18.8%) | 28 (17.9%) | |
| 2013 | 62 (20.4%) | 28 (18.6%) | |
| Neoadjuvant chemotherapy | 0.74 | ||
| Not performed | 298 (98.0%) | 151 (97.4%) | |
| Performed | 6 (2.0%) | 4 (2.6%) | |
| Surgical type | 0.88 | ||
| Abdominal | 270 (88.8%) | 140 (90.3%) | |
| Vaginal | 14 (4.6%) | 6 (3.9%) | |
| Laparoscopica | 20 (6.6%) | 9 (5.8%) | |
| Trachelectomy type | 0.55 | ||
| Simple/modified radical | 62 (20.5%) | 36 (23.2%) | |
| Radical | 242 (79.5%) | 119 (76.8%) | |
| Lymphadenectomy | 0.33 | ||
| Sentinel/biopsy | 82 (27.0%) | 49 (31.6%) | |
| Systematic | 222 (73.0%) | 106 (68.4%) | |
| Adjuvant treatment | 0.75 | ||
| Not performed | 275 (90.5%) | 138 (89.0%) | |
| CCRT | 2 (0.7%) | 2 (1.3%) | |
| Chemotherapy | 27 (8.9%) | 15 (9.7%) |
Number (%) is shown. Univariate analysis with logistic regression analysis for P values.
Laparoscopic trachelectomy including laparoscopic-assisted surgery.
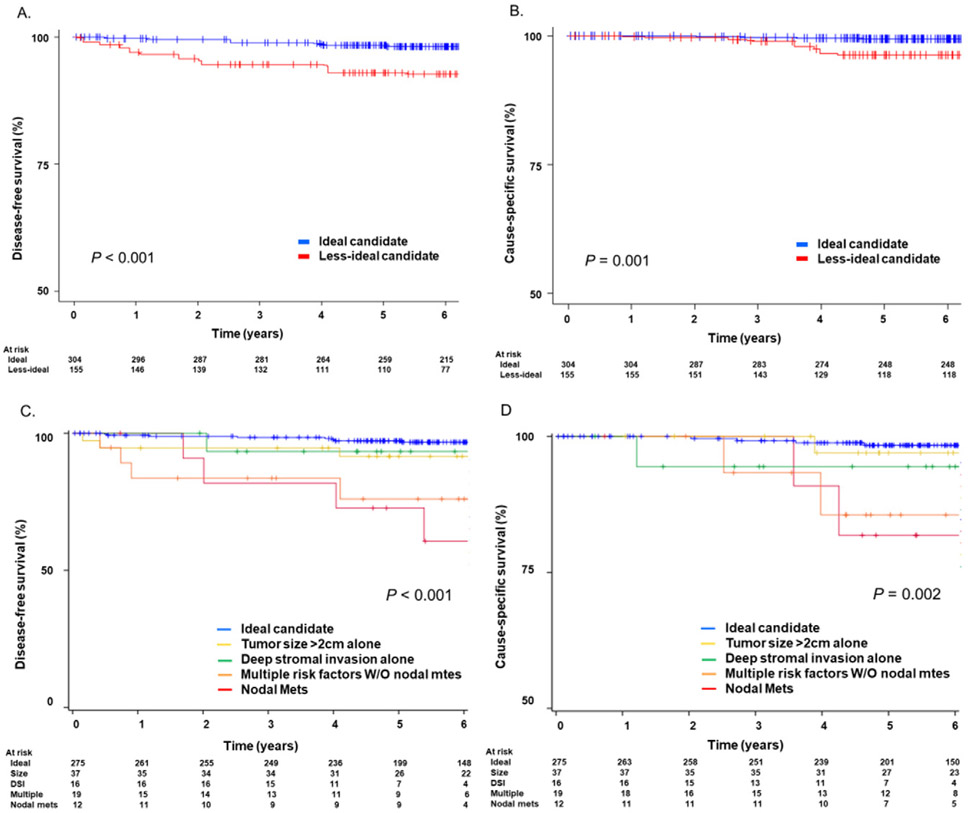
The median follow-up time was 6.2 (IQR, 4.8–7.4) years for the ideal candidate group and 5.9 (IQR4.4–7.6) years for the less-ideal candidates. In the ideal and less-ideal groups (n = 304 and n = 155, respectively), cervical cancer recurrences were noted in 9 (3.3%) and 12 (14.3%) women, and deaths in 4 (1.5%) and 6 (7.1%) women, respectively. The IPTW-adjusted Kaplan-Meier curves demonstrated a significant four-fold increased risk of recurrence (5-year DFS rates: 85.5% versus 95.5%; HR 3.93 95% CI 1.99–7.74, P < 0.001; Fig. 2A) and cause-specific death (5-year CSS rates: 92.5% versus 98.6%; HR 5.47, 95% CI 1.68–17.8, P = 0.001; Fig. 2B) for the less-ideal group compared with those in the ideal group. Of note, among 12 recurrences in the less-ideal candidate group, 6 (50%) recurrences occurred in the first 1 year after surgery.
Fig. 2.
Survival curves: ideal candidates versus less-ideal candidates. P values were derived from IPTW-adjusted log-rank test. A) disease-free survival and B) cause-specific survival are shown between the ideal and less-ideal candidate groups. Y-axis was truncated to 50–100%. Risk-stratified survival curves are shown for C) disease-free survival and D) cause-specific survival between the ideal versus less-ideal candidate groups.
3.4. Sensitivity analyses
Recurrence risks between the ideal and less-ideal candidates were compared using PS-IPTW analysis in various subgroups (Fig. 3). Among 339 women aged <40 years (adjusted 5-year DFS rates: 85.5% versus 97.7%; HR 6.42), 302 women with tumor size ≤2 cm (81.0% versus 96.9%; HR 6.62), 325 women who underwent abdominal surgery (85.8% versus 97.4%; HR 5.76), 266 women who underwent nodal dissection (83.7% versus 97.4%; HR 5.31), and 316 women who underwent surgery alone (84.8% versus 95.8%; HR 3.38), and 265 women with squamous tumors (83.5% versus 94.5%; HR 2.93), the less-ideal candidate group had significantly decreased DFS compared with the ideal candidate group (all, P < 0.05). Specifically, survival difference between the two groups was largest among six subgroups when tumor size was ≤2 cm.
Fig. 3.
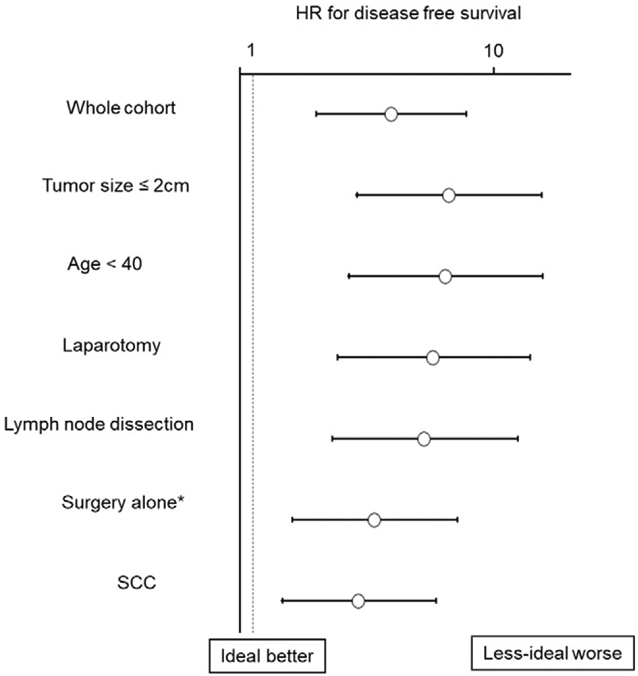
Forest plots for HR for disease-free survival (IPTW models). Cox proportional hazard regression models for analysis. In each subgroup analysis, IPTW was fitted to compare the ideal and less-ideal candidate groups. HR represents the less-ideal candidate group versus the ideal candidate group. In all the subgroups, the less-ideal group had significantly decreased disease-free survival compared with that of the ideal group. X-axis was transformed to log10 scale. Circles represent IPTW-HR, and bars represent 95% confidence intervals. Abbreviations: HR, hazard ratio; IPTW, inverse probability of treatment weighting; SCC, squamous cell carcinoma. *Trachelectomy alone without neoadjuvant chemotherapy or postoperative treatment.
3.5. Less-ideal candidates: tumor factors patterns
Another sensitivity test was performed by stratifying the pattern of tumor factors in the less-ideal group. Candidates in the less-ideal group were categorized into four subgroups: those with tumor size >2 cm alone (n = 37), those with deep stromal invasion alone (n = 16), those with multiple risk factors without nodal metastasis (n = 19), and those with nodal metastasis regardless of tumor size (n = 12). Comparisons with the ideal group demonstrated that patients with tumor size >2 cm (21.6% versus 3.2%) and those with deep stromal invasion (43.8% versus 3.2%) were more likely to receive postoperative chemotherapy, and those with nodal metastasis were more likely to receive postoperative concurrent chemoradiation therapy (25.0% versus 0.4%) (all, P < 0.05; Supplemental Table S1).
Women with single risk factor had a similar recurrence risk to those with ideal tumor characteristics; tumor size >2 cm (5-year DFS rates: 91.3% versus 97.2%; HR, 2.17, 95% CI 0.28–12.2, P = 0.46) and deep stromal invasion (92.8% versus 97.2%; HR, 2.48, 95%CI, 0.67–9.16, P = 0.17). However, women with multiple risk factors regardless of no nodal metastasis; large tumor size, deep stromal invasion, or high-risk histology (81.3% versus 97.2%; HR, 10.9, 95% CI, 3.35–35.6, P < 0.001) and those with nodal metastasis (71.5% versus 97.2%; HR, 11.5, 95% CI, 3.55–37.5, P < 0.001) had significantly increased recurrence risk compared with those with ideal tumor characteristics (Fig. 2C). CSS was similar between the large tumor group and the ideal candidate group (P = 0.58), however, women with nodal metastasis had significantly poorer CSS than ideal candidates (P < 0.001) (Fig. 2D).
4. Discussion
4.1. Principal findings
Our study found that tumor factors are the key to provide successful treatment through fertility-sparing trachelectomy for early-stage cervical cancer. When tumors were small (≤2 cm),and when they do not present nodal metastasis, deep stromal invasion, or high-risk histology types, oncologic outcome after fertility-sparing trachelectomy was decent with 5-year recurrence rate being 2.8%. In contrast, when tumors were large, had nodal metastasis, deep stromal invasion, or high-risk histology types, the recurrence risk were considerably high (5-year rate, 16.6%).
4.2. Results and clinical implication
4.2.1. Nodal metastasis
Fertility-sparing trachelectomy is a relatively new treatment approach, and its strategic algorithm for the treatment remains controversial. To date, there is no definitive guideline for women with early-stage cervical cancer who wish to undergo fertility-sparing trachelectomy in Japan. Therefore, we evaluated the ideal candidate based on tumor factors for recurrence. In previous studies, women with early-stage cervical cancer with histopathologically confirmed nodal metastasis had a higher risk of disease recurrence [3,16]. Except that with isolated small local relapse, the prognosis of recurrent cervical cancer is grim [17]. Our study suggests that regardless of adjuvant chemotherapy or concurrent chemoradiation, these women with nodal metastasis had considerable recurrence and poor survival.
Small lymph node metastasis may be difficult to identify on preoperative work up and larger nodal metastasis on preoperative imaging are probably not the ideal trachelectomy. Therefore, we propose that the performance of nodal evaluation at the time of fertility-sparing trachelectomy should be strongly considered and women with nodal metastasis should not be treated as ideal candidates for this procedure.
4.2.2. Deep stromal invasion
According to JSOG treatment guideline for cervical cancer, women in intermediate risk are defined as having at least one risk factor (deep stromal invasion, LVSI, and large tumor) and without nodal/parametrial involvement [6]. In our study, women with single risk factor, such as deep stromal invasion alone or larger tumor size alone, had similar survival to those with ideal group. However, women with multiple risk factors were associated with increased risk of recurrence and had similar hazard ratio to that of nodal metastasis group. This finding supports that the criteria of intermediate risk group in cervical cancer according to the NCCN guideline require at least two factors [5] and recommends postoperative treatment [18].
4.2.3. Tumor size
Tumor size is one of the predictors of survival outcome [19]. The NCCN guidelines recently suggested that selected women with early-stage cervical cancer with a tumor size of 2–3.9 cm have to be carefully evaluated for a fertility-sparing trachelectomy [5]. This recommendation reflects the recent increasing interest on fertility-sparing trachelectomy for a large tumor size in the United States [20]. Our study showed non-inferior cervical cancer disease recurrence in women with tumor size >2 cm and ideal candidates (tumor size ≤2 cm). Therefore, our results may partly support the recent change in the NCCN guidelines.
Women with large tumors of 2–3.9 cm can indeed “technically” be offered a trachelectomy, although prior studies have shown that approximately half of the women in early-stage cervical cancer with large tumors may receive postoperative adjuvant therapy due to pathologic risk factors [21,22]. In fact, approximately half of the group with tumor size >2 cm (45.1%) had multiple risk factors of disease recurrence in our study. Therefore, some gynecologists are proposing neoadjuvant chemotherapy followed by fertility-sparing surgery for such tumor size. However, this option is performed only in few centers, and remains largely in the experimental stage [23].
Our study showed that one-fifth of the women with large tumor size received postoperative chemotherapy. Ovarian function is supposedly preserved by adjuvant chemotherapy as opposed to radiation-based therapy. Moreover, limited data implies that oncologic outcomes are comparable between radiation-based and chemotherapy-based therapy for high- and intermediate-risk cervical cancer [18,24]. Therefore, while the current standard for adjuvant therapy remains concurrent chemoradiotherapy, a role of adjuvant chemotherapy for the purpose of fertility preservation merits further investigation [25].
4.2.4. Histology type
There are some arguments associated with specific histological subtypes and a risk of recurrence in early-stage cervical cancer. Prior studies have shown that histological types did not affect the survival for stage I disease but stage II with adenocarcinoma exhibited a significantly worse prognosis compared with that of squamous cell carcinoma [26,27]. However, these studies lacked the details of histological subtypes of adenocarcinoma. The variants of adenocarcinoma as gastric type or adenoma malignum, although rare in the United States and European countries, are rather common in Japan, where 20% of adenocarcinomas show an aggressive phenotype [28]. In addition, neuroendocrine tumors are associated with rapid and widespread metastasis that results in poor survival even in the early-stage disease [29]. While data are limited for these histological subtypes due to rarity, it is paramount for clinicians to exclude these histological subtypes while considering candidates for trachelectomy.
4.3. Strengths and limitations
Strength of this study is that this is the largest study conducted to identify ideal candidates with early-stage cervical cancer for fertility-sparing trachelectomy. Statistical approach with PS-IPTW enhanced the statistical rigor of our findings, and adequate follow-up of approximately six years strengthened our interpretation of survival analysis. Sensitivity analysis strengthened the robustness of the study findings.
Several limitations must be acknowledged. First, unmeasured bias inherent to the nature of retrospective studies exists. For instance, there were inadequate details regarding detailed decision-making process for treatment planning, adenocarcinoma subtypes, surgeon's experience for performing trachelectomy, surgical volume, and surveillance protocol, all of which may impact the outcome. Additionally, only 21 centers agreed to share their data, but it covered 87.2% of all patients who had planned fertility-sparing trachelectomy from 2009 to 2013 in Japan. Moreover, regardless of LVSI status, 30 women with IA stage were included in our study and they may be considered for non-radical surgery, such as cone biopsy and nodal assessment.
Lastly, sample size remained limited for performing further sensitivity analysis. Recent studies showed that minimally-invasive radical hysterectomy was associated with increased mortality compared with abdominal radical hysterectomy for early-stage cervical cancer [30,31]. In our study, laparoscopic trachelectomy was performed only in 21 cases, and examining the safety of minimally-invasive trachelectomy was not feasible because of the small number of participants. However, another study demonstrated that minimally-invasive trachelectomy has non-inferior survival rates compared with that of abdominal trachelectomy [32].
4.4. Conclusions
Proposed candidate criteria for fertility-sparing trachelectomy for early-stage cervical cancer in this study clearly distinguished survival outcome with considerably favorable survival when women meet the criteria compared with those who do not. The increase in the number of ideal candidates during the study period implied that surgeons are likely to consider tumor characteristics in their decision-making process. Specific guidelines for the use of fertility-sparing trachelectomy in women with early-stage cervical cancer are being actively developed in Japan. Our study team proposed that the features of the ideal candidate, identified in this study, be used as the future framework for developing guidelines in Japan.
This study team also endorses that establishing the multidisciplinary approach as well as infrastructure setup particularly for experienced gynecologic oncological surgeons and gynecologic pathologists for intraoperative frozen section to evaluate nodal status as well as surgical margin would be the key to provide sufficient care to reproductive-aged women who wish to have fertility-sparing trachelectomy.
Supplementary Material
HIGHLIGHTS.
A retrospective study was performed for 393 women underwent fertility-sparing trachelectomy for stage I-II cervical cancer.
Women without tumor size>2cm, deep stromal invasion, nodal metastasis, or high-risk histology may be ideal candidates.
Less-ideal candidates had approximately four-fold higher recurrence risk and mortality compared with ideal candidates.
Adjuvant chemotherapy for women with large tumors may have benefit for the purpose of fertility preservation.
Acknowledgments
We thank all the Japanese Gynecologic Oncology Group participating sites for this study. Especially, Keio University School of Medicine, Kyushu University School of Medicine, Kitano Hospital, Sapporo medical University, Osaka University Graduate School of Medicine, Tohoku University, Kyoto Prefectural University of Medicine, Hyogo Cancer Center, University of The Ryukyus, Osaka International Cancer Institute, Niigata University School of Medicine, Gifu University Graduate School of Medicine, Yamagata University, Fukushima Medical University Hospital, Kanazawa University, Ehime University, Teikyo University, Akita University Graduate School of Medicine, and Yamaguchi University Graduate School of Medicine.
Funding support
None.
Footnotes
Disclosure statement
Honorarium, Chugai, textbook editorial expense, Springer, and investigator meeting attendance expense, VBL therapeutics (K.M.); honorarium, Chugai, Astrazenca (T.E.); and none for others.
Ethical committee approval
Japan Society of Obstetrics and Gynecology (2018-68), and Tokai ethical committee (17R093).
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ygyno.2019.11.021.
References
- [1].Projected Cancer Statistics, Cancer information service, https://ganjoho.jp/en/public/statistics/short_pred.html, 2018. Accessed date: 4 September 2019.
- [2].Peters WA 3rd, Liu PY, Barrett RJ 2nd, Stock RJ, Monk BJ, Berek JS, et al. , Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix, J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol 18 (2000) 1606–1613. [DOI] [PubMed] [Google Scholar]
- [3].Monk BJ, Wang J, Im S, Stock RJ, Peters WA 3rd, Liu PY, et al. , Rethinking the use of radiation and chemotherapy after radical hysterectomy: a clinical-pathologic analysis of a Gynecologic Oncology Group/Southwest Oncology Group/Radiation Therapy Oncology Group trial, Gynecol. Oncol 96 (2005) 721–728. [DOI] [PubMed] [Google Scholar]
- [4].Querleu D, Morrow CP, Classification of radical hysterectomy, Lancet Oncol. 9 (2008) 297–303. [DOI] [PubMed] [Google Scholar]
- [5].Cervical cancer. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines), https://www.nccn.org/professionals/physician_gls/pdf/cervical.pdf, Accessed date: 20 August 2019.
- [6].Ebina Y, Mikami M, Nagase S, Tabata T, Kaneuchi M, Tashiro H, et al. , Japan Society of Gynecologic Oncology guidelines 2017 for the treatment of uterine cervical cancer, Int. J. Clin. Oncol 24 (2019) 1–19. [DOI] [PubMed] [Google Scholar]
- [7].Cui RR, Chen L, Tergas AI, Hou JY, St Clair CM, Neugut AI, et al. , Trends in use and survival associated with fertility-sparing trachelectomy for young women with early-stage cervical cancer, Obstet. Gynecol 131 (2018) 1085–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Machida H, Mandelbaum RS, Mikami M, Enomoto T, Sonoda Y, Grubbs BH, et al. , Characteristics and outcomes of reproductive-aged women with early-stage cervical cancer: trachelectomy vs hysterectomy, Am. J. Obstet. Gynecol 219 (461) (2018) e1–e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sedlis A, Bundy BN, Rotman MZ, Lentz SS, Muderspach LI, Zaino RJ, A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage IB carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: a Gynecologic Oncology Group Study, Gynecol. Oncol 73 (1999) 177–183. [DOI] [PubMed] [Google Scholar]
- [10].Retherford RD NOaRM, Late marriage and less marriage in Japan, Popul. Dev. Rev 27 (2001) 65–102. [Google Scholar]
- [11].Wright JD, NathavithArana R, Lewin SN, Sun X, Deutsch I, Burke WM, et al. , Fertility-conserving surgery for young women with stage IA1 cervical cancer: safety and access, Obstet. Gynecol 115 (2010) 585–590. [DOI] [PubMed] [Google Scholar]
- [12].Bhatla N, Aoki D, Sharma DN, Sankaranarayanan R, Cancer of the cervix uteri, Int. J. Gynaecol. Obstet 143 (Suppl. 2) (2018) 22–36. [DOI] [PubMed] [Google Scholar]
- [13].Austin PC, Stuart EA, Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies, Stat. Med 34 (2015) 3661–3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Joffe MM, Rosenbaum PR, Invited commentary: propensity scores, Am. J. Epidemiol 150 (1999) 327–333. [DOI] [PubMed] [Google Scholar]
- [15].von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies, BMJ (Clin. Res. Ed.) 335 (2007) 806–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Macdonald OK, Chen J, Dodson M, Lee CM, Gaffney DK, Prognostic significance of histology and positive lymph node involvement following radical hysterectomy in carcinoma of the cervix, Am. J. Clin. Oncol 32 (2009) 411–416. [DOI] [PubMed] [Google Scholar]
- [17].Taarnhoj GA, Christensen IJ, Lajer H, Fuglsang K, Jeppesen MM, Kahr HS, et al. , Risk of recurrence, prognosis, and follow-up for Danish women with cervical cancer in 2005-2013: a national cohort study, Cancer 124 (2018) 943–951. [DOI] [PubMed] [Google Scholar]
- [18].Matsuo K, Shimada M, Yokota H, Satoh T, Katabuchi H, Kodama S, et al. , Effectiveness of adjuvant systemic chemotherapy for intermediate-risk stage IB cervical cancer, Oncotarget 8 (2017) 106866–106875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Park JY, Kim DY, Kim JH, Kim YM, Kim YT, Nam JH, Outcomes after radical hysterectomy according to tumor size divided by 2-cm interval in patients with early cervical cancer, Ann. Oncol 22 (2011) 59–67. [DOI] [PubMed] [Google Scholar]
- [20].Matsuo K, Machida H, Mandelbaum RS, Mikami M, Enomoto T, Roman LD, et al. , Trachelectomy for stage IB1 cervical cancer with tumor size >2 cm: trends and characteristics in the United States, J. Gynecol. Oncol 29 (2018) e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wethington SL, Sonoda Y, Park KJ, Alektiar KM, Tew WP, Chi DS, et al. , Expanding the indications for radical trachelectomy: a report on 29 patients with stage IB1 tumors measuring 2 to 4 centimeters, Int. J. Gynecol. Cancer 23 (2013) 1092–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Li J, Wu X, Li X, Ju X, Abdominal radical trachelectomy: is it safe for IB1 cervical cancer with tumors >/= 2 cm? Gynecol. Oncol 131 (2013) 87–92. [DOI] [PubMed] [Google Scholar]
- [23].Robova H, Rob L, Halaska MJ, Pluta M, Skapa P, Review of neoadjuvant chemotherapy and trachelectomy: which cervical cancer patients would be suitable for neoadjuvant chemotherapy followed by fertility-sparing surgery? Curr. Oncol. Rep 17 (2015) 446. [DOI] [PubMed] [Google Scholar]
- [24].Matsuo K, Shimada M, Aoki Y, Sakamoto M, Takeshima N, Fujiwara H, et al. , Comparison of adjuvant therapy for node-positive clinical stage IB-IIB cervical cancer: systemic chemotherapy versus pelvic irradiation, Int. J. Cancer 141 (2017) 1042–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Waimey KE, Smith BM, Confino R, Jeruss JS, Pavone ME, Understanding fertility in young female cancer patients, J. Women’s Health (Larchmt) 24 (2015) 812–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Spoozak L, Lewin SN, Burke WM, Deutsch I, Sun X, Herzog TJ, et al. , Microinvasive adenocarcinoma of the cervix, Am. J. Obstet. Gynecol 206 (2012) (80.e1–6). [DOI] [PubMed] [Google Scholar]
- [27].Shimada M, Nishimura R, Nogawa T, Hatae M, Takehara K, Yamada H, et al. , Comparison of the outcome between cervical adenocarcinoma and squamous cell carcinoma patients with adjuvant radiotherapy following radical surgery: SGSG/TGCU Intergroup Surveillance, Mol. Clin. Oncol 1 (2013) 780–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Nishio S, Mikami Y, Tokunaga H, Yaegashi N, Satoh T, Saito M, et al. , Analysis of gastric-type mucinous carcinoma of the uterine cervix - an aggressive tumor with a poor prognosis: a multi-institutional study, Gynecol. Oncol 153 (2019) 13–19. [DOI] [PubMed] [Google Scholar]
- [29].Viswanathan AN, Deavers MT, Jhingran A, Ramirez PT, Levenback C, Eifel PJ, Small cell neuroendocrine carcinoma of the cervix: outcome and patterns of recurrence, Gynecol. Oncol 93 (2004) 27–33. [DOI] [PubMed] [Google Scholar]
- [30].Ramirez PT, Frumovitz M, Pareja R, Lopez A, Vieira M, Ribeiro R, et al. , Minimally invasive versus abdominal radical hysterectomy for cervical cancer, N. Engl. J. Med 379 (2018) 1895–1904. [DOI] [PubMed] [Google Scholar]
- [31].Melamed A, Margul DJ, Chen L, Keating NL, Del Carmen MG, Yang J, et al. , Survival after minimally invasive radical hysterectomy for early-stage cervical cancer, N. Engl. J. Med 379 (2018) 1905–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Matsuo K, Chen L, Mandelbaum RS, Melamed A, Roman LD, Wright JD, Trachelectomy for reproductive-aged women with early-stage cervical cancer: minimally invasive surgery versus laparotomy, Am. J. Obstet. Gynecol 220 (469) (2019) e1–e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.