Abstract
A major goal of rehabilitation strategies after spinal cord injury (SCI) is to enhance the recovery of function. One possible avenue to achieve this goal is to strengthen the efficacy of the residual neuronal pathways. Non-invasive repetitive transcranial magnetic stimulation (rTMS) has been used in patients with motor disorders as a tool to modulate activity of residual cortical, subcortical, and the corticospinal pathway to promote functional recovery. Here, we review a series of studies published during the last decade that used rTMS in the acute and chronic stages of para- and tetraplegia in humans with complete and incomplete SCI. rTMS has been applied over the arm and leg representations of the primary motor cortex to treat three main consequences of SCI: sensory and motor function impairments, spasticity, and neuropathic pain. Though some studies demonstrated that consecutive sessions of rTMS improve aspects of particular functions, other studies did not show similar effects. We discuss how rTMS stimulation parameters and post-injury reorganization in the corticospinal tract, motor cortical and spinal cord circuits might be critical factors in understanding the advantages and disadvantages of using rTMS in SCI patients. The available data highlight the limited information on the use of rTMS after SCI and the need to further understand the pathophysiology of neuronal structures affected by rTMS to maximize the potential beneficial effects of this technique in humans with SCI.
Keywords: Plasticity, motor control, primary motor cortex, corticospinal tract, reorganization, non-invasive brain stimulation
Introduction
In the U.S. around 12,000 people suffer from spinal cord injury (SCI) every year (https://www.nscisc.uab.edu). SCI impairs sensory and motor function affecting numerous daily life activities such as walking, grasping, eating, writing, and several other body functions. More than 60% of individuals with SCI develop symptoms of spasticity1 and over 80% suffer from chronic neuropathic pain in the paralyzed body parts below the lesion.2 Altogether, these SCI-induced impairments result in severe decline in the patient’s quality of life.
Anatomical and electrophysiological studies have demonstrated that extensive reorganization takes place in the corticospinal tract, primary motor cortex (M1), and spinal cord in animals and humans after SCI.3,4 These neuronal pathways are largely involved in the control of voluntary movements in mammals4 and, during the last decade, have been prominent targets for investigating injury-induced plasticity and motor recovery in animals and humans with SCI.3,5,6 Since one of the major goals of rehabilitation strategies after SCI is to enhance the recovery of sensory and motor function it is critical to further understand methodologies available in humans to access and/or alter activity in the corticospinal tract, M1, and spinal cord structures. In this review we will focus on the use repetitive transcranial magnetic stimulation (rTMS).
Single TMS pulses have been used as a non-invasive and painless method to stimulate the brain of intact conscious human subjects through the scalp.7 TMS has been used most extensively in the corticospinal system since the output of the motor cortex can be easily assessed in the form of a motor evoked potential (MEP) by using surface electromyographic (EMG) recording electrodes. Changes in the amplitude, latency, and threshold of MEPs have been used to make inferences about corticospinal transmission after SCI. Resting motor threshold (RMT) is usually defined as the minimum stimulus intensity required to elicit MEPs greater than 50 μV peak-to-peak amplitude in at least 5 out of 10 consecutive trials in the relaxed muscle while active motor threshold (AMT) is usually defined as the minimal stimulus intensity able to evoke MEPs bigger than 100–200 μV peak-to-peak amplitude in at least 5 out of 10 consecutive trials during a small isometric voluntary contraction.8 During measurements of threshold, the initial cortical elements activated by TMS are likely to be large diameter myelinated axons but MEPs are evoked after a sequence of synaptic relays that can occur at the level of cortex and spinal cord. TMS was first used in patients with SCI in the early 1990’s9,10 and since then an increasing number of studies in the field of motor control have used TMS to assess the impact of the injury on transmission in motor cortical and corticospinal pathways including the efficacy of synaptic transmission.7,11
When single TMS pulses are applied repeatedly, referred to as rTMS, at times long-lasting changes in the excitability of the corticospinal tract, M1, and spinal cord structures can be elicited resulting in significant improvements in aspects of sensory and motor function in patients with motor disorders.12 This review examines how during the last decade rTMS has been used over the arm and leg representations of the M1 in the acute and chronic stage of humans with complete and incomplete para- and tetraplegia. Overall, rTMS has been used to target three main consequences of SCI: a) sensory and motor function impairments, b) spasticity, and c) neuropathic pain. We will discuss the impact of methodological parameters of rTMS and the extensive reorganization taking place in the corticospinal tract, M1, and spinal cord after SCI; critical factors to consider for understanding the advantages and disadvantages of using rTMS in humans with SCI.
Effects of rTMS on sensory and motor function after SCI
Neurorehabilitation programs improve motor outcomes by enhancing activity in neuronal pathways involved in the generation of voluntary movement.13 Evidence has shown that the effects of rTMS depend, at least in part, on the activity in N-Methyl-D-Aspartate (NMDA) receptors resembling mechanisms involved in long-term potentiation and depression of synaptic transmission.14 Therefore, it seems sensible to expect that rTMS could also be a viable strategy to enhance sensory and motor function in patients with SCI. rTMS can be used in a variety of arrangements which might be geared towards increasing or decreasing brain activity. Generally high frequency rTMS (≥ 5 Hz) increases corticospinal and M1 excitability whereas low frequency rTMS (≤ 1 Hz) decrease it.14 Evidence has shown that both types of stimulation also cause changes in the excitability of spinal neuronal circuits15–17 and even low frequency rTMS can affect efficiency of corticomotoneuronal synapses.18 A more specific pattern of rTMS, known as theta burst stimulation (TBS), which consists of a burst of 3 pulses at 50 Hz repeated every 200 ms, uses high frequency protocols to either increase or decrease activity in motor cortical circuits.19 For example, TBS intermittently delivered for 2 seconds (intermittent TBS, iTBS) often results in increments in motor cortical excitability, whereas uninterrupted TBS delivered for 40 seconds (continuous TBS, cTBS) often suppresses motor cortical excitability. Although the results from using different forms of rTMS are variable it is also highlighted that rTMS may represent a useful technique to promote the ability to achieve measurable improvements in behaviors after central nervous system injury.14 Indeed, a large number of studies have applied rTMS over the arm and leg motor representations in the M1 in healthy controls and in patients with motor disorder such as stroke, Parkinson’s disease, dystonia, and multiple sclerosis to modulate motor cortical and spinal physiological outcomes, voluntary motor output, and motor learning processes.12
To date, three studies have examined the effects of rTMS over the M1 on sensory and motor function in patients with SCI.6,20,21 Belci et al.20 used 10 Hz high frequency rTMS over the hand representation of the M1 in 4 patients with chronic incomplete SCI. Patients participated in 5 days of sham rTMS over the occipital cortex followed by 5 days of real rTMS over the area of the M1 innervating thenar muscles (see parameters of rTMS in Table 1). After the real rTMS sessions, sensory and motor function assessed by the American Spinal Injury Association (ASIA) scale was improved. In addition, the mean time to complete a Nine-Hole-Peg-Test decreased by ~10% and the electrical perceptual threshold (EPT) decreased by ~15%. These improvements persisted for at least 3 weeks. Neurophysiological assessment revealed that the duration of the cortical silent period (a measurement of intracortical inhibition) elicited by TMS during a small voluntary contraction in thenar muscles was decreased by > 30% during the period of real rTMS but had returned to baseline after 3 weeks.
Table 1.
Patients information and rTMS parameters
| SCI patients | rTMS parameters | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Target function/symptoms | Number of patients | Age (years) | Level | ASIA scale | C/I | Time after SCI (years) | Etiology | Site within M1 | Frequency (Hz) | Number of pulses | Intensity | Number of sessions |
| Sensory and motor function | ||||||||||||
| Belci et al., 2004 | 4 | 43.8±13.2 | C5 | D | I | 1.3–8 | NR | Hand | 10 | 720 (2 p/10 s) | 90% RMT (Hand) | 5 |
| Kuppuswamy et al., 2011 | 15 | 39.7±9.8 | C2-T3 | A-D | C,I | 10.3±6.9 | T,NT | Hand/Arm | 5 | 900 (10 p/10 s) | 80% AMT (Hand/Arm) | 5 |
| Benito et al., 2012 | 17 | 37.3±14.1 | C4-T12 | D | I | 0.6±0.3 | T,NT | Leg | 20 | 1800 (40 p/30 s) | 90% RMT (Upper limb) | 15 |
| Spasticity | ||||||||||||
| Kumru et al., 2010 | 15 | 36.2±15.8 | C5-T12 | C,D | I | 0.6±0.3 | T,NT | Leg | 20 | 1600 (40 p/30 s) | 90% RMT (BB) | 5 |
| Benito et al., 2012 | 17 | 37.3±14.1 | C4-T12 | D | I | 0.6±0.3 | T,NT | Leg | 20 | 1800 (40 p/30 s) | 90% RMT (Upper limb) | 15 |
| Neuropathic pain | ||||||||||||
| André-Obadia et al., 2006 | 1 | 46 | Cervical | NR | NR | NR | NR | Hand | 1,20 | 1600 | 90% RMT (ADM) | 1 |
| Defrin et al., 2007 | 11 | 54.6±6.6 | T4-T12 | NR | C,I | >1 | T | Leg | 5 | 500 (50 p/30 s) | 115% RMT (APB) | 10 |
| Kang et al., 2009 | 11 | 54.8±13.7 | C6-T11 | A-D | C,I | 5.0±5.4 | NR | Hand | 10 | 1000 (50 p/60 s) | 80% RMT (FDI) | 5 |
| Jetté et al., 2013 | 16 | 50.3±9.3 | C1-L4 | A,C,D | C,I | 7.8±8.3 | NR | Hand/Leg | 10 | 2000 (40 p/30 s) | M1 Hand; 90% RMT (FDI) | 1 |
| M1 Leg; 110% RMT (FDI) | ||||||||||||
| Yılmaz et al., 2013 | 16 | 38.6±6.5 | T4-L1 | A-D | C,I | >1 | NR | Leg | 10 | 1500 (30 p/30 s) | 110% RMT (FPB) | 10 |
ADM: abductor digiti minimi, AMT: active motor treshold, APB: abductor pollicis brevis, ASIA: American Spinal Injury Association, BB: biceps brachii, C/I: complete injury/incomplete injury, FDI: first dorsal interosseous, FPB: Flexor pollicis brevis, M1: primary motor cortex, NR: not reported, NT: nontrauma, RMT: resting motor threshold, rTMS: repetitive transcranial magnetic stimulation, SCI: spinal cord injury, T: trauma
More recently, Kuppuswamy et al.6 used 5 Hz high frequency rTMS over the hand and arm representation of the M1 in 15 patients with chronic incomplete and complete SCI. The study used a single-blind, randomized, sham-stimulation controlled, and crossover design. Patients participated in 5 days of sham rTMS over the vertex and 5 days of real rTMS over the motor presentation of the upper limb muscle showing the lowest RMT (see rTMS parameters in Table 1). In contrast to Belci’s study, rTMS did not elicit changes in sensory and motor function assessed by the ASIA scale and in most of the neurophysiological assessments including the RMT, MEP amplitude, or in the duration of the cortical silent period. Only the AMT measured in the first dorsal interosseous muscle increased at 72 and 120 hours after real rTMS compared with baseline. In the last study, Benito et al.21 examined the effects of 20 Hz high frequency rTMS over the leg representation of the M1 in 17 patients with sub-acute incomplete SCI. The study also used a double-blind, randomized, sham-stimulation controlled, and crossover design but 15 sessions of sham rTMS over the vertex and 15 sessions of rTMS over the same area were tested (see Table 1). As in the Belci’s study, rTMS elicited significant improvements in motor function assessed by the ASIA scale. Gait function (assessed by 10-meter walking and Timed Up and Go test) was improved for at least 2 weeks. However, neurophysiological testing was not conducted in this study.
In summary, three studies have been conducted to investigate the effects of rTMS on sensory and motor function in patients with SCI. It is difficult to compare the results across studies because of differences in stimulation parameters (intensity, frequency, and number of pulses), number of sessions, region of the M1 targeted, type of patients, and outcome measurements. From a functional point of view, the improvements reported in sensory and motor function are limited and variable and appear to be present when higher rTMS intensities were used. From a physiological point of view, changes in motor threshold and cortical silent period are also limited and variable and the neuronal mechanisms underlying the few improvements remain to be tested. Thus, the overall range of parameters that have been used to date do not appear to be sufficient to engage prominent and consistent changes in sensory and motor function in patients with SCI. This is important to consider when designing future interventions using rTMS after SCI. It was noted that all studies used high frequency rTMS over the hand and/or leg representations of the M1 in patients with sub-acute and/or chronic para- and tetraplegia. Therefore, spinal motoneurons of the muscles targeted by rTMS were at times located above or below the level of the injury. Recent evidence demonstrated differences in reorganization in the corticospinal and other descending pathways according to the distance from the injury site.22,23 Also, high frequency rTMS affects activity of spinal cord circuitry when using higher stimulus intensities than those used in the present studies.15,16 Thus, consideration of the muscle tested regarding the injury level and the stimulus intensity might contribute to enhance the effects of rTMS protocols.
Effects of rTMS on spasticity after SCI
More than 60% of individuals with chronic SCI develop symptoms of spasticity including muscle spasms, increased tone, hyperreflexia, and involuntary movements.1 In humans and nonhuman primates, corticospinal cells exert modulation over a large group of spinal interneurones.24 Therefore, it is possible that activating corticospinal neurons by rTMS may also induce long-lasting changes in spinal neuronal circuitries. Indeed, evidence has shown that a train of high frequency (≥ 5 Hz) rTMS pulses resulted in changes in the size of the H-reflex in healthy controls.15,16 Repeated sessions of high frequency rTMS are also effective in decreasing spasticity in patients with other motor disorders including multiple sclerosis and stroke.25 Furthermore, recent evidence has shown that baclofen, a gamma-aminobutyric acid-B (GABAB) receptor agonist commonly used to relieve spasticity related to SCI, selectively maintains use-dependent modulation of largely subcortical but not cortical GABAB neuronal pathways in patients with SCI, which may contribute to the effects of baclofen on spasticity in patients with SCI.26 Since the neuronal mechanisms contributing to spasticity are in part mediated by spinal cord pathways, which can be accessed by rTMS protocols, it seems possible that rTMS could also be a viable strategy to decrease spasticity after SCI.
Two studies have reported the effect of rTMS over the leg representation of the M1 on spasticity in patients with incomplete SCI.21,27 Kumru et al.27 used 20 Hz high frequency rTMS over the leg representation of the M1 in 15 patients with sub-acute and chronic incomplete SCI. The study used a double-blind, randomized, sham-stimulation controlled design. Patients participated in 5 days of sham rTMS over the vertex and 5 days of real rTMS over the same region (see Table 1). Neurophysiological assessment revealed that rTMS had no effects on the excitability of spinal reflexes including the Hoffmann (H-) reflex, tendon tap, and withdrawal reflexes. Nevertheless, the clinical assessment [Modified Ashworth Scale, Visual Analogue Scale (VAS) of subjective spastic sensation, Modified Pen Spasm Frequency Scale, and Spinal Cord Association Tool for Spasticity] demonstrated a significant decline in leg spasticity after the real rTMS sessions for 1 week. Benito et al.21 (study described in the previous section) found a decrease in the Modified Ashworth Scale after real rTMS for 2 weeks. No neurophysiological testing was conducted.
In summary, two studies have reported the effects of rTMS on spasticity in patients with incomplete SCI. It is important to note that these studies used similar stimulation parameters (intensity, frequency, and number of pulses), region of the M1 targeted, type of patients, and outcome measurements. Although the number of sessions was different and patients were tested in the more acute phase after SCI, all patients had an incomplete SCI, and all studies reported some reduction in the clinical symptoms of spasticity. The neurophysiological mechanisms underlying these improvements were either not tested or the electrophysiological measurements examined remained unchanged. Also, a concern is, at times there is a discrepancy between changes in spinal reflexes and clinical measurements of spasticity. As noted in the previous section, both studies used 20 Hz of high frequency rTMS and here it was used over the leg representation of the M1 over repeated sessions. Overall, ranges of parameters that have been used appear to be sufficient to engage some changes in the degree of spasticity after SCI. It is important to consider this information when designing future studies using rTMS after SCI. As noted above, higher stimulus intensities as those used in the present studies elicited changes in electrophysiological measurements of spinal cord function,15,16 which might in part contribute to the lack of effects observed here.
Effects of rTMS on neuropathic pain after SCI
Neuropathic pain is another major problem that affects over 80% of patients with SCI.2 Evidence has shown that chronic neuropathic pain after SCI is associated with structural and functional changes of both gray and white matter, which involve a number of brain structures related to pain perception and modulation.28 Although a variety of brain regions are affected and this may also vary according to the characteristics of the neuropathic pain, rTMS has been applied only over the M1 for the relief of neuropathic pain in patients with SCI.
Five studies have reported the effect of high and low frequency rTMS over the arm and leg representations of the M1 on neuropathic pain in patients with SCI.29–33 André-Obadia et al.29 used low (1 Hz) and high frequency (20 Hz) rTMS over the hand representation of the M1 in 14 patients with chronic neuropathic pain but only one of them had SCI. Defrin et al.30 used 5 Hz high frequency rTMS over the leg representation of the M1 in 11 patients with chronic thoracic SCI. This is the first study that systematically investigated the analgesic effects of rTMS on a larger number of SCI patients using multiple measurements of pain sensation. The study used a double-blind, randomized, sham-stimulation controlled design. In the study, patients participated in 10 days of sham rTMS over the vertex or in 10 days of real rTMS over the hand motor cortical presentation (see rTMS parameters in Table 1). The VAS of pain sensation was attenuated after real and sham rTMS sessions. However, real rTMS sessions decreased pain sensations assessed by the McGill Pain Questionnaire and increased the heat-pain threshold by 4°C for 6 weeks. Kang et al.31 used 10 Hz high frequency rTMS over the hand representation of the M1 in 11 patients with chronic complete and incomplete SCI. The study used a blinded, randomized, sham-stimulation crossover design. Patients participated in 5 days of sham rTMS over the hand M1 and 5 days of real rTMS over the same region (see table 1). Real rTMS did not change the averaged pain sensation tested in multiple body parts by the numeric rating scale (NRS). Although, the worst pain of all the body parts’ scores declined for 1 week after the real rTMS sessions. Jetté et al.32 used 10 Hz high frequency rTMS over the hand or leg representations of the M1 in 16 patients with chronic complete and incomplete SCI. The study used a double-blinded, randomized, sham-stimulation crossover design. Patients received 1 session of sham rTMS by locating a coil in a counterbalance manner over the hand and leg M1 and 2 sessions of real rTMS by locating the coil over the same regions (rTMS parameters in Table 1). The NRS for pain decreased to a similar extent after real rTMS but not sham rTMS when applied to the hand or leg representations of the M1. These effects returned to the baseline 3 to 4 days after the rTMS sessions. MEP amplitude, map area, and volume of the first dorsal interosseous muscle increased after rTMS over the hand representation of the M1. No correlation was found between changes in pain sensation and MEP amplitude. Recently, Yılmaz et al.33 also used 10 Hz high frequency rTMS over the leg representation of the M1 in 16 patients with chronic complete and incomplete SCI. A double-blind, randomized, sham-stimulation controlled design was used in this study. Patients participated in 10 days of real rTMS over the vertex or in 10 days of sham rTMS over the same region (see rTMS parameters in Table 1). After real and sham rTMS the VAS was decreased. However, only real rTMS resulted in sustained reduction on the VAS for 6 weeks.
In summary, five studies have reported the effects of rTMS on neuropathic pain in patients with complete and incomplete SCI. These studies used different stimulation parameters (intensity, frequency, and number of pulses), number of sessions, region of the M1 targeted, type of patients, and outcome measurements tested. All studies reported some reductions in the clinical symptoms of pain regardless of the area of the M1 that was stimulated using high frequency rTMS over the hand and/or leg representations of the M1. The differences across stimulation parameters used in these studies were more pronounced than those used in the studies reviewed in the previous sections. Another consideration is that a number of measurements included here involved qualitative self-rating outcomes, which were not used in the studies reviewed in the previous sections. Due to the variety of brain regions involved in different types of neuropathic pain deciding the site of stimulation will be an important factor to consider in future studies.
Considerations and limitations for using rTMS after SCI
All the studies reviewed, regardless of the consequence of SCI that was targeted, used high frequency rTMS over the hand and/or leg representations of the M1 in patients with sub-acute and chronic para- and tetraplegia. We propose that consideration of rTMS stimulation parameters in light of the post-injury reorganization taking place in the neuronal pathways that can be affected by rTMS might contribute to enhance the potential beneficial effects of rTMS after SCI. We suggest that the following areas are important to consider, including their limitations, when using rTMS in patients with SCI:
a. Effects of rTMS frequency on the temporal organization of corticospinal descending volleys.
In an intact system, a single shock over the motor cortex evokes temporally organized descending volleys in the corticospinal tract with frequencies of ~700 Hz which can be recorded from the epidural space and surface EMG recordings in animals and humans.34 An early volley is due to direct stimulation of the corticospinal neuron at or near the initial segment (D-wave) and later volleys, at periodic intervals of 1.5 ms, likely reflect transsynaptic activation of corticospinal neurons by intracortical circuits and are therefore termed indirect (I-) waves. Pharmacological evidence indicates that motor circuits involved in the generation of the early I-wave (I1) are less sensitive to the administration of a gamma-aminobutyric acid-A (GABAA) agonist (lorazepam) than the motor circuits generating the later I-waves.35 When rTMS is applied over the M1 evidence from epidural recordings showed that TMS-induced descending volleys in conscious human subjects relate to the pattern of rTMS.36 A high frequency (5 Hz) rTMS train of pulses was found to increase the amplitude of the D-wave and the later I-waves, but not the I1 wave (Figure 1A). Indeed, the number of I-waves evoked by single-pulse TMS was increased after 5 Hz rTMS and additionally I4 and I5 waves were recruited. Accordingly, the increase in excitability obtained by 5 Hz rTMS was interpreted as the result of augmenting activity in the later I-wave circuits. Low frequency (1 Hz) rTMS applied for 15 min also results in changes in the activity in the later I-waves. One Hz rTMS was found to decrease the amplitude of later I-waves without changing the I1 wave (Figure 1B).
Figure 1.
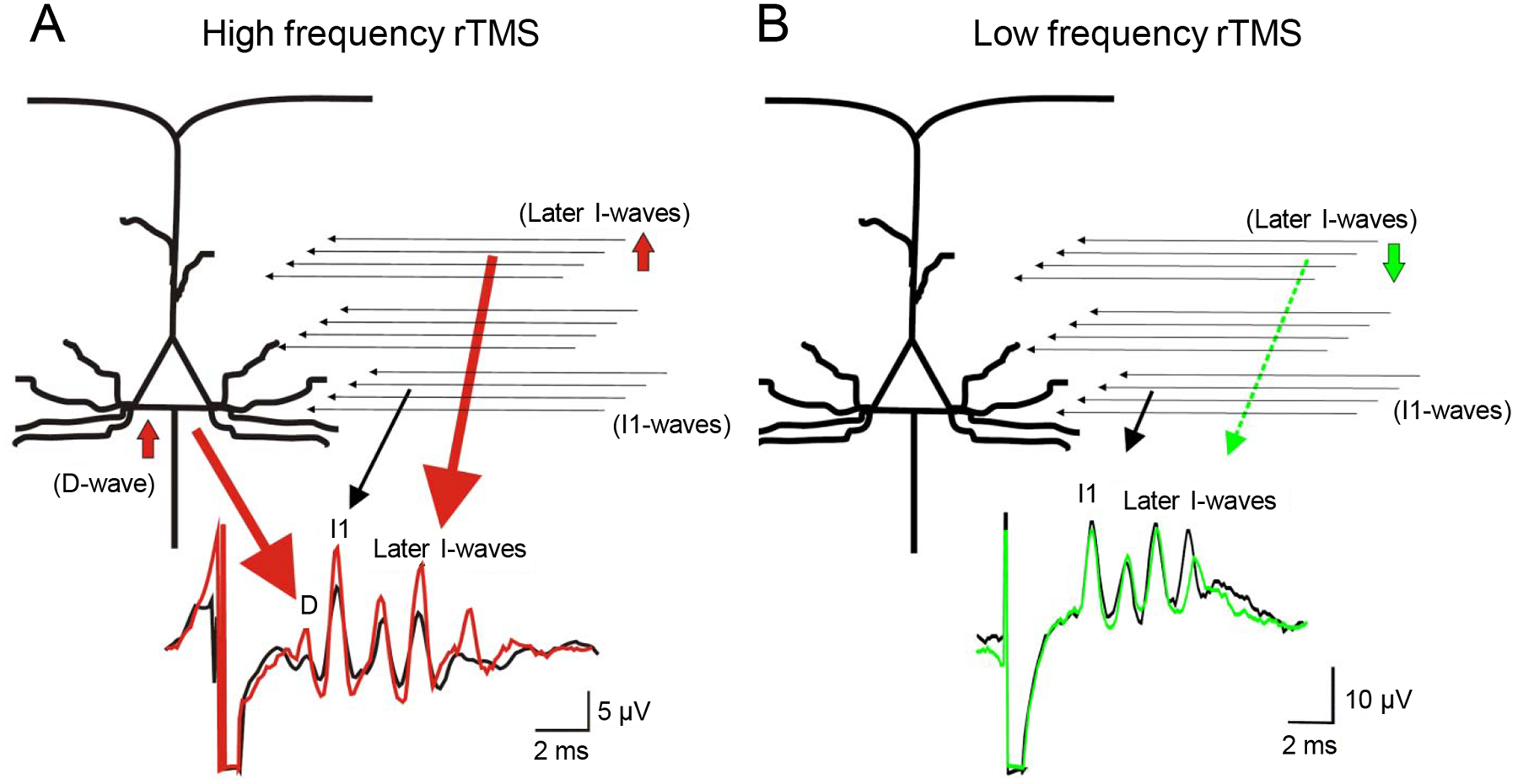
Schematic illustration of the effects of high (A) and low (B) frequencies rTMS on corticospinal descending volleys. Top diagrams represent possible sites and structures of central circuits activated by TMS. Horizontal arrows represent excitatory inputs to the corticospinal cells from excitatory interneurons. Bottom trances indicate the epidural volleys elicited by TMS before (black) and after 5 Hz (red) and 1 Hz rTMS (green), respectively. Note that after 5 Hz rTMS the amplitude of D-wave is increased, the amplitude of the I3-wave is increased and a late I4-wave appears, and that after 1 Hz rTMS the amplitude of the later I-wave is reduced. (Modified with permission from Di Lazzaro et al., 2010).36
At present, considerable evidence indicates that transmission in the corticospinal pathway is impaired after SCI. However, the way in which corticospinal volleys descends from the cerebral cortex to reach spinal motoneurones remains unknown after SCI in animals and humans. Most of the current results indicate that this is impaired. Animal studies showed that injury-induced sprouting from injured and uninjured corticospinal axons occurs days and weeks after injury near and away from the injury site.22,37 It was proposed that remodeling of corticospinal neurons after SCI may occur in two phases: initially injured corticospinal axons contact neurons in a non-specific fashion which is then followed by refinement of the established connections.22 There is partial loss and demyelination of corticospinal axons and sprouts from other descending pathways38 interacting with the corticospinal tract. In agreement, human studies demonstrated that MEPs elicited by TMS, representing an index of corticospinal transmission, show longer latencies and higher thresholds in patients with SCI compared to healthy controls.11 Patients with SCI also showed delayed central conduction times compared to healthy controls.5 Human SCI may extend longitudinally 1–5 cm39 and is characterized by disrupted myelin sheaths extending throughout segments adjacent to the lesion.40
The temporal organization of descending corticospinal volleys in humans can be examined by using a paired-pulse TMS paradigm over the M1 and recordings from surface EMG electrodes instead of the epidural electrodes.41 Using this methodology, recent results showed that the latency of the later I-waves targeting an intrinsic finger muscle are delayed at rest in patients with chronic incomplete cervical SCI.42 The ability to recruit later I-waves is a factor that appears to contribute to the after-effects of rTMS.43 In healthy controls, Hamada and colleagues found that iTBS and cTBS were effective in individuals in whom the anterior to posterior current in the brain favorably evoked later I-waves, but not effective in individuals in whom later I-waves were not preferentially elicited.43 Therefore, the ability to recruit later I-waves might be especially critical in patients with SCI. On one side, since both high- and low-frequency rTMS modulate the later I-waves circuits, one could speculate that both strategies could be used in patients with SCI. On the other side, the non-uniformity of conduction velocities from axonal loss and demyelination of the injured spinal cord region, which likely disrupt the temporal dispersion of corticospinal descending volleys and particularly the later I-waves,42 may influence the effectiveness of rTMS. More insight into the pathophysiology of the corticospinal tract after SCI is needed to provide answers to these questions. The extent to which changes in corticospinal volleys organization after SCI, at rest and during voluntary activity, will affect the effectiveness of rTMS protocols remains to be determined. Other factors that may contribute to the variability after rTMS in SCI patients14 and that have not been considered in previous studies include comparisons between stimulation parameters (number of pulses, intensity and frequency of stimulation), age, gender, time of the day, activity level, and medications also need further exploration.
b. Effects of rTMS frequency on the excitability of corticospinal and motor cortical circuits.
In patients with some asymmetric motor disorders such as stroke, high frequency rTMS has been used in the M1 contralateral to a paretic limb to enhance corticospinal activity in the more affected limb whereas low frequency rTMS has been shown to be effective when targeting the less affected limb.12 It remains unclear to which extent these considerations will be relevant for patients with SCI since the majority of injuries to the spinal cord in humans result in bilateral damage. Furthermore, crossed interactions between the limbs that involve the corticospinal pathway are impaired in patients with SCI regardless of the magnitude of motor recovery.23,44 Also, while evidence has shown that the excitability of intracortical inhibitory circuits, including the cortical silent period, is decreased at rest in patients with SCI compared to healthy controls;45,46 this appears to be different during voluntary contraction. Barry and colleagues26 showed that patients with chronic cervical SCI were able to decrease the magnitude of short-interval intracortical inhibition to the same extent as healthy controls during small levels of voluntary contraction compared to rest (Figure 2). It is unlikely that these effects were related to the recovery state of the patients since other subcortical physiological measurements in the same patients were affected by the injury. Therefore, task-dependent changes in corticospinal and motor cortical function after SCI make the extrapolation of whether using low or high frequency rTMS to increase and/or decrease excitability difficult. Consequently, the potential benefits of using high and low frequency rTMS still remains open.
Figure 2.
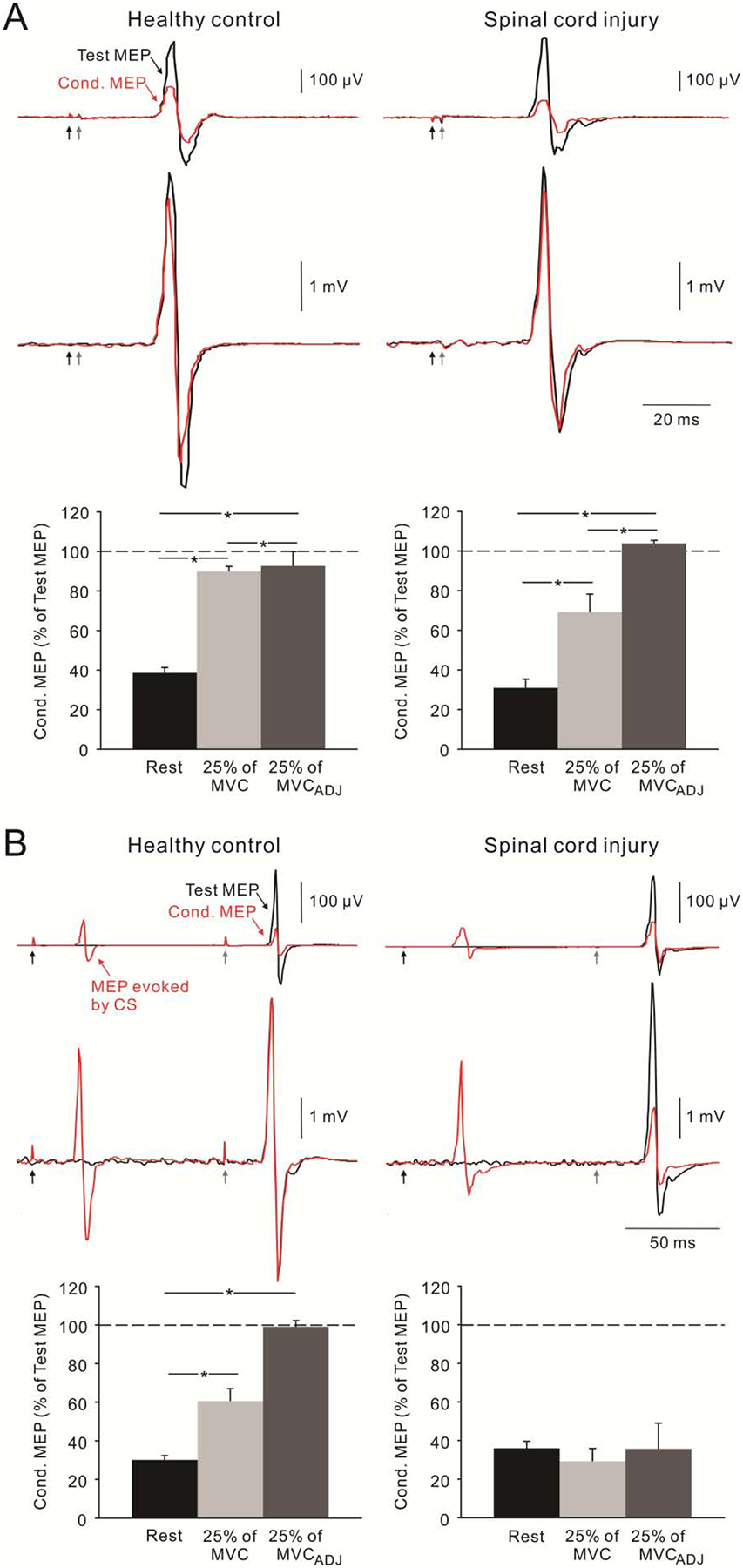
The effect of voluntary contraction on short-interval intracortical inhibition (SICI, A) and long-interval intracortical inhibition (LICI, B) in healthy controls and patients with SCI. Traces show MEPs of first dorsal interosseous muscle elicited at rest (top) and during 25% of maximal voluntary contraction (MVC, bottom). Black and red traces represent test MEP and conditioned (Cond.) MEP, respectively. Conditioning stimulation (CS, black arrows) preceded test stimulation (gray arrows) by 2 ms for SICI and 100 ms for LICI. On each bar graph, 25% of MVCADJ represents the condition in which the TMS intensity was adjusted so that the size of test MEP during 25% MVC was matched to rest. Note that LICI was decreased during voluntary contraction compared to at rest in healthy controls but not in SCI patients. SICI was decreased during voluntary contraction in both subject groups. (Modified with permission from Barry et al., 2013).26
c. Effects of rTMS frequency and intensity on spinal cord circuits.
Even though some studies reported no effect of rTMS applied over the M1 on the excitability of subcortical pathways,47,48 other studies have found positive effects.16–17 Perez and collaborators16 applied 15 trains of 20 pulses at 5 Hz at intensities between 75 to 120% of the RMT of the tibialis anterior muscle and reported a decrease in the size of the soleus H-reflex at stimulus intensities ranging from 92% to 120% (Figure 3). In this study, the authors proposed that the effects of rTMS in increasing the level of presynaptic inhibition at the terminals of Ia afferent fibers could be a potential mechanisms mediating the H-reflex suppression. Also, studies using 5 Hz at intensities at 90 and 100 % of the RMT of the first dorsal interosseous and soleus muscles showed increased in the size of MEPs elicited by transcranial electrical stimulation and by cervicomedullary stimulation,18,49 suggesting that rTMS affected the subcortical structures. At rTMS frequencies of 1 Hz different results have been reported. Valero-Cabré et al.17 applied 600 pulses of 1 Hz rTMS at 90% of RMT of the flexor carpi radialis (FCR) muscle and reported a lasting decrease in threshold and an increase in size of the FCR H-reflex. Whereas, Touge et al.48 found no effect of rTMS on the size of the FCR H-reflex after 600 stimuli at 1 Hz and 95% of RMT of an intrinsic finger muscle. Recently, Taube et al.,18 also showed that 1 Hz rTMS changed the efficiency of corticospinal synaptic transmission. Although the variability of the effects of rTMS on H-reflex size might be in part related to methodological aspects, the results indicate that a relatively high intensity of rTMS is likely to induce changes in spinal cord excitability.15,16 This is in agreement with the results observed in the three studies described in patients with SCI, where intensities of ~90% of RMT resulted in some reduction in spasticity scores. TMS studies have reported that AMT and RMT are increased in patients with incomplete SCI regardless of the time since the injury. Thus, it is possible that this deficit is a result of the reduced numbers of corticospinal axons reaching the pool of motoneurons.50 The higher TMS threshold in patients may also indicate that stronger stimulus intensities were used in this population compared to healthy controls. Therefore, since low and high frequency rTMS might be able to access subcortical circuits depending on the stimulus intensity, we speculate that both may potentially be of value in the treatment of spasticity.
Figure 3.
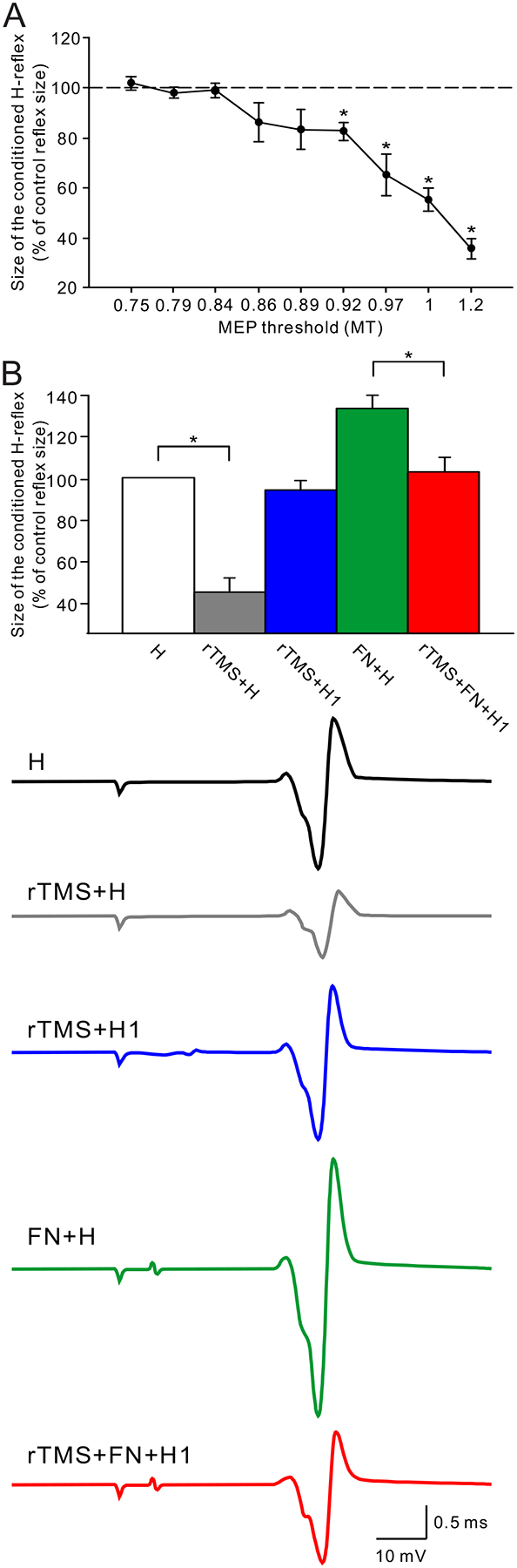
A. The effect of 5 Hz rTMS over M1 on the soleus H-reflex. Note that the H-reflex was suppressed when the rTMS intensity was higher than 0.92 times the resting motor evoked potential (MEP) threshold (MT), and that the suppression of the H-reflex was gradually increased according to the rTMS intensity. B. The effect of rTMS on the heteronymous Ia facilitation of the soleus H-reflex. Traces show the control H-reflex (H, black), the H-reflex conditioned by rTMS (rTMS+H, gray), the size adjusted rTMS-conditioned H-reflex (rTMS+H1, blue), the H-reflex with preceded femoral nerve stimulation (FN+H, green), and the size adjusted rTMT-conditioned H-reflex with preceded femoral nerve stimulation (rTMS+FN+H1). Note that rTMS suppressed the soleus H-reflex (gray bar), and that the heteronymous Ia facilitation of the H-reflex (green bar) was attenuated by the conditioning rTMS (red bar). (Modified with permission from Perez et al., 2005).16
Subcortical circuits targeting the corticospinal tracts play an important role in the control of skilled motor behaviors.51 Indeed, a recent study showed that the involvement of premotoneuronal subcortical pathways controlling a precision grip between the thumb and index finger was impaired in patients with incomplete cervical SCI (Figure 4).52 During voluntary activity, sensory feedback needs to be filtered to become functionally relevant53 and spinal circuits may provide fast ongoing filtering needed to shape corticospinal output to control motor skills54 highlighting the possible role of spinal targets in motor recovery after SCI.5 Therefore, the effects of rTMS on subcortical circuitry might provide the effective approach to enhance recovery after SCI.
Figure 4.
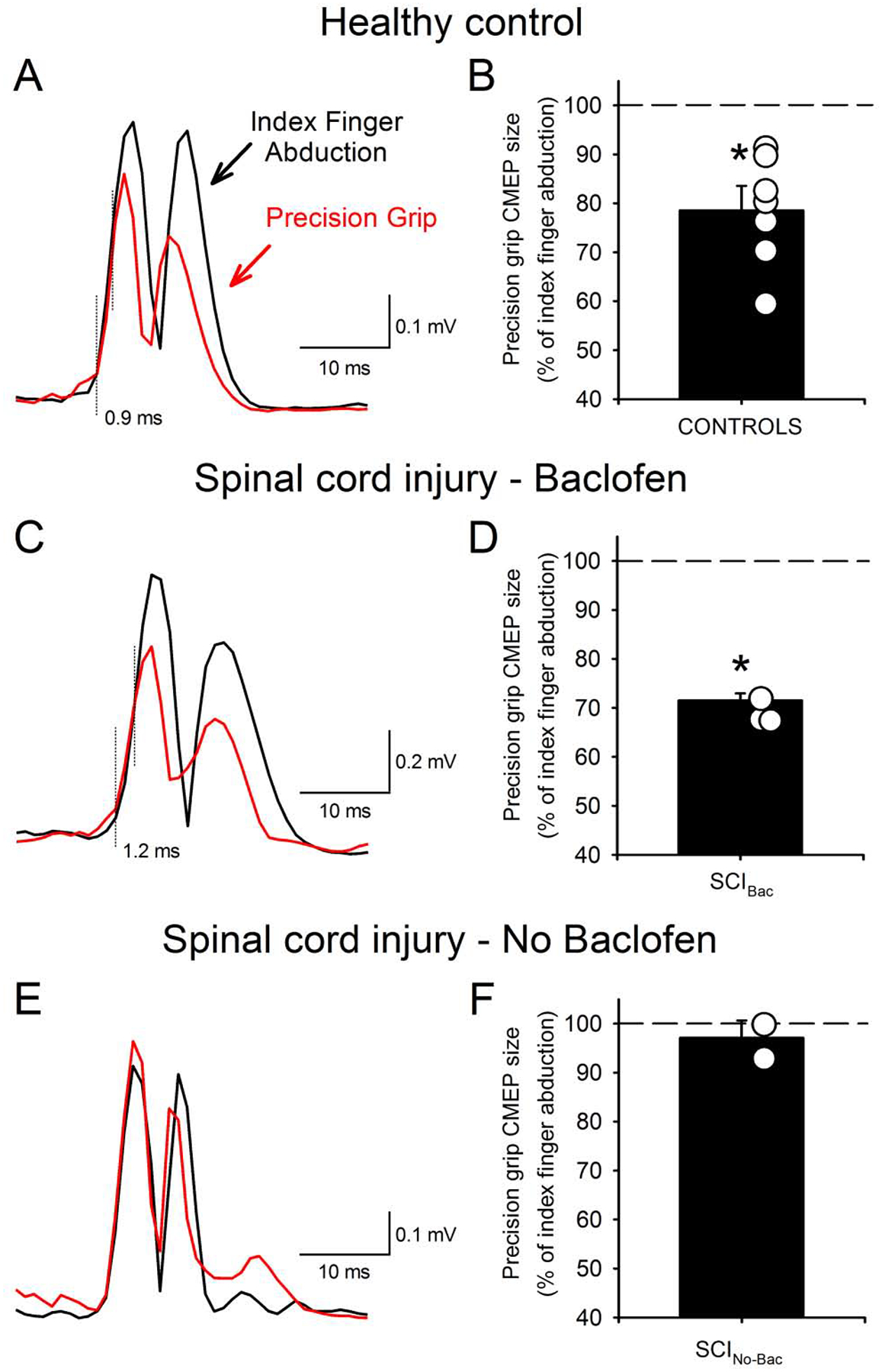
Motor evoked potentials elicited by cervicomedullary stimulation (CMEPs) during index finger abduction (black trace) and precision grip (red traces) in healthy controls and patients with SCI. Rectified traces of CMEPs are illustrated (A, C, E). The dotted vertical lines indicated the approximate time of CMEP onset and the CMEP responses diverge. Left figures represent data from the mean of all subjects (black bars) and individual subjects (open circles) (B, D, F). Note that the size of CMEPs decreased during precision grip compared to index finger abduction in healthy controls and in SCI patients with taking baclofen but remains unchanged in SCI patients without taking baclofen. (Modified with permission from Bunday et al., 2014).52
d. Effects of baclofen intake on rTMS effectiveness.
The majority of patients with SCI take baclofen to reduce the symptoms of spasticity.55 It was shown that baclofen has limited effects on voluntary motor output,56,57 decreases contractile properties of motor units of partially paralyzed muscles,58 and has side effects such as drowsiness and drug tolerance59 after SCI. Recent results indicate that baclofen selectively maintains use-dependent modulation of largely subcortical but not cortical GABAB neuronal pathways tested by TMS in patients with chronic incomplete cervical SCI.26 It was proposed that to some extent baclofen restores activity in subcortical mediated spinal circuits. Therefore, this might be a critical factor to consider during assessment of the effectiveness of rTMS. The published studies in patients with SCI did not separate the patients by baclofen intake and while rTMS had some benefits in reducing spasticity the effects in sensory and motor function were more variable and limited.
e. Effects of rTMS site of stimulation.
We will focus in this section on neuropathic pain since although a variety of brain regions are involved in different types of neuropathic pain it seems that rTMS has been mainly used over the M1 to obtain analgesic effects in patients with neurological disorders.60 It is proposed that rTMS over the M1 attenuates pain sensation through an orthodromic effect on thalamic nuclei61,62 resulting in the plastic changes in the somatosensory cortex.63 Enhanced GABA release in the thalamic nuclei by the stimulation of the M1 is thought to contribute to the inhibition of hyperalgesia.62 As the neuropathic pain after SCI generally appears in the paralyzed body parts below the level of injury,64 the motor cortical representation within M1 targeted by rTMS may be a critical factor for SCI-related pain treatment. A previous study in patients with various neurological disorders including SCI demonstrated that rTMS over the motor representation in the M1 corresponding to the painful body part attenuated neuropathic pain.60 However, Jetté et al.32 showed that rTMS applied to both hand and leg M1 showed similar analgesic effects in paraplegic and tetraplegic patients. Although, little is known regarding the extent of reorganization taking place at different regions of the M1 in humans with SCI most of the available evidence indicates that representations in M1 are enlarged and shifted.50,65,66 Conceivably, the reorganization in motor representations after SCI could affect not only the area of the M1 that will be stimulated but also interaction between motor cortical representations.66
Chronic neuropathic pain following SCI involves reorganization in a number of cortical and subcortical structures, which may also be valuable in the treatment of neuropathic pain and could potentially be targeted by rTMS. For example, reorganization in the somatosensory cortex was found to be more prominent in a patient with SCI with pain compared to a patient without pain67 and atrophy has been reported in the dorsolateral prefrontal cortex (DLPFC) in patients with chronic pain conditions including individuals with SCI.28 When rTMS is applied over the DLPFC, low frequency rTMS affected analgesia68 and high frequency rTMS resulted in the attenuation of capsaicin-induced tonic pain in healthy subjects.69 Therefore, since low and high frequency rTMS might be able to access pain-related structures, both may be of potential value in the treatment of neuropathic pain after SCI. Neuropathic pain after SCI is also affected by psychological factors.70,71 Therefore, relationship of neuropathic pain after SCI with the level of injury, etiology, completeness of SCI and psychosocial factors and rTMS aftereffects remains to be explored.
Clinical implications
So far, a limited number of studies have used rTMS in patients with SCI and the overall magnitude of improvements in sensory and motor function, spasticity, and neuropathic pain was limited and variable. This might be related, at least to some extent, to the large range of different methodologies, from stimulation parameters to type of patients tested used across studies. In parallel, there is a deficit in our understanding of the neuronal changes taking place at multiple levels in the central nervous system which can be affected by rTMS including corticospinal, cortical, and spinal cord elements. Besides the limitations, rTMS appears to be a promising approach to facilitate recovery after SCI. We propose, however, that a better characterization of the changes taking place in neuronal structures affected by rTMS will provide critical information for enhancing the efficacy of existing rTMS protocols. For example, the corticospinal tract is a major descending pathway contributing to the control of voluntary movements,2,72 and a prominent target for investigating injury-induced plasticity and motor recovery after SCI.2,72 It can be easily accessed by rTMS. Thus, we suggest that this is one of the neuronal structures that need to be better characterized after injury. Similarly, we suggest better characterizing the changes in the activity in spinal cord circuitry after SCI, because these as well can be accessed by rTMS.
A main conclusion from this review is that knowledge on the pathophysiology of residual neuronal pathway actively involved in the generation of voluntary movement may represent a critical approach to guide and enhance the efficacy of rTMS plasticity-induced protocols to promote motor recovery after SCI.
Acknowledgements:
This work was supported by The National Institute of Neurological Disorders and Stroke, NIH (R01 NS076589 to M.A.P.), Veteran Affairs (3397626 to M.A.P.), and the Paralyzed Veterans of America (2955 to T.T.).
Abbreviations:
- AMT
active motor threshold
- ASIA
American Spinal Injury Association
- cTBS
continuous theta burst stimulation
- DLPFC
dorsolateral prefrontal cortex
- EMG
electromyographic
- EPT
electrical perceptual threshold
- FCR
flexor carpi radialis
- GABA
gamma-aminobutyric acid
- GABAA
gamma-aminobutyric acid-A
- GABAB
gamma-aminobutyric acid-B
- H-reflex
Hoffman reflex
- iTBS
intermittent theta burst stimulation
- M1
primary motor cortex
- MEP
motor evoked potential
- NMDA
N-Methyl-D-Aspartate
- NRS
numeric rating scale
- RMT
resting motor threshold
- rTMS
repetitive transcranial magnetic stimulation
- SCI
spinal cord injury
- TBS
theta burst stimulation
- TMS
transcranial magnetic stimulation
- VAS
visual analog scale
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
We have no conflicts of interest to disclose.
References
- 1.Adams MM, Hicks AL. Spasticity after spinal cord injury. Spinal Cord, 2005; 43, 577–586. [DOI] [PubMed] [Google Scholar]
- 2.Moreno-Duarte I, Morse LR, Alam M, Bikson M, Zafonte R, Fregni F. Targeted therapies using electrical and magnetic neural stimulation for the treatment of chronic pain in spinal cord injury. Neuroimage. 2014; 85, 1003–1013. [DOI] [PubMed] [Google Scholar]
- 3.Oudega M, Perez MA. Corticospinal reorganization after spinal cord injury. J Physiol (Lond), 2012; 590, 3647–3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lemon RN. Descending pathways in motor control. Annu Rev Neurosci, 2008; 31, 195–218. [DOI] [PubMed] [Google Scholar]
- 5.Bunday KL, Perez MA. Motor recovery after spinal cord injury enhanced by strengthening corticospinal synaptic transmission. Curr Biol, 2012; 22, 2355–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuppuswamy A, Balasubramaniam AV, Maksimovic R, Mathias CJ, Gall A, Craggs MD, Ellaway PH. Action of 5Hz repetitive transcranial magnetic stimulation on sensory, motor and autonomic function in human spinal cord injury. Clin Neurophysiol, 2011; 122, 2452–2461. [DOI] [PubMed] [Google Scholar]
- 7.Baker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet, 1985; 1, 1106–1107. [DOI] [PubMed] [Google Scholar]
- 8.Rothwell JC, Hallett M, Berardelli A, Eisen A, Rossini P, Paulus W. Magnetic stimulation: motor evoked potentials. The International Federation of Clinical Neurophysiology. Electroencephalogr Clin Neurophysiol Suppl, 1999; 52, 97–103. [PubMed] [Google Scholar]
- 9.Levy WJ Jr, Amassian VE, Traad M, Cadwell J. Focal magnetic coil stimulation reveals motor cortical system reorganized in humans after traumatic quadriplegia. Brain Res, 1990; 510, 130–134. [DOI] [PubMed] [Google Scholar]
- 10.Topka H, Cohen LG, Cole RA, Hallett M. Reorganization of corticospinal pathways following spinal cord injury. Neurology, 1991; 41, 1276–1283. [DOI] [PubMed] [Google Scholar]
- 11.Ellaway PH, Catley M, Davey NJ, Kuppuswamy A, Strutton P, Frankel HL, Jamous A, Savic G. Review of physiological motor outcome measures in spinal cord injury using transcranial magnetic stimulation and spinal reflexes. J Rehabil Res Dev, 2007; 44, 69–76. [DOI] [PubMed] [Google Scholar]
- 12.Ridding MC, Rothwell JC. Is there a future for therapeutic use of transcranial magnetic stimulation? Nat Rev Neurosci, 2007; 8, 559–567. [DOI] [PubMed] [Google Scholar]
- 13.Nudo RJ. Functional and structural plasticity in motor cortex: implications for stroke recovery. Phy Med Rehabil Clin N Am, 2003; 14, 57–76. [DOI] [PubMed] [Google Scholar]
- 14.Ridding MC, Ziemann U. Determinants of the induction of cortical plasticity by non-invasive brain stimulation in healthy subjects. J Physiol (Lond), 2010; 588, 2291–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berardelli A, Inghilleri M, Rothwell JC, Romeo S, Currà A, Gilio F, Modugno N, Manfredi M. Facilitation of muscle evoked responses after repetitive cortical stimulation in man. Exp Brain Res, 1998; 122, 79–84. [DOI] [PubMed] [Google Scholar]
- 16.Perez MA, Lungholt BK, Nielsen JB. Short-term adaptations in spinal cord circuits evoked by repetitive transcranial magnetic stimulation: possible underlying mechanisms. Exp Brain Res, 2005; 162, 202–212. [DOI] [PubMed] [Google Scholar]
- 17.Valero-Cabré A, Oliveri M, Gangitano M, Pascual-Leone A. Modulation of spinal cord excitability by subthreshold repetitive transcranial magnetic stimulation of the primary motor cortex in humans. Neuroreport, 2001; 12, 3845–3848. [DOI] [PubMed] [Google Scholar]
- 18.Taube W, Leukel C, Nielsen JB, Lundbye-Jensen J. Repetitive activation of the corticospinal pathway by means of rTMS may reduce the efficiency of corticomotoneuronal synapses. Cereb Cortex, In press. [DOI] [PubMed] [Google Scholar]
- 19.Huang Y-Z, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron, 2005; 45, 201–206. [DOI] [PubMed] [Google Scholar]
- 20.Belci M, Catley M, Husain M, Frankel HL, Davey NJ. Magnetic brain stimulation can improve clinical outcome in incomplete spinal cord injured patients. Spinal Cord, 2004; 42, 417–419. [DOI] [PubMed] [Google Scholar]
- 21.Benito J, Kumru H, Murllo N, Costa U, Medina J, Tormos JM, Pascual-Leone A, Vidal J. Motor and gait improvement in patients with incomplete spinal cord injury induced by high-frequency repetitive transcranial magnetic stimulation. Top Spinal Cord Inj Rehabil, 2012; 18, 106–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bareyre FM, Kerschensteiner M, Raineteau O, Mettenleiter TC, Weinmann O, Schwab ME. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci, 2004; 7, 269–277. [DOI] [PubMed] [Google Scholar]
- 23.Bunday KL, Oudega M, Perez MA. Aberrant crossed corticospinal facilitation in muscles distant from a spinal cord injury. PLoS One, 2013; 8, e76747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pierrot-Deseilligny E, Burke D. The circuitry of the human spinal cord: its role in motor control and movement disorders. 1st ed Cambridge University Press; 2005. [Google Scholar]
- 25.Mori F, Koch G, Foti C, Bernardi G, Centonze D. The use of repetitive transcranial magnetic stimulation (rTMS) for the treatment of spasticity. Prog Brain Res, 2009; 175, 429–439. [DOI] [PubMed] [Google Scholar]
- 26.Barry MD, Bunday KL, Chen R, Perez MA. Selective effects of baclofen on use-dependent modulation of GABAB inhibition after tetraplegia. J Neurosci, 2013; 33, 12898–12907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumru H, Murillo N, Samso JV, Valls-Sole J, Edwards D, Pelayo R, Valero-Cabre A, Tormos JM, Pascual-Leone A. Reduction of spasticity with repetitive transcranial magnetic stimulation in patients with spinal cord injury. Neurorehabil Neural Repair, 2010; 24, 435–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoon EJ, Kim YK, Shin HI, Lee Y, Kim SE. Cortical and white matter alterations in patients with neuropathic pain after spinal cord injury. Brain Res, 2013; 1540, 64–73. [DOI] [PubMed] [Google Scholar]
- 29.André-Obadia N, Peyron R, Mertens P, Mauquiè F, Leurent B, Garcia-Larrea L. Transcranial magnetic stimulation for pain control. Double-blind study of different frequencies against placebo, and correlation with motor cortex stimulation efficacy. Clin Neurophysiol, 2006; 117, 1536–1544. [DOI] [PubMed] [Google Scholar]
- 30.Defrin R, Grunhaus L, Zamir D, Zeilig G. The effect of a series of repetitive transcranial magnetic stimulations of the motor cortex on central pain after spinal cord injury. Arch Phys Med Rehabil, 2007; 88, 1574–1580. [DOI] [PubMed] [Google Scholar]
- 31.Kang BS, Shin HI, Bang MS. Effect of repetitive transcranial magnetic stimulation over the hand motor cortical area on central pain after spinal cord injury. Arch Phys Med Rehabil, 2009; 90, 1766–1771. [DOI] [PubMed] [Google Scholar]
- 32.Jetté F, Côté I, Meziane HB, Mercier C. Effect of single-session repetitive transcranial magnetic stimulation applied over the hand versus leg motor area on pain after spinal cord injury. Neurorehabil Neural Repair, 2013; 27, 636–643. [DOI] [PubMed] [Google Scholar]
- 33.Yılmaz B, Kesikburun S, Yas Ar E, Tan AK. The effect of repetitive transcranial magnetic stimulation on refractory neuropathic pain in spinal cord injury. J Spinal Cord Med, In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Di Lazzaro V, Oliviero A, Profice P, Saturno E, Pilato F, Insola A, Mzzone P, Tonali P, Rothwell JC. Comparison of descending volleys evoked by transcranial magnetic and electric stimulation in conscious humans. Electroencephalogr clin Neurophysiol, 1998; 109, 397–401. [DOI] [PubMed] [Google Scholar]
- 35.Di Lazzaro V, Oliviero A, Meglio M, Cioni B, Tamburrini G, Tonali P, Rothwell JC. Direct demonstration of the effect of lorazepam on the excitability of the human motor cortex. Clin Neurophysiol, 2000; 111, 794–799. [DOI] [PubMed] [Google Scholar]
- 36.Di Lazzaro V, Profice P, Pilato F, Dileone M, Oliviero A, Ziemann U. The effects of motor cortex rTMS on corticospinal descending activity. Clin Neurophysiol, 2010; 121, 464–473. [DOI] [PubMed] [Google Scholar]
- 37.Fouad K, Pedersen V, Schwab ME, Brösamle C. Cervical sprouting of corticospinal fibers after thoracic spinal cord injury accompanies shifts in evoked motor responses. Curr Biol, 2001; 11, 1766–1770. [DOI] [PubMed] [Google Scholar]
- 38.Ballermann M, Fouad K. Spontaneous locomotor recovery in spinal cord injured rats is accompanied by anatomical plasticity of reticulospinal fibers. Eur J Neurosci, 2006; 23, 1988–1996. [DOI] [PubMed] [Google Scholar]
- 39.Bedrock GM. Some pertinent observations on the pathology of traumatic spinal paralysis. Paraplegia, 1963; 1, 215–227. [DOI] [PubMed] [Google Scholar]
- 40.Guest JD, Hiester ED, Bunge RP. Demyelination and Schwann cell responses adjacent to injury epicenter cavities following chronic human spinal cord injury. Exp Neruol, 2005; 192, 384–393. [DOI] [PubMed] [Google Scholar]
- 41.Ziemann U, Tergau F, Wassermann EM, Wischer S, Hildebrandt J, Paulus W. Demonstration of facilitatory I wave interaction in the human motor cortex by paired transcranial magnetic stimulation. J Physiol (Lond), 1998; 511, 181–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cirillo J, Perez MA. Temporal dispersion of corticospinal volleys is impaired in humans with spinal cord injury. Soc Neurosci Abst, 2013. [Google Scholar]
- 43.Hamada M, Murase N, Hasan A, Balaratnam M, Rothwell JC. The role of interneuron networks in driving human motor cortical plasticity. Cereb Cortex, 2013; 23, 1593–1605. [DOI] [PubMed] [Google Scholar]
- 44.Bunday KL, Perez MA. Impaired crossed facilitation of the corticospinal pathway after cervical spinal cord injury. J Neurophysiol, 2012; 107, 2901–2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saturno E, Bonato C, Miniussi C, Di Lazzaro V, Callea L. Motor cortex changes in spinal cord injury: a TMS study. Neurol Res, 2008; 30, 1084–1085. [DOI] [PubMed] [Google Scholar]
- 46.Roy FD, Zewdie ET, Gorassini MA. Short-interval intracortical inhibition with incomplete spinal cord injury. Clin Neurophysiol, 2011; 122, 1387–1395. [DOI] [PubMed] [Google Scholar]
- 47.Modugno N, Nakamura Y, McKinnon CD, Filipovic SR, Bestmann S, Berardelli A, Rothwell JC. Motor cortex excitability following short trains of repetitive magnetic stimuli. Exp Brain Res, 2001; 140, 453–459. [DOI] [PubMed] [Google Scholar]
- 48.Touge T, Gerschlager W, Brown P, Rothwell JC. Are the after-effects of low-frequency rTMS on motor cortex excitability due to changes in the efficacy of cortical synapses? Clin Neurophysiol, 2001; 112, 2138–2145. [DOI] [PubMed] [Google Scholar]
- 49.Quartarone A, Bagnato S, Rizzo V, Morgante F, Sant’angelo A, Battaglia F, Messina C, Siebner HR, Girlanda P. Distinct changes in cortical and spinal excitability following high-frequency repetitive TMS to the human motor cortex. Exp Brain Res, 2005; 161, 114–124. [DOI] [PubMed] [Google Scholar]
- 50.Perez MA. Transcranial magnetic stimulation and spinal cord injury In: Chen R, Rothwell JC, editors. Cortical connectivity: Brain stimulation for assessing and modulating cortical connectivity and function. Berlin: Springer; 2012. p323–336. [Google Scholar]
- 51.Takei T, Seki K. Spinal premotor interneurons mediate dynamic and static motor commands for precision grip in monkeys. J Neurosci, 2013; 33, 8850–8860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bunday BL, Tazoe T, Rothwell JC, Perez MA. Subcortical control of precision grip after human spinal cord injury. J Neurosci, 2014; 34, 7341–7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Prut Y, Perlmutter SI. Firing properties of spinal interneurons during voluntary movement. I. State-dependent regularity of firing. J Neurosci, 2003; 23, 9600–9610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Perez MA, Lungholt BKS, Nielsen JB. Presynaptic control of groupIa afferents in relation to acquisition of a visuo-motor skill in healthy humans. J Physiol (Lond), 2005; 568, 343–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aydin G, Tomruk S, Keleş I, Demir SO, Orkun S. Transcutaneous electrical nerve stimulation versus baclofen in spasticity: clinical and electrophysiologic comparison. Am J Phys Med Rehabil, 2005; 84, 584–592. [DOI] [PubMed] [Google Scholar]
- 56.Burke D, Andrews CJ, Knowles L. The action of a GABA derivative in human spasticity. J Neurol Sci, 1971; 14, 199–208. [DOI] [PubMed] [Google Scholar]
- 57.Domingo A, Al-Yahya AA, Asiri Y, Eng JJ, Lam T. A systematic review of the effects of pharmacological agents on walking function in people with spinal cord injury. J Neurotrauma, 2012; 29, 865–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thomas CK, Häger-Ross CK, Klein CS. Effects of baclofen on motor units paralysed by chronic cervical spinal cord injury. Brain, 2010; 133, 117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rösche J Treatment of spasticity. Spinal Cord, 2002; 40, 261–262. [DOI] [PubMed] [Google Scholar]
- 60.Fregni F, Freedman S, Pascual-Leone A. Recent advances in the treatment of chronic pain with non-invasive brain stimulation techniques. Lancet Neurol, 2007; 6, 188–191. [DOI] [PubMed] [Google Scholar]
- 61.Garcia-Larrea L, Peyron R, Mertens P, Gregoire MC, Lavenne F, Le Bars D, Convers P, Mauguiere F, Sindou M, Laurent B. Electrical stimulation of motor cortex for pain control: A combined PET-scan and electrophysiological study. Pain, 1999; 83, 259–273. [DOI] [PubMed] [Google Scholar]
- 62.Rokyta R, Fricová J. Neurostimulation method in the treatment of chronic pain. Physiol Res, 2012; 61, S23–S31. [DOI] [PubMed] [Google Scholar]
- 63.Houzé B, Bradley C, Magnin M, Garcia-Larrea L. Changes in Sensory Hand Representation and Pain Thresholds Induced by Motor Cortex Stimulation in Humans. Cereb Cortex, 2013; 23, 2667–2676. [DOI] [PubMed] [Google Scholar]
- 64.Yezierski RP. Spinal cord injury: a model of central neuropathic pain. Neurosignals, 2005; 14, 182–193. [DOI] [PubMed] [Google Scholar]
- 65.Corbetta M, Burton H, Sinclair RJ, Conturo TE, Akbudak E, McDonald JW. Functional reorganization and stability of somatosensory-motor cortical topography in a tetraplegic subject with late recovery. Proc Natl Acad Sci USA, 2002; 99, 17066–17071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tazoe T, Perez MA. Reorganization of corticospinal maps in distal and proximal upper-limb muscles after tetraplegia. Soc Neurosci Abst, 2013. [Google Scholar]
- 67.Wrigley PJ, Press SR, Gustin SM, Macefield VG, Gandevia SC, Cousins MJ, Middleton JW, Henderson LA, Siddall PJ. Neuropathic pain and primary somatosensory reorganization following spinal cord injury. Pain, 2009; 141, 52–59. [DOI] [PubMed] [Google Scholar]
- 68.Krummenacher P, Candia V, Folkers G, Schedlowski M, Schönbächler G. Prefrontal cortex modulates placebo analgesia. Pain, 2010; 148, 368–374. [DOI] [PubMed] [Google Scholar]
- 69.Fierro B, De Tommaso M, Giglia F, Giglia G, Palermo A, Brighina F. Repetitive transcranial magnetic stimulation (rTMS) of the dorsolateral prefrontal cortex (DLPFC) during capsaicin-induced pain: modulatory effects on motor cortex excitability. Exp Brain Res, 2010; 203, 31–38. [DOI] [PubMed] [Google Scholar]
- 70.Craig A, Tran Y, Siddall P, Wijesuriya N, Lovas L, Bartrop R, Middleton J. Developing a model of associations between chronic pain, depressive mood, chronic fatigue and self-efficacy in people with spinal cord injury. J Pain, 2013; 14, 911–920. [DOI] [PubMed] [Google Scholar]
- 71.Craig A, Rodrigues D, Tran Y, Guest R, Bartrop R, Middleton J. Developing an algorithm capable of discriminating depressed mood in people with spinal cord injury. Spinal Cord, In press. [DOI] [PubMed] [Google Scholar]
- 72.Hill MR, Noonan VK, Sakakibara BM, Miller WC, the SCIRE Research Team. Quality of life instruments and definitions in individuals with spinal cord injury: a systematic review. Spinal Cord, 2010; 48, 438–450. [DOI] [PMC free article] [PubMed] [Google Scholar]


