Abstract
Introduction
Cognitive impairment and psychopathology caused by brain hypoxia and the traumatic impact of critical illness are common in cardiac arrest survivors and can lead to negative consequences of everyday life functioning, and further impact mental health in relatives. Most studies have dealt with the mere survival rate after cardiac arrest and not with long-term consequences to mental health in cardiac arrest survivors. Importantly, we face a gap in our knowledge about suitable screening tools in the early post-arrest phase for long-term risk prediction of mental health problems. This study aims to evaluate the efficacy of a novel screening procedure to predict risk of disabling cognitive impairment and psychopathology 3 months after cardiac arrest. Furthermore, the study aims to evaluate long-term prevalence of psychopathology in relatives.
Methods and analyses
In this multicentre prospective cohort study, out-of-hospital cardiac arrest survivors and their relatives will be recruited. The post-arrest screening includes the Montreal Cognitive Assessment (MoCA), the Hospital Anxiety and Depression Scale (HADS), the Impact of Event Scale-Revised (IES-R) and the Acute Stress Disorder Interview (ASDI) and is conducted during hospitalisation. In a subsample of the patients, functional MRI is done, and cortisol determination collected. At 3-month follow-up, the primary study outcomes for 200 survivors include the Danish Affective Verbal Learning Test-26 (VAMT-26), Delis-Kaplan Executive Function System tests (trail making, colour-word interference, word and design fluency), Rey’s Complex Figure and Letter-number sequencing subtest of Wechsler Adult Intelligence Scale-IV, HADS and IES-R. For the relatives, they include HADS and IES-R.
Ethics and dissemination
The study is approved by the local regional Research Ethics Committee (H-18046155) and the Danish Data Protection Agency (RH-2017-325, j.no.05961) and follows the latest version of the Declaration of Helsinki. The results will be published in peer-reviewed journals and may impact the follow-up of cardiac arrest survivors.
Keywords: mental health, cardiology, neurology, anxiety disorders, depression and mood disorders, magnetic resonance imaging
Strengths and limitations of this study.
A strength of the study is the prospective design and consistent follow-up with the use of standardised and validated measurement tools.
A limitation of the study is the differential loss to follow-up, which can introduce bias and challenge the internal validity of the study.
A strength of the study is the multicentre approach. This will improve statistical power and generalisability of the results.
A strength of the study is its potential to contribute to a better referral from cardiac care to targeted rehabilitation services.
Introduction
The global incidence of cardiac arrest with assumed primary cardiac cause is 55/100 000 inhabitants.1 In Europe, approximately 275 000 people with all-rhythm cardiac arrests are treated by the emergency medical systems (EMS) each year.2 Out-of-hospital cardiac arrest (OHCA) is a significant cause of global mortality.3 However, in recent years the survival rates have improved especially in developing countries due to advances in the chain of survival.1 At present, the majority of published OHCA studies focus on the acute prehospital and intensive care treatment of OHCA sufferers. Far fewer studies have investigated the period from early recovery to long-term return to everyday life. The existing literature on this period highlights two critical challenges for cardiac arrest survivors in the post-arrest recovery process, first diminished neurocognitive functions and second disabling emotional difficulties.4–7 In these survivors, long-term cognitive impairment and psychopathology constitute a major personal and family burden, as well as a public health and economic concern.8–15 Mild to moderate cognitive sequelae are reported in up to 50% of survivors.6 16 Transient or permanent memory loss, reduced visual–motor skills, attention deficit and executive impairment are the most dominant cognitive impairment found in this population.5 6 17 18 Importantly, impaired cognitive functioning at 3-month post-arrest has been found to correlate with worse physical and mental health-related quality of life (HRQoL) and not being able to return to work around the first year after OHCA.19 Cardiac-induced cerebral hypoxia cause a diffuse injury that may damage the functional integrity of the brain20 and cause cognitive impairment.21 22 Resting state functional MRI (fMRI) is an informative imaging method that assays functional integrity and level of communication within the brain.23 In a recent study, patients with increased within-network and decreased between-network functional connectivity in the acute phase after cardiac arrest survival had a favourable outcome (FO) 1 year later compared with patients with a non-favourable outcome (nFO),13 suggesting higher functional integrity in patients with an FO. This increased within-network functional connectivity was also observed in a smaller study of cardiac arrest patients with good outcome at hospital discharge.14 To date, no reliable cognitive screening or imaging method for use during the in-patient hospitalisation that can detect risk of long-term cognitive impairment in cardiac arrest patients exists. A post-arrest screening model may contribute to prompt initiation of relevant follow-up and targeted cognitive rehabilitation.
A cardiac arrest is a severe and traumatising life event and long-term post-arrest psychopathology is prevalent.5 24–26 Processing near-death experiences, coping with prolonged preoccupation with somatic symptoms and fear of a second cardiac arrest is a burden to many cardiac arrest patients.5 24 Up to 61% of cardiac arrest patients experience anxiety, up to 45% depression and up to 27% post-traumatic stress disorder (PTSD).5 In particular, anxiety and depression have been found to negatively impact HRQoL and physical health in patients up to several years after survival.27 Furthermore, a cardiac arrest yields the highest prevalence of PTSD among cardiac disease categories,24 and PTSD is reported to double the overall risk of mortality, recurrence of a new cardiac event and causing long-term diminished mental health and quality of life.28 29 Little is currently known about the role of acute emotional reactions for developing long-term psychopathology in cardiac arrest survivors, but high acute stress reactions in other patient populations appear to be associated with worse long-term outcomes.30–32 Elucidating the role of acute emotional reactions may serve to support and advice the patients about future challenges and to initiate targeted psychological interventions.
Being the close relative of a cardiac arrest survivor can cause enduring psychological strains.33–35 Research indicates that these psychological strains are caused by a burden of witnessing the cardiac arrest, having to care for the patient and from the emotional stress of living with someone who is at risk of another cardiac arrest.36 At 1 year post-arrest, 40% of the close relatives of patients with brain injury are still experiencing a high impact of the cardiac event and display a more severe traumatic stress level than the patient (S Armand, unpublished data, March 2020), and after 2 years these caregivers show a higher level of trauma-related stress than that observed in the general population.37 As a growing number of patients are surviving cardiac arrest, it is crucial to focus more attention on psychological challenges in relatives in the aftermath after surviving cardiac arrest. The overall aim of the current study is therefore to evaluate and test a novel screening procedure during hospitalisation for its ability to predict at-risk patients for disabling cognitive impairment and psychopathology at 3 months. The incidence of psychopathology in close relatives 3-month post-arrest will also be explored. Overall, we expect that the screening procedure will be able to identify at-risk patients for disabling cognitive impairment and psychopathology at 3-month follow-up. In particular that:
Hypothesis 1: Lower level of cognitive impairment during hospitalisation (screening) is positively associated with cognitive outcome at 3-month follow-up.
Hypothesis 2: The strength of discrete resting-state networks in the brain assessed with fMRI during hospitalisation (screening) is positively associated with cognitive outcome at 3-month follow-up.
Hypothesis 3: Higher level of acute emotional reactions during hospitalisation (screening) is positively associated with psychopathology outcome at 3-month follow-up.
Hypothesis 4: Higher level of cognitive impairment and emotional reactions during hospitalisation in patients is positively associated with psychopathology outcomes in relatives at 3-month follow-up.
Methods and analysis
Study design, setting and population
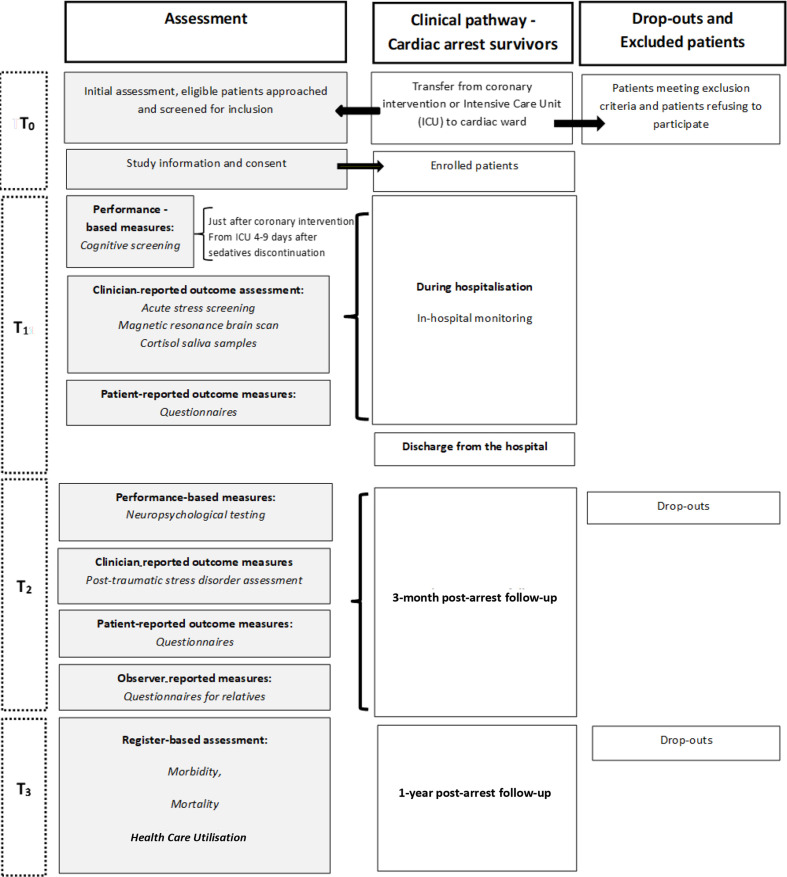
The REcovery after cardiac arrest surVIVAL (REVIVAL) study is designed as a multicentre, prospective cohort study in which first-time OHCA survivors are followed for up to 1 year after the event (flowchart presented in figure 1). The study settings are three highly specialised heart centres at university hospitals in Denmark: Rigshospitalet and Herlev-Gentofte Hospital in the Capital Region of Denmark and Odense University Hospital in the Southern Region of Denmark. All cardiac arrest patients will be approached for study participation and approximately 250 in-hospital patients (≥18 years of age) with a first-time OHCA admission diagnosis will be recruited, starting 1 January 2018 and ending 31 December 2021. Only patients with a presumed cardiac cause for their cardiac arrest as defined by Utstein template will be included.38 Both cardiac arrests as primary and secondary diagnosis will be included. Furthermore, the closest relatives will be identified and included during hospitalisation for a 3-month follow-up. Patients are excluded if they have a serious not treatable other somatic or psychiatric illness or previous cerebrovascular events or traumatic brain injury and those unable to speak and understand Danish. The cognitive screening and neuropsychological testing require the patients to follow verbal instructions and to see the tasks presented. Therefore, patients with solid hearing or visual impairments are excluded. In a substudy exploring resting state fMRI and stress reactivity, respectively, 40 patients without contraindications to MR will be included from Rigshospitalet. The REVIVAL study is described in accordance with the current Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement (Guidelines for reporting observational studies).39
Figure 1.
Flowchart of study assessment. T0: Study inclusion, T1: During hospitalisation, T2: 3-month follow-up, T3: 1-year follow-up.
Patient selection and recruitment
All the patients and relatives are selected and recruited in the three Danish cardiology wards. OHCA survivors transferred from the intensive care units (ICUs) to the ward will be included 4 to 9 days after termination of analgo-sedatives to ensure no residual sedation used during therapeutic hypothermia. The survivors from the coronary care units who undergo percutaneous coronary intervention after a brief cardiac arrest without ICU admission will be included after intervention in the cardiac catheterisation laboratory (figure 1). This subgroup of cardiac arrest survivors will be included although they are awake or only had a brief period of coma after admission to the hospital and presumably recover earlier to their premorbid cognitive functioning than the critically ill patients. The patients and relatives at the included sites follow the same protocol. A trained cardiac nurse will collect most of the clinical data from the patients’ charts. The same nurse will approach eligible patients during hospital admission on the cardiology ward and invite them to participate in the study. The patients will be assessed clinically and provided with oral as well as written information about the study. The patients are given the opportunity to read and consider the study information leaflet carefully. If the patients agree to participate, they will be asked to provide written informed consent in consultation with their closest relative prior to inclusion. This is justified in ethical issues as some patients with cognitive impairment may not feel empowered to refuse participation.
Collection of data and measures
Screening model during hospitalisation
A team of certified nurses with a background in cardiology will screen the patients. Administration and interpretation of data will take place under supervision by a trained psychologist.40
Cognition
The cognitive screening is conducted using the Danish version of the Montreal Cognitive Assessment tool (MoCA), V.7.0.41 The MoCA is a brief cognitive screening test designed to identify mild cognitive impairment. The MoCA covers six cognitive domains: attention, visuospatial construction, executive functioning, memory, language and orientation (table 1) and is rated from 0 to 30. A cut-off ≥26 is considered as normal cognitive function level. In the summary score, a level of education ≤12 years is given an extra point as education level has shown to decrease the overall score.41 The MoCA is suggested for use in the post-cardiac arrest settings,21 42 however, it remains to be evaluated in a Danish patient population with possible hypoxic-ischaemic brain injury. The MoCA has shown high level of internal reliability with Cronbach’s α=0.83 in detection of mild cognitive impairment.41
Table 1.
The cognitive assessment
| Target cognitive domain | During hospitalisation | 3-month follow-up |
| MoCA | Neuropsychological tests | |
| Attention | Number sequence Letter list |
D2 (visual attention) (trail making+stroop) |
| Visuospatial construction | Cube copying | Rey’s figure |
| Episodic memory | Verbal memory test | VAMT-26 |
| Working memory | Serial subtraction | Letter-number sequence |
| Executive functioning | Trail making B Clock drawing Abstraction |
Trailmaking B D-KEFS colour-word interference |
| Psychomotor processing speed | Trail making B | Trail making A and B |
| Language | Naming Repeating Word mobilisation |
|
| Orientation | Orientation |
D-KEFS, Delis–Kaplan Executive Function System; MoCA, Montreal Cognitive Assessment; VAMT-26, Danish Affective Verbal Learning Test-26.
Mood and delirium
As symptoms of delirium often are subtle but still can have an impact on cognition, the cognitive screening is initiated with a rapid clinical assessment of delirium, using 4AT scale.43 Furthermore, the mood state and functional independence of the patients are collected (table 2). If the study nurse is in doubt regarding patients functioning, an informed nurse or relative is asked.44
Table 2.
Outcome domains, measurement instruments, time of measurement and quantity for the cardiac arrest survivors
| Outcome domains and measurement instruments | Time of measure | Type of quantity |
| Sociodemographic variables | ||
| Age | T0 | Continuous |
| Sex | T0 | Binary |
| Marital status, type of occupation, employment status, living situation | T0 | Categorical |
| Medical variables | ||
| Known IHD, hypertension, previous MI, PCI or CABG, chronic heart failure, diabetes mellitus, COPD and chronic kidney disease | T0 | Binary |
| Clinical variables related to the cardiac arrest | ||
| Place of OHCA, aetiology of cardiac arrest, initial rhythm, TTM, medication during ICU stay, LVEF | T0 | Categorical |
| Bystander witnessed cardiac arrest, bystander performed CPR, use of AED, shockable rhythm, awake at arrival to hospital, TTM, intubated, medication during ICU, delirium at ICU | T0 | Binary |
| Time to ROSC, intubation time, length of stay at ICU | T0 | Continuous |
| Conscious state | ||
| GCS | T0 | Categorical |
| Neurological outcome | ||
| CPC | T1 | Categorical |
| Length of stay at hospital | T1 | Continuous |
| Performance-based variables | ||
| Delirium score | ||
| 4AT | T1 | Categorical |
| Functional independence | ||
| Barthel Index-20 | T1 | Categorical |
| Cognitive status | ||
| MoCA | T1 | Binary |
| Brain activity while resting | ||
| rsfMRI | T1 | Continuous |
| Neuropsychological outcome | ||
| VAMT-26, D- KEFS trail-taking, D-KEFS colour-word interference, D-KEFS design fluency, Rey’s complex figure and Letter-number sequencing: subtest of WAIS-IV | T2 | Binary |
| Cortisol awakening response | T1 | Continuous |
| Patient-reported outcome measures | ||
| POMS | T1 | |
| HADS, IES-R, CSS | T1, T2 | Continuous |
| B-IPQ, FSS, HeartQoL, SF-12, PSQI, CISS, BRIEF-A, ECR-R, AMCQ, CES-S, MTEQ, PTGQ, ACQ, NDEQ | T2 | Continuous |
| Lifestyle changes, health profile | T2 | Categorical |
| Register based | ||
| Depression, anxiety, dementia, chronical fatigue syndrome and heart failure, mortality and healthcare utilisation | T3 | Continuous |
ACQ, Attribution; AED, automated external defibrillator; AMCQ, Autobiographical Memory Characteristics Questionnaire; B-IPQ, Brief Illness Perception Scale; BRIEF-A, Behavior Rating Inventory of Executive Functions, adult version; CABG, coronary artery bypass surgery; CES-S, Centrality of Events–Short; CISS, Coping Inventory for Stressful Situations; COPD, chronic obstructive pulmonary disease; CPC, Cerebral Performance Category; CPR, cardiopulmonary resuscitation; CSS, Crisis Support Scale; D- KEFS, Delis–Kaplan Executive Function System; ECR-R, Experience in Close Relationships; FSS, Fatigue Severity Scale; GCS, Glasgow Coma Scale; HADS, Hospital Anxiety and Depression Scale; ICU, intensive care unit; IES-R, Impact of Event Scale-Revised; IHD, ischaemic heart disease; LVEF, left ventricular ejection fraction; MI, myocardial infarction; MoCA, Montreal Cognitive Assessment; MTEQ, Memory of Event Scale; NDEQ, Near-death Experience Questionnaire; OHCA, out-of-hospital cardiac arrest; PCI, percutaneous coronary intervention; POMS, Profile of Mood States; PSQI, Pittsburgh Sleep Quality Inventory; PTGQ, Post-Traumatic Growth Questionnaire; ROSC, return of spontaneous circulation; rsfMRI, resting state functional magnetic resonance imaging; SF-12, 12-item Short Form Survey; T0, pre-arrest, medical and clinical data; T1, during hospitalisation; T2, 3 months follow-up; T3, 1 year follow-up; TTM, targeted temperature management; VAMT-26, Danish Affective Verbal Learning Test-26.
Resting state fMRI
Patients who meet inclusion criteria for MRI will undergo structural and functional brain imaging on a 3 Tesla Siemens Prisma MRI scanner at Rigshospitalet. Trained personnel will perform MRI scans; during transport and scanning, a trained nurse will monitor the patients. We will obtain high-resolution structural T1-weighted and T2-weighted images and T2*-weighted BOLD fMRI resting state scans (~10 min of resting state fMRI). During resting state fMRI, patients are asked to lie still with eyes closed and let their mind wander freely. Resting state fMRI data will be processed using canonical brain imaging tools, for example, SPM45 and Conn23 to establish region-to-region connectivity estimates while accounting for motion, physiological and other noise sources. Resting state networks will be defined based on a priori connectivity network descriptions.46
Psychopathology
Self-rated symptoms of depression and anxiety and trauma reactions will be collected using the Hospital Anxiety and Depression Scale (HADS-D and HADS-A)47 and the Impact of event Scale-revised (IES-R).48 The Hospital Anxiety and Depression Scale (HADS) is a valid and internally consistent 14-item instrument to measure anxiety and depression. Each item is scored from 0 to 3 with a summary score between 0 and 21 for either anxiety or depression. Scores of 11 and above indicate the probable presence of a mood disorder. HADS has shown a mean α of 0.83 and 0.82 for the HADS-A and HADS-D, respectively.49 Impact of Event Scale-revised (IES-R) measures distress caused by a traumatic event is a widely used measures to assess traumatic stress symptoms with a maximum score of 88. The IES-R consist of 22 items measuring subjective distress caused by a traumatic event scored on a Likert scale from 0 (not at all) to 4 (extremely) and includes subscales for avoidance, intrusions and hyperarousal. The IES-R has shown high internal consistency with coefficient alphas ranging from 0.84 to 0.85 for avoidance, 0.87 to 0.92 for intrusion and 0.79 to 0.90 for hyperarousal.48
Acute stress disorder
The Acute Stress Disorder Structured Interview (ASDI) is a 19-item clinical interview, which investigates the incidence and severity of acute stress responses operated as acute stress disorder (ASD) in DSM-5 in the month following trauma exposure.50 51 ASD is divided into five symptom clusters: intrusive memories or revival, negative mood, dissociation, avoidance and arousal. To meet the criteria for ASD, the patient must have experienced a traumatic event (in this case a cardiac arrest) during the past month (criteria A) as well as the presence or deterioration of at least nine symptoms independent of the associated category after the onset of episode (Criterion B), which should occur within 3 days to 1 month (Criterion C).52
Cortisol awakening response
At the same day as the structured clinical interview (ie, ASDI) is performed, saliva samples for determination of the cortisol awakening response (CAR) will be obtained from eligible patients. Five samples are collected during awakening and three saliva samples will also be collected during the day at 12, at 18 and at 23 o’clock. The total amount of saliva per patient is 16 mL. After collecting the saliva, the samples will be stored and analysed at the Department of Clinical Biochemistry, Rigshospitalet, Glostrup.
Sociodemographic variables and several clinical prehospital and inhospital data were obtained from electronic medical records (see table 2).
At 3-month follow-up
The patients included at Rigshospitalet and Herlev-Gentofte Hospital will undergo a detailed and individual neuropsychological assessment at the Neurobiology Research Unit at Rigshospitalet, and patients included at Odense University Hospital will be assessed with a similar test battery at the University of Southern Denmark. The cognitive assessment of the patient’s cognitive functions will be given, administered and interpreted by a clinical and trained psychologist or psychology student under supervision. The patients and their relatives will furthermore complete a package of self-reported questionnaires.
The neuropsychological assessment
The tests used are a carefully selected neuropsychological test battery comprising some of the same subcomponents as for the MoCA; attention, visuospatial construction, executive functioning, episodicmemory, working memory and psychomotor processing speed. The tests used are all validated in clinical settings for a variety of populations and have shown good test–retest reliability. To address a source of bias, all tests are conducted by a psychologist, who is kept blind of the clinical data as well as the results of the previous brief cognitive screening. A detailed outline of the neuropsychological tasks includes Danish Affective Verbal Learning Test-26 (VAMT-26)53 and Delis-Kaplan Executive Function System tests (D-KEFS) comprising trail making, colour-word interference, design fluency and word fluency54 together with the Rey’s complex figure test55 and Letter-number sequencing test from the Wechsler Adult Intelligence Scale-IV (WAIS-IV)56 (table 1).
Psychopathology in patients
Furthermore, the patients will repeat the self-reported questionnaires identical to the questionnaires completed during hospitalisation: HADS-D, HADS-A and IES-R.
Psychopathology in relatives
The relatives are also asked to complete the HADS-D, HADS-A and IES-R. The questionnaires for both patients and their relatives are sent via e-mail 2 weeks before the 3 months follow-up.
At 1-year follow-up
The 1-year follow-up is designed as register-based (table 2). To investigate whether baseline data are associated with morbidity: depression, anxiety, dementia, chronical fatigue syndrome or heart failure and with mortality and healthcare utilisation, the collected data will be linked with data from national administrative registers; the Danish National Patient Register,57 the Danish Civil Registration System,58 the Danish National Prescription Registry,59 the Danish education registers60 and the Danish registers on personal income and transfer payments.61
Primary and secondary study outcome measures
Primary outcome measures for patients
The primary outcome is whether the patients present an FO or an nFO at 3-month follow-up. To elucidate cognition and psychopathology separately, primary outcomes will be established for each of these domains.
Cognition
As primary cognitive outcome, the patients will be divided into two groups, FO and nFO, based on their performance in the neuropsychological tasks. The subset with an nFO is defined as minimum 1.5 SD under the norm or reference data53–55 62 on minimum one test or 1 SD on two or more tests. The rest of the patients fall into the FO group.
Psychopathology
As primary psychopathology outcome, the patients will be divided into two groups, FO and nFO, based on their scores on the HADS and IES-R. The subset with an nFO is defined one or more scores above cut-off for psychopathology on the HADS and IES-R: HADS-D and HADS-A >8 and IES-R >24. The rest of the patients fall into the FO group.
Secondary outcome measures for patients
Secondary outcomes for patients are self-reported measures of sleep quality, fatigue, executive functioning and HRQoL at 3 months of follow-up (table 2). A detailed description of the secondary outcomes is described in the online supplemental material 1. Secondary outcomes for patients also include register-based information of morbidity: depression, anxiety, dementia, chronical fatigue syndrome or heart failure, mortality and healthcare utilisation at 1-year post-arrest (table 2).
bmjopen-2020-038633supp001.pdf (67.2KB, pdf)
Primary outcome measures for relatives
The primary outcomes for the relatives include HADS-D, HADS-A and IES-R47 63 (table 3).
Table 3.
Outcome domains, measurement instruments and measurement time for the relatives
| Outcome domain | Measurement instruments | Time |
| Demographic variables and psychiatric medical history | T2 | |
| Health-related quality of life | SF-12 | |
| Anxiety and depression | HADS | |
| Distress caused by a traumatic event | IES-R | |
| Experience in close relationships | ECR-R | |
| Social support after a crisis | CSS | |
| Major depression | MDI | |
| The extent to which an event is viewed as being central to one’s identity | CES-S | |
| Cognitive decline reported by informants (relatives or close friends) | IQ-CODE |
CES-S, Centrality of Event short; CSS, The Crisis Support Scale; ECR-R, Experience in Close Relationships; HADS, Hospital Anxiety and Depression Scale; IES-R, Impact of Event Scale-Revised; IQ-CODE, The Informant Questionnaire on Cognitive Decline in the Elderly; MDI, Major Depression Inventory; SF-12, 12-item Short Form Survey; T2, 3-month follow-up.
Secondary outcome measures for relatives
Secondary outcome measures for relatives include self-reported HRQoL, experience of cognitive changes in the patient after the cardiac arrest, social support, major depression, the quality in the relationship with the cardiac arrest survivor and the extent to which the cardiac arrest is viewed as being central to one’s identity (table 3).
Several exploratory outcomes will also be collected (figure 1).
Data analysis plan
The collected sociodemographic data will be presented as means±SD or percentages, respectively, and group differences will be calculated by t-tests (continuous data) or χ2 (categorical data). Appropriate regression models will be used to examine the associations between screening performance during hospitalisation and the primary and secondary study outcomes. In all model’s relevant covariates, for example, sex, age, comorbidity, time to return of spontaneous circulation, coma duration time, time at ICU, time of hospitalisation will be included (table 2). A formal power calculation of sample size has not been performed due to the several aims and potential analyses of this study. With no comparative patient groups and only few small existing studies, we aim for a sample size of 200 cardiac arrest survivors at 3-month follow-up for the primary outcome to be statistically and clinically significant.
Patient and public involvement
As a patient involvement method, two theme-based sessions involving direct patient feedback have been carried out in the early pilot and design phase of the study. The aim of these sessions was to (1) identify patient preferences regarding the cognitive screening procedure (figure 2) and (2) develop the research priorities including implementation of possible changes based on the patient’s feedback. Data derived from the theme-based patient feedback sessions provides the basis for the cognitive screening procedure in the REVIVAL study. To ensure further patient involvement, we plan to engage the patients in the planning phase of disseminating the results.
Figure 2.
Direct patient feedback.
Discussion
To the best of our knowledge, the present study will be the largest study evaluating and testing a novel screening procedure for cognitive impairment and emotional reactions during hospitalisation in a population of OHCA survivors. As the incidence of cardiac arrest survival is increasing, establishing a standardised approach to screening in OHCA survivors will be critical in the future. Following the aims of the study and to strengthen the standardisation of the results, only patients with a presumed cardiac cause for their cardiac arrest as defined by Utstein template will be included. Since a common single aetiology of cardiac arrest is respiratory failure, it could be considered to include this population in a future study. Although cognitive and mental health outcomes in OHCA survivors may be comparable with other medical populations,7 64 65 the study does not contain a comparative arm as it does not aim to investigate a specific intervention. Instead, the study seeks to investigate OHCA survivors after standard treatment in a naturalistic setting. Due to the nature of the study and the vulnerable state of the patients, differential loss to follow-up is expected in the study. To elucidate data missing not at random, we plan to conduct phone calls to non-responders at 3-month follow-up regarding their withdrawal from the study. We expect that results from the REVIVAL study will inform an early screening procedure of OHCA survivors in clinical settings as well as inform future targeted rehabilitation in survivors who are likely to develop protracted cognitive impairment and psychopathology.
Ethics and dissemination
There is little to no discomfort for the patients and their relatives in this study. Due to the weakened constitution of the patients, the neuropsychological test at 3-month post-arrest could be experienced as taxing and can be divided into 2 days. The study complies with the Declaration of Helsinki and has been approved by the Danish National Committee on health research ethics (H-18046155) and the Danish Data Protection Agency (RH-2017-325, I-Suite no: 05961). Patients and their relatives will receive oral and written information about the study and inclusion will require obtained written consent for all participants before enrolment. Results from this study will be disseminated at regional, national and international conferences and in peer-reviewed journals.
Supplementary Material
Footnotes
Contributors: Department of Cardiology, Centre for Cardiac, Vascular, Pulmonary and Infectious Diseases, Copenhagen University Hospital Rigshospitalet (MKW, SKB, and CH) and Neurobiology Research Unit, Rigshospitalet (DSS, PMF and GMK) has full access to all the data in the study and takes responsibility for the integrity of data. MKW, SKB, CH and DSS contributed to the study concept and design. MKW, SKB, CH, SA, JEM, PMF, GMK and DSS contributed to the data acquisition. Analysis will be performed by MKW, SKB, CH, OE and DSS; MKW and DSS drafted the manuscript with critical input from SKB, CH, SA, JEM, OE, TBR, PMF and GMK. All authors approved the final version of the manuscript.
Funding: The REVIVAL study is funded by independent research grants from the following non-profit or governmental agencies: The Research Foundation of Copenhagen University Hospital, Rigshospitalet, Denmark (GNT 38-A2015 and GNT A5638), The Danish Knowledge Centre for Rehabilitation and Palliative Care (REHPA) (GNT 34 177 058), The Helsefonden, Denmark (GNT 18-B-0235), The Danish Heart Association (GNT 18-R124-A8454-22099, and from both The Research Committee and the Research Committee in clinical nursing, The Heart Centre, Copenhagen University Hospital, Rigshospitalet, Denmark (GNT Not Applicable).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Porzer M, Mrazkova E, Homza M, et al. Out-Of-Hospital cardiac arrest. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2017;161:348–53. 10.5507/bp.2017.054 [DOI] [PubMed] [Google Scholar]
- 2.Myat A, Song K-J, Rea T. Out-Of-Hospital cardiac arrest: current concepts. Lancet 2018;391:970–9. 10.1016/S0140-6736(18)30472-0 [DOI] [PubMed] [Google Scholar]
- 3.Berdowski J, Berg RA, Tijssen JGP, et al. Global incidences of out-of-hospital cardiac arrest and survival rates: systematic review of 67 prospective studies. Resuscitation 2010;81:1479–87. 10.1016/j.resuscitation.2010.08.006 [DOI] [PubMed] [Google Scholar]
- 4.Elliott VJ, Rodgers DL, Brett SJ. Systematic review of quality of life and other patient-centred outcomes after cardiac arrest survival. Resuscitation 2011;82:247–56. 10.1016/j.resuscitation.2010.10.030 [DOI] [PubMed] [Google Scholar]
- 5.Green CR, Botha JA, Tiruvoipati R. Cognitive function, quality of life and mental health in survivors of our-of-hospital cardiac arrest: a review. Anaesth Intensive Care 2015;43:568–76. 10.1177/0310057X1504300504 [DOI] [PubMed] [Google Scholar]
- 6.Moulaert VRMP, Verbunt JA, van Heugten CM, et al. Cognitive impairments in survivors of out-of-hospital cardiac arrest: a systematic review. Resuscitation 2009;80:297–305. 10.1016/j.resuscitation.2008.10.034 [DOI] [PubMed] [Google Scholar]
- 7.Cronberg T, Lilja G, Horn J, et al. Neurologic function and health-related quality of life in patients following targeted temperature management at 33°C vs 36°C after out-of-hospital cardiac arrest: a randomized clinical trial. JAMA Neurol 2015;72:634–41. 10.1001/jamaneurol.2015.0169 [DOI] [PubMed] [Google Scholar]
- 8.Whitehead L, Perkins GD, Clarey A, et al. A systematic review of the outcomes reported in cardiac arrest clinical trials: the need for a core outcome set. Resuscitation 2015;88:150–7. 10.1016/j.resuscitation.2014.11.013 [DOI] [PubMed] [Google Scholar]
- 9.Perez CA, Samudra N, Aiyagari V. Cognitive and functional consequence of cardiac arrest. Curr Neurol Neurosci Rep 2016;16:70. 10.1007/s11910-016-0669-y [DOI] [PubMed] [Google Scholar]
- 10.Sakusic A, O'Horo JC, Dziadzko M, et al. Potentially modifiable risk factors for long-term cognitive impairment after critical illness: a systematic review. Mayo Clin Proc 2018;93:68–82. 10.1016/j.mayocp.2017.11.005 [DOI] [PubMed] [Google Scholar]
- 11.Davies SE, Rhys M, Voss S, et al. Psychological wellbeing in survivors of cardiac arrest, and its relationship to neurocognitive function. Resuscitation 2017;111:22–5. 10.1016/j.resuscitation.2016.11.004 [DOI] [PubMed] [Google Scholar]
- 12.Wachelder EM, Moulaert VRMP, van Heugten C, et al. Life after survival: long-term daily functioning and quality of life after an out-of-hospital cardiac arrest. Resuscitation 2009;80:517–22. 10.1016/j.resuscitation.2009.01.020 [DOI] [PubMed] [Google Scholar]
- 13.Descatha A, Dumas F, Bougouin W, et al. Work factors associated with return to work in out-of-hospital cardiac arrest survivors. Resuscitation 2018;128:170–4. 10.1016/j.resuscitation.2018.05.021 [DOI] [PubMed] [Google Scholar]
- 14.Lilja G, Nielsen N, Bro-Jeppesen J, et al. Return to work and participation in society after out-of-hospital cardiac arrest. Circ Cardiovasc Qual Outcomes 2018;11:e003566. 10.1161/CIRCOUTCOMES.117.003566 [DOI] [PubMed] [Google Scholar]
- 15.van Wijnen HGFM, Rasquin SMC, van Heugten CM, et al. The impact of cardiac arrest on the long-term wellbeing and caregiver burden of family caregivers: a prospective cohort study. Clin Rehabil 2017;31:1267–75. 10.1177/0269215516686155 [DOI] [PubMed] [Google Scholar]
- 16.Cronberg T, Lilja G. Cognitive decline after cardiac arrest--It is more to the picture than hypoxic brain injury. Resuscitation 2015;91:A3–4. 10.1016/j.resuscitation.2015.03.002 [DOI] [PubMed] [Google Scholar]
- 17.van Heugten C, Gregório GW, Wade D. Evidence-Based cognitive rehabilitation after acquired brain injury: a systematic review of content of treatment. Neuropsychol Rehabil 2012;22:653–73. 10.1080/09602011.2012.680891 [DOI] [PubMed] [Google Scholar]
- 18.Mędrzycka-Dąbrowska WA, Czyż-Szybenbejl K, Kwiecień-Jaguś K, et al. Prediction of cognitive dysfunction after resuscitation - a systematic review. Postepy Kardiol Interwencyjnej 2018;14:225–32. 10.5114/aic.2018.78324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moulaert VRM, van Heugten CM, Winkens B, et al. Early neurologically-focused follow-up after cardiac arrest improves quality of life at one year: a randomised controlled trial. Int J Cardiol 2015;193:8–16. 10.1016/j.ijcard.2015.04.229 [DOI] [PubMed] [Google Scholar]
- 20.Barkhof F, Haller S, Rombouts SARB. Resting-State functional MR imaging: a new window to the brain. Radiology 2014;272:29–49. 10.1148/radiol.14132388 [DOI] [PubMed] [Google Scholar]
- 21.Nolan JP, Soar J, Cariou A, et al. European resuscitation Council and European Society of intensive care medicine guidelines for post-resuscitation care 2015: section 5 of the European resuscitation Council guidelines for resuscitation 2015. Resuscitation 2015;95:202–22. 10.1016/j.resuscitation.2015.07.018 [DOI] [PubMed] [Google Scholar]
- 22.Hinduja A, Gupta H, Yang JD, et al. Hypoxic ischemic brain injury following in hospital cardiac arrest - lessons from autopsy. J Forensic Leg Med 2014;23:84–6. 10.1016/j.jflm.2014.02.003 [DOI] [PubMed] [Google Scholar]
- 23.Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect 2012;2:125–41. 10.1089/brain.2012.0073 [DOI] [PubMed] [Google Scholar]
- 24.Vilchinsky N, Ginzburg K, Fait K, et al. Cardiac-disease-induced PTSD (CDI-PTSD): a systematic review. Clin Psychol Rev 2017;55:92–106. 10.1016/j.cpr.2017.04.009 [DOI] [PubMed] [Google Scholar]
- 25.Gamper G, Willeit M, Sterz F, et al. Life after death: posttraumatic stress disorder in survivors of cardiac arrest--prevalence, associated factors, and the influence of sedation and analgesia. Crit Care Med 2004;32:378–83. 10.1097/01.CCM.0000108880.97967.C0 [DOI] [PubMed] [Google Scholar]
- 26.Kamphuis HCM, De Leeuw JRJ, Derksen R, et al. A 12-month quality of life assessment of cardiac arrest survivors treated with or without an implantable cardioverter defibrillator. Europace 2002;4:417–25. 10.1053/eupc.2002.0258 [DOI] [PubMed] [Google Scholar]
- 27.Moulaert VRMP, Wachelder EM, Verbunt JA, et al. Determinants of quality of life in survivors of cardiac arrest. J Rehabil Med 2010;42:553–8. 10.2340/16501977-0547 [DOI] [PubMed] [Google Scholar]
- 28.Wilder Schaaf KP, Artman LK, Peberdy MA, et al. Anxiety, depression, and PTSD following cardiac arrest: a systematic review of the literature. Resuscitation 2013;84:873–7. 10.1016/j.resuscitation.2012.11.021 [DOI] [PubMed] [Google Scholar]
- 29.Edmondson D, Richardson S, Falzon L, et al. Posttraumatic stress disorder prevalence and risk of recurrence in acute coronary syndrome patients: a meta-analytic review. PLoS One 2012;7:e38915. 10.1371/journal.pone.0038915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Visser E, Gosens T, Den Oudsten BL, et al. The course, prediction, and treatment of acute and posttraumatic stress in trauma patients: a systematic review. J Trauma Acute Care Surg 2017;82:1158–83. 10.1097/TA.0000000000001447 [DOI] [PubMed] [Google Scholar]
- 31.Bryant RA, Moulds ML, Guthrie RM. Acute stress disorder scale: a self-report measure of acute stress disorder. Psychol Assess 2000;12:61–8. 10.1037/1040-3590.12.1.61 [DOI] [PubMed] [Google Scholar]
- 32.Wessa M, Rohleder N, Kirschbaum C, et al. Altered cortisol awakening response in posttraumatic stress disorder. Psychoneuroendocrinology 2006;31:209–15. 10.1016/j.psyneuen.2005.06.010 [DOI] [PubMed] [Google Scholar]
- 33.van Wijnen HG, Rasquin SM, van Heugten CM, et al. The impact of cardiac arrest on the long-term wellbeing and caregiver burden of family caregivers: a prospective cohort study. Clin Rehabil 2017;31:1267–75. 10.1177/0269215516686155 [DOI] [PubMed] [Google Scholar]
- 34.Zimmerli M, Tisljar K, Balestra G-M, et al. Prevalence and risk factors for post-traumatic stress disorder in relatives of out-of-hospital cardiac arrest patients. Resuscitation 2014;85:801–8. 10.1016/j.resuscitation.2014.02.022 [DOI] [PubMed] [Google Scholar]
- 35.Haywood K, Dainty KN. Life after cardiac arrest: The importance of engaging with the 'forgotten patient'. Resuscitation 2018;128:A1–2. 10.1016/j.resuscitation.2018.04.034 [DOI] [PubMed] [Google Scholar]
- 36.Moulaert V. Life after survival of a cardiac arrest: the brain is the heart of the matter, 2014. [Google Scholar]
- 37.Van't Wout Hofland J, Moulaert V, van Heugten C, et al. Long-Term quality of life of caregivers of cardiac arrest survivors and the impact of witnessing a cardiac event of a close relative. Resuscitation 2018;128:198–203. 10.1016/j.resuscitation.2018.03.016 [DOI] [PubMed] [Google Scholar]
- 38.Perkins GD, Jacobs IG, Nadkarni VM, et al. Cardiac arrest and cardiopulmonary resuscitation outcome reports: update of the Utstein resuscitation registry templates for out-of-hospital cardiac arrest: a statement for healthcare professionals from a task force of the International liaison Committee on resuscitation (American heart association, European resuscitation Council, Australian and New Zealand Council on resuscitation, heart and stroke Foundation of Canada, InterAmerican heart Foundation, resuscitation Council of southern Africa, resuscitation Council of Asia); and the American heart association emergency cardiovascular care Committee and the Council on cardiopulmonary, critical care, perioperative and resuscitation. Circulation 2015;132:1286–300. 10.1161/CIR.0000000000000144 [DOI] [PubMed] [Google Scholar]
- 39.Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. PLoS Med 2007;4:e297–54. 10.1371/journal.pmed.0040297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Czyż-Szypenbej K, Mędrzycka-Dąbrowska W, Sak-Dankosky N. Neurocognitive Testing-Do we lack in expertise? Crit Care Med 2019;47:e530–1. 10.1097/CCM.0000000000003726 [DOI] [PubMed] [Google Scholar]
- 41.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA : A Brief Screening. J Am Geriatr Soc 2005:695–9. [DOI] [PubMed] [Google Scholar]
- 42.Boyce LW, Goossens PH, Moulaert VR, et al. Out-Of-Hospital cardiac arrest survivors need both cardiological and neurological rehabilitation! Curr Opin Crit Care 2019;25:240–3. 10.1097/MCC.0000000000000609 [DOI] [PubMed] [Google Scholar]
- 43.Bellelli G, Morandi A, Davis DHJ, et al. Validation of the 4AT, a new instrument for rapid delirium screening: a study in 234 hospitalised older people. Age Ageing 2014;43:496–502. 10.1093/ageing/afu021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hsueh I-P, Lin J-H, Jeng J-S, et al. Comparison of the psychometric characteristics of the functional independence measure, 5 item Barthel index, and 10 item Barthel index in patients with stroke. J Neurol Neurosurg Psychiatry 2002;73:188–90. 10.1136/jnnp.73.2.188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Statistical parametric mapping. Available: www.fil.ion.ucl.ac.uk/spm/
- 46.Raichle ME. The brain's default mode network. Annu Rev Neurosci 2015;38:433–47. 10.1146/annurev-neuro-071013-014030 [DOI] [PubMed] [Google Scholar]
- 47.Snaith RP. The hospital anxiety and depression scale. Health Qual Life Outcomes 2003;1:29 10.1186/1477-7525-1-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Creamer M, Bell R, Failla S. Psychometric properties of the Impact of Event Scale - Revised. Behav Res Ther 2003;41:1489–96. 10.1016/j.brat.2003.07.010 [DOI] [PubMed] [Google Scholar]
- 49.Bjelland I, Dahl AA, Haug TT, et al. The validity of the hospital anxiety and depression scale. An updated literature review. J Psychosom Res 2002;52:69–77. 10.1016/s0022-3999(01)00296-3 [DOI] [PubMed] [Google Scholar]
- 50.Bryant RA, Harvey AG, Dang ST, et al. Assessing acute stress disorder: psychometric properties of a structured clinical interview. Psychol Assess 1998;10:215–20. 10.1037/1040-3590.10.3.215 [DOI] [Google Scholar]
- 51.American Psychiatric Diagnosis and statistical manual of mental disorders, DSM-5. 5 th Washington, DC: American Psychiatric Association, 2013. [Google Scholar]
- 52.Bryant RA, Friedman MJ, Spiegel D, et al. A review of acute stress disorder in DSM-5. Depress Anxiety 2011;28:802–17. 10.1002/da.20737 [DOI] [PubMed] [Google Scholar]
- 53.Jensen CG, Hjordt LV, Stenbæk DS, et al. Development and psychometric validation of the verbal affective memory test. Memory 2016;24:1208–23. 10.1080/09658211.2015.1087573 [DOI] [PubMed] [Google Scholar]
- 54.Delis DC, Kaplan E, Kramer JH. Delis-Kaplan executive function system : The psychological Corporation. San Antonio, TX, 2001. [Google Scholar]
- 55.Somerville J, Tremont G, Stern RA. The Boston qualitative scoring system as a measure of executive functioning in Rey-Osterrieth complex figure performance. J Clin Exp Neuropsychol 2000;22:613–21. 10.1076/1380-3395(200010)22:5;1-9;FT613 [DOI] [PubMed] [Google Scholar]
- 56.Wechsler DW. Adult intelligence scale. third edit. San Antonio, TX: The Psychological Corporation, 1997. [Google Scholar]
- 57.Lynge E, Sandegaard JL, Rebolj M. The Danish national patient register. Scand J Public Health 2011;39:30–3. 10.1177/1403494811401482 [DOI] [PubMed] [Google Scholar]
- 58.Pedersen CB. The Danish civil registration system. Scand J Public Health 2011;39:22–5. 10.1177/1403494810387965 [DOI] [PubMed] [Google Scholar]
- 59.Kildemoes HW, Sørensen HT, Hallas J. The Danish national prescription registry. Scand J Public Health 2011;39:38–41. 10.1177/1403494810394717 [DOI] [PubMed] [Google Scholar]
- 60.Jensen VM, Rasmussen AW. Danish education registers. Scand J Public Health 2011;39:91–4. 10.1177/1403494810394715 [DOI] [PubMed] [Google Scholar]
- 61.Baadsgaard M, Quitzau J. Danish registers on personal income and transfer payments. Scand J Public Health 2011;39:103–5. 10.1177/1403494811405098 [DOI] [PubMed] [Google Scholar]
- 62.Wechsler D. WAIS-III administration and scoring manual. Antonio, TX: Psychol Corp, 1997. [Google Scholar]
- 63.Weiss DS, Marmar CR. The impact of event Scale-Revised. in: assessing psychological trauma and PTSD: a practioners Handbook. New York: Guilford Press, 1997: 399–411. [Google Scholar]
- 64.Jackson JC, Girard TD, Gordon SM, et al. Long-Term cognitive and psychological outcomes in the awakening and breathing controlled trial. Am J Respir Crit Care Med 2010;182:183–91. 10.1164/rccm.200903-0442OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cronberg T, Lilja G, Rundgren M, et al. Long-Term neurological outcome after cardiac arrest and therapeutic hypothermia. Resuscitation 2009;80:1119–23. 10.1016/j.resuscitation.2009.06.021 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-038633supp001.pdf (67.2KB, pdf)