Abstract
Chronic lymphatic leukaemia (CLL) is the most common leukaemia in the Western world. Ibrutinib, a tyrosine kinase inhibitor, is the treatment of choice on relapse or p53-dysfunction. Richter’s transformation to diffuse large B cell lymphoma is most often seen. However, transformation to other aggressive lymphomas as plasmablastic lymphoma (PBL) does occur. PBL is an extremely aggressive lymphoma and is usually treated using a CHOP-like regimen (cyclophosphamide, doxorubicin, vincristine and prednisone/dexamethasone), but with poor outcome. The only curative treatment is allogeneic stem cell transplant (ASCT).
We report on a case of CLL treated with ibrutinib that underwent transformation to PBL. Due to high expression of CD138, we added daratumumab to the chemotherapy with a good, but transitory response. The case did not make it to an ASCT. Targeting CD138 by daratumumab may be added to chemoimmune therapy for PBL.
Keywords: haematology (incl blood transfusion), chemotherapy
Background
Chronic lymphatic leukaemia (CLL) is the most common leukaemia in the Western world with an incidence 4.1/100 000, causing 5000 mortalities in USA yearly.1 A 2012 Norwegian study reported an age-adjusted incidence of 3.8/100 000, median age at diagnosis was 70 years and men/women ratio was 1.43.2
Chemoimmune therapy with fludarabine, cyclophosphamide, bendamustine or chlorambucil in combination with an anti-CD20 antibody has been the recommended first line treatment, depending on age and comorbidity.3 On relapse or resistant disease, the signal pathway inhibitors ibrutinib and idelalisib are the treatment of choice. Signal pathway inhibitors are preferred in first line for patient with p53 dysfunction (del(17 p) and/or TP53 mutation).4 About 5%–15% of patients undergoing treatment may transform (Richter’s transformation (RT)) to more aggressive lymphomas, usually diffuse large B cell lymphoma (DLBCL).5 Prognosis for the latter is dependent on whether the DLBCL is clonally related to CLL (true transformation) or unrelated (de novo DLBCL). In RT DLBCL is the most prevalent phenotype, but other phenotypes occur as well, in particular Hodgkin lymphoma,6 and some more feared than others due to their aggressive nature like plasmablastic lymphoma (PBL).7–9
PBL was described in 199710 and is a rare, but aggressive lymphoma thought to be related to DLBCL, defined by WHO as a non-Hodgkin B cell malignancy that exhibits immunological and morphological features of plasma cell differentiation.11 PBL is most frequently found in immunocompromised patients and is associated with viral infections like human immunodeficiency virus (HIV) and Epstein-Barr virus (EBV). Actually, 63% of all PBL cases reported as of 2015 were HIV-associated, and only 3% of PBL evolved in patients with haematological malignancies. Of the latter, 50% and 30% were transformation from CLL and follicular lymphoma, respectively. Patients with transformation to PBL were mainly men at a median age of 62 years.12 PBL that arises in patients with CLL is associated with poor prognosis and has been difficult to treat. We report on transformation to PBL in a patient with CLL on ibrutinib treatment as third line treatment in chemoimmune therapy-resistant disease.
Case presentation
Our patient was a man diagnosed with high-risk λ restricted IgM+ CLL (Immunoglobulin Heavy Variable 4-39 with 100% homology with germ line and heterozygous del(13q14)) in 2010 at the age of 53 years. At diagnosis he was anti-EBV IgG antibody positive. Within 9 months of diagnosis treatment was indicated due to progressive Binet stage B. He was included in the HOVON 68 CLL Study13 and received six courses of fludarabine and cyclophosphamide. At the end of the treatment, he was in complete remission, but was positive for minimal residual disease by flow cytometry (0.03%). In 2013 he was diagnosed with prostate cancer (T3bN1), and following hormone therapy he received radiation therapy with a curative intent.
In 2015, 4 years after start of first-line therapy, he showed signs of relapse of CLL. One year later, his disease status was progressive Binet stage B. Genetic analysis similarities heterozygous del(13q14), del(6q23) and TP53 mutation (c.329G>C, p.Arg110Pro). He received second-line treatment with fludarabine, cyclophosphamide and rituximab in six courses. By the end of the treatment he was in partial remission with persistent lymphadenopathy and bone marrow involvement (15% of the cellularity), and ibrutinib was initiated with a prompt resolution of lymphadenopathy and unsustained lymphocytosis. Following 24 months of treatment with ibrutinib he was hospitalised with malaise, B symptoms, persistent coughing with sputum, gastrointestinal bleeding and anaemia. CT scan disclosed multiple pathological lymph nodes above and below the diaphragm with a large intra-abdominal conglomerate with central necrosis residing close to the intestine, but the CT scan did not disclose any osteolytic lesions. Blood tests revealed hypercalcaemia, increased lactate dehydrogenase and acute renal failure interpreted as tumour lysis syndrome which was treated with rasburicase, zoledronic acid and fluid infusion with good effect. Serum protein electrophoresis unravelled hypogammaglobulinaemia (IgG <1.08 g/L and IgA 0.1 g/L) and monoclonal IgM λ of 3 g/L. Ig κ light chain was 2.9 and Ig λ light chain 2280 mg/L.
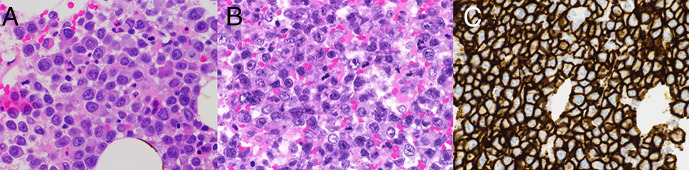
Biopsy confirmed plasmablastic malignancy with the following immunophenotype (figure 1): CD138+, λ+, MUM1+, CD65+, CD56±, CD45±, CD79a±, Bcl-2 weak±, c-Myc± (<40%), Cyclin D1±, EMA±, CD10±, CD30−/(+approximately 5%), Pax-5−, CD 20−, CD5−, CD3−, CD123−, Bcl-6−, EBV(ISH)−. The same light chain restriction and clonal Ig-rearrangement as in his CLL was confirmed, which proved a true clonal transformation. Our diagnostic workup and the clinical presentation was interpreted as PBL and not plasmablastic plasma cell myeloma.
Figure 1.
(A) H&E-stained section (× 60) from bone marrow showing large cells with plasmablastic differentiation with abundant cytoplasm, eccentrically placed nuclei and prominent nucleoli. (B) H&E-stained section (× 60) from lymph node showing the same large atypical lymphoid cells with plasmablastic features as seen in the bone marrow. (C) Immunohistochemical study showed the atypical lymphoid cells were positive for CD138. Example from bone marrow (× 40) shown here.
Based on biopsy results and our patient’s general condition a modified COP regimen (cyclophosphamide, vincristine and dexamethasone) was given. Rituximab was omitted due to lack of CD20 expression, and doxorubicin due to increased risk of toxicity. An induction phase (cycle 1) with COP regimen was given. Etoposide and daratumumab were both added from cycle 2. However, etoposide was omitted for the remaining three cycles due to liver toxicity. We considered venetoclax due to expression of B-cell lymphoma 2 (BCL-2) by the tumour cells. However, the choice fell down on daratumumab due to high expression of CD138 (figure 1C), and its rather favourable adverse event profile. Apart for etoposide, our patient withstood the treatment remarkably well, and he was discharged for outpatient follow-up. Treatment response was compelling in terms of clinical improvement and tumour regression (figure 2), and an allogeneic stem cell transplant (ASCT) was planned for as consolidation therapy.
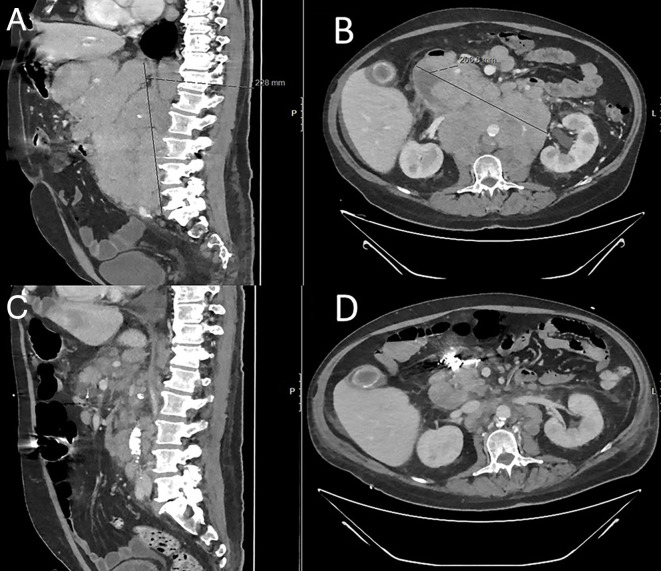
Figure 2.
(A, B) CT scan at admission disclosed multiple pathological lymph nodes above and under the diaphragm with a large intra-abdominal conglomerate residing close to the intestine, with central necrosis. (C, D) CT scan evaluation after two cycles with daratumumab+COEP (cyclophosphamide, vincristine, etoposide and dexamethasone) shows regression of thoracic and abdominal lymph nodes.
Outcome and follow-up
One month later our patient was readmitted due to dyspnoea, reduced general condition and acute renal failure. Control CT scan revealed progressive disease, and the patient developed septicaemia. He succumbed within a short time.
Discussion
RT is a transformation from B cell malignancies like CLL to more aggressive lymphomas like DLBCL or Hodgkin lymphoma, though transformation to other aggressive lymphomas such as PBL does occur.
It is difficult to discern PBL from plasmablastic plasma cell myeloma due to similar morphology and immunophenotype.11 14 This distinction may be important with regard to choice of chemotherapy regimens. Our patient had a low level monoclonal IgM λ gammopathy when presenting with the aggressive lymphoproliferative disease. A monoclonal gammopathy was not present when he was diagnosed with CLL. No osteolytic lesions were disclosed by a CT scan. Furthermore, acute renal failure and hypercalcaemia was related to tumour lysis syndrome which resolved quickly. Martinez et al presented a case series of plasmablastic transformation of low-grade B cell lymphomas.7 Three of the cases were transformation from CLL. In two of the cases the transformed tumour cells were EBV−, and all three cases had low level monoclonal gammopathy. The similarities with our case are striking.
Our patient with chemoimmune therapy-resistant disease received ibrutinib as third-line treatment, underwent RT to PBL which is a very aggressive lymphoma usually treated with CHOP-like regimens, though found difficult to treat.7 The only effective treatment is ASCT where 10%–20% can expect sustained remission if transplanted following good response to induction treatment.14 We have reported on an increased incidence of RT after 2003 during the chemoimmune therapy era both in treated and treatment-naive patients.6 However, we reported a rather low incidence of RT, compared with reports on RT in patients treated with signal pathway inhibitors.15
Our patient had PBL with strong CD138 and weak CD20 expression which suggested a case for chemoimmune therapy with daratumumab, not rituximab. The initial response was very good. Daratumumab has proven to be efficacious treating CD138-positive multiple myeloma.16
However, our patient had a very rapid relapse, and he did not make it to an ASCT. It is well known that remission on treatment with CHOP-like regimens in clonally related RT is often limited. ASCT may provide sustained remission in RT, if the aggressive PBL is responsive to chemoimmune therapy and the transplant is performed in remission.17 To achieve remission targeting multiple targets like CD138 and BCL-2 would be an interesting strategy in a high-grade malignancy like PBL.
Learning points.
Transformation of chronic lymphatic leukaemia (CLL) is becoming more frequent and usually transforms to diffuse large B cell lymphoma but can transform to aggressive lymphomas like plasmablastic lymphoma (PBL).
PBL is difficult to treat and has poor prognosis. Sustained remission is seen in 10%–20% of cases on allogeneic stem cell transplant (ASCT).
To warranty ASCT adequate remission is necessary. This can be achieved treating PBL with CHOP-like regimens including targeted therapy.
Acknowledgments
The authors thank Kristina Anderson of the Department of Haematology for her valuable input and Irene Langmyhr of the Department of Pathology at Oslo University Hospital for her contribution regarding pathology samples provided for this publication.
Footnotes
Contributors: KM, UMB, EBT and GET contributed with valuable inputs regarding original ideas, design and supervision of this article.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Next of kin consent obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Jemal A, Siegel R, Ward E, et al. . Cancer statistics, 2007. CA Cancer J Clin 2007;57:43–66. 10.3322/canjclin.57.1.43 [DOI] [PubMed] [Google Scholar]
- 2.Tjønnfjord GE, Ly BE, Johannesen TB, et al. . Chronic lymphocytic leukaemia in Norway--incidence and prognostic markers at diagnosis. Tidsskr Nor Laegeforen 2012;132:2056–9. 10.4045/tidsskr.11.1349 [DOI] [PubMed] [Google Scholar]
- 3.Hallek M, Fischer K, Fingerle-Rowson G, et al. . Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet 2010;376:1164–74. 10.1016/S0140-6736(10)61381-5 [DOI] [PubMed] [Google Scholar]
- 4.Byrd JC, Furman RR, Coutre SE, et al. . Targeting Btk with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med 2013;369:32–42. 10.1056/NEJMoa1215637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.NCCN Continuing Education NCCN Guidelines® Insights - Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma, Version 2, 2019. Available: https://education.nccn.org/node/84832 [Accessed 31 Jul 2020].
- 6.Lenartova A, Randen U, Johannesen TB, et al. . Richter syndrome epidemiology in a large population based chronic lymphocytic leukemia cohort from Norway. Cancer Epidemiol 2019;60:128–33. 10.1016/j.canep.2019.04.002 [DOI] [PubMed] [Google Scholar]
- 7.Martinez D, Valera A, Perez NS, et al. . Plasmablastic transformation of low-grade B-cell lymphomas: report on 6 cases. Am J Surg Pathol 2013;37:272–81. 10.1097/PAS.0b013e31826cb1d1 [DOI] [PubMed] [Google Scholar]
- 8.Robak T, Urbańska-Ryś H, Strzelecka B, et al. . Plasmablastic lymphoma in a patient with chronic lymphocytic leukemia heavily pretreated with cladribine (2-CdA): an unusual variant of Richter's syndrome. Eur J Haematol 2001;67:322–7. 10.1034/j.1600-0609.2001.00592.x [DOI] [PubMed] [Google Scholar]
- 9.Pan Z, Xie Q, Repertinger S, et al. . Plasmablastic transformation of low-grade CD5+ B-cell lymphoproliferative disorder with Myc gene rearrangements. Hum Pathol 2013;44:2139–48. 10.1016/j.humpath.2013.04.008 [DOI] [PubMed] [Google Scholar]
- 10.Delecluse HJ, Anagnostopoulos I, Dallenbach F, et al. . Plasmablastic lymphomas of the oral cavity: a new entity associated with the human immunodeficiency virus infection. Blood 1997;89:1413–20. 10.1182/blood.V89.4.1413 [DOI] [PubMed] [Google Scholar]
- 11.Swerdlow SH, Campo E, Pileri SA, et al. . The 2016 revision of the world Health organization classification of lymphoid neoplasms. Blood 2016;127:2375–90. 10.1182/blood-2016-01-643569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castillo JJ, Bibas M, Miranda RN. The biology and treatment of plasmablastic lymphoma. Blood 2015;125:2323–30. 10.1182/blood-2014-10-567479 [DOI] [PubMed] [Google Scholar]
- 13.Geisler CH, van T' Veer MB, Jurlander J, et al. . Frontline low-dose alemtuzumab with fludarabine and cyclophosphamide prolongs progression-free survival in high-risk CLL. Blood 2014;123:3255–62. 10.1182/blood-2014-01-547737 [DOI] [PubMed] [Google Scholar]
- 14.Wierda WG, Byrd JC, Abramson JS, et al. . NCCN guidelines insights: chronic lymphocytic Leukemia/Small lymphocytic lymphoma, version 2.2019. J Natl Compr Canc Netw 2019;17:12–20. 10.6004/jnccn.2019.0002 [DOI] [PubMed] [Google Scholar]
- 15.Maddocks KJ, Ruppert AS, Lozanski G, et al. . Etiology of ibrutinib therapy discontinuation and outcomes in patients with chronic lymphocytic leukemia. JAMA Oncol 2015;1:80–7. 10.1001/jamaoncol.2014.218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu T, Xing L, Lin L, et al. . An immune based, Anti-CD138 targeting antibody for the treatment of multiple myeloma. Blood 2018;132:5617 10.1182/blood-2018-99-119112 [DOI] [Google Scholar]
- 17.Allan JN, Furman RR. Current trends in the management of Richter's syndrome. Int J Hematol Oncol 2018;7:IJH09. 10.2217/ijh-2018-0010 [DOI] [PMC free article] [PubMed] [Google Scholar]