Abstract
Background
Data on the outcomes of patients with coronavirus disease 2019 (COVID-19) requiring Intensive Care Unit (ICU) care in Poland are limited. There are no data on critically ill patients with COVID-19 who did not meet criteria for ICU admission.
Material/Methods
We analyzed patients admitted to the ICU and those ineligible for ICU admission in a large COVID-19-dedicated hospital, during the first 3 months of the pandemic in Poland. Data from 67 patients considered for ICU admissions due to COVID-19 infection, treated between 10 March and 10 June 2020, were reviewed. Following exclusions, data on 32 patients admitted to the ICU and 21 patients ineligible for ICU admission were analyzed.
Results
In 38% of analyzed patients, symptoms of COVID-19 infection occurred during a hospital stay for an unrelated medical issue. The mean age of ICU patients was 62.4 (10.4) years, and the majority of patients were male (69%), with at least one comorbidity (88%). The mean admission APACHE II and SAPS II scores were 20.1 (8.1) points and 51.2 (15.3) points, respectively. The Charlson Comorbidity Index and Clinical Frailty Scale were lower in ICU patients compared with those disqualified: 5.9 (4.3) vs. 9.1 (3.5) points, P=0.01, and 4.7 (1.7) vs. 6.9 (1.2) points, P<0.01, respectively. All ICU patients required intubation and mechanical ventilation. ICU mortality was 67%. Hospital mortality among patients admitted to the ICU and those who were disqualified was 70% and 79%, respectively.
Conclusions
Patients with COVID-19 requiring ICU admission in our studied population were frail and had significant comorbidities. The outcomes in this group were poor and did not seem to be influenced by ICU admission.
MeSH Keywords: Comorbidity, COVID-19, Intensive Care Unit, Mortality
Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a novel strain of coronavirus that causes coronavirus disease 2019 (COVID-19) in humans [1]. On March 11, 2020, the World Health Organization (WHO) declared COVID-19 a pandemic [2]. Just over 2 months later, there were 5.5 million COVID-19 cases, and 350,000 deaths reported worldwide [3]. Based on the available data, 23% to 32% of hospitalized patients required Intensive Care Unit (ICU) admission, and the case fatality rate among patients in the ICU ranged from 26% to 78% [4–8]. The first available systematic review and meta-analysis of outcomes of patients admitted to ICU with COVID-19 was based on data from 24 studies with a total of 10,150 patients and demonstrated an ICU mortality rate of 41.6%. Moreover, meta-regression by the month of publication revealed a significant reduction in the reported mortality rates over time [9].
The first positive case of COVID-19 in Poland was diagnosed on March 4, 2020. The Polish Ministry of Health announced a state of epidemiological threat, which was followed by a state of COVID-19 epidemic on March 20, 2020. As a response to the pandemic threat, selected multidisciplinary hospitals were converted into designated infectious disease centers [7]. Likely due to the prompt adoption of social distancing and complete lockdown throughout the country (described in detail by Pinkas et al. [10]), the spread of the disease was not as rapid as in Western Europe and the United States [3]. On April 12, 2020, 6,674 cases and 232 deaths were confirmed in Poland [7], and by May 31, 2020, those values had increased to 23,786 cases and 1,064 deaths [11]. At the same time, Italy was facing one of the worst outbreaks in the world, with more than 100,000 cases, leading to more than 12,000 deaths. Moreover, the standard operational capacity of ICU beds was exceeded by 40% [12].
It has been confirmed that a significant proportion of patients with severe COVID-19 infection require ICU admission and their mortality is alarmingly high. However, information describing the demographics, clinical courses, and outcomes of these patients is limited.
The primary objective of this study was to analyze patients admitted to the ICU in a multidisciplinary hospital located in the Silesian region of Poland. This hospital was transformed into a COVID-19-dedicated infectious hospital in the first 3 months of the COVID-19 pandemic. The secondary objective was to analyze the patients who were referred but ultimately ineligible for ICU admission during the same time period because their ICU treatment was regarded as medically or ethically inappropriate.
Material and Methods
We conducted a retrospective observational cohort study in critically ill adult patients with confirmed COVID-19 infection referred for admission to the Department of Anesthesiology and Intensive Therapy (i.e., ICU) in Provincial Specialist Hospital in Tychy (PSHT), in the Silesian District of Poland. Following the decision of the Silesian authorities, the ICU of PSHT was transformed into a COVID-19-dedicated unit on March 12, 2020. The whole hospital was subsequently transformed into a COVID-dedicated unit on March 27, 2020. Our analysis covered 3 consecutive months (from 10 March until 10 June 2020).
Laboratory-confirmed SARS-Cov-2 infection was defined as a positive result of real-time reverse transcriptase-polymerase chain reaction (RT-PCR) assay from nasal and pharyngeal swabs or lower respiratory tract aspirates. Demographic, clinical, and laboratory data, as well as details on patients’ treatment and outcomes, were retrospectively analyzed. PSHT management approved the use of patients’ data for scientific purposes. Due to the retrospective and anonymous nature of the study, the Ethical Committee of the Medical University of Silesia in Katowice waived the need for consent of the patients to participate in the study.
Two cohorts of patients were analyzed in the same period: (i) patients referred and admitted to the ICU, and (ii) patients referred but ultimately ineligible for ICU admission because their ICU treatment was considered medically and ethically inappropriate by the ICU team.
Each patient referred for the potential ICU admission was assessed by the ICU physician on call. The number of details included in such consultation varied, but the conclusion at the end was always clear. Patients were either considered “too ill for ICU care,” “too good for ICU care,” or “eligible for ICU admission.”
Variables analyzed for all patients included: patient flow, demographic data, and general condition of each patient at the time of admission to the ICU or ineligibility for admission, as well as the outcomes. Comorbidities were assessed separately and in a combined form as the Charlson Comorbidity Index [13]. Clinical Frailty Scale was retrospectively assessed in all patients based on data available in medical records [14]. Patients were categorized as frail or nonfrail when they received ≥5 points or <5 points, respectively, on a scale of 1 to 9, as described by Fronczek et al. [15].
Variables assessed in patients admitted to the ICU also included admission APACHE II and SAPS II score, ICU treatment details, and selected laboratory data at admission and during the first 3 days of ICU stay.
Data were compared between patients who were admitted or not admitted to the ICU. Among patients admitted to the ICU, data on survivors and non-survivors were also compared.
Analyses were performed with the use of Dell Statistica, version 13. Demographic data were presented using descriptive statistics methods and compared using Mann-Whitney tests. For comparison of qualitative variables, Fischer exact test was used. For all calculations, statistical significance was accepted at a significance level of P<0.05.
Results
Between March 10 and June 10, 2020, 67 adults with suspected or confirmed COVID-19 infection were referred for ICU admission in PSHT. In this group, 32 patients (48%) were admitted to the ICU, while 35 patients (52%) were not eligible for ICU treatment and remained in the other departments of PSHT. Figure 1 shows the number of ICU beds occupied by COVID-19 patients each day.
Figure 1.
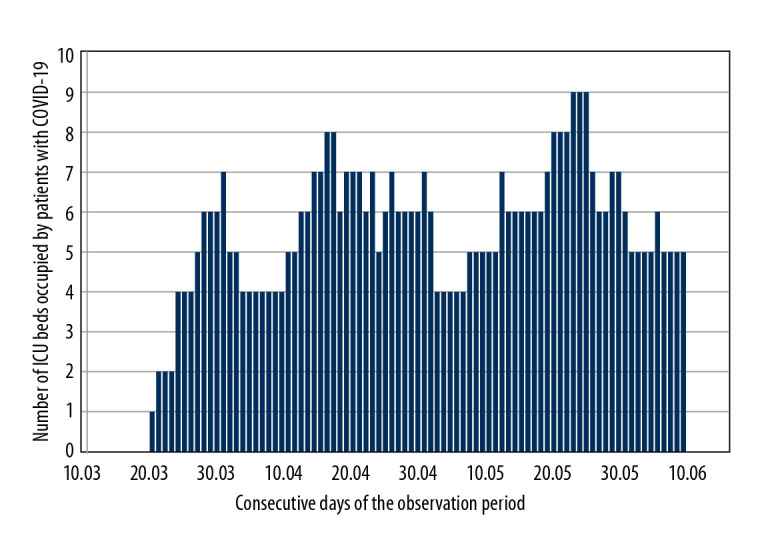
Number of Intensive Care Unit beds occupied by patients with coronavirus disease 2019 (COVID-19) infection in the consecutive days of the observation period.
All 32 patients admitted to the ICU had confirmed COVID-19. Among the 35 patients not eligible for ICU admission, 21 with confirmed COVID-19 were considered “too ill” for ICU care, 4 with confirmed COVID-19 were considered “too good” for ICU care, and 10 were further confirmed COVID-19 negative and were considered “too ill” for ICU care.
Patients with COVID-19 whose condition was too good for ICU care and patients COVID-10 negative who were too ill for ICU care were excluded from the study.
Therefore, 53 patients with confirmed COVID-19 were finally analyzed and compared: (i) patients admitted to the ICU (n=32) and (ii) patients not eligible for admission because they were too ill for ICU care (n=21).
Before COVID-19 infection, 31 of the 32 patients admitted to the ICU (97%) were functioning independently at home, and 1 patient was a nursing home resident (3%). Overall, 24 patients were admitted to the ICU from other locations in PSHT: the Emergency Department (n=2), surgical ward (n=1), and medical wards (n=21). The remaining 8 patients (25%) were admitted to the ICU directly from other hospitals, either from another ICU (n=5), the medical ward (n=1), or the Emergency Department (n=2). Among the 21 patients not eligible for ICU treatment, only 12 patients were functioning independently at home, and 9 patients (43%) were either nursing home residents (n=7) or were receiving palliative therapy at home (n=2).
Among the 32 patients hospitalized in the ICU, symptoms of COVID-19 infection occurred at home in 16 patients. In contrast, in the remaining 8 patients (25%), symptoms appeared during hospitalization in other hospitals; therefore, these patients were immediately transported to PSHT for further treatment when they had tests confirmed as positive for COVID-19. In the group of 21 patients not qualified for ICU treatment, symptoms of COVID-19 infection occurred at home (or in a nursing home) in 9 patients, while in the remaining 12 patients (57%), symptoms appeared during hospitalization for other reasons (with the same immediate transfer to PSHT).
Among the 32 patients admitted to the ICU, the mean time of hospitalization in PSHT before ICU admission was 2.1 (2.8) days (from 0 to 9 days), with the mean total time of hospitalization being 4.0 (3.8) days (from 0 to 17 days). Among the 21 patients not eligible for ICU treatment, the corresponding time of hospitalization in PSHT (before ICU disqualification) was 4.0 (4.7) days (from 0 to 15 days). The mean total time of hospitalization was not analyzed in this group. Seven patients (33%) were reffered to the ICU but were considered ineligible for ICU treatment immediately after admission to PSHT. Among patients in this group who died, the mean time between the refusal of ICU treatment and the patient’s death was 3.2 (4.0) days (from 0 to 15 days). However, 2 patients died immediately after being considered ineligible for ICU treatment.
Among the 32 patients admitted to the ICU, 22 were men (69%). The mean age was 62.4 (10.4) years (from 36 to 82 years), and the mean body mass index was 29.5 (7.3) kg/m2 (from 22.5 to 55.4 kg/m2). The mean admission APACHE II score was 20.1 (8.1) points, and the mean SAPS II score was 51.2 (15.3) points. Among the 21 patients ineligible for ICU treatment, 13 were men (62%). The mean age was 72.2 (12.3) years (from 49 to 90 years). The admission ICU scores could not be assessed. All 53 patients (both admitted and disqualified) showed clinical features of COVID-19 infection upon admission or when they were considered ineligible for ICU admission. However, in some cases, the PCR test result was not yet available.
A comparison of demographic parameters and comorbidities in patients eligible and ineligible for ICU treatment are presented in Table 1.
Table 1.
Demographic data and medical status on Intensive Care Unit admission.
| Variables | Admitted (n=32) | Not admitted D (n=21) | p | |
|---|---|---|---|---|
| Age (years) | 62.4 (10.4) | 72.2 (12.3) | <0.01 | |
| Male gender | 22 (69%) | 13 (62%) | 0.41 | |
| Comorbidities | Coronary artery disease | 12 (38%) | 13 (62%) | 0.10 |
| Past myocardial infarction | 8 (25%) | 7 (33%) | 0.55 | |
| Heart failure | 14 (44%) | 13 (62%) | 0.26 | |
| Peripheral vascular disease | 14 (44%) | 6 (29%) | 0.39 | |
| Arterial hypertension | 20 (63%) | 16 (76%) | 0.37 | |
| COPD | 3 (9%) | 3 (14%) | 0.67 | |
| Diabetes | 17 (53%) | 10 (48%) | 0.78 | |
| Chronic renal failure | 6 (19%) | 3 (14%) | 1.00 | |
| Previous cerebral stroke | 4 (13%) | 10 (48%) | 0.01 | |
| Chronic neurological disorders | 6 (19%) | 11 (52%) | 0.02 | |
| Dementia | 3 (9%) | 9 (43%) | 0.01 | |
| Systemic autoimmune diseases | 5 (16%) | 1 (5%) | 0.38 | |
| Cancer | 4 (13%) | 4 (19%) | 0.70 | |
| None | 4 (13%) | 0 (0%) | 0.14 | |
| Charlson Comorbidity Index | 5.9 (4.3) | 9.1 (3.5) | 0.01 | |
| Clinical Frailty Scale (points) | 4.7 (1.7) | 6.9 (1.2) | <0.01 | |
| Clinical Frailty Scale ≥5 points | 17 (53%) | 20 (95%) | <0.01 |
Numerical data are presented as mean (SD). COPD – chronic obstructive pulmonary disease
For 31 of the 32 patients admitted to the ICU, the main reason for admission was respiratory failure. In these 31 patients, respiratory failure was accompanied by circulatory failure or shock (n=19, 59%), renal failure (n=5, 16%), impaired consciousness (n=11, 34%), or severe metabolic abnormalities (n=10, 31%). Shock was the main reason for ICU admission in 1 patient.
Eight patients (25%) were already intubated upon admission to the ICU, with a mean prior intubation time of 35.1 (40.1) h, while the remaining 24 patients required intubation and initiation of mechanical ventilation within 1 h of ICU admission. Hemodynamic status, respiratory status, selected biochemical parameters in the initial period of ICU treatment, and comparisons of these data in survivors and non-survivors of ICU stays are presented in Table 2. The corresponding laboratory results are presented in Table 3.
Table 2.
Hemodynamic and respiratory data on Intensive Care Unit (ICU) admission and during ICU treatment.
| Variables | All patients (n=32) | Survivors (n=9) | Non-survivors (n=18) | p | |
|---|---|---|---|---|---|
| ICU admission | Heart rate (beats/min.) | 93 (24) | 99 (29) | 92 (25) | 0.60 |
| Systolic blood pressure (mmHg) | 117 (26) | 116 (34) | 116 (26) | 0.96 | |
| Oxygen saturation (%) | 67 (20) | 72 (16) | 63 (22) | 0.33 | |
| paO2 (mmHg) | 78 (37) | 80 (29) | 77 (44) | 0.64 | |
| FiO2 (1) | 0.77 (0.20) | 0.79 (0.21) | 0.77 (0.2) | 0.69 | |
| paO2/FiO2 ratio (1) | 106 (52) | 103 (42) | 105 (59) | 0.96 | |
| paCO2 (mmHg) | 47 (26) | 43 (10) | 52 (34) | 0.49 | |
| HCO3 (mmol/L) | 23 (4.1) | 23 (3.1) | 22 (4.7) | 0.98 | |
| pH (1) | 7.33 (0.13) | 7.36 (0.10) | 7.30 (0.14) | 0.35 | |
| Lactate (mmol/L) | 2.7 (2.8) | 1.9 (0.6) | 3.4 (3.5) | 0.21 | |
| Day 1 | paO2 (mmHg) | 68 (27) | 80 (19) | 63 (32) | 0.06 |
| FiO2 (1) | 0.78 (0.17) | 0.66 (0.2) | 0.82 (0.12) | 0.05 | |
| paO2/FiO2 ratio (1) | 92 (51) | 129 (55) | 75 (47) | 0.02 | |
| Lactate (mmol/L) | 3.0 (3,3) | 1.9 (0.6) | 4.0 (4.2) | 0.08 | |
| PEEP (cmH2O) | 13.5 (17.3) | 9.9 (2.0) | 10.7 (1.9) | 0.37 | |
| Body temperature (°C) | 36.5 (1.6) | 36.5 (1.3) | 36.6 (1.9) | 0.50 | |
| Urine output (mls/24 hours) | 1839 (1133) | 1656 (891) | 1708 (827) | 0.76 | |
| Day 2 | paO2 (mmHg) | 75 (23) | 90 (25) | 67 (17) | 0.03 |
| FiO2 (1) | 0.72 (0.18) | 0.67 (0.18) | 0.75 (0.19) | 0.28 | |
| paO2/FiO2 ratio (1) | 117 (64) | 151 (76) | 93 (38) | 0.07 | |
| Lactate (mmol/L) | 2.5 (1.5) | 2.3 (1.1) | 2.8 (1.9) | 0.61 | |
| Day 3 | paO2 (mmHg) | 83 (35) | 92 (17) | 77 (41) | 0.02 |
| FiO2 (1) | 0.67 (0.16) | 0.63 (0.2) | 0.70 (0.14) | 0.34 | |
| paO2/FiO2 ratio (1) | 134 (76) | 160 (74) | 107 (56) | 0.05 | |
| Lactate (mmol/L) | 2.2 (0.88) | 2.1 (0.9) | 2.3 (0.87) | 0.55 |
FiO2 – fraction of inspired oxygen; HCO3 – bicarbonate; PaO2 – arterial partial pressure of oxygen, PEEP – positive end-expiratory pressure. Only the worst or the worst recorded values of paO2, lactate, PEEP and body temperature are presented for day 1, 2 and 3. Sample size might be smaller due to non-survivors or missing data in day 1, 2 and 3. Numerical data are presented as mean (SD).
Table 3.
First recorded laboratory results in all patients and in survivors or non-survivors of the Intensive Care Unit stay.
| Variables | All patients (n=32) | Survivors (n=9) | Non-survivors (n=18) | p | |
|---|---|---|---|---|---|
| Full blood count | WBC (103/μl) | 12.2 (4.9) | 10.9 (5.1) | 12.5 (3.8) | 0.40 |
| Haemoglobin (g/dl) | 12.3 (1.7) | 11.8 (2.0) | 12.5 (1.7) | 0.46 | |
| Haematocrit (%) | 37.2 (5.4) | 35.1 (5.5) | 38.3 (5.6) | 0.22 | |
| Lymphocytes (%) | 8.2 (6.6) | 8.9 (7.6) | 7.9 (6.4) | 0.98 | |
| Neutrophils (%) | 84.7 (15.3) | 77.9 (25.9) | 87.4 (7.8) | 0.62 | |
| Other | Sodium (mmol/L) | 139 (4.6) | 137 (3.5) | 139 (3.9) | 0.13 |
| Potassium (mmol/L) | 4.1 (0.7) | 3.8 (0.6) | 4.4 (0.6) | 0.03 | |
| Urea (mmol/L) | 8.9 (4.2) | 7.0 (2.1) | 9.9 (4.3) | 0.04 | |
| Creatinine (mmol/L) | 101 (44) | 88 (19) | 107 (52) | 0.72 | |
| CRP (mg/l) | 157 (91) | 170 (82) | 156 (99) | 0.52 | |
| PCT (ng/ml) | 1.1 (2.2) | 0.36 (0.21) | 1.53 (2.85) | 0.27 | |
| Bilirubin (mg/dl) | 9.4 (6.3) | 8.0 (3.5) | 11.0 (7.7) | 0.43 | |
| INR (1) | 1.3 (0.3) | 1.3 (0.11) | 1.4 (0.4) | 0.91 | |
| APTT (s) | 37 (10) | 36 (4.6) | 38 (13) | 0.36 | |
| D-dimer (μg/l) | 4804 (7964) | 1231 (843) | 7265 (10027) | 0.08 |
Numerical data are presented as mean (SD). APTT – activated partial thromboplastin time; CRP-C – reactive protein; INR – international normalized ratio; PCT – procalcitonin; WBC – white blood count.
Among the 32 patients admitted to the ICU, 18 patients died during the ICU stay, 9 were discharged from the ICU, and 5 were still in the ICU on the last day of the observation period. COVID-19 infection was confirmed in all patients during hospitalization. Thus, the ICU case fatality rate in patients with confirmed COVID-19 infection and a completed ICU stay was 67%.
During ICU stay, invasive ventilation was used in all patients (100%), while 31 patients required infusion of catecholamines (97%), 15 patients (47%) underwent a tracheostomy, and 6 patients (19%) required renal replacement therapy. One patient (aged 47, with no comorbidities and isolated severe hypoxemic respiratory failure) was transferred to the nearest extracorporeal membrane oxygenation (ECMO) referral center for COVID-19 patients in Cracow (90 km from PSHT). In this case, venovenous ECMO was instituted in PSHT by the ECMO retrieval team.
In 27 patients who completed their ICU stay during the observation period, the mean ICU stay was 12.7 (9.7) days (range, 1 h to 41.4 days). Of the 9 patients discharged from the ICU, 6 were transferred directly to various departments of other hospitals and 3 were discharged to various departments of PSHT. Among the patients discharged to other PSHT departments, 2 patients were discharged home (after another 14 and 6 days of treatment in PSHT), and 1 patient died after 5 days of treatment in the medical ward of PSHT. Thus, the PSHT case fatality rate among ICU patients with confirmed COVID-19 infection and a completed ICU stay was 70%.
Antiviral treatment was administered according to the directions of the attending specialist in infectious diseases. Patients were given antiviral therapy in various combinations and drug regimens, with each patient receiving 1 to 4 drugs. Treatment with 1 drug was used in 2 patients. Two drugs were used simultaneously in the treatment of 14 patients; 3 or 4 drugs were used simultaneously in 16 patients. Patients were given chloroquine (n=32; 100%), lopinavir/ritonavir (n=20; 63%), ribavirin (n=13; 41%), oseltamivir (n=5; 16%), tocilizumab (n=2; 6%), and convalescent plasma (n=7; 22%).
Among the 21 patients ineligible for ICU treatment, 15 patients died in various PSHT departments, 4 patients were discharged from PSHT to other hospitals in stable condition, and 2 patients were still hospitalized in the medical departments of PSHT on the last day of the observation period. Thus, the hospital case fatality rate in patients not admitted to the ICU with confirmed COVID-19 infection and completed hospital stay was 79%.
Discussion
Patients with COVID-19 requiring ICU admission in the studied population were elderly, frail, and had significant comorbidities. ICU mortality in this group of patients was excessively high, and routine admission ICU scoring systems could therefore underestimate the risk of death. The outcomes in this group were poor and did not seem to be influenced by ICU admission.
In our cross-sectional study, we analyzed the first 3 months of the ICU activity in a large multidisciplinary hospital located in the Silesian region of Poland, which was transformed into a COVID-19-dedicated hospital (i.e., only patients with suspected or confirmed COVID-19 infection were admitted). The Silesian region of Poland is an industrial area with a high population density. The region includes only 3.9% of the territory of Poland, but it holds 11.9% of the country’s population [16].
Importantly, due to its geographical location, PSHT was the busiest COVID-19 multidisciplinary hospital operating in the Silesian region. Local authorities dedicated it entirely to admitting and providing services for COVID-19 patients, in accordance with regulations issued by the Polish government at the beginning of the pandemic. Therefore, in our study, we likely managed to analyze at least half of the patients requiring ICU admissions due to COVID-19 infection in the Silesian region of Poland in the first period of the pandemic.
According to the data on the website of the Republic of Poland [11], the course of the pandemic in Poland generally did not resemble the dynamics previously observed in China, Italy, Spain, France, the United Kingdom, or the United States [5,6,8,17,18]. The number of new ICU cases did not increase rapidly, likely due to the early introduction of extremely restrictive rules for social distancing in Poland. Our findings support this observation. Figure 1 shows a relatively constant number of ICU beds occupied by patients with COVID-19 infection during the first 3 months of the pandemic.
These facts do not provide a sense of security because the outcomes observed in our group of ICU patients were generally poor. Therefore, it is necessary to compare our data to the results obtained in other countries and geographical regions. The results of our study are undoubtedly influenced by the profound differences in patient populations and indications for ICU admission in Poland, relative to other countries [19]. The interpretation of demographic indices and ICU outcomes across countries must be performed with extreme caution [20].
In Poland, the strategy of COVID-19-dedicated hospitals was introduced by the Polish Ministry of Health at the very beginning of the pandemic. A similar approach was also used in China [17]. More than one-third of patients in our analyzed cohort were not admitted to the hospital due to COVID-19 infection but were first hospitalized for an unrelated medical issue. We cannot exclude that the situation in the Silesian region of Poland was unique. It is possible that some of these patients were already hospitalized before the introduction of COVID-dedicated hospitals, while others were probably not screened with nasopharyngeal swabs at hospital admission.
The mean age of the 32 patients admitted to the ICU with COVID-19 infection was 62.4 years and was therefore similar to the ICU population admitted with COVID-19 infection in Northern Italy [5,21,22]. The majority of patients were men (69%). This finding is consistent with almost all recently reported studies [5,6,8,17], in which the comparative percentages were 82%, 58%, 60%, and 73%, respectively. The WHO has reported that 63% of deaths related to COVID-19 in Europe occurred among men [23]. The reason for this is still unclear.
The majority of patients in our cohort had multiple comorbidities before their admission to the ICU. Patients with these comorbidities were previously reported to be at higher risk for severe disease or death from COVID-19. Recent studies from China, the United States, Italy, and the United Kingdom revealed that patients who required ICU care were more likely to have chronic coexisting diseases in comparison with patients who did not require ICU admission [5,6,17,24]. However, these comorbidities may be differently distributed among individual patients. Therefore, we aimed to assess our cohort with the use of the Charlson Comorbidity Index and the Clinical Frailty Scale. These tools were selected because the Charlson Comorbidity Index is currently the most extensively validated single measure of multiple chronic illnesses [13,25]. Similarly, the Clinical Frailty Scale is recognized as a simple tool used to categorize patients as frail or nonfrail [15]. This issue was important, because we were aiming for a single value to determine the degree of multiple comorbidities and frailty.
The mean Charlson Comorbidity Index was 5.9 (4.3) points for the admitted group, and 9.1 (3.5) points for patients who were not admitted (P=0.01) [13,25]. To date, there has only been one report evaluating the Charlson Comorbidity Index in COVID-19 patients, in which the median score was 4 points among 5,700 patients in the New York City area. However, the study was not restricted to ICU patients [8]. In this situation, it is unsurprising that the Clinical Frailty Scale was higher in the group ineligible for ICU admission (6.9 [124] vs. 4.7 [1.7] points, P<0.01). Ineligible patients were older, had more comorbidities, and were more frequently categorized as frail (95% vs. 53%, P<0.01) compared with patients admitted to the ICU. Interestingly, among patients not eligible for ICU treatment, as many as 43% were residing in long-term facilities or nursing homes (or undergoing palliative therapy at home), while there was only 1 (3%) such patient among those admitted to the ICU.
According to the most recent Intensive Care National Audit and Research Centre (ICNARC) report, 13% of critically ill COVID-19 patients requiring ICU admission in the United Kingdom were dependent upon or staying in long-term facilities [18]. The corresponding percentage in the United States was 25% [26]. The number and percentage of such patients among those considered too ill for the ICU was not reported [18]. Generally, information on patients ineligible for ICU care during the COVID-19 pandemic and their outcomes is lacking. This is difficult to explain because the problem of limited ICU resources has been explored in detail and frequently discussed, and guidelines centered on this issue have already been published [27,28].
The mean length of ICU stay for our patients was similar to other studies in which the mean times were 9 and 12 days, respectively [18,29]. ICU mortality was 67%. ICU mortality varied enormously across different studies, from 16% [6] to 44% [18], 62% [30], or 76% [8]. These differences may be associated with methodological issues. In a large Italian study by Grasselli et al. [5], ICU mortality was only 26%, but 58% of patients were still in the ICU when the study analysis was completed. In a first study performed in a COVID-19-dedicated hospital in Warsaw, Poland, ICU mortality was 59% [7].
The reasons for these differences remain unclear, and we expect that they are complex. This issue was first analyzed in a systematic review with a meta-analysis of all observational studies, concentrating on ICU patients with COVID-19 infection [9]. In the 24 studies that were selected, 10.150 patients were recruited between 16 December 2019 and 28 May 2020. Reported case fatality rates were even more diverse, ranging from 0% (in small case series) to 84.6%. The authors emphasized that COVID-19 treatment has been very challenging, with a large number of patients requiring advanced respiratory support. Many patients were treated with the use of high-flow nasal oxygen and noninvasive mechanical ventilation outside the ICU area, and patients admitted to ICU could have been disproportionately sicker [9]. Therefore, mortality could be primarily linked to the stage of the disease at which the patient was admitted to the ICU.
The similar mortality among patients admitted and not admitted to the ICU was surprising. We also could not find any comparative data in the medical literature. The only factor differentiating the groups was the use of invasive mechanical ventilation. All patients admitted to the ICU were either already invasively ventilated, or invasive ventilation was administered shortly after admission, while patients not admitted to the ICU usually remained on high-flow oxygen therapy.
The mean admission APACHE II score in our cohort of patients admitted to the ICU was 20 points, with a corresponding ICU mortality of 67%. A similar mean admission APACHE score (19 points) was observed in a South Korean study, but the corresponding ICU mortality was only 30% [31]. This striking difference may have been because comorbidities generally do not have a strong influence on the results of the APACHE II score, and the Korean population consisted mainly of middle-aged women, which is exceptionally unusual for COVID-19 patients [31]. A previous Chinese study reported a mean APACHE II score of 17 and ICU mortality of 62% [30]. The most recent ICNARC database (from 20 May) shows a mean APACHE II score of 14 points, with a corresponding mortality of 51%. This is important because a relatively low APACHE II score does not explain such a high case fatality rate. This is also a sign that in COVID-associated ICU admissions, ICU mortality may be significantly underestimated (and the same may also be true for other available admission ICU scores).
Almost all analyzed patients developed hypoxemic respiratory failure and were already mechanically ventilated at ICU admission or were intubated in the first hour following ICU admission. Recent data from the United Kingdom, Hong Kong, the United States, South Korea, Italy, and Spain also indicate a high need (from 72% to 98%) for early invasive ventilation in this group [5,18,26,29,31,32]. Interestingly, in our study, patients did not receive noninvasive ventilation before ICU admission or intubation, while such a sequence was relatively common in China and Italy [6,17,21]. This finding indicates that patients analyzed in our study were transferred to the ICU relatively late, and an early intubation strategy, considered as a life-saving intervention by Barrasa et al. [32], was performed. The high percentage of mechanically ventilated patients may be explained both by the severity of the disease and by the different organization of the health care system in our hospital, where invasive techniques are usually only applied in the ICU. Almost all ventilated patients fulfilled the Berlin criteria of mild to severe acute respiratory distress syndrome [33], with the PaO2/FiO2 ratio being the lowest during the first 24 h following ICU admission. Only 1 patient needed ECMO support, which was unsurprising because, in this particular disease, relatively rare ECMO usage has previously been reported [5,32].
The antiviral treatment administered to our patients did not follow a particular pattern. This confusing picture may stem from the fact that during the enrollment period for our study (March 10 to June 10, 2020), no specific antiviral treatment for COVID-19 had been proven to be safe and effective [34]. It was not until the end of the period (on May 22, 2020) that Beigel et al. [35] published a large prospective randomized trial and proved that Remdesivir was superior to placebo in shortening the time to recovery in adults hospitalized with COVID-19 with evidence of lower respiratory tract infection. Therefore, patient management in our study focused on supportive care, adequate oxygenation, sophisticated ventilation, and careful fluid management [36]. During our enrollment period, various combinations of antiviral drugs had been encouraged in the medical literature, with Remdesivir, tocilizumab, and convalescent plasma appearing to be the most promising agents [30–32,37]. Additionally, treatment protocols were variable, often temporary, and hard to compare between countries. A study published in May 2020 demonstrated that patients taking hydroxychloroquine alone or in combination with macrolide were at higher risk of death and ventricular arrhythmias in comparison with other patients [38]. Based on the available evidence, on July 4, 2020, WHO accepted the recommendation from the Solidarity Trial’s International Steering Committee to immediately discontinue the trial’s hydroxychloroquine and lopinavir/ritonavir arms [39].
Our study had some limitations. First, it was a retrospective, observational study, and the sample size was relatively small. ICU admission prognostic scores (APACHE II and SAPS II) were not available for patients ineligible for ICU admission. We did not have the exact information on the disease duration from the onset of symptoms. Information on the ethical and medical reasons behind the decision on ICU admission widely varied, so they could not be systematically detailed. Similar outcomes observed among patients admitted and not admitted to the ICU should not be generalized to the whole population based on a single-center, retrospective study. Also, patients referred for ICU admission were analyzed without a broader context because they were not compared with the remaining patients with COVID-19 hospitalized in PSHT at the same time. These significant deficiencies are balanced, however, by the timeliness and importance of the collected data. It needs to be mentioned that our study was prone to bias. The information was based on data obtained from the Department of Anesthesiology and Intensive Therapy; however, some patients may not have been referred for ICU admission and not assessed by the ICU team. Therefore, the total number of patients ineligible for ICU admission may have been higher than presented in the study. Additionally, decisions regarding ICU admission were taken when our knowledge of COVID-19 infection was less advanced than it is currently. These decisions were also made by different physicians in very different circumstances. Therefore, it cannot be excluded that for some patients, different physicians could have made different decisions on ICU admission or ineligibility for ICU admission.
Conclusions
Patients with COVID-19 requiring ICU admission in our studied population were frail and had significant comorbidities. The outcomes in this group were generally poor and did not seem to be influenced by ICU admission.
Acknowledgments
The authors wish to thank Jolanta Cieśla for editorial help in preparing the manuscript and Patryk Korecki for statistical support.
Footnotes
Conflict of interest
None.
Source of support: Departmental sources
References
- 1.Gujski M, Raciborski F, Jankowski M, et al. Epidemiological analysis of the first 1389 cases of COVID-19 in Poland: A preliminary report. Med Sci Monit. 2020;26:e924702. doi: 10.12659/MSM.924702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization Regional Office for Europe. WHO announces COVID-19 outbreak a pandemic. 2019. http://www.euro.who.int/en/health-topics/health-emergencies/coronavirus-covid-19/news/news/2020/3/who-announces-covid-19-outbreak-a-pandemic.
- 3.World Health Organization. Coronavirus disease (Covid-19). Situation Report – 128. 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200527-covid-19-sitrep-128.pdf?sfvrsn=11720c0a_2.
- 4.Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington state. JAMA. 2020;323:1612–14. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323:1574–81. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nowak B, Szymański P, Pańkowski I, et al. Clinical characteristics and short-term outcomes of coronavirus disease 2019: Retrospective, single-center experience of designated hospital in Poland. Pol Arch Intern Med. 2020;130:407–11. doi: 10.20452/pamw.15361. [DOI] [PubMed] [Google Scholar]
- 8.Richardson S, Hirsch JC, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–59. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Armstrong RA, Kane AD, Cook TM. Outcomes from intensive care in patients with COVID-19: A systematic review and meta-analysis of observational studies. Anaesthesia. 2020 doi: 10.1111/anae.15201. [Online ahead of print] [DOI] [PubMed] [Google Scholar]
- 10.Pinkas J, Jankowski M, Szumowski Ł, et al. Public health interventions to mitigate early spread of SARS-CoV-2 in Poland. Med Sci Monit. 2020;26:e924730. doi: 10.12659/MSM.924730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.2020. https://www.gov.pl/web/koronawirus/wykaz-zarazen-koronawirusem-sars-cov-2 [in Polish]
- 12.Immovilli P, Morelli N, Antonucci E, et al. COVID-19 mortality and ICU admission: The Italian experience. Crit Care. 2020;24:228. doi: 10.1186/s13054-020-02957-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 14.Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–95. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fronczek J, Polok KJ, Nowak-Kózka I, et al. Frailty is associated with an increased mortality among patients ≥80 years old treated in polish ICUs. Anaesthesiol Intensive Ther. 2018;50:245–51. doi: 10.5603/AIT.a2018.0032. [DOI] [PubMed] [Google Scholar]
- 16.Knapik P, Trejnowska E, Knapik M, et al. Young adults among patients admitted to Polish intensive care units in the Silesian ICU registry. Med Sci Monit. 2019;25:5727–37. doi: 10.12659/MSM.913852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guan W, Ni Z, Hu Yu, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–20. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.ICNARC report on COVID-19 in critical care. 2020. https://www.icnarc.org/DataServices/Attachments/Download/593555ce-709c-ea11-9126-00505601089b.
- 19.Weigl W, Adamski J, Goryński P, et al. Mortality rate is higher in Polish intensive care units than in other European countries. Intensive Care Med. 2017;43:1430–32. doi: 10.1007/s00134-017-4804-2. [DOI] [PubMed] [Google Scholar]
- 20.Knapik P, Krzych Ł, Weigl W, et al. Mortality rate in Polish intensive care units is lower than predicted according to the APACHE II scoring system. Intensive Care Med. 2017;43:1745–46. doi: 10.1007/s00134-017-4883-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piva S, Filippini M, Turla F, et al. Clinical presentation and initial management critically ill patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in Brescia, Italy. J Crit Care. 2020;58:29–33. doi: 10.1016/j.jcrc.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Remuzzi A, Remuzzi G. COVID-19 and Italy: What next? Lancet. 2020;395:1225–28. doi: 10.1016/S0140-6736(20)30627-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization. COVID-19 weekly surveillance report. 2020. https://www.euro.who.int/en/health-topics/health-emergencies/coronavirus-covid-19/weekly-surveillance-report.
- 24.Docherty AB, Harrison EM, Green CA, et al. Features of 20 133 UK patients in hospital with COVID-19 using the ISARIC WHO clinical characterisation protocol: Prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173:676–82. doi: 10.1093/aje/kwq433. [DOI] [PubMed] [Google Scholar]
- 26.Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in critically ill patients in the Seattle region – case series. N Engl J Med. 2020;382:2012–22. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joebges S, Biller-Andorno N. Ethics guidelines on COVID-19 triage – an emerging international consensus. Crit Care. 2020;24:201. doi: 10.1186/s13054-020-02927-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Emanuel EJ, Persad G, Upshur R, et al. Fair allocation of scarce medical resources in the time of Covid-19. N Engl J Med. 2020;382:2049–55. doi: 10.1056/NEJMsb2005114. [DOI] [PubMed] [Google Scholar]
- 29.Ling L, So C, Shum HP, et al. Critically ill patients with COVID-19 in Hong Kong: A multicentre retrospective observational cohort study. Crit Care Resusc. 2020;22(2):119–25. doi: 10.51893/2020.2.oa1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–81. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hong KS, Lee KH, Chung JH, et al. Clinical features and outcomes of 98 patients hospitalized with SARS-CoV-2 infection in Daegu, South Korea: A brief descriptive study. Yonsei Med J. 2020;61:431–37. doi: 10.3349/ymj.2020.61.5.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barrasa H, Rello J, Tejada S, et al. SARS-CoV-2 in Spanish intensive care units: Early experience with 15-day survival in Vitoria. Anaesth Crit Care Pain Med. 2020 doi: 10.1016/j.accpm.2020.04.001. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: The Berlin definition. JAMA. 2012;307:2526–33. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 34.Zhai P, Ding Y, Wu X, et al. The epidemiology, diagnosis and treatment of COVID-19. Int J Antimicrob Agents. 2020;55:105955. doi: 10.1016/j.ijantimicag.2020.105955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19 – preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMc2022236. [Online ahead of print] [DOI] [PubMed] [Google Scholar]
- 36.Alhazzani W, Møller MH, Arabi YM, et al. Surviving sepsis campaign: Guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19) Crit Care Med. 2020;46(5):854–87. doi: 10.1007/s00134-020-06022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rajendran K, Krishnasamy N, Rangarajan J, et al. Convalescent plasma transfusion for the treatment of COVID-19: Systematic review. J Med Virol. 2020 doi: 10.1002/jmv.25961. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mehra MR, Desai SS, Ruschitzka F, Patel AN. Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: A multinational registry analysis. Lancet. 2020 doi: 10.1016/S0140-6736(20)31180-6. [Online ahead of print] [RETRACTED] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.World Health Organization. “Solidarity” clinical trial for COVID-19 treatments. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov/solidarity-clinical-trial-for-covid-19-treatments.


