Abstract
Aneurysms are uncommon, but potentially life-threatening abnormalities of the pulmonary arteries. Aneurysm of the main pulmonary artery (MPA) defined as MPA diameter over 40 mm was reported in 1 : 14 000 autopsies. The most frequent location is the main pulmonary artery (89% of cases), whereas the maximum described diameter is 106–170 mm. Clinical manifestations are usually nonspecific or asymptomatic. Right heart failure symptoms, pulmonary regurgitation, trachea or bronchi compression or pulmonary emboli caused by enlarged MPA are the most commonly described clinical manifestations. Pulmonary artery aneurysm dissection is an uncommon complication but associated with a high mortality rate. Unfortunately, guidelines regulating the optimal time for the surgical intervention still have not been developed. We present the history of 76-year-old patient suffering from an aneurysm of the pulmonary artery (74 × 61 mm), as well as mitral and aortic valve disease, who was successfully operated on in our hospital.
Keywords: pulmonary artery aneurysm, pulmonary hypertension
Abstract
Tętniaki są rzadkimi, ale potencjalnie zagrażającymi życiu patologiami tętnicy płucnej. Częstość ich występowania szacuje się na 1 : 14 000 autopsji. Zgodnie z definicją tętniaka pnia tętnicy płucnej rozpoznaje się, gdy średnica naczynia przekracza 40 mm. Najczęstszą jego lokalizacją jest pień płucny (89% przypadków), podczas gdy największy opisany dotąd tętniak miał średnicę 106–170 mm. Objawy są najczęściej niespecyficzne lub choroba przebiega bezobjawowo. Prawokomorowa niewydolność serca, niedomykalność zastawki płucnej, ucisk na tchawicę, oskrzela lub zator płucny spowodowany przez ucisk powiększającego się tętniaka są najczęstszymi objawami zgłaszanymi przez chorego. Rozwarstwienie tętniaka tętnicy płucnej jest bardzo rzadkim powikłaniem, ale obarczonym wysoką śmiertelnością. Niestety dotychczas nie zostały opracowane żadne wytyczne dotyczące postępowania u pacjentów z tą chorobą. Przedstawiamy przypadek 76-letniego pacjenta z tętniakiem tętnicy płucnej (74 × 61 mm), wadą zastawki aortalnej i mitralnej, który został zoperowany w naszym ośrodku.
Introduction
Aneurysm and pseudoaneurysm are uncommon, but potentially life-threatening abnormalities of the pulmonary arteries. Aneurysm of the main pulmonary artery (MPA) defined as MPA diameter over 40 mm was reported in 1 : 14 000 autopsies [1, 2]. By definition, an aneurysm is a local dilatation of a blood vessel, involving all three layers of the vessel wall: the tunica intima, tunica media, tunica adventitia [3]. Otherwise, pseudoaneurysm does not involve all walls of the artery and it poses a higher risk of rupture [3]. MPA aneurysm based on the recent literature is defined in men as a diameter larger than 43.4 mm and in women larger than 40.4 mm, on average 40 mm or the PA diameter exceeds 1.5 times the normal one [4]. The maximum described diameter of the MPA was 106–170 mm [4, 5]. The frequency of a pulmonary artery aneurysm (PAA) does not depend on age or gender [6]. It occurs more commonly than aortic aneurysm in younger patients [2, 6]. The most frequent location is the main pulmonary artery – 89% and only 11% (or from another data 20–30%) of the PAAs are situated on the pulmonary artery (PA) peripheral branches [2, 7]. Clinical manifestations of PAAs are usually nonspecific or asymptomatic [8]. Generally, they may lead to right heart failure symptoms, secondary to pulmonary regurgitation, trachea or bronchi compression or pulmonary emboli from the enlarged MPA [9, 10]. Patients complain about hemoptysis, hoarseness, cough, dyspnea, chest pain, heart palpitations or syncope [3, 11].
The etiology of PAA may be congenital or acquired, as is shown in Table I [3, 7]. PAA are also divided into:
Table I.
Etiology of pulmonary artery aneurysm
| Congenital conditions | Acquired conditions | ||
|---|---|---|---|
| Etiology | Example | Etiology | Example |
| Heart defects | Ductus arteriosus Atrial septal defects Ventricular septal defects Hypoplastic aortic valve |
Infectious | Endocarditis Tuberculosis Syphilis Pyogenic bacteria Pneumonia |
| Connective tissue abnormality | Ehlers – Danlos syndrome Marfan syndrome Cystic medial necrosis |
Vasculitis | Behcet’s disease |
| Pulmonary artery hypertension | |||
| Chronic pulmonary embolism | |||
| Acute or chronic inflammatory lung disease | Bronchiectasis and pulmonary fibrosis Interstitial lung diseases COPD |
||
| Neoplasm | Metastasis Primary lung cancer |
||
| Iatrogenic | Cardiac surgery Chest tube placement Lung biopsy PA catheter placement PA arteriography Lung resection Radiation in the past |
||
COPD – chronic obstructive pulmonary disease, PA – pulmonary artery.
- High-pressure:
- people with pulmonary hypertension connected with congenital heart diseases,
- people with another pulmonary hypertension;
Pulmonary hypertension is described as the most important cause of the PAA [4, 13]. The prevalence of the PAA in patients with pulmonary hypertension ranges from 1.3% to 24% [4].
Diagnosis
During physical examination, systolic combined with diastolic murmur might be revealed due to a pulmonary valve defect. Signs of right ventricular or atrial hypertrophy might be observed in the electrocardiogram. Chest X-ray illustrates a dilatation of PA, hilar enlargement, lung nodule or pulmonary mass. An accurate echocardiogram concentrating on the RV outflow tract/PA view provides early diagnosis. In the diagnosis of peripheral aneurysm, echocardiography is of limited value. The echocardiographic reference value of the MPA dilatation is 26 mm [9]. A cardiac magnetic resonance imaging scan or cardiac computed tomography angiogram is the gold standard to confirm the diagnosis and provide additional information about size, number, location and extent of PAA. Dilatation of the MPA is identified when the diameter of the artery on the computed tomography (CT) or on the magnetic resonance images (MRI) is greater than 29 mm in men and 27 mm in women [14] while an interlobar artery is over 17 mm [3, 15].
Treatment
Due to infrequent incidence of PAA, there is a lack of guidelines regulating the optimal time for the surgical intervention. Most PAA seem to be relatively benign, especially when they remain asymptomatic. On the other hand, PAA dissection is associated with poor prognosis. There is still a lack of well-distinguished risk factors of PAA dissection and rupture. As the result of case reports analysis, the following high risk predictors were established: fast progression of PAA diameter (> 2 mm per year), tissue weakness due to infection and/or pregnancy, PA diameter > 75 mm or systolic PA pressure > 50 mm Hg [4]. Symptomatic patients or patients with accelerated growth of the PAA, with pulmonary hypertension (defined as RVSP > 35 mm Hg) should be operated on [12, 13]. Asymptomatic patients with the PAA diameter 80 mm or greater also should be referred for cardiac surgery [9, 12, 13]. Hence the question arises when to choose a risky procedure and when to choose a conservative treatment. This question still remains unanswered. Ergo, the scheme of the management proposed by Reisenauer [12] seems to be appropriate (Figure 1).
Figure 1.
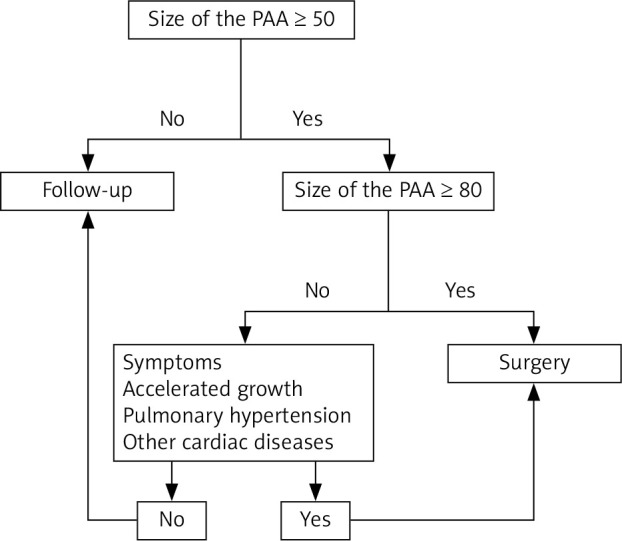
Algorithm for management for pulmonary artery aneurysm
Conservative treatment should be chosen in asymptomatic patients with no concomitant pulmonary hypertension and stable size dimensions of PAA. In the case of vasculitis or infectious etiology, causal treatment should be assigned. Interventional treatment such as coil embolization and vascular plugs are treatment option for iatrogenic PAA causes and for small branches [16, 17]. Thus, surgical treatment remains the treatment of choice [16, 17]. The operations are performed through a full medial sternotomy or a minimally invasive access such as left anterior minithoracotomy. The procedure needs extracorporeal circulation (ECC) to be applied [17, 18]. Aneurysmorrhaphy and aneurysmectomy and repair or replacement of the right ventricular outflow tract are the most common surgical techniques [12–17]. Gore-Tex or Dacron tubes, homografts or xenografts (porcine aortic grafts or bovine jugular conduits) are used for replacement of the pulmonary artery [17]. The armamentarium also includes lobectomy, bilobectomy, pneumonectomy, and, in the case of severe pulmonary hypertension, heart-lung transplantation [16, 17].
Outcomes
The PA diameter larger than 55 mm in patients with the high-pressure PAA is associated with higher risk of sudden, unexpected death, compression of the left main coronary artery, right PA thrombosis, lung compression and atelectasis [4]. PAA dissection is a rare complication (2.9%) [4], but with fatal prognosis. The mortality rate associated with rupture of the PAA was reported as 50–100% [3, 19] and is secondary to intrapulmonary hemorrhage [20].
Data regarding surgical outcomes are poor, because of the infrequency of PAA. However, perioperative morbidity is similar to that of the repair of aneurysms of the ascending aorta [17]. Significant postoperative problems include ventilation difficulties resulting from a tendency of the bronchi to collapse, atelectasis of the lung, and postoperative effusion in the former cavity of the aneurysm, which together may extend mechanical ventilation time and increase the risk for lung injury [17].
Case report
A seventy-six-year-old patient suffering from an aneurysm of the pulmonary artery, after transcutaneous mitral commissurotomy, with mitral and aortic valve disease, with stable coronary disease, with permanent atrial fibrillation, arterial hypertension, type two diabetes treated with metformin, chronic obstructive pulmonary disease and thyroid nodular goiter, was admitted to the clinic with the aim of heart defect diagnostics.
The aneurysm of the pulmonary artery was first identified in 1998 during mitral stenosis diagnostics. At that time, the patient was qualified for transcutaneous mitral valvuloplasty by the Inoue method and transcutaneous angioplasty of the significantly stenotic branch of the left anterior descending coronary artery with the implantation of a DES stent. The PAA described in the echocardiography examination was left without any intervention.
In 2005, the patient was again referred to the clinic in order to evaluate the possible progression of the PAA. Then, the PAA with diameter of 49 × 59 was described in the tomography evaluation with the maximal measurement of 69 mm. The diameter of the left pulmonary artery was 24 mm and the measurement of the right pulmonary artery was also 24 mm. The patient did not report any ailments and after excluding the presence of significant left heart failure was qualified for further aneurysm observation.
In 2006, the patient was once more hospitalized due to unstable angina and ventricular arrhythmia in the form of non-sustained ventricular tachycardia. The performed coronarography pictured again the left anterior descending artery and coronary artery bypass grafting. Both arteries underwent revascularization with the implantation of two DES stents. Significant progression of the PAA and the mitral valve disease were not diagnosed. Additionally, moderate aortic stenosis was described. The patient, in a good condition, without angina or ventricular arrhythmia, was discharged from the clinic.
After hospital discharge, the patient continued to experience gradual and slow deterioration of the exercise intolerance. During his visit to the clinic in 2017, he complained about dyspnea after walking 100 m and orthopnea. He still reported angina pectoris class 2 according to CCS. During the hospital admission, a loud systolic murmur above the heart was diagnosed. It was dominating over the base of the heart and radiating to the carotid arteries. Moreover, no audible second tone of the heart was detected. Echocardiography evaluation described the significantly changed aortic bicuspid valve with gradients GA 64.5/37.1 mm Hg (Figures 2, 3) and moderate mitral re-stenosis with the fibrosis of the leaflet’s edges, crevice opening of commissures. Gradients through the mitral valve were assessed at 21.5/7.2 mm Hg (Figure 4). The mitral valve area was planimetrically difficult to evaluate, estimated at 1.3–1.5 cm2 (Figure 5). Mitral regurgitation was described as minor. Total cardiac contractility remained unchanged, LVEF about 65%. The ECHO examination showed a huge possibility of pulmonary hypertension (RVSP > 60 mm Hg) (Figures 6, 7).
Figure 2.

Echocardiographic five chamber view showing elevated gradient across aortic valve (64.5/37.1 mm Hg)
Figure 4.

Four chamber cardiac echocardiography showing elevated gradient across mitral valve (21.5/7.2 mm Hg)
Figure 5.

Echocardiographic parasternal short axis view demonstrated stenosis of mitral valve – mitral valve area 1.4 cm2
Figure 6.

Parasternal short cardiac echocardiography image demonstrating large dilatation of pulmonary artery
Figure 3.

Echocardiographic parasternal short axis view revealing stenosis of bicuspid aortic valve – aortic valve area 1.1 cm2
Figure 7.

echocardiographic five chamber view showing increased tricuspid regurgitation peak gradient suggesting pulmonary hypertension
Computed tomography pictured supravalvular aneurysm before the division of the pulmonary trunk of size 74 × 61 mm, widening of the left pulmonary artery of 34 × 28 mm, and boundary dimensions of the right pulmonary artery estimated at 25 mm (Figures 8–10).
Figure 8.

Computed tomography scan showing a large aneurysm of the main pulmonary artery in longitudinal – section
Figure 10.
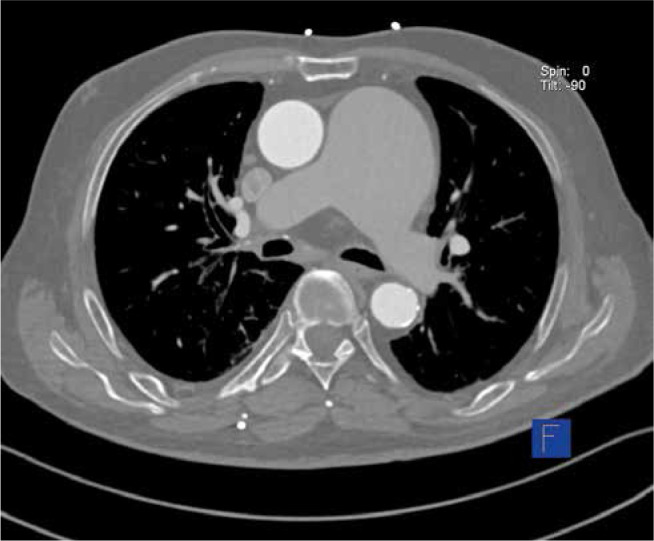
Computed tomography scan revealing a large aneurysm of the main pulmonary artery in cross – section
Figure 9.

Three-dimensional reconstruction of the heart and the massively dilated main pulmonary artery
Progression of the pulmonary artery aneurysm and valve disease with the presence of aortic stenosis was revealed by performed diagnostics. At the meeting of the Heart Team, the patient was qualified for cardiosurgical treatment due to life indications, despite the elevated risk (EuroSCORE II – 7.34%). The performed coronarography, with the aim of preoperative evaluation of the coronary arteries, showed significant stenosis of the anterior descending branch.
In hypothermia, in cardiopulmonary bypass, plastic surgery of the pulmonary trunk and the left pulmonary artery was performed, a mechanical valve was implanted in the aortic orifice (St. Jude Medical 23 mm) and in the mitral one (St. Jude Medical 29 mm) as well as the venous coronary bypass graft to the left anterior descending artery. The left atrial appendage also was closed. The postoperative period included complications in the form of permanent atrial fibrillation with advanced atrioventricular block requiring implantation of a heart stimulation system. We observed the post-pericardiotomy syndrome with pericarditis and left side pleuritis, which was relieved by the use of anti-inflammatory treatment consisting of colchicine and nonsteroidal anti-inflammatory drugs. During the postoperative period, the patient also required antibiotic therapy due to the acute exacerbation of chronic obstructive pulmonary disease. After 38 days, the patient was discharged in a generally good condition.
Discussion
PAA is infrequently diagnosed and seems to be a relatively benign condition. The clinical manifestations are nonspecific and rare, even those with large PAA diameters up to 70 mm. The dissection of the PA, opposite to ascending aortic aneurysm, is rare. Probably, the asymptomatic course of the disease with the estimated high risk of operation preponderated in the case of the patient years ago, qualifying him for conservative treatment. PA of a maximal diameter 69 mm was left without any intervention.
The presented patient was successfully conservatively treated for a dozen years. The presence of the left heart valve disease, especially the significant aortic stenosis, necessitated surgical treatment. It is highly probable that the patient’s symptoms concerning the deterioration of exercise intolerance as well as angina pectoris were secondary to the left heart valve disease.
It is worth mentioning that aneurysms are quite frequently observed among individuals with long-lasting pulmonary hypertension, but it has not yet been proved that treatment lowering the pulmonary artery pressure could significantly affect the lack of its progression [2]. In the case of the patient in question, the most probable mechanism of dilatation PA was pulmonary hypertension secondary to the left heart valve disease.
There is no doubt that further research determining factors of the PAA progression risk and its rupture is needed. During patient qualification for the procedure, it is crucial to take the risk of cardiac surgery into consideration. Amongst available treatment methods for the aforementioned patient, given the extensiveness of the operation, it was decided that the most appropriate one would be repair of the aneurysm in order to prevent the artery from widening in the future.
In a Japanese study it was observed that the PA aneurysm area, in comparison to non-PAA areas, showed increased EP4 receptor (for the cyclooxygenase-2-dependent PGE2) expression [2]. The increased expression of that receptor is also described in non-striated muscle cells as well as in macrophages in abdominal aortic aneurysm areas [21–23]. PGE2, via the EP4 receptor, increases the activity of metalloproteinase 2 and the production of interleukin 6, causing increased degradation of elastic fibers redounding to the AAA progression [21, 22]. Nonsteroidal anti-inflammatory drugs and selective COX-2 inhibitors suppress PGE2 synthesis. However, on the grounds of the adverse drug reactions (an increased risk of gastrointestinal bleeding – NSAID, cardiovascular events – coxibs) those drugs ought not to be used at length. An interesting goal of pharmacotherapy inhibiting the progression of the aneurysms would be a selective EP4 receptor antagonist. Perhaps the use of the aforementioned receptor antagonist will be one of the treatment options in the future, but it would involve further research.
Conclusions
Identification of the etiology is a crucial step in qualification for treatment. Pulmonary artery dilatation over 80 mm, occurrence of symptoms, accelerated growth of aneurysm, pulmonary hypertension and concomitant other cardiac disease should be taken into consideration in the decision-making process about surgical treatment.
Biography

Disclosure
The authors report no conflict of interest.
References
- 1.Deterling RA Jr, Clagett OT. Aneurysm of the pulmonary artery: review literature and report of a case. Am Heart J 1947; 34: 471-499. [DOI] [PubMed] [Google Scholar]
- 2.Akagi S, Nakamura K, Sarashina T, et al. Progression of pulmonary artery dilatation in patients with pulmonary hypertension coexisting with a pulmonary artery aneurysm. J Cardiol 2018; 71: 517-522. [DOI] [PubMed] [Google Scholar]
- 3.Park HS, Chamarthy MR, Lamus D, et al. Pulmonary artery aneurysms: diagnosis and endovascular therapy. Cardiovasc Diagn Ther 2018; 8: 350-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duijnhouwer AL, Navarese EP, Van Dijk APJ, et al. Aneurysm of the pulmonary artery, a systematic review and critical analysis of current literature. Congenit Heart Dis 2016; 11: 102-109. [DOI] [PubMed] [Google Scholar]
- 5.Kanaoka K, Horii M, Nagato H, Kaneda K. A giant pulmonary artery aneurysm. Eur Heart J Cardiovasc Imaging 2018; 19: 236. [DOI] [PubMed] [Google Scholar]
- 6.Gallego P, Rodríguez-Puras MJ, Serrano Gotarredona P, et al. Prevalence and prognostic significance of pulmonary artery aneurysms in adults with congenital heart disease. Int J Cardiol 2018; 270: 120-125. [DOI] [PubMed] [Google Scholar]
- 7.Valente T, Abu-Omar A, Sica G, et al. Acquired peripheral pulmonary artery aneurysms: morphological spectrum of disease and multidetector computed tomography angiography findings–cases series and literature review. Radiol Medica 2018; 123: 664-675. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y, Gilman MD, Humphrey KL, et al. Pulmonary artery pseudoaneurysms: clinical features and CT findings. Am J Roentgenol 2017; 208: 84-91. [DOI] [PubMed] [Google Scholar]
- 9.Sheikhzadeh S, De Backer J, Gorgan NR, et al. The main pulmonary artery in adults: a controlled multicenter study with assessment of echocardiographic reference values, and the frequency of dilatation and aneurysm in Marfan syndrome. Orphanet J Rare Dis 2014; 9: 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Butto F, Lucas RV, Edwards JE. Pulmonary arterial aneurysm. A pathologic study of five cases. Chest 1987; 91: 237-241. [DOI] [PubMed] [Google Scholar]
- 11.Vistarini N, Aubert S, Gandjbakhch I, Pavie A. Surgical treatment of a pulmonary artery aneurysm. Eur J Cardiothoracic Surg 2007; 31: 1139-1141. [DOI] [PubMed] [Google Scholar]
- 12.Reisenauer JS, Said SM, Schaff HV, et al. Outcome of surgical repair of pulmonary artery aneurysms: a single-center experience with 38 patients. Ann Thorac Surg 2017; 104: 1605-1610. [DOI] [PubMed] [Google Scholar]
- 13.Kuwaki K, Morishita K, Sato H, et al. Surgical repair of the pulmonary trunk aneurysm. Eur J Cardiothoracic Surg 2000; 18: 535-539. [DOI] [PubMed] [Google Scholar]
- 14.Truong QA, Massaro JM, Rogers IS, et al. Reference values for normal pulmonary artery dimensions by noncontrast cardiac computed tomography the Framingham heart study. Circ Cardiovasc Imaging 2012; 5: 147-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nguyen ET, Silva CIS, Seely JM, et al. Pulmonary artery aneurysms and pseudoaneurysms in adults: findings at CT and radiography. AJR Am J Roentgenol 2007; 188: 126-134. [DOI] [PubMed] [Google Scholar]
- 16.Gupta M, Agrawal A, Iakovou A, et al. Pulmonary artery aneurysm: a review. Pulm Circ 2020; 10: 2045894020908780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kreibich M, Siepe M, Kroll J, et al. Aneurysms of the pulmonary artery. Circulation 2015; 131: 310-316. [DOI] [PubMed] [Google Scholar]
- 18.Rahman S, Cook G, Zheng J, Rehman A. Minimally invasive pulmonary artery aneurysm repair through a left anterior minithoracotomy–an alternative approach. Innov Technol Tech Cardiothorac Vasc Surg 2019; 14: 353-356. [DOI] [PubMed] [Google Scholar]
- 19.Ishimoto S, Sakurai H, Higure R, et al. Pulmonary artery pseudoaneurysm secondary to lung inflammation. Ann Thorac Cardiovasc Surg 2018; 24: 154-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeLima LG, Wynands JE, Bourke ME, Walley VM. Catherter-induced pulmonary artery false aneurysm and rapture: case report and review. J Cardiothorac Vasc Anesth 1994; 8: 70-75. [DOI] [PubMed] [Google Scholar]
- 21.Yokoyama U, Ishiwata R, Jin MH, et al. Inhibition of EP4 signaling attenuates aortic aneurysm formation. PLoS One 2012; 7: e36724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mamun A, Yokoyama U, Saito J, et al. A selective antagonist of prostaglandin E receptor subtype 4 attenuates abdominal aortic aneurysm. Physiol Rep 2018; 6: e13878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dilmé JF, Solà-Villà D, Bellmunt S, et al. Active smoking increases microsomal pgesynthase-1/pge-receptor-4 axis in human abdominal aortic aneurysms. Mediators Inflamm 2014; 2014: 316150. [DOI] [PMC free article] [PubMed] [Google Scholar]


