Abstract
Benzodiazepines and SSRIs are considered as standard treatment options for anxiety and depression, hallmarks of Post-Traumatic Stress Disorder (PTSD), although their use is often limited by adverse effects. While promising evidence emerged with β-adrenergic receptor (β-AR) antagonists (or ‘β-blockers’) and PTSD relief, efficacy issues dampened the excitement. However, we believe it is premature to completely eliminate a beneficial role of β-blockers. Our previous work has suggested that social defeat (SD) results in anxiety-like and depression-like behaviors in rats. Here, using the SD paradigm, we examined the effect of several β-adrenergic receptor antagonists (propranolol, nadolol, bisoprolol) on these behaviors in rats. Following acclimatization, Sprague-Dawley rats received no treatment (for control groups) or treated with; propranolol (50 mg/kg/day in water), or nadolol (18 mg/kg/day in rats’ chow), or bisoprolol (15 mg/kg/day in water). The treatment lasted for 36 days, following which rats were subjected to SD/control exposures (1 week). Later, anxiety-like and depression-like behaviors, social interaction and learning-memory function tests were conducted. SD rats exhibited anxiety- and depression-like behavior as well as learning-memory impairment. Propranolol and nadolol protected SD rats from exhibiting anxiety-or depression-like behaviors. Bisoprolol treatment did not mitigate SD-induced behavioral impairments in rats. Nadolol, propranolol or bisoprolol have no effect in attenuating SD-induced memory function tests. These results suggest that certain ‘β-blockers’ have the potential to mitigate the negative psychological effects of traumatic events.
Keywords: PTSD, Social stress, Depression, Anxiety, Beta-blockers
1. Introduction
Traumatic experiences are often reported to cause post-traumatic stress disorder (PTSD) [1–3]. Benzodiazepines and SSRIs are considered as standard treatment options [4]. Although clinically useful, their use can also produce serious side effects [5]. Thus, there remains an important need for improving PTSD treatment, and finding preventive pharmacological interventions. Promising evidence has emerged from studies reporting that administration of a non-selective β-adrenergic receptor (β-AR) antagonist, propranolol, which blocks epinephrine/norepinephrine signaling [4], may also offer PTSD relief [6]. Furthermore, propranolol was proven to be effective in eliminating stage fright [7], improving anxiety-related cognitive dysfunction [8], and in reducing emotional arousal [9]. However, in a double-blind study with propranolol and atenolol, positive effects were not observed in phobic subjects [10]. Animal studies also reported mixed results on the usefulness of propranolol in PTSD [11]. Thus, methodological limitations of the studies and efficacy issues have dampened the excitement [12, 13].
However, it is premature to disregard the role of β-AR antagonists in PTSD treatment, especially considering their beneficial role in the treatment of numerous psychiatric ailments including anxiety disorders [14–18], schizophrenia [19], autism [20], aggression [21], and various forms of fearful behaviors [7, 22–26]. It is also important to note that β-AR blockers are relatively safe drugs that are taken by hundreds of millions daily for a myriad of diseases/disorders ranging from cardiovascular disorders such as hypertension, heart failure and angina, to glaucoma and migraines. However, a consensus on the potential usefulness of β-ARs cannot be reached as a comprehensive screening of different β-adrenergic receptor antagonists on various conditions including anxiety, depression and cognitive impairment, is lacking. The rodent model of stress/trauma from social defeat (SD) [27–29] provides a reasonable paradigm to examine effect of different β-adrenergic receptor antagonists on PTSD-relevant behaviors. The SD model resembles societal stress in humans and represents an ethologically valid stressor, as it induces long-lasting physiologic and behavioral changes [30]. Rodent exhibit increased anxiety-like and depression-like behaviors, impaired memory, social avoidance, and decreased locomotor and exploratory activity following consecutive social defeat exposures [31–33]. Using the SD model of stress, we asked two important questions; first, whether propranolol, nadolol, or bisoprolol treatments exert protective effect on stress-induced anxiety-, depression-like behaviors and memory impairments? And, what is the degree and extent of protective effect, if any, on anxiety-, depression-like behaviors and learning-memory functions? Second, is there any response selectivity or superiority of efficacy amongst three different beta-blockers, propranolol, nadolol and bisoprolol? We focused on these three beta-blockers because propranolol is a lipophilic non-selective β1-AR and β2-AR antagonist which crosses the blood-brain barrier (BBB) easily; nadolol is also a non-selective β1-AR and β2-AR antagonist, but very hydrophilic and crosses the BBB to a considerably lesser extent than propranolol [34, 35]. Bisoprolol has both lipid and water-soluble properties and thus some BBB permeability, but is the most selective β1-AR antagonist in clinical use.
Our previous work has suggested that social defeat (7 days of repeated stress) results in anxiety-like and depression-like behaviors in rats [36, 37]. Here, using the social defeat paradigm, we examined the effect of β-AR antagonists, propranolol, nadolol, and bisoprolol, on these behaviors in rats. Male Sprague Dawley rats were treated with either propranolol, nadolol, or bisoprolol or with normal food and water for 36 days. Following treatment, the rats were subjected to 7 days of SD. After conclusion of the SD protocol, the effect of the different beta-blockers was examined using a variety of established behavioral tests.
2. Methods
2.1. Animals
All experiments were conducted in accordance with the NIH guidelines for the use of experimental animals and approved animal protocol by the Institution Animal Care and Use Committee (IACUC). Male Long-Evans retired breeders (400–500 g) and male Sprague Dawley (275–300 g) rats were purchased from Harlan (Indianapolis, IN). Upon arrival at the animal housing facility, rats were randomly selected, grouped in cages and acclimatized for one week before any treatment. Rats were housed in plastic cages (14×18×18 inches) and kept in a room on a 12h light/dark cycle at 23°C, 60% humidity, with food and tap water available ad libitum.
2.2. Experimental Design
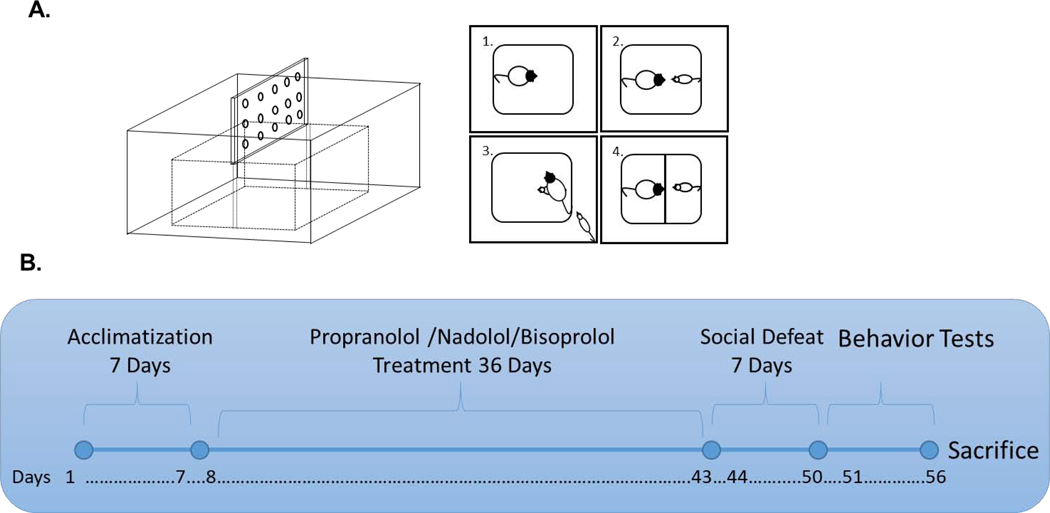
Rats were assigned randomly into eight groups (n=8–12 per group). 1. Control (CON), 2. Nadolol (NAD), 3. Propranolol (Prop), 4. Bisoprolol (BIS), 5. Social defeat (SD), 6. Nadolol with social defeat (NAD-SD), 7. Propranolol with social defeat (Prop-SD), 8. Bisoprolol with social defeat (BIS-SD). After acclimatization for 7 days, CON and SD rats received regular rat chow and tap water ad libitum. Nadolol and NAD-SD groups received nadolol-mixed rat chow (18 mg/kg/day) and tap water ad libitum for 36 days. Propranolol and Prop-SD groups received standard rat chow and propranolol-treated water (50 mg/kg/day) ad libitum for 36 days. Bisoprolol and BIS-SD groups received bisoprolol treated water (15 mg/kg/day) and standard rat chow ad libitum for 36 days. Following 36 days of treatment, groups 5–8 were subjected to 3 social defeat exposures daily for 7 days, while rats in CON, Prop, NAD, and BIS groups were handled gently in a similar room. Following conclusion of social defeat protocol, rats were subjected to different rodent behavior tests as described below. At first light-dark (LD) test was conducted followed by elevated plus-maze (EPM) test, conducted on the same day with a rest period of 2h between the two tests. Forced swim test (FST), social interaction (SI), Short-term memory (STM) and long-term memory (LTM) were conducted over a period of 4 days with one test per day. The experimental design is summarized in Fig. 1B.
Fig. 1: Illustration of the social defeat apparatus and schematic representation of the experimental design:
Social defeat apparatus consists of a Plexiglas cage layered with standard rat bedding. A perforated Plexiglas sheet is used as a partition to separate between the resident and the intruder rats following social defeat (A). An aggressive male LE rat considered as a resident (panel#1, A) is met with a Sprague-Dawley male rat considered as an intruder (panel#2, A). This leads to aggressive behavior with the resident rat pouncing over the intruder rat resulting in social defeat (panel#3, A). After defeat, a perforated plexiglass partition is placed inside the cage to prevent direct physical contact between the LE and intruder for the remainder of the 30-min session (panel#4, A). Rats were assigned into 8 groups, n=8–12 rats per group. Groups: Control (CON), Social defeat (SD), Propranolol (Prop), Nadolol (NAD), Bisoprolol (BIS), Propranolol with social defeat (Prop-SD), Nadolol with social defeat (NAD-SD), and Bisoprolol with social defeat (BIS-SD). After acclimatization for 7 days, CON and SD rats received regular rat chow and tap water ad libitum. Rats in NAD and NAD-SD groups received nadolol-mixed rat chow (18 mg/kg/day) and tap water ad libitum for 36 days. Rats in Prop and Prop-SD group received standard rat chow and propranolol-treated water (50 mg/kg/day) ad libitum for 36 days. Rats in BIS and BIS-SD group received bisoprolol treated water (15 mg/kg/day) and standard rat chow ad libitum for 36 days. Following 36 days of drugs treatment, four groups were subjected to 3 social defeat exposures daily for 7 days, while the control rats did not. After conclusion of social defeat protocol, rats were subjected to different rodent behavior tests (B).
2.3. Drug Treatment
Nadolol was mixed with rat chow (18 mg/kg/day), propranolol (50 mg/kg/day) and bisoprolol (15 mg/kg/day) were dissolved in water, separately. Body weight and the amount of food and water consumed by rats in each cage were measured daily. Propranolol hydrochloride was obtained from Sigma Aldrich (cat # P0084), nadolol and bisoprolol were also obtained from Sigma Aldrich (nadolol cat # N1892, bisoprolol cat # 50787). The doses and route of administration were chosen based on previous studies in rodents [38–41]. In previous work, these compounds have produced significant cardiovascular changes (such as decreases in heart rate and/or blood pressure) in rats. Furthermore, the authors have reported dosing regimens between 14 days and 42 days with comparable and consistent changes in respiratory and inflammatory parameters in murine models of asthma [42–45]. The difference of whether the drug was placed in chow or the drinking water was for consistency with these published studies. The bioavailability and half-life of all 3 drugs make them suitable for oral administration. Both methods of drug delivery (through drinking water and chow) are comparable means of achieving oral administration, since they would be subject to the same metabolic pathways, including the first pass effect through the liver.
2.4. Social Defeat Paradigm
The social defeat model was originally developed by Miczek [46], it is a widely accepted rodent model of PTSD [27, 28, 47, 48]. The model involves aggressive encounters between a large Long-Evans (LE) male rat (resident) and a smaller Sprague-Dawley male rat (intruder). The social defeat paradigm consists of 3 encounters between an aggressive male LE rat (resident) and the Sprague-Dawley male rat (intruder), daily for 7 consecutive days. Each session lasts for 30 min. Notably, each day, the intruder encounters different LE resident rat. Social defeat is indicated by the intruder surrendering or acquiring a supine position for ~5s. Once the intruder rat is defeated, a perforated plexiglass partition is placed inside the cage to prevent direct physical contact between the LE and intruder preventing injury from persistent attacks while allowing visual, auditory and olfactory interactions for the remainder of the 30-min session. Control rats were placed behind the plexiglass partition in a fresh cage for 30 min daily in the absence of the resident rat. The social defeat apparatus is illustrated in Fig.1A.
2.5. Anxiety-like behavior tests
2.5.1. Light/dark (LD) test
The LD apparatus consists of two compartments; a light compartment (27 X 27 X 27 cm) and a dark compartment (27 X18 X 27 cm), connected through a single opening (7 X 8 cm) between the two compartments. The rats were placed individually in the light compartment and their movements between the two compartments were recorded for 5 minutes. Less time spent in the lit area is an indication of anxiety-like behavior [49].
2.5.2. Elevated plus maze (EPM) test
The EPM apparatus consists of two open and two closed arms (10 X 50 cm) that intersects to create a plus shape. The EPM apparatus is elevated from the floor by 100 cm. The rats were placed individually in the middle between the four arms. Within the 5-minute test duration, rat’s movement between the arms and the time spent in each arm were recorded. Less time spent in open arms is an indicator of anxiety-like behavior [49].
2.6. Social interaction (SI) test
The social interaction apparatus consists of three-compartments connected via sliding partitions. The middle compartment (25 X 35 X 35 cm) is the habituation compartment. The other two end compartments (25 X 50 X 35 cm) contained a wire cup. A naïve novel Sprague Dawley male rat, from a different litter, was placed under one of the wire cups and the second cup was left empty. The test consisted of two sessions: habituation session and sociability session. During the habituation session, the test rat was placed in the middle empty compartment for 5 minutes for habituation. In the second “sociability” session, the test rat encountered a novel rat in one compartment and an empty cup in the other compartment. The sociability session lasted for 10 minutes. The time spent sniffing and interacting with each cup, the time spent in each compartment, and the number of entries into each compartment were recorded. Normal sociability in rats is indicated by their preference to spend more time interacting with another rat (novel) rather than with the empty cup [50–52].
2.7. Depression-like behavior test - forced swim test (FST)
The apparatus consists of a cylindrical Plexiglas tank (24 cm in diameter and 30 cm in height) filled with water (25 °C). The rats were placed individually in the tank and their mobility was recorded for 5 minutes. After struggling in water for some time, the rats assume an immobile posture. Longer immobility time in the water is an indication of depression-like behavior [27].
2.8. Memory Function Tests
Radial Arm Water Maze (RAWM) Test-
The standard RAWM procedures were performed as published previously by us [27, 47, 49]. RAWM is a black circular pool filled with water at room-temperature, with six V-shaped arms, which creates six swim paths from one open central area. Experiments were performed in a dimly lit room. The test began with 12 practice trials. The purpose of the practice trials was to help the rat learn and memorize the location of the platform. Each rat was randomly assigned a goal arm, which contains a hidden black platform near the end of the arm. The rats were released at an arm other than the goal arm, and the rat must swim and locate the platform, which is submerged about 1 cm under the water. The rats were allowed a maximum time of 1 minute for each learning trial or memory test. An error was counted when the rat’s entire body enters more than halfway into an arm other than the goal arm. An error was also counted if the rat’s entire body enters more than half of the goal arm but fails to approach the platform. The number of errors ranged from 1 to 7 as the rat can swim into 7 arms within 1 minute. If the rat failed to locate the platform within 1 minute, the rat was manually guided to the platform and was scored with 7 errors. Once the rat reached the platform, the rat was allowed 15 seconds sitting time on the platform before the next trial began. STM was conducted 30 min after the last practice trial, and LTM was conducted 24 h after conclusion of STM.
2.9. Data Analysis
Data are expressed as mean ±SEM. Significance was determined by one-way ANOVA applying Tukey’s post-hoc test (GraphPad Software, Inc. San Diego, CA). Furthermore, Two-tailed t test was applied for comparing between two groups. For the social interaction analysis two-way ANOVA statistics was conducted. For all other behavioral measurements included in Fig. 2, 3 and 5, one-way ANOVA and t test were used. A value of P< 0.05 was considered significant. All data sets passed when subjected to normality tests using Kolmogorov-Smirnov and Shapiro-Wilk analysis using GraphPad Prism® to justify use of ANOVA analysis.
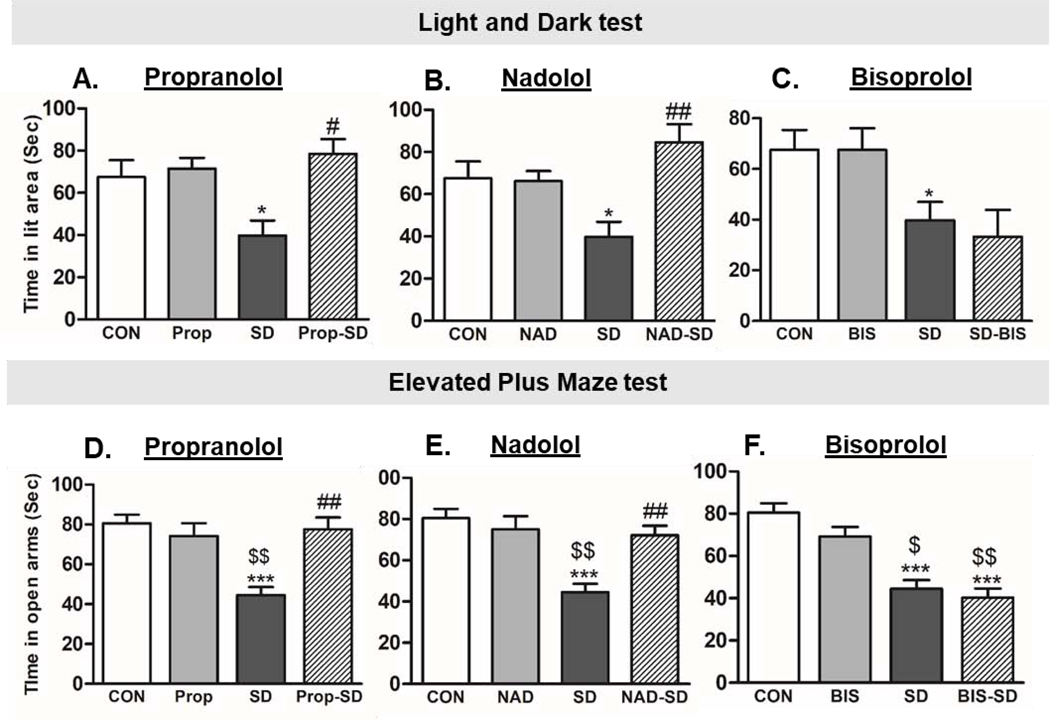
Fig 2. Examination of anxiety-like behavior:
Total time spent in the lit compartment in light-dark (LD) test (A, B, and C), and in the open arms in the elevated plus maze (EPM) test (D, E, and F) was used to measure anxiety-like behavior. Group designations: rats not subjected to social defeat (CON: control); rats subjected to social defeat (SD); rats treated with propranolol alone (Prop), rats treated with propranolol and then subjected to SD (Prop-SD), rats treated with nadolol (NAD), rats treated with nadolol and then subjected to SD (NAD-SD), rats treated with bisoprolol (BIS), rats treated with bisoprolol and then subjected to SD (BIS-SD). (*) Indicates significantly different from CON, P<0.05. (#) Indicates significantly different from SD, P<0.05. ($) Indicates significantly different from Prop, NAD, or BIS, P<0.05. Data were analyzed using one-way ANOVA for comparison between all the groups, and t-tailed t test for comparison between each two groups. Bars represent means ± SEM, n =8–12 rats/group.
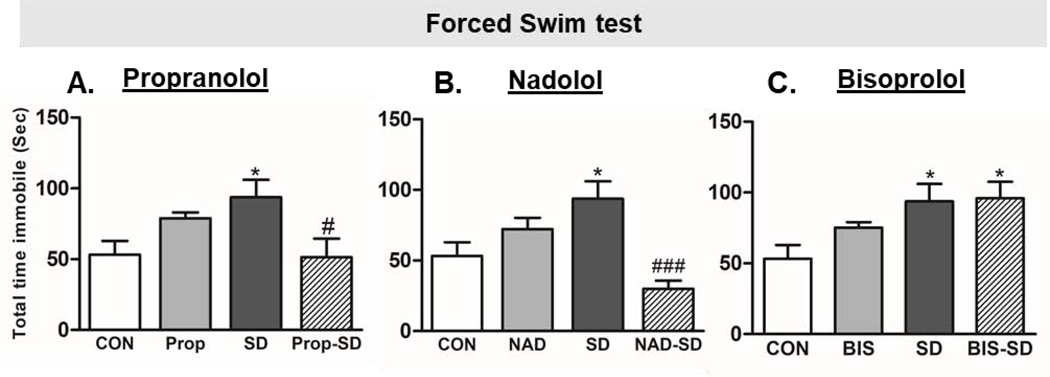
Fig. 3: Examination of depression-like behavior:
Total immobility time in the Forced swim test (FST) was used to examine depression-like behavior in rats. Group designations: rats not subjected to social defeat (CON: control); rats subjected to social defeat (SD); rats treated with propranolol alone (Prop), rats treated with propranolol and then subjected to SD (Prop-SD), rats treated with nadolol (NAD), rats treated with nadolol and then subjected to SD (NAD-SD), rats treated with bisoprolol (BIS), rats treated with bisoprolol and then subjected to SD (BIS-SD). (*) Indicates significantly different from CON, P<0.05. (#) Indicates significantly different from SD P<0.05. Data were analyzed using one-way ANOVA for comparison between all the groups, and t-tailed t test for comparison between each two groups. Bars represent means ± SEM, n =8–12 rats/group.
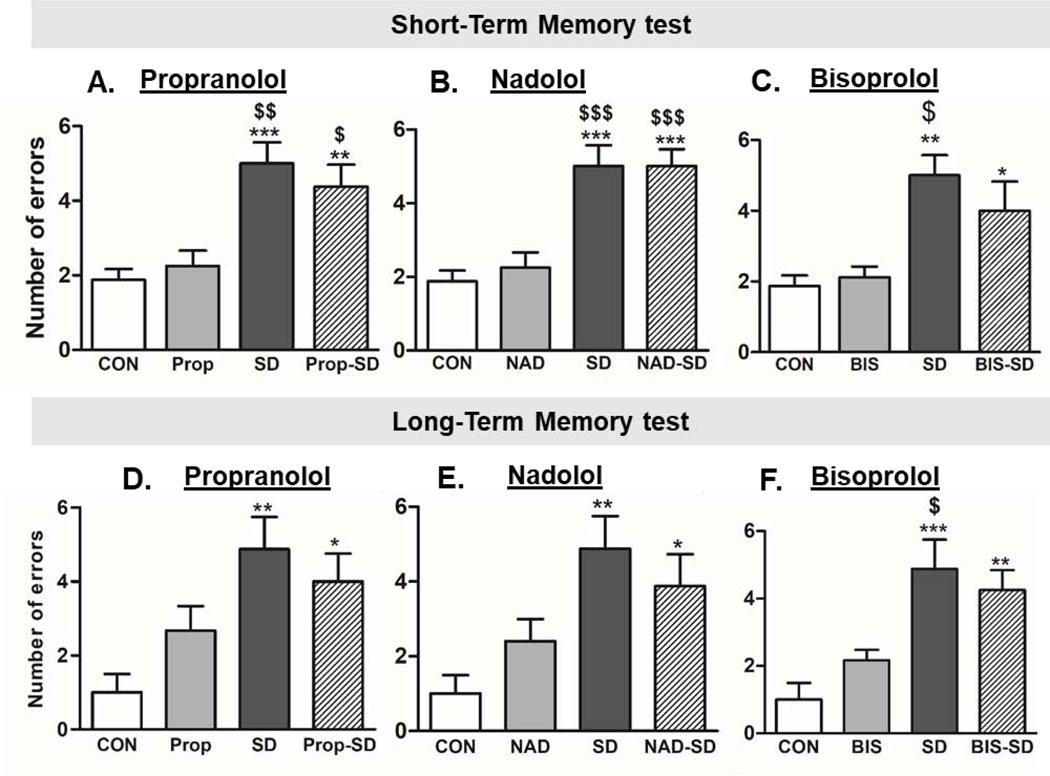
Fig. 5: Examination of memory function:
Number of errors made in the short-term memory (STM) test (A, B, and C) and long-term memory (LTM) test (D, E, and F) examined in radial arm water maze (RAWM) apparatus. Group designations: rats not subjected to social defeat (CON: control); rats subjected to social defeat (SD); rats treated with propranolol alone (Prop), rats treated with propranolol and then subjected to SD (Prop-SD), rats treated with nadolol (NAD), rats treated with nadolol and then subjected to SD (NAD-SD), rats treated with bisoprolol (BIS), rats treated with bisoprolol and then subjected to SD (BIS-SD). (*) Indicates significantly different from CON, ($) Indicates significantly different from corresponding bet-blocker treatment, P<0.05; Data were analyzed using one-way ANOVA for comparison between all the groups, and t-tailed t test for comparison between each two groups. Bars represent means ± SEM, n =8–12 rats/group.
3. Results
3.1. General body parameters:
Daily food and water intake were measured in all rats. All groups consumed comparable amounts of food. CON: 19±1.12 g/day, SD: 20±1.76 g/day, Prop-SD: 20±0.86 g/day, NAD-SD: 19.4±0.91 g/day, BIS-SD: 22±0.55 g/day. Water intake also remained unchanged in all groups with each group consuming 34–52 ml water per day. Body weight gain also was comparable in all groups CON: 301±7.5 g, SD: 295±5.0 g, Prop-SD: 305±4.0, NAD-SD: 308±6.2, BIS-SD: 298±6.0 (Table 1). Thus, no significant changes were observed in total weight gain, food intake or water intake.
Table 1. Examination of general body parameters.
Daily food intake, water intake, and body weight was measured in all groups. Abbreviations: Propranolol (Prop), Nadolol (NAD), Bisoprolol (BIS). Group designations: rats not subjected to social defeat (CON: control); rats subjected to social defeat (SD); rats treated with propranolol and then subjected to SD (Prop-SD), rats treated with nadolol and then subjected to SD (NAD-SD), rats treated with bisoprolol and then subjected to SD (BIS-SD).
| CONTROL | SOCIAL DEFEAT (SD) | PROPRANOLOL + SD | NADOLOL + SD | BISOPROLOL + SD | |
|---|---|---|---|---|---|
| DAILY FOOD INTAKE (g) | 19±1.12 | 20±1.76 | 20±0.86 | 19.4±0.91 | 22±0.55 |
| DAILY WATER INTAKE (ml) | 34±2.42 | 34.5±2.11 | 45±1.12 | 40±3.22 | 52±1.02 |
| BODY WEIGHT GAIN (g) | 301±7.5 | 295±5.0 | 305±4.0 | 308±6.2 | 298±6.0 |
3.2. Analysis of Anxiety-like behavior:
In light/dark (LD) test, one-way ANOVA following Tukey’s multiple comparison test was performed. Furthermore, t test was performed to analyze the statistical difference between the two groups. SD rats spent significantly less time in the lit area as compared to CON rats ( SD:39.79 ± 7.16 sec, CON: 67.50 ± 7.96 sec, t(41)= 2.52, p= 0.0157 ) (Fig. 2A, B, and C), suggesting that SD rats exhibited anxiety-like behavior, less time spent in the lit area of the LD apparatus during a 5-min session is indicative of high anxiety-like behavior [53]. Interestingly, rats treated with propranolol before social defeat spent significantly greater time in lit area as compared to social defeat rats (Prop-SD: 78.54 ± 6.92 sec; SD: 39.79 ± 7.16 sec, t(30)= 3.72, p= 0.0008). Notably, rats treated with propranolol alone spent similar time in lit area as compared to CON rats (Prop: 71.50 ± 5.06 sec; CON: 67.50 ±7.96 sec, t(28)= 0.24, p= 0.808) (Fig. 2A). Similarly, rats treated with nadolol before social defeat spent significantly greater time in lit area as compared to social defeat rats (NAD-SD: 84.63 ± 8.54 sec, SD: 39.79 ± 7.16 sec, t(25)= 3.62, p= 0.0013). Notably, rats treated with nadolol alone spent similar time in lit area as compared to CON rats (NAD: 66.17 ± 4.81 sec; CON: 67.50 ± 7.96 sec, t(28)= 0.82, p= 0.935) (Fig. 2B). However, pretreatment with bisoprolol did not improve the time spent in lit area by BIS-SD rats (BIS-SD: 33.33 ± 10.53 sec; SD: 39.79 ± 7.16 sec, t(26)= 0.51, p= 0.615). Notably, rats treated with bisoprolol alone spent similar time in lit area as compared to CON rats (BIS: 67.50 ± 7.96 sec; CON: 67.50 ± 7.96 sec, t(28)= 0.00, p= 1.000) (Fig. 2C).
In the elevated-plus maze test (EPM), one-way ANOVA following Tukey’s multiple comparison test was performed. Additionally, t test was performed to analyze the statistical difference between each two groups. SD rats spent significantly less time in the open arms of the EPM apparatus when compared to CON rats (SD: 44.50 ± 4.14 sec; CON: 80.50 ± 4.42 sec, t(22)= 5.19, p< 0.0001) (Fig. 2D, E, and F). Less time spent in the open arms during a 5-min session of EPM test is an indicative of high anxiety-like behavior [53]. Interestingly, the time spent in the open arms by Prop-SD rats was significantly greater than SD rats (Prop-SD: 77.63 ± 5.89 sec, SD: 44.50 ± 4.14 sec, t(14)= 4.60, p=0.0004). Rats treated with propranolol alone spent similar time in open arms as compared to CON rats (Prop: 74.13 ± 6.47 sec; CON: 80.50 ± 4.42 sec, t(22)= 0.82, p= 0.419) (Fig. 2D). Similarly, rats in NAD-SD group spent significantly longer time in open arms when compared to SD rats (NAD-SD: 72.13 ± 4.58 sec, SD: 44.50 ± 4.14 sec, t(14)= 4.47, p=0.0005). Rats treated with nadolol alone spent similar time in open arms as compared to CON rats (NAD: 75.00 ± 6.37 sec; CON: 80.50 ± 4.42 sec, t(22)= 0.71, p= 0.4839) (Fig. 2E). However, pretreatment with bisoprolol did not improve the time spent in open arms by BIS-SD rats (BIS-SD: 40.25 ± 4.41 sec; SD: 44.50 ± 4.14 sec, t(14)= 0.71, p= 0.494). Notably, rats treated with bisoprolol alone spent similar time in open arms as compared to CON rats (BIS: 69.13 ± 4.63 sec; CON: 80.50 ± 4.42 sec, t(22)= 1.600, p= 0.1227) (Fig. 2F).
3.3. Analysis of depression-like behavior:
In the forced swim test (FST), one-way ANOVA following Tukey’s multiple comparison test was performed. Additionally, t test was performed to analyze the statistical difference between each two groups. SD rats spent significantly longer time being immobile when compared to CON rats (SD: 93.80 ± 12.25 sec; CON: 53.17 ± 9.73 sec, t(25)= 2.50, p= 0.0193) (Fig. 3A, B, C), a sign of increased depression like behavior [53]. Interestingly, immobility time exhibited by Prop-SD rats was significantly less than SD rats (Prop-SD: 51.44 ± 13.09 sec; SD: 93.80 ± 12.25 sec; t(29)= 2.36, p= 0.0255), suggesting that pretreatment with propranolol protected against depression-like behavior induced by SD. Notably, rats treated with propranolol alone spent longer time being immobile in FST as compared to CON rats, however, the difference was insignificant (Prop: 78.88 ± 4.21 sec; CON: 53.17 ± 9.73 sec, t(18)= 2.06, p= 0.054) (Fig. 3A). Similarly, rats in NAD-SD group, spent significantly less time being immobile as compared to SD rats (NAD-SD: 29.89 ± 5.86 sec; SD: 93.80 ± 12.25 sec, t(22)= 3.855, p= 0.0009), suggesting that pretreatment with nadolol protected the rats from the detrimental effect of social defeat on depression-like behavior. Remarkably, CON rats and NAD rats spent comparable time being immobile in FST test (NAD: 72.25 ± 8.05 sec; CON: 53.17 ± 9.73 sec, t(18)= 1.40, p= 0.179) (Fig. 3B). On the other hand, rats in BIS-SD group and SD group spent similar time being immobile (BIS-SD: 95.89 ± 11.71 sec; SD: 93.80 ± 12.25 sec; t(22)= 0.114, p= 0.910), suggesting that bisoprolol did not protect the rats from the detrimental effect of social defeat in forced swim test (Fig. 3C).
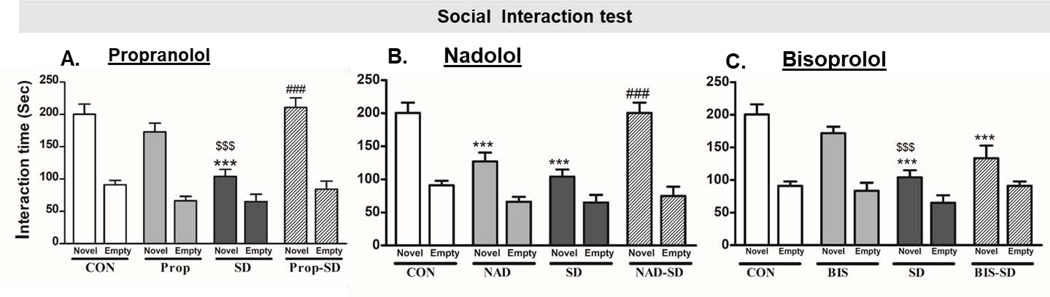
3.4. Analysis of social interaction:
In social interaction test, two-way ANOVA was used to compare between each two groups. While t-test was used to analyze the difference between the interaction time with empty cup versus the interaction time with novel rat in the same group. The CON rats spent (91.14 ± 6.68 sec) interacting with the empty cup and (200.28 ± 15.59 sec) interacting with the novel rat (p< 0.0001). While SD rats spent (65.00 ± 11.53 sec) interacting with the empty cup and (104.12 ± 10.80 sec) interacting with the novel rat (p< 0.05) (Fig. 4A, B, and C). The preference for interaction with the novel rat rather than the empty cup was significantly less in SD rats, when compared to CON rats (F(1,13)= 34.61, p<0.0001). The results suggest that SD affected the high sociability behavior in rats. Interestingly, the preference for interaction with the novel rat (210.88 ± 14.90 sec) rather than the empty cup (84.25 ±12.48, p<0.0001) was significantly greater in Prop-SD (210.88 ± 14.9 0 sec) as compared to SD rats (104.12 ± 10.80 sec, F(1,13)= 34.86, p<0.05). Rats treated with propranolol showed significant preference to interact with novel rat (173.00 ± 13.47 sec) rather than the empty cup (66.21 ± 7.02) (p<0.0001). (Fig. 4A). Similarly, rats in NAD-SD group showed greater preference to interact with novel rat (200.30 ± 15.59 sec) rather than the empty cup (74.71 ±14.13, p<0.0001) when compared to SD rats (104.1 ± 10.80 sec, F(1,13)= 11.97, p= 0.004). ). Rats treated with nadolol alone showed significant preference to interact with novel rat (127.00 ± 13.35 sec) rather than the empty cup (66.14 ± 7.55) (p<0.01) (Fig. 4B). Unexpectedly, nadolol treated rats showed less preference to interact with novel rat (127.00 ± 13.35 sec) when compared to CON rats (200.28 ± 15.59 sec, p<0.001) (Fig. 4B). On the other hand, the preference of BIS-SD treated rats to interact with novel rat (133.43 ± 19.40 sec) rather than the empty cup (91.14 ± 6.68 sec, p<0.05), were comparable to the SD group (interacting with novel rat= 104.12 ± 10.80 sec, F(1,13)= 3.56, p> 0.05) (Fig. 4C). Though, rats treated with bisoprolol showed significant preference to interact with novel rat (171.7 ± 10.08 sec) rather than the empty cup (83.43 ± 12.43 sec, p<0.00). The data suggests that pretreatment with the non-selective β-blockers; propranolol and nadolol, protected the rats from social defeat associated social interaction deficits.
Fig. 4: Examination of social interaction behavior using the three-compartment paradigm test.
Bars represent the time the rats spent interacting with the novel rat or with the empty cup in seconds (sec) ± SEM. Group designations: rats not subjected to social defeat (CON: control); rats subjected to social defeat (SD); rats treated with propranolol alone (Prop), rats treated with propranolol and then subjected to SD (Prop-SD), rats treated with nadolol (NAD), rats treated with nadolol and then subjected to SD (NAD-SD), rats treated with bisoprolol (BIS), rats treated with bisoprolol and then subjected to SD (BIS-SD). (*) Indicates significantly different from CON, P<0.05. (#) Indicates significantly different than from SD, P<0.05. ($) Indicates significantly different from Prop, NAD or BIS, P<0.05. Data were analyzed using two-way ANOVA test. Bars represent means ± SEM, n =8–12 rats/group.
3.5. Analysis of learning-memory function:
Short-term memory (STM) and long-term memory (LTM) function tests were conducted in the RAWM apparatus. For statistical analysis, one-way ANOVA followed by Tukey’s multiple comparison test was performed. Furthermore, t-test were performed to analyze the statistical difference between each two groups. In STM test, rats in SD group made more errors as compared CON rats (CON:1.87 ± 0.29; SD: 5.00 ± 0.57, t(14)= 4.89, p=0.0002) (Fig. 5A, B, C), suggesting that exposure to social defeat altered STM. However, treatment with beta-blockers did not affect the SD-induced short-term memory impairment. For example, rats treated with propranolol before SD sessions (Prop-SD) made comparable errors in STM as compared to SD rats that did not receive treatment (Prop-SD: 4.37 ± 0.59, SD: 5.00 ± 0.57, t(14)=0.76, p=0.4). Notably, rats treated with propranolol alone made similar errors as CON group (CON:1.87 ± 0.29; Prop: 2.25± 0.41, t(14)= 0.74, p= 0.47) (Fig. 5A). Similarly, rats treated with NAD followed by SD made comparable errors as compared to SD rats (NAD-SD: 5.00 ± 0.46, SD: 5.00 ± 0.57, t(14)= 0.00, p= 1.00). Remarkably, rats treated with nadolol alone made similar errors as CON group (CON:1.87 ± 0.29; NAD: 2.25± 0.41, t(14)= 0.074, p= 0.471) (Fig. 5B). Rats treated with BIS followed by SD made comparable errors as compared to SD rats (BIS-SD: 4.00 ± 0.82, SD: 5.00 ± 0.57,t(14)= 1.00, p= 0.334). While rats treated with bisoprolol alone (without SD) made similar errors as CON rats (CON:1.87 ± 0.29; BIS: 2.12± 0.29, t(14)=0.59, p= 0.558) (Fig. 5C).
In long-term memory (LTM) test, rats in SD group made more errors as compared CON rats (CON:1.00 ± 0.50; SD: 4.88 ± 0.88, t(14)= 3.84, p= 0.0018) (Fig. 5D, E, F) suggesting that exposure to social defeat altered LTM. However, treatment with beta-blockers did not affect the SD-induced long-term memory impairment. For example, rats treated with propranolol before SD sessions (Prop-SD) made comparable errors in LTM as compared to SD rats that did not receive treatment (Prop-SD: 4.00 ± 0.76, SD: 4.88 ± 0.88, t(14)= 0.76, p= 0.462). Notably, rats treated with propranolol alone made similar errors as CON group (CON:1.00 ± 0.50; Prop: 2.67± 0.67, t(12)= 0.205, p= 0.0635) (Fig. 5D). Similarly, rats treated with NAD followed by SD made comparable errors as compared to SD rats (NAD-SD: 3.88 ± 0.85, SD: 4.88 ± 0.88, t(14)= 0.82, p= 0.527). Remarkably, rats treated with nadolol alone made similar errors as CON group (CON:1.00 ± 0.50; NAD: 2.40± 0.67, t(11)= 1.77, p= 1.105) (Fig. 5E). Rats treated with BIS followed by SD made comparable errors as compared to SD rats (BIS-SD:4.25 ± 0.59, SD: 4.88 ± 0.88, t(14)= 0.59, p= 0.563). While rats treated with bisoprolol alone (without SD) made similar errors as control rats in LTM test (CON:1.00 ± 0.50; BIS: 2.17± 0.31, t(12)= 1.82, p= 0.0931) (Fig. 5F). The memory function tests data suggested that social defeat caused STM and LTM deficits in rats, however, pretreatment with bet-blockers did not protect rats from SD-induced memory impairment.
4. Discussion
β-adrenergic receptor antagonists or ‘β-blockers’ are competitive pharmacologic inhibitors of catecholamine actions. The first-generation β-blockers, such as propranolol and nadolol, produce equal blockade of β1 and β2 adrenergic receptors. The second-generation agents exhibit higher affinity for β1 receptors and are thus termed selective β1-blockers. The extent of selectivity of these agents, including metoprolol, bisoprolol, and atenolol, varies [54]. However, the selectivity of β-blockers varies by compounds, with bisoprolol having the highest selectivity for β1ARs among the clinically available β1-AR antagonists [55]. Aside from their receptor selectivity, β-blockers also vary in their lipophilicity and ability to cross the blood-brain barrier (BBB). In the 3 compounds we used, propranolol has high BBB penetration, nadolol has very low BBB penetration, and bisoprolol has intermediate ability to cross the BBB.
We observed an attenuation of social defeat-induced anxiety-like behavior with propranolol and nadolol treatment, but not with bisoprolol. The finding that social defeat increases anxiety-like behavior is consistent with previous studies conducted in both mice and rats [29, 36, 56–58]. The data suggests that the anxiety-like behavior-mitigating effect was only produced by the non-selective β1-AR and β2-AR antagonists, propranolol and nadolol. The failure of bisoprolol, a highly preferential β1-AR antagonist, to attenuate the social defeat-induced anxiety suggest a potential role for β2-ARs in mediating the anxiety response. The fact that propranolol treatment mitigated social defeat-induced anxiety-like behavior also is in agreement with a previous report in which stress-induced anxiety-like behavior and c-Fos activation were prevented by propranolol pretreatment in a social defeat model in mice [59], although propranolol treatment and social defeat protocols were different from the present study. Similarly, propranolol and nadolol but not bisoprolol treatment mitigated social defeat-induced depression-like behavior as well as social interaction in rats, again suggesting a potential role for β2-ARs in mediating the anxiety response. Stress-induced depression-like behavior has been reported to be reversed by beta-blockers in several rodent studies [60] and several lines of evidence suggest the involvement of β-blockers in the pathophysiology of depression [61]. Additionally, rats treated with propranolol alone exhibited insignificant increase in immobility time in FST as compared to CON rats. This observation may be attributed perhaps to propranolol’s high lipophilic characteristics, and greater blood-brain barrier permeability. Prolonged treatment with propranolol (36 days) by decreasing noradrenaline release, might cause fatigue, which is potentially reflected by slight increase in immobility time in this group of rats. Furthermore, rats treated with nadolol alone also showed reduction in time of interaction with novel rat as compared to CON rats, which also might be due to prolonged nadolol treatment on the peripheral β-adrenergic receptors. Therefore, the regimen for propranolol and nadolol treatment might need some adjustment in future studies.
Given their large differences in their lipophilic properties, an unexpected finding in our study is the comparable effectiveness of nadolol and propranolol in attenuating the behavioral impairments. These results would imply a possible role for peripheral (non-CNS) β2-ARs in mediating anxiety and depression like responses. However, this finding remains speculative as it is likely some nadolol crossed the BBB at the doses used. Nevertheless, it would seem unlikely that both drugs achieved comparable blockade on central β2-ARs, yet both were of equal effectiveness.
While anxiety-and depression-like behavior as well as social interaction test results obtained in the present study were intriguing, the learning-memory function analysis conducted in the RAWM test, were quite surprising. The SD rats exhibited significant learning-memory deficits but neither propranolol, nor nadolol or bisoprolol had any mitigating effect on learning-memory function in rats. There are reports suggesting norepinephrine (NE) is a modulator of attention processes [62], especially visuo-spatial attention [63–65]. Therefore, blockade of NE by any of the ‘β-blockers’ could disrupt attention-promoting processes [66]. Interestingly, Prop-SD and NAD-SD rats committed as many if not more errors as SD rats in the RAWM test which measures spatial memory and working memory. This also might imply that working memory which is considered vital for reasoning and decision-making [67] is not modulated by β-blockers and hence may not be protected or improved with β-blocker treatment. Interestingly, propranolol and nadolol treatment without SD do not impair these processes in the STM and LTM tests in the RAWM apparatus, as all groups (CON, SD, Prop, NAD and BIS) made comparable errors. Perhaps, in the absence of stress-induced hyper-responsiveness of adrenergic signaling, β-blockade does not modify adrenergic signaling and therefore does not elicit a behavioral response. It may be hypothesized that prior treatment of propranolol and nadolol prevented recall of social defeat-induced fearful and traumatic memories thus inhibiting expression of anxious and melancholic emotional responses, without affecting declarative and/or spatial/working learning-memory process.
Conclusion
Clinically, in individuals who have already suffered from PTSD, propranolol and/or nadolol may not be as effective in disrupting fearful memory reconsolidation processes, as permanent erasure or weakening of a longstanding fear memory by beta-blockers seems unlikely. However, there is the potential of these drugs to perhaps be administered before exposure of a potentially traumatic event in the high-risk careers of defense forces, airline and other industries. Studies have argued that targeting memory reconsolidation period is unlikely to work considering that only weak and recently formed memories are susceptible to erasure [68–70]. This pilot study was conducted to see if the drugs could meet the ‘lower bar’ of prevention, as many drugs have failed because the pharmacological intervention was started after too much damage had already occurred (for example, this late intervention has been hypothesized a major contributor for the failure of several Alzheimer’s therapies. If these observations hold up under further study, future experiments will test the potential of the drugs as a treatment for established PTSD. Given that beta-blockers are very well studied and safe in the majority of patients, and have no abuse potential, we think it is imperative to examine their preventive therapeutics. Detailed time-courses and neurobehavioral pharmacology studies will address the mechanistic consequences of beta-adrenergic receptor blockade.
Highlights.
Propranolol and nadolol protected SD rats from exhibiting anxiety-or depression-like behaviors.
Bisoprolol treatment did not mitigate SD-induced behavioral impairments in rats.
Nadolol, propranolol or bisoprolol have no effect in attenuating SD-induced memory function tests.
Certain ‘β-blockers’ have the potential to mitigate the negative psychological effects of traumatic events.
Acknowledgement
Funding for this research was provided by NIH grants awarded to Samina Salim (2R15MH09391802) and Richard Bond (1R01AI110007).
Abbreviations:
- PTSD
post-traumatic stress disorder
- SD
social defeat
- LD
light and dark test
- EPM
elevated plus maze test
- STM
short term memory
- LTM
long term memory
- FST
forced swim test
Footnotes
Disclosure of interest
The authors report no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Owens GP, et al. , Review of assessment and treatment of PTSD among elderly American armed forces veterans. International Journal of Geriatric Psychiatry: A journal of the psychiatry of late life and allied sciences, 2005. 20(12): p. 1118–1130. [DOI] [PubMed] [Google Scholar]
- 2.Reeves RR, Parker JD, and Konkle-Parker DJ, War-related mental health problems of today’s veterans: new clinical awareness. Journal of Psychosocial Nursing and Mental Health Services, 2016. 43(7): p. 18–28. [DOI] [PubMed] [Google Scholar]
- 3.Yehuda R, Current status of cortisol findings in post-traumatic stress disorder. Psychiatric Clinics of North America, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Baldwin DS, et al. , Evidence-based pharmacological treatment of anxiety disorders, post-traumatic stress disorder and obsessive-compulsive disorder: a revision of the 2005 guidelines from the British Association for Psychopharmacology. Journal of Psychopharmacology, 2014. 28(5): p. 403–439. [DOI] [PubMed] [Google Scholar]
- 5.Berger W, et al. , Pharmacologic alternatives to antidepressants in posttraumatic stress disorder: a systematic review. Progress in neuro-psychopharmacology and biological psychiatry, 2009. 33(2): p. 169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brunet A, et al. , Effect of post-retrieval propranolol on psychophysiologic responding during subsequent script-driven traumatic imagery in post-traumatic stress disorder. Journal of psychiatric research, 2008. 42(6): p. 503–506. [DOI] [PubMed] [Google Scholar]
- 7.Brantigan CO, Brantigan TA, and Joseph N, Effect of beta blockade and beta stimulation on stage fright. The American journal of medicine, 1982. 72(1): p. 88–94. [DOI] [PubMed] [Google Scholar]
- 8.Faigel HC, The effect of beta blockade on stress-induced cognitive dysfunction in adolescents. Clinical pediatrics, 1991. 30(7): p. 441–445. [DOI] [PubMed] [Google Scholar]
- 9.Grillon C, et al. , Effects of the beta-blocker propranolol on cued and contextual fear conditioning in humans. Psychopharmacology, 2004. 175(3): p. 342–352. [DOI] [PubMed] [Google Scholar]
- 10.Fagerström O, Hugdahl K, and Lundström N, Effect of beta-receptor blockade on anxiety with reference to the three-systems model of phobic behavior. Neuropsychobiology, 1985. 13(4): p. 187–193. [DOI] [PubMed] [Google Scholar]
- 11.Giustino TF, Fitzgerald PJ, and Maren S, Revisiting propranolol and PTSD: memory erasure or extinction enhancement? Neurobiology of learning and memory, 2016. 130: p. 26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muravieva EV and Alberini CM, Limited efficacy of propranolol on the reconsolidation of fear memories. Learning & Memory, 2010. 17(6): p. 306–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noyes R Jr, Beta-blocking drugs and anxiety. Psychosomatics, 1982. 23(2): p. 155–170. [DOI] [PubMed] [Google Scholar]
- 14.Becker A, Oxprenolol and propranolol in anxiety states. A double-blind comparative study. South African medical journal= Suid-Afrikaanse tydskrif vir geneeskunde, 1976. 50(16): p. 627–629. [PubMed] [Google Scholar]
- 15.Kathol RG, et al. , Propranolol in chronic anxiety disorders: A controlled study. Archives of General Psychiatry, 1980. 37(12): p. 1361–1365. [DOI] [PubMed] [Google Scholar]
- 16.Meibach R, Mullane J, and Binstok G, A placebo-controlled multicenter trial of propranolol and chlordiazepoxide in the treatment of anxiety. Current therapeutic research, 1987. 41(1): p. 65–76. [Google Scholar]
- 17.Wheatley D, Comparative effects of propranolol and chlordiazepoxide in anxiety states. The British Journal of Psychiatry, 1969. 115(529): p. 1411–1412. [DOI] [PubMed] [Google Scholar]
- 18.Turner P, Granville-Grossman K, and Smart J, Effect of adrenergic receptor blockade on the tachycardia of thyrotoxicosis and anxiety state. The Lancet, 1965. 286(7426): p. 1316–1318. [DOI] [PubMed] [Google Scholar]
- 19.Yorkston N, et al. , Propranolol in the control of schizophrenic symptoms. Br Med J, 1974. 4(5945): p. 633–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ratey JJ, et al. , Brief report: open trial effects of beta-blockers on speech and social behaviors in 8 autistic adults. Journal of Autism and Developmental Disorders, 1987. 17(3): p. 439–446. [DOI] [PubMed] [Google Scholar]
- 21.Fleminger S, Greenwood RR, and Oliver DL, Pharmacological management for agitation and aggression in people with acquired brain injury. Cochrane Database of Systematic Reviews, 2003(1). [DOI] [PubMed] [Google Scholar]
- 22.Brewer C, Beneficial effect of beta-adrenergic blockade on” exam. nerves”. The Lancet, 1972. 300(7774): p. 435. [DOI] [PubMed] [Google Scholar]
- 23.Drew P, Barnes J, and Evans S, The effect of acute beta-adrenoceptor blockade on examination performance. British journal of clinical pharmacology, 1985. 19(6): p. 783–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clark DB and Agras WS, The assessment and treatment of performance anxiety in musicians. The American journal of psychiatry, 1991. 148(5): p. 598. [DOI] [PubMed] [Google Scholar]
- 25.Elman MJ, et al. , The effect of propranolol versus placebo on resident surgical performance. Transactions of the American Ophthalmological Society, 1998. 96: p. 283. [PMC free article] [PubMed] [Google Scholar]
- 26.Dyck J. and Chung F, A comparison of propranolol and diazepam for preoperative anxiolysis. Canadian journal of anaesthesia, 1991. 38(6): p. 704–709. [DOI] [PubMed] [Google Scholar]
- 27.Patki G, et al. , Depression, anxiety-like behavior and memory impairment are associated with increased oxidative stress and inflammation in a rat model of social stress. Brain Res, 2013. 1539: p. 73–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patki G, et al. , Novel mechanistic insights into treadmill exercise based rescue of social defeat-induced anxiety-like behavior and memory impairment in rats. Physiol Behav, 2014. 130: p. 135–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Solanki N, et al. , Modulating Oxidative Stress Relieves Stress-Induced Behavioral and Cognitive Impairments in Rats. Int J Neuropsychopharmacol, 2017. 20(7): p. 550–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hollis F. and Kabbaj M, Social defeat as an animal model for depression. ILAR journal, 2014. 55(2): p. 221–232. [DOI] [PubMed] [Google Scholar]
- 31.Koolhaas J, et al. , The temporal dynamics of the stress response. Neuroscience & Biobehavioral Reviews, 1997. 21(6): p. 775–782. [DOI] [PubMed] [Google Scholar]
- 32.Meerlo P, et al. , Changes in behaviour and body weight following a single or double social defeat in rats. Stress, 1996. 1(1): p. 21–32. [DOI] [PubMed] [Google Scholar]
- 33.Tidey JW and Miczek KA, Acquisition of cocaine self-administration after social stress: role of accumbens dopamine. Psychopharmacology, 1997. 130(3): p. 203–212. [DOI] [PubMed] [Google Scholar]
- 34.McAinsh J. and Cruickshank JM, Beta-blockers and central nervous system side effects. Pharmacology & therapeutics, 1990. 46(2): p. 163–197. [DOI] [PubMed] [Google Scholar]
- 35.van Stegeren AH, et al. , Memory for emotional events: differential effects of centrally versus peripherally acting β-blocking agents. Psychopharmacology, 1998. 138(3–4): p. 305–310. [DOI] [PubMed] [Google Scholar]
- 36.Patki G, et al. , Moderate treadmill exercise rescues anxiety and depression-like behavior as well as memory impairment in a rat model of posttraumatic stress disorder. Physiology & behavior, 2014. 130: p. 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patki G, et al. , Witnessing traumatic events and post-traumatic stress disorder: Insights from an animal model. Neurosci Lett, 2015. 600: p. 28–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dreyfuss J, Shaw JM, and Ross JJ, Absorption of the β-adrenergic-blocking agent, nadolol, by mice, rats, hamsters, rabbits, dogs, monkeys, and man: An unusual species difference. Xenobiotica, 1978. 8(8): p. 503–508. [DOI] [PubMed] [Google Scholar]
- 39.Greenblatt DJ, et al. , Cognitive effects of β-adrenergic antagonists after single doses: Pharmacokinetics and pharmacodynamics of propranolol, atenolol, lorazepam, and placebo. Clinical Pharmacology & Therapeutics, 1993. 53(5): p. 577–584. [DOI] [PubMed] [Google Scholar]
- 40.Laser A, et al. , Long-term beta-blocker treatment prevents chronic creatine kinase and lactate dehydrogenase system changes in rat hearts after myocardial infarction. Journal of the American College of Cardiology, 1996. 27(2): p. 487–493. [DOI] [PubMed] [Google Scholar]
- 41.Sibley P, et al. , Preclinical toxicologic evaluation of nadolol, a new β-adrenergic antagonist. Toxicology and applied pharmacology, 1978. 44(2): p. 379–389. [DOI] [PubMed] [Google Scholar]
- 42.Callaerts-Vegh Z, et al. , Effects of acute and chronic administration of beta-adrenoceptor ligands on airway function in a murine model of asthma. Proc Natl Acad Sci U S A, 2004. 101(14): p. 4948–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thanawala VJ, et al. , beta-Blockers have differential effects on the murine asthma phenotype. Br J Pharmacol, 2015. 172(20): p. 4833–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin R, et al. , Changes in beta 2-adrenoceptor and other signaling proteins produced by chronic administration of ‘beta-blockers’ in a murine asthma model. Pulm Pharmacol Ther, 2008. 21(1): p. 115–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nguyen LP, et al. , Chronic exposure to beta-blockers attenuates inflammation and mucin content in a murine asthma model. Am J Respir Cell Mol Biol, 2008. 38(3): p. 256–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miczek KA, A new test for aggression in rats without aversive stimulation: differential effects of d-amphetamine and cocaine. Psychopharmacology (Berl), 1979. 60(3): p. 253–9. [DOI] [PubMed] [Google Scholar]
- 47.Patki G, et al. , Moderate treadmill exercise rescues anxiety and depression-like behavior as well as memory impairment in a rat model of posttraumatic stress disorder. Physiol Behav, 2014. 130: p. 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wood SK, et al. , Depressive and cardiovascular disease comorbidity in a rat model of social stress: a putative role for corticotropin-releasing factor. Psychopharmacology (Berl), 2012. 222(2): p. 325–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vollert C, et al. , Exercise prevents sleep deprivation-associated anxiety-like behavior in rats: potential role of oxidative stress mechanisms. Behav Brain Res, 2011. 224(2): p. 233–40. [DOI] [PubMed] [Google Scholar]
- 50.Toth I. and Neumann ID, Animal models of social avoidance and social fear. Cell Tissue Res, 2013. 354(1): p. 107–18. [DOI] [PubMed] [Google Scholar]
- 51.Smith CJ, et al. , Social Novelty Investigation in the Juvenile Rat: Modulation by the mu-Opioid System. J Neuroendocrinol, 2015. 27(10): p. 752–64. [DOI] [PubMed] [Google Scholar]
- 52.Eagle AL, Fitzpatrick CJ, and Perrine SA, Single prolonged stress impairs social and object novelty recognition in rats. Behav Brain Res, 2013. 256: p. 591–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu H, et al. , Behavioral and cognitive impact of early life stress: Insights from an animal model. Prog Neuropsychopharmacol Biol Psychiatry, 2017. 78: p. 88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brixius K, et al. , Nebivolol, bucindolol, metoprolol and carvedilol are devoid of intrinsic sympathomimetic activity in human myocardium. British journal of pharmacology, 2001. 133(8): p. 1330–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baker JG, The selectivity of beta-adrenoceptor antagonists at the human beta1, beta2 and beta3 adrenoceptors. Br J Pharmacol, 2005. 144(3): p. 317–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Berton O, et al. , Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science, 2006. 311(5762): p. 864–868. [DOI] [PubMed] [Google Scholar]
- 57.Krishnan V, et al. , Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell, 2007. 131(2): p. 391–404. [DOI] [PubMed] [Google Scholar]
- 58.Kinsey SG, et al. , Repeated social defeat causes increased anxiety-like behavior and alters splenocyte function in C57BL/6 and CD-1 mice. Brain, behavior, and immunity, 2007. 21(4): p. 458–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wohleb ES, et al. , β-Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. Journal of Neuroscience, 2011. 31(17): p. 6277–6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hymel KA, et al. , Stress-induced increases in depression-like and cocaine place-conditioned behaviors are reversed by disruption of memories during reconsolidation. Behavioural pharmacology, 2014. 25(5 and 6): p. 599–608. [DOI] [PubMed] [Google Scholar]
- 61.Pandey SC, et al. , Beta-adrenergic receptor subtypes in stress-induced behavioral depression. Pharmacol Biochem Behav, 1995. 51(2–3): p. 339–44. [DOI] [PubMed] [Google Scholar]
- 62.Mueller D, Porter JT, and Quirk GJ, Noradrenergic signaling in infralimbic cortex increases cell excitability and strengthens memory for fear extinction. Journal of neuroscience, 2008. 28(2): p. 369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Petersen SE and Posner MI, The attention system of the human brain: 20 years after. Annual review of neuroscience, 2012. 35: p. 73–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Posner MI, Orienting of attention. Quarterly journal of experimental psychology, 1980. 32(1): p. 3–25. [DOI] [PubMed] [Google Scholar]
- 65.Reynaud AJ, et al. , Atomoxetine improves attentional orienting in a predictive context. Neuropharmacology, 2019. [DOI] [PubMed] [Google Scholar]
- 66.Gray CL, et al. , Immediate post-defeat infusions of the noradrenergic receptor antagonist propranolol impair the consolidation of conditioned defeat in male Syrian hamsters. Physiol Behav, 2015. 152(Pt A): p. 56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Diamond A, Executive functions. Annual review of psychology, 2013. 64: p. 135–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Milekic MH and Alberini CM, Temporally graded requirement for protein synthesis following memory reactivation. Neuron, 2002. 36(3): p. 521–525. [DOI] [PubMed] [Google Scholar]
- 69.Parsons RG and Ressler KJ, Implications of memory modulation for post-traumatic stress and fear disorders. Nature neuroscience, 2013. 16(2): p. 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Suzuki A, et al. , Memory reconsolidation and extinction have distinct temporal and biochemical signatures. Journal of Neuroscience, 2004. 24(20): p. 4787–4795. [DOI] [PMC free article] [PubMed] [Google Scholar]