Background:
Thyroid tumors are the most frequent neoplasm of the endocrine system. The major treatment is surgical intervention followed by radioiodine therapy. The sodium/iodide symporter (NIS) has positive expression in thyroid carcinomas with good prognoses and plays a critical role in radioiodine therapy response. Low expression of NIS always leads to tumor recurrence or treatment failure. Redifferentiation therapy is more tumor specific than chemotherapy. Peroxisome proliferator–activated receptor gamma (PPARγ) agonists and retinoids are two types of redifferentiating agents. In this study, we examined whether the PPARγ agonist rosiglitazone and retinoid X receptor (RXR) agonist bexarotene could increase NIS expression and exhibit anticancer activity in human thyroid cancer cells.
Methods:
Using a TCGA data set, we analyzed the expression of NIS (SLC5A5), PPARγ, and RXR in clinical thyroid tumors and assessed their correlations with the relapse-free survival (RFS) of thyroid tumor patients. Moreover, two human thyroid cancer cell lines, differentiated thyroid papillary BCPAP cells and follicular follicular thyroid cancer-131 cells, were treated with different concentrations of the PPARγ agonist rosiglitazone alone or in combination with the RXR agonist bexarotene. Cell growth was analyzed by the MTT assay. NIS protein expression was determined by Western blotting.
Results:
From analysis of the TCGA data set, we found that thyroid tumors have lower expression of both NIS (SLC5A5) and PPARγ than nontumor controls. Higher expression levels of NIS, PPARγ, and RXR are associated with higher RFS in patients with thyroid tumors. Moreover, rosiglitazone treatment reduced cell growth and increased NIS protein expression in thyroid cancer cells under normoxic or hypoxic conditions. In addition, bexarotene potentiated the effects of rosiglitazone on cell growth and NIS protein expression.
Conclusion:
Our results suggest that the combination of PPARγ and RXR agonists has potential as a chemotherapeutic strategy for thyroid cancer.
Keywords: Peroxisome proliferator–activated receptor gamma, Retinoid X receptor, Sodium/iodide symporter, Thyroid cancer
1. INTRODUCTION
Most thyroid cancers are highly curable, but the cure rate depends on patient age, tumor size cell type, and disease extent. In particular, an increase in the size of a thyroid nodule raises concerns about its malignancy.1 The first choice of treatment is usually surgery to partially or totally remove the thyroid gland (thyroidectomy). Treatment with radioactive iodine (I-131) after thyroidectomy is recommended for large tumors, and cancer has spread outside the thyroid. There are factors that influence radioactive iodine (RAI) therapy uptake by thyroid cancer, including patient preparation, administered activity, and scanning protocols.2
The majority of thyroid cancers, including papillary thyroid cancer (PTC), follicular thyroid cancer (FTC), and Hürthle cell cancer, are well-differentiated malignancies originating from thyroid follicular cells.3 Thyroid cancer incidence increased, on average, 3.6% per year from 1974 to 2013, and this change was primarily related to increases in papillary thyroid cancer.4 PTC is the most common histologic type, particularly in iodine-sufficient areas. Compared with the long natural history and excellent prognosis of most patients with differentiated thyroid cancer, those with advanced thyroid neoplasms, especially dedifferentiated thyroid cancer, have a poor prognosis even when treated with high-dose radioiodine therapy.3,5
Tumor hypoxia correlates with aggressive disease. Hypoxia has been demonstrated to induce a dedifferentiated, stem cell–like phenotype in neuroblastoma and breast tumor cells.6 In addition, tumor conditions, including hypoxia, acidosis, and a range of treatments, can trigger epithelial–mesenchymal transition (EMT).6 EMT has been connected with differentiation status.7 Although EMT has been described as a differentiation event in development, EMT is indicated as a dedifferentiation event in cancer.7 These changes are caused by the action of several transcription factors, including Snail1 (Snail), Snail2 (Slug), Twist, ZEB1, and ZEB2, which are potent repressors of epithelial gene expression.8 Tumor hypoxia and dedifferentiation might be implicated in the subsequent development of resistance to those same treatments.9
One hallmark of dedifferentiation in thyroid cancer is impairment of sodium/iodide symporter (NIS) function.10 The NIS (SLC5A5) is a key basolateral plasma membrane glycoprotein that mediates active iodide uptake into thyroid follicular cells in the first step of biosynthesis of iodine-containing thyroid hormones. The inactivating mutations in the SLC5A5 gene lead to deficient iodide accumulation into the thyroid follicular cells, and this is an uncommon cause of dyshormonogenetic congenital hypothyroidism.11
Redifferentiation therapy is more tumor specific than chemotherapy and is associated with fewer complications and better quality of life. Some redifferentiating agents have been studied, including retinoids, aromatic fatty acids, histone deacetylase inhibitors, resveratrol, and PPARγ agonists.12,13 The PPARγ agonist rosiglitazone was shown to induce redifferentiation and inhibit proliferation in thyroid cancer cells.14,15 PPARγ forms a heterodimer with RXR that regulates the transcription of various genes, such as those involved in adipogenesis, inflammation, cell cycle control, and apoptosis.16 However, the combined effect of PPARγ and RXR agonists on thyroid cancer cells is still unclear.
2. METHODS
2.1. TCGA RNA sequencing expression data analysis and relapse-free survival analysis
The RNA sequencing expression data were obtained from the TCGA and GTEx projects. The gene expression levels of NIS (SLC5A5), PPARγ, and RXR in normal tissues (gray box) and tumor tissues (red box) from thyroid tumors were analyzed by using a box plot. The box plots were generated by the GEPIA website and software (http://gepia.cancer-pku.cn/).17 |Log2FC| cutoff: 1; p value cutoff: 0.01, T number: 512, N number: 337. The relapse-free survival (RFS) analysis was analyzed by the Kaplan–Meier (KM) plotter website and software (https://kmplot.com/analysis/). The KM plotter online database (http://kmplot.com/analysis/) combined with the GEO (Affymetrix microarrays only), EGA, and TCGA databases are handled by a PostgreSQL server. The patient groups were compared by a KM survival plot, and the hazard ratios with 95% confidence intervals and log rank p values were calculated using online software as described previously.18 In the present study, the expression of the specific genes NIS (SLC5A5), PPARγ, and RXR and the RFS of thyroid cancer patients were evaluated by KM analysis. Patients were classified as having high expression or low expression, with the median risk score as the threshold value.
2.2. Cancer cell lines and cell culture
Human papillary thyroid cancer BCPAP cells were cultured in RPMI 1640 (with l-glutamate) supplemented with fetal bovine serum (10%), penicillin/streptomycin (2%), and amphotericine B (1%). Human follicular thyroid cancer FTC-133 cells were cultured in Dulbecco’s Modified Eagle Medium, Nutrient Mixture F-12 (1:1) supplemented with fetal bovine serum (10%), penicillin/streptomycin (2%), and amphotericine B (1%). To evaluate the effect of hypoxia, cancer cells were incubated in the same conditions but in a hypoxic incubator (Precision Scientific, Winchester, VA) with 1% O2, 5% CO2, and 94% N2.
2.3. Cell growth and viability
For PPARγ agonist treatments, thyroid cancer cells were treated with 5, 10, 20, or 40 μM rosiglitazone (RGZ) (Sigma-Aldrich, St. Louis, MO) for 24, 48, or 72 hours. For RXR agonist treatment, thyroid cancer cells were treated with 10 nM, 100 nM, 1 μM, or 10 μM bexarotene (Sigma-Aldrich) for 24, 48, or 72 hours. Cell growth and viability were determined by the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay.
2.4. Western blot analysis
Whole cell lysate was prepared by resuspending cells in M-PER protein extraction reagent (Thermo Scientific/Pierce, Rockford, IL) according to the manufacturer’s instructions. Cell lysates were centrifuged at 14,000 × g for 10 minutes, and the supernatant was collected. Protein concentration was measured using the Bradford assay (Bio-Rad Laboratories, Hercules, CA). An aliquot of protein lysate (20 μg) from each sample was mixed with 10× Laemmli sample buffer (Bio-Rad Laboratories), and proteins were separated in 10% SDS-polyacrylamide gels. After transferring the proteins to a nitrocellulose membrane, the membrane was blocked with 5% skimmed milk for 30 minutes at room temperature. The proteins were detected with antibodies against short and long forms of hypoxia inducible factor-1α (BD Biosciences, Franklin Lakes, NJ, USA), NIS (Sigma-Aldrich), RXR (Sigma-Aldrich), E-cadherin (Cell Signaling, Danvers, MA, USA), N-cadherin (Cell Signaling) Snail (Cell Signaling), Slug (Abcam, Cambridge, UK), vimentin (Sigma-Aldrich), thyroglobulin (Abcam), and β-actin (Sigma-Aldrich) overnight at 4°C. After three washes, the blots were subsequently incubated with peroxidase-conjugated secondary antibodies (Sigma) for 1.5 hrs at room temperature. The blots were visualized with an enhanced chemiluminescence kit (Pierce) and Amersham Hyperfilm ECL (GE Healthcare).
2.5. Statistical analysis
To compare two groups in the study, Student’s t test was used. To compare multiple groups, analysis of variance was used. Statistical significance was defined as a p < 0.05.
3. RESULTS
3.1. The gene expression levels of NIS, PPARγ, and RXR were lower in thyroid tumors than in nontumor tissues, and their expression was associated with poor RFS in patients with thyroid tumors
In this study, using TCGA data set, we found that the gene expression levels of NIS, PPARγ, and RXR were lower in thyroid tumors than in nontumor tissues, and their expression was associated with poor RFS in patients with thyroid tumors. Moreover, treatment with the PPARγ agonist rosiglitazone reduced cell growth and increased NIS protein expression in two cancer cell lines under normoxic or hypoxic conditions. In addition, the RXR agonist bexarotene potentiated the effects of rosiglitazone on cell growth and NIS protein expression.
To explore the role of NIS, PPARγ, and RXR in the clinic, we analyzed the RNA sequencing data from the TCGA data set and found that the gene expression levels of NIS, PPARγ, and RXR in thyroid tumor samples were lower than those in nontumor tissues, though the difference in RXR expression did not reach statistical significance (Fig. 1A). Moreover, KM survival analysis revealed that lower gene expression levels of these three genes, especially NIS and RXR, in thyroid cancer were correlated with poorer RFS rates in patients with thyroid cancer (Fig. 1B). These data suggest that lower gene expression of these three genes might be associated with higher recurrence rates for human thyroid cancer.
Fig. 1.
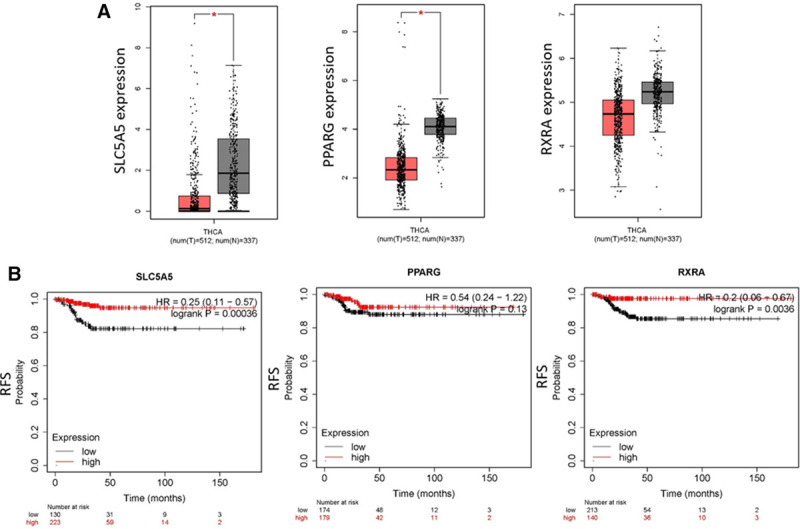
The gene expression levels of sodium/iodide symporter (NIS; SLC5A5), peroxisome proliferator–activated receptor gamma (PPARγ), and retinoid X receptor (RXR) in thyroid tumors and Kaplan–Meier (KM) survival analyses of relapse-free survival (RFS) of patients with thyroid tumors. A, The RNA sequencing expression data were obtained from the TCGA and GTEx projects. The gene expression levels of NIS (SLC5A5), PPARγ, and RXR in normal tissues (gray box) and tumor tissues (red box) from thyroid tumors were analyzed by using a box plot. The box plots were generated by the GEPIA website and software (http://gepia.cancer-pku.cn/). |Log2FC| cutoff: 1; p value cutoff: 0.01, T number: 512, N number: 337. B, The KM RFS was analyzed by the KM plotter website and software (https://kmplot.com/analysis/).
3.2. The PPARγ agonist rosiglitazone inhibited the cell growth of thyroid cancer cells under normoxic or hypoxic conditions
To evaluate the effect of the PPARγ agonist on cell growth, we treated two human thyroid cancer cell lines, BCPAP cells (PTC cells) and FTC-133 cells (FTC cells), with rosiglitazone under normoxic and hypoxic conditions, and cell growth was measured by MTT assay. As shown in Figure 2, rosiglitazone inhibited the cell growth of BCPAP and FTC-133 cells. BCPAP cells were much more sensitive to rosiglitazone than FTC-133 cells under normoxic conditions. However, FTC-133 cells were much more sensitive to rosiglitazone than BCPAP cells under hypoxic conditions. These results indicate that the PPARγ agonist rosiglitazone may inhibit the cell growth of thyroid cancer cells under normoxic and hypoxic conditions.
Fig. 2.
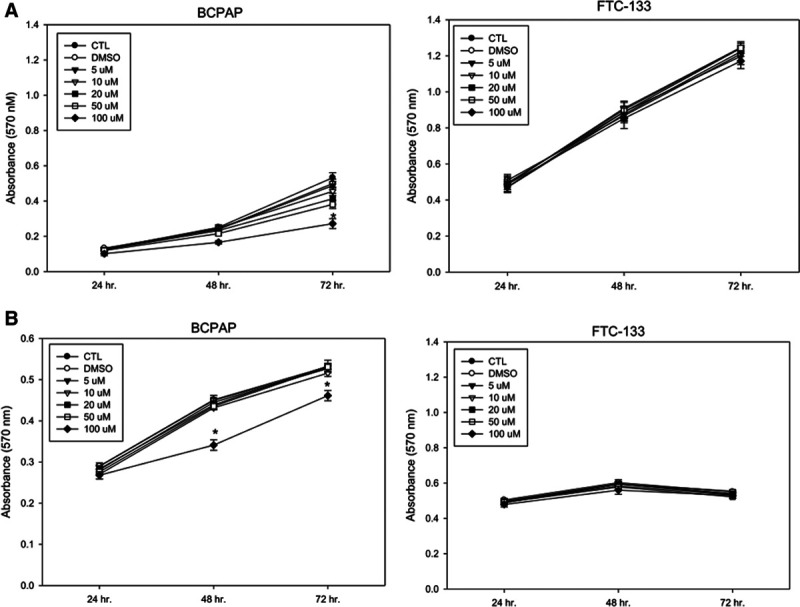
Effect of rosiglitazone on the cell growth of BCPAP and follicular thyroid cancer (FTC)-133 cells. The two thyroid cancer cell lines were treated with different concentrations of rosiglitazone under normoxic (A) or hypoxic (B) conditions for 24, 48, and 72 h. Cell growth was analyzed by the MTT assay. *p < 0.05.
3.3. Rosiglitazone increased NIS expression and repressed EMT-related protein expression in thyroid cancer cells under normoxic or hypoxic conditions
The ability of thyroid follicular epithelial cells to accumulate iodide via NIS has been exploited to successfully treat most thyroid cancers. Lower or deficient expression of NIS always leads to tumor recurrence or treatment failure.16 The dedifferentiation process by which NIS loses its function could involve multiple steps, including a series of genetic mutations or rearrangements. The expression of NIS allows RAI therapy to be a highly effective management strategy for PTC, especially in metastatic PTC patients. Multiple agents have been investigated in attempts to restore NIS expression and to enhance RAI uptake in RAI-refractory PTC patients.18
To examine whether the PPARγ agonist rosiglitazone affects the expression of NIS in thyroid cancer cells, we treated the two thyroid cancer cell lines with 10 or 50 μM rosiglitazone under normoxic and hypoxic conditions for 24 hours. The results revealed that these rosiglitazone treatments might increase the protein expression of NIS in BCPAP and FTC-133 cancer cells under normoxic and hypoxic conditions (Fig. 3A).
Fig. 3.
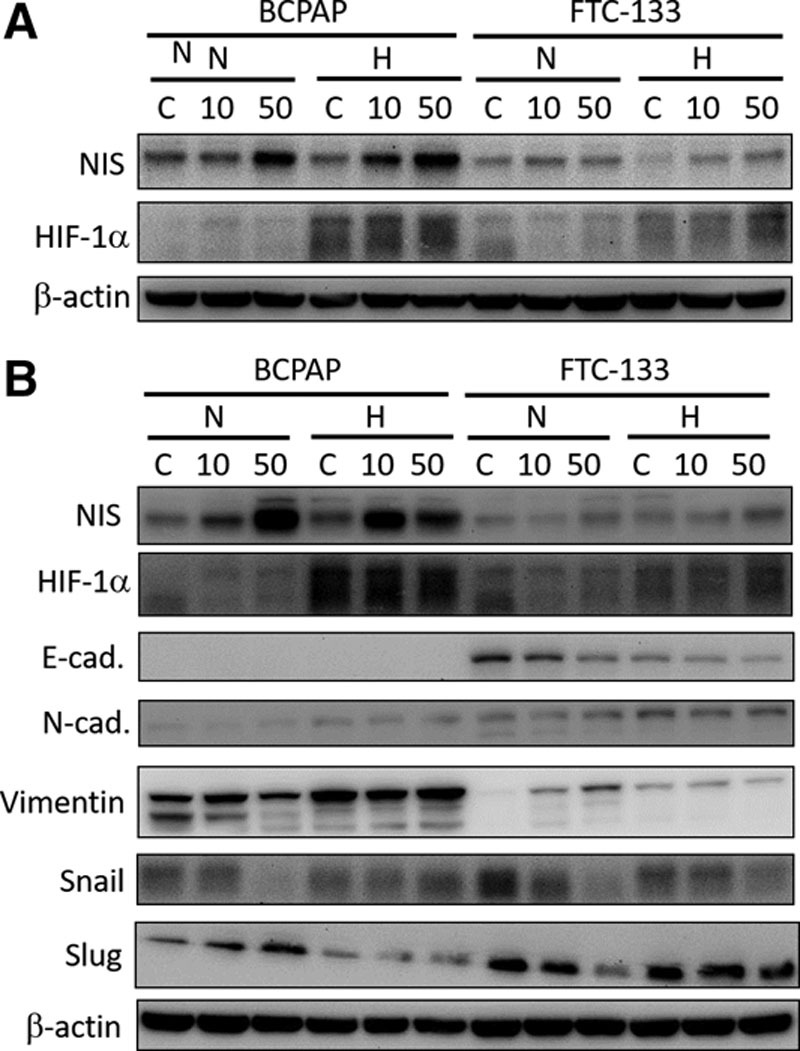
Effect of rosiglitazone treatment on the protein expression of sodium/iodide symporter (NIS) and epithelial–mesenchymal transition -related proteins in BCPAP and follicular thyroid cancer (FTC)-133 cells. A, The two thyroid cancer cell lines were treated with 0, 10, or 50 μM rosiglitazone under normoxic (N) or hypoxic (H) conditions for 24 h. The protein levels were detected by Western blot analysis. B, The two thyroid cancer cell lines were treated with 0, 10, or 50 μM rosiglitazone under N or H conditions for 72 h. The protein levels were detected by Western blot analysis. HIF, hypoxia-inducible factors.
Moreover, we explored whether the PPARγ agonist rosiglitazone affects EMT-related protein expression in thyroid cancer cells and found that Snail expression was reduced in BCPAP cancer cells under normoxic conditions (Fig. 3B). Moreover, the expression of Snail was decreased in FTC-133 cells under normoxic and hypoxic conditions after treatment with 10 or 50 μM rosiglitazone for 72 hours (Fig. 3B). The expression of Slug was decreased in FTC-133 cells under normoxic conditions (Fig. 3B). However, there were no consistent changes in the other EMT-related proteins, such as vimentin, E-cadherin, and N-cadherin, between the thyroid cancer cells (Fig. 3B). These results revealed that the PPARγ agonist rosiglitazone might increase NIS expression and repress Snail or Slug expression, which suggests that the PPARγ agonist can induce redifferentiation of thyroid cancer cells under normoxic or hypoxic conditions.
3.4. Rosiglitazone combined with the RXR agonist bexarotene inhibited the cell growth of thyroid cancer cells
To evaluate the combined effect of rosiglitazone and the RXR agonist, we treated BCPAP and FTC-133 cells with rosiglitazone combined with or without the RXR agonist bexarotene at various concentrations under normoxic and hypoxic conditions for 24–72 hours. Fig. 4 shows the synergetic effect on the growth inhibition of BCPAP and FTC-133 cells after treatment with 10 or 50 μM rosiglitazone combined with 0.1–10 μM bexarotene under hypoxic conditions for 48–72 hours but no synergetic effect under normoxic conditions. FTC-133 cells were much more sensitive to 10 or 50 μM rosiglitazone combined with 0.1–10 μM bexarotene than BCPAP cells under hypoxic conditions for 48–72 hours (Fig. 4). The cell morphology was also observed in BCPAP and FTC-133 cells after treatment with rosiglitazone combined with bexarotene at 72 hours under normoxic or hypoxic conditions. As shown in Figure 5, the cell morphology was round and sparse in treated cells, especially under hypoxic conditions, and this phenomenon further confirmed the results shown in Figure 4. These results suggest that the combination treatments with rosiglitazone and bexarotene might significantly inhibit the cell growth of the two thyroid cancer cell lines, especially under hypoxic conditions.
Fig. 4.
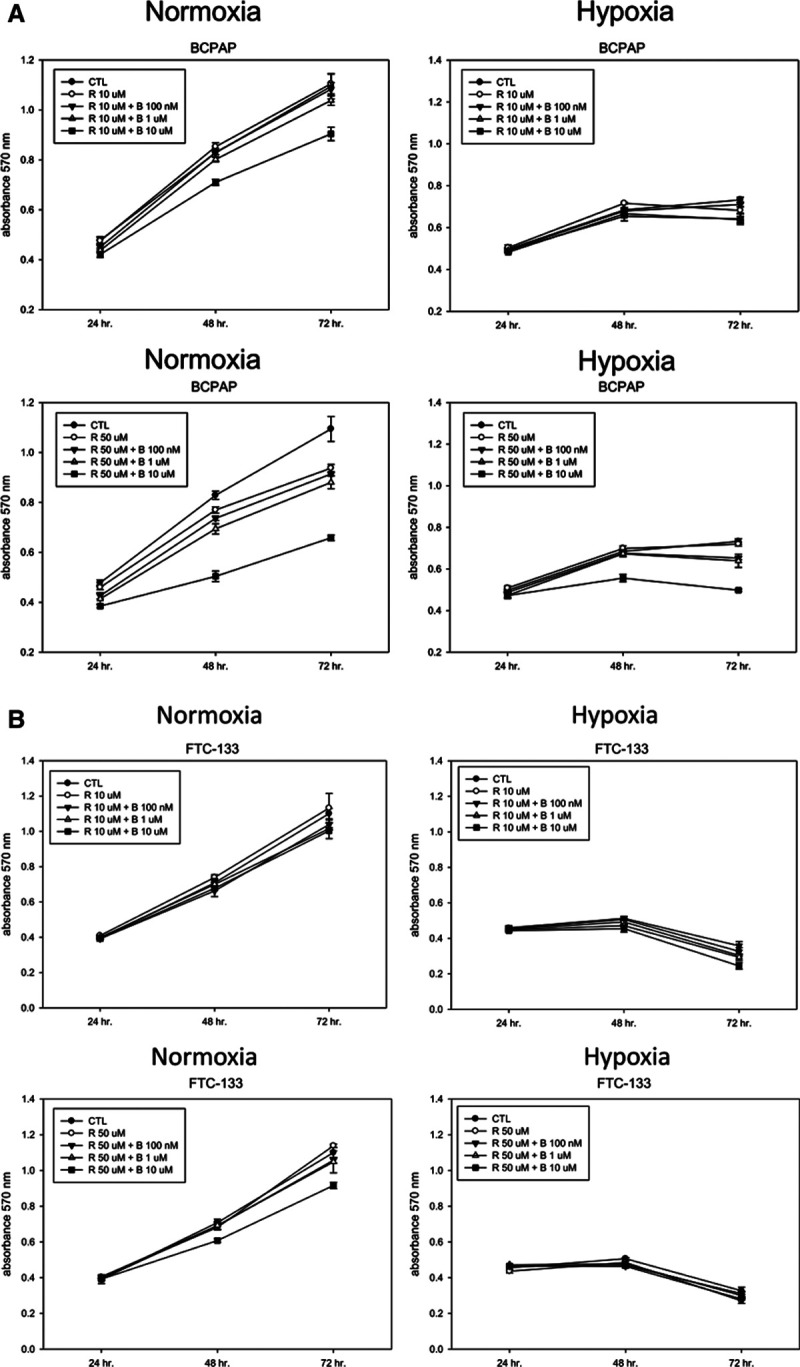
Effect of rosiglitazone (R) alone or combined with bexarotene on the cell growth of BCPAP and follicular thyroid cancer (FTC)-133 cells. A, Thyroid cancer BCPAP cells were treated with 10 μM R alone or in combination with different concentrations of bexarotene (B) under normoxic or hypoxic conditions for 24, 48, and 72 h. Cell growth was analyzed by the MTT assay. *p < 0.05. B, Thyroid cancer FTC-133 cells were treated with 50 μM R alone or in combination with different concentrations of bexarotene (B) under normoxic or hypoxic conditions for 24, 48, and 72 h. Cell growth was analyzed by the MTT assay. *p < 0.05. CTL, control.
Fig. 5.
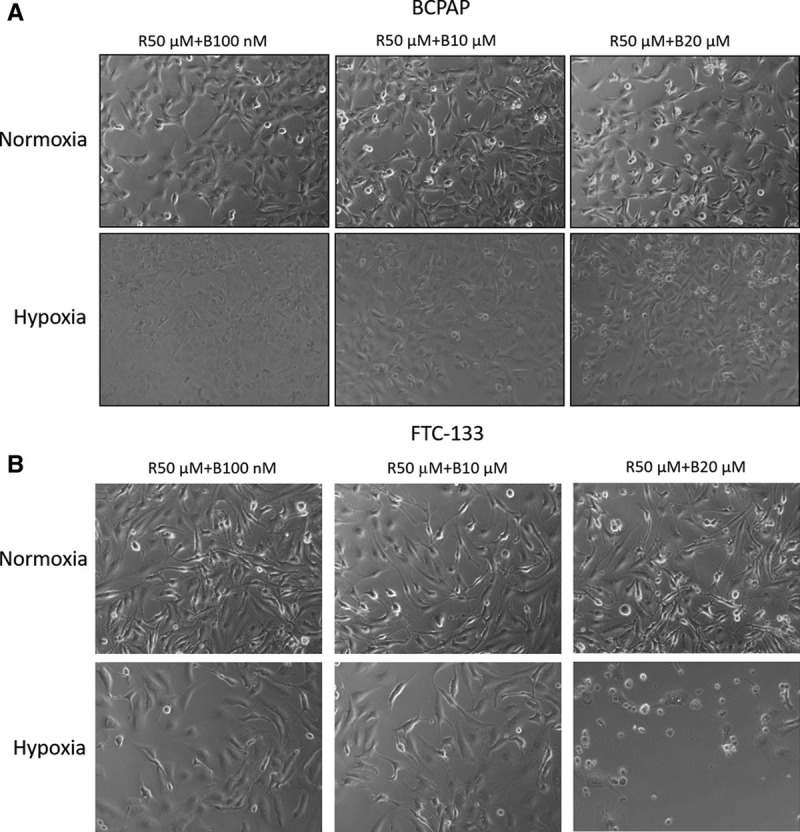
Effects of rosiglitazone alone or combined with bexarotene on the cell morphology of BCPAP and follicular thyroid cancer (FTC)-133 cells. A, Thyroid cancer BCPAP cells were treated with 50 μM rosiglitazone (R) alone or in combination with different concentrations of bexarotene (B) under normoxic or hypoxic conditions for 72 h. The cell morphology was observed. B, Thyroid cancer FTC-133 cells were treated with 50 μM R alone or in combination with different concentrations of bexarotene (B) under normoxic or hypoxic conditions for 72 h. The cell morphology was observed.
3.5. Effects of rosiglitazone combined with bexarotene on the protein expression of NIS and thyroglobulin in thyroid cancer cells under normoxic or hypoxic conditions
To evaluate the effect of rosiglitazone combined with bexarotene on the protein expression of NIS and the other redifferentiation-related protein thyroglobulin, we treated the two cancer cell lines with rosiglitazone with or without bexarotene at different concentrations for 24 hours under normoxic and hypoxic conditions. As shown in Figure 6, we found that the protein expression levels of NIS and thyroglobulin were increased in BCPAP and FTC-133 cells after combination treatments with rosiglitazone and bexarotene under normoxic conditions. However, the increased expression of thyroglobulin was not observed in the two thyroid cancer cell lines under hypoxic conditions. These results suggest that combination treatments with rosiglitazone and bexarotene might upregulate NIS protein expression in the two thyroid cancer cell lines but not thyroglobulin expression.
Fig. 6.
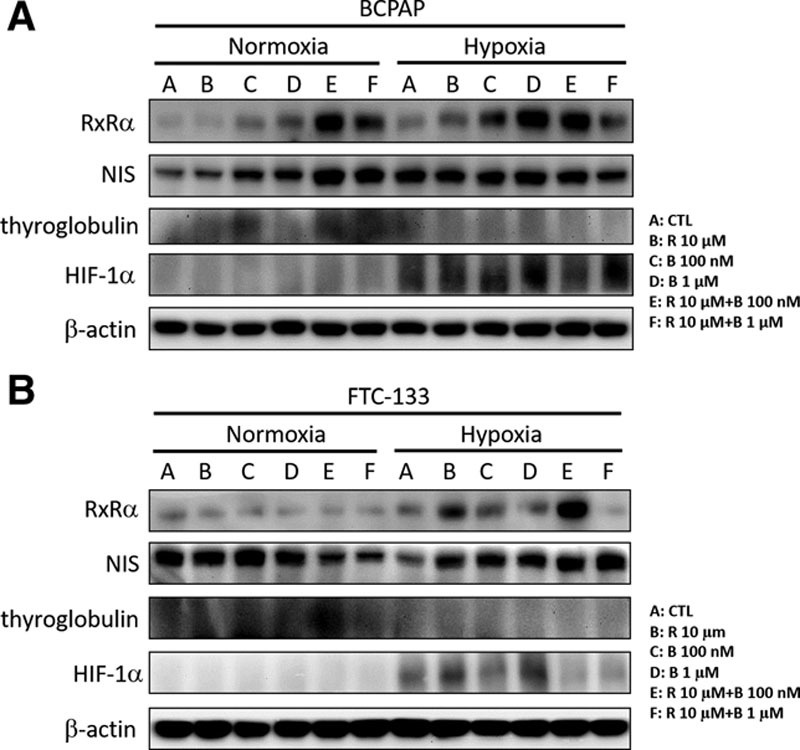
Effects of rosiglitazone alone or combined with bexarotene on the protein expression of sodium/iodide symporter (NIS) and thyroglobulin in BCPAP and follicular thyroid cancer (FTC)-133 cells. A, Thyroid cancer BCPAP cells were treated with 10 μM rosiglitazone (R) alone or in combination with different concentrations of bexarotene (B) under normoxic or hypoxic conditions for 24 h. The protein levels were detected by Western blot analysis. B, Thyroid cancer FTC-133 cells were treated with 10 μM R alone or in combination with different concentrations of bexarotene (B) under normoxic or hypoxic conditions for 24 h. The protein levels were detected by Western blot analysis. CTL, control; HIF, hypoxia-inducible factors.
4. DISCUSSION
In the clinic, after surgery involving total thyroidectomy and ipsilateral lobectomy with or without lymph node dissection, treatment with radioactive iodine is recommended to prevent tumor recurrence.10,19 Differentiation of thyroid cancer influences the effect of radioiodine therapy on thyroid cancers.19 Decreased expression of the NIS or impairment of NIS function is one of the hallmarks of dedifferentiation in thyroid cancer.15 Well-differentiated thyroid cancers express much higher levels of NIS than less differentiated cancers and are more susceptible to radioiodine uptake.20 Moreover, redifferentiation therapy is proposed as a more tumor-specific therapeutic strategy than chemotherapy.10 In the present study, we investigated the effect of redifferentiation agents on NIS expression and assessed their anticancer activity in human thyroid cancer cells.
Our first finding from the analysis using TCGA dataset18 indicated that NIS gene expression is significantly lower in thyroid tumors than in nontumor tissues (Fig. 1). The KM survival analysis revealed that higher NIS expression is correlated with better RFS of patients with thyroid tumors, suggesting that the strategy for upregulating NIS expression in thyroid tumors might improve the therapeutic outcome and reduce the recurrence of thyroid cancer.
To test this hypothesis, we treated two human thyroid cancer cell lines with the PPARγ agonist rosiglitazone alone or combined with the RXR agonist bexarotene and demonstrated that combination treatments with PPARγ and RXR agonists may increase NIS expression and inhibit the growth of thyroid cancer cells. Consistent with a previous report that rosiglitazone induces redifferentiation and inhibits proliferation in anaplastic thyroid cancer cells,13 our results revealed that rosiglitazone inhibits cell growth in FTC-133 cells more than in BCPAP cells under hypoxic conditions (Fig. 2). It was demonstrated in anaplastic thyroid cancer cells that rosiglitazone inhibits anchorage-dependent and anchorage-independent growth, represses cell migration, and increases the apoptosis rate.14 These complex biological effects of rosiglitazone on BCPAP and FTC-133 cells need to be further investigated. Moreover, rosiglitazone-induced growth inhibition was associated with cell cycle arrest and changes in cell cycle regulators, such as an increase in cyclin-dependent kinase inhibitors p21 (cip1) and p27 (kip1), a decrease in cyclin D1, and inactivation of Rb protein.12 Rosiglitazone-induced apoptosis was associated with decreases in Bcl-XL expression and caspase-3 and caspase-7 activation.13 The detailed mechanisms for rosiglitazone-induced growth inhibition or cell death need to be further dissected in different types of thyroid cancer cells.
The EMT mechanism in cancer is involved in the dedifferentiation process.7 Regulation of cell–cell and cell–extracellular matrix interactions is important for development, regeneration, tumor progression, and, particularly, invasion and metastasis.8,9 Reduced cell–cell adhesiveness is associated with loss of contact inhibition of proliferation, thereby allowing escape from growth control signals.8,9 We examined the expression levels of the EMT-related proteins Snail, Slug, and vimentin and the adhesion molecules E-cadherin and N-cadherin in thyroid cancer cells after treatment with rosiglitazone. We observed downregulation of the EMT-related proteins Snail and Slug in rosiglitazone-treated thyroid cancer cells under normoxic and/or hypoxic conditions (Fig. 4), though the expression of the adhesion molecules E-cadherin and N-cadherin was not obviously changed in either treated cell line. These results suggest that downregulation of Snail might be associated with the PPARγ agonist-induced redifferentiation of thyroid cancer cells under normoxic or hypoxic conditions.
PPARγ forms a heterodimer with RXR that regulates the transcription of various genes, such as those involved in cell cycle control and apoptosis.14 We used the PPARγ agonist rosiglitazone and the RXR agonist bexarotene alone or in combination to investigate the growth inhibition of thyroid cancer cells. The present study revealed that bexarotene potentiates the effects of rosiglitazone when administered in combination by inhibiting the growth of thyroid cancer cells under hypoxic conditions but not under normoxic conditions (Fig. 5). These results suggest that rosiglitazone combined with bexarotene induces the growth inhibition of thyroid cancer cells.
In addition, our results clearly demonstrated that rosiglitazone can upregulate the expression of NIS in the two thyroid cancer cell lines under normoxic and hypoxic conditions (Fig. 3). In addition, combination treatments with rosiglitazone and bexarotene can enhance NIS expression in thyroid cancer cells (Fig. 6). It becomes interesting to investigate whether rosiglitazone-activated PPARγ and bexarotene-activated RXR are able to directly interact at the promoter regions of the NIS (SLC5A5) gene and trans-activate gene expression.
We noted that one of the limitations of this study is that the findings were only obtained from experiments in cultured cancer cell lines. We did not generate an animal model of thyroid cancer and did not perform immunohistochemical assays for gene expression in the study. These results should be further verified in animals and validated in an independent cohort.
In conclusion, the present study revealed that bexarotene potentiates the effects of rosiglitazone when administered in combination by inhibiting thyroid cancer cell proliferation under hypoxic conditions or by increasing the expression of NIS and redifferentiation under normoxic conditions. Our findings suggest that the combination treatment of PPARγ agonists and RXR agonists has potential as a chemotherapeutic strategy for thyroid cancer.
ACKNOWLEDGMENTS
This research was supported by the Ministry of Science and Technology (MOST 107-2314-B-008-002) and Taipei Veterans General Hospital research fund (V103C-206). Technical support provided by Ting-Kuei Lee was greatly appreciated.
Footnotes
Conflicts of interest: The authors declare that they have no conflicts of interest related to the subject matter or materials discussed in this article.
REFERENCES
- 1.Yun KJ, Ha J, Kim MH, Seo YY, Kim MK, Kwon HS, et al. Comparison of natural course between thyroid cancer nodules and thyroid benign nodules. Endocrinol Metab (Seoul). 2019;34:195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schneider DF, Chen H. New developments in the diagnosis and treatment of thyroid cancer. CA Cancer J Clin. 2013;63:374–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liao S, Shindo M. Management of well-differentiated thyroid cancer. Otolaryngol Clin North Am. 2012;45:1163–79. [DOI] [PubMed] [Google Scholar]
- 4.Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in thyroid cancer incidence and mortality in the United States, 1974-2013. JAMA. 2017;317:1338–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Durante C, Costante G, Filetti S. Differentiated thyroid carcinoma: defining new paradigms for postoperative management. Endocr Relat Cancer. 2013;20:R141–54. [DOI] [PubMed] [Google Scholar]
- 6.Mohlin S, Wigerup C, Jögi A, Påhlman S. Hypoxia, pseudohypoxia and cellular differentiation. Exp Cell Res. 2017;356:192–6. [DOI] [PubMed] [Google Scholar]
- 7.Wang H, Unternaehrer JJ. Epithelial-mesenchymal transition and cancer stem cells: at the crossroads of differentiation and dedifferentiation. Dev Dyn. 2019;248:10–20. [DOI] [PubMed] [Google Scholar]
- 8.Dongre A, Weinberg RA. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol. 2019;20:69–84. [DOI] [PubMed] [Google Scholar]
- 9.Redfern AD, Spalding LJ, Thompson EW. The Kraken Wakes: induced EMT as a driver of tumour aggression and poor outcome. Clin Exp Metastasis. 2018;35:285–308. [DOI] [PubMed] [Google Scholar]
- 10.Aashiq M, Silverman DA, Na’ara S, Takahashi H, Amit M. Radioiodine-refractory thyroid cancer: molecular basis of redifferentiation therapies, management, and novel therapies. Cancers. 2019;111382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martín M, Bernal Barquero CE, Geysels RC, Papendieck P, Peyret V, Masini-Repiso AM, et al. Novel sodium/iodide symporter compound heterozygous pathogenic variants causing dyshormonogenic congenital hypothyroidism. Thyroid. 2019;29:1023–6. [DOI] [PubMed] [Google Scholar]
- 12.Wong KP, Lang BH. New molecular targeted therapy and redifferentiation therapy for radioiodine-refractory advanced papillary thyroid carcinoma: literature review. J Thyroid Res. 2012;2012:818204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hong CM, Ahn BC. Redifferentiation of radioiodine refractory rifferentiated thyroid cancer for reapplication of I-131 therapy. Front Endocrinol. 2017;8:260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aiello A, Pandini G, Frasca F, Conte E, Murabito A, Sacco A, et al. Peroxisomal proliferator-activated receptor-gamma agonists induce partial reversion of epithelial-mesenchymal transition in anaplastic thyroid cancer cells. Endocrinology. 2006;147:4463–75. [DOI] [PubMed] [Google Scholar]
- 15.Antonelli A, Ferrari SM, Fallahi P, Berti P, Materazzi G, Minuto M, et al. Thiazolidinediones and antiblastics in primary human anaplastic thyroid cancer cells. Clin Endocrinol (Oxf). 2009;70:946–53. [DOI] [PubMed] [Google Scholar]
- 16.Chandra V, Huang P, Hamuro Y, Raghuram S, Wang Y, Burris TP, et al. Structure of the intact PPAR-gamma-RXR- nuclear receptor complex on DNA. Nature. 2008;456:350–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45(W1):W98–W102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagy Á, Lánczky A, Menyhárt O, Győrffy B. Validation of miRNA prognostic power in hepatocellular carcinoma using expression data of independent datasets. Sci Rep. 2018;8:9227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Nostrand D. Radioiodine refractory differentiated thyroid cancer: time to update the classifications. Thyroid. 2018;28:1083–93. [DOI] [PubMed] [Google Scholar]
- 20.Liu J, Liu Y, Lin Y, Liang J. Radioactive iodine-refractory differentiated thyroid cancer and redifferentiation therapy. Endocrinol Metab (Seoul). 2019;34:215–25. [DOI] [PMC free article] [PubMed] [Google Scholar]


