Background:
Neoadjuvant chemoradiotherapy (CRT) followed by an esophagectomy is the standard treatment for locally advanced esophageal cancer, but remains a great challenge for elderly patients. Therefore, we aim to evaluate the efficacy of definitive CRT in elderly patients with esophageal cancer.
Methods:
From December 2007 to October 2017, 40 esophageal cancer patients aged ≥70 years receiving definitive CRT were retrospectively analyzed. All patients received cisplatin-based chemotherapy. Ten patients received standard doses of cisplatin 20 mg/m2 and fluorouracil (5-FU) 800 mg/m2 for 4 days, during the first and fifth weeks of radiotherapy. Eighteen patients received modified doses of cisplatin 16 to 18 mg/m2 and 5-FU 600 to 800 mg/m2. Twelve patients received lower doses of cisplatin 10 to 12 mg/m2 and 5-FU 400 to 600 mg/m2. The endpoints were overall survival (OS), tumor response rate, and treatment compliance.
Results:
The 3-year OS rate was 28.8% The 3-year OS rates for patients receiving standard, modified, and lower doses were 12.5%, 53.8%, and 0.0%, respectively (p = 0.05). There were 87.5% of patients completing the scheduled radiotherapy dose, along with two cycles of concurrent chemotherapy. The response rate (clinical complete response and partial response rate) was 70.0%. Multivariate analysis revealed that no statistical difference was found in the OS among three groups of chemotherapy dosage. The treatment response was the only independent prognostic factor to OS (p < 0.001).
Conclusion:
Definitive CRT with dose modification is a feasible, safe, and reasonable treatment for elderly esophageal cancer patients. Achieving a better compliance to CRT via an optimal dose modification of chemotherapy may provide better clinical outcomes and would be the treatment goal for elderly esophageal cancer patients.
Keywords: Aged, Chemoradiotherapy, Esophageal neoplasms
1. INTRODUCTION
Worldwide, esophageal cancer is the eighth most common form of cancer,1 and the sixth leading cause of cancer-related death.2 Esophagectomy is a mainstay of curative treatment for esophageal cancer. However, primary esophagectomy alone is associated with a high local-regional recurrence rate in locally advanced esophageal cancer patients. Moreover, esophagectomy is also considered a major surgery with potential postoperative morbidity and mortality.3–5 Neoadjuvant concurrent chemoradiotherapy (CCRT) followed by esophagectomy has become the standard treatment due to a better survival rate when compared with esophagectomy alone for patients with potentially operable locally advanced esophageal cancer.6,7 Studies have validated the efficacy of the two commonly used neoadjuvant CCRT regimens: (1) paclitaxel and carboplatin with concurrent radiotherapy (41.4 Gy in 23 fractions, 5 days/wk),7 and (2) cisplatin and fluorouracil (5-FU) with concurrent radiotherapy (50.4 Gy in 28 fractions, 5 days/wk).8
Although neoadjuvant CCRT followed by esophagectomy is the standard treatment for locally advanced esophageal cancer, this trimodality treatment is a clinical challenge particularly for elderly patients due to the risk of post esophagectomy complications and morbidities. There have been studies reporting that esophagectomy in elderly patients may potentially increase the risk of postoperative complications.9–11 Definitive CRT has been the alternative treatment for patients with medically inoperable or unresectable disease. The recommended chemotherapy regimen for definitive CRT is (1) cisplatin and 5-FU12,13 or (2) oxaliplatin and 5-FU.14 Both regimens are administered concurrently with radiotherapy (50 Gy in 25 fractions, 5 days/wk).
There have been few clinical studies analyzing the chemotherapy dose modification of definitive CRT for elderly esophageal cancer patients. The compliance of definitive CRT with standard dose chemotherapy is also a major concern for elderly patients. The main purpose of this retrospective study was to evaluate the efficacy and feasibility of modified definitive CRT in elderly esophageal cancer patients aged ≥70 years.
2. METHODS
2.1. Patients
The inclusion criteria for this retrospective study were as follows: (1) pathology-proven esophageal squamous cell carcinoma (ESCC), (2) age ≥70 years, (3) at stages II, III, IV, (4) adequate performance status with a Karnofsky performance score ≥60% or Eastern Cooperative Oncology Group ≤2, and (5) patients with medically inoperable or unresectable disease. Patients diagnosed with distant metastasis, synchronous double cancer, or previously treated esophageal cancer were excluded. All patients underwent complete pretreatment staging workup, including a comprehensive medical history report, clinical physical examination, esophageal tumor biopsy, complete blood cell count, serologic evaluation of liver and renal functions, chest X-ray, esophagogastroduodenoscopy (EGD), chest computed tomography scan (CT), and fluorodeoxyglucose positron emission tomography/CT (FDG-PET/CT). All patients were staged based upon the American Joint Committee on Cancer (AJCC) 7th edition staging system.
2.2. Chemotherapy
In our institution, chemotherapy involving four cycles of cisplatin 20 mg/m2 and 5-FU 800 mg/m2 for 4 days has been verified as a definitive CRT regimen for patients <70 years of age.15 However, the compliance of standard CRT was suboptimal particularly for elderly patients. To improve the treatment compliance of standard CRT in elderly patients, we introduced chemotherapy dose modifications. Patients aged ≥70 years were stratified into three groups according to their physician’s choice, which was based upon a comprehensive evaluation of the patient’s performance status, liver and renal functions, and comorbidity. All patients in this study received two cycles of concurrent chemotherapy during the first and fifth weeks of radiotherapy, in addition to two cycles at the eighth and eleventh weeks after radiotherapy was completed. Standard dose chemotherapy consists of cisplatin 20 mg/m2 and 5-FU 800 mg/m2 for 4 days in each cycle. Modified dose chemotherapy consists of cisplatin 16 to 18 mg/m2 and 5-FU 600 to 800 mg/m2 for 4 days in each cycle. Lower dose chemotherapy consists of cisplatin 10 to 12 mg/m2 and 5-FU 400 to 600 mg/m2 for 4 days in each cycle.
2.3. Radiotherapy
All patients underwent CT simulation in a supine position with their arms placed above their heads. A customized vacuum bag was used for immobilization. The CT images were taken at a 5-mm thickness from the neck to the thorax for upper and middle thoracic tumors or to thorax and abdomen for lower thoracic tumors. The gross tumor volume (GTV), clinical target volume (CTV), planning target volume (PTV), and organs at risk (OARs) were delineated on the CT simulation images. An EGD and chest CT scan were obtained to localize the esophageal tumor and metastatic lymph nodes prior to CCRT. The GTV was defined as the gross tumor of the esophagus and positive lymph nodes based upon chest CT or FDG-PET/CT scans. The CTV was delineated from the GTV plus a margin of 0.5 to 1 cm radially, a 5-cm margin cephalically and caudally, and covered the mediastinal and supraclavicular lymph nodes for upper or middle thoracic tumors, or the celiac trunk lymph nodes for lower thoracic tumors. The PTV was defined as CTV plus a margin of 5 mm to cover the daily setup error and internal organ motion. All radiotherapy plans were performed with the intensity-modulated radiotherapy (IMRT) technique, using a dynamic multi-leaf linear accelerator and 6-MV photon energy (Varian 2100EX with a 120-leaf Millennium multileaf collimator; Varian Oncology Systems, Palo Alto, CA, USA). The Eclipse planning system (versions 6.5 to 7.2.24; Varian Medical Systems Inc.) was used for treatment planning. A total dose of 50 to 50.4 Gy was prescribed to the PTV. A 95% PTV volume should covered by a 100% prescribed dose. The dose constraints for an organ at risk in our institution for esophageal CCRT are maximum dose for the spinal cord <45 Gy, the whole lung V20 (volume receiving >20 Gy) <20%, the mean lung dose <20 Gy; the V30 for the heart <30%, the mean heart dose <30 Gy; the mean liver dose <30 Gy; the mean dose for each kidney <18 Gy; and the maximum dose for the stomach <50 Gy.
2.4. Toxicity, treatment compliance, and tumor response assessment
Throughout the treatment course, acute adverse events including hematologic toxicity and non-hematologic toxicity were evaluated and recorded. Adverse events were graded according to the Common Terminology Criteria for Adverse Events (CTCAE) v3.0. The patient’s body weight was recorded for purpose of nutritional status evaluation every week during treatment. Treatment compliance was evaluated separately with regard to radiotherapy and chemotherapy. Chemotherapy compliance was defined as a patient receiving at least four cycles of chemotherapy, while radiotherapy compliance was defined as completion of total dose within a 5% deviation of the scheduled dose. CCRT compliance was defined as completion of a scheduled radiotherapy dose with at least two cycles of concurrent chemotherapy. All patients received a treatment response evaluation at the second month after completing the definitive CRT by an EGD and chest CT scan. A post-treatment FDG-PET/CT scan was optional and not routinely arranged. Tumor response was retrospectively reviewed and graded according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. Patients who did not respond to definitive CRT would receive subsequent systemic therapy or a best supportive care according to individual performance status.
2.5. Statistical analysis
All patient outcomes were retrospectively reviewed in July 2019. The endpoints were OS, tumor response rate, and treatment compliance. The OS was calculated from the date of diagnosis to the date of death from any cause, or the date of last follow-up. Survival times were estimated using the Kaplan-Meier method, and the log-rank test was implemented for comparison between the groups. A Cox regression model was used for multivariate analysis. Statistical analyses were performed using SPSS software, version 23. A p value <0.05 was considered statistically significant. The study was approved by the Institutional Review Board of Taichung Veterans General Hospital.
3. RESULTS
From December 2007 to October 2017, 40 eligible ESCC patients were included for this retrospective study. Ten patients received standard dose, 18 patients received modified dose, and 12 patients received lower dose chemotherapy. At the last follow-up in July 2019, seven patients were still alive. The median follow-up time was 10 months (range, 3.0-127.3). Table 1 summarizes the patients’ characteristics. The median age of all patients was 74 years (range, 70-91). Thirty-three (82.5%) patients were male. Thirty-five (87.5%) patients were diagnosed with stage III or IV ESCC. The combined clinical complete and partial response rate was 70.0% (28/40). Table 2 lists the CRT-related adverse events. Grade 3 or 4 leucopenia was the most common (35%) severe adverse event among all patients. Patients who underwent modified dose chemotherapy experienced the least body weight loss. Patients administered a standard dose had more hematological toxicity, esophagitis and renal function impairment. Patients given a lower dose displayed the least hematological toxicity but the most body weight loss. Only one patient had grade 3 radiation pneumonitis. There were no grade 5 adverse events.
Table 1.
Patient characteristics (n = 40)
| Standard dose (n = 10) | Modified dose (n = 18) | Lower dose (n = 12) | |
|---|---|---|---|
| Median age (range) | 74 (70-91) | 73 (70-81) | 82 (71-86) |
| Performance status | |||
| ECOG 1 | 3 | 5 | 0 |
| ECOG 2 | 7 | 13 | 12 |
| Gender | |||
| Male | 8 | 16 | 9 |
| Female | 2 | 2 | 3 |
| Tumor location | |||
| Upper thoracic | 2 | 3 | 3 |
| Middle thoracic | 7 | 8 | 3 |
| Lower thoracic | 1 | 7 | 6 |
| AJCC stage | |||
| I | 0 | 0 | 0 |
| II | 2 | 2 | 1 |
| III | 6 | 14 | 10 |
| IV | 2 | 2 | 1 |
| Response | |||
| CR + PR | 6 | 15 | 7 |
| SD + PD | 4 | 3 | 5 |
AJCC = American Joint Committee on Cancer; CR = complete response; ECOG = Eastern Cooperative Oncology Group; PD = progression disease; PR = partial response; SD = stable disease.
Table 2.
Adverse events in each group
| Standard dose (n = 10) | Modified dose (n = 18) | Lower dose (n = 12) | |
|---|---|---|---|
| Leukopenia | |||
| Grade 0-2 | 6 (60%) | 11 (61%) | 9 (75%) |
| Grade 3-4 | 4 (40%) | 7 (39%) | 3 (25%) |
| Anemia | |||
| Grade 0-2 | 8 (80%) | 16 (89%) | 10 (83%) |
| Grade 3-4 | 2 (20%) | 2 (11%) | 2 (17%) |
| Thrombocytopenia | |||
| Grade 0-2 | 9 (90%) | 16 (89%) | 11 (91.7%) |
| Grade 3-4 | 1 (10%) | 2 (11%) | 1(8.3%) |
| Esophagitis | |||
| Grade 0-2 | 8 (80%) | 17 (94.4%) | 12 (100%) |
| Grade 3-4 | 2 (20%) | 1 (5.6%) | 0 (0%) |
| Radiation pneumonitis | |||
| Grade 0-2 | 10 (100%) | 18 (100%) | 11 (91.7%) |
| Grade 3-4 | 0 (0%) | 0 (0%) | 1(8.3%) |
| Liver enzyme elevation | |||
| Grade 0-2 | 10 (100%) | 18 (100%) | 12 (100%) |
| Grade 3-4 | 0 (0%) | 0 (0%) | 0 (0%) |
| Creatinine elevation | |||
| Grade 0-2 | 8 (80%) | 18 (100%) | 12 (100%) |
| Grade 3-4 | 2 (20%) | 0 (0%) | 0 (0%) |
| Weight loss | |||
| <10% | 8 (80%) | 17 (94.4%) | 9 (75%) |
| ≥10% | 2 (20%) | 1 (5.6%) | 3 (25%) |
The 3-year OS rate for all patients was 28.8%, while the 3-year OS rates for standard, modified, and lower dose chemotherapy were 12.5%, 53.8%, and 0.0%, respectively (p = 0.05; Fig. 1). Table 3 respectively summarizes the compliance rate of chemotherapy, radiotherapy, and CCRT, as well as the response rate in each group. The compliance rates of the scheduled chemotherapy, radiotherapy, and CCRT were 62.5% (25/40), 90% (36/40), and 87.5% (35/40), respectively. The most common reason for treatment incompliance was treatment-related adverse events. In subgroup analysis, the modified arm had the highest compliance rate in all aspects and the highest response rate. However, the standard arm experienced the lowest chemotherapy compliance rate.
Fig. 1.
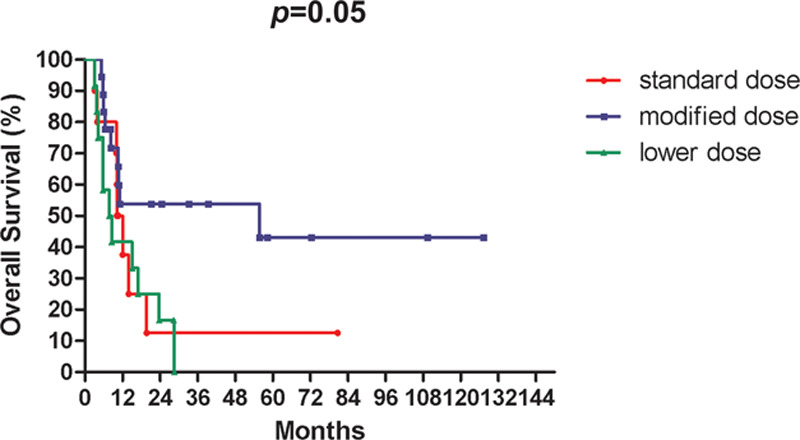
Kaplan-Meier survival curves for overall survival among different chemotherapy dosage.
Table 3.
Treatment compliance and treatment response in each group
| Standard dose (n = 10) | Modified dose (n = 18) | Lower dose (n = 12) | |
|---|---|---|---|
| Chemotherapy compliance | |||
| ≥4 cycles | 4 (40%) | 14 (78%) | 7 (58%) |
| <4 cycles | 6 | 4 | 5 |
| Radiotherapy compliance | |||
| <5% dose deviation | 9 (90%) | 18 (100%) | 9 (75%) |
| ≥5% dose deviation | 1 | 0 | 3 |
| CCRT compliance | |||
| Complete | 9 (90%) | 17 (94%) | 9 (75%) |
| Not complete | 1 | 1 | 3 |
| Response | |||
| CR + PR | 6 (60%) | 15 (83%) | 7 (58%) |
| SD + PD | 4 | 3 | 5 |
CCRT = concurrent chemoradiotherapy; CR = complete response; PD = progression disease; PR = partial response; SD = stable disease.
Table 4 summarizes the univariate analysis. Patients who had either complete or partial response after CRT displayed significantly better OS than those with stable disease or progressive disease (p < 0.001). Patients who received modified doses of CRT tended to have better OS rates than other groups (p = 0.05). Table 5 summarizes the multivariate analysis. The type of chemotherapy regimens used did not reveal statistical differences in OS (hazard ratio [HR] = 1.16; 95% confidence interval [CI] = 0.70-1.92; p = 0.57). There were no significant differences in OS with regard to stage (HR = 1.60; 95% CI = 0.74-3.48; p = 0.23) or age ≥75 years (HR = 1.29; 95% CI = 0.58-2.90; p = 0.53). Treatment response was the independent prognostic factor for OS (HR = 0.22; 95% CI = 0.10-0.49; p < 0.001). From the outcomes of the elderly ESCC patients in this study, we demonstrate that patients receiving a modified dose of chemotherapy experienced acceptable toxicities, better treatment compliance and trend for better OS than patients in the other two groups.
Table 4.
Univariate analysis for OS
| Parameter | OS (%) | p |
|---|---|---|
| Age | ||
| 70-74 (n = 20) | 37.9 | 0.71 |
| ≥75 (n = 20) | 18.2 | |
| Gender | ||
| Male (n = 33) | 26.8 | 0.88 |
| Female (n = 7) | 38.0 | |
| AJCC stage | ||
| I-III (n = 35) | 31.8 | 0.07 |
| IV (n = 5) | 0.0 | |
| Response | ||
| CR + PR (n = 28) | 42.0 | <0.001 |
| SD + PD (n = 12) | 0.0 | |
| Chemotherapy | ||
| Standard dose (n = 10) | 12.5 | 0.05 |
| Modified dose (n = 18) | 53.8 | |
| Lower dose (n = 12) | 0.0 | |
AJCC = American Joint Committee on Cancer; CR = complete response; OS = overall survival; PD = progression disease; PR = partial response; SD = stable disease.
Table 5.
Multivariate analysis for OS
| Variables | HR (95%CI) | p |
|---|---|---|
| Age | 1.29 (0.58-2.87) | 0.53 |
| Gender | 0.97 (0.34-2.77) | 0.96 |
| Stage | 1.60 (0.74-3.48) | 0.23 |
| Response | 0.22 (0.10-0.49) | <0.001 |
| Chemotherapy dosage | 1.16 (0.70-1.92) | 0.57 |
CI = confidence interval; HR = hazard ratio.
4. DISCUSSION
It is well known that treatment decisions for cancers often largely depend on the patient’s age, performance status, and individual comorbidity. Several studies have indicated that old age may alter a physician’s treatment decisions and further affect the clinical outcomes in different cancers.16–18 One of the possible strategies that have been implemented to decrease toxicity and increase the treatment compliance is to modify the chemotherapy dose.19,20 However, there have been few clinical studies on the optimal dose modification for chemotherapy while providing definitive CRT to elderly esophageal cancer patients.
For decades, randomized controlled trials which validated the feasibility of definitive CRT using different regimens were mainly composed of esophageal cancer patients <75 years of age. These studies have shown a similar 3-year OS rate ranging from 20% to 30%.12–15,21 However, these studies did not demonstrate further subgroup analysis for their elderly patients. There were only retrospective studies highlighting the survival outcomes of the elderly patients. Jingu’s study was one of the largest database studies and reviewed 196 patients aged >80 years who were treated with CRT, which showed a 3-year OS rate of approximately 30% and 10% for stage III, IV diseases, respectively.22 However, the chemotherapy regimen, radiotherapy dose and toxicity profile were not provided in their database. Tougeron et al23 reported a better 2-year OS rate of 35.5% from 109 esophageal cancer patients who were >70 years of age and treated with cisplatin-based definitive CRT. Zhao et al24 showed the highest 2-year OS rate of 48.1% from 52 ESCC patients aged more than 75 years and treated with cisplatin-based definitive CRT. When compared with Tougeron’s study, we had a similar OS rate in ESCC patients aged ≥70 years (2-year OS: 32.4%; 3-year OS: 28.8%). However, we had a worse OS rate in patients aged ≥75 years (n = 20; 2-year OS: 27.3%; 3-year OS: 18.2%) when compared with Zhao’s study, which may be due to the selection bias from small patient numbers.
In our study, leucopenia was the most common side effect seen in patients. Overall, 35% of patients developed grade 3 or 4 leukopenia. There have been two studies using similar cisplatin-based definitive CRT regimen as ours, showing comparable toxicity profiles. In Tougeron’s study,23 24.8% of patients suffered from more than grade 2 leukopenia, and 30.3% of patients required chemotherapy dose reduction due to adverse events. In Huang’s study,25 the outcomes of 46 ESCC patients aged >65 years were compared between two concurrent chemotherapy regimens (cisplatin-5-FU vs platinum-taxane), with the results showing there were no significant differences in OS or progression-free survival rates. The cisplatin-5-FU arm resulted in significantly less grade 3 or 4 leukopenia (25.0% vs 63.6%, p = 0.019). However, there was a range of variation in concurrent chemotherapy dosage in Huang’s study, and it did not evaluate the impact of the dosage difference on the outcomes or toxicity profiles. Acute adverse events and malnutrition are the major challenges for physical to treat elderly esophageal cancer patients with definitive CRT. How to balance the treatment response and toxicity during CRT is crucial for treatment compliance and may relate to the clinical outcomes in elderly esophageal cancer patients. It is also known that closely monitoring one’s nutritional status and the timely giving of nutritional supplements during CRT is necessary and helpful for elderly patients.26
In our subgroup analysis, we found a higher 3-year OS rate of 53.8% in patients treated with modified dose chemotherapy than those who were administered standard dose and lower dose chemotherapy. This result also corresponds to our findings that patients in the modified group showed both better treatment compliance and a higher response rate than patients in other two groups (Table 3). The combined clinical complete and partial response rate was 70.0% in our study, which is compatible with the studies mentioned above using a similar CRT regimen (60%-80%).15,23,25 The multivariate analysis also revealed that post-CRT response was the prognostic factor for OS, which also corresponds to the conclusions from previous studies.23,24 Based upon our findings, the reason why the modified group revealed better outcomes can be interpreted that modified concurrent chemotherapy regimen results in less toxicity (Table 2) and better treatment compliance (Table 3), which may lead to therapeutic benefits. The need for chemotherapy modification in elderly patients was also noted in Tougeron’s study, in which 53.2% of patients required dose reduction during their scheduled CRT course.23 The importance of treatment compliance was also shown in Hsieh’s study, in which completion of at least four cycles of chemotherapy was a prognostic factor for OS.15 Although patients receiving a lower dose chemotherapy experienced less toxicity during CCRT, the insufficient chemotherapy intensity may lead to poorer treatment outcomes.
There were some limitations in this retrospective study. First, a retrospective study with a small number of patients meant that we were unable to perform adjustments to multiple potential confounding factors and detect differences in this study. Second, each elderly patient had individual comorbidity, which may have led to a bias when evaluating the benefits of therapy. Third, five patients in our study were diagnosed as stage IV disease, which may lead to poorer clinical outcomes. The main advantage of this study is its high overall CCRT compliance rate of 87.5%, in which the modified arm offered the best results. We have demonstrated the feasibility of dose modification to definitive CRT and the subsequent better outcomes for elderly ESCC patients. A large prospective or randomized study is still needed to validate the optimal modification strategy for definitive CRT in elderly esophageal cancer patients.
In conclusion, definitive CRT using dose modification is a feasible, safe, and reasonable treatment for elderly esophageal cancer patients. Achieving a better compliance to definitive CRT via an optimal dose modification of chemotherapy may provide better clinical outcomes and would be a treatment goal for elderly esophageal cancer patients.
ACKNOWLEDGMENTS
This study was supported by Taichung Veterans General Hospital.
Footnotes
Conflicts of interest: The authors declare that they have no conflicts of interest related to the subject matter or materials discussed in this article.
REFERENCES
- 1.Stewart BW, Wild CP. World Cancer Report. 2014France: International Agency for Research on Cancer; [Google Scholar]
- 2.Di Pardo BJ, Bronson NW, Diggs BS, Thomas CR, Jr, Hunter JG, Dolan JP. The global burden of esophageal cancer: a disability-adjusted life-year approach. World J Surg. 2016;40:395–401. [DOI] [PubMed] [Google Scholar]
- 3.Cijs TM, Verhoef C, Steyerberg EW, Koppert LB, Tran TC, Wijnhoven BP, et al. Outcome of esophagectomy for cancer in elderly patients. Ann Thorac Surg. 2010;90:900–7. [DOI] [PubMed] [Google Scholar]
- 4.Bailey SH, Bull DA, Harpole DH, Rentz JJ, Neumayer LA, Pappas TN, et al. Outcomes after esophagectomy: a ten-year prospective cohort. Ann Thorac Surg. 2003;75:217–22; discussion 222. [DOI] [PubMed] [Google Scholar]
- 5.Atkins BZ, Shah AS, Hutcheson KA, Mangum JH, Pappas TN, Harpole DH, Jr, et al. Reducing hospital morbidity and mortality following esophagectomy. Ann Thorac Surg. 2004;78:1170–6. [DOI] [PubMed] [Google Scholar]
- 6.Gebski V, Burmeister B, Smithers BM, Foo K, Zalcberg J, Simes J; Australasian Gastro-Intestinal Trials Group Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: a meta-analysis. Lancet Oncol. 2007;8:226–34. [DOI] [PubMed] [Google Scholar]
- 7.van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, et al. ; CROSS Group Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–84. [DOI] [PubMed] [Google Scholar]
- 8.Tepper J, Krasna MJ, Niedzwiecki D, Hollis D, Reed CE, Goldberg R, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol. 2008;26:1086–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rahamim JS, Murphy GJ, Awan Y, Junemann-Ramirez M. The effect of age on the outcome of surgical treatment for carcinoma of the oesophagus and gastric cardia. Eur J Cardiothorac Surg. 2003;23:805–10. [DOI] [PubMed] [Google Scholar]
- 10.Sabel MS, Smith JL, Nava HR, Mollen K, Douglass HO, Gibbs JF. Esophageal resection for carcinoma in patients older than 70 years. Ann Surg Oncol. 2002;9:210–4. [DOI] [PubMed] [Google Scholar]
- 11.Adam DJ, Craig SR, Sang CT, Cameron EW, Walker WS. Esophagectomy for carcinoma in the octogenarian. Ann Thorac Surg. 1996;61:190–4. [DOI] [PubMed] [Google Scholar]
- 12.Cooper JS, Guo MD, Herskovic A, Macdonald JS, Martenson JA, Jr, Al-Sarraf M, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA. 1999;281:1623–7. [DOI] [PubMed] [Google Scholar]
- 13.Minsky BD, Pajak TF, Ginsberg RJ, Pisansky TM, Martenson J, Komaki R, et al. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol. 2002;20:1167–74. [DOI] [PubMed] [Google Scholar]
- 14.Conroy T, Galais MP, Raoul JL, Bouché O, Gourgou-Bourgade S, Douillard JY, et al. ; Fédération Francophone de Cancérologie Digestive and UNICANCER-GI Group Definitive chemoradiotherapy with FOLFOX versus fluorouracil and cisplatin in patients with oesophageal cancer (PRODIGE5/ACCORD17): final results of a randomised, phase 2/3 trial. Lancet Oncol. 2014;15:305–14. [DOI] [PubMed] [Google Scholar]
- 15.Hsieh HY, Yeh HL, Hsu CP, Lin JC, Chuang CY, Lin JF, et al. Feasibility of intensity-modulated radiotherapy for esophageal cancer in definite chemoradiotherapy. J Chin Med Assoc. 2016;79:375–81. [DOI] [PubMed] [Google Scholar]
- 16.Dale DC. Poor prognosis in elderly patients with cancer: the role of bias and undertreatment. J Support Oncol. 2003;1(4 Suppl 2):11–7. [PubMed] [Google Scholar]
- 17.Bouchardy C, Rapiti E, Blagojevic S, Vlastos AT, Vlastos G. Older female cancer patients: importance, causes, and consequences of undertreatment. J Clin Oncol. 2007;25:1858–69. [DOI] [PubMed] [Google Scholar]
- 18.Steyerberg EW, Neville B, Weeks JC, Earle CC. Referral patterns, treatment choices, and outcomes in locoregional esophageal cancer: a population-based analysis of elderly patients. J Clin Oncol. 2007;25:2389–96. [DOI] [PubMed] [Google Scholar]
- 19.Wasil T, Lichtman SM. Clinical pharmacology issues relevant to the dosing and toxicity of chemotherapy drugs in the elderly. Oncologist. 2005;10:602–12. [DOI] [PubMed] [Google Scholar]
- 20.Repetto L. Greater risks of chemotherapy toxicity in elderly patients with cancer. J Support Oncol. 2003;1(4 Suppl 2):18–24. [PubMed] [Google Scholar]
- 21.Bedenne L, Michel P, Bouché O, Milan C, Mariette C, Conroy T, et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol. 2007;25:1160–8. [DOI] [PubMed] [Google Scholar]
- 22.Jingu K, Numasaki H, Toh Y, Nemoto K, Uno T, Doki Y, et al. Chemoradiotherapy and radiotherapy alone in patients with esophageal cancer aged 80 years or older based on the Comprehensive Registry of Esophageal Cancer in Japan. Esophagus. 2020;17:223–9. [DOI] [PubMed] [Google Scholar]
- 23.Tougeron D, Di Fiore F, Thureau S, Berbera N, Iwanicki-Caron I, Hamidou H, et al. Safety and outcome of definitive chemoradiotherapy in elderly patients with oesophageal cancer. Br J Cancer. 2008;99:1586–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao Q, Hu G, Xiao W, Chen Y, Shen M, Tang Q, et al. Comparison of definitive chemoradiotherapy and radiotherapy alone in patients older than 75 years with locally advanced esophageal carcinoma: a retrospective cohort study. Medicine (Baltimore). 2017;96:e7920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang C, Huang D, Zhu Y, Xie G, Wang H, Shi J, et al. Comparison of a concurrent fluorouracil-based regimen and a taxane-based regimen combined with radiotherapy in elderly patients with esophageal squamous cell carcinoma. Transl Oncol. 2020;13:100736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cotogni P, Pedrazzoli P, De Waele E, Aprile G, Farina G, Stragliotto S, et al. Nutritional therapy in cancer patients receiving chemoradiotherapy: should we need stronger recommendations to act for improving outcomes? J Cancer. 2019;10:4318–25. [DOI] [PMC free article] [PubMed] [Google Scholar]


