Abstract
Background and Aims
Programmatic colorectal cancer (CRC) screening increases uptake, but the design and resources utilized for such models are not well known. We characterized program components and participation at each step in a large program that used mailed fecal immunochemical testing (FIT) with opportunistic colonoscopy.
Methods
Mixed-methods with site visits and retrospective cohort analysis of 51-75-year-old adults during 2017 in the Kaiser Permanente Northern California integrated health system.
Results
Among 1,023,415 screening-eligible individuals, 405,963 (40%) were up to date with screening at baseline, and 507,401 of the 617,452 not up-to-date were mailed a FIT kit. Of the entire cohort (n = 1,023,415), 206,481 (20%) completed FIT within 28 days of mailing, another 61,644 (6%) after a robocall at week 4, and 40,438 others (4%) after a mailed reminder letter at week 6. There were over 800,000 medical record screening alerts generated and about 295,000 FIT kits distributed during patient office visits. About 100,000 FIT kits were ordered during direct-to-patient calls by medical assistants and 111,377 people (11%) completed FIT outside of the automated outreach period. Another 13,560 (1.3%) completed a colonoscopy, sigmoidoscopy, or fecal occult blood test unrelated to FIT. Cumulatively, 839,463 (82%) of those eligible were up to date with screening at the end of the year and 12,091 of 14,450 patients (83.7%) with positive FIT had diagnostic colonoscopy.
Conclusions
The >82% screening participation achieved in this program resulted from a combination of prior endoscopy (40%), large initial response to mailed FIT kits (20%), followed by smaller responses to automated reminders (10%) and personal contact (12%).
Keywords: Colorectal Cancer Screening, Fecal Immunochemical Tests, Mailed Fecal Tests
Abbreviations used in this paper: COVID-19, coronavirus disease 2019; CRC, colorectal cancer; FIT, fecal immunochemical testing; KPNC, Kaiser Permanente Northern California
What You Need to Know.
Background
- Colorectal cancer screening rates in the United States may be plateauing at <65%
- Mailed fecal immunochemical testing (FIT) can increase screening rates
Findings
- About 61% of those mailed a FIT responded to automated outreach with a pre-letter, FIT kit, automated call and reminder postcard, yielding an overall screening rate of 70%
- Personalized telephone outreach and reminders during clinic visits gave an additional 12% percentage point increase in overall screening
Implications for patient care
- Automated FIT outreach provides an efficient way to reach most people eligible for CRC screening
- Attention is needed for individuals requiring repeated personal contacts
Most deaths from colorectal cancer (CRC) are preventable with screening, but many eligible people are not up-to-date on screening.1 , 2 In 2006, Kaiser Permanente Northern California (KPNC) began an organized program of annual mailed fecal immunochemical testing (FIT) combined with opportunistic colonoscopy. That approach increased screening dramatically: the proportion of its members up-to-date with screening doubled from about 40% to more than 80%,3 , 4 accompanied by a 52% decrease in CRC mortality.3 Health systems wishing to replicate this approach lack detailed information about the program components and required resources. Prior reports noted that extensive service delivery infrastructure (eg, program management and quality assurance activities) and navigation staff were needed to increase screening uptake,5 multiple methods of outreach and in-reach increased screening participation, and multicomponent approaches were more effective than individual components.6 Screening outreach has become more important with precipitous drops in uptake because of the coronavirus disease 2019 (COVID-19) pandemic. However, few studies have examined the specific program components, resources required, and screening outcomes of simultaneous use of multiple strategies in a well-defined population to serve as a model for informing such approaches.7
We sought to characterize the program components, resources needed, and incremental participation at each step in the screening process over a 1-year period (2017) in an established KPNC program that primarily uses mailed FIT for persons due for screening with colonoscopy on request.
Methods
Study Design
The study used a mixed methods sequential explanatory design to assess increases in CRC screening uptake with a population-based programmatic approach. We thus evaluated screening program quantitative data in tandem with qualitative data including direct ethnographic observations of screening processes. The KPNC Institutional Review Board approved this study and waived the requirement for individual informed consent.
Setting
We used data from KPNC, a large integrated health care delivery organization with 15 health service areas that serve approximately 4.5 million members in urban, suburban, and semirural regions in California. Each service area has its own leadership, primary care offices, and gastroenterology departments. KPNC’s members are similar sociodemographically to the rest of Northern California, except at extremes of income, but are less likely to have >5 doctor visits per year or report being in poor health.8
Screening Program
Overviews of the CRC screening program have been published previously.3 , 9 , 10 Before 2006, KPNC relied on visit-based physician requests for CRC screening (ie, opportunistic screening), predominantly using flexible sigmoidoscopy and guaiac fecal occult blood tests. Following pilot testing in 2006, KPNC established a direct-to-patient annual mailed FIT outreach program for those not up to date with screening, without the need for a face-to-face office visit. Screening up to date was defined as receipt of colonoscopy within 10 years, sigmoidoscopy within 5 years, or FIT within the same calendar year. Completed tests are analyzed by an automated OC-Sensor Diana (Polymedco Inc, Cortland Manor, NY) with a cutoff level of >20 μg hemoglobin per gram of stool for a positive result. Patients with a positive test are directed to have follow-up colonoscopy. Screening colonoscopy in place of FIT is available by request.
Framework
The overall FIT-based screening program involves 6 core functions: (1) central management of FIT-based screening, (2) automated FIT outreach, (3) local FIT outreach, (4) local FIT in-reach, (5) central processing of completed FIT kits, and (6) local follow-up of FIT results.
Data Collection
We began by creating detailed process maps of the entire FIT-based CRC screening program, from the identification of those due for screening, to the completion of diagnostic colonoscopies for those with positive tests. This required a review of program components and site visits to primary care offices, gastroenterology departments, and the regional laboratory. Data collection methods included field notes, ethnographic observations, and interviews with program leaders. Centralized FIT outreach activities were determined at the regional level (across all of KPNC); however, for greater granularity, we measured local outreach and in-reach activities in a single KPNC service area; although some details of in-reach differ between service areas, global resource use is similar across service areas. Staff positions were described using job titles (eg, clinical lead, project manager) and training level (eg, physician, medical assistant).
Screening Cohort
We identified a cohort of KPNC health plan members who were CRC screening-eligible in 2017, as defined earlier. The program targets people who are due for screening and are 51–75 years old on December 31 of each calendar year.
Statistical Analyses
For the quantitative analysis, we summarized the cohort characteristics and the percentages of people who were eligible for screening and completed each step of the screening process. We also examined the percentages who were mailed a FIT kit, completed a FIT (after initial outreach, robocall reminder, mailed reminders, and telephone outreach), and completed colonoscopy after a positive FIT.
Results
Core Functions
Central management of fecal immunochemical testing–based screening: Oversight of the CRC screening program was ensured by a population health management team including a part-time clinical leader (physician), full-time lead project manager, part-time data analyst health educator, and a part-time operations manager. This group managed all automated outreach and provided assistance and materials to the service areas. They often test changes to outreach procedures in parallel with existing procedures (A/B testing), and only proceed to widespread implementation if they observe increases in FIT completion. They were supported by the Information Technology Division, which maintains a Patient Reminder, Outreach Management & Population Tracker (PROMPT) system and the Population Health Management Division, which coordinates all population-level preventive activities. PROMPT was developed by KPNC as a custom-built add-on to the EPIC-based electronic health record system. A Consumer Report Services group manages approximately 800–1000 complaints and questions annually (primarily people ineligible for FIT or requesting a new FIT kit). A communications department regularly updates information materials (materials available on request).
Automated fecal immunochemical testing outreach: Prenotices, automated FIT kit mailings, and the coordination of robocall and letter reminders were performed by an outside vendor (Figure 1). Each year, the PROMPT system identifies people eligible for FIT outreach. Letters are then mailed near the anniversary of the prior year’s FIT completion date, or birthday or half birthday for those who had not completed FIT previously. The vendor sends preletters (touch 1) to eligible individuals 1 week before the arrival of mailed FIT kits (touch 2). The FIT kit includes information materials personalized with the primary care provider, pictorial instructions, and a prepaid envelope addressed to a central laboratory. Four weeks after mailing the FIT kits, a robocall (touch 3) with interactive voice response is made, and after 6 weeks, a reminder letter (touch 4) is sent to nonrespondents. These automated steps are tailored for those identified as Black or Hispanic; the communications department created paper information materials with messages that resonated more strongly with these groups during a series of focus groups. Active users of an online patient portal receive electronic messages (ie, e-Alert) 3 days before mailings and are only sent a reminder letter by mail if the electronic message is not opened.
Figure 1.
Delivery of centralized, automated FIT outreach, local outreach, and local in-reach in 2017. Eligible people are identified by the Patient Reminder, Outreach Management, Population Tracking (PROMPT) system. At 56 days after the FIT kit mailing, the names of nonresponders are transferred to responsible primary care practices for local outreach. Local outreach occurs primarily within 5 weeks of transfer. All FIT completed in 2017 that were not within 91 days of a FIT mailing were assumed to be caused by local FIT in-reach.
Local fecal immunochemical testing outreach: The names of nonrespondents are automatically sent to their primary care office 8 weeks after FIT mailings for further local follow-up. Medical assistants make telephone calls or send partially personalized electronic messages or mailings when possible (touch 5) to nonrespondents to complete and return the test. This activity occurs primarily between 8 and 13 weeks after the automated processes to minimize redundancy.
Local fecal immunochemical testing in-reach: Throughout the year, those ages 51–75 who attend office visits and are not up to date with screening may receive reminders and be offered a FIT kit (in-reach) at the visit. The PROMPT system alerts medical assistants during the “rooming” process, showing which step of the outreach process has been completed, allowing staff to, if possible, leverage upcoming delivery of FIT kits rather than hand out additional kits in the office. If the patient cannot recall receiving a mailed FIT kit and no record exists of a completed test, a kit is given to the patient at the time of the visit.
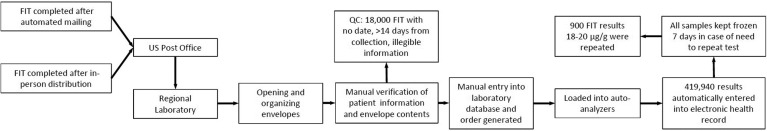
Laboratory processing of completed fecal immunochemical testing kits: Whether received by mail or in-person, all tests were completed at home and returned in prepaid envelopes to a designated central laboratory where staff review the contents for completeness (Figure 2). Tests with no date or illegible information were submitted to the laboratory’s Client Services Department. If the information obtained allowed further processing, the required test information is manually entered into a laboratory database, an order placed, and a label generated for subsequent automated processing. If the test cannot be processed, a new FIT kit is mailed to the member with an explanation of the error. Laboratory technicians load specimens onto automated analyzers, with a maximum processing speed of 250 tests per hour. Analyses were repeated for all borderline positive results (between 18 and 20 μg/g), with the highest result retained. Specimens with >14 days between collection and receipt are analyzed and referred to Client Services if negative; given that stool hemoglobin concentrations decline over time, negative results >14 days old trigger a request for a new test and are not reported.
Figure 2.
Central processing and analysis of completed FIT. QC, quality control.
Local follow-up of fecal immunochemical testing results: Negative test results are sent electronically to patients and primary care providers in addition to a postcard to patients (Figure 3). All positive results are posted in PROMPT. Primary care providers or their staff contact patients individually to explain the need for diagnostic colonoscopy and make an electronic referral to the gastroenterology department. Each primary care department has a medical assistant assigned to track results. In most cases, gastroenterology departments have a designated nurse practitioner or similar who ensures follow-up of patients with a positive FIT, in addition to a designated medical assistant scheduler who contacts patients each day to explain the colonoscopy procedure and schedule appointments. In some areas, this staff member calls members with a positive FIT result directly, without waiting for primary care referral.11 Patients scheduled for colonoscopy are given a prescription and standardized instructions for bowel preparation to be picked up from the local pharmacy. All FIT-positive colonoscopies are considered preventive examinations, and therefore have no or limited copayments.
Figure 3.
Follow-up of FIT results, stratified by normal or “negative” results ≤20 μg/mL and abnormal or “positive” results >20 μg/mL. GI, gastroenterology.
For negative examinations (ie, no biopsy), the endoscopist adds to the patient’s medical record an indication for “average-risk screening in 10 years.” For positive colonoscopy examinations (ie, polyp or mass), the endoscopist enters “pending pathology results.” Once pathology results are available, the relevant guideline-recommended rescreening or surveillance interval is entered into PROMPT. If the lesion was cancerous, the primary care provider is notified, and an e-referral is made to a colorectal surgeon. Referral is made to medical oncology if stage IV disease is found.
A system-wide goal was set for 80% of patients with a positive FIT to be reached by telephone and complete their colonoscopy (if indicated) within 28 days of the positive test. Primary care and gastroenterology departments receive regular performance reports about screening up-to-date and colonoscopy follow-up status for FIT-positive patients, respectively.
Screening Participation in the 2017 Cohort
Of 1,237,448 KPNC health plan members who were 51–75 years old in 2017, 1,023,415 (83%) were continuously enrolled in 2016 and 2017 and were included (Figure 1). In the beginning of 2017, 405,963 (40%) of the 1,023,415 were up to date with screening from a prior colonoscopy or sigmoidoscopy. At the end of the 2017 calendar year, a total of 839,463 (82%) members were screening up to date. Compared with those who completed screening, those who did not were younger and less likely to have completed a FIT in 2016 (Table 1 ).
Table 1.
Characteristics of Kaiser Permanente Northern California Cohort Members Ages 51–75 in 2017 With Continuous Enrollment in 2016–2017, Stratified into 4 Groups Based on Screening Status at the Beginning of 2017 and the Completion of Screening Tests During 2017
| Characteristic | Up to date with screening beginning 2017 | Completed FIT within 8 wk of a mail date | Completed FIT or colonoscopy at another time in 2017 | No documented screening test by end of 2017 | Overall cohort |
|---|---|---|---|---|---|
| Total | 405,963 | 308,563 | 124,937 | 183,952 | 1,023,415 |
| Age, y | |||||
| 51–64 | 217,901 (54) | 194,797 (63) | 95,122 (76) | 141,865 (77) | 649,685 (63) |
| 65–75 | 188,062 (46) | 113,766 (37) | 29,815 (24) | 42,087 (23) | 373,730 (37) |
| Sex | |||||
| Male | 191,368 (47) | 143,750 (47) | 56,796 (45) | 89,538 (49) | 481,451 (47) |
| Female | 214,595 (53) | 164,813 (53) | 68,141 (54) | 94,414 (51) | 541,963 (53) |
| Race/ethnicity | |||||
| Non-Hispanic White | 236,467 (58) | 166,967 (54) | 64,100 (51) | 91,318 (50) | 558,852 (55) |
| Black | 28,641 (7) | 18,936 (6) | 9169 (7) | 13,950 (8) | 70,696 (7) |
| Asian or Pacific Islander | 64,050 (16) | 62,187 (20) | 22,924 (18) | 28,288(15) | 177,449 (17) |
| Hispanic | 52,064 (13) | 40,882 (13) | 20,675 (17) | 30,454 (17) | 144,075 (14) |
| Other | 18,530 (5) | 11,257 (4) | 4058 (3) | 4539 (2) | 38,384 (4) |
| Unknown | 6211 (2) | 8334 (3) | 4011 (3) | 15,403 (8) | 33,959 (3) |
| Completed a FIT in 2016 | 86,302 (21) | 286,179 (93) | 76,566 (61) | 46,659 (25) | 495,706 (48) |
NOTE. All values are n (%).
FIT, fecal immunochemical test.
Outcomes of automated outreach: The screening pathway and incremental participation for those eligible for mailed FIT at the beginning of 2017 is shown in Figure 1. A total of 507,401 were mailed a FIT kit and 206,481 (41%) completed the test within 4 weeks. Among those eligible who were not mailed a kit, many completed screening before their mail date, some had previously refused participation, and others were excluded because of serious illness (as documented by a physician or because residing in a skilled nursing facility or hospice). Of 300,920 members who received a robocall reminder at Week 4, 61,644 (20%) completed FIT, and a further 40,438 (13%) completed the test within 2 weeks after a reminder letter. Almost all of those who completed a FIT during the automated outreach had completed a FIT the year prior (93%; Table 1). At this point, through a combination of prior colonoscopy and mailed outreach efforts, 70% of the population was screening up to date.
Local outreach and in-reach: During local outreach to nonresponders (n = 198,838), primarily between 8 and 13 weeks after automated mailings, 42,753 (4.2%) completed a FIT. A further 27,631 (2.7%) of those mailed a FIT completed a test by the end of 2017, presumably through local in-reach. Those who completed a FIT or colonoscopy outside of the automated outreach were younger and less likely to have completed a FIT the year prior than those who responded to automated outreach (Table 1). In 2017, more than 800,000 PROMPT patient alerts for CRC screening occurred and nearly 295,000 additional FIT kits were given directly to patients during clinic visits. Approximately 100,000 additional FIT kits were ordered through direct-to-patient calls from medical assistants. The central laboratory attempted to contact approximately 18,000 members by telephone or secure email to obtain missing information, allowing them to process about half of these samples.
Follow-up for positive test results: Of the 419,940 patients who completed a FIT in 2017, 14,450 (3.4%) were FIT-positive and 6203 (42%) received a colonoscopy within 30 days of the result date and 11,738 (81%) within 6 months. Of those who received a colonoscopy within 1 year, 7301 (60%) had ≥1 adenoma resected, 1028 (8.5%) had an advanced adenoma (ie, advanced neoplasia on histology), and 335 (2.7%) were diagnosed with CRC.
Discussion
In a population of more than 1 million people with a 40% screening rate at baseline, centralized, automated outreach resulted in a 30 percentage point increase in screening within 8 weeks. Subsequent clinic-based outreach via personalized telephone calls and messages, and in-reach through visit-based reminders, resulted in an additional 12 percentage point increase in coverage, yielding an overall 82% screening participation rate.
CRC screening rates in the United States are well short of the 80% goal set by the National Colorectal Cancer Round Table.2 , 12 Effective programs are needed to increase uptake, particularly if expansion of lower eligibility age is more widely adopted. Our study findings are consistent with randomized trials showing that FIT mailings provide a 28 percentage point increase in screening compared with opportunistic screening alone.13 In another meta-analysis, various types of patient navigation increased screening uptake by 17 percentage points, and patient reminders by 3 percentage points,6 although inconsistent implementation can diminish effectiveness.14 In a 4-arm randomized trial that added automated electronic health record–linked FIT mailings to usual care, then mailings, and finally nurse navigation,15 each addition provided added benefit: usual care to automated mailings increased participation from 26% to 51%, with telephone assistance increasing participation further to 58%, and navigation to 65%, although that study required informed consent, limiting the representativeness of its participants. Our study shows that high rates of screening completion can be achieved on a much larger scale among a diverse, community-based population. The proactive delivery of screening using opt-out principles likely contributes to the high screening rates achieved.16 KPNC offered colonoscopy in addition to the mailed program, which over time contributes to the high screening rates. A potential pitfall of using multiple strategies is overscreening (patients already up to date who nonetheless complete a FIT). Anecdotally, overscreening is rare because the electronic health record add-on, PROMPT, is continuously updated across all sites. However, the use of multiple outreach strategies can create tension between providers and patients who do not want CRC screening and resent repeated reminders.
People can encounter multiple barriers to CRC screening.12 Systematically mailing FIT without charge along with reminders addresses multiple structural barriers. However, potential participants often still express feelings of fear, negative past experiences with the health system, and fatalism.17 In-person contact with a trusted provider may explain why automated outreach alone may not be sufficient for reaching screening rates >80%. Repeat nonparticipants in the KPNC program are less intrinsically motivated and more often disgusted by stool collection for FIT.18 FIT outreach may be particularly relevant with limitations in access to colonoscopy and population fears of in-person visits because of COVID-19.19
KPNC invested significant resources to track tests with incomplete information and to ensure timely completion of colonoscopy following positive FIT results.20 These quality criteria, often overlooked by guidelines,21 are critical to effective screening. Additional follow-up was necessary for 4% of tests received at the laboratory, including 2% that could not be processed. Error rates as high as 20% have been observed.22 The fewer errors in the program studied may be caused by automated processes including preprinted labels in contrast to handwritten identifiers and dates. KPNC also distributes illustrated (wordless) FIT instructions created with input from patient focus groups, which has decreased laboratory recall for specimens with incomplete data. KPNC investments in tracking systems, patient support and navigation resources, and endoscopy capacity increased colonoscopy completion for positive FIT within 30 days from 9% to 34% between the 2006–2008 period and 2013–2016.11
Strengths of this study include its mixed methods approach to provide a complete picture of programmatic CRC screening in a large, diverse population. A primary study limitation is that we did not have a comparison group or time-point to evaluate the precise effects of implementing the various screening program components described. However, several screening components have been shown to be effective in smaller randomized trials of a single intervention. Our results are from a single, large integrated health system, which may limit applicability for smaller programs or individual practices; however, many of the most effective elements (ie, mailed outreach, and telephone reminders) are commonly used. We do not have information about reasons for nonparticipation; some members may have made informed decisions not to be screened. Furthermore, we described an established program and did not show the steps needed to build and launch the program. Finally, precise cost information was not available because of difficulty identifying true costs versus charges and high variability in personnel costs across settings.
In conclusion, this study showed mailed FIT with automated outreach and targeted personalized outreach and in-reach increased screening participation to 82%. Substantial resources were used for laboratory quality control and the follow up of positive FIT. High-quality CRC screening can be achieved on a large scale, but attention is needed for individuals requiring repeated personal contacts.
Acknowledgments
The authors thank all of their Kaiser Permanente Northern California clinical and administrative partners who provided valuable insights and data, in particular, Eryn Eby, Molly Landau, Matthew Petrie, and Tasha Morales.
CRediT Authorship Contributions
Kevin Selby, MD, MAS (Conceptualization: Equal; Data curation: Supporting; Writing – original draft: Lead; Writing – review & editing: Equal);
Christopher D. Jensen, PhD (Conceptualization: Equal; Data curation: Equal; Project; administration: Lead; Writing – original draft: Equal; Writing – review & editing: Equal);
Theodore R. Levin, MD (Conceptualization: Supporting; Funding acquisition: Supporting; Resources: Equal; Writing – review & editing: Equal);
Jeffrey K. Lee, MD, MAS (Conceptualization: Supporting; Data curation: Supporting; Writing – review & editing: Supporting);
Joanne E. Schottinger, MD (Conceptualization: Supporting; Funding acquisition: Equal; Writing – review & editing: Supporting);
Wei K. Zhao, MPH (Data curation: Equal; Formal analysis: Lead; Writing – review & editing: Supporting);
Douglas A. Corley, MD, PhD (Conceptualization: Equal; Funding acquisition: Equal; Project administration: Equal; Writing – review & editing: Equal);
Chyke A. Doubeni, MBBS, MPH (Conceptualization: Equal; Funding acquisition: Equal; Project administration: Equal; Writing – review & editing: Equal)
Footnotes
Conflicts of interest This author discloses the following: Chyke A. Doubeni is a member of the US Preventive Services Task Force (USPSTF) and authors topics on UpToDate. This article does not necessarily represent the views and policies of the USPSTF or UpToDate. The other authors disclose no conflicts.
Funding This study was conducted within the National Cancer Institute–funded Population-based Research to Optimize the Screening Process (PROSPR) consortium (U54 CA163262) and SCOLAR (1R01CA213645-01A1). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Siegel R.L., Miller K.D., Fedewa S.A., et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67:177–193. doi: 10.3322/caac.21395. [DOI] [PubMed] [Google Scholar]
- 2.Wender R.C. A letter from the National Colorectal Cancer Roundtable. Dig Dis Sci. 2015;60:594–595. doi: 10.1007/s10620-015-3602-3. [DOI] [PubMed] [Google Scholar]
- 3.Levin T.R., Corley D.A., Jensen C.D., et al. Effects of organized colorectal cancer screening on cancer incidence and mortality in a large, community-based population. Gastroenterology. 2018;155:1383–1391. doi: 10.1053/j.gastro.2018.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehta S.J., Jensen C.D., Quinn V.P., et al. Race/ethnicity and adoption of a population health management approach to colorectal cancer screening in a community-based healthcare system. J Gen Intern Med. 2016;31:1323–1330. doi: 10.1007/s11606-016-3792-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Subramanian S., Tangka F.K.L., Hoover S., et al. Costs of planning and implementing the CDC’s Colorectal Cancer Screening Demonstration Program. Cancer. 2013;119(Suppl 15):2855–2862. doi: 10.1002/cncr.28158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dougherty M., Crockett S., Brenner A.T., et al. Evaluation of interventions intended to increase colorectal cancer screening rates in the United States: a systematic review and meta-analysis. JAMA Intern Med. 2018;178:1645–1658. doi: 10.1001/jamainternmed.2018.4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carethers J.M., Doubeni C.A. Causes of socioeconomic disparities in colorectal cancer and intervention framework and strategies. Gastroenterology. 2020;158:354–367. doi: 10.1053/j.gastro.2019.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gordon N. Similarity of the Adult Kaiser Permanente membership in Northern California to the insured and general population in Northern California: statistics from the 2011 California Health Interview Survey. 2015. https://divisionofresearch.kaiserpermanente.org/projects/memberhealthsurvey/SiteCollectionDocuments/chis_non_kp_2011.pdf Available at:
- 9.Jensen C.D., Corley D.A., Quinn V.P., et al. Fecal immunochemical test program performance over 4 rounds of annual screening: a retrospective cohort study. Ann Intern Med. 2016;164:456–463. doi: 10.7326/M15-0983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levin T.R., Jamieson L., Burley D.A., et al. Organized colorectal cancer screening in integrated health care systems. Epidemiol Rev. 2011;33:101–110. doi: 10.1093/epirev/mxr007. [DOI] [PubMed] [Google Scholar]
- 11.Selby K., Jensen C.D., Zhao W.K., et al. Strategies to improve follow-up after positive fecal immunochemical tests in a community-based setting: a mixed-methods study. Clin Transl Gastroenterol. 2019;10 doi: 10.14309/ctg.0000000000000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.American Cancer Society . American Cancer Society; Atlanta: 2017. Colorectal cancer facts & figures 2017-2019. [Google Scholar]
- 13.Jager M., Demb J., Asghar A., et al. Mailed outreach is superior to usual care alone for colorectal cancer screening in the USA: a systematic review and meta-analysis. Dig Dis Sci. 2019;64:2489–2496. doi: 10.1007/s10620-019-05587-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coronado G.D., Petrik A.F., Vollmer W.M., et al. Effectiveness of a mailed colorectal cancer screening outreach program in community health clinics: the STOP CRC cluster randomized clinical trial. JAMA Intern Med. 2018;178:1174–1181. doi: 10.1001/jamainternmed.2018.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Green B.B., Wang C.-Y., Anderson M.L., et al. An automated intervention with stepped increases in support to increase uptake of colorectal cancer screening: a randomized trial. Ann Intern Med. 2013;158(5 Part 1):301–311. doi: 10.7326/0003-4819-158-5-201303050-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mehta S.J., Khan T., Guerra C., et al. A randomized controlled trial of opt-in versus opt-out colorectal cancer screening outreach. Am J Gastroenterol. 2018;113:1848–1854. doi: 10.1038/s41395-018-0151-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones R.M., Devers K.J., Kuzel A.J., et al. Patient-reported barriers to colorectal cancer screening: a mixed-methods analysis. Am J Prev Med. 2010;38:508–516. doi: 10.1016/j.amepre.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gordon N.P., Green B.B. Factors associated with use and non-use of the fecal immunochemical test (FIT) kit for colorectal cancer screening in response to a 2012 outreach screening program: a survey study. BMC Public Health. 2015;15:546. doi: 10.1186/s12889-015-1908-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Issaka R.B., Somsouk M. Colorectal cancer screening and prevention in the COVID-19 era. JAMA Health Forum. 2020 doi: 10.1001/jamahealthforum.2020.0588. e200588-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doubeni C.A., Gabler N.B., Wheeler C.M., et al. Timely follow-up of positive cancer screening results: a systematic review and recommendations from the PROSPR Consortium. CA Cancer J Clin. 2018;68:199–216. doi: 10.3322/caac.21452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Population health . National Quality Forum; Washington, DC: 2012. prevention measures. [Google Scholar]
- 22.Wang A., Rachocki C., Shapiro J.A., et al. Low literacy level instructions and reminder calls improve patient handling of fecal immunochemical test samples. Clin Gastroenterol Hepatol. 2019;17:1822–1828. doi: 10.1016/j.cgh.2018.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]