Abstract
COVID-19 is a present-day complex pandemic infection with unpredictable levels of morbidity and mortality in various global populations. COVID-19 is associated with the different comorbidities with its change in biological function such as causing heart dysfunction via deregulating ACE-2 receptor, gastrointestinal risk via causing vomiting, diarrhea, and abdominal pain, chronic kidney disease via proteinuria and hematuria, diabetes mellitus, liver injury via increasing ALT, AST and bilirubin level, lung injury, CNS risk, ocular risk, and cancer risk. In this, we are focused on the COVID-19 connected with male infertility. Some of the studies show that the patients of COVID-19 are associated with impaired spermatogenesis. Impaired spermatogenesis via COVID-19 decreases the level of testosterone by disturbing cytokines such as TNF-α, IL-4, IL-6, and IL-12 and further, attenuates the sperm count. COVID-19 is causing inflammation via TNF-α and interferons. IL-4 plays an eminent role in the activation of the JAK-STAT pathway and leads to the disturbing pro-inflammatory cytokine as well as further cause’s male infertility. Th2 activates the IL-4 through IgG and IgE and mediates apoptosis with the triggering of STAT signaling. The activated STAT signaling augments Batf/Irf4, and the Bach2/Batf pathway. On the other hand, SARS-CoV-2 is activating the level of Th2 cells. So, we hypothesized that the augmented Th2 cells would disturb the level of IL-4, JAK-STAT signaling, Batf/Irf4, and Bach2/Batf pathway. The disturbed IL-4 decreases the level of the ACE-2 with the inflammation. This further leads to male infertility in COVID-19 patients. So, in this hypothesis, we focused on the role of IL-4 in COVID-19 patients associated with male infertility via Th2 cells and JAK-STAT signaling.
Keywords: COVID-19, Interleukin-4, Inflammation, Male infertility, JAK-STAT, Signaling
1. Introduction
Several reports suggest that coronavirus disease of 2019 (COVID-19) might damage male fertility. Since December 2019, the infection has spread worldwide, leading to severe acute respiratory syndrome (SARS) named "COVID-19" by the World Health Organization (WHO) (Organization, 2020) and it has been declared as a global pandemic by WHO (Organization, 2020). Angiotensin-converting enzymes 2 (ACE2) receptors play a crucial role in the pathogenesis of COVID-19 and cells that show a high level of ACE2 expression has the potential to be targeted and damaged by the virus (Fan et al., 2020a). Numerous studies detected high ACE2 expression levels in testicular cells, mainly in seminiferous duct cells, spermatogonia, Leydig cell, and Sertoli cells (Fan et al., 2020a; Shen et al., 2020; Wang and Xu, 2020). Based on the previous studies, it is evident that the testis could be a potential target for the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). At the level of testicular cells, four main cell types; seminiferous duct cells, spermatogonia, Leydig cells, and Sertoli cells, shows a higher rate of mRNA expression in ACE2 (Fan et al., 2020a; Shen et al., 2020; Wang and Xu, 2020). Due to the viral entry into these cells, the process of spermatogenesis could be affected, which might generate risk to male fertility. Interestingly, the testicular expression of ACE2 is age-related and the highest expression of ACE2 has been recorded in younger groups than old patients (Shen et al., 2020).
Interleukin -4 (IL-4), a pro-inflammatory cytokine has been found to use the receptor IL-4R and is seen to activate quite common pathways in signaling. The use of Janus kinase (JAKs) by IL-4 has also been seen. A variety of other different signaling molecules found activated, which plays an essential role in regulating proliferation induced by IL-4 (Jiang et al., 2000). The locus of IL-4 has been found to undergo a series of methylation-demethylation, during the process of differentiation of T-helper (Th) cells. It has been observed that the locus of IL-4 is demethylated quite specifically in the T-helper type 2 (Th2) cells but hypermethylated in the case of T cells which are naïve in nature (Lee et al., 2002). IL-4 mediates many specific functions, including fine-tuning the Th2 immune response through its ability to initiate (TH2 cell proliferation), perpetuate (Th2 cytokine production, Immunoglobulin E (IgE) synthesis, and eosinophil and alternatively activated macrophage activation), or shut off (suppression of IL-13–mediated processes) the allergic response. This is activated through multiple signaling pathways (signal transducer and activator of transcription 6 (STAT6) and insulin receptor substrate 2 (IRS-2), downstream of its type I receptor (Wills-Karp and Finkelman, 2008). A couple's failure in accomplishing pregnancy regardless of having unprotected ordinary intercourse is characterized as infertility. Infertility due to males (Male Factor) has been found to contribute to almost half of all the infertility cases (Bisht et al., 2017). Studies have shown, inflammatory cytokines playing a significant role in spermatogenesis regulation by cell interaction. An imbalance in its level may disturb its stability which may contribute to infertility in men (Syriou et al., 2018). The T helper cells type 2 produce a variety of cytokines that regulate anti-inflammation. These T-helper cells type 2 are involved in promoting a humoral model of immune response to counter the extracellular pathogens. IL-4 is considered a major cytokine regulating anti-inflammation (Opal and DePalo, 2000). Studies have also shown that in case of chronic pain, which is widespread, a decrease in the levels of anti-inflammatory cytokines is seen along with the analgesic activity of h2 cytokine (Üçeyler et al., 2006). Interleukin-4 has shown significant inhibition of cytokines derived from pro-inflammatory monocytes including IL-8, IL-6, IL-1, tumor Necrosis Factor-α (TNF-α). Furthermore, the ability of IL-4 to suppress the cytotoxic activity of macrophage, the action involving the killing of the parasite, and nitric oxide (NO) production which derived from macrophage activity has also been seen in studies (Opal and DePalo, 2000).
Cluster of differentiation 4 (CD4+) T cells after activation start synthesizing cytokines. After the cytokines are secreted, they differentiate and grow by autocrine signaling pathway and as a result of which naïve T cells are seen to differentiate into effector cells. The different types of T cells can be distinguished by the type of cytokine they secrete. T-helper type 1 (Th1) cells secrete interleukin-2 (IL-2), TNF, and interferon-gamma (IFN-γ). On the other hand, Th2 cells synthesize various kinds of cytokines – IL-4, IL-5, IL-6, and IL-13. The Th1 cells are important for cell-mediated immunity. In contrast, the Th2 cells help in B-cell activation and the subsequent production of antibodies and also are involved in class switching of immunoglobulin M (IgM) antibodies to of immunoglobulin G (IgG) and IgE antibodies. The cytokine, IL4 is a polypeptide that weights 15-KD with multiple effects on different cells. It consists of a heterodimer receptor with an α subunit and a γ subunit. The binding of the cytokine IL-4 with its receptor results in the proliferation and the cell differentiation of T cells into Th 2 cells (Paul, 1997; Choi and Reiser, 1998). IL-4 has a regulatory function and the early treatment with this cytokine improves the condition in case of a few autoimmune diseases with the increase in the number of Th2 cells (Choi and Reiser, 1998). In this hypothesis, we focused on the role of the IL-4 in COVID-19 associated male infertility.
2. COVID-19 pandemic
The novel COVID-19 which is caused due to the SARS-CoV-2 emerged as a global pandemic causing calamitous repercussions for the healthcare system and human beings worldwide (Sathishkumar Vinayagam, 2020; Venugopal et al., 2020). The initial outbreak of the COVID-19 was started in December 2019 (Wu and McGoogan, 2020). The COVID-19 had attained its peak by the mid of March 2020 after which the World Health Organization had declared it a pandemic. The reasons behind this vast spread of COVID-19 infection is due to the rapid urbanization and an increase in International travels compared to the earlier days (Balachandar et al., 2020). Another reason is due to its incubation period, as when the SARS cases were analyzed the viral shedding was high when the patients have infected with the disease and are in their advanced stage. Whereas, COVID-19 disease can be transmitted in the early stages when the affected patients are still in the asymptomatic stage (Wilder-Smith et al., 2005; Rothe et al., 2020; Wilder-Smith et al., 2020). The coronavirus is a type of virus that mainly targets the human respiratory system as its survival point in the human host system. This is the third outbreak of the coronaviruses among the human beings, where the previous outbreaks were the SARS and the Middle East respiratory syndrome (MERS) which also had a global threat among the public (Rothan and Byrareddy, 2020). The SARS-CoV-2 is a single-stranded positive-sense RNA virus, which comes under the betacoronavirus genus of the Coronaviridae family (Vellingiri et al., 2020). The SARS-CoV-2 has a 99 % sequence similarity with its ancestral SARS virus. Hence its genome also contains two untranslated regions (UTRs) with 5′-cap structure and 3′-poly-A tail region along with an open reading frame (ORF) which encodes a polyprotein (Harapan et al., 2020). The virus survives inside the human host cell mainly due to its structural proteins which are the Spike (S) protein, Envelope (E) protein, Membrane (M) protein, and Nucleocapsid (N) protein and the accessory proteins including the ORF 3a, 7 and 8 which has a major role in receptor binding and viral replication inside the human host body (Chan et al., 2020; Fan et al., 2020b). The symptoms of the COVID-19 affected patients vary from one to another, such as the mild, moderate, and severe form of symptoms. The period for the onset of the COVID-19 infection ranges from 3 to 21 days where the approximate median period of the symptoms is on the 14th day (Li et al., 2020). In most cases, the patients who are elder than 70 years are at huge risk for the severity of the disease whereas the patients below 70 years of age are at less risk for this disease. The most common symptoms of COVID-19 disease are fever, cough, fatigue, headache, dyspnoea, diarrhea (Huang et al., 2020; Wang et al., 2020). Another peculiarity of this disease is that the COVID-19 patients showed anosmia, high levels of leukocytes, increased levels of pro-inflammatory cytokines, and abnormality in the respiratory system (Rothan and Byrareddy, 2020). Hence it is high time to find a cure or vaccine to treat this deadly infection before huge damage has been caused among the public worldwide.
3. COVID-19 and inflammation
The COVID-19 patients who were critical have common features including sudden deterioration of disease after onset, low levels of lymphocytes (NK cells), higher inflammatory parameters including C-reactive protein (CRP) and pro-inflammatory cytokines (IL-6, TNF-α, IL-8) (Mahalaxmi et al., 2020), atrophy of spleen and lymph nodes, reduced lymphocytes, vasculitis, and hypercoagulation. Inflammatory cytokine storm (CS) was widespread in patients with severe COVID-19. The CS refers to the excessive and uncontrolled release of pro-inflammatory cytokines. In the case of SARS and MERS, severe inflammatory cell infiltration, and CS led to acute lung injury (Channappanavar and Perlman, 2017; Chousterman et al., 2017). Several accumulating evidence revealed that severe COVID-19 patients resemble SARS and MERS in their cytokine profiles. Huang et al. reported increased cytokine levels including IL-1B, IL- 1RA, IL-7, IL-8, IL-9, IL-10, fibroblast growth factor (FGF), granulocyte-macrophage colony-stimulating factor (GM-CSF), IFNγ, granulocyte colony-stimulating factor (G-CSF), interferon-γ-inducible protein (IP10), monocyte chemoattractant protein (MCP1), macrophage inflammatory protein 1 alpha (MIP1A), platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF) were increased, among which IL-2, IL-7, IL-10, G-CSF, IP10, MCP1, MIP1A, TNF-α were higher in severe patients (Conti et al., 2020; Huang et al., 2020). The most prominent markers of COVID-19 is lymphocytopenia, which is one of the most important diagnostic criteria in COVID-19 patients in China (NHC, 2020) and reduced levels of T-cells and NK cells were observed. Other important assays such as biochemical and blood count including lymphocyte and CRP levels, may also be an important biomarker to detect COVID-19 (Iyer et al., 2020). Siddiqu and Mehra (2020) suggested the use of immune-modulatory agents to reduce systemic inflammation (Siddiqi and Mehra, 2020). The pathophysiology of COVID-19 infection has aggressive inflammatory responses causing damage to the airways (Wong et al., 2004). Viral infection in immune cells like monocytes and macrophages can result in aberrant cytokine production, even if the viral infection is not productive (Tseng et al., 2005). COVID-19 specific CD4 + T cells express IFNγ, TNF, and IL-2, which suggests that patients with SARS-CoV infection exhibit a Th1 cell response and mainly use cellular immunity to control the infection (Shin et al., 2019). Individuals with COVID-19 exhibit an abundance of the interleukins IL-1, IL-2, IL-6, IL-7, 1L-8, granulocyte colony-stimulating factor (GCSF), interferon γ-induced protein 10 (IP10), monocyte chemoattractant protein-1 (MCP1), macrophage inflammatory protein (MIP1A), and TNF-α. The degree of SARS-CoV-2 targeting these cells remains poorly defined. Therapies inhibiting viral infection and regulation of dysfunctional immune responses can synergize to resists pathologies at multiple steps (Yang et al., 2020) (represented in Table 1 ). At the same time, the relationship between immune dysfunction and outcome of disease severity in patients with COVID-19 serves for vaccine development and evaluation. Understanding these immune dysfunctions is essential to guide the application of appropriate immune-modulatory treatments (Florindo et al., 2020). Therefore, as CS occurs in critical COVID-19 patients leading to ARDS and organ damage, anti-inflammatory treatment may be applied (Prompetchara et al., 2020). A deeper understanding of the implications of COVID-19 in patients with immune-mediated inflammatory disease and the effects of anti-cytokine and other immunosuppressive therapies is urgently needed to guide clinicians in the care of patients.
Table 1.
COVID-19 causes inflammation via deregulating inflammatory markers.
| Viral infection | Inflammatory markers | Outcome | Reference |
|---|---|---|---|
| COVID-19 | low levels of lymphocytes (NK cells), higher inflammatory parameters including CRP and pro-inflammatory cytokines (IL-6, TNF-α, IL-8) | Inflammation | (Mahalaxmi et al., 2020) |
| COVID-19 | increased cytokine levels including IL-1B, IL- 1RA, IL-7, IL-8, IL-9, IL-10, FGF,GM-CSF, IFNγ, G-CSF,IP10,MCP1, MIP1A,PDGF, VEGF were increased, among which IL-2, IL-7, IL-10, G-CSF, IP10, MCP1, MIP1A, TNF-α were higher | Inflammation | (Conti et al., 2020; Huang et al., 2020) |
| COVID-19 | Increased level of IFNγ, TNF, and IL-2 | Inflammation | (Shin et al., 2019) |
| COVID-19 | Increased level of IL-1, IL-2, IL-6, IL-7, 1L-8, GCSF,IP10,MCP1, MIP1A and TNF-α | Inflammation | (Yang et al., 2020) |
4. IL-4 and its role in anti-inflammation
IL-4 shows its anti-inflammatory nature by causing an up-regulation of the inhibitors of certain cytokines and scavenging the receptors (Schuerwegh et al., 2003). Studies on IL-4 have also shown that IL-4 is involved in the enhancement of Th2 mediated immunity. It causes repression in the signaling mediated by IL-12 and inhibits the Th1 mediated response. IL-4 production has also shown a marked increase throughout pregnancy. Progesterone has been shown to induce the same, and both IL-4 and progesterone have been found to act together to inhibit Th1 mediated response during pregnancy (Chatterjee et al., 2014). IL-4 reduces the production of TNF-α, IL-1, prostaglandin E2, and antagonistic to the actions of IFN-γ. Thereby it activates and actuating the ability of monocytes of humans in the oxidation of LDL and also reducing inflammation (Bhattacharjee et al., 2013). Studies involving analysis of IL-4 found an increase in the expression of genes like chitinase-like 3 (Chil3), Fibronectin 1 (Fn1), Resistin-like molecule alpha/FIZZ (Retnla), with apoptotic cells induced the tissue repair program in macrophages of lungs and gut which are some of the classical genes involved in anti-inflammation (Bosurgi et al., 2017). The cytokine association in the testis' physiology and its pathological condition is deficient.
5. Th1/Th2 theory and its theory in SARS-CoV-2
A theory was hypothesized back in the 80 s that different cytokines were expressed by the T-helper cells and this theory was further applied to human immunological studies, wherein the T helper cells – Th1 and Th2 produced different immunological response. The first-ever Th cells that were reported were mouse Th1 and Th2 cells. IFN-gamma cytokine was secreted by Th1 cells, while IL-4 was secreted by Th2 cells (Mosmann et al., 1986). It was observed that Th1 cells were involved in cellular immunity (or the type-1 pathway) – fighting viruses, stimulating hypersensitivity reactions, and eliminating cancer cells. On the other hand, Th2 cells were involved in the humoral immunity (or the type-2 pathway) – causing a rise in the antibodies to fight foreign organisms. These two pathways may act antagonistically to each other while causing down-regulation of each other (Kidd, 2003).
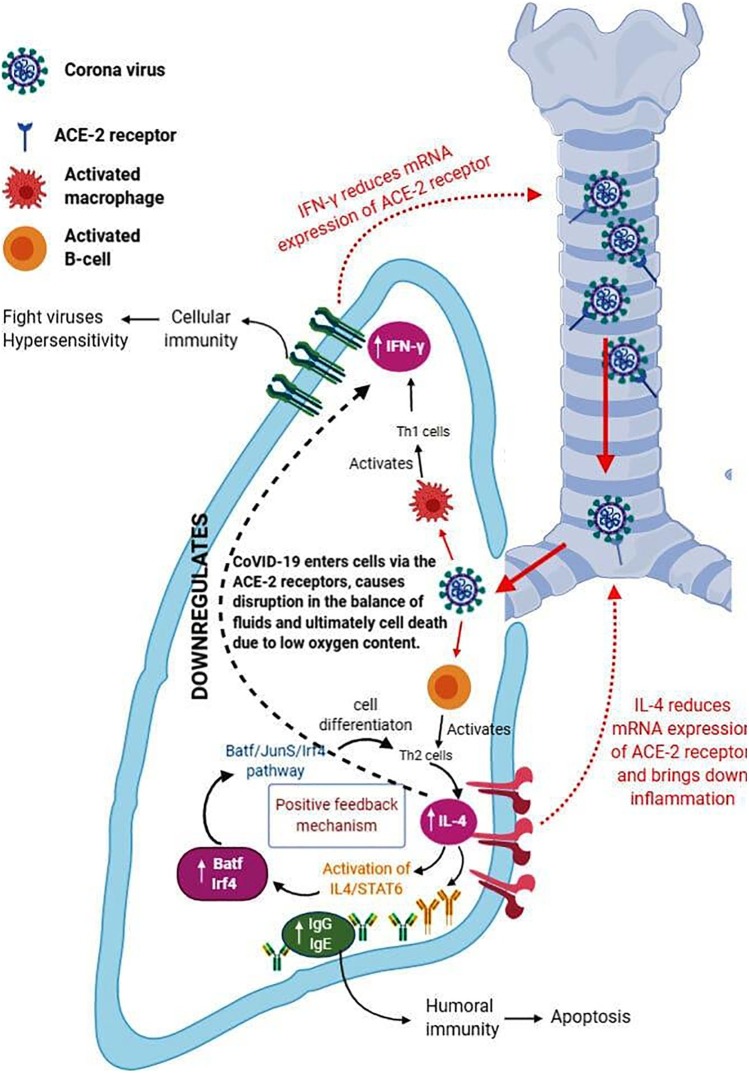
IL-4 cells are generated from Th2 cells. These interleukins block the pathway of Th1 immune response and trigger the reactions of Th2 cells. It was found that in cases of autoimmune conditions and cases of hyperactive immunity reactions, there is an increased production of Th1 cells. In cases of high intense care, Th2 cells were found to have been present in high amounts in COVID-19 patients (Prompetchara et al., 2020). Due to the difference in the molecular weight of the SARS-CoV-2 spike protein, it was observed that the pathway or mechanism of immune response differed in different patients. The spike protein which weighs more than 70 kDa activates the Th1 inflammatory response through the macrophages. The other proteins that weight less than 70 kDa activate the Th2 cells and initiate the activation of the B-cell receptor. Due to the uncontrollable surge in the viral particles, as a result of pro-inflammatory response (a result of termination by the Th1 cells) a condition called AICD (Activated Induced Cell Death) induced by the B-cells. Due to this, the process of apoptosis is increased dramatically, releasing many pro-inflammatory cytokines that cause lymphopenia and producing the interleukin IL-10. This causes a shift in the immune response and causes COVID-19 sepsis, deteriorating the immune system (Kaviyarasi Renu and Abilash, 2020) (represented in Fig. 1 ).
Fig. 1.
Role of cytokine, IL-4 in COVID-19 associated inflammation pathway.
Indicates the entry of the Coronavirus into the cells of the lung tissue. The spike proteins of the coronavirus bind themselves to the ACE-2 receptor present throughout the respiratory tract and are an easy target for viruses. Upon entering the cells, the virus having spike proteins more than 70k Da of weight activates the macrophages. The activated macrophages further activate the cellular immunity via the Th1 cell pathway (activating IFN-γ)
(indicated by bold black arrow).On the other hand, the virus having spike proteins less than 70k Da of weight activates the B-cells and produces antibodies via the Th2 cell pathway (activating IL-4) (indicated by bold black arrow). A positive feedback pathway is seen in which the increase in IL-4 causes the increase in the cell differentiation of Th2 cells by the Batf/JunD/Irf4 pathway.
Often, it is seen that IL-4 cytokine blocks or down-regulates IFN-γ (indicated by black dotted arrow), so that the Th2 pathway takes place and more number of Th2 cells differentiate.
The cytokines - IL-4 and IFN-γ, together down-regulate the mRNA expression of ACE-2 receptors so that fewer virus particles bind to the receptors (indicated by red dotted arrow). ACE-2 receptors help in the balancing of fluids. When this is disrupted by the virus, there is an accumulation of fluid in the lungs. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
6. IL-4 form Th cells and regulation of immune responses
Among all the interleukins secreted, IL-2 and IL-4 were considered crucial for the development of naive CD+ T cells, activated by the presence of antigens, into Th1 and Th2 cells (Nelms et al., 1999). While IL-2 is important for Th1 cell development, Il-4 is important for Th2 cell development (Seder, 1994; Mosmann and Sad, 1996; Paludan, 1998). Il-4 is involved in the up-regulation of its receptor expression, inhibition of IL-12 secretion, and also the down-regulation of beta-2 subunit expression present on the receptor of IL-12. IL-4 is responsible for the shift of the immune from Th1 to Th2, which occurs by self-activation of the receptors present on Th1 cells (Szabo et al., 1995; Breit et al., 1996). One of the functions of Il-4 in the production of IgG and IgE antibodies (Boehm et al., 1997; Paludan, 1998). IL-4 is seen to be down-regulating the activity of IFN-gamma and vice versa. While IFN-gamma blocks the production of IgE and IgG1 antibodies, IL-4 blocks the secretions of the IgG2A antibody (Cuff et al., 1998; Kaviyarasi Renu and Abilash, 2020). In the intronic region (which is the hypersensitive site of DNase I) of the IL4 gene, a site known as Gata3-binding site is present that is highly important for producing the interleukin IL-4 by the CD4+ T cells (Tanaka et al., 2011). In Th2 cells, the deletion or the absence of IL4 IE site may result in low production of IL-4.
When BTB domain and CNC homolog 2 (Bach2) expressions are suppressed in the case of the naïve CD4+ T cells that are lacking Bach-2 genes, there is an increased production of IL-4 and also results in the induction of basic leucine zipper transcription factor (Batf) and Interferon regulatory factor 4 (Irf4). Bach2 down-regulates the expression of Batf and Irf4 genes. Therefore in the case of Bach2-deficient CD4 T cells, there is a surge in the expression of Batf and Irf4. These two expressions form an activated complex and cause a rise in the expression of IL4. This forms a positive feedback mechanism that induces Th2 differentiation of cells. The increase in the Th2 production can be attributed to the recruitment of the Batf/JunD/Ifr4-containing activator protein 1 (AP-1) complex. In the presence of Bach2, an active complex of Bach2 –Batf is formed that binds to AP1 that is present within rad50 hypersensitive site 6 (RHS6). This interferes with the activation of the AP1 complex that contains Batf/JunD/Ifr4 and results in the reduction of the production of Th2. Therefore, in Bach2 deficient cells, Th2 cytokine synthesis is highly augmented. It was also found out that by the activation of the Il-4/Stat6 pathway, the expression of Batf, Batf3, Irf4 increased and also caused the suppression of Bach2. It was thus stated that the presence of such a network between IL-4, Batf/Irf4, and Bach2/Batf complexes causes the differentiation of Th2 cells and also controls the Th2 type response of the immune system (Kuwahara et al., 2016) (represented in fig, 1).
7. IL-4 and JAK-SAT signaling
JAK-STAT pathway plays an important role for IL-4 signaling. IL-4 has two types of the receptor such as IL-4R-I and IL-4R-II. Type-I has been found to induce JAK1 and JAK3 activation and finally, the activation of STAT6. The JAK1, JAK2, and also Tyrosine Kinase 2 (TYK2) are linked to the type II receptors and also stimulate the activation of STAT6 (Busch-Dienstfertig and González-Rodríguez, 2013). Studies that involved characterizing the JAK1 tissue (Knock-outs) led to the confirmation of it playing a significant role in response to cytokine IL-4 (Schindler et al., 2007). Studies have also found that there is an increase in the activity of STAT6 and the mRNA levels of IL-4 by the induction of Ras. Evidence about the positive regulation of the STAT6 activity as well as the IL-4 activity by Ras/Erk family has been seen in similar studies. All these contribute to the notion of a cross-talk being present in functional aspects between the IL-4, JAK1, and STAT6 with Ras/Erk, all of which lead to IL-4 transcription regulation (So et al., 2007).
IL-4 has been found to regulate the expression of IL-8 induced by TNF-α at the level of transcription, and this involves nuclear factor kappa B (NF-κB) and STAT6 (Raingeaud and Pierre, 2005). Mice models that were STAT6-deficient have shown that the JAK-STAT pathway is the mediator of a major segment of the IL-4 response(Ji et al., 2015). Differential regulation of gene and IL-4 concerned studies has shown IL-4 stimulating the activation of JAK1. The regulation of STAT3, as well as STAT6 and their subsequent activation by JAK1 in monocytes induced by IL-4, have also been documented. The use of IL-4Rα and JAK1 and also STAT3 or STAT6 cascade by IL-4 to regulate gene expression of significant inflammatory genes like CD36 (scavenger receptor), 15LO, and MAOA has been studied as well (Bhattacharjee et al., 2013).
STAT6 activation has been well noted in the case of an IL-4 induction. It involves STAT6 associating with SH2 domains of cytokine receptors in its TP (tyrosine-phosphorylated) regions. Followed by this, the phosphorylation of STAT6 on Tyr-641 is seen. The dimerization of STAT6 monomers is the next step. These dimers have been seen to translocate. The STAT6 nuclear translocation is dependent on its phosphorylation and also its dimerization (Bhattacharjee et al., 2013). Mice studies have also shown that mice with STAT6 knocked out led to the impairment of IL-4 signaling and the subsequent process involved with it like polarisation of Th2 (Heim, 1999). The studies have exposed the significant function of IL-4 using the JAK-STAT6 pathway which regulates the target genes in lymphocytes which are connected to Th2 (Bao et al., 2013). The Hippocampus of aged rats had shown that a decrease in the JAK1 and STAT6 phosphorylation occurred when the concentration of IL-4 decreased due to age factor (Maher et al., 2005).
The inhibition of the activation of STAT6, which is dependent on IL-4 by both SOCS-1 and 3 has been shown in studies. In contrast to this, a protein containing Src Homology 2 (SH2)-cytokine-induced and suppressors of cytokine signaling (SCOS-2) has been seen to up-regulate the same process. The IL-4 signaling down-regulation due to SCOS-1 as well as SCOS-3 due to JAK1 receptor activity inhibition has also been studied. The role of the motif-Thr-Phe present in the domain-Pre-SH2, in regulating the JAK-STAT IL-4 signaling inhibition mediated by SCOS-3 has also been found in studies (Haque et al., 2000). IL-4 is also involved in the activation of STAT5, although it is a prototypical stimulus for STAT6 (Villarino et al., 2017). RNA interference (RNAi) of STAT6 and its over-expression has also indicated to the fact of the involvement of JAK-STAT and IL-4 induction dependency. Cytochrome P450 family 2 subfamily E member 1 (CYP2E1) proximal promoter region contains a binding site for STAT6 has also been revealed by mutagenesis (Wang et al., 2010). C-C motif chemokine ligand 26 (CCL26), being up-regulated by IL-4 in the skin and the involvement of JAK1, JAK2, and STAT6 pathway in the skin has also been seen in studies (Bao et al., 2012) (represented in Fig. 2 ).
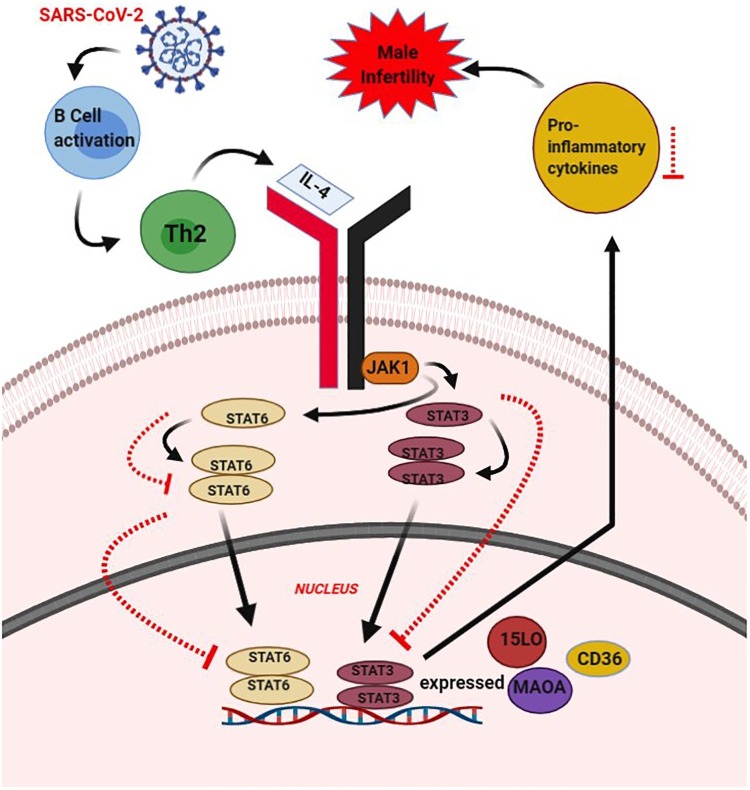
Fig. 2.
IL-2 mediated JAK-STAT signaling pathway in COVID-19 associated male infertility.
This figure shows IL-4 JAK-STAT Pathway and their effect on male infertility, during the event of infection by SARS-CoV-2. The entry of SARS-CoV2 will lead to the supposed activation of B-cells and these wills up-regulate the Th2 cells. The Th2 cell alters the level of IL-4. The IL-4 alteration leads to STAT6 or STAT 3 being stimulated and dimer formation is seen. These dimers translocate to the nucleus and undergo transcription. Genes like CD36, 15 LO, and MAOA are seen to get expressed. IL-4 alteration causes the deregulation of proinflammatory cytokines. In the case of the upregulation of proinflammatory cytokines, it will result in male inflammation. Here, it is hypothesized that infection by SARS-CoV-2 will cause a down-regulation of IL-4, which is anti-inflammatory. This will cause a down-regulation of STAT6 or STAT3 (indicated by red dotted arrow). This will up-regulate the levels of pro-inflammatory cytokines and cause male infertility. Overall, the deregulation of the IL-4 level alters the pathway involved in the STAT and causes male infertility upon COVID-19 infection. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
8. COVID-19 and reproductive system
In theory, highly expressed ACE2 or trans-membrane protease serine 2 (TMPRSS) in an organ is vulnerable to infection (Lukassen et al., 2020). Notably, the ACE2 receptors are also present in the female reproductive systems such as in the ovaries and uterus(Vaz-Silva et al., 2009; Reis et al., 2010). Angiotensin II (Ang II) in the endometrium is required for the proper functioning of the menstrual cycle as if there are any changes in its levels would lead to endometrial hyperplasia (Ahmed et al., 1995). Also, the ACE2 is expressed in the syncytiotrophoblast, cytotrophoblast, endothelium, and vascular smooth muscle of the placental villi, maternal stroma, and also in the endothelium and smooth muscles of the umbilical cord (Valdes et al., 2006). Recently a newborn born to a mother with COVID-19 infection had high levels of IgM, which makes the speculations that the infection may be spread to the newborn via intrauterine infection during pregnancy (Yu and Chen, 2020). It has also been reported that a breastfeeding mother could pass on the infection via breastfeeding as the COVID-19 was positive in the breast milk samples (Yu and Chen, 2020). Gene ontology analysis showed that genes associated with viral reproduction and transmissions were highly enriched in ACE2-positive spermatogonia, but the genes associated with male gamete generation were down-regulated. Intercellular junction and immune-related genes were greater in ACE2-positive Leydig and Sertoli cells, but genes related to mitochondria and reproduction were lessened. Also, Fan and colleagues found that ACE2 was highly expressed in renal tubular cells, Leydig cells, and cells in the seminiferous ducts of the testis (Wu et al., 2020). In a recent, study reported by Pan and colleagues cannot definitively rule out the presence of SARS-CoV-2 in the seminal fluid during acute infection with severe COVID-19 symptoms (Fan et al., 2020a; Pan et al., 2020). Human placental RAS has a vital part in placental vascular development and during early pregnancy is noted to be up-regulated (Pringle et al., 2011). La Pena et al. noted, in early pregnancy, a high level of ACE2 mRNA located in syncytiotrophoblasts and villous stroma in the placenta was noted, also reported ACE2 regulated the release of Ang-1–7, which was beneficial for vasodilation in the maternal-fetal circulation and favorable for virus spread (Isela et al., 2018). Sun et al., proposed theoretical public health concern for COVID-19 in mildly symptomatic and asymptomatic women to impact both a current pregnancy and future reproduction validated by several studies. The first is the association between the COVID-19 infection and disrupted ACE2 expression, which can dysregulate the ACE2 Ang-(1-7) axis. The next one is an association between dysregulated ACE2 Ang-(1-7) axis and impaired maternal and fetal health (Sun and Yeh, 2020). Based on these findings, the authors hypothesized that COVID-19 could harm in general, the cardiovascular adaptation of mothers, proper hemodynamic regulation of the placenta, fetal growth and long-term cardiovascular health, and also reproductive health of females. Moreover, COVID-19/ACE2 may also disturb both the male and female reproductive functions, resulting in infertility, menstrual disorder, and fetal distress (Jing et al., 2020). Thus it is possible that the SARS-CoV-2 could also cause problems related to the reproductive system in the affected individuals.
9. Male infertility in COVID-19
There are current safety concerns about the effects of COVID-19, especially on male reproductive health (male infertility and testosterone deficiency). SARS-CoV-2 viral spike (S) protein attaches to the ACE2 receptors and engaging the cellular serine protease (TMPRSS2) for S protein priming, and also they both are existing in the testis (Hoffmann et al., 2020), raising the concern regarding infection of the testes and possible sexual transmission. ACE2 receptors are established at higher concentrations in the testes and confirmed the presence of ACE2, angiotensin (1-7), and its MAS receptors in the testicles, unambiguously in Leydig and Sertoli cells (Reis et al., 2010). These particular receptors are noted on developing sperm, production of testosterone, the male sex hormone. According to a recent study, it was observed that in 81 reproductive-aged men who have infected with COVID-19 the ratio of testosterone to luteinizing hormone was significantly reduced which was accompanied by C-reactive protein levels when compared to COVID-19 unaffected healthy individuals (Ma et al., 2020; Shen et al., 2020; Wu et al., 2020). Fan et al. (2020) and Shen et al. (2020) found high ACE2 expression in testis (both germ cells and somatic cells) suggesting potential tropism of COVID-19 to testicular tissues (Shen et al., 2020; Wu et al., 2020). Pan and colleagues have done the semen analysis for thirty-four men diagnosed with COVID-19 infection that had only mild symptoms or were getting recovered from the acute infections, and the results revealed that there was no presence of SARS-CoV-2 in the semen of the patients (Pan et al., 2020; Shen et al., 2020). The study showed another remarkable and unique clinical observation that 17.6 % (6/34) of men with COVID-19 infection described scrotal discomfort. This particular clinical sign and symptom need further studies to apprehend the pathophysiology along with the reproductive sequelae in men. As more than 80 % of individuals with COVID-19 disease are asymptomatic, the reproductive effects for the infected men could be positive/ or unidentified. Zhao et al. (2003), noted the presence of SARS-CoV in the testicular epithelial cells and the Leydig cells (Zhao et al., 2003). In contrast, Ding et al. (2004), reported direct infection in other organs excluding the testicles (Ding et al., 2004). Song et al. (2020) analyzed 12 semen samples from survived COVID-19 patients and testicular biopsies from a dead COVID-19 patient. The results of these samples did not show the presence of SARS-CoV-2 RNA and specify that the virus does not infect the testes or male genital tract directly even in the acute phase (Song et al., 2020).
10. Inflammation and male infertility
The ACE2 receptor provides a cellular and biochemical pathway for the SARS-CoV-2 to infect a wide array of human cells. ACE2 is present in tissues, several cell types including epithelial cells in the respiratory path and enter the cells to complete its replication cycle this direct pathological mechanism of infection and cell damage increases ACE2 expression as a potential entry route for the viral infiltration(Fan et al., 2020a). According to a recent study, it has been reported that organs with higher expression of ACE2 or TMPRSS2 (the respiratory, cardiovascular, digestive, and urinary system) are more prone to acquire the SARS-CoV-2 infection (Zou et al., 2020). Evidence suggests the renin-angiotensin-aldosterone system (RAAS) components, specifically ACE2, appears to play a vital role in male reproduction. The ACE2 receptors have a crucial role in testicular regulation such as the steroidogenesis and spermatogenesis; also there is an increased expression of ACE2 mRNA in four main testicular cells which are seminiferous duct cells, spermatogonia, Leydig cells, and Sertoli cells (Fan et al., 2020a; Shen et al., 2020; Wang and Xu, 2020). These cells affected by the virus hinder the process of spermatogenesis and could cause problems in male fertility. Research shows that the testicular expression of ACE2 by COVID-19 is age-related as the young male patients are at greater threat of testicular damage than elder patients (Shen et al., 2020). In an earlier study, where six male patients with SARS-CoV infection, there testis autopsy report revealed orchitis, and the histopathological reports showed inflammation in the seminiferous tubules and deposition of IgG immunoglobulins in seminiferous tubules, interstitium, and Sertoli cells (Xu et al., 2006). The reports point out that testicular impairment was caused by inflammatory and immunological responses rather than viral infiltration. As both SARS-CoV and the SARS-CoV-2 share 96 % of sequence similarity and also the entry route for both the virus via ACE2 receptors, it is possible that testicular injury may also occur in COVID-19 affected patients due to ACE2 receptor or secondary immunological and inflammatory responses.
It has been reported that the cytokine storm is a major hallmark in the severely affected COVID-19 patients (Tay et al., 2020). The proper functioning of cytokines required to maintain proper testicular function and regulation of the male reproductive system (Loveland et al., 2017), hence in COVID-19 as cytokine storm causes the major issues this may also have future implications in the male fertility. A recent study has shown that a subgroup of individuals with severe COVID-19 might show a secondary cytokine storm syndrome (hemophagocytic lymphohistiocytosis). This is a less acknowledged, hyperinflammatory syndrome described by persistent fulminant fever, and serious hypercytokinemia with multi-organ failure. These patients show a particular serum blood cytokine profile with cytopenia and hyperferritinemia. The mortality rate of these patients could be sizably improved with immunomodulatory therapy (IL-6 antagonist) (Mehta et al., 2020; Tveito, 2020) as follow-up studies of recovered male patient's reproductive function is a prerequisite to study these possibilities of the treatment. Recently, Schroeder et al. (2020) had reported that male COVID-19 affected patients showed low levels of testosterone along with increased levels of inflammatory cytokines such as IL-2 and IFN-γ (Schroeder et al., 2020). It was reported that testosterone has a sex-specific and protective effect on vascular aging by mitigating oxidative stress and inflammation (Moreau et al., 2020). This piece of evidence shows that the cytokine storm might also have an imperative role in causing damage to the male reproductive system (represented in Fig. 3 ).
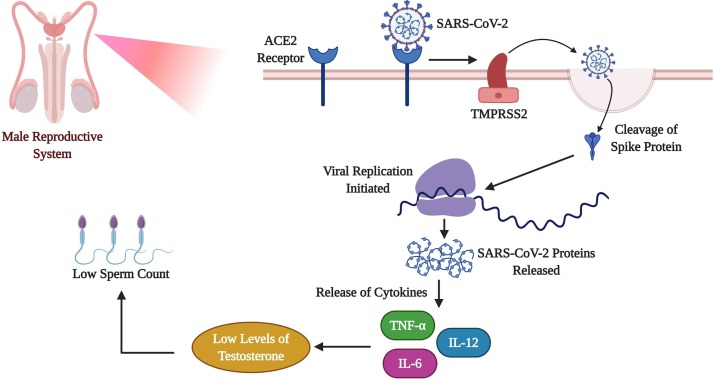
Fig. 3.
Impact of COVID-19 on Male Reproductive System.
This figure depicts the effect of COVID-19 in male infertility. The ACE2 receptors have a crucial role in testicular regulation. Once the COVID-19 enters the human system the ACE2 receptors which are present in the male reproductive system get attached to it. Further, the spike (S) protein of the SARS-CoV-2 gets cleaved with the help of TMPRSS2. This leads to viral genome replication and the release of various cytokines and the development of cytokine storm. On the other hand, this storm in the male reproductive system reduces the levels of testosterone which hinders the production of sperm, which might also reduce the sperm count causing male infertility.
11. IL-4 and male infertility
Studies have shown that infertile patients due to unexplained causes have an increased IL-4 concentration in serum (Syriou et al., 2018). Numerous studies have shown that the seminal plasma levels of IL-4 in the infertile group of people were quite significantly low when compared to the normal group of people (Yigitbasi et al., 2010). A significant decrease in the levels of IL-4 was seen in infertile male groups as compared to normal groups in a study concerning male infertility (Zhang and Gao, 2004). Infertility induced by varicocele and studies involving the same and the expression analysis showed a down-regulation in the levels of TNF. This down-regulation was supposed to be due to the anti-inflammatory nature of IL-4 (Hassanpour et al., 2017). Studies have shown that an increase in IL-4 concentration levels in semen inhibits the expression of cytokines, pro-inflammatory which is being released from macrophages or Th1 lymphocytes or the monocytes (Vicari and Calogero, 2001). Cadmium-induced damage to testes of rats saw a reduction in the levels of IL-4 and an increase in cytokines pro-inflammatory in action (Al-Azemi et al., 2010). Infertility studies involving infertile males from Iraq showed that Il-4 induced macrophage activation also saw a reduction in the levels of cytokines that are pro-inflammatory in action (Al-Assaf et al., 2013) (represented in Fig. 2).Th2 mediated cytokine IL-4 maintains the testes' immune level and spermatogenesis normally (Klein et al., 2016). There is a DC subsets presence in the seminomas in humans. There is an increased number of CD11c + myeloid DC (mDC) in the testes tumor patients. This CD11c + myeloid DC (mDC) expresses the immune tolerance cytokine such as IL-4. The mediated immune response via IL-4+ mediates testicular germ cell tumors escape of immune through the suppression of the immune level (Loveland et al., 2017).
12. IL-4 protects form the COVID-19 associated male infertility – A hypothesis
Based on the above evidence, we have hypothesized that, once the SARS-CoV-2 enters into the system, it activates the Th1 cells further mediates the level of the IFN-γ, and mediates the cellular immunity. On the other hand, it activates the Th2 cells which mediate the IL-4 level through the activation JAK-STAT6 pathway, augments the IgG, IgE, and mediates the humoral immunity and apoptosis. It also mediates the IL-4, Batf/Irf4, and Bach2/Batf pathway. The proven evidence shows that SARS-CoV-2 augments the level of the Th2 cells (Kaviyarasi Renu and Abilash, 2020). COVID-19 augments of the Th1/Th17 and antibody production. Also, it shows that a high level of Th2 needs intensive care (Kaviyarasi Renu and Abilash, 2020). So, this shows that increased Th2 cells mediate the level of the IL-4 level and alters the inflammation via the JAK-STAT6 pathway. This mediates the level of the dysregulation of the IgG, IgE and mediates the apoptosis and further causes male infertility associated with the COVID-19.
13. Recommendation
The COVID-19 causes not only respiratory-related illnesses but also a multi-organ damage, the reproductive organs are also at risk of this viral infection. Thus a few suggestions or guidelines might help to prevent the infection from causing any severe damage to the reproductive system. The following recommendations will be helpful to overcome the issue:
-
•
The male patients who are infected with COVID-19 must have a follow-up survey to ensure that there is no colossal damage in their reproductive system.
-
•
Considering the absence of information about male richness and the proposal that couples think about holding back to get pregnant, sperm freezing might be helpful - emotionally and biologically - during the COVID-19 pandemic.
-
•
Social distancing proposals are still set up, so visiting a specialist's office or clinic to talk about sperm freezing isn't suggested, or maybe conceivable, right now. Mail-in sperm testing and freezing alternatives, similar to the Legacy pack, are a significant choice to save your ripeness from the wellbeing and solace of your own home.
-
•
Females who have not yet attained their menopause and have acquired COVID-19 disease must check their ovaries' life and quality in their future.
-
•
Females who are affected with SARS-CoV-2 infection after their recovery must check their fertility status, menstrual cycle and if planning to become pregnant should try to postpone it till their reports are normal.
-
•
Both men and females should get their hormone profiles checked after getting recovered from the SARS-CoV-2 infection to avoid any future complications.
-
•
Men recovered from COVID-19 disease must have food rich in sperm production such as high content of zinc, garlic, dark chocolates, walnuts, etc. these foods will enable them to regulate sperm production and quantity naturally.
-
•
Similarly, female recovered from COVID-19 disease must have a high intake of food which are rich in antioxidants, vitamin D such as green leafy vegetables, etc. as these kinds of food will enable to regulate their fertility quality and reduce any damage naturally.
-
•
Pregnant women must be careful during this pandemic situation and must avoid any unnecessary traveling to prevent from this SARS-CoV-2 infection.
14. Conclusion
Several studies suggest that SARS-CoV-2 infection may suspect to have long-term effects on male and female reproductive function. COVID-19 may affect some pregnant women and children and additional studies are needed to assess the effects of SARS-CoV-2 on human infertility. The immense impact of the COVID-19 pandemic is inestimable because the epidemic is still unchecked throughout the globe. Although data are limited and incomplete at this time, there is justifiable concern that the reproductive consequences of the novel corona-virus may have lasting effects for male reproduction and some pregnant women and children. As COVID-19 related concerns are negatively impacting people's wellbeing, a considerable amount of research is needed in the future to pinpoint the exact effects of this viral infection on infertility and its related issues
Funding
The work was supported by the "VIT SEED GRANT" and ICMR-National Task Force Project [F.No.5/7/482/2010-RBMH&CH]. The author Kaviyarasi Renu is grateful to ICMR for providing financial assistance in the form of a Senior Research Fellowship (SRF). The author Dr. VB would like to thank Bharathiar University for providing the necessary infrastructure facility and Project funded and supported by MHRD-RUSA 2.0 – BEICH to carry out this manuscript.
Conflicts of Interest Statement
The authors whose names are listed immediately below certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Kaviyarasi Renu, Mohana Devi Subramanian, Rituraj Chakraborty, Haritha Myakala, Mahalaxmi Iyer, Geetha Bharathi, Kamalakannan Siva, Balachandar Vellingiri, Abilash V.G.
The authors whose names are listed immediately below report the following details of affiliation or involvement in an organization or entity with a financial or non-financial interest in the subject matter or materials discussed in this manuscript. Please specify the nature of the conflict on a separate sheet of paper if the space below is inadequate.
Acknowledgment
The authors would also like to thank the VIT authorities and Bharathiar University for providing all the facilities to carry out this work.
References
- Ahmed A., et al. Localization of the angiotensin ii and its receptor subtype expression in human endometrium and identification of a novel high-affinity angiotensin ii binding site. J. Clin. Invest. 1995;96:848–857. doi: 10.1172/JCI118131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Assaf A.I.S., et al. Evaluation of anti-sperm antibodies and some cytokines profile in seminal plasma of iraqi infertile males. Al-Mustansiriyah J. Pharmaceut. Sciences (AJPS) 2013;13:155–163. [Google Scholar]
- Al-Azemi M., et al. Lithium protects against toxic effects of cadmium in the rat testes. J. Assist. Reprod. Genet. 2010;27:469–476. doi: 10.1007/s10815-010-9426-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balachandar V., et al. Covid-19: emerging protective measures. Eur. Rev. Med. Pharmacol. Sci. 2020;24:3422–3425. doi: 10.26355/eurrev_202003_20713. [DOI] [PubMed] [Google Scholar]
- Bao L., et al. Il-4 regulates chemokine ccl26 in keratinocytes through the jak1, 2/stat6 signal transduction pathway: implication for atopic dermatitis. Mol. Immunol. 2012;50:91–97. doi: 10.1016/j.molimm.2011.12.008. [DOI] [PubMed] [Google Scholar]
- Bao L., et al. The involvement of the jak-stat signaling pathway in chronic inflammatory skin disease atopic dermatitis. Jak-Stat. 2013;2:e24137. doi: 10.4161/jkst.24137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee A., et al. Il-4 and il-13 employ discrete signaling pathways for target gene expression in alternatively activated monocytes/macrophages. Free Radic. Biol. Med. 2013;54:1–16. doi: 10.1016/j.freeradbiomed.2012.10.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisht S., et al. Oxidative stress and male infertility. Nat. Rev. Urol. 2017;14:470–485. doi: 10.1038/nrurol.2017.69. [DOI] [PubMed] [Google Scholar]
- Boehm U., et al. Cellular responses to interferon-γ. Annu. Rev. Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- Bosurgi L., et al. Macrophage function in tissue repair and remodeling requires il-4 or il-13 with apoptotic cells. Science. 2017;356:1072–1076. doi: 10.1126/science.aai8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breit S., et al. A strict requirement of interleukin‐4 for interleukin‐4 induction in antigen‐stimulated human memory t cells. Eur. J. Immunol. 1996;26:1860–1865. doi: 10.1002/eji.1830260829. [DOI] [PubMed] [Google Scholar]
- Busch-Dienstfertig M., González-Rodríguez S. Il-4, jak-stat signaling, and pain. Jak-Stat. 2013;2:e27638. doi: 10.4161/jkst.27638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.F.-W., et al. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting wuhan. Emerg. Microbes Infect. 2020;9:221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channappanavar R., Perlman S. Semin. Immunopathol. Springer; 2017. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology; pp. 529–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee P., et al. Regulation of the anti-inflammatory cytokines interleukin-4 and interleukin-10 during pregnancy. Front. Immunol. 2014;5:253. doi: 10.3389/fimmu.2014.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi P., Reiser H. Il-4: role in disease and regulation of production. Clin. Exp. Immunol. 1998;113:317. doi: 10.1046/j.1365-2249.1998.00690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chousterman B.G., et al. Semin. Immunopathol. Springer; 2017. Cytokine storm and sepsis disease pathogenesis; pp. 517–528. [DOI] [PubMed] [Google Scholar]
- Conti P., et al. Induction of pro-inflammatory cytokines (il-1 and il-6) and lung inflammation by coronavirus-19 (covi-19 or sars-cov-2): anti-inflammatory strategies. J. Biol. Regul. Homeost. Agents. 2020;34:1. doi: 10.23812/CONTI-E. [DOI] [PubMed] [Google Scholar]
- Cuff C.A., et al. Lymphotoxin α3 induces chemokines and adhesion molecules: insight into the role of ltα in inflammation and lymphoid organ development. J. Immunol. 1998;161:6853–6860. [PubMed] [Google Scholar]
- Ding Y., et al. Organ distribution of severe acute respiratory syndrome (sars) associated coronavirus (sars‐cov) in sars patients: implications for pathogenesis and virus transmission pathways. J. Pathol. 2004;203:622–630. doi: 10.1002/path.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan C., et al. Ace2 expression in kidney and testis may cause kidney and testis damage after 2019-ncov infection. MedRxiv. 2020 [Google Scholar]
- Fan W., Holmes edward C., yong-zhen zhang, et al. A new coronavirus associated with human respiratory disease in china. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florindo H.F., et al. Immune-mediated approaches against covid-19. Nat. Nanotechnol. 2020:1–16. doi: 10.1038/s41565-020-0732-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque S.J., et al. Identification of critical residues required for suppressor of cytokine signaling-specific regulation of interleukin-4 signaling. J. Biol. Chem. 2000;275:26500–26506. doi: 10.1074/jbc.275.34.26500. [DOI] [PubMed] [Google Scholar]
- Harapan H., et al. Coronavirus disease 2019 (covid-19): a literature review. J. Infect. Public Health. 2020 doi: 10.1016/j.jiph.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassanpour H., et al. Comparative expression analysis of hsp70, hsp90, il-4, tnf, kitlg and kit-receptor gene between varicocele-induced and non-varicocele testes of dog. Int. J. Fertil. Steril. 2017;11:148. doi: 10.22074/ijfs.2017.5020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim M.H. The jak-stat pathway: cytokine signalling from the receptor to the nucleus. J. Recept. Signal Transduct. Res. 1999;19:75–120. doi: 10.3109/10799899909036638. [DOI] [PubMed] [Google Scholar]
- Hoffmann M., et al. Sars-cov-2 cell entry depends on ace2 and tmprss2 and is blocked by a clinically proven protease inhibitor. Cell. 2020 doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., et al. Clinical features of patients infected with 2019 novel coronavirus in wuhan, china. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isela S.-R., et al. Changes in trophoblasts gene expression in response to perchlorate exposition. Toxicol. In Vitro. 2018;50:328–335. doi: 10.1016/j.tiv.2018.04.006. [DOI] [PubMed] [Google Scholar]
- Iyer M., et al. Covid-19: an update on diagnostic and therapeutic approaches. BMB Rep. 2020;53:191. doi: 10.5483/BMBRep.2020.53.4.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji X., et al. Acute nitrogen dioxide (no2) exposure enhances airway inflammation via modulating th1/th2 differentiation and activating jak-stat pathway. Chemosphere. 2015;120:722–728. doi: 10.1016/j.chemosphere.2014.10.039. [DOI] [PubMed] [Google Scholar]
- Jiang H., et al. Il-4/il-13 signaling beyond jak/stat. J. Allergy Clin. Immunol. 2000;105:1063–1070. doi: 10.1067/mai.2000.107604. [DOI] [PubMed] [Google Scholar]
- Jing Y., et al. Potential influence of covid-19/ace2 on the female reproductive system. Mol. Hum. Reprod. 2020 doi: 10.1093/molehr/gaaa030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaviyarasi Renu P.L.P., Abilash V. Coronaviruses pathogenesis, comorbidities and multi-organ damage–a review. Life Sci. 2020;225 doi: 10.1016/j.lfs.2020.117839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd P. Th1/th2 balance: the hypothesis, its limitations, and implications for health and disease. Altern. Med. Rev. 2003;8:223–246. [PubMed] [Google Scholar]
- Klein B., et al. Specific immune cell and cytokine characteristics of human testicular germ cell neoplasia. Hum. Reprod. 2016;31:2192–2202. doi: 10.1093/humrep/dew211. [DOI] [PubMed] [Google Scholar]
- Kuwahara M., et al. Bach2–batf interactions control th2-type immune response by regulating the il-4 amplification loop. Nat. Commun. 2016;7:1–13. doi: 10.1038/ncomms12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D.U., et al. Th2 lineage commitment and efficient il-4 production involves extended demethylation of the il-4 gene. Immunity. 2002;16:649–660. doi: 10.1016/s1074-7613(02)00314-x. [DOI] [PubMed] [Google Scholar]
- Li Q., et al. Early transmission dynamics in wuhan, china, of novel coronavirus–infected pneumonia. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loveland K.L., et al. Cytokines in male fertility and reproductive pathologies: immunoregulation and beyond. Front. Endocrinol. (Lausanne) 2017;8:307. doi: 10.3389/fendo.2017.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukassen S., et al. Sars‐cov‐2 receptor ace 2 and tmprss 2 are primarily expressed in bronchial transient secretory cells. EMBO J. 2020;39 doi: 10.15252/embj.20105114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L., et al. Effect of sars-cov-2 infection upon maL.e gonadal function: a single center-based study. MedRxiv. 2020 [Google Scholar]
- Mahalaxmi I., et al. Covid‐19 and olfactory dysfunction: a possible associative approach towards neurodegenerative diseases. J. Cell. Physiol. 2020 doi: 10.1002/jcp.29937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher F., et al. Downregulation of il-4-induced signalling in hippocampus contributes to deficits in ltp in the aged rat. Neurobiol. Aging. 2005;26:717–728. doi: 10.1016/j.neurobiolaging.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Mehta P., et al. Correspondence covid-19: consider cytokine storm syndromes and. Lancet. 2020;6736:19–20. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau K.L., et al. Sex differences in vascular aging in response to testosterone. Biol. Sex Differ. 2020;11:1–14. doi: 10.1186/s13293-020-00294-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T.R., Sad S. The expanding universe of t-cell subsets: Th1, th2 and more. Immunol. Today. 1996;17:138–146. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- Mosmann T.R., et al. Two types of murine helper t cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- Nelms K., et al. The il-4 receptor: signaling mechanisms and biologic functions. Annu. Rev. Immunol. 1999;17:701–738. doi: 10.1146/annurev.immunol.17.1.701. [DOI] [PubMed] [Google Scholar]
- Nhc, N.H.C.O.T.P.S.R.O.C., 2020. The diagnosis and treatment plan for 2019-ncov (the seventh trial edition).
- Opal S.M., Depalo V.A. Anti-inflammatory cytokines. Chest. 2000;117:1162–1172. doi: 10.1378/chest.117.4.1162. [DOI] [PubMed] [Google Scholar]
- Organization W.H. 2020. Coronavirus Disease (covid-19)-events As They Happen.https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen [Google Scholar]
- Paludan S. Interleukin-4 and interferon-gamma: the quintessence of a mutual antagonistic relationship. Scand. J. Immunol. 1998;48:459–468. doi: 10.1046/j.1365-3083.1998.00435.x. [DOI] [PubMed] [Google Scholar]
- Pan F., et al. No evidence of sars-cov-2 in semen of males recovering from covid-19. Fertil. Steril. 2020 doi: 10.1016/j.fertnstert.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul W.E. Interleukin 4: signalling mechanisms and control of t cell differentiation. Ciba Found. Symp. Wiley Online Library. 1997:208–219. doi: 10.1002/9780470515280.ch14. [DOI] [PubMed] [Google Scholar]
- Pringle K., et al. The expression and localization of the human placental prorenin/renin-angiotensin system throughout pregnancy: Roles in trophoblast invasion and angiogenesis? Placenta. 2011;32:956–962. doi: 10.1016/j.placenta.2011.09.020. [DOI] [PubMed] [Google Scholar]
- Prompetchara E., et al. Immune responses in covid-19 and potential vaccines: lessons learned from sars and mers epidemic. Asian Pac. J. Allergy Immunol. 2020;38:1–9. doi: 10.12932/AP-200220-0772. [DOI] [PubMed] [Google Scholar]
- Raingeaud J., Pierre J. Interleukin-4 downregulates tnfα-induced il-8 production in keratinocytes. FEBS Lett. 2005;579:3953–3959. doi: 10.1016/j.febslet.2005.06.019. [DOI] [PubMed] [Google Scholar]
- Reis A.B., et al. Angiotensin (1–7) and its receptor mas are expressed in the human testis: implications for male infertility. J. Mol. Histol. 2010;41:75–80. doi: 10.1007/s10735-010-9264-8. [DOI] [PubMed] [Google Scholar]
- Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (covid-19) outbreak. J. Autoimmun. 2020 doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothe C., et al. Transmission of 2019-ncov infection from an asymptomatic contact in germany. N. Engl. J. Med. 2020;382:970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathishkumar Vinayagam S.K. Sars-cov-2 and coagulation disorders in different organs. Life Sci. 2020 doi: 10.1016/j.lfs.2020.118431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler C., et al. Jak-stat signaling: from interferons to cytokines. J. Biol. Chem. 2007;282:20059–20063. doi: 10.1074/jbc.R700016200. [DOI] [PubMed] [Google Scholar]
- Schroeder M., et al. The majority of male patients with covid-19 present low testosterone levels on admission to intensive care in hamburg, germany: A retrospective cohort study. medRxiv. 2020 [Google Scholar]
- Schuerwegh A., et al. Influence of pro-inflammatory (il-1α, il-6, tnf-α, ifn-γ) and anti-inflammatory (il-4) cytokines on chondrocyte function. Osteoarthr. Cartil. 2003;11:681–687. doi: 10.1016/s1063-4584(03)00156-0. [DOI] [PubMed] [Google Scholar]
- Seder R.A. Acquisition of lymphokine-producing phenotype by cd4+ t cells. J. Allergy Clin. Immunol. 1994;94:1195–1202. doi: 10.1016/0091-6749(94)90332-8. [DOI] [PubMed] [Google Scholar]
- Shen Q., et al. The ace2 expression in sertoli cells and germ cells may cause male reproductive disorder after sars‐cov‐2 infection. J. Cell. Mol. Med. 2020 doi: 10.1111/jcmm.15541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H.-S., et al. Immune responses to middle east respiratory syndrome coronavirus during the acute and convalescent phases of human infection. Clin. Infect. Dis. 2019;68:984–992. doi: 10.1093/cid/ciy595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqi H.K., Mehra M.R. Covid-19 illness in native and immunosuppressed states: a clinical–therapeutic staging proposal. J. Heart Lung Transplant. 2020;39:405. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So E.-Y., et al. Ras/erk pathway positively regulates jak1/stat6 activity and il-4 gene expression in jurkat t cells. Mol. Immunol. 2007;44:3416–3426. doi: 10.1016/j.molimm.2007.02.022. [DOI] [PubMed] [Google Scholar]
- Song C., et al. Detection of 2019 novel coronavirus in semen and testicular biopsy specimen of covid-19 patients. medRxiv. 2020 [Google Scholar]
- Sun B., Yeh J. Mild and asymptomatic covid-19 infections: implications for maternal, fetal, and reproductive health. Front. Reprod. Health. 2020;2 doi: 10.3389/frph.2020.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syriou V., et al. Cytokines and male infertility. Eur. Cytokine Netw. 2018;29:73–82. doi: 10.1684/ecn.2018.0412. [DOI] [PubMed] [Google Scholar]
- Szabo S.J., et al. Developmental commitment to the th2 lineage by extinction of il-12 signaling. Immunity. 1995;2:665–675. doi: 10.1016/1074-7613(95)90011-x. [DOI] [PubMed] [Google Scholar]
- Tanaka S., et al. The enhancer hs2 critically regulates gata-3-mediated il4 transcription in th 2 cells. Nat. Immunol. 2011;12:77–85. doi: 10.1038/ni.1966. [DOI] [PubMed] [Google Scholar]
- Tay M.Z., et al. The trinity of covid-19: immunity, inflammation and intervention. Nat. Rev. Immunol. 2020:1–12. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng C.-T.K., et al. Severe acute respiratory syndrome and the innate immune responses: modulation of effector cell function without productive infection. J. Immunol. 2005;174:7977–7985. doi: 10.4049/jimmunol.174.12.7977. [DOI] [PubMed] [Google Scholar]
- Tveito K. Cytokine storms in covid-19 cases? Tidsskr. Den Nor. Legeforening. 2020 doi: 10.4045/tidsskr.20.0239. [DOI] [PubMed] [Google Scholar]
- Üçeyler N., et al. Reduced levels of antiinflammatory cytokines in patients with chronic widespread pain. Arthritis Rheum. 2006;54:2656–2664. doi: 10.1002/art.22026. [DOI] [PubMed] [Google Scholar]
- Valdes G., et al. Distribution of angiotensin-(1-7) and ace2 in human placentas of normal and pathological pregnancies. Placenta. 2006;27:200–207. doi: 10.1016/j.placenta.2005.02.015. [DOI] [PubMed] [Google Scholar]
- Vaz-Silva J., et al. The vasoactive peptide angiotensin-(1–7), its receptor mas and the angiotensin-converting enzyme type 2 are expressed in the human endometrium. Reprod. Sci. 2009;16:247–256. doi: 10.1177/1933719108327593. [DOI] [PubMed] [Google Scholar]
- Vellingiri B., et al. Covid-19: a promising cure for the global panic. Sci. Total Environ. 2020 doi: 10.1016/j.scitotenv.2020.138277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venugopal A., et al. Novel wastewater surveillance strategy for early detection of covid–19 hotspots. Curr. Opin. Environ. Sci. Health. 2020 doi: 10.1016/j.coesh.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicari E., Calogero A. Effects of treatment with carnitines in infertile patients with prostato-vesiculo-epididymitis. Hum. Reprod. 2001;16:2338–2342. doi: 10.1093/humrep/16.11.2338. [DOI] [PubMed] [Google Scholar]
- Villarino A.V., et al. Mechanisms and consequences of jak–stat signaling in the immune system. Nat. Immunol. 2017;18:374. doi: 10.1038/ni.3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Xu X. Scrna-seq profiling of human testes reveals the presence of the ace2 receptor, a target for sars-cov-2 infection in spermatogonia, leydig and sertoli cells. Cells. 2020;9:920. doi: 10.3390/cells9040920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., et al. Il-4-mediated transcriptional regulation of human cyp2e1 by two independent signaling pathways. Biochem. Pharmacol. 2010;80:1592–1600. doi: 10.1016/j.bcp.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Wang W., et al. Updated understanding of the outbreak of 2019 novel coronavirus (2019‐ncov) in wuhan, china. J. Med. Virol. 2020;92:441–447. doi: 10.1002/jmv.25689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilder-Smith A., et al. Asymptomatic sars coronavirus infection among healthcare workers, singapore. Emerg. Infect. Dis. 2005;11:1142. doi: 10.3201/eid1107.041165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilder-Smith A., et al. Can we contain the covid-19 outbreak with the same measures as for sars? Lancet Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30129-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills-Karp M., Finkelman F.D. Untangling the complex web of il-4–and il-13–mediated signaling pathways. Sci. Signal. 2008;1:pe55. doi: 10.1126/scisignal.1.51.pe55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C., et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin. Exp. Immunol. 2004;136:95–103. doi: 10.1111/j.1365-2249.2004.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., Mcgoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (covid-19) outbreak in china: summary of a report of 72 314 cases from the chinese center for disease control and prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- Wu F., et al. A new coronavirus associated with human respiratory disease in china. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., et al. Orchitis: a complication of severe acute respiratory syndrome (sars) Biol. Reprod. 2006;74:410–416. doi: 10.1095/biolreprod.105.044776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., et al. Covid-19: immunopathogenesis and immunotherapeutics. Signal Transduct. Target. Ther. 2020;5:1–8. doi: 10.1038/s41392-020-00243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yigitbasi T., et al. Eotaxin and interleukin-4 levels and their relation to sperm parameters in infertile men/infertil erkeklerde eotaksin ve interlökin-4 düzeyleri ve sperm parametreleri ile iliskisi. Türkiye Klinikleri. Tip Bilimleri Dergisi. 2010;30:1441. [Google Scholar]
- Yu Y., Chen P. Coronavirus disease 2019 (covid-19) in neonates and children from china: a review. Front. Pediatr. 2020;8:287. doi: 10.3389/fped.2020.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Gao J. Determination of il-1beta, il-4 and il-10 contents in the seminal plasma of infertile patients and its clinical value. Zhonghua nan ke xue. National journal of andrology. 2004;10:851–854. [PubMed] [Google Scholar]
- Zhao J., et al. Clinical pathology and pathogenesis of severe acute respiratory syndrome. Zhonghua shi yan he lin chuang bing du xue za zhi= Zhonghua shiyan he linchuang bingduxue zazhi. Chin. J. Exper. Clin. Virol. 2003;17:217–221. [PubMed] [Google Scholar]
- Zou X., et al. Single-cell rna-seq data analysis on the receptor ace2 expression reveals the potential risk of different human organs vulnerable to 2019-ncov infection. Front. Med. 2020:1–8. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]