Abstract
A 13-year-old boy reported acute horizontal binocular diplopia and headache. Ten days before these symptoms he suffered from a gastrointestinal infection. Ophthalmological examination revealed bilateral ophthalmoparesis and diffuse hyporeflexia. Magnetic resonance imaging of the brain was normal. Lumbar puncture revealed albumin-cytological dissociation. There were no anti-GQ1b antibodies, but serum anti-GM1 antibodies were detected. He received intravenous immunoglobulins and had fully recovered two weeks later. Miller Fisher syndrome and its atypical variants are uncommon in childhood; nevertheless, they should be considered in the differential diagnosis of bilateral acute ophthalmoparesis.
Keywords: acute ophthalmoparesis, Miller Fisher syndrome, anti-GQ1b antibody syndromes, antiganglioside antibodies, bilateral sixth nerve palsy, childhood diplopia
(In keeping with the format of a clinical pathologic conference, the abstract and key words appear at the end of the article.)
1. Case report
A 13-year-old boy was referred for acute horizontal binocular diplopia. His psychomotor development was normal, and his past medical history was unremarkable. He denied head trauma or exposure to toxins. Ten days before, he had experienced diarrhea and a low-grade fever, as well as asthenia. On admission, temperature was 99.3°F, and other vital signs were normal. Cardiopulmonary auscultation and abdominal examination were normal.
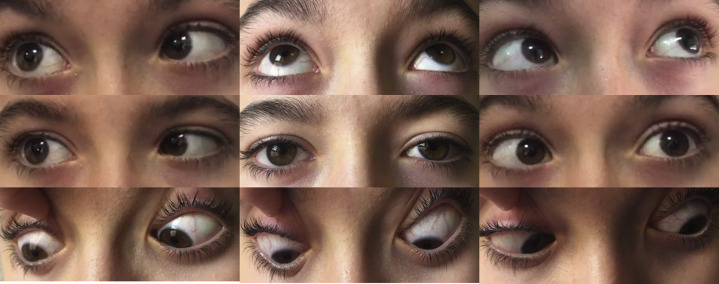
His visual acuity was 20/20 in each eye. Ocular examination revealed a dilated pupil that reacted poorly to light in the left eye. No relative afferent pupillary defect was observed. There was a complex pattern of extrinsic ocular motility abnormalities. He had an incomitant esophoria measuring 12 prism diopters at distance and 4 prism diopters at near associated with limited abduction of both eyes. In addition, there was a left ptosis (Fig. 1 ). He did not relate diurnal variation. Slit lamp biomicroscopy, intraocular pressure, and funduscopy were normal in both eyes.
What is your initial diagnosis? What is the differential diagnosis at this point? What additional examinations and ancillary test would your order?
Fig. 1.
Ocular motility examination. Limitation of abduction in both eyes with mild internal ophthalmoplegia and ptosis in the left eye.
2. Comments by R. Michael Siatkowski, MD
This patient presents with acute onset of bilateral ophthalmoplegia after a presumed viral illness. The photo shows clear abduction deficits in each eye, as well as an adduction deficit in the right eye. Left-sided ptosis and anisocoria are not easily appreciable in these pictures, but we are told that there is an efferent pupillary defect OS.
Bilateral diffuse ophthalmoplegia, whether complete or incomplete, may result from problems anywhere from the brainstem to the neuromuscular junction. From a topical diagnosis standpoint, these include intrinsic brainstem disease such as stroke or demyelination; infectious or noninfectious meningitis; ischemic, inflammatory, or autoimmune disorders of the cranial nerves; cavernous sinus or orbital lesions; and myasthenia gravis or botulism.
We are not told whether this patient has any motor or sensory problems that could occur with brainstem or autoimmune disease, but he has no evidence of optic neuropathy, proptosis, or other signs that would be expected with orbital involvement. The presence of anisocoria and/or abnormally reacting pupils is not consistent with myasthenia. Wernicke disease or other vitamin deficiency may present similarly, but this patient's history (or lack thereof) makes it unlikely. Diabetes mellitus has rarely been reported to cause bilateral simultaneous cranial neuropathies, but this is generally in type 2 patients who are several decades older than this patient.
Thus, the differential at this point is brainstem disease, some type of meningitis, a cavernous sinus mass, or inflammatory/autoimmune cranial neuropathies. In a previously healthy teenage boy, the most common entities would be a low-grade brainstem tumor, a parasellar mass (craniopharyngioma, pituitary adenoma) extending into the cavernous sinus, or parainfectious cranial nerve inflammation (idiopathic postviral, Miller Fisher syndrome [MFS] or variant thereof). The appropriate initial workup would consist of neurologic examination, magnetic resonance imaging of the brain and posterior fossa with and without gadolinium, and lumbar puncture. If there is any question that the anisocoria may be physiologic or the asymmetric pupillary reactivity is minimal, serum acetylcholine receptor antibodies should be drawn as well (or, alternatively, an edrophonium or prostigmine test if available), as myasthenia is always a great masquerader.
3. Case report (continued)
The clinical diagnosis of incomplete left third nerve with pupillary involvement and bilateral abducens nerve and palsies was made.
Neurological examination revealed no abnormalities in consciousness or corticospinal tract signs. There was no dysmetria, ataxia, limb muscles weakness, or sensory deficits. Deep tendon reflexes were diminished. Cranial nerve examination was normal except the previously described ocular motility disturbances.
Brain and orbit magnetic resonance imaging were unremarkable. Routine biochemistry and hemogram tests were also normal. Serologic tests for syphilis, tuberculosis, Borrelia, cytomegalovirus, Epstein-Barr virus, herpes simplex virus type 1 and 2, varicella-zoster virus, and adenovirus were negative. Edrophonium testing was also negative.
What supplemental testing would you order? What would be the final diagnosis? Is there any treatment available?
4. Comments by R. Michael Siatkowski … (continued)
With normal neuroimaging and a negative edrophonium test, autoimmune/inflammatory cranial neuropathies rise to the top of the differential diagnosis. As the patient is systemically well, infectious meningitis is unlikely, but lumbar puncture is required to assess for noninfectious inflammation. The abnormal deep tendon reflexes in the setting of parainfectious multiple cranial neuropathies make the Miller Fisher variant of Guillain-Barre syndrome a likely diagnosis. This syndrome likely results from diffuse cross-reactivity of antimicrobial or antiviral antibodies with neural sheathes and/or axons. Although ataxia is present in the classic cases, it does not always occur or may be extremely subtle or short-lived; thus, the eponym's triad of ophthalmoplegia, areflexia or hyporeflexia, and ataxia are better considered as a spectrum of clinical findings rather than an obligate requisite diagnostic combination. Serum GQ1b antibodies are present in 80–90% of cases, but there are reports of antibodies to a number of other gangliosides, so a more comprehensive panel should be performed if the GQ1b testing is negative.
Although there are no clinical trials to support any treatment strategies, management is similar to that for classic Guillain-Barre syndrome, namely intravenous immunoglobulins or plasmapheresis. Steroids have not been proven to be useful in Guillain-Barre syndrome, so they would not be appropriate in this case. Fortunately, the prognosis for these patients is generally good, with improvement beginning a few weeks after symptom onset and complete or near-complete recovery within several months. Relapses occur only in 2–3% of patients.
5. Case report (continued)
A spinal tap was performed. The opening pressure was 11 cm of H20. Cerebrospinal fluid content revealed no cells, slightly elevated protein (54 mg/dl), glucose of 67 mg/dl, sterile cultures, and negative serologies. Full ganglioside antibodies serum test was performed (GM1, GM2, GM3, GD1a, GD1b, GD3, GT1a, GT1b, GQ1b, and antisulfatide antibodies). The patient had IgM antibodies against GM1. Electromyography did not show nerve conduction abnormalities.
The patient was diagnosed with acute ophthalmoparesis without ataxia, a variant of MFS. Our patient had bilateral abducens nerve palsy plus an incomplete third cranial nerve palsy with pupillary involvement presumably related to a prior gastrointestinal infection, associated with anti-GM1 autoantibody.
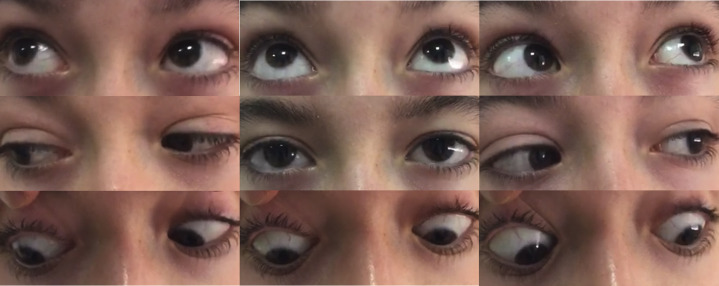
Intravenous immunoglobulin therapy was started, 400 mg/kg daily for 5 days. He experienced progressive and complete recovery of ocular motility deficits and deep tendon reflexes (Fig. 2 ).
Fig. 2.
After treatment, there was complete recovery of extraocular motility.
6. Discussion
The clinical spectrum of the anti-GQ1b antibody syndrome encompasses a broad group of polyneuropathies with overlapping clinical features. The acute onset of the triad of extrinsic ophthalmoplegia, ataxia, and hypoeflexia or aeflexia characterizes MFS.5 Clinical variants of anti-GQ1b antibody syndrome include MFS—complete and atypical forms—Guillain-Barré syndrome, Bickerstaff brainstem encephalitis, or polyneuritis cranialis.14 , 21 , 25 These conditions are uncommon during childhood.18 , 19 Nearly 90% of the reported cases have serum anti-GQ1b antibody titers, which seems to be closely related to ophthalmoplegia.3 , 6
Polyneuropathies are preceded by upper respiratory tract infection in 75% of patients and gastrointestinal infection in only the 4%.17 , 20 , 22 The most common pathogen related to MFS is Campylobacter jejuni.13 , 28 , 30 Other infectious agents also related are cytomegalovirus, parvovirus,1 and Haemophilus influenzae.26 Recently severe acute respiratory syndrome coronavirus-2 infection has been reported to produce an acute Miller Fisher–like syndrome.8
Acute ophthalmoplegia accounts for an incomplete form of MFS that might also be associated with hyporeflexia or areflexia.17 , 29 Diagnosis is generally based on clinical features, lack of abnormalities in consciousness or corticospinal tract signs, absence of limb weakness, monophasic illness pattern, and the absence of any identifiable alternative diagnosis. Moreover, there may be supportive findings such as cerebrospinal fluid albumin-cytological dissociation, electrophysiological abnormalities, and serum anti-GQ1b autoantibodies.25 Just few cases have been previously reported in adults,29 even less in childhood.7 , 10 , 11 , 15 , 23 , 24 , 27 There were serum anti-GQ1b antibodies in all the cases, and occasionally antibodies against ganglioside complex.
Antiganglioside antibodies are strongly related to MFS, being present in approximately 80% of patients.3 They induce an autoimmune cross-reaction due to a molecular similarity between host ganglioside protein and surface pathogen epitopes.9 , 30 Clinical manifestations of spectrum anti-GQ1b syndrome depend on the different expression sites of the target molecules in the nervous system. GQ1b and GT1a antigens are mainly located in paranodal regions of the extramedullary portion of the oculomotor, trochlear, and abducens cranial nerves.2 , 6 Fukami and coworkers described a significant association between anti-GQ1b or anti-GT1a titers and ophthalmoplegia, but it can also appear related to other ganglioside autoantibodies, as in the present case.6 On the other hand, anti-GM1, anti-GM1b, and anti-GD1 have been related to limb weakness.6 MFS and its variants are usually self-limiting, and spontaneous recovery without residual deficits occurs in most cases; however, medical supportive care may be necessary. Corticosteroids, intravenous immunoglobulin, or plasmapheresis are recommended to speed up the recovery of symptoms.
Acute ophthalmoplegia in childhood is a mayor diagnostic challenge for clinicians. The most frequent clinical picture is bilateral abducens nerve palsy.17 , 29 It also may present as pure horizontal, pure vertical, or with mixed palsy pattern, as intrinsic ophthalmoplegia, or ptosis without limited eyed movements. Anti-GQ1b or anti-GT1a have been identified in all cases. In addition, serum anti-GM1 was also detected in two cases.1 , 4 Our patient would be the first described one associated with isolated anti-GM1 positive titers.
Some potentially dangerous diseases may present with multiple or bilateral acute nerve palsies. Therefore, pituitary apoplexy, mesencephalic ischemia, neuromuscular junction disorders, cavernous sinus disease, trauma, drug toxicity, infections, metastatic or paraneoplastic diseases, and autoimmune diffuse polyneuropathies should be ruled out.12 , 16
To conclude, MFS and especially its atypical forms may be potentially severe and challenging to diagnosis, so a high clinical suspicion is needed. This case underlines the relevance of a complete screening of all the antiganglioside antibody complex.
7. Method of literature search
PubMed literature search was performed for relevant articles related to bilateral abducens palsy and Miller Fisher spectrum disorders, using the following terms: Miller Fisher syndrome, acute ophthalmoparesis without ataxia, anti-GQ1b antibody syndromes, and antiganglioside antibodies. Additional articles were collected from the reference list of articles previously obtained from the original PubMed literature search.
8. Disclosure
Declaration of conflicting interests.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Author contributions: R.-R.S. and G.-O.C. contributed to conceptualization, supervision, validation, writing the original draft, and reviewing and editing the same. M.S., O.-C.J.V., and S.R.M. contributed to supervision, validation, and reviewing and editing the article.
Peter Savino and Helen Danesh-Meyer, Editors
References
- 1.Canavese C., Mancini S., Tocchet A., et al. Acute unilateral ophthalmoparesis associated with anti-GQ1b and GM1 antibodies after parvovirus infection in a 10-year-old girl. Eur J Paediatr Neurol. 2018;22(1):213–214. doi: 10.1016/j.ejpn.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Chiba A., Kusunoki S., Obata H., et al. Serum anti-GQ1b IgG antibody is associated with ophthalmoplegia in Miller Fisher syndrome and Guillain-Barré syndrome: clinical and immunohistochemical studies. Neurology. 1993;43(10):1911–1917. doi: 10.1212/wnl.43.10.1911. [DOI] [PubMed] [Google Scholar]
- 3.Chiba A., Kusunoki S., Shimizu T., et al. Serum IgG antibody to ganglioside GQ1b is a possible marker of Miller Fisher syndrome. Ann Neurol. 1992;31(6):677–679. doi: 10.1002/ana.410310619. [DOI] [PubMed] [Google Scholar]
- 4.De Bruyn A., Poesen K., Bossuyt X., et al. Clinical spectrum of the anti-GQ1b antibody syndrome: a case series of eight patients. Acta Neurol Belg. 2019;119(1):29–36. doi: 10.1007/s13760-019-01093-8. [DOI] [PubMed] [Google Scholar]
- 5.Fisher M. An unusual variant of acute idiopathic polyneuritis (syndrome of ophthalmoplegia, ataxia and areflexia) N Engl J Med. 1956;255(2):57–65. doi: 10.1056/NEJM195607122550201. [DOI] [PubMed] [Google Scholar]
- 6.Fukami Y., Wong A.H., Funakoshi K., et al. Anti-GQ1b antibody syndrome: anti-ganglioside complex reactivity determines clinical spectrum. Eur J Neurol. 2016;23(2):320–326. doi: 10.1111/ene.12769. [DOI] [PubMed] [Google Scholar]
- 7.Guisset F., Ferreiro C., Voets S., et al. Anti-GQ1b antibody syndrome presenting as acute isolated bilateral ophthalmoplegia: report on two patients and review of the literature. Eur J Paediatr Neurol. 2016;20(3):439–443. doi: 10.1016/j.ejpn.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Gutiérrez-Ortiz C., Méndez A., Rodrigo-Rey S., et al. Miller Fisher syndrome and polyneuritis cranialis in COVID-19. Neurology. 2020;95(5):e601–e605. doi: 10.1212/WNL.0000000000009619. [DOI] [PubMed] [Google Scholar]
- 9.Jacobs B., Endtz H., van der Meché F., et al. Serum anti-GQ1b IgG antibodies recognize surface epitopes on Campylobacter jejuni from patients with Miller Fisher syndrome. Ann Neurol. 1995;37(2):260–264. doi: 10.1002/ana.410370218. [DOI] [PubMed] [Google Scholar]
- 10.Jindal G., Parmar V.R., Gupta V.K. Isolated ptosis as acute ophthalmoplegia without ataxia, positive for anti-GQ1b immunoglobulin G. Pediatr Neurol. 2009;41(6):451–452. doi: 10.1016/j.pediatrneurol.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 11.Kauser H., Jain P., Sharma S., Aneja S. Complete bilateral ophthalmoplegia with unilateral facial palsy in a child with anti-GQ1b syndrome. Indian J Pediatr. 2015;82(2):192–194. doi: 10.1007/s12098-014-1514-4. [DOI] [PubMed] [Google Scholar]
- 12.Keane J.R. Bilateral ocular paralysis. Arch Neurol. 2007;64(2):178. doi: 10.1001/archneur.64.2.178. [DOI] [PubMed] [Google Scholar]
- 13.Koga M., Gilbert M., Li J., et al. Antecedent infections in Fisher syndrome: A common pathogenesis of molecular mimicry. Neurology. 2005;64(9):1605–1611. doi: 10.1212/01.WNL.0000160399.08456.7C. [DOI] [PubMed] [Google Scholar]
- 14.Koga M., Kishi M., Fukusako T., et al. Antecedent infections in Fisher syndrome: sources of variation in clinical characteristics. J Neurol. 2019;266(7):1655–1662. doi: 10.1007/s00415-019-09308-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuroki S., Saida T., Nukina M., et al. Three patients with ophthalmoplegia associated with Campylobacter jejuni. Pediatr Neurol. 2001;25(1):71–74. doi: 10.1016/s0887-8994(01)00281-8. [DOI] [PubMed] [Google Scholar]
- 16.Lee M.S., Galetta S.L., Volpe N.J., Liu G.T. Sixth nerve palsies in children. Pediatr Neurol. 1999;20(1):49–52. doi: 10.1016/s0887-8994(98)00090-3. [DOI] [PubMed] [Google Scholar]
- 17.Lee S.H., Lim G.H., Kim J.S., et al. Acute ophthalmoplegia (without ataxia) associated with anti-GQ1b antibody. Neurology. 2008;71(6):426–429. doi: 10.1212/01.wnl.0000324266.95814.74. [DOI] [PubMed] [Google Scholar]
- 18.Lin J.J., Hsia S.H., Wang H.S., et al. Clinical variants of Guillain-Barré syndrome in children. Pediatr Neurol. 2012;47(2):91–96. doi: 10.1016/j.pediatrneurol.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 19.Lo Y.L. Clinical and immunological spectrum of the Miller Fisher syndrome. Muscle Nerve. 2007;36(5):615–627. doi: 10.1002/mus.20835. [DOI] [PubMed] [Google Scholar]
- 20.Mori M., Kuwabara S., Fukutake T., et al. Clinical features and prognosis of Miller Fisher syndrome. Neurology. 2001;56(8):1104–1106. doi: 10.1212/wnl.56.8.1104. [DOI] [PubMed] [Google Scholar]
- 21.Odaka M., Yuki N., Hirata K. Anti-GQ1b IgG antibody syndrome: clinical and immunological range. J Neurol Neurosurg Psychiatry. 2001;70(1):50–55. doi: 10.1136/jnnp.70.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pellegrini F., Prosdocimo G., Barton J.J. The pescatorial sixth. Surv Ophthalmol. 2016;61(2):248–254. doi: 10.1016/j.survophthal.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 23.Sato K., Yoshikawa H. Bilateral abducens nerve paresis associated with anti-GQ1b IgG antibody. Am J Ophthalmol. 2001;131(6):816–818. doi: 10.1016/s0002-9394(00)00956-9. [DOI] [PubMed] [Google Scholar]
- 24.Vanden Eijnden S., Borza D., Caudron V., Cavatorta E. Isolated 6th nerve palsy in a child associated with asymptomatic Chlamydia pneumoniae infection and elevated anti-GQ1b antibody. Eur J Pediatr. 2001;160(6):400. doi: 10.1007/s004310100742. [DOI] [PubMed] [Google Scholar]
- 25.Wakerley B.R., Uncini A., Yuki N. Guillain-Barré and miller fisher syndromes - New diagnostic classification. Nat Rev Neurol. 2014;10(9):537–544. doi: 10.1038/nrneurol.2014.138. [DOI] [PubMed] [Google Scholar]
- 26.Wakerley B.R., Yuki N. Polyneuritis cranialis—subtype of Guillain–Barré syndrome? Nat Rev Neurol. 2015;11:664. doi: 10.1038/nrneurol.2015.115. [DOI] [PubMed] [Google Scholar]
- 27.Weber P., Regeniter A., Kaiser H. Acute ophthalmoparesis in a child with anti-GQ1 b IgG antibody. Neuropediatrics. 2003;34(5):274–275. doi: 10.1055/s-2003-43253. [DOI] [PubMed] [Google Scholar]
- 28.Yuki N., Koga M. Bacterial infections in Guillain-Barré and Fisher syndromes. Curr Opin Neurol. 2006;19(5):451–457. doi: 10.1097/01.wco.0000245367.36576.e9. [DOI] [PubMed] [Google Scholar]
- 29.Yuki N., Odaka M., Hirata K. Acute ophthalmoparesis (without ataxia) associated with anti-GQ1b IgG antibody: Clinical features. Ophthalmology. 2001;108(1):196–200. doi: 10.1016/s0161-6420(00)00420-6. [DOI] [PubMed] [Google Scholar]
- 30.Yuki N., Taki T., Takahashi K., et al. Molecular mimicry between GQ1b ganglioside and lipopolysaccharides of Campylobacter jejuni isolated from patients with Fisher's syndrome. Ann Neurol. 1994;36(5):791–793. doi: 10.1002/ana.410360517. [DOI] [PubMed] [Google Scholar]